Abstract
In this prospective study, our aim was to determine the clinical and radiographic outcomes of the surgical treatment of primary complex problem femoral and humeral shaft fractures treated by a new technique called “sandwich technique.” A total of 45 patients with comminuted, complex and/or osteopoenic fractures of the femur or humerus (30 femoral and 15 humeral fractures) were treated using this technique. The patients were followed up for a mean of 25 months. In 24 (85.7%) of 28 patients with femoral fractures and in 14 (93.3%) of 15 patients with humeral fractures, union was achieved within 3–6 months of the operation (mean: 4.5 months). The total union rate was 88.4%. The pseudoarthrosis rate was 12%. There was no infection or implant failure seen during the follow-up period. The cortical allograft struts can be used to provide collateral support to weakened osteopoenic/osteoporotic bone. This technique provides a union rate of about 88% in osteoporotic and/or complex primary humerus or femur fractures.
Résumé
Etude prospective de l’évolution clinique et radiographique des fractures complexes de l’humérus et du fémur avec une technique appelée “sandwich technique”. 30 fractures du fémur et 15 de l’humérus avaient été traitées avec un suivi moyen de 25 mois. Pour 24 des 28 patients opérés du fémur et 14 des 15 opérés de l’humérus, la consolidation était obtenue en 3 à 6 mois après l’opération, en moyenne 4,5 mois. Le taux total de consolidation était de 88,4% et celui des pseudarthroses de 12%. Il n’y a pas eu d’infection ni d’échec d’implant durant ce suivi. Les baguettes d’allogreffes corticales peuvent être utilisés pour renforcer les os ostéopéniques ou ostéoporotiques. Cette technique permet 88% de consolidation pour les fractures primaires mais complexes de l’humérus et du fémur.
Introduction
Today, the surgical treatment of osteoporotic comminuted femoral and humeral diaphyseal or metaphyseal fractures has several challenges despite newly developed fixation systems and the end result is often pseudoarthrosis.
When dealing with such complex fractures, the surgeon should provide the optimum mechanical stabilisation, biological stimulation of healing, and early joint motion to optimise function [18, 21]. This issue is also important for the treatment of fractures in osteoporotic patients.
Surgical stabilisation of complex, comminuted humeral and femoral shaft fractures can be difficult to achieve if severe osteopenia is present. Significant problems arise when the patient has established osteopoenia/osteoporosis. Inadequate screw purchase into the adjacent cortices can result in poor fracture stabilisation, mechanical failure, and non-union. To facilitate primary bone healing, one must ensure rigid fracture stabilisation through firm bone contact and compression of the bone ends. Sufficient mechanical stability is essential to reduce strain at the fracture site and allow biological repair [16]. In an effort to provide improved screw fixation, cortical strut allografts can be used as a way of anchoring the screw to the opposite cortex, thus “sandwiching” the host shaft. The allograft struts provide stability to the fracture site, and they can incorporate [4] and ultimately increase the femoral or humeral bone stock [1, 7, 9, 13, 14, 21].
Similar techniques have been described for periprosthetic fractures, hip revisions, or complex non-unions [5, 11, 12, 14, 19, 22].
The primary aim of this study was to determine the clinical and radiographic outcomes of the surgical treatment of primary complex problem femoral and humeral shaft fractures treated by a new technique called “sandwich technique.” In this technique, dual onlay strut allografts combined with allogenous or autogenous cancellous grafts are wrapped with cables besides the standard fixation systems, either plates or intramedullary nails.
Materials and methods
Between 1999 and 2003, a total of 45 patients with comminuted, complex and/or osteopoenic fractures of the femur or humerus (30 femoral and 15 humeral fractures) were treated using this technique and constituted our study material. Eight (18%) of the femoral fractures were periprosthetic type (two fractures were Vancouver type B1 and six fractures were type C). In these patients, only fracture fixation was performed without revision of to the prostheses as they were stable.
The female-to-male ratio was 2:1 and mean age was 66 years (range: 15–92 years). The fracture was on the right side in 24 patients and on the left side in 21 patients. Assessment of plain radiographs revealed severe osteoporosis in 31 (69%) patients.
All of the patients were followed up until fracture union or until re-operation. The patients’ demographic characteristics and the distribution of fractures according to the AO classification are shown in Table 1. The distribution of implants and allograft options used are summarised in Table 2.
