Abstract
The anatomy of the proximal femur was studied in 35 specimens using quantitative computed tomography (QCT) and compared with anatomical sections studied by plane radiography and gross dissection. We found the primary supporting structure of the femoral head to be the primary compressive strut, which is a dense column of trabecular bone projecting from the pressure buttress of the medial femoral neck to the epiphyseal scar. Trabecular bone mushroomed from the epiphyseal scar and terminated at right angles to the cortex of the femoral head. We believe the primary compressive strut is the predominant load-bearing structure connecting the femoral head to the femoral neck, as many specimens lacked continuity of the head cortex to the femoral neck. Based on the CT number, the primary compressive strut had similar bone density to cortical structures such as the lesser trochanter, calcar femorale and posterior lateral femoral cortex. Ward’s triangle lacked structural integrity in many cases, and we doubt the significance of tensile trabculae for sharing load. Surgical techniques such as femoral fracture fixation, resurfacing hip arthroplasty and allograft transplantation may benefit from this knowledge.
Résumé
L’anatomie de l’extrémité supérieure du fémur a été étudiée chez 35 sujets à partir d’études de type scanner et comparée avec des sections anatomiques, des radiographies et des dissections. Nous avons étudié les différentes structures qui supporte la tête fémorale. Les coupes scanner montrent que la densité osseuse de ces structures est identique à celle du trochanter et de la corticale de la face postérieure du fémur. Les techniques chirurgicales concernant l’extrémité supérieure du fémur, fixation de fractures, resurfaçage, arthroplastie de resurfaçage et al. logreffes doivent bénéficier de ce travail anatomique et radiologique.
Introduction
Numerous studies have attempted to define the bony architecture of the proximal femur in terms of human function [6, 8, 24]. According to Wolf’s law, the anatomical form corresponds to mechanical stress patterns realised within the bone [25]. Previous authors have applied engineering concepts to this analysis, suggesting the presence of both compressive and tensile forces within the proximal femur. This would enhance the value of such areas of the greater trochanter and the superior femoral neck. However, simplistic two-dimensional anatomical and force models may not adequately predict the stresses present in such a three-dimensional structure [3, 5, 19, 21]. Additionally, there is a misunderstanding in the contemporary literature regarding the identity of the calcar femorale, which is often believed to disappear completely through middle age [9].
Quantitative computed tomograhy (QCT) is an excellent tool for the study of the distribution of cortical and trabecular bone in the human body. The cross-sectional presentation of QCT gives a clear unambiguous picture of the internal anatomic structure [14]. The ability to reformat axial data to form an image in one plane within the data set further elucidates the anatomy. By using appropriate calibrations, the digital nature of QCT allows calculations of bone mineral density [7, 10, 15, 22, 23]. Elke et al. used QCT to assess the three-dimensional cancellous structure of the proximal femur and focused on the trabecular structure of the epiphyseal plate in the subchondral area of the femoral head [6].
We wanted to investigate the anatomy of the proximal femur and determine the most substantial and persistent structures over a large spectrum of anatomical specimens. Specifically, we wanted to evaluate the structures of the proximal femur and discover if we could correlate anatomical structure and morphology with bone density, which is likely to explain mechanical properties of the bone. Our hypothesis was that the most important structures will have the greatest bone density and will be aligned in a fashion to carry the predominant loads of the hip joint. We used a combination of quantitative computed tomography, plain radiography and gross dissection to define the femoral anatomy and then compared these findings with the existing literature.
Materials and methods
Demographics The proximal morphology of the proximal femur was studied in 35 specimens. This included 22 cadaver femora in 9 males and 2 females with a mean age of 62 years (range, 21 to 83 years) and 6 live subjects (12 femora). The latter comprised three males and three females with a mean age of 46 years (range, 26 to 71). An Investigational Review Board had approved participation in this study based on the apparent risks and goals.
