Abstract
Manipulation under anesthesia (MUA) has been used to speed up the recovery of frozen shoulder, which is said to be a self-limiting process. We would like to elucidate the short- and long-term results of the treatment of frozen shoulders by manipulation under anesthesia and compare the results of idiopathic, post-trauma and post-surgery frozen shoulders. We applied an adjusted Constant score (Constant score after excluding the 25 points allocated for the assessment of muscle strength) to assess all patients. In our series, 47 cases with 51 frozen shoulders were collected and evaluated retrospectively. The adjusted Constant score at pre-manipulation was on average 22.8±4.9 (10–31) points. The score from the 3-week follow-up was 52.6±9.2 (31–67) points on average. The score from the averaged 82-month follow-up was on average 70.1±6.2 (54–75) points, with 23 shoulders scored for a maximum point number of 75. The score at the early and late follow-ups was significantly lower for the post-surgery group (63.2±6.7) when compared to the other two groups (P<0.001). Our results revealed that manipulation under anestheia is a very simple and noninvasive procedure for shortening the course of an apparently self-limiting disease and can improve shoulder function and symptoms within a short period of time. However, we found less improvement in post-surgery frozen shoulders, especially in residual pain and limited range of motion (ROM), which may be influenced by the initial injury or initial surgery. Although less improvement in pain and ROM was noted, manipulation is still a good and simple way to treat post-surgery frozen shoulders.
Résumé
La manipulation sous anesthésie est utilisée pour hâter la récupération des épaules gelées qui sont considérées comme un processus d’auto-limitation. Nous avons étudié les résultats à court et long terme de ces manipulations en comparant les résultats des épaules gelées idiopathiques, post-traumatiques et post-chirugicales avec un score de Constant modifié (en excluant les 25 points alloués à la force musculaire). Nous avons évalué rétrospectivement 51 épaules gelées chez 47 patients. Le score de Constant modifié était avant la manipulation de 22,8±4,9 (10–31). Le score à 3 semaines était de 52,6±9,2 (31–67). Le score à 82 mois de recul moyen était de 70,1±6,2 (54–75), avec 23 épaules au score maximum de 75 points. Le score au recul précoce et tardif était significativement plus faible dans le proupe post-chirurgie (63,2±6,7) que dans les autres groupes (P<0,001). Nos résultats montrent que la manipulation sous anesthésie est une méthode non invasive éfficace pour améliorer rapidement la fonction de l’épaule. Il y a moins d’amélioration dans les cas post-chirurgicaux notamment au niveau des douleurs résiduelles et de l’amplitude de mobilité mais la manipulation reste quand même un bon traitement dans ces cas.
Introduction
Adhesive capsulitis of the shoulder (frozen shoulder) is characterized by a gradual increase in stiffness and pain. This self-limiting disorder has three stages lasting up to 1 to 3 years [9, 20] and does not re-occur on the same shoulder [8]. However, it can sometimes result in a minor contracture [2]. Its etiology is still unknown, and many treatment regimens, including conservative treatment, nerve blocks and distension, manipulation under anesthesia and open or arthroscopic, with or without MUA, have been used to hasten the recovery time [7, 10, 14, 18, 21, 23]. However, the benefit has yet to be determined [1].
Excellent results of manipulation under anesthesia have been reported by many authors [6, 11, 13, 14, 17], but only a few have focused on the secondary frozen shoulder [11, 13]. In our study, we tried to elucidate the short- and long-term results of frozen shoulders after manipulation under anesthesia and to compare the results of idiopathic, post-trauma and post-surgery frozen shoulders.
Materials and methods
Selection of patients
We adopted the criteria by Shaffer et al. for selection for the diagnosis of frozen shoulders [22]. It consisted of (1) at least a 1-month history of pain and stiffness of the shoulder; (2) documented restriction of both passive and active gleno-humeral and scapulo-thoracic motion of equal or less than 100 degrees of elevation, and less than 50% of external rotation, as compared to the contralateral side; (3) the intraoperative characteristic feeling of tissue breakdown during manipulation. The patients were managed conservatively with medications and stretching techniques first, with manipulation started in 3 months time if pain and loss of motion were reported. Between 1992 and 2003, 251 patients with frozen shoulders were treated in our hospital. In this study, patients were excluded if they had (1) a history of cancer, diabetes or rheumatic disease, (2) rotator cuff tear or residual tear after repair, (3) recent fracture, (4) severe neurologic deficit of the involved upper extremity, (5) loss of follow-ups or incomplete preoperative data and (6) a range of motion <80% was regained. After selection, 47 cases with 51 frozen shoulders were collected and evaluated retrospectively. Fifty-one shoulders were separated into three groups according to the system of Zuckerman et al. [24]. Twenty-six shoulders were classified as an idiopathic group as there was no significant trauma to the shoulder prior to the onset of symptoms. Twelve patients were classified as the after-trauma group (under non-operative treatment). The remaining 13 patients were classified as the after-surgery group. In the after-trauma group, six had proximal humeral nondisplaced fracture, three had distal clavicle nondisplaced fracture, one had an AC injury (type III) and two had glenoid nondisplaced fracture. In the after-surgery group, eight patients had RCT open repair, two had proximal humeral fracture, two had AC injury (type V), and one had distal clavicle fracture.
