Abstract
Fifteen patients with infectious spondylitis were treated by percutaneous endoscopic discectomy and drainage (PEDD) and associated appropriate parenteral antibiotics. Infectious spondylitis was diagnosed clinically, on the basis of elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) values, and on roentgenographic and magnetic resonance imaging (MRI) findings. Causative bacteria were identified in 13 (86.7%) of 15 biopsy specimens. Systemic antibiotics were administered according to sensitivity analyses of pathogens. All patients reported immediate back pain relief except for two, who required anterior debridement and fusion one week and two weeks later, respectively. Two other patients with recurrent infection and intractable pain underwent surgical intervention at one month and eight months after PEDD, respectively. The remaining 11 patients recovered uneventfully after full-course, specific, antimicrobial therapy. No surgery related complications or side effects were observed during at least 12 months of follow-up. In conclusion, PEDD can provide retrieval of sufficient specimens and has high diagnostic efficacy, thereby enabling prompt and appropriate antibiotic therapy to the offending pathogens. It can be considered an effective alternative for treating uncomplicated spondylitis.
Résumé
Résumé 15 patients présentant une discospondylite infectieuse ont été traités par chirurgie percutanée, drainage et antibio thérapie parentérale adaptée. Le diagnostic de discospondylite infectieuse repose sur l’élévation de la vitesse de sédimentation et de la protéine C réactive, le diagnostic est également confirmé par l’étude radiographie et l’IRM. On a pu faire le diagnostic bactériologique dans 13 cas (86,7%) sur 15 biopsies. L’antibio thérapie a été mise en place en fonction de l’antibiogramme. Tous les patients ont bénéficié d’une disparition rapide de la douleur en dehors de deux patients qui ont nécessité un traitement chirurgical par voie antérieure avec greffe à une et deux semaines après le début du traitement. Deux autres patients présentant une récidive de l’infection, avec récidive des douleurs ont nécessité une reprise chirurgicale à un et huit mois, les 11 patients restant ayant été guéris après l’antibio thérapie. Nous n’avons pas à déplorer de complications secondaires à la chirurgie, dans les 12 mois pendant lesquels nous avons suivis les patients. Le drainage et la chirurgie percutanée est un traitement efficace pour ces sujets. Il permet une antibio thérapie adaptée et, constitue pour nous, une alternative de choix.
Introduction
Pyogenic spondylitis is the most common spinal infection that can occur spontaneously in immunocompromised patients, owing to haematogenous spread from other inflammatory foci or following diagnostic and therapeutic procedures. Tuberculous spondylitis continues to be a major problem in underdeveloped and developing countries and is resurgent in the Western world [6, 14, 18, 20]. The typical clinical symptoms and signs of infectious spondylitis, either pyogenic or tuberculous spondylitis, include severe back pain with or without sciatica. The initial radiographic sign is commonly a collapsed intervertebral disc space with or without subtle erosion of the vertebral endplate. In more aggressive cases osteolysis can result in substantial shortening and compression of the vertebral body, finally producing instability. However, the disc is relatively preserved until late in the tuberculous infection process, despite extensive bony destruction [2, 10, 14, 16].
Surgical intervention is usually reserved for patients with failed antibiotics therapy, progressive spinal deformity or instability, epidural abscesses, or neurological impairment [4, 13, 16, 21]. However, peri-operative morbidity increases with anterior debridement or combined anterior and posterior surgery, especially in elderly patients or those in poor general condition. Thus, early diagnosis and prompt application of appropriate antibiotic therapy for cultured organisms are crucial for the successful treatment of infectious spondylitis and prevention of further morbidity.
Identifying the offending pathogen is critical to administration of the correct antibiotics for non-operative treatment. However, spinal biopsy for bacteriologic diagnosis has a variable rate of reported success in patients with spondylitis [5, 8, 19, 23, 24]. In addition to inadequate amount of biopsy tissue, a high degree of radiation exposure is another concern [15, 17]. In this study, percutaneous endoscopic discectomy and drainage (PEDD) was used to treat 15 patients with infectious spondylitis. Clinical outcomes were retrospectively reviewed to evaluate the diagnostic and therapeutic values of this minimally invasive procedure.