Anatomical analysis All cadaver femora were dissected free of soft tissue and studied in detail. In order to elucidate the morphology of the proximal femur, the specimens were sectioned in 10-mm-thick slices in various planes. This included sectioning parallel to the femoral shaft (three femora), parallel to the ante version plane (one femur) and oblique to the ante version plane (three femora). Very soft trabecular bone and marrow elements were gently removed with a curette. More dense trabecular bone with definite structural capability was left in place.
Imaging analysis Anterior/posterior radiographs were done on 29 femora. QCT scans were then obtained through the proximal portions of femurs, which were supported by a rigidly constructed fixture attached to the QCT table. Four specimens were scanned in-situ within the cadaver pelvis, and three additional femurs (two embalmed and one dry) were scanned in a pelvis simulation water tank. Six in-vivo femurs were studied in healthy patients. All computed tomography scans were performed with a General Electric C.T. 9800 model (General Electric Medical Systems, Milwaukee, Wisconsin). Contiguous 1.5-mm scans were performed on all samples from the level of the mid-lesser trochanter to the superior margin of the femoral head. These images were reconstructed using the “Bone Detail” algorithm with a 12-cm field of view (pixel size =0.25 mm ). Separate reconstructions were performed for right and left samples in the cadaver and phantom studies. From the axial image set, an axial image was chosen that included the greater trochanter, neck and inferior portion of the femoral head. A paraxial reformation plane bisecting the femoral head and neck (anteversion plane) was prescribed from this image using standard reformation software. Additional oblique images were prescribed from either the axial or paraxial reformatted images. The paraxial and oblique reformatted images were generally 1-pixel (0.25 mm) thick.We quantified bone density using the most obvious morphological features that coud be determined from the reformatted computed tomograms. In addition, we were able to make generalisations from comparing the various factors such as the age, gender and CT number of the specific regions examined. The current study was part of a larger research effort aimed at using quantitative computed tomography to assess bone density [13]. In that process, we discovered that simply using the CT number was not always straightforward, as the resulting density could be influenced by the presence of red and yellow marrow. There appeared to be a linear relationship though between the CT number and comparison with a known standard of bone density that we had constructed from ashed mineral bone by weight and volume. A typical CT scan analysis revealed assessment of the bone density in Hounsfield units, demonstrating the progressive nature of the most dense to least dense structures of the proximal femur (Table 1).
Table 1.
Typical relative bone density of the anatomical structures of the proximal femur is demonstrated
| Feature CT | Number (Hounsfield units) |
|---|---|
| Pressure buttress | 1,500 HU |
| Medial cortex of the femoral shaft | 1,300 HU |
| Lateral cortex of the femoral shaft | 1,000 HU |
| Superior cortex of the femoral neck | 600 HU |
| Calcar femorale | 400 HU |
| Primary compressive strut | 400 HU |
| Posterior lateral cortex | 350 HU |
| Lesser trochanter | 350 HU |
| Cortex of the femoral head | 300 HU |
(Key: 10 HU equals an increase in the density of water by 0.1%)
Gross anatomical analysis Gross anatomical correlation was obtained by sectioning three femurs perpendicular to the axis of the shaft with 1-cm-thick sections and a fourth femur parallel to the plane of anteversion. These bone slices were then radiographed using a mammographic imaging system using the mammographic imaging protocol. The mammographic technique gives a resolution in the order of 25 microns compared to that of conventional CT scanning, which is about 500 microns. Three additional femurs were sectioned in the oblique plane bisecting the anteversion plane of the neck. In all dissected specimens, a curette was used to remove all cancellous bone that yielded to light pressure. What remained was dense trabecular and cortical bone, which comprises the primary support structure of the proximal femur.
