Abstract
The purpose of this study was to evaluate the biomechanical effect of a hat type cervical intervertebral fusion cage (HCIFC). In this in vitro biomechanical study, 48 goat cervical spines (C2-5) were tested in flexion, extension, axial rotation, and lateral bending with a nondestructive stiffness method using a nonconstrained testing apparatus, and three-dimensional displacement was measured. Autologous iliac bone and cervical spine intervertebral fusion cage were implanted according to manufacturers’ information after complete discectomy (C3-4). Eight spines in each of the following groups were tested: intact, autologous iliac bone graft, Harms cage, SynCage C, carbon cage, and HCIFC. The mean apparent stiffness values were calculated from the corresponding load-displacement curves. Additionally, cage volume and volume-related stiffness were determined. The stiffness of the SynCage C was statistically greatest in all directions. After implantation of the HCIFC, flexion stiffness increased compared with that of the intact motion segment. There was no significant difference in stiffness between the HCIFC and carbon cage. The stiffness of the HCIFC was statistically higher than that of the Harms cage in axial rotation and significantly lower in flexion, extension, and lateral bending. Volume-related stiffness of all cages was higher than that of iliac bone graft. The Harms cage was highest in volume-related stiffness in all directions. The HCIFC can provide enough primary stability for cervical intervertebral fusion.
Résumé
Le but de cette étude est d’évaluer les effets biomécaniques de HCIFC. Méthode: une étude biomécanique a été conduite sur 48 colonnes cervicales de chèvres de C2 à C5, testées en flexion extension, rotation axiale, bending avec mesure des déplacements tridimensionnels. Une greffe d’os iliaque, une cage intervétébrale ont été implantés pour discectomie (C3-C4). 8 colonnes dans chaque groupe ont été testées. Résultats: la résistance de la Syncage C est statistiquement plus importante dans toutes les directions après l’implantation de la HCIFC raideur en flexion, la résistance en flexion est augmentée si on la compare à un segment atteint. Il n’y a pas de différence dans la résistance entre la HCIFC et la cage en carbone. La résistance de la HCIFC est statistiquement plus importante que la Harms cage en rotation axiale et significativement moins importante en flexion, extension et en bending latéral. La résistance est plus importante avec toutes les cages si on la compare à une greffe iliaque. La Harms cage semble donner le meilleur résultat dans toutes les directions. En conclusion: la HCIFC semble donner une bonne stabilité primaire pour une fusion inter cervical vertébral.
Introduction
Anterior decompression and interbody fusion are widely accepted as surgical treatments for patients with cervical spondylosis. Tricortical iliac crest bone graft, formerly the gold standard, is associated with high donor site morbidity. Additional problems such as pseudarthrosis, graft collapse with kyphotic deformity, and graft extrusion have led to a rapid increase in the use of a cervical spine interbody fusion cage (CSIFC) as an adjunct to spondylodesis [1–8]. Interbody fusion cages are being developed in the quest for interspace structural stability during bony fusion. They have been promoted with the aim of providing immediate strong anterior column support. Several interbody designs have been developed. According to Weiner and Fraser [8], these designs can be subdivided into three groups: screw (horizontal cylinder), box, or cylinder (vertical ring) designs. Kandziora et al. [6] found that the cages with box and cylinder designs are biomechanically better than cages with a screw design. The aim of this study was to evaluate the biomechanical effect of a hat type cervical intervertebral fusion cage (HCIFC) by comparing it to a tricortical iliac bone graft, Harms cage, SynCage C, and carbon cage.
Materials and methods
Spine preparation In this study, 48 cervical spines (C2-C5) of 2-year-old adult male goats were used for biomechanical testing. En bloc specimens were stored at −20°C until they were thawed in a water bath at 25°C. The motion segment C3-C4 was isolated and superficial musculature was removed. Care was taken to preserve all ligaments. Each specimen was radiographically screened to exclude abnormalities that might compromise the mechanical properties of the goat cervical spine.To simulate essential clinical features, a complete discectomy of C3-C4 with resection of the anterior longitudinal ligament was performed. The end plates were shaved using a curette. Cervical interbody fusion cages were implanted according to the manufacturers’ information.