Table 1.
The patients’ demographic characteristics and the distribution of fractures according to the AO classification
| ID | Age | Sex | Site | FX | AO type/Vancouver type | Osteoporosis |
|---|---|---|---|---|---|---|
| FHM | 88 | F | L | F | 31a3 | + |
| NC | 92 | M | L | F | 31a2 | + |
| MK | 76 | F | R | F | 32a2.1 | + |
| EK | 69 | F | L | F | 32b3.1 | + |
| MD | 39 | M | R | F | 32a2.1 | − |
| AB | 83 | F | R | F | 31a3 | + |
| SS | 74 | F | R | F | 31a3 | + |
| NB | 78 | F | L | F | 32c2 | + |
| MAK | 15 | M | R | F | 32c2 | − |
| GE | 64 | F | R | F | 31a2 | + |
| AK | 70 | F | L | F | 32a1 | + |
| RL | 52 | M | L | F | 31a3 | − |
| SK | 88 | M | R | F | 31a3 | + |
| HM | 76 | F | R | F | 32b2.1 | + |
| MH | 74 | F | L | F | 32b1.1 | + |
| OE | 74 | M | R | F | 32a2 | + |
| SK | 66 | M | R | F | 32a2.1 | − |
| FTA | 79 | F | L | F | 31a3 | + |
| BK | 37 | F | L | F | 31a1 | − |
| AK | 79 | F | R | F | 32b2.1 | + |
| BD | 26 | M | L | F | 32b1 | − |
| HB | 85 | M | L | F | 31a2 | + |
| RS | 87 | F | R | F | 33c2 / C | + |
| NK | 60 | M | R | F | 33a1 / C | + |
| PB | 79 | F | L | F | 32a2 / B1 | + |
| MVY | 89 | M | L | F | 32A2 / B1 | + |
| NS | 61 | F | R | F | 32A1 / C | + |
| AA | 88 | F | R | F | 32A1 / C | + |
| RB | 73 | F | L | F | 32B1 / C | + |
| NL | 82 | F | R | F | 32A3 / C | + |
| OB | 42 | M | L | H | 12a2 | − |
| SO | 77 | F | L | H | 12a2 | + |
| HD | 53 | F | R | H | 12c1 | + |
| ZM | 43 | M | L | H | 12b3 | − |
| BV | 85 | F | R | H | 12c3 | + |
| TK | 78 | M | R | H | 11a3 | + |
| AA | 46 | F | R | H | 12b2 | − |
| ZA | 48 | M | L | H | 12c1 | − |
| FGG | 57 | F | L | H | 12a1 | + |
| SG | 54 | F | R | H | 11a3 | + |
| BC | 22 | F | L | H | 12b2 | − |
| ZY | 83 | F | R | H | 12a1 | + |
| NB | 75 | F | R | H | 12c1 | − |
| SS | 68 | F | L | H | 12a3 | − |
| AV | 37 | F | R | H | 12b3 | − |
Table 2.
The distribution of implants and allograft options
| Plate | Intramedullary nail | Allogenous strut | Allogenous spongiosa | Autogenous spongiosa | |
|---|---|---|---|---|---|
| Femur | 22 | 8 | 30 | 26 | 4 |
| Humerus | 15 | 0 | 15 | 11 | 4 |
| Total | 37 | 8 | 45 | 37 | 8 |
Surgical technique Under general anaesthesia, the patient was positioned in a semi-sitting position with the involved arm free draped for humeral fractures and in a lateral decubitus position with the involved limb uppermost and free draped for femoral fractures. Preoperative antibiotics were administered in the form of cefazolin 2 g intravenously and continued for 24 h postoperatively. Thromboprophylaxis was used for femoral fractures only. In humeral fracture cases, the radial nerve was identified between the brachialis and brachioradialis muscles distally and protected throughout the operation.