Patients and methods
Fifteen patients (nine male and six female; ages 27–88 years) with infectious spondylitis were treated by PEDD procedures between January 2002 and February 2005 in one medical centre. The patients’ medical records, including outpatient and emergency room notes, admission notes, in-patient progress and nursing notes, discharge summaries, procedure notes, surgical reports, radiology reports, pathology reports, and microbiology laboratory results, were reviewed. The microbiology reports comprised microscopy and culture findings and any specific pathogens identified by either method. All patients presented with intractable back pain requiring narcotic pain control and bed rest. Of 15 patients, 11 had serious medical problems unsuited to major surgery. Infectious spondylitis was diagnosed on the basis of clinical examination, including elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) values, and roentgenographic and magnetic resonance imaging (MRI) findings.
Fifteen patients with clinical symptoms and comparable imaging studies met the inclusion criteria. Infected levels were L2–3 in four patients, L3–4 in one, L4–L5 in seven, and L5–S1 in three. The criteria included: (1) early stage spondylitis; (2) complicated spondylitis in patients of advanced age and/or with a compromising medical condition. Patients with an infection associated with epidural abscess resulting in neurological deficit were excluded.
Clinical outcome was assessed by careful physical examination, regular serological tests, and imaging studies during admission and at least every three months after discharge, to determine whether continued conservative treatment or change to surgical intervention was required.
Technique
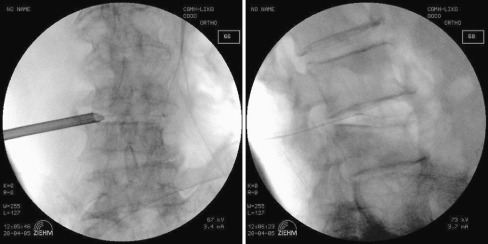
The patient was situated prone on a radiolucent frame suitable for fluoroscopy. All procedures were performed under local anaesthesia with conscious sedation similar to that used for standard lumbar discography. Under fluoroscopic guidance the target site was located, and the entry site was marked on the skin at a point 8–12 cm from the midline. Following sterile preparation, draping, and local anaesthesia, a spinal needle was inserted directly into the center of the targeted disc. A guide wire was introduced through the spinal needle into the central disc space, and the spinal needle was then withdrawn. After a small stab wound incision (about 1 cm) had been created, a dilator and cannulated sleeve were guided over the wire and passed sequentially into the disc center. Fluoroscopy was repeated in two orthogonal planes to verify the correct position of the endoscope tip. The tissue dilator was then removed, and the cutting tool was inserted. The cutting tool, a cylindrical sleeve with a serrated edge at its distal end, was used to harvest a core of impacted biopsy specimen. Discectomy forceps were then inserted through the cannulated sleeve to extract additional tissue from the infected disc. Percutaneous debridement was conducted in a piecemeal fashion by manipulating the biopsy forceps and shaver into different positions to withdraw as much tissue as possible under fluoroscopic monitoring. Following biopsy and debridement procedures, normal saline solution was used for irrigation. Finally, a drainage tube was inserted into the debrided disc space and connected to a negative-pressure Hemovac (Fig. 1). The biopsy specimen contained disc material and parts of vertebral endplates of adjacent vertebrae. Each biopsy was examined for micro-organisms and examined histopathologically.
Fig. 1.
A drainage tube is inserted through the trocar for further drainage under intra-operative fluoroscopy
Results
The most prominent clinical sign of infectious spondylitis was spinal pain, which was detected in all 15 patients. Thirteen patients (86.7%) reported immediate relief of back pain following PEDD. Of these 13 patients, two experienced recurrent intractable pain with progressive kyphotic deformity, identified radiologically, underwent surgical treatment at one month and eight months after PEDD, respectively. The remaining two patients, who had persistent infection and severe back pain, underwent anterior debridement with accompanied autograft interbody fusion one week and two weeks after PEDD, respectively.