Cervical spine interbody fusion cages Based on a previous study, we designed a new kind of cage named the hat type cervical intervertebral fusion cage (HCIFC) shown in Fig. 1. The HCIFC made of Polyetheretherketone (PEEK) is box-shaped with superior and inferior oval surfaces and wedge-like with anterior height greater than posterior. Superior and inferior hat edges are extended thus increasing the surface area and decreasing the thickness of the wall. There are two titanium needles at the diagonal corner of the cage to judge the position. The HCIFC is divided into three sizes of 16 mm/14 mm, 14 mm/12 mm, and 12 mm/10 mm according to width/depth and three sizes of 6 mm, 7 mm, and 8 mm according to height. Cages are shown in Fig. 2. The height, width, and depth of the CSIFCs used in this study are depicted in Table 1. To allow comparison between the different cage designs, cages of similar height, width, and depth were used. The volumes of the cages were determined by a water displacement technique, according to the Archimedes principle.
Fig. 1.
Superior, lateral, and interior view of the HCIFC
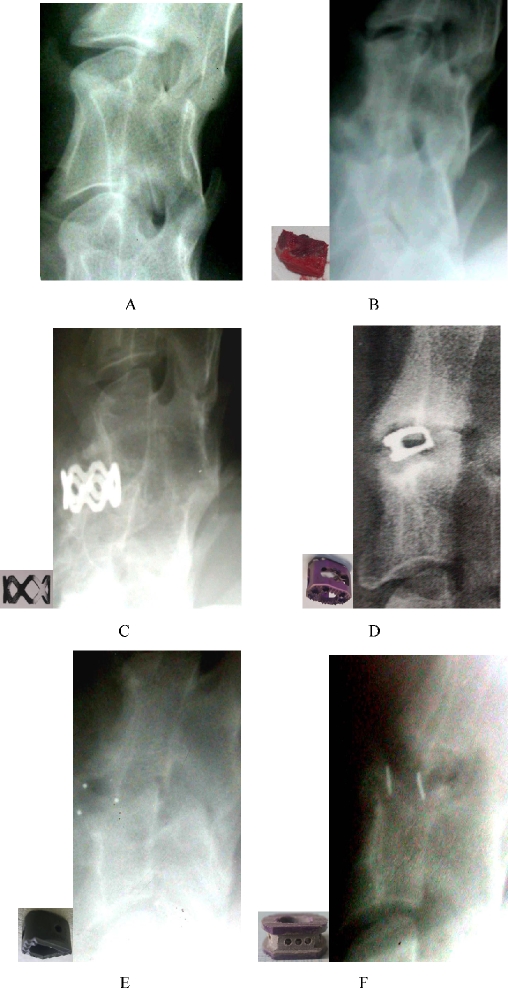
Fig. 2.
Intact cervical spine, iliac bone graft, and cages. a Intact cervical spine. b Iliac bone graft. c Harms cage. d SynCage C. e Carbon cage. f HCIFC
Table 1.
Height, width, and depth of the cages
| Group | Cage | Type | Company | Material | Height (mm) | Width (mm) | Depth (mm) |
|---|---|---|---|---|---|---|---|
| B | Iliac bone graft | – | – | Bone | 8 | 14 | 14 |
| C | Harms cage | Cylinder | DePuy AcroMed | Titanium | 8 | 14 | 14 |
| D | SynCage C | Box | AO | Titanium | 7 | 15 | 12 |
| E | Carbon cage | Box | DePuy AcroMed | Carbon | 8 | 16 | 13 |
| F | HCIFC | Box | – | PEEK | 8 | 14 | 12 |
Test setup Testing was performed by a nondestructive flexibility method using a nonconstrained testing apparatus described elsewhere in detail [9]. Pure bending moments were applied using a system of cables and pulleys to induce flexion, extension, left and right lateral bending, and left and right axial rotation, correspondingly. Tension was applied to the cables with a uniaxial testing machine (Instron 4411, High Wycombe, UK). Moments were calculated by multiplying the applied force by the radius of the pulley on the spine-testing fixture.Three-dimensional displacement of each motion segment was measured using a protractor. Two marker points were made on C3 and C4. Angular displacement of the upper vertebrae in relation to the lower vertebrae was determined from a marker position.