Clinical assessment
X-rays made at the anteroposterior plane in internal rotation and abduction, at the axillary plane, and in the supraspinatous-tunnel view were taken to exclude other shoulder disorders. Ultrasound was also performed for all patients. The subjective symptoms and objective findings of all shoulders were graded according to the adjusted Constant score (the Constant score after excluding the 25 points for the assessment of muscle strength) [5]. The maximum score for pain is 15, with 15 points representing no pain and 0 points being severe, constant pain. The ability to carry out daily activities is assigned a maximum of 20 points. Flexion, abduction, external rotation and internal rotation are each given a maximum of 10 points (total maximum score for motion: 40 points). The maximum score was therefore 75 points.
Technique
All patients received general anesthesia for the procedure, using an intravenous barbiturate given by anesthetists. The technique used for manipulation was started by gradual forward elevation in the sagittal plane to the maximum possible extent while the scapula was fixed. Next, passive external rotation was performed in 0° of abduction, followed by external rotation in 90° of abduction. Lastly, internal rotation at 90° of abduction and cross-body adduction were performed. Care was taken not to fracture the humerus during manipulation. External rotation forces were applied very carefully by two thumbs. A full range of motion was always achieved. The shoulder joint was injected with 3 ml of mucaine and 1 ml of steroid. To avoid intraoperative complications, 6 patients (6/57, 10.5%) with refractory frozen shoulders (<80% of the range of motion was regained) then underwent arthroscopic release and were not included in our series.
Postoperative treatment
All patients received immediate continuous passive exercise in the ward soon after the procedure. After discharge, exercise training with a physiotherapist in our outpatient clinic was continued until the range of movement was satisfactory.
After the operation, each patient was followed at 3 weeks, 3 months, 6 months and 1 year, and then at a final review. Additional visits were arranged if indicated. Each patient had a special chart with detailed records of their personal data, the mechanism and associated condition of the injury, classification of the type of frozen shoulder, course of management (including the time of treatment, operation course, presence of early and late complications and management of complications) and the course of functional recovery. Measurement of shoulder function was done at each follow-up visit, and all the evaluations and records were done by senior staff. The early follow-up period was 3 weeks on average, while the late follow-up period was 82 months (range: 18-153 months).
Statistical analysis
The Kruskal-Wallis test was used to determine the score difference among the three groups. If a difference was noted, Bonferroni correction was applied for multiple comparisons. All analyses were performed with the use of Statistical Analysis System software (version 6.12; SAS, Cary, NC).
Results
The average age of the patients at the time of presentation was 53.9 years old (range: 38 to 79 years old). The group was composed of 16 male and 33 female patients. The average duration from the onset of the disease to manipulation was 6.8 months, with a range from 3 to 18 months. The minimum follow-up period was 18 months. There were 35 right-sided fractures and 16 left-sided fractures. Four patients had both shoulders involved, but from different occasions.
Shoulder stiffness noted during anesthesia and the typical rasping noise occurring during manipulation confirmed the clinical diagnosis of frozen shoulders. All patients had complete functional assessments. These results are summarized in Table 1. The adjusted Constant score at pre-manipulation was on average 22.8±4.9 (10–31) points. The average follow-up period was 82 months (range: 18–153 months). The adjusted Constant score from early follow-up was 52.6±9.2 (31–67) points on average with 13 shoulders scoring less than 50 points; the adjusted Constant score from the late follow-up was on average 70.1±6.2 (54–75) points, with 23 shoulders scoring a maximum score of 75. However, the results are influenced by the natural course of the disorder if the follow-up period is longer than 12 months. There were no complications after manipulation. Significant improvement was seen 3 weeks after manipulation, and we found a very good early response to manipulation with a rise in the adjusted Constant score from the preoperative median of 30.4% to a post–manipulation of 70.2%. In our study, the adjusted Constant score at the 12-month follow-up was on average 69.4 (50–75), which was close to the late follow-up of 70.1 (54–75).
