Abstract
Tendon attachment to interconnected porous calcium hydroxyapatite ceramics (IP-CHA) with cultured bone marrow stromal cells (BMSC) was analysed. The purpose of this study was to evaluate whether BMSC in IP-CHA could augment the tendon attachment to IP-CHA histologically and biomechanically. Eighteen Japanese white rabbits were used. Cultured BMSCs were subcultured in IP-CHA. The grafted tendon and IP-CHA with BMSC complex were implanted in a bone defect of the knee [BMSC(+) group]. In the contralateral knee, a tendon and IP-CHA without BMSC complex were implanted [BMSC(-) group]. Histological findings of the interface between the tendon and IP-CHA were similar in the two groups 3 weeks after the operation. However, 6 weeks after the operation, more abundant bone formation around the tendon was observed in the BMSC(+) group. The direct apposition of the tendon to bone in pores and collagen fibre continuity between the tendon and fibrous tissue in pores were observed. In biomechanical evaluation, the maximum pull-out load of the tendon from the IP-CHA in the BMSC(+) group was significantly higher than that in the BMSC(-) group 6 weeks after the operation. BMSCs cultured in IP-CHA could augment tendon attachment to IP-CHA.
Résumé
Le propos de cette étude est de déterminer si l’utilisation de culture cellulaire de moelle osseuse (BMSC) avec l’utilisation de calcium d’hydroxyapatite (IP-CHA) augmente la résistance de l’attachement osseux du tendon sur le plan histologique et biomécanique. Dix-huit lapins blancs du Japon ont été utilisés pour ce travail. Les tendons greffés avec hydroxyapatite et culture de la cellule de moelle osseuse ont été implantés dans une defect au niveau du genou. Sur le genou opposé, le tendon a été implanté sans culture cellulaire. Sur le plan histologique les résultats sont identiques dans les deux groupes, 3 semaines après intervention, cependant 6 semaines après l’intervention la progression de la formation osseuse est plus importante autour du tendon ayant bénéficié d’une culture cellulaire de moelle osseuse. Il en est de même en ce qui concerne la résistance biomécanique du tendon qui est significativement plus importante dans le groupe culture cellulaire, 6 semaines après l’intervention.
Introduction
Hydroxyapatite ceramics have superior biocompatibility and have been widely used as an artificial bone substitute in orthopaedics. Interconnected porous calcium hydroxyapatite ceramic (IP-CHA) has good osteoconductivity because the interconnection of pores allows cells to invade deeply into the material [11]. Recently, IP-CHA has been used in studies of bone and joint regeneration by tissue engineering because the interconnected pores work as a scaffold for cultured cells and growth factors [1, 3, 4, 6, 12]. We have been doing animal studies to regenerate a bone defect including tendon attachment using IP-CHA as a scaffold. We have already reported that IP-CHA could have a bioactive interface to the tendon by conducting fibrous tissue and bone including vessels in the pores in contact with the tendon [7]. In this study, for the regeneration of a bone defect including tendon attachment, IP-CHA was used as a scaffold, and cultured BMSCs were used as a cell source. Our hypothesis was that BMSCs cultured in IP-CHA could enhance bone formation around the grafted tendon and that this would result in the achievement of a more mature interface both histologically and biomechanically. The purpose of this study was to determine whether BMSCs in IP-CHA could augment the tendon attachment to IP-CHA.
Materials and methods
IP-CHA
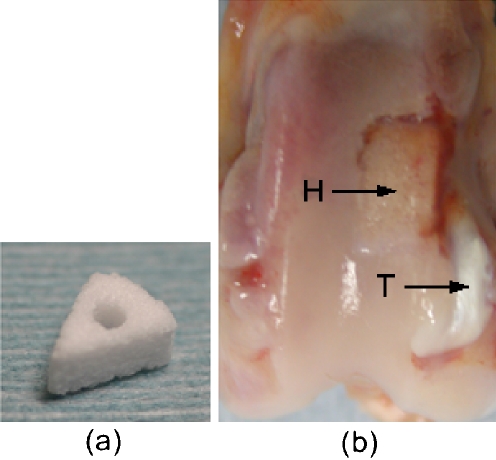
The IP-CHA used in this study is commercially manufactured (Toshiba Ceramics Co., Ltd., Tokyo, Japan) and allowed for clinical use as a bone substitute in Japan. It is made from a slurry of hydroxyapatite [Ca12(PO4)6(OH)2] by the “foam-gel” technique. We analysed its porous structure and reported it in the previous study [7]. The summary of the analysis was described again. Photomicrographs were taken using a scanning electron microscope (JSM-6330F, JEOL Ltd., Tokyo), and mercury porosimetry (Autopore 9420, Micromeritics Instrument Corp, GA) was performed to characterise the porous structure of the IP-CHA. SEM photomicrographs revealed regularly lined pores, thin walls and interconnecting holes (Fig. 1a). The pore diameter was 100–250 μm. Mercury porosimetry demonstrated that the total porosity was 63.6%, and most of the interpore connections ranged from 10 to 100 μm, with a maximum peak at 40 μm (Fig. 1b). The shape of the IP-CHA used in this study was a 1/8 cylinder (radius: 7 mm; height: 3 mm; angle: 45°) with a 1.5-mm diameter hole (Fig. 2a).