Study protocol Eight spines were assigned randomly to be tested in each of the following groups: intact, autologous tricortical iliac bone graft, Harms cage (titanium mesh cylinder, DePuy AcroMed, Raynham, MA, USA), SynCage C (titanium box, Synthes, Paoli, PA, USA), carbon cage (carbon box, DePuy AcroMed, Raynham, MA, USA), and HCIFC (PEEK box).Specimens were kept moist during tests. For testing, C2 and C5 were mounted in pots using polymethylmethacrylate. The lower pot was attached rigidly to the base of the testing apparatus. This test setup resulted in a compressive preload of 25 N from the weight of the upper fixation pot, which represents the average weight of the goat head. Moments were applied in a quasistatic manner in increments of 1–6 Nm. At each step, to minimise viscoelastic response, the specimen was allowed to relax for 60 s before data were recorded. Test modes were flexion, extension, left and right axial rotation, and left and right lateral bending. Specimens were preconditioned using three cycles of 6-Nm load with a 1.2-mm/s velocity of the traverse bar. The fourth cycle was measured. The mean apparent stiffness values in the elastic zone were calculated from the corresponding load-displacement curves. Volume-related stiffness was determined by dividing stiffness results by cage volume.
Statistical analysis Statistical analysis was performed using the analysis of variance (ANOVA) Tukey test. Statistically significant differences were defined at a 95% confidence level. The values are given as mean±standard deviation. Statistical evaluation was supported by SPSS software (8.0).
Results
Volume of cages
The volume of autologous tricortical iliac bone was 1.44±0.01 cm3, Harms cage 0.1±0.01 cm3, and SynCage C 0.26±0.01 cm3. The volume of the carbon cage was 0.28±0.01 cm3 and HCIFC 0.23±0.01 cm3.
Stiffness tests
Table 2 summarises the stiffness of the CSIFCs normalised with respect to the intact motion segment during flexion, extension, rotation, and bending. The stiffness of SynCage C was significantly the greatest in all directions (p<0.05). No significant difference was found between the HCIFC and carbon cage of box design. In comparison with the tricortical iliac bone, the flexion stiffness of the HCIFC was significantly less (p<0.001), and the rotation stiffness was significantly greater (p<0.05). The stiffness of the HCIFC with box design was less than the Harms cage with cylinder design in flexion, extension, and bending (p<0.05), but significantly greater in rotation (p<0.001).
Table 2.
The stiffness of intact cervical spine, iliac bone graft, and cages (mean±SD)
| Group, n | Volume (cm3) | Stiffness (Nm/°) | |||||
|---|---|---|---|---|---|---|---|
| Flexion | Entension | R Rotation | L Rotation | R Bend | L Bend | ||
| A, 8 | 0.85±0.23 | 1.82±0.27 | 3.02±0.28a | 2.97±0.25a | 1.18±0.31 | 1.25±0.32 | |
| B, 8 | 1.44±0.01 | 2.40±0.29b | 2.10±0.29 | 2.66±0.34b | 2.82±0.30b | 1.54±0.29 | 1.44±0.28 |
| C, 8 | 0.10±0.01 | 1.80±0.26c | 2.41±0.24d | 2.14±0.26 | 2.31±0.31 | 2.05±0.25d | 2.13±0.27d |
| D, 8 | 0.26±0.01 | 2.97±0.32e | 3.07±0.33e | 4.18±0.41e | 4.09±0.42e | 2.75±0.30e | 2.83±0.25e |
| E, 8 | 0.28±0.01 | 1.28±0.31f | 1.86±0.33 | 3.18±0.30a | 3.32±0.27a | 1.24±0.28 | 1.23±0.29 |
| F, 8 | 0.23±0.01 | 1.30±0.26f | 1.91±0.27 | 3.19±0.28a | 3.35±0.24a | 1.26±0.31 | 1.26±0.32 |
aGroups B and C, p<0.05
bGroup C, p<0.05
cGroups A, E and F, p<0.05
dGroups A, B, E, and F, p<0.05
eOther groups, p<0.05
fGroup A, p<0.05
Volume-related stiffness
Table 3 summarises the volume-related stiffness results for the cervical spine interbody fusion cages normalised with respect to the bone graft during flexion, extension, rotation, and bending. In comparison with bone graft, all the cages increased significantly in volume-related stiffness. The volume-related stiffness was significantly the greatest for the Harms cage (p<0.001 ). The SynCage C was higher than the HCIFC and carbon cage in the volume-related stiffness.
