Abstract
Diffusion-weighted MRI (DWI) has been proposed as a method to evaluate the integrity of white matter tracts in the spinal cord. The integrity of the spinal cord reflects the exact stage of traumatic injury. The purpose of this study was to evaluate the diagnostic value of DWI in SCIWORA in the thoracic spine. A total of five patients with thoracic SCIWORA underwent MRI and DWI within 48 h of injury. DWIs were obtained with a single-shot fast spin-echo (SSFSE) sequence; TI- and T2-weighted images were obtained with spin echo. Consistency among the clinical presentation, outcome, MRI and DWI was analysed. There was complete injury in one patient and partial in four patients. Four patients showed hypointense T1-weighted signal and hyperintense T2-weighted signal, and one patient had no changes on MRI. All patients showed hyperintense signal on DWI. Two patients made good recoveries (ASIA grades D and E), one had a moderate recovery (ASIA grade C), and two showed minimal or no improvement (ASIA grade A or B) in neurological function. Patients with no cord changes on MRI showed abnormal signals on DWI. It is likely that in the future DWI may provide important information complimentary to conventional MRI and allow a better prognostic evaluation of recovery from SCIWORA.
Résumé
L’IRM en séquence de diffusion (DWI) a été proposé pour évaluer l’intégrité de la substance blanche au sein de la moelle spinale dont l’état reflète exactement les lésions traumatiques. L’étude présentée évalue la valeur diagnostique de la DWI dans les syndromes de traumatisme médullaire sans anomalie radiographique (SCIWORA) au niveau du rachis thoracique. Cinq patients avec un tel syndrome ont eu une IRM et une DWI dans les 48 h suivant le traumatisme. L’aspect clinique, l’évolution, l’IRM et la DWI ont été analysé. Il y avait une lésion complète chez un patient et des lésions partielles chez quatre. En IRM quatre patients avaient un signal hypointense en T1 et un signal hyperintense en T2 et un patient n’avait pas de modification visible. Tous les patients avaient un signal hyperintense en DWI. Deux patients avaient une bonne récupération neurologique (grade ASIA D et E), un avait une récupération modérée (grade ASIA C) et deux avaient une récupération minime ou nulle (grade ASIA A ou B). Les patients sans anomalie à l’IRM avaient des signaux anormaux en DWI. Dans le futur la DWI pourra apporter des informations complémentaires à l’IRM conventionnelle et permettre une meilleure évaluation du pronostic des traumatismes médullaires sans anomalie radiographique.
Introduction
Spinal cord injury without radiographic abnormality (SCIWORA) is a syndrome describing spinal cord injury without evidence of fracture or dislocation of the spine on plain radiographs or computerised tomography (CT) studies [4, 8]. The incidence, pathogenesis and severity of SCIWORA are different for various age groups due to anatomical and biomechanical differences in the spine [17, 19]. Conventional magnetic resonance imaging (MRI) remains the best clinical method for the evaluation of traumatic injury to the spinal cord. MRI is helpful in both the diagnosis and prognosis of SCIWORA because of the better contrast resolution, absence of bony artifacts and multiplanar imaging [4]. Moreover, it provides adequate information about neural and extraneural injuries and identifies, for example, epidural heamatomas and significant disc herniations, requiring surgical intervention.
Although the degree of spinal cord compression, amount of heamorrhage and site of the injury are well visualised with MRI, the integrity of white matter tracts within the spinal cord are not well demonstrated. Diffusion-weighted MRI (DWI) has been proposed as a method to evaluate the integrity of white matter tracts in the spinal cord. DWI measures the free diffusion or Brownian motion of water molecules, thereby providing information beyond the anatomical or spatial resolution of conventional MRI techniques. The diffusion of water in the spinal cord is thought to be affected both by diffusion barriers formed by cell membranes and myelin sheaths and by its location (intracellular or extracellular). Therefore, DWI has the potential to detect axotomy, myelin disruption and axonal swelling. However, there have been no previous studies using DWI to examine SCIWORA. This study highlights the diagnostic value of DWI in SCIWORA in the thoracic spine.