Results
The proximal femur sustains loads through the pressure buttress of the medial femoral neck, which is the most substantial structure of this region (Table 1). From bone mass information derived from the CT images, the predominant loading structure from the femoral head to the pressure buttress is the primary compressive strut. This structure is a dense trabecular mass that extends from the epiphyseal scar, which approximates the center or nucleus of the femoral head, to the pressure buttress of the femoral neck. The angle of the longitudinal trabecular structure of the primary compressive strut is typically 25° to the axis of the femoral shaft (Figs. 1, 2, 3 and 4).
Fig. 1.
Coronal section of anatomical proximal femoral specimen reveals: a trabecular mushroom of femoral head; b primary compressive strut; c pressure buttress of the medial femoral neck; d Ward’s triangle
Fig. 2.
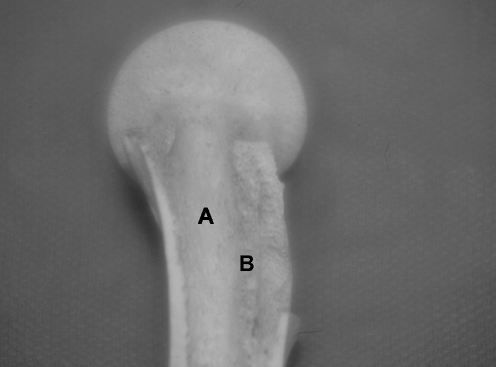
Anterior-posterior view of dissected anatomical specimen demonstrates: a primary compressive strut; b pressure buttress of the medial femoral neck; c calcar femorale
Fig. 3.
Proximal femoral head/neck specimen after all soft cancellous bone is removed reveals: a trabecular mushroom; b medial femoral neck cortex. Note the “dumbbell” extensions toward the anterior and posterior walls of the acetabulum
Fig. 4.
Lateral view of dissected anatomical specimen reveals: a pressure buttress of the medial femoral neck; b calcar femorale
At the level of the epiphyseal scar, trabecular bone radiated or “mushroomed” from the nucleus of the femoral head terminating at right angles to the thin cortical shell of the femoral head (Fig. 4). Transverse cuts through the femoral head revealed areas of increased density that would oppose the anterior, superior and posterior articular surfaces of the acetabulum, while the segment near the cotyloid notch was less dense. These structures mirror the “dumbbell”-shaped extensions defined by the model of Elke et al. [6]. A dense trabecular support of the superior femoral neck cortex was noted to extend along the epiphyseal scar to the primary compressive strut. A more variable support extended from the medial epiphyseal scar to the inferior femoral head. In many cases, we found a lack of structural continuity between the cortex of the femoral head and the femoral neck. Our conclusion was that loading must extend from the femoral head through the primary compressive strut to the femoral shaft and that the femoral head does not significantly load through the superior or inferior femoral neck cortex.
The calcar femorale is a bony projection that extends laterally from the posterior medial femoral neck cortex at about the mid-level of the lesser trochanter (Figs. 2 and 3). This structure was seen in all specimens and formed a posterior base of the primary compressive strut. The most dense bone, also known as the “femoral spur,” originated from the medial cortex and diminished as it fanned to the lateral cortex, being absent in osteoporotic specimens.
In general, trabecular bone in the region of the greater trochanter was variable and of low density. There was little consistency of the trabecular architecture, and therefore, no definite structural orientation was described. The central radiolucency of the proximal femur, generally known as Ward’s triangle, was lateral to the hemi-cylindrical dense trabecular bone of the primary compressive strut. It was bounded superiorly by the superior cortex of the femoral neck.
Using the reformation planes, the anatomy of the proximal femur revealed the femoral helix or anterior twist of the metaphysis in this region. Though variable in the amount of anteversion between specimens, this important feature could be identified with QCT. Interestingly, the gross anatomical specimens best demonstrated the significant anterior flexion and rotation of the femoral metaphysis in relation to the femoral shaft axis. The hemi-cylindrical column of the dense medial femoral neck, primary compressive strut and trabecular “mushroom” in the femoral head made a gradually consistent twist starting at the lesser trochanter and extending to the subchondral bone of the head.