Fig. 1.
(a) SEM photomicrographs of IP-CHA (×80): Many thin-walled spherical pores with diameters of about 100–250 μm were present at high density, and interconnecting holes connected neighboring pores. (b) Log differential intrusion volume versus diameter curves by mercury porosimetry: The vertical axis shows the number of pores, while the size of interconnecting pores is plotted on the horizontal axis. Most of the interpore connections ranged from 10 to 100 μm, with a maximum peak at 40 μm
Fig. 2.
(a) IP-CHA: The shape of the IP-CHA used in this study was a 1/8 cylinder (radius: 7 mm; height: 3 mm; angle: 45°) with a 1.5-mm diameter hole. (b) Macroscopic finding. H: IP-CHA; T: tendon
BMSC
Eighteen Japanese white rabbits (average weight, 2.5 kg) were used in this study. For anesthesia, 1 ml of pentobarbital (50 mg/ml; Nembutal, Dainippon Sumitomo Pharma Co., Ltd., Osaka, Japan) was administered intravenously, and 5 ml of ketamine (50 mg/ml; Ketalar, Sankyo Yell Yakuhin Co., Ltd., Tokyo) was administered intramuscularly. Bone marrow cells (6 ml) were obtained by aspiration from tibiae and divided into three 100-mm dishes in 8 ml of standard medium. The medium consisted of Dulbecco’s modified eagle medium (Invitrogen Corp., CA) containing 10% foetal bovine serum (Sigma-Aldrich Corp., MO) and 5% antibiotic-antimycotic mixed stock solution (Nacalai tesque Inc., Kyoto). Cells were incubated at 37°C in 5% CO2 for 2 weeks. Adherent cells were used as BMSCs in this study. BMSCs were treated with trypsin to produce a cell suspension and concentrated by centrifugation at 1,500 rpm for 5 min at 4°C. The IP-CHA was soaked in a concentrated cell suspension (2–5×106 cells/ml) in a CO2 incubator for 2 h. The IP-CHA was then transferred into a six-well plate for subculture. BMSCs were subcultured with IP-CHA in the same medium (without osteoinductive factors) for 1 week before use as implants.
Surgical procedure
The left knee joint was exposed using the lateral parapatellar approach, and by dislocating the patella medially, the long digitorum extensor tendon attached to the lateral femoral condyle was identified. The tendon was cut 3 cm distally from the femoral attachment and was reflected proximally. The muscle tissue attached to the tendon was removed. A 1/8 cylindrical wedge bone defect (radius: 8 mm; height: 3 mm; angle: 45°) was created on the lateral femoral condyle proximal to the tendon attachment. A 1.5-mm hole was made from the bone defect to the medial side of the femur using a drill. The tendon was trimmed so that it could pass through the 1.5-mm hole in the IP-CHA block. After placing the IP-CHA block with BMSCs in the bone defect, the end of the tendon was pulled to the medial side of the femur through the holes in the IP-CHA and femur. The IP-CHA block was fixed by pulling the tendon medially. The tendon was then sutured to the medial collateral ligament. The medially dislocated patella was reduced, and after confirming that the IP-CHA block was fixed in the bone defect in all ranges of motion and that the bone defect did not hinder the gliding movements of the patella, the wound was closed. Using the same procedures, an IP-CHA block without BMSCs and the tendon complex was fixed to a bone defect in the right knee. Hind limbs were not externally immobilised postoperatively. For histological analysis, six rabbits were killed 3 and 6 weeks after the operation, and for biomechanical analysis, six rabbits were killed 6 weeks after the operation by administering a lethal dose of pentobarbital intravenously. The distal part of the femur was harvested (Fig. 2b). This animal study was performed in accordance with the “Principles of laboratory animal care” (NIH publication No. 85-23, revised 1985), and the rules and regulations of the Research Facilities for Laboratory Animal Science, Hiroshima University.
Histological analysis
The harvested distal part of the femur was fixed in 10% neutral-buffered formalin. The excised bone was decalcified in formic acid, dehydrated through an ethanol series, and embedded in paraffin. Sections, 5 μm thick, perpendicular to the joint surface and to the long axis of the tendon in IP-CHA were made. For histological analysis, sections at the center of the IP-CHA hole were used. Sections were stained with hematoxylin and eosin or azan. Histological examination was performed under a light microscope (DM IRB, Leica, Germany). Histological findings of the IP-CHA with the BMSC model [BMSC(+) group] and the IP-CHA without cells model [BMSC(-) group] were compared. The interface between the tendon and IP-CHA was evaluated. For quantitative analysis, the area of the bone around the tendon was measured on the sections stained with hematoxylin and eosin by image-analysing software (Scion Image Beta 4.0.2, Scion Corp., MD).