Table 3.
The volume-related stiffness of iliac bone graft and cages (mean±SD)
| Group, n | Volume-related stiffness(Nm/° per cm3) | |||||
|---|---|---|---|---|---|---|
| Flexion | Extension | R Rotation | L Rotation | R Bend | L Bend | |
| B, 8 | 1.67±0.20 | 1.46±0.21 | 1.85±0.24 | 1.96±0.21 | 1.07±0.20 | 1.00±0.19 |
| C, 8 | 18.03±2.62a | 24.10±2.43a | 21.43±2.61a | 23.10±3.06a | 20.50±2.50a | 21.30±2.72c |
| D, 8 | 11.41±1.22b | 11.79±1.27b | 16.09±1.56b | 15.72±1.61b | 10.59±1.15b | 10.89±0.96b |
| E, 8 | 4.58±1.09c | 6.63±1.18c | 11.34±1.07c | 11.85±0.98c | 4.43±1.01c | 4.39±1.04c |
| F, 8 | 5.64±1.13c | 8.28±1.18c | 13.87±1.21c | 14.56±1.03c | 5.48±1.35c | 5.50±1.40c |
aOther groups, p<0.001
bOther groups except C, p<0.05
cGroup B, p<0.05
Discussion
Animal model
Recently, anatomical, biomechanical, and bone mineral density evaluation of the sheep and goat cervical spine has shown good comparability with the human spine. In spine research, the sheep or goat spine is frequently used as a model for the human spine [10, 11]. Anatomical evaluation has demonstrated an average disc space height of 6 mm in the goat or sheep cervical spine, which is approximately 1 mm higher than in the human cervical spine [12]. Additionally, similar upper end plate parameters of C4 and C5 in both species were observed. Therefore, human implants fit in the goat or sheep cervical spine.
Wilke et al. [10] were the first to describe biomechanical analogies between goat and human spines by testing goat spines and comparing the data to the literature. Although much data is available from in vitro and in vivo tests of the normal human cervical spine, comparison remains difficult because of the widely varied test setups. Kandziora et al. [6] compared both species biomechanically using the same test setup. Although significant differences were observed between the two species, especially for rotation parameters, range of motion and stiffness, data was largely comparable in their craniocaudal trends. These researchers concluded that if the sheep animal model is used, motion segments C2-C3 and C3-C4 were the most suitable for biomechanical tests.
Bone mineral density has an influence on the primary stability of cervical spine fixation techniques. In particular, a linear correlation was observed between the axial compression strength of cages and bone mineral density [13]. Currently, no information is available comparing the bone mineral density of severely degenerated human cervical spine motion segments, for which surgery is indicated, with that of the goat cervical spine. However, in a study comparing bone mineral density values between human and goat cervical spines, no significant difference could be registered with regard to bone mineral density between the two species [11]. The least percentile difference was found for C4. Standard deviations for bone mineral density of the human cervical spine were four times greater than for that of the goat cervical spine. Therefore, the goat animal model, in particular the motion segment C3-C4, seems to allow a quasi “bone mineral density-independent” biomechanical evaluation of human size implants.