Discussion
We were able to identify that the anatomical connection of the femoral head to the femoral neck occurs through a dense trabecular buttress (primary compressive strut) that arises from the pressure buttress of the medial femoral neck and extends to the epiphyseal scar of the femoral head. From the epiphyseal scar of the femoral head, trabecular bone radiates or “mushrooms” to the articular surface of the femoral head. Elke et al. made similar findings of the trabecular bone of the femoral head and noted the “dumbbell” distribution of the dense cancellous bone in the femoral head so as to abut the anterior and posteriour columns of the acetabulum [6] (Fig. 4). We found the tensile trabecular bone in Ward’s triangle to be of unknown mechanical significance, disappearing with greater levels of osteoporosis. We conclude that the primary load-bearing structures of the proximal femur are the pressure buttress of the femoral neck, the primary compressive strut and the trabecular “mushroom” of the femoral head. This conclusion is strengthened by the observation that the cortical shell of the femoral head is often discontinuous with the cortex of the femoral neck. More importantly, the primary compressive strut exhibited a relative CT number comparable to lesser cortical structures such as the calcar femorale, lesser trochanter and posterior lateral cortex of the femoral neck.
The three-dimensional aspects of the femoral anatomy are important in understanding the biomechanical loading of the hip joint. Since the axis of the femoral shaft is directed 10 degrees lateral to the longitudinal axis of the body, the orientation of the trabecular buttress of the femoral neck (typically 25 degrees from the axis of the femoral shaft) is approximately 15–20 degrees away from the longitudinal axis. Thus, there is excellent agreement between the orientation of this buttress and the direction of the primary joint reaction forces determined by biomechancial analysis [2]. Another finding is that the trabecular buttress is perpendicular to the epiphysis. Thus, the component of sheer force across the epiphysis is zero with normal weight-bearing.
From a biomechanical point of view, most authors have argued that bone mass distribution and mechanical properties reflect a specific loading history or function [5, 10]. In the proximal femur, earlier biomechanical models attempted to explain trabecular distribution based on the single-limb stance phase of walking, resulting from either a static load or a single typical load to which the bone is exposed [1, 10, 21]. However, the properties of this complex structure cannot be simply defined by a two-dimensional cantilever bending approach. Our work supports the three-dimensional nature of the proximal femur as suggested over the years by several anatomists [8, 12].
Prior finite element models of the proximal femur have attempted to create bone anisotropy of trabecular orientation to study applied stresses. Using these techniques, Carter was able to develop a scenario where mechanical stresses and ultimately bone morphology were determined by the cumulative influence of many loads from multiple directions [5]. It is interesting that the remodelling trends predicted in that model followed identically with the morphological changes seen in aging as identified by our study, that is, loss of bone in the arcuate system and Ward’s triangle with increasing prominence of bone in the area of the primary compressive strut. After the anatomical findings of Humphrey in 1858, the finite element model found that the principle trabecular orientations were perpendicular to the joint surface in the femoral head, and all were directed to the centre of the femoral head [12]. This would define the trabecular “mushroom” configuration as identified in our study and parallels the findings of Elke et al. [6].
Other mechanical studies have emphasised the importance of the trabecular bone in the proximal femur. Brown noted a prominent stiffness elevation of 160 to 400% in regions traversed by the primary trabecular systems [3]. Martens et al. found that by removing the trabecular bone from the proximal femur, they weakened the structure by approximately 50% [16]. With aging, there is a significant loss in tensile strength of cortical and cancellous bone due to atrophy. Diaphyseal cortical bone adjusts to these changes with net periosteal accretion and endosteal bone absorption, which tends to enlarge the bone cross-section [20]. The mechanical effect of this geometrical enlargement is to increase structural stiffness, which compensates for loss of bone strength. Beck et al. were able to show that the femoral neck section moduli in the elderly were on the average within 14% of young values in females and within 6% in males [1]. In the proximal femoral metaphysis, periosteal changes are not seen and hence the increasing weakness of this area with age. In metaphyseal areas, it is likely that trabecular bone strength is affected more rapidly with osteoporosis. As previously demonstrated by others, complete replacement of Ward’s triangle with yellow marrow is seen in certain specimens [11]. We would argue the limited importance of the tensile arcuate trabeculae located in the superior femoral neck as suggested by the trajectory theory of Wolff [25]. In fact, previous anatomical studies have shown this system to be severely atrophic in the valgus femoral neck and with postmenopausal osteoporosis [8, 11].