Table 1.
Results of the adjusted Constant score before, 3 weeks after and 82 months after surgery in the three groups
| Timing | Score (points) | Idiopathic | Groups | P value | |
|---|---|---|---|---|---|
| Post-trauma | Post-surgery | P value | |||
| Before | Pain | 4.23±3 | 4.17±3.5 | 3.1±3.2 | 0.5263 |
| Activity | 7.2±1.8 | 7.2±2.1 | 4.5±2.4 | <0.01* | |
| ROM | 12.2±2.4 | 12.5±2.4 | 12.8±2.2 | 0.6634 | |
| Total | 23.6±4.2 | 23.8±5.1 | 20.3±4.4 | 0.1355 | |
| Post-surgery | Pain | 12.5±2.9 | 12.9±3.3 | 10±3.5 | 0.0495 |
| Activity | 14.2±2 | 14.8±1.3 | 11.1±2.5 | <0.01* | |
| 3 weeks | ROM | 29.6±3.3 | 28.3±3 | 20.9±3.4 | <0.01* |
| Total | 56.3±6.8 | 56.1±5.2 | 42±8.4 | <0.01* | |
| Post-surgery | Pain | 14.4±1.6 | 14.9±1.4 | 11.1±2.1 | <0.01* |
| Activity | 18.9±1.7 | 19.5±1.2 | 18.3±1.6 | 0.0964 | |
| 82 months | ROM | 38.91.9 | 39.2±1.3 | 33.7±3.6 | <0.01* |
| Total | 72.3±4.4 | 73.3±2.5 | 63.2±6.7 | <0.01* | |
*P<0.01, significantly lower scores in the post-surgery group
The values are given as the mean and the standard deviation
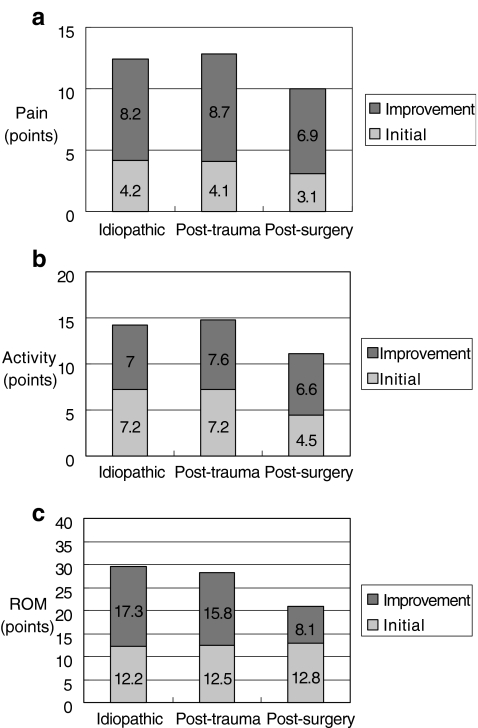
There was no difference in the baseline preoperative scores among the group except in activity (Table 1, Fig. 1b). For the post-surgery group at the late follow-ups, the mean pain score was 11.1±2.1, which was significantly lower than the other two groups (Table 1). The mean ROM score was 20.9±3.4 and 33.7±3.6 at the early and late follow-ups, respectively, which was also significantly lower than the other two groups (Table 1). However, activity of daily living at the late follow-ups did not differ significantly among these three groups (Table 1). Finally, the total adjusted Constant score at the early and late follow-ups was significantly lower for the post-surgery group (63.2±6.7) when compared to the other two groups (Table 1). The results of post-trauma and idiopathic frozen shoulders were similar at early and late follow-ups (Table 1).
Fig. 1.
a Improvement in pain 3 weeks after surgery; b improvement in activity 3 weeks after surgery; c improvement in ROM 3 weeks after surgery
Four shoulders took 1 year or more before reaching a satisfactory outcome, i.e., a spontaneous recovery. These four patients did not show much improvement in the range of motion at the 3-week follow-up. Three of them were from the post-surgery group and one from the idiopathic group. So far, we have still been unable to identify any features correlated with a poor outcome. Hence, the reason behind this failure to recover is still unknown.
In this series, there were no complications, including iatrogenic neurologic injury, fracture and dislocation, noted.