Materials and methods
All MRI studies were performed on a 1.5-T Intera Master MRI system (Intera Master, Philips, The Netherlands). TI- and T2-weighted images were obtained with spin echo. All patients underwent spin-echo TI (TR 500 s; TE 15 s) and T2 (TR 4,800 s; TE 120 s) (matrix size 256×256; slice thickness 3 mm; slice gap 3 mm; FOV 230 mm for the axial plane and 250 mm for the sagittal plane).
DWI were obtained with a single-shot fast spin-echo (SSFSE) sequence in which motion-probing gradients were applied in the cephalocaudal direction with b values of 0 and 400 s/mm2. Other scanning parameters for DWI were as follows: TR 5,000 s, TE 100 s, imaging matrix 128×128, slice thickness 3 mm, slice gap 0, NEX 1 and FOV 230 mm for the sagittal plane. No cardiac gating and respiratory compensation were used.
Out of a total of five patients with SCIWORA treated between June 2004 and June 2005, there were four cases of road traffic accidents (RTAs) and one case of sports-related injury (SRI) in this study. The ages ranged from 6 to 45 years (mean, 27.8 years). There were three males and two females. The lesions were all located in the thoracic area. All patients underwent MRI and DWI within 48 h of injury, although the time interval between injury and MRI was variable. All patients underwent MRI of the spine in the sagittal, axial and coronal planes with TI and T2. Patients reporting within 8 h of injury were put on methylprednisolone infusion at doses of 30 mg/kg body weight for the first hour, followed by 5.4 mg/kg body weight for the next 23 h.
Results
Clinical data
Analysis of the clinical presentation revealed cord injury at the time of admission to be complete in one patient and partial in four patients. The clinical features of the patients are given in Table 1. Four patients showed hypointense T1-weighted signal and hyperintense T2-weighted signal, and one patient had no changes on MRI. All patients showed hyperintense signal on DWI. The assessment of the ASIA grade [1] revealed that two patients had good recoveries (ASIA grades D and E), one had a moderate recovery (ASIA grade C), and two showed minimal or no improvement (ASIA grades A or B) in neurological function. All patients were followed up at 1-, 3- and 6-month intervals.
Table 1.
Summary of patient demographics, ASIA Impairment Scale and MR imaging
| Case no. | Age (years) | Sex | Cause of injury* | Injury level | ASIA Scale before treatment | MRI** | DWI** | ASIA Scale after treatment*** |
|---|---|---|---|---|---|---|---|---|
| 1 | 6 | F | SRI | T10 | C | − | + | D |
| 2 | 26 | M | RTAs | T7-8 | B | + | + | B |
| 3 | 27 | M | RTAs | T9 | D | + | + | E |
| 4 | 35 | F | RTAs | T6 | A | + | + | A |
| 5 | 45 | M | RTAs | T9-10 | B | + | + | C |
*Road traffic accidents (RTAs), sports-related injury (SRI)
**(−) means no changes on image, (+) means valuable finding on image
***ASIA Scale 6 months after treatment
A follow-up DWI performed approximately 6 months after the injury demonstrated abnormalities in all patients with no reversion. A 26-year-old male (case 2; Table 1) injured in a road traffic accident, showed the lesion site extending to the adjacent area (Figs. 1, 2, 3). One patient (case 4; Table 1) underwent laminectomy and spinal decompression operations 2 weeks after injury.
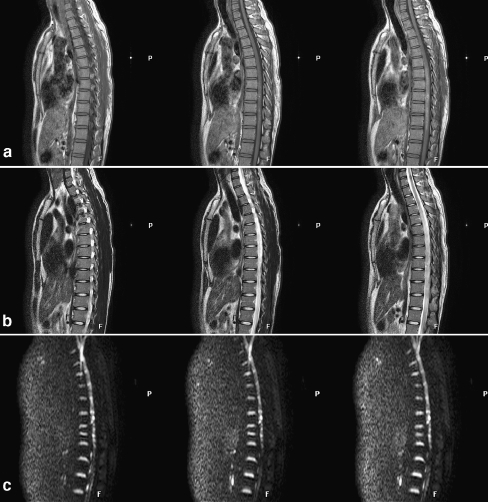
Fig. 1.
