Abstract
Local delivery of gentamicin is an accepted method of infection prophylaxis in the surgery of open fractures. However, the few reports of studies into the effect of locally applied gentamicin on osteoblasts used inadequate methods. In our study, we used the well-characterised C2C12 cell line with reproducible differentiation pathway into the osteoblast lineage. We investigated the viability, cell number, alkaline phosphatase activity, and the expression of osteogenic genes of C2C12 cells after exposure to gentamicin at concentrations of 12.5–800 μg/ml for 48 h. Exposure of C2C12 cells to gentamicin (12.5–800 mg/ml) for 48 h showed no significant changes in the cell number, but cell viability was decreased by one-third at the tested concentrations of 200–800 μg/ml. The alkaline phosphatase activity was significantly decreased by one-third to one-half at any tested concentration (12.5–800 μg/ml) of gentamicin. Any tested concentration of gentamicin up to 800 μg/ml for 48 h did not inhibit or decrease the osteogenic gene expression of osterix and alkaline phosphatase of the C2C12 cells. In conclusion, gentamicin at high concentrations as achieved by local application reduced cellular viability and alkaline phosphatase activity in vitro and therefore may be detrimental for bone healing and repair in vivo.
Résumé
La délivrance locale de gentamycine est une méthode usuelle de prophylaxie de l’infection dans la chirurgie des fractures ouvertes. Il y a peu d’études fiables sur les effets de la gentamycine sur les ostéoblastes. Dans cette étude est utilisée la lignée C2C12 avec reproductibilité des voies de différenciation dans la lignée ostéoblastique. Nous avons étudié la viabilité, le nombre de cellules, l’activité phosphatase alcaline et l’expression des gènes ostéogèniques des cellules C2C12 après exposition pendant 48 heures à des concentrations de gentamycine de 12,5 μg/ml à 800 μg/ml. Il n’y avait pas de modification significative du nombre de cellules mais la viabilité était diminuée d’un tiers pour les concentrations de 200 à 800 μg/ml. L’activité phosphatase alcaline était diminuée d’un tiers à la moitié pour toutes les concentrations étudiées. Dans aucun cas il n’y avait diminution ou inhibition de l’expression génique de l’osterix ou de la phosphatase alcaline des cellules C1C12. En conclusion, la gentamycine à haute concentration réduit la viabilité cellulaire et l’activité phosphatase alcaline in vitro, ce qui est peut-être néfaste pour la cicatrisation osseuse in vivo.
Introduction
Local application of gentamicin for prophylaxis of bone and arthroplasty infection is an accepted method of therapy [10, 22]. Meanwhile, antibiotic-loaded bone graft substitutes are being increasingly investigated in order to prevent infection of open fractures and to enhance bone healing [1]. Reports on anti-infective coatings of orthopaedic implants were recently published [4, 6, 7]. However, only two studies have been reported that looked at the in vitro effect of gentamicin on bone and osteogenesis [11, 19]. They used primary cell cultures derived from human bone or rodent bone tissue which may not guarantee reproducibility because of donor variability and different developmental stages. Second, osteogenic differentiation of these cells is questionable. Moreover, the cells become senescent after a relatively short time in culture and have a slow rate of proliferation; only limited measurements can be done with one batch of cells.
The aim of our study was to investigate the influence on osteogenic function of preosteoblastic C2C12 cells which are well characterised and have been proven to give reproducible induction of the pathway to the osteoblast lineage with the expression of osteogenic marker and genes [14].
In this study cellular viability, cell number, alkaline phosphatase (ALP) activity, and gene expression of osterix (OSX) and alkaline phosphatase were measured. We applied gentamicin for 48 h, which should reflect the twofold time period of perioperative infection prophylaxis in orthopaedic surgery. We decided to use gentamicin concentrations up to 800 μg/ml to mimic clinical settings because measurement of the in vivo elution of gentamicin around polymethylmethacrylate (PMMA) beads showed levels of 400–600 μg/ml on the 1st day [24].