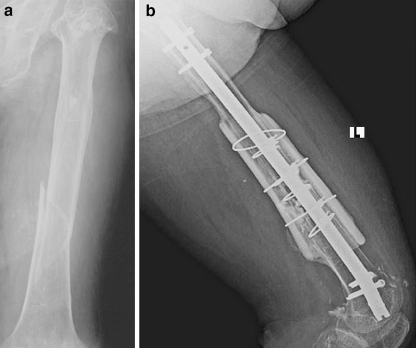
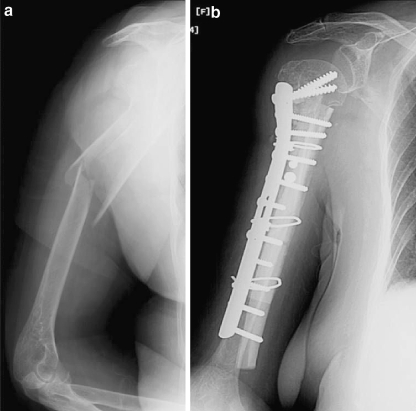
After exposure of the fracture site, fracture fragments were reduced and fixed with either plate and screws or intramedullary nail. The fracture site was then filled with autogenous or allogenous cancellous chip grafts. Usually four cables were placed around the bone away from the fracture site (two proximal and two distal to the fracture site) and two or rarely three allogenous freeze-dried or fresh-frozen strut grafts placed over the medial and lateral aspects of the femur or humerus. All bone bank allograft policies and procedures for donor screening as well as serological and microbiological testing meet current standards established by the American Association of Tissue Banks. All grafts were prepared from tissue produced from cadaver donors using sterile surgical techniques. All allografts were subjected to low-dose (1.0–1.8 megarads) gamma radiation. The length of cortical strut allografts ranged from 10 to 20 cm. Excessive stripping of soft tissues was avoided to preserve the periosteal circulation. Again, autogenous or allogenous cancellous chips were placed around the fracture site between the cortical strut allografts. Then cables were squeezed, secured, and fixed. Any available autogenous or allogenous cancellous bone grafts were placed between the ends of the strut and host bone or along the entire length of the strut if enough bone was available (Fig. 1). With the stability of the reconstruction ensured, the surgical area is irrigated with normal saline. Following introduction of drains, closure of the wound was performed in the standard fashion (Figs. 2, 3).
Fig. 1.

The fracture fragments were reduced and fixed with either plate and screws or intramedullary nail. Then the fracture site is filled with autogenous or allogenous cancellous chip grafts. Usually four cables were placed around the bone away from the fracture site (two proximal and two distal to the fracture site) and two or rarely three allogenous freeze-dried or fresh-frozen strut grafts were placed over the medial and lateral aspects of the femur or humerus. The cables were then squeezed, secured, and fixed. Any available autogenous or allogenous cancellous bone grafts were placed between the ends of the strut and host bone or along the entire length of the strut
Fig. 2.
A case example of femoral fracture and its treatment
Fig. 3.
A case example of humeral fracture and its treatment
Postoperatively, immediate movement of the joint is encouraged to improve function. The patients with femoral fractures were instructed to walk without bearing weight until fracture union. No means of external immobilisation were used.
Serial radiographs and sometimes multislice reformatted computed tomography (CT) scans were used to define fracture union and union of the allograft to the host bone. Union was defined as cortical continuity as seen on both the anteroposterior and the lateral radiographs. The presence of cortical bridging was required for a diagnosis of union for both the fracture itself and allograft to the host bone, although this could not always be visualised circumferentially because of the presence of hardware. In such conditions, CT scans were used.
Results
At the most recent clinical follow-up, two patients treated for femoral fracture had died of unrelated medical causes. Thus, 43 patients were included for clinical and radiological study. The average postoperative follow-up period was 25 months (SD ±10.9 months).
In 24 (85.7%) of 28 patients with femoral fractures and in 14 (93.3%) of 15 patients with humeral fractures, union was achieved within 3–6 months of the operation (mean: 4.5 months). The total union rate was 88.4%. The pseudoarthrosis rate was 12% (four patients with femoral fractures and one patient with humeral fracture). Two of four patients with femoral fractures who developed pseudoarthrosis and one patient with pseudoarthrosis of the humerus fracture were re-operated using the same technique and union was achieved in all of them. One patient with a femoral fracture who developed pseudoarthrosis underwent total hip replacement with calcar replacement and one patient refused revision surgery.