A 26-year-old male injured in a road traffic accident showed hypointense T1-weighted signal (a) and hyperintense T2-weighted signal (b) at the lesion site. DWI revealed an area of hyperintensity at the lesion site (c)
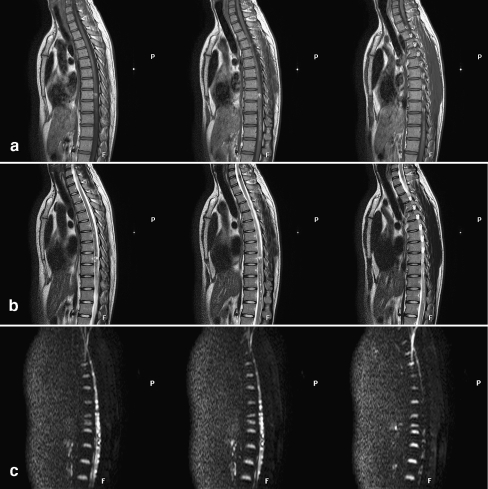
Fig. 2.
MRI performed 6 months after injury showed hypointense T1-weighted signal (a) and hyperintense T2-weighted signal (b); the lesion site extended to the adjacent area; the bright area on DWI persisted (c)
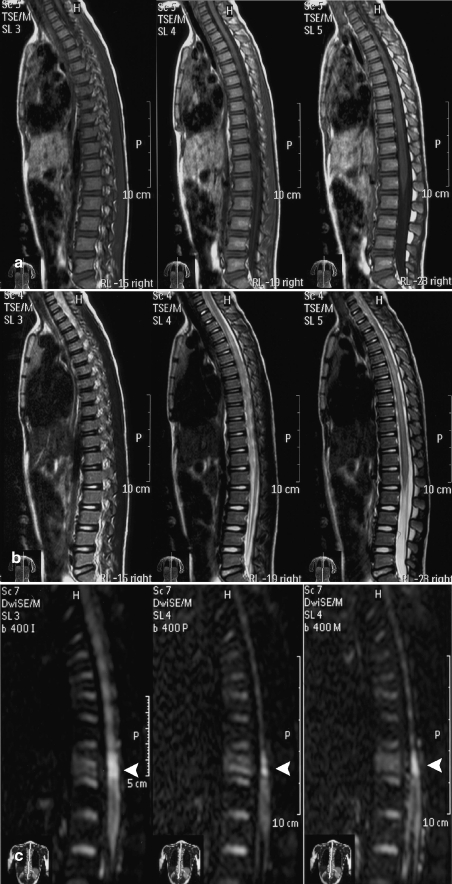
Fig. 3.