The other morphological issue is the substantial anatomical variability that many authors have demonstrated regarding such proximal femoral features as shaft-head offset, centre-column-diaphyseal (CCD) angle, metaphyseal flare index and calcar/cortical ratio [18–20]. Each patient has individual heredity, developemental and constitutional factors that determine the shape, bone density and mechanical integrity of the proximal femur. With aging and osteoporosis, the “champagne flute” appearance of the metaphyseal canal becomes more tubular, while there is marked atrophy of internal structures [18]. Despite this general trend, it is very difficult to take an individual feature such as the CCD angle and predict bone strength or the likelihood of femoral neck fracture. Nonetheless, we find our anatomical correlation of the persistence of the primary compressive and femoral head trabecular mushroom in ostoeoporotic specimens to be important. This is contrasted with the progressive atrophy of other structures such as Ward’s triangle, calcar femorale and the superior acuate trabecular complex.
For the surgeon, this information can be quite helpful. Chandler et al. have emphasised the need to line up the trabecular structure of the femoral head with the lines of loading stress when an allograft is used for acetabular reconstruction [4]. Placement of internal fixation for a femoral neck fracture can be optimised by placing a screw into the dense trabecular structure of the primary compressive strut and the center of the femoral head. For prosthetic implant design, as most of the stress transfer goes down the primary compressive strut to the pressure buttress of the medial femoral neck, it is logical to interface the femoral stem with the pressure buttress [17]. Finally, for hip resurfacing devices, any condition that weakens the primary compressive strut will expose this reconstruction to potential fracture.
Based on an anatomical study complimented by quantitive computed tomography, we have identified the primary loading structures of the proximal femur to be the trabecular “mushroom” above the epiphyseal scar, the primary compressive strut and the pressure buttress of the medial femoral neck. The femoral head does not significantly load through the superior or inferior femoral neck extensions. The primary compressive strut is a substantial trabecular structure that had a relative CT number comparable to lesser cortical structures. We believe that the proximal femur has a complex three-dimensional nature that must be considered for accurate theoretical models. Surgical techniques such as femoral fracture fixation, resurfacing hip arthroplasty and allograft transplantation will benefit from this knowledge.