Materials and methods
Cell culture
The murine C2C12 cells [2, 14, 25] were plated on polystyrene of 24- and 96-well tissue culture plates at a density of 25,000 and 10,000 cells, respectively, and cultured at 37°C in 95:5% air:CO2 for 24 h. The cells were then incubated with various concentrations of gentamicin (12.5, 25, 50, 100, 200, 400, and 800 μg/ml) for 48 h and subsequently for 24 h without any antibiotic. The medium was changed every 2 days and consisted of a 1:1 solution of Ham’s F12 and Dulbecco’s modified Eagle’s medium (DMEM/Ham’s F12), supplemented with heat-inactivated 10% calf serum without any antibiotics (Biochrom, Berlin, Germany).
The first measurement was done after 48 h exposure to gentamicin. After subsequent incubation for 24 h without any antibiotic the second measurement was performed representing a state of recovery after antibiotic exposure.
Addition of 300 ng bone morphogenetic protein-2 (BMP-2) per ml low mitogen medium for 48 h induced the osteogenic pathway for the measurement of alkaline phosphatase activity and the gene expression. The low mitogen media consisted of DMEM/Ham’s F12 with 2% heat-inactivated calf serum without any antibiotics.
Antibiotic
Gencin (Curasan, Kleinostheim, Germany) contains 0.04 g/ml gentamicin sulphate solution. Concentrations of 12.5–800 μg/ml were prepared by dilution with DMEM/Ham’s F12 culture medium. All solutions were prepared under sterile conditions and used within 2 h.
Cell viability
Cell viability was measured by staining with the colorimetric reagent WST-1 (Böhringer Mannheim, Ingelheim, Germany), which is designed for the nonradioactive quantitation of cellular viability. The tetrazolium salt WST-1 is cleaved to formazan by the succinate-tetrazolium reductase system, a component of the respiratory chain of the mitochondria, and is active only in viable cells [12]. Assay cells were rinsed with 1× phosphate-buffered saline and incubated with 0.1 ml DMEM/Ham’s F12 culture medium, supplemented with 10% heat-treated foetal calf serum and 10 μl WST-1 reagents at 37°C for 30 min. The absorbance of the resulting formazan in the supernatant was measured at 450 nm with a microplate reader (SLT, Crailsheim, Germany).
Cell number
To measure the cell number by electronic counting the cells were rinsed twice with phosphate-buffered saline and detached by treatment with 0.2 ml trypsin-ethylenediaminetetraacetate (0.25% trypsin, 1 mmol/l ethylenediaminetetraacetate, Gibco Life Technologies, Karlsruhe, Germany). After 5 min, 0.6 ml DMEM/Ham’s F12 culture medium, supplemented with 10% heat-treated foetal calf serum, was added to stop the reaction [8].
The automatic cell analyser system CASY 1 TTC (Schärfe System, Reutlingen, Germany) was used to measure the amount of viable cells. Experimentally, 100-μl aliquots of the cell cultures were diluted in 10 ml phosphate-buffered saline and three 400-μl aliquot samples were analysed by the system. Cell number was calculated automatically with the Casystat software (Schärfe System, Reutlingen, Germany).
Alkaline phosphatase
After confluency the cells were exposed to various gentamicin concentrations for 48 h in low mitogen medium and simultaneously stimulated with BMP-2 as described before. Then the cells were rinsed twice with phosphate-buffered saline and incubated with 0.1 ml of lysis buffer I (0.1 M glycine, 1% NP-40, 1 mM MgCl2, 1 mM ZnCl2) for 1 h at room temperature. Then 0.1 ml of 10 ml lysis buffer II (0.1 M glycine, 1 mM MgCl2, 1 mM ZnCl2) supplemented with 20 mg p-nitrophenol phosphate substrate was added to each well. The absorbance of p-nitrophenol was measured at 405 nm with a microplate reader (SLT, Crailsheim, Germany) [14]. All chemicals were purchased from Sigma-Aldrich (Munich, Germany) unless otherwise mentioned.