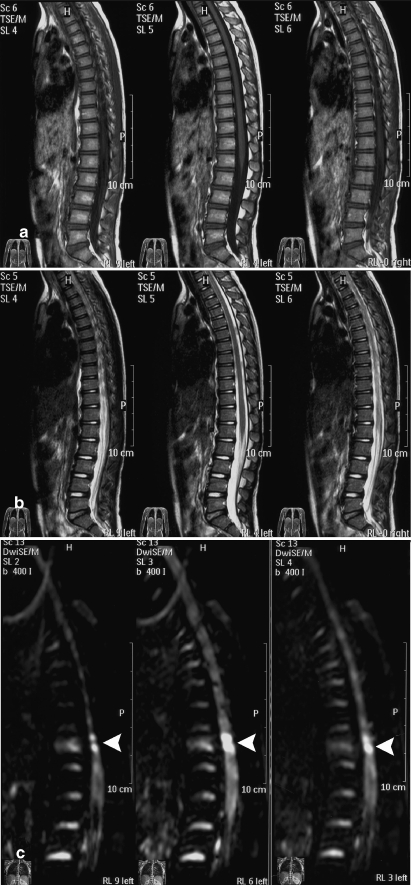
A 6-year-old female with a sports-related injury (hyperextension of the spine). No conspicuous abnormal signal intensity was seen on T1- (a) and T2-weighted images (b). However, DWI (c) revealed an area of hyperintensity at a low thoracic level (white arrow
Individual case
A 6-year-old female (case 1; Table 1) was transferred to the emergency room of our hospital following a sports-related injury (hyperextension of the spine). A neurological examination revealed hip flexors (0), knee extensors (0), ankle dorsiflexors (0), long toe extensors (3), ankle plantar flexors (0), absence of sensation of touch and a reduced sensation to pinprick below the umbilicus; voluntary anal contraction was noted. The cerebrospinal fluid analysis was normal. Radiographs of the thoracic spine showed no compressive deformity of the vertebrae or intervertebral disk space narrowing. No abnormal signal intensity was seen on TI- and T2-weighted images. However, DWI revealed an area of hyperintensity at a low thoracic level (Fig. 3). Treatment was started immediately after the diagnosis of spinal cord injury. Methylprednisolone was infused at doses of 30 mg/kg body weight for the first hour, followed by 5.4 mg/kg body weight for the next 23 h. MR imaging performed 2 weeks after injury showed normal results on T1-weighted images and T2-weighted images. The bright area on DWI persisted (Fig. 4). A follow-up MR imaging performed approximately 6 months after injury showed normal results on T1- and T2-weighted images. The bright area on DWI persisted (Fig. 5). The muscle weakness in both legs improved 6 months after treatment: hip flexors (0), knee extensors (3), ankle dorsiflexors (3), long toe extensors (3) and ankle plantar flexors (0); the sensory scores remained unchanged.
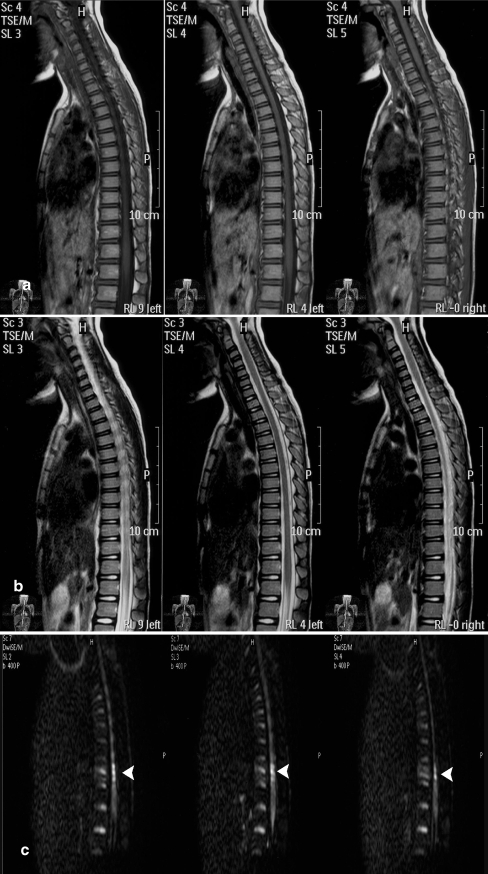
Fig. 4.
MR imaging performed 2 weeks after injury showed normal on T1- (a) and T2-weighted images (b). The bright area on DWI (c) persisted (white arrow)
Fig. 5.