At the most recent follow-up, all patients had a functional range of motion. Apart from one patient who refused re-operation, adequate radiographic graft union and incorporation were present in all patients. Bony bridging of more than 50% of the contact area between the grafts and host bone was seen at the latest follow-up.
In 3 of 15 patients with humeral fractures, iatrogenic radial nerve injury was observed. All three were explored and mechanical irritation of cables on the radial nerve was seen. After exploration, all three patients recovered from radial nerve palsy.
There was no infection or implant failure seen during the follow-up period.
Discussion
To our knowledge, there is no study in the literature that has examined the use of cortical allograft struts for the purposes of treating primary complex and/or osteopoenic humeral or femoral shaft fractures. There are some examples using a similar technique in nonunions [15, 21]. Hornicek et al. [15] treated ten established humeral shaft non-unions with a similar surgical technique and achieved excellent results with a union rate of 100%. We also have experience with 24 patients (15 femur, 8 humerus) with non-union treated by the same surgical technique and a similar rate of union.
The use of cortical allograft struts in the stabilisation of fractures is not a novel treatment idea. The use of cortical onlay allografts has emerged as a very successful treatment option for periprosthetic femoral fractures around the inserted implant. Cortical onlay strut allografts can act as biological plates, either alone or in combination with other internal fixation devices, to stabilise the fracture [3, 6]. In addition to conferring mechanical stability, they may enhance fracture healing and increase bone stock. If appropriately selected and prepared, allograft struts can be customised to fit almost any femur or humerus. Several studies have shown that cortical strut allografts unite consistently and reliably with union rates ranging from 89 to 99% when used in the treatment of periprosthetic femoral fractures [2, 5, 8, 11, 12, 14, 17, 19]. Cortical strut allograft augmentation has also been implemented to help manage periprosthetic humeral fractures following total elbow arthroplasty [19].
There is a dynamic change in allograft biomechanics during the incorporation and remodelling process. The histological and mechanical response to onlay strut allografts has been well documented in the canine model [1, 9, 10]. A zone of highly vascularised mesenchymal tissue forms at the host-graft junction. Osteoclasts subsequently create cutting cones in the graft, which is then invaded by vascular buds. As the graft remodels, it is at its weakest and is vulnerable to mechanical failure unless the fracture has already healed. The construction must therefore be secure enough during the incorporation period, 4–6 months, to ensure that the fracture unites before the allograft struts weaken. This is the rationale for the use of rigid internal fixation (either plates or intramedullary devices) in our series. Stable fixation, with as many cables or wires as necessary, is required. This is facilitated by good apposition of the struts to the native bone. Autograft or morselised allograft or demineralised bone matrix may enhance both fracture union and strut-to-host bone union.
The potential drawbacks of this surgical technique include extensive periosteal stripping of the soft tissue to secure the compression plate laterally and the allograft strut medially. Theoretically, this should compromise the blood supply to the host shaft, but it has not been shown to be clinically important. However, extensive stripping could be minimised by using a long plate with a shorter cortical allograft strut. Another potential drawback, which comes with the use of any donor allograft material, is the risk of infection to the recipient. It is recognised that operations involving allografts involve numerous factors that increase the risk of infection such as a large surgical incision, extensive soft tissue dissection, prolonged time of exposure, and increased blood loss. Therefore, the risk of infection associated with allograft usage is likely to be related to factors other than the contamination of the allograft [20]. Maximum care in surgical procedures will diminish the infection rate.
We conclude that humeral or femoral cortical allograft struts can be used to provide collateral support to weakened osteopoenic/osteoporotic bone. The main advantages of this method include decreased morbidity, rigid stabilisation assuring union, decreased hospital stay, and rapid return to independence. This technique provides a union rate of about 88% in osteoporotic and/or complex primary humeral or femoral fractures. The risk of iatrogenic radial nerve lesions must be borne in mind during humeral procedures.