Stiffness tests
Primary stability in cervical spine interbody fusion is determined essentially by the biomechanical effects of the fixation devices. Cage size has a significant influence on the biomechanical behavior of different cages [14]. Therefore, to allow comparison between the different cage designs, cages of similar height, width, and depth were used in this study.
The results of this study show that any cage implantation increased flexion stiffness over that of the intact motion segment, whereas extension and bending stiffness remained unchanged. Rotation stiffness did not decrease after implantation of a CSIFC except for the Harms cage. As compared with the intact motion segment, extension and bending stiffness significantly increased with the Harms cage of cylinder design and SynCage C of box design. After implantation of the HCIFC, flexion stiffness significantly increased, and extension, bending, and rotation stiffness were unchanged. The HCIFC can provide adequate primary stability for cervical interbody fusion.
Volume-related stiffness
The ideal biological environment for spondylodesis is influenced by several factors including optimal grafting techniques, maximum graft filling of the intervertebral space, mechanical protection of the graft in the intervertebral space, and optimal surface contact area of graft and vertebral body [8]. However, as cage volume increases, graft volume decreases. Therefore, some kind of competition exists between cage and graft volume. Several studies have documented that if graft volume increases, the chance for bony fusion also increases [15]. In conclusion, one important biological factor for a fusion cage is having the smallest possible cage volume, which allows the maximum graft filling of the intervertebral space [8]. However, cages also must provide adequate mechanical stability. In an attempt to take these two factors into consideration during this study, the volume-related stiffness was determined by dividing stiffness results by cage volume. This parameter should allow the interpretation of biomechanical test results from a more biological point of view. The higher the volume-related stiffness is, the less implant is needed for mechanical stabilisation. Therefore, sufficient space remains to be filled by bone graft, which in turn might lead to a better biological environment for bony fusion. Secondary stability in cervical spine interbody fusion is determined biologically, resulting from the amount of bony fusion. Further in vivo studies with different cage designs are necessary to determine whether the volume-related stiffness parameter is able to predict secondary stability in cervical spine interbody fusion.
In the study, the Harms cage and SynCage C are made of titanium, and the carbon cage is produced by carbon fiber. The HCIFC is made of Polyetheretherketone (PEEK) which has successfully replaced glass, stainless steel, and other metals in a growing range of medical applications. The material’s exceptional combination of properties allows designers and engineers to create cost-effective, innovative parts that require outstanding wear, heat, electrical and chemical resistance. For surgical instruments that require extensive repeat use, this inherently pure polymer can withstand repeated autoclave sterilization cycles in which temperatures typically reach high levels. This semicrystalline material maintains high mechanical strength, excellent stress cracking resistance, and hydrolytic stability in the presence of hot water, steam, solvents, and chemicals. The volume-related stiffness was significantly highest with the Harms cage. With this cage, the least implant is needed for mechanical stabilisation, which might lead to sufficient space for bone graft and better interbody fusion. The volume-related stiffness of the HCIFC was higher, which indicates that less PEEK is needed for stabilisation. The HCIFC can provide a better environment for interbody fusion.