MR imaging performed 6 months after injury showed normal on T1WI (a) and T2WI (b). The bright area on DWI (c) persisted (white arrow)
Discussion
Burke [2] first reported SCIWORA in 1974; Pang and Wilberger [18] defined it as a clinicoradiological entity that presents as acute spinal cord injury with normal radiographic and CT scan findings. However, if a negative MRI is included for the diagnosis, then the incidence decreases [21]. Gupta [9] reported that the incidence of SCIWORA was 10% of all spinal injuries. All levels of spinal cord injuries are susceptible to SCIWORA. The cervical cord is most frequently affected, followed by the thoracic and lumbosacral regions [5, 19]. Infants and children up to 8 years and the elderly above the age of 60 years are more prone to develop SCIWORA [22]. The mechanism underlying adult SCIWORA is the compression of the cord during sudden hyperflexion anteriorly with the posterior longitudinal ligament and mildly protruded discs and posteriorly with the laminae and ligamentum flavum [9, 11, 21]. Spinal stenosis and intervertebral disc disease are important conditions in the adult for the development of SCIWORA [10, 11]. Traction to the upper part of the injured spinal cord by forward translation along with compression of the lower part of the injured spinal cord segment between modest disc bulge and the edges of the laminae has also been a suggested mechanism for SCIWORA [3, 11]. Unlike the adult spine, the vertebral column of children is malleable and enables vertebral segments to twist, glide and separate from each other to some extent without sustaining damage. The malleability allows self-reducing intersegmental displacement to occur with external forces. This displacement stretches the spinal cord past its tolerance or causes the cord to impact the opposing bony surfaces of the spinal canal, leading to serious neurological injury [20]. The presentation of patients in these case studies showed road traffic accidents and sports-related injury were the two causes of SCI in our study.
SCIWORA is less common in the thoracic than in the cervical region, because the thorax and abdomen protect the thoracic vertebrae from forced excessive flexion or extension [19]. In a meta-analysis of a patient population already diagnosed with SCIWORA syndrome, as described in the literature, 74% of the spinal injuries were located in the cervical spine and 26% in the thoracic spine [12]. However, the patients with thoracic SCIWORA had sustained marked impact injury, and their spinal cord damage was severe. The long-term neurological outcome was poor. In Pang’s series, 7 out of 12 cases of thoracic SCIWORA were associated with crush injuries [19]. In Walsh’s series, all patients had suffered severe impact, such as crush injuries, motor vehicle accidents or falls from a height [25]. In our study, one case of peadiatric SCIWORA was unique in that the patient suffered severe neurological impairment, but the primary injury was subtle. The mechanism of injury may be due to the onset of the spasm in the spinal artery caused by hyperextension of the thoracic spine.
Conventional MRI remains the best clinical imaging modality for the evaluation of traumatic injury to the spinal cord parenchyma. An absence of bone artifacts, better contrast resolution, the feasibility of multiplanar imaging and selection of different pulse sequences make MRI a better choice over conventional radiography or CT in the diagnosis of spinal trauma [15]. Matsumara et al. [14] were the first to report a correlation between SCIWORA and magnetic resonance (MR) findings based on revelations of MR in acute and delayed phases. Grabb and Pang [8] reported a definite correlation between abnormal MR findings and the severity of neurological injury, and they put forward their classification to prognosticate SCIWORA on the basis of MRI appearances: (1) worse if there is intramedullary heamatoma occupying more than 50% of the cross-sectional area of the spinal cord, (2) intermediate if the contusions are minor and associated with oedema, (3) better in patients where there is cord oedema, and (4) the best when there are no changes in the spinal cord. After oedema subsides, improvement can be seen in patients [4, 8, 21].
Conventional MRI techniques for evaluation of acute spinal cord injury generally include TI and T2. These techniques are limited, however, in that their contrast primarily reflects changes in the water content or heamorrhage. One study using a rat spinal cord contusion model showed that the degree of neurological recovery following contusion injury did not correlate with the MRI findings [6]. Another study with the rat contusion model demonstrated that the water content and average T2 may not change significantly in areas of acute injury [7]. With conventional MRI, it is difficult to test the integrity of the axons within the white matter tracts of the spinal cord, so the exact stage of traumatic injury is often difficult to characterise by conventional MRI. In our study, the peadiatric patient in whom MRI showed no changes made a limited recovery. Clinical presentation and outcome in this patient were not well correlated with the MRI findings. DWI, however, may provide a greater insight into the nature and severity of an injury by evaluating factors that reflect the integrity of the white matter.