Causative bacteria were identified in 13 (86.7%) of 15 biopsy specimens. Systemic antibiotics and anti-tuberculous or anti-fungal chemotherapy were administered according to sensitivity studies for identified pathogens. Six patients were infected with Staphylococcus aureus, three cases of which were oxacillin-resistant and three oxacillin sensitive. Two patients had spinal tuberculous spondylitis, and five had Streptococcus viridans, Prevotella spp, Enterococcus faecalis, Pseudomonas aeruginosa, and Candida albicans infections. Two patients with negative biopsy findings underwent surgical exploration for persistent back pain and spinal deformity at one week and eight months after PEDD, respectively. Short-term broad-spectrum antibiotics were administrated after PEDD in these two patients. One patient had chronic inflammation histologically by open debridement but still no phatogens were identified. The other patient with tuberculous spondylitis confirmed eight months after PEDD had a history of anti-tuberculous chemotherapy for pulmonary tuberculosis.
Overall, four of 15 patients underwent anterior debridement and fusion with autograft because the pathogens or progressive infection had not been identified. Extensive osteolytic destruction of the vertebral body, with spinal instability or kyphotic deformity, was observed in these four patients. Eleven patients responded to PEDD and were successfully treated with at least a six-week course of parenteral antibiotics therapy or full-course antimicrobial chemotherapy (Figs. 2, 3 and 4). Elevated CRP values returned to normal range within seven days to five weeks in these patients. Elevated ESR irregularly decreased to half of the original pretreatment values within three to nine weeks. However, no surgery related complications or side effects were noted during at least 12 months of follow-up (average 26.5 months; range 12–48 months). Table 1 presents a summary of the patients’ data, including age, gender, involved level, medical disorder, and clinical outcome after PEDD. Table 2 presents the changes in serological values before and after PEDD.
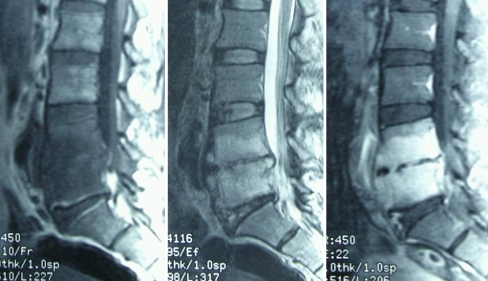
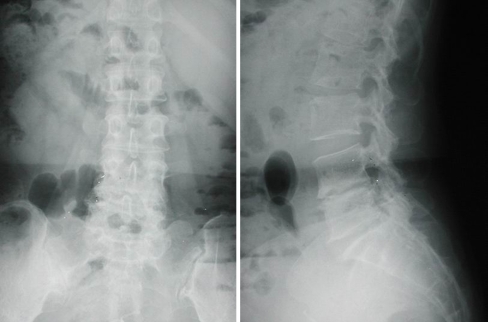
Fig. 2.
A 65-year-old woman sustained infectious spondylitis and was treatment with percutaneous endoscopic discectomy and drainage. Preoperative radiography showed narrowing of the L4–L5 disc space and endplate erosion
Fig. 3.
Sagittal MRI with contrast demonstrated L4–L5 infectious spondylitis
Fig. 4.
Postoperative radiography obtained at 2-year follow-up showed collapse of the L4–L5 disc space and spontaneous fusion
Table 1.
Patients’ demographic data and clinical outcomes (M male, F female, DM diabetes mellitus, HTN hypertension, TB tuberculosis, CAD coronary artery disease, COPD chronic obstructive pulmonary disease, ESRD end-stage renal disease, ARF acute renal failure, AIDS acquired immunodeficient syndrome, OSSA oxacillin-sensitive Staphylococcus aureus, ORSA oxacillin-resistant Staphylococcus aureus)
| Case no. | Age (years) | Gender | Level | Major medical disease | Pain relief | Culture | Pathology by PEDD | Surgery | Pathology by surgery |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 68 | M | L5–S1 | DM and HTN | None | Negative | Inflammation | 1 week later | Inflammation |
| 2 | 79 | F | L4–5 | DM and HTN | Immediate | TB | Positive | None | |
| 3 | 44 | F | L4–5 | Immediate | Prevotella | Positive | None | ||
| 4 | 82 | M | L4–5 | Pulmonary TB | Immediate | Negative | Inflammation | 8 months later | TB |
| 5 | 79 | M | L2–3 | CAD and COPD | None | Candida | Positive | 2 weeks later | |
| 6 | 56 | M | L5–S1 | Immediate | OSSA | Positive | 1 month later | ||
| 7 | 79 | M | L2–3 | CAD | Immediate | Streptococcus | Positive | None | |
| 8 | 60 | F | L2–3 | Immediate | TB | Positive | None | ||
| 9 | 54 | M | L4–5 | Immediate | ORSA | Positive | None | ||
| 10 | 52 | F | L5–S1 | ESRD | Immediate | ORSA | Positive | None | |
| 11 | 75 | M | L3–4 | Liver cirrhosis | Immediate | OSSA | Positive | None | |
| 12 | 55 | M | L4–5 | ARF | Immediate | Enterococcus faecalis | Positive | None | |
| 13 | 27 | M | L4–5 | AIDS | Immediate | Pseudomonas aeruginosa | Positive | None | |
| 14 | 88 | F | L2–3 | DM and HTN | Immediate | OSSA | Positive | None | |
| 15 | 65 | F | L4–5 | DM and hepatitis | Immediate | ORSA | Positive | None |
Table 2.