References
- 1.Beck TJ, Looker AC, Ruff CB, Sievanen H, Wahner HW (2000) Structural trends in the aging femoral neck and proximal shaft: analysis ot the Third National Health and Nutrition Examination Survey dual-energy X-ray absorptiometry data. J Bone Miner Res 15:2297–2304 [DOI] [PubMed]
- 2.Bergmann G, Rohlmann A, Graichen F (1989) Invivo messung der Hüftgelenksbelastung: Teil: Krankengymnastik. Z Orthop 127:672–679 [DOI] [PubMed]
- 3.Brown TD, Ferguson AB (1980) Mechanical property distributions in the cancellous bone of the human proximal femur. Acta Scand Ortho 51:429–437 [DOI] [PubMed]
- 4.Chandler HP (1995) Structural grafting of the acetabulum. Orthopaedics 18:863–864 [DOI] [PubMed]
- 5.Carter DR, Orr TE, Fyhrie DP (1989) Relationships between loading history and femoral cancellous bone architecture. J Biomech 22:231–244 [DOI] [PubMed]
- 6.Elke RPE, Cheal EJ, Simmons C, Poss R (1995) Three-dimensional anatomy of the cancellous structures within the proximal femur from computed tomography data. J Orthop Res 13:513–523 [DOI] [PubMed]
- 7.Esses SI, Lotz JC, Hayes WC (1989) Biomechanical properties of the proximal femur determined in-vitro by single-energy quantitative computed tomography. J Bone Miner Res 4:715–722 [DOI] [PubMed]
- 8.Garden RS (1961) The structure and function of the proximal end of the femur. J Bone Jt Surg 43B:576–589
- 9.Griffin JB (1982) The calcar femorale redefined. Clin Ortho Rel Res 164:211–214 [PubMed]
- 10.Hayes WC, Snyder BD (1981) Toward a quantitative formulation of Wolff’s law in trabecular bone. In: Cowin SC (ed) Mechanical properties of bone. American Society of Mechanical Engineers, New York, pp 43–68
- 11.Hedlund LR, Gallagher JC (1989) The effect of age and menopause on bone mineral density of the proximal femur. J Bone Miner Res 4:639–642 [DOI] [PubMed]
- 12.Humphrey GM (1958) A treatise of the human skeleton. Macmillan and Co., Cambridge
- 13.Jacobson D (1991) Bone morhpometry and mineral density measurement using quantitative computed tomography. PhD Disertation, Medical College of Wisconsin, Milwaukee, Wisconsin, p 244–261
- 14.Kerr R, Resnick D, Pineda C (1988) CT analysis of proximal femoral trabecular pattern simulating skeletal pathology. J Comput Assist Tomogr 12:277–280 [DOI] [PubMed]
- 15.Lotz JC, Gerhart TN, Hayes WC (1989) Mechanical properties of trabecular bone from the proximal femur: a quantitative CT study. J Comput Assist Tomogr 14:107–114 [DOI] [PubMed]
- 16.Martens M, Van Audekercke R, Delport P, De Meester P, Mulier JC (1983) The mechanical characteristics of cancellous bone at the upper femoral region. J Biomechanics 16:971–983 [DOI] [PubMed]
- 17.Noble PC, Alexander JW, Lindahl LJ, Yew DT, Granberry WM, Tullos HS (1988) The anatomic basis of femoral component design. Clin Ortho Rel Res 235:148–165 [PubMed]
- 18.Noble PC, Box GG, Kamaric E, FinkMJ, Alexander JW, Tullos HS (1995) The effect of aging on the shape of the proximal femur. Clin Orthop 316:31–44 [PubMed]
- 19.Phillips JP, Williams JF, Melick RA (1975) Prediction of the strength of the neck of femur from its radiological appearance. Biomed Eng 10:367–372 [PubMed]
- 20.Ruff CB, Hayes WC (1983) Cross-sectional geometry of Pecos Pueblos femora and tibia: a biomechanical investigation. I. Method and general patterns of variation. Am J Phys Anthropol 60:359–381 [DOI] [PubMed]
- 21.Rybicki EF, Simonen FA, Weis EB (1972) On the mathematical analysis of stress in the human femur. J Biomech 5:203–215 [DOI] [PubMed]
- 22.Sartoris DJ, Resnick D, Bielecki D, Gershuni D, Meyers M (1988) Computed tomography with multiplanar reforamation and three-dimensional image reconstruction in the preoperative evaluation of adult hip disease. Int Orthop(SICOT) 12:1–8 [DOI] [PubMed]
- 23.Snyder SM, Schneider E (1991) Estimation of mechanical properties of cortical bone by computed tomography. J Orthop Res 9:422–431 [DOI] [PubMed]
- 24.Ward FO (1838) Outlines of human osteology. Henry Renshaw, London, pp 370
- 25.Wolff J (1870) Uber die innere Architecktur der Knochen und ihre Bedentung fur die Frage vom Knochenwachstum. Virchow Arch Path Anat 50:389 [DOI]