Because of its unique contrast mechanism, DWI promises to add to the diagnostic specificity and sensitivity of MRI in the spinal cord. Based on the ability to depict the microscopic motion of water protons, DWI can be used to sensitise image contrast to microstructural changes and thus can provide important information complimentary to conventional MRI [13]. In this study, four patients showed hypointense T1-weighted signal and hyperintense T2-weighted signal, and one patient had no changes on MRI. However, all patients showed hyperintense signal on DWI. In the case of the peadiatric patient, MRI showed no changes, but DWI revealed an area of hyperintensity at a low thoracic level. A follow-up MR imaging performed approximately 6 months after injury showed normal results on T1- and T2-weighted images. The bright area on DWI persisted, and the patient made a limited recovery.
DWI can be used to evaluate injury severity and treatment effects. Changes on DWI in spinal cord white matter have been correlated with behavioral recovery following cervical lateral funiculus lesions and the transplantation of fibroblasts genetically modified to express brain-derived neurotrophic factor (BDNF) [24]. Nevo et al. [16] showed that DWI was sensitive spinal cord damage, and reduced anisotropy loss was observed if neuroprotective therapy was applied. The animal research demonstrated that DWI was sensitive for the evaluation of spinal cord injury and the outcome after neuroprotection and regeneration, but successful application to humans is still awaited. In our study, three patients had good and moderate recoveries of neurological function, but the DWI did not revert in the follow-up period. In contrast to the results of the previous study, however, we could not find a correlation between DWI and clinical outcome. This outcome might be explained by the potential drawbacks for DWI in the presence of heamorrhagic components of traumatic tissue damage [23]. Moreover, MR imaging of ex vivo formaldehyde-fixed spinal cord specimens in the animal experiment might be responsible for the discrepancy. Clearly, further clinical studies of the prognostic value of DWI in SCI are required.
The potential for recovery in SCIWORA is related to the initial injury. There is also a report correlating timing with the potential for recovery [4]. We believe that the DWI results will contribute to an early treatment in SCIWORA, possibly leading to subsequent clinical improvement. However, the possibility of false-positive DWI in spinal cord injury should be considered. A spinal cord lesion, which could lead to hyperintensity on DWI, might include myelitis, neoplasms and multiple sclerosis, although those have not been fully investigated using DWI. As to the DWI technique, it can be used for emergency DWI study because it has an acceptable imaging time without requiring cardiac gating or a respiratory compensation technique.
The study highlights that DWI has diagnostic value in SCIWORA. Patients with no cord changes on MRI showed abnormal signals on DWI. Clinical presentation and outcome in these patients did not correlate well with the MRI findings. It is likely that in the future DWI may provide important information complimentary to conventional MRI and allow a better prognostic evaluation of recovery from SCIWORA.