Reverse transcriptase polymerase chain reaction (RT-PCR)
After cell confluency the BMP-2-induced osteogenic pathway was initiated and the cells were exposed to different concentrations of gentamicin (25, 100, 400, 800 μg/ml). The C2C12 cells’ mRNA was isolated with the NucleoSpin isolation kit (Macherey-Nagel, Dueren, Germany). RNA isolation was performed as described in the manufacturer’s manual. Incubation was performed with Superscript Reverse Transcriptase II (Invitrogen, Carlsbad, CA, USA) as per the manufacturer’s recommendation. The control housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was included to normalise the data. RT-PCR was done in 30 μl of 10× PCR buffer containing 1 μl of each cDNA, 1 μl (5 pmol/μl) of each primer (Operon Biotechnolgies, Cologne, Germany), and 0.3 μl Taq (Amersham, Braunschweig, Germany) (see Table 1). The primers (see Table 1) were selected according to the literature [5, 13]. Conditions for amplification of the genes were 94°C for 3 min followed by 25 cycles at 55°C for 45 s for GAPDH, for ALP 45 cycles at 55°C and for OSX 30 cycles at 55°C, and finally 72°C for 3 min.
Table 1.
Primer sequences used for RT-PCR
| Gene | Sequence | Size (bp) | Accession number |
|---|---|---|---|
| OSX | Forward 5′-CCCTTCTCAAGCACCAATGG-3′, reverse 5′-AAGTAGGCAGCTGGGGGTTC-3′ | 394 | NM_130458.5 |
| ALP | Forward 5′-GCCATGACATCCCAGAAAGA-3′, reverse 5′-GAGGCATACGCCATCACATG-3′ | 374 | NM_007431.1 |
| GAPDH | Forward 5′-CCACCCAGAAGACTGTGGATGGC-3′, reverse 5′-CATGTAGGCCATGAGGTCCACCAC-3′ | 446 | NM_001001978 |
Statistical methods
Data were evaluated with the software SPSS 12.0 for Windows, Lead Technologies, Chicago, IL, USA. We used the analysis of variance (ANOVA) test with post hoc analysis for statistical analysis, and a 95% (p<0.05) confidence level was adopted for statistical significance. All experiments were done twice with a minimum of three samples for each concentration and parameter.
Results
Cell viability and number
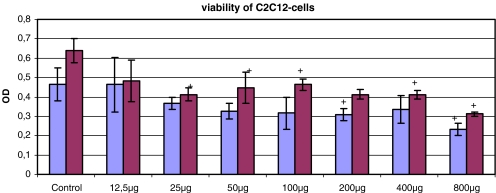
The cell viability of C2C12 cells measured by the WST-1 test was negatively influenced by the exposure to gentamicin for 48 h. In the first measurement the absorbance of the WST-1 test was decreased by one-third at concentrations of 200–800 μg/ml versus control. Concentrations below 200 μg/ml gentamicin showed values comparable to the control. The second measurement after subsequent 24-h incubation without any antibiotic revealed decreased absorbance of the WST-1 test at concentrations of 800–25 mg/ml (Fig. 1).
Fig. 1.
WST-1 test of C2C12 cells after exposure to gentamicin for 48 h (first bar) and subsequent incubation for 24 h without any antibiotic (second bar) (*p<0.05 to control)
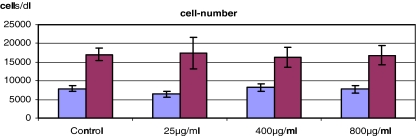
The cell number showed no significant changes after exposure of C2C12 cells to gentamicin for 48 h and after subsequent incubation without any antibiotic for 24 h (Fig. 2).
Fig. 2.
C2C12 cell number after exposure to gentamicin for 48 h (first bar) and subsequent incubation for 24 h without any antibiotic (second bar) (each value not significant versus control, p>0.05 to control)
The alkaline phosphatase activity
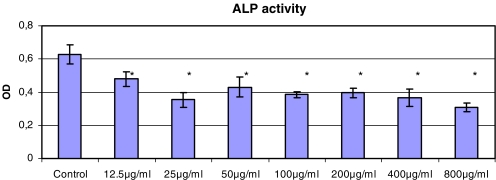
The alkaline phosphatase activity was significantly decreased by one-third to one-half versus control at any concentration of gentamicin (12.5–800 μg/ml) as shown in Fig. 3. Even the lowest concentration as achieved for example after systemic application of gentamicin (12.5 μg/ml), resulted in a decrease of absorbance in the measurement of alkaline phosphatase.
Fig. 3.