Discussion
Untreated frozen shoulders usually resolve in their natural course within 1 to 3 years. The condition of frozen shoulder can cause severe pain that may require medications for several months and sometimes even results in some restrictive motions, implying that the disease itself does not always have a successful long-term outcome [2, 22]. However, many authors have reported that this period of disability can be minimized by manipulation under anesthesia [6, 11, 13, 14, 17].
This study reported our experience with the short- and long-term effects of treating 51 cases of idiopathic, post-trauma and post-surgery frozen shoulders by manipulation under anesthesia over a mean follow-up period of 82 months. There was no recurrence in our long-term follow-ups. Four out of 47 patients (8.5%) had both shoulders involved. Diabetic patients were excluded from our study due to the fact that diabetes alone is a predisposing factor for secondary frozen shoulders. Besides, our number of diabetic patients was not sufficient for making a definite conclusion, and hence further evaluation would be needed.
To avoid intraoperative complications, six cases (10.5%) with refractory frozen shoulders that underwent open or arthroscopic release were excluded in our series. We did not see any intraoperative or postoperative complications following manipulation. However, shoulder dislocation, rotator cuff tear or brachial plexus palsy after manipulation were reported [3]. Despite the fact that the Constant scoring system was excellent in assessing shoulder functions, some authors still stated that the assessment of muscle strength prior to manipulation is almost impossible because most patients cannot abduct their shoulders to 90 degrees, and this would therefore result in false scores [4, 17, 18]. This is part of the reason we adopted the adjusted Constant score instead to assess and evaluate our patients.
An excellent early response to manipulation was found in short-term follow-ups, with a rise in the adjusted Constant score from a preoperative median of 30.4 to 70.2% at post–manipulation. This has never occurred in untreated frozen shoulders with an improvement over such a short period of time. In the series of Othman, the adjusted Constant score at the early follow-up was on average 54.9 points (28–75) [17], which was close to our average of 52.6 points (31–67). As for our long-term follow-ups, the scores were even better than those at the early follow-ups, therefore representing spontaneous recovery (Table 1).
Some authors stated that manipulation alone should be sufficient for the majority of patients [15–17], with the arthroscope reserved for combined rotator cuff tear, sub-acromial decompression and refractory shoulder stiffness [12, 18, 19]. Due to the restricted volume of the glenohumeral joint, an arthroscope often damages the articular surface when entering the joint space. In our series, six cases of refractory frozen shoulders (10.5%) were converted to arthroscopic release (four in the idiopathic groups, one in post-trauma and one in post-surgery). Manipulation alone was sufficient for 89.4% of frozen shoulders in our series.
Some authors stated that manipulation for patients with secondary frozen shoulders should be avoided due to its poor results [11, 13]. On the contrary, our patients with post-trauma and post-surgery frozen shoulders gained much improvement. Similar improvement was also noted in the pain and activity of daily living among the three groups at early follow-up (Fig. 1a,b). However, significantly lower total scores were found at both early and late follow-ups in the post-surgery group. The activity of daily living and ROM (Table 1) at early follow-ups mainly accounted for the low scores, while pain and ROM (Table 1) contributed to the low scores at late follow-ups. In the series of Holloway et al. [12], less improvement was also found in postoperative frozen shoulders after arthroscopic release. It was thought to be due to the initial trauma and operation. Therefore, outcomes in postoperative frozen shoulders, either with manipulation or arthroscopic release, were not as good as those from the idiopathic group. Interestingly, the activity of daily living at the late follow-ups did not differ significantly among these three groups (Table 1). On the contrary, the results of post-trauma and idiopathic frozen shoulders were similar at early and late follow-ups due to lower energy trauma and nonoperative treatment.
We concluded that manipulation under anesthesia when initial conservative management failed speeds up the recovery of idiopathic, post-trauma and post-surgery frozen shoulders and improves shoulder function and symptoms within a short period of time. In treating post-surgery frozen shoulders with manipulation, the results of the activity of daily living were shown to be as good as those for idiopathic frozen shoulder. Although less improvement in pain and ROM was noted, manipulation is still a good and simple way to treat post-surgery frozen shoulders.