References
- 1.American Spinal Injury Association (2002) International standards for neurological classification of SCI. American Spinal Injury Association, Chicago
- 2.Burke DC (1974) Traumatic spinal paralysis in children. Paraplegia 11(4):268–276 [DOI] [PubMed]
- 3.Chen TY, Lee ST, Lui TN et al (1997) Efficacy of surgical treatment in traumatic central cord syndrome. Surg Neurol 48(5):435–440 [DOI] [PubMed]
- 4.Dare AO, Dias MS, Li V (2002) Magnetic resonance imaging correlation in pediatric spinal cord injury without radiographic abnormality. J Neurosurg 971 {Suppl]:33–39 [DOI] [PubMed]
- 5.Dickman CA, Zabramski JM, Hadley MN et al (1991) Pediatric spinal cord injury without radiographic abnormalities: report of 26 cases and review of the literature. J Spinal Disord 4(3):296–305 [DOI] [PubMed]
- 6.Falconer JC, Narayana PA, Bhattacharjee MB et al (1994) Quantitative MRI of spinal cord injury in a rat model. Magn Reson Med 32(4):484–491 [DOI] [PubMed]
- 7.Ford JC, Hackney DB, Alsop DC et al (1994) MRI characterization of diffusion coefficients in a rat spinal cord injury model. Magn Reson Med 31(5):488–494 [DOI] [PubMed]
- 8.Grabb PA, Pang D (1994) Magnetic resonance imaging in the evaluation of spinal cord injury without radiographic abnormality in children. Neurosurgery 35(3):406–414 [DOI] [PubMed]
- 9.Gupta SK, Rajeev K, Khosla VK et al (1999) Spinal cord injury without radiographic abnormality in adults. Spinal Cord 37(10):726–729 [DOI] [PubMed]
- 10.Hendey GW, Wolfson AB, Mower WR et al (2002) Spinal cord injury without radiographic abnormality: results of the National Emergency X-Radiography Utilization Study in blunt cervical trauma. J Trauma 53(1):1–4 [DOI] [PubMed]
- 11.Kothari P, Freeman B, Grevitt M et al (2000) Injury to the spinal cord without radiological abnormality (SCIWORA) in adults. J Bone Joint Surg Br 82(7):1034–1037 [DOI] [PubMed]
- 12.Launay F, Leet AI, Sponseller PD (2005) Pediatric spinal cord injury without radiographic abnormality: a meta-analysis. Clin Orthop Relat Res (433):166–170 [DOI] [PubMed]
- 13.Le Bihan D, Mangin JF, Poupon C et al (2001) Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 13(4):534–546 [DOI] [PubMed]
- 14.Matsumura A, Meguro K, Tsurushima H et al (1990) Magnetic resonance imaging of spinal cord injury without radiologic abnormality. Surg Neurol 33(4):281–283 [DOI] [PubMed]
- 15.Mirvis SE, Geisler FH, Jelinek JJ et al (1988) Acute cervical spine trauma: evaluation with 1.5-T MR imaging. Radiology 166(3):807–816 [DOI] [PubMed]
- 16.Nevo U, Hauben E, Yoles E et al (2001) Diffusion anisotropy MRI for quantitative assessment of recovery in injured rat spinal cord. Magn Reson Med 45(1):1–9 [DOI] [PubMed]
- 17.Osenbach RK, Menezes AH (1989) Spinal cord injury without radiographic abnormality in children. Pediatr Neurosci 15(4):168–174 [DOI] [PubMed]
- 18.Pang D, Wilberger Jr JE (1982) Spinal cord injury without radiographic abnormalities in children. J Neurosurg 57(1):114–129 [DOI] [PubMed]
- 19.Pang D, Pollack IF (1989) Spinal cord injury without radiographic abnormality in children-the SCIWORA syndrome. J Trauma 29(5):654–664 [DOI] [PubMed]
- 20.Pang D (2004) Spinal cord injury without radiographic abnormality in children, 2 decades later. Neurosurgery 55(6):1325–1342 [DOI] [PubMed]
- 21.Saruhashi Y, Hukuda S, Katsuura A et al (1998) Clinical outcomes of cervical spinal cord injuries without radiographic evidence of trauma. Spinal Cord 36(8):567–573 [DOI] [PubMed]
- 22.Scher AT (1976) Cervical spinal cord injury without evidence of fracture or dislocation. An assessment of the radiological features. S Afr Med J 50(25):962–965 [PubMed]
- 23.Schwartz ED, Hackney DB (2003) Diffusion-weighted MRI and the evaluation of spinal cord axonal integrity following injury and treatment. Exp Neurol 184(2):570–589 [DOI] [PubMed]
- 24.Schwartz ED, Shumsky JS, Wehrli S et al (2003) Ex vivo MR determined apparent diffusion coefficients correlate with motor recovery mediated by intraspinal transplants of fibroblasts genetically modified to express BDNF. Exp Neurol 182(1):49–63 [DOI] [PubMed]
- 25.Walsh JW, Stevens DB, Young AB (1983) Traumatic paraplegia in children without contiguous spinal fracture or dislocation. Neurosurgery 12(4):439–445 [DOI] [PubMed]