ALP activity of C2C12 cells after exposure to gentamicin for 48 h (*p<0.05 to control)
Semiquantitative analysis of the gene expression
Osterix is essential for developing a bony skeleton [18] whereby ALP is necessary for building up the extracellular matrix [3, 21].
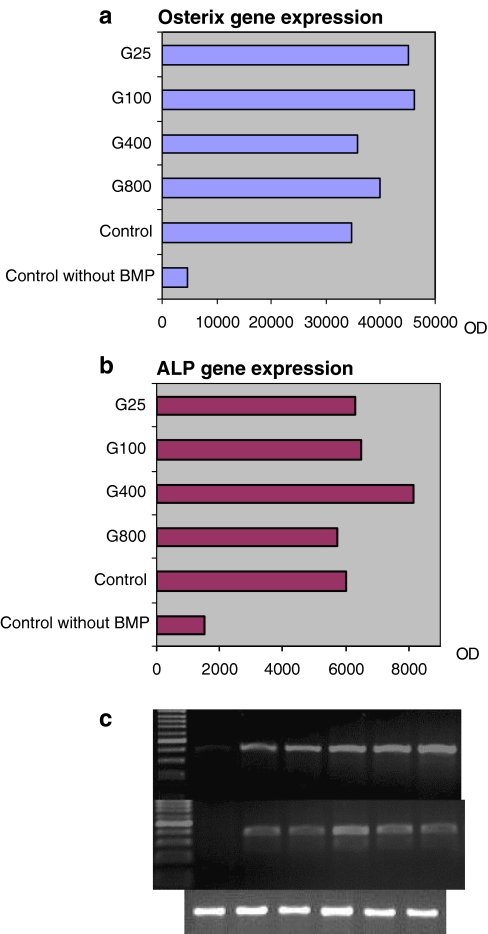
The expression of the osteogenic gene osterix of the C2C12 cells was not hampered after exposure to various concentrations of gentamicin (800, 400, 100, and 25 μg/ml) for 48 h (Fig. 4a).
Fig. 4.
Semiquantitative analysis with measurement of the optical density (OD) of the a osterix gene and b alkaline phosphatase gene PCR products adopted to the housekeeping gene GAPDH, c gel electrophoresis of the PCR products (osterix-alkaline phosphatase-GAPDH)
The gene expression of alkaline phosphatase was also not negatively influenced by exposure to various concentrations of gentamicin (Fig. 4b). The control cells without BMP-2 induction showed firmly decreased expression of osteogenic genes. The gel electrophoresis of the PCR products is shown in Fig. 4c.
Discussion
We have shown that the cell number of C2C12 cells was not affected after exposure to gentamicin indicating no toxic influences on the cell membrane. The cell viability was negatively influenced at concentrations above 200 μg/ml. The alkaline phosphatase activity, an early expressed marker for osteogenic differentiation, was significantly decreased at any concentration of gentamicin compared with the antibiotic-free control.
However, mRNA expression of the osteogenic genes like osterix and alkaline phosphatase was not inhibited by cell exposure to gentamicin for 48 h. It seems that gentamicin influenced the viability and osteogenic marker of the C2C12 cells but did not inhibit or decrease cell number or the expression of osteogenic genes.
From the clinical point of view, the efficacy of local antibiotic therapy has been reported in several clinical studies on open fractures and osteomyelitis. However, despite the favourable experience of several authors, the clinical role of local antibiotic therapy has only been evaluated to a limited extent [9, 17, 20].
A successful reduction in the rate of infection of open tibial fracture was reported using tobramycin-loaded cement beads in open wounds. Mean times to union in cases with local tobramycin application were longer (though not statistically significant) than those in the cases without this treatment, although the severity of soft tissue damage associated with the open fracture was almost equal in both groups. This clinical observation might suggest an adverse effect on osteogenic differentiation of cells in the presence of locally applied antibiotics [15].
McKee et al. used tobramycin-loaded calcium sulphate pellets in the treatment of 25 patients with infected long bone defects and non-unions. Infection was eradicated in 23 of 25 patients (92%) and union was achieved in 5 of 7 non-unions treated only with calcium sulphate without autologous bone grafting. However, eight patients developed sterile draining sinuses that healed after pellet resorption, which could not be definitely attributed to the antibiotic. They concluded that further investigation was necessary to establish clinical safety [16].