Changes in serological values before and after PEDD
| Case no. | Highest preoperative CRP | Time to normal value (weeks) | Highest preoperative ESR | Time to half of original pretreatment value (weeks) |
|---|---|---|---|---|
| 1 | 25 | 26 | ||
| 2 | 97 | 5 | 38 | 9 |
| 3 | 15 | 2 | 60 | 6 |
| 4 | 60 | 77 | ||
| 5 | 182 | 129 | ||
| 6 | 239 | 125 | ||
| 7 | 147 | 4 | 57 | 7 |
| 8 | 18 | 2 | 57 | 6 |
| 9 | 49 | 3 | 95 | 6 |
| 10 | 65 | 4 | 85 | 8 |
| 11 | 11 | 1 | 35 | 3 |
| 12 | 127 | 3 | 116 | 5 |
| 13 | 20 | 1 | 52 | 3 |
| 14 | 31 | 2 | 33 | 4 |
| 15 | 96 | 3 | 69 | 5 |
Discussion
The primary goals in treating infectious spondylitis are to make a diagnosis, isolate the organism responsible, and prescribe effective antibiotic therapy. Conservative therapy is adequate for mild cases and those detected early. Surgical intervention is indicated for patients with significant neurological deficit, large epidural abscesses, extensive vertebral body destruction, severe kyphotic deformity or spinal instability, or failed antibiotic therapy [4, 13, 16, 21]. Diagnosis and treatment can be delayed when patient complaints are relatively non-specific. Imaging study and a high degree of suspicion can alert surgeons to a correct diagnosis; however, tissue must be obtained for confirmation by biopsy. Needle aspiration is recommended for the isolation of offending pathogens. However, the aspirate is often inadequate and, sometimes, no organism is cultured [3, 5, 7, 8].
Percutaneous endoscopic discectomy was first employed for treating uncomplicated herniated discs in the early 1980s. Recently, numerous minimally invasive, percutaneous, endoscopic procedures for lumbar disc herniation have been developed for spinal surgery, with clinical outcomes comparable to those of conventional open surgery [11, 25]. The minimal invasiveness and simplicity of the technique led the authors to apply it in treating infectious spondylitis in select cases. Obtaining a sufficient amount of material for microbiological examination directly from the infected region is commonly possible. Additionally, eradication and irrigation of infected and necrotic tissue from a disc and even an epidural space facilitate a fenestration similar to open debridement [1, 9].
In cases of spinal infection the reported accuracy of spinal biopsy varies from 36–76%, according to the organism isolated [5, 8, 19, 23, 24]. Fouquet et al. obtained bacteriological diagnosis in nine (36%) of 25 patients biopsied, using a Mazabraud trocar [5]. Staatz et al. reported 16 (76%) positive cultures for 21 patients, using CT-guided, percutaneous, catheter drainage [23]. Rankine et al., who analysed 20 patients undergoing percutaneous spinal biopsy for spinal infection, isolated an organism in six of 12 patients not taking antibiotics, whereas an organism was isolated in only two of eight patients taking antibiotics [19]. The results of percutaneous needle biopsy may be negative for various reasons, including inadequate amount of specimen, sampling error, or previous antibiotic therapy. Causative pathogens were identified in 13 (86.7%) of 15 patients in this series. The positive culture rate is comparable to that obtained with open biopsy and superior to that from needle aspiration. A sufficient amount of specimen can be obtained through PEDD under local anaesthesia, avoiding the potential morbidity associated with an extensive surgical procedure, and achieves a high diagnostic rate.