References
- 1.Baslund B, Thomsen BS, Jensen EM (1990) Frozen shoulder. Current concepts. Scand J Rheumatol 19:321–325 [DOI] [PubMed]
- 2.Binder AI, Bulgen DY, Hazleman BL, Roberts S (1984) Frozen shoulder: a long-term prospective study. Ann Rheum Dis 43:361–364 [DOI] [PMC free article] [PubMed]
- 3.Birch R, Jessop J, Scott G (1991) Brachial plexus palsy after manipulation of the shoulder. J Bone Joint Surg 73B:172 [DOI] [PubMed]
- 4.Conboy BC, Morris RW, Kiss J, Carr AJ (1996) An evaluation of the Constant-Murley shoulder assessment. J Bone Joint Surg 78B:229–232 [PubMed]
- 5.Constant CR, Murley AHG (1987) A clinical method of function assessment of the shoulder. Clin Orthop 214:160–164 [PubMed]
- 6.Dodenhoff R, Levy O, Wilson A, Copeland S (2000) Manipulation under anesthesia for primary frozen shoulder: effect on early recovery and return to activity. J Shoulder Elbow Surg 9:23–26 [DOI] [PubMed]
- 7.Fareed DO, Gallivan WR Jr (1989) Office management of frozen shoulder syndrome: treatment with hydraulic distension under local anaesthesia. Clin Orthop 242:177–183 [PubMed]
- 8.Goldberg BA, Scarlat MM, Harryman DT (1999) Management of the stiff shoulder. J Ortho Sci 4(6):462–471 [DOI] [PubMed]
- 9.Grey R (1978) The natural history of idiopathic frozen shoulder. J Bone Joint Surg (A) 60:564 [PubMed]
- 10.Haines J, Hargadon E (1982) Manipulation as the primary treatment of the frozen shoulder. J R Coll Surg Edinb 27:271–275 [PubMed]
- 11.Hill J, Bogumill H (1988) Manipulation in the treatment of frozen shoulder. Orthopaedics 11:1255–1260 [DOI] [PubMed]
- 12.Holloway GB, Schenk T, Williams GR, Ramsey ML, Iannotti JP (2001) Arthroscopic capsular release for the treatment of refractory postoperative or post-fracture shoulder stiffness. J Bone Joint Surg (A) 83:1682–1687 [DOI] [PubMed]
- 13.Janda DH, Hawkins RJ (1993) Shoulder manipulation in patients with adhesive capsulitis and diabetes mellitus: a clinical note. J Shoulder Elbow Surg 2:36–38 [DOI] [PubMed]
- 14.Kivimaki J, Pohjolainen T (2001) Manipulation under anesthesia for frozen shoulder with and without steroid injection. Arch Phys Med Rehab 82:1188–1190 [DOI] [PubMed]
- 15.Neviaser RJ, Neviaser TJ (1987) The frozen shoulder. Diagnosis and management. Clin Orthop 223:59–64 [PubMed]
- 16.Ogilvie-Harris DJ, Biggs DJ, Fitsialos DP, Mackay M (1995) The resistant frozen shoulder. Clin Orthop 319:238–248 [PubMed]
- 17.Othman A, Taylor G (2002) Manipulation under anaesthesia for frozen shoulder. Int Orthop 26:268–270 [DOI] [PMC free article] [PubMed]
- 18.Patel VR, Singh D, Calvert PT, Bayley JIL (1999) Arthroscopic subacromial decompression: Results and factors affecting outcome. J Shoulder Elbow Surg 8:231–237 [DOI] [PubMed]
- 19.Ramsier EW, Debrunner HU, Pfirrmann C (1992) Shoulder dislocation and periarthrosis humeroscapularis. Z Unfallchir. Versicherungsmedizin 85:111–116 [PubMed]
- 20.Reeves B (1975) The natural history of the frozen shoulder syndrome. Scand J Rheumatol 4:193–196 [DOI] [PubMed]
- 21.Roubal P, Dobritt D, Placzek J (1996) Glenohumeral gliding manipulation following interscalene brachial plexus block in patients with adhesive capsulitis. JOSPT 24:66–77 [DOI] [PubMed]
- 22.Shaffer B, Tibone JE, Kerlan RK (1992) Frozen shoulder. A long-term follow-up. J Bone Joint Surg (A) 74:738–746 [PubMed]
- 23.Warner JJ, Allen A, Marks PH, Wong P (1997) Arthroscopic release of postoperative capsular contracture of the shoulder. J Bone Joint Surg (A) 79:1151–1158 [DOI] [PubMed]
- 24.Zuckerman JD, Cuomo F, Rokito S (1994) Definition and classification of frozen shoulder: a consensus approach. J Shoulder Elbow Surg 3:Z.72 [DOI] [PubMed]