HCIFC design
Cages should retain interbody distraction and should be resistant to subsidence into the adjacent vertebrae to foster a biological environment that guarantees the desired quality of osseous fusion [8]. The authors of several clinical studies have demonstrated that all kinds of interbody fusion cages induced decrease in disc space height during the postoperative period. Two of the factors that influence the subsidence tendency of an intervertebral implant are the shape of the implant, especially its contact area at the implant-end plate interface, together with preparation of the end plates [16]. A small surface area and destructive preparation of the end plates increase the subsidence risk. We designed the hat type cervical intervertebral fusion cage (HCIFC) characterised by superior and inferior hat edges extended thus increasing the surface area and decreasing the thickness of the wall compared with the carbon cage. The hat edge of the HCIFC surface, by increasing the contact area with the end plate, can decrease the subsidence tendency. Additionally, the HCIFC with a thinner wall has a lower stress-shielding effect on the incorporated bone graft than the carbon cage. The authors assumed that the lower the stress-shielding effect on a graft, the greater the possibility for interbody fusion [17]. Better interbody fusion can be found in the HCIFC, which needs to be proven by in vivo study of the HCIFC.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s00264-006-0264-y
References
- 1.Brooke NS, Rorke AW, King AT et al (1997) Preliminary experience of carbon fibre cage prostheses for treatment of cervical spine disorders. Br J Neurosurg 11:221–227 [DOI] [PubMed]
- 2.Kumaresan S, Yoganandan N, Pintar FA (1997) Finite element analysis of anterior cervical spine interbody fusion. Biomed Mater Eng 7:221–230 [PubMed]
- 3.Majd ME, Vadhva M, Holt RT (1999) Anterior cervical reconstruction using titanium cages with anterior plating. Spine 24:1604–1610 [DOI] [PubMed]
- 4.Matge G (1998) Anterior interbody fusion with the BAK-cage in cervical spondylosis. Acta Neurochir 140:1–8 [DOI] [PubMed]
- 5.Munoz FLO, de las Heras BG, Lopez VC et al (1998) Comparison of three techniques of anterior fusion in single-level cervical disc herniation. Eur Spine J 7:512–516 [DOI] [PMC free article] [PubMed]
- 6.Kandziora F, Schollmeier G, Scholz M et al (2002) Influence of cage design on interbody fusion in a sheep cervical spine model. J Neurosurg 96(3 Suppl):321–332 [DOI] [PubMed]
- 7.Matge G (2002) Cervical cage fusion with 5 different implants: 250 cases. Acta Neurochir (Wien) 144:539–549, discussion 50 [DOI] [PubMed]
- 8.Weiner BK, Fraser RD (1998) Spine update lumbar interbody fusion cages. Spine 23:634–640 [DOI] [PubMed]
- 9.Kandziora F, Pflugmacher R, Scholz M et al (2001) Comparison between sheep and human cervical spines: an anatomic, radiographic, bone mineral density, and biomechanical study. Spine 26:1028–1037 [DOI] [PubMed]
- 10.Goh JC, Wong HK, Thambyah A et al (2000) Influence of PLIF cage size on lumbar spine stability. Spine 25:35–39 [DOI] [PubMed]
- 11.Kim KW, Ha KY, Moon MS et al (1999) Volumetric change of the graft bone after intertransverse fusion. Spine 24:428–433 [DOI] [PubMed]
- 12.Wilke HJ, Kettler A, Claes LE (1997) Are sheep spines a valid biomechanical model for human spines? Spine 22:2365–2374 [DOI] [PubMed]
- 13.Cain CC, Fraser RD (1995) Bony and vascular anatomy of the normal cervical spine in the sheep. Spine 20:759–765 [DOI] [PubMed]
- 14.Pait GT, Killefer JA, Arnautovic KI (1996) Surgical anatomy of the anterior cervical spine: the disc space, vertebral artery, and associated bony structures. Neurosurgery 39:769–776 [DOI] [PubMed]
- 15.Jost B, Cripton PA, Lund T et al (1998) Compressive strength of interbody cages in the lumbar spine: the effect of cage shape, posterior instrumentation, and bone density. Eur Spine J 7:132–141 [DOI] [PMC free article] [PubMed]
- 16.Wilke HJ, Kettler A, Goetz C et al (2000) Subsidence resulting from simulated postoperative neck movements: an in vitro investigation with a new cervical fusion cage. Spine 25(21):2762–2770 [DOI] [PubMed]
- 17.Kanayama M, Cunningham BW, Haggerty CJ et al (2000) In vitro biomechanical investigation of the stability and stress-shielding effect of lumbar interbody fusion devices. J Neurosurg (2 Suppl) 93:259–265 [DOI] [PubMed]