Two culture results of debrided infective disc tissue were not false negative as no pathogens were identified in one patient despite open biopsy and debridement one week after PEDD. This negative finding may have resulted from previous empirical antibiotic therapy. Tuberculous spondylitis in the other patient was confirmed eight months later following surgical intervention; this patient had a past history of pulmonary tuberculosis and had undergone continuous anti-tuberculous chemotherapy.
Haaker et al. treated 16 patients with lumbar disc infection, using percutaneous lumbar discectomy. Diagnostic biopsy identified the specific infection in 45% of cases, and only three patients were treated surgically. The authors concluded that percutaneous lumbar discectomy is a very useful, minimally invasive procedure for conservative treatment of lumbar discitis [8]. With improved instruments and techniques, extensive debridement and eradication of the infected tissue can be achieved. Combined with good drainage and full-course antimicrobial therapy, it allowed favourable outcomes in 11 patients in this study. Relief of intradisc pressure, irrigation of inflammatory factors, adequate debridement, and a minimally invasive procedure preserving adequate stability contribute to immediate back pain relief. Moreover, with a postoperative negative-pressure Hemovac, the pathogens and infected tissue can continue to be sucked out.
Progressive symptoms occurred in four patients due to poorly controlled infection in complicated spondylitis or significant mechanical back pain after PEDD. Of these four patients, two had intractable back pain and underwent surgical treatment for active infection within two weeks of PEDD. In the other two operatively treated patients the symptoms were attributed to instability or kyphotic deformity caused by extensive osteolytic destruction of the vertebral body. When more extensive bone destruction is identified, open debridement and bone grafting can provide adequate stability and relief of symptoms. These factors should be considered as exclusion criteria for this procedure. With strict clinical selection, PEDD and associated full-course antimicrobial treatment can achieve a better outcome.
As arthroscopic lavage has proven effective and results in lower morbidity in the treatment of septic arthritis in large diarthrodial joints, PEDD can also be successfully employed to treat early stage infectious spondylitis [12, 22]. Early diagnosis of the aggressive infectious process, with prompt and appropriate antibiotic therapy, is crucial to successful treatment of spondylitis and prevention of its dreadful complications. This technique produces less morbidity than open treatment and, in this study, effectively relieved patients’ symptoms, provided a bacteriological diagnosis, and facilitated the eradication of infection.
On the basis of analytical results in this study, the authors propose that PEDD is an effective alternative to spinal biopsy and should be considered prior to extensive anterior surgery for infectious spondylitis in carefully selected cases. It is especially recomended for patients with early stage spinal infection or serious medical problems.
Acknowledgments
S.-C.Y performed the surgery, prepared this article, and reviewed the references; T.-S.F. performed the surgery, designed the study, and analysed the data; P.-L.L., C.-C.N., L.-H.C. and W.-J.C. revised the article for intellectual content.