References
- 1.Allan DG, Lavoie GJ, McDonald S et al (1991) Proximal femoral allografts in revision hip arthroplasty. J Bone Joint Surg Br 73:235–240 [DOI] [PubMed]
- 2.Barden B, Ding Y, Fitzek JG et al (2003) Strut allografts for failed treatment of periprosthetic femoral fractures. Good outcome in 13 patients. Acta Orthop Scand 74(2):146–153 [DOI] [PubMed]
- 3.Brady OH, Garbuz DS, Masri BA et al (1999) The treatment of periprosthetic fractures of the femur using cortical onlay allograft struts. Orthop Clin North Am 30:249–257 [DOI] [PubMed]
- 4.Burchardt H (1987) Biology of bone transplantation. Orthop Clin North Am 18:187–196 [PubMed]
- 5.Chandler HP, King D, Limbird R et al (1993) The use of cortical allograft struts for fixation of fractures associated with well-fixed total joint prostheses. Semin Arthroplasty 4:99–107 [PubMed]
- 6.Chandler HP, Tigges RP (1997) The role of allografts in the treatment of periprosthetic femoral fractures. J Bone Joint Surgery Am 79:1422–1432
- 7.Dave DJ, Koka SR, James JE (1995) Mennen plate fixation for fracture of the femoral shaft with ipsilateral total hip and knee arthroplasties. J Arthroplasty 10:113–115 [DOI] [PubMed]
- 8.Dennis MG, Simon JA, Kummer FJ et al (2000) Fixation of periprosthetic femoral shaft fractures occurring at the tip of the stem. A biomechanical study of 5 techniques. J Arthroplasty 15:523–528 [DOI] [PubMed]
- 9.Emerson RH Jr, Malinin TI, Cuellar AD et al (1992) Cortical strut allografts in the reconstruction of the femur in revision total hip arthroplasty. A basic science and clinical study. Clin Orthop 285:35–44 [PubMed]
- 10.Gresham RB (1964) The free-dried cortical bone homograft: a roentgenographic and histologic evaluation. Clin Orthop 37:194–201 [PubMed]
- 11.Gross AE, Wong PK, Hutchison CR et al (2003) Onlay cortical strut grafting in revision arthroplasty of the hip. J Arthroplasty 18(Suppl 1):104–106 [DOI] [PubMed]
- 12.Haddad FS, Duncan CP, Berry DJ et al (2002) Periprosthetic femoral fractures around well-fixed implants: use of cortical onlay allografts with or without a plate. J Bone Joint Surg Am 84:945–950 [PubMed]
- 13.Head WC, Wagner RA, Emerson RH Jr et al (1993) Restoration of femoral bone stock in revision total hip arthroplasty. Orthop Clin North Am 24:697–703 [PubMed]
- 14.Head WC, Malinin TI, Mallory TH et al (1998) Onlay cortical allografting for the femur. Orthop Clin North Am 29:307–312 [DOI] [PubMed]
- 15.Hornicek FJ, Zych GA, Hutson JJ et al (2001) Salvage of humeral nonunions with onlay bone plate allograft augmentation. Clin Orthop 386:203–209 [DOI] [PubMed]
- 16.Kumar A, Sadiq SA (2002) Nonunion of the humeral shaft treated by internal fixation. Int Orthop 26:214–216 [DOI] [PMC free article] [PubMed]
- 17.Parvizi J, Rapuri VR, Purtili JJ et al (2004) Treatment protocol for proximal femoral periprosthetic fractures. J Bone Joint Surg Am 86:8–16 [DOI] [PubMed]
- 18.Pugh DMW, McKee MD (2003) Advances in the management of humeral nonunion. J Am Acad Orthop Surg 11:48–59 [DOI] [PubMed]
- 19.Sanchez-Sotelo J, O’Driscoll S, Morrey BF (2002) Periprosthetic humeral fractures after total elbow arthroplasty: treatment with implant revision and strut allograft augmentation. J Bone Joint Surg Am 84:1642–1650 [DOI] [PubMed]
- 20.Tomford WW, Thongphasuk J, Mankin HJ et al (1990) Frozen musculoskeletal allografts: a study of the clinical incidence and causes of infection associated with their use. J Bone Joint Surg Am 72:1137–1143 [PubMed]
- 21.Van Houwelingen AP, McKee MD (2005) Treatment of osteopenic humeral shaft nonunion with compression plating, humeral cortical allograft struts, and bone grafting. J Orthop Trauma 19(1):36–42 [DOI] [PubMed]
- 22.Wang JW, Weng LH (2003) Treatment of distal femoral nonunion with internal fixation, cortical allograft struts, and autogenous bone-grafting. J Bone Joint Surg Am 85:436–440 [DOI] [PubMed]