Osteogenic differentiation in the presence of antibiotics for infection prophylaxis is essential for bone remodelling and repair after trauma or joint replacement surgery. Recently, antibiotic-loaded bone graft substitutes have been developed to improve perioperative infection prophylaxis [1, 16]. To date the effect of antibiotics on the osteogenic function and differentiation pathway remains unclear.
Few studies of the in vitro effects of gentamicin on bone and osteogenesis have been reported. Unfortunately, these studies were done with primary cells derived from human or rodent trabecular bone which may not guarantee reproducibility. Moreover, there was no investigation of the expression of bone-related genes after exposure to gentamicin. Isefuku and coworkers described the toxic effects on osteoblast-like cells when exposed for 4 days to more than 100 μg/ml gentamicin. Synthesis rates of alkaline phosphatase, collagen I, DNA content, and 3H-thymidine incorporation were decreased, suggesting toxic damage to these cells [11]. Pedersen and Lund [19] observed a dose-dependent decrease in the release of previously incorporated calcium 45 and alkaline phosphatase activity in mouse calvaria exposed to various concentrations of gentamicin (20–320 μg/ml) for 2 days.
The results of our study show that gentamicin negatively influenced the viability and the alkaline phosphatase activity of C2C12 cells. In contrast, the data indicates an unaffected mRNA expression of osterix and alkaline phosphatase genes even at the highest concentration of 800 μg/ml, which could be explained by the bactericidal activity of gentamicin. It has the ability to bind procaryotic ribosomes which cause inhibition of protein synthesis and in consequence bacterial death [23].
In conclusion, gentamicin at high concentrations as achieved by local application, reduced cellular viability and alkaline phosphatase activity in vitro and therefore may be detrimental for bone healing and repair in vivo.
Acknowledgements
BMP-2 was kindly provided by Prof. W. Sebald (Biocentre, University of Würzburg, Germany).
We thank M. Kunz, S. Jatzke, T. Schilling and A. Heymer for assistance in laboratory work and K. Schreyer for assistance in preparing the manuscript. The first author was funded by the non-commercial ENDO-Verein e.V., Hamburg, Germany.
References
- 1.Beardmore AA, Brooks DE, Wenke JC, Thomas DB (2005) Effectiveness of local antibiotic delivery with an osteoinductive and osteoconductive bone-graft substitute. J Bone Joint Surg Am 87:107–112 [DOI] [PubMed]
- 2.Blau HM, Chiu CP, Webster C (1983) Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell 32:1171–1180 [DOI] [PubMed]
- 3.Boyan BD, Schwartz Z, Bonewald LF, Swain LD (1989) Localization of 1,25-(OH)2D3-responsive alkaline phosphatase in osteoblast-like cells (ROS 17/2.8, MG 63, and MC 3T3) and growth cartilage cells in culture. J Biol Chem 264:11879–11886 [PubMed]
- 4.Campbell AA, Song L, Li XS, Nelson BJ, Bottoni C, Brooks DE, DeJong ES (2000) Development, characterization, and anti-microbial efficacy of hydroxyapatite-chlorhexidine coatings produced by surface-induced mineralization. J Biomed Mater Res 53:400–407 [DOI] [PubMed]
- 5.Celil AB, Hollinger JO, Campbell PG (2005) Osx transcriptional regulation is mediated by additional pathways to BMP2/Smad signaling. J Cell Biochem 95:518–528 [DOI] [PubMed]
- 6.Darouiche RO, Farmer J, Chaput C, Mansouri M, Saleh G, Landon GC (1998) Anti-infective efficacy of antiseptic-coated intramedullary nails. J Bone Joint Surg Am 80:1336–1340 [DOI] [PubMed]