References
- 1.Bavinzski G, Schoeggl A, Trattnig S, Standhardt H, Dietrich W, Reddy M, Al-Schameri R, Horaczek A (2003) Microsurgical management of postoperative disc space infection. Neurosurg Rev 26:102–107 [DOI] [PubMed]
- 2.Carragee EJ (1997) Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am 79:874–880 [DOI] [PubMed]
- 3.Digby JM, Kersley JB (1979) Pyogenic non-tuberculous spinal infection: an analysis of thirty cases. J Bone Joint Surg Br 61:47–55 [DOI] [PubMed]
- 4.Emery SE, Chan DP, Woodward HR (1989) Treatment of hematogenous pyogenic vertebral osteomyelitis with anterior debridement and primary bone grafting. Spine 14:284–291 [PubMed]
- 5.Fouquet B, Goupille P, Jattiot F, Cotty P, Lapierre F, Valat JP, Amouroux J, Benatre A (1992) Discitis after lumbar disc surgery. Features of “aseptic” and “septic” forms. Spine 17:356–358 [DOI] [PubMed]
- 6.Fraser RD, Osti OL, Vernon-Roberts B (1987) Discitis after discography. J Bone Joint Surg Br 69:26–35 [DOI] [PubMed]
- 7.Gebhard JS, Brugman JL (1994) Percutaneous discectomy for the treatment of bacterial discitis. Spine 19:855–857 [DOI] [PubMed]
- 8.Haaker RG, Senkal M, Kielich T, Kramer J (1997) Percutaneous lumbar discectomy in the treatment of lumbar discitis. Eur Spine J 6:98–101 [DOI] [PMC free article] [PubMed]
- 9.Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ (2000) Hematogenous pyogenic spinal infections and their surgical management. Spine 25:1668–1679 [DOI] [PubMed]
- 10.Hadjipavlou AG, Katonis PK, Gaitanis IN, Muffoletto AJ, Tzermiadianos MN, Crow W (2004) Percutaneous transpedicular discectomy and drainage in pyogenic spondylodiscitis. Eur Spine J 13:707–713 [DOI] [PMC free article] [PubMed]
- 11.Hermantin FU, Peters T, Quartararo L, Kambin P (1999) A prospective, randomized study comparing the results of open discectomy with those of video-assisted arthroscopic microdiscectomy. J Bone Joint Surg Am 81:958–965 [DOI] [PubMed]
- 12.Ivey M, Clark R (1985) Arthroscopic debridement of the knee for septic arthritis. Clin Orthop 199:201–206 [PubMed]
- 13.Krodel A, Sturz H, Siebert CH (1991) Indications for and results of operative treatment of spondylitis and spondylodiscitis. Arch Orthop Trauma Surg 110:78–82 [DOI] [PubMed]
- 14.Maiuri F, Iaconetta G, Gallicchio B, Manto A, Briganti F (1997) Spondylodiscitis. Clinical and magnetic resonance diagnosis. Spine 22:1741–1746 [DOI] [PubMed]
- 15.Nawfel RD, Judy PF, Silverman SG, Hooton S, Tuncali K, Adams DF (2000) Patient and personnel exposure during CT fluoroscopy-guided interventional procedures. Radiology 216:180–184 [DOI] [PubMed]
- 16.Ozuna RM, Delamarter RB (1996) Pyogenic vertebral osteomyelitis and postsurgical disc space infections. Orthop Clin North Am 27:87–94 [PubMed]
- 17.Paulson EK, Sheafor DH, Enterline DS, McAdams HP, Yoshizumi TT (2001) CT fluoroscopy–guided interventional procedures: techniques and radiation dose to radiologists. Radiology 220:161–167 [DOI] [PubMed]
- 18.Perronne C, Saba J, Behloul Z, Salmon-Ceron D, Leport C, Vilde JL, Kahn MF (1994) Pyogenic and tuberculous spondylodiskitis (vertebral osteomyelitis) in 80 adult patients. Clin Infect Dis 19:746–750 [DOI] [PubMed]
- 19.Rankine JJ, Barron DA, Robinson P, Millner PA, Dickson RA (2004) Therapeutic impact of percutaneous spinal biopsy in spinal infection. Postgrad Med J 80:607–609 [DOI] [PMC free article] [PubMed]
- 20.Rohde V, Meyer B, Schaller C, Hassler WE (1998) Spondylodiscitis after lumbar discectomy. Incidence and a proposal for prophylaxis. Spine 23:615–620 [DOI] [PubMed]
- 21.Sapico FL (1996) Microbiology and antimicrobial therapy of spinal infections. Orthop Clin North Am 27:9–13 [PubMed]
- 22.Smith MJ (1986) Arthroscopic treatment of the septic knee. Arthroscopy 2:30–34 [DOI] [PubMed]
- 23.Staatz G, Adam GB, Keulers P, Vorwerk D, Gunther RW (1998) Spondylodiskitic abscesses: CT-guided percutaneous catheter drainage. Radiology 208:363–367 [DOI] [PubMed]
- 24.Vinicoff PG, Gutschik E, Hansen SE, Karle A, Rieneck K (1998) CT-guided spinal biopsy in spondylodiscitis. Ugeskr Laeger 160:5931–5934 [PubMed]
- 25.Yeung AT, Tsou PM (2002) Posterolateral endoscopic excision for lumbar disc herniation: surgical technique, outcome, and complications in 307 consecutive cases. Spine 27:722–731 [DOI] [PubMed]