- 7.DeJong ES, DeBerardino TM, Brooks DE, Nelson BJ, Campbell AA, Bottoni CR, Pusateri AE, Walton RS, Guymon CH, McManus AT (2001) Antimicrobial efficacy of external fixator pins coated with a lipid stabilized hydroxyapatite/chlorhexidine complex to prevent pin tract infection in a goat model. J Trauma 50:1008–1014 [DOI] [PubMed]
- 8.Hendrich C, Noth U, Stahl U, Merklein F, Rader CP, Schutze N, Thull R, Tuan RS, Eulert J (2002) Testing of skeletal implant surfaces with human fetal osteoblasts. Clin Orthop 394:278–289 [DOI] [PubMed]
- 9.Huddleston PM, Steckelberg JM, Hanssen AD, Rouse MS, Bolander ME, Patel R (2000) Ciprofloxacin inhibition of experimental fracture healing. J Bone Joint Surg Am 82:161–173 [DOI] [PubMed]
- 10.Ince A, Seemann K, Frommelt L, Katzer A, Lohr JF (2004) One-stage revision of shoulder arthroplasty in the case of periprosthetic infection (in German). Z Orthop Ihre Grenzgeb 142:611–617 [DOI] [PubMed]
- 11.Isefuku S, Joyner CJ, Simpson AH (2003) Gentamicin may have an adverse effect on osteogenesis. J Orthop Trauma 17:212–216 [DOI] [PubMed]
- 12.Ishiyama M, Tominaga H, Shiga M, Sasamoto K, Ohkura Y, Ueno K (1996) A combined assay of cell viability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet. Biol Pharm Bull 19:1518–1520 [DOI] [PubMed]
- 13.Jadlowiec J, Koch H, Zhang X, Campbell PG, Seyedain M, Sfeir C (2004) Phosphophoryn regulates the gene expression and differentiation of NIH3T3, MC3T3-E1, and human mesenchymal stem cells via the integrin/MAPK signaling pathway. J Biol Chem 279:53323–53330 [DOI] [PubMed]
- 14.Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T (1994) Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol 127:1755–1766 [DOI] [PMC free article] [PubMed]
- 15.Keating JF, Blachut PA, O’Brien PJ, Meek RN, Broekhuyse H (1996) Reamed nailing of open tibial fractures: does the antibiotic bead pouch reduce the deep infection rate? J Orthop Trauma 10:298–303 [DOI] [PubMed]
- 16.McKee MD, Wild LM, Schemitsch EH, Waddell JP (2002) The use of an antibiotic-impregnated, osteoconductive, bioabsorbable bone substitute in the treatment of infected long bone defects: early results of a prospective trial. J Orthop Trauma 16:622–627 [DOI] [PubMed]
- 17.Miclau T, Edin ML, Lester GE, Lindsey RW, Dahners LE (1998) Effect of ciprofloxacin on the proliferation of osteoblast-like MG-63 human osteosarcoma cells in vitro. J Orthop Res 16:509–512 [DOI] [PubMed]
- 18.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B (2002) The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108:17–29 [DOI] [PubMed]
- 19.Pedersen JG, Lund B (1988) Effects of gentamicin and monomer on bone. An in vitro study. J Arthroplasty 3(Suppl):S63–S68 [DOI] [PubMed]
- 20.Perry AC, Prpa B, Rouse MS, Piper KE, Hanssen AD, Steckelberg JM, Patel R (2003) Levofloxacin and trovafloxacin inhibition of experimental fracture-healing. Clin Orthop 414:95–100 [DOI] [PubMed]
- 21.Sakano S, Murata Y, Miura T, Iwata H, Sato K, Matsui N, Seo H (1993) Collagen and alkaline phosphatase gene expression during bone morphogenetic protein (BMP)-induced cartilage and bone differentiation. Clin Orthop Relat Res 292:337–344 [PubMed]
- 22.Steinbrink K, Frommelt L (1995) Treatment of periprosthetic infection of the hip using one-stage exchange surgery (in German). Orthopade 24:335–343 [PubMed]
- 23.Sundin DP, Sandoval R, Molitoris BA (2001) Gentamicin inhibits renal protein and phospholipid metabolism in rats: implications involving intracellular trafficking. J Am Soc Nephrol 12:114–123 [DOI] [PubMed]
- 24.Wahlig H, Dingeldein E, Buchholz HW, Buchholz M, Bachmann F (1984) Pharmacokinetic study of gentamicin-loaded cement in total hip replacements. Comparative effects of varying dosage. J Bone Joint Surg Br 66:175–179 [DOI] [PubMed]
- 25.Yaffe D, Saxel O (1977) Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270:725–727 [DOI] [PubMed]