Biomechanical analysis
Biomechanical analysis was performed 6 weeks after the operation. Soft tissue other than the tendon was removed from the distal part of the femur. To eliminate the effects of the area where the tendon passed through the bone tunnel of the femur, the bone and tendon were cut using a high-speed electric cutter with a thin circular blade at the medial margin of the IP-CHA block. The tendon was detached from its attachment site at the lateral femoral condyle, and while exercising caution to avoid damaging the tendon itself, the tendon was detached from the surrounding soft tissue up to the area where the tendon came in contact with the edge of the IP-CHA. Furthermore, the tendon was detached from the lateral wall of the IP-CHA up to the area where the tendon came in contact with the hole of the IP-CHA carefully using a magnifier for the exact measurement of adhesive strength between the tendon and the hole of the IP-CHA.
The distal end of the femoral shaft was soaked in 0.9% saline solution, and biomechanical examination was performed within 1 h of harvesting. The femoral shaft was fixed using resin in a cylindrical container having a diameter of 10 mm. The maximum pull-out load of the tendon from the IP-CHA was measured by a materials testing machine (1840NT/500, Aikoh Engineering Co., Ltd., Osaka, Japan). The distal femur fixed in the cylindrical container was clamped to the load cell. The tendon was directly fixed 3 mm from the inlet of the IP-CHA using a clamp attached to the bottom of the apparatus. The direction of the load was set to the long axis of the tendon in the IP-CHA. The distal femur was pulled in an upward direction at a rate of 2.0 mm/min, and the maximum pull-out load and failure site were recorded (Fig. 3).
Fig. 3.
Biomechanical analysis: The distal femur fixed in the cylindrical container was clamped to the load cell. The tendon was directly fixed 3 mm from the inlet of the IP-CHA using a clamp attached to the bottom of the apparatus. The distal femur was pulled in an upward direction at a rate of 2.0 mm/min
Statistical analysis
Wilcoxon signed-ranks tests were performed using statistical software (StatView 5.0, SAS Institute Inc., NC). Statistical significance was set at P<0.05.
Results
Histological findings
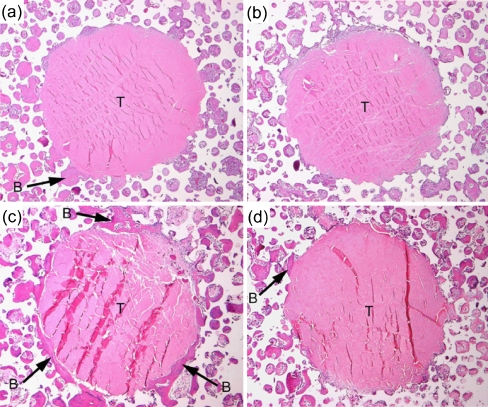
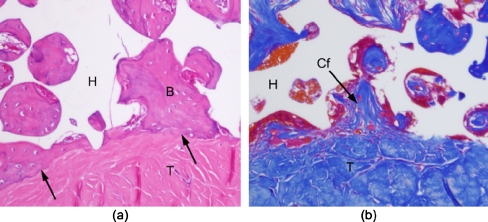
Three weeks after the operation, abundant fibrous tissue with vessels was observed in all the pores of the IP-CHA in both the BMSC(+) and BMSC(-) groups. Although the grafted tendon passed through the deep portion of the IP-CHA, vascular, highly cellular fibrous tissue could be found within the pores in contact with the tendon in both groups. Bone formation was observed in the pores of IP-CHA close to the host bone. A little bone formation was observed around the tendon in both groups 3 weeks after the operation. Six weeks after the operation, bone formation around the tendon had increased further compared with 3 weeks. Extensive proliferation of bone around the tendon was observed, particularly in the BMSC(+) group, at 6 weeks (Fig. 4). In both groups, the direct apposition of grafted tendon to bone was observed in some pores of the IP-CHA (Fig. 5a), and collagen fibre continuity was observed between the tendon and the fibrous tissue in some pores (Fig. 5b). Neither a fibrocartilagenous layer nor penetrating fibres from bone to tendon were observed in the interface between the tendon and the bone in pores of the IP-CHA.
Fig. 4.
(a–d) Typical histological images of the tendon-IP-CHA interface (a) BMSC(+) group 3 weeks after operation. (b) BMSC(-) group 3 weeks after operation. (c) BMSC(+) group 6 weeks after operation. (d) BMSC(-) group 6 weeks after operation (B: bone; T: tendon; hematoxylin and eosin staining, original magnification ×50). Vascular, highly cellular fibrous tissue and bone were observed within the pores in contact with the tendon. The amount of bone around the tendon increased 6 weeks after the operation compared with 3 weeks after the operation. Extensive proliferation of bone was observed, particularly in the BMSC(+) group 6 weeks after the operation
Fig. 5.
Histological images of the interface 6 weeks after operation. (a) Direct apposition of the tendon to bone within some pores of the IP-CHA was observed in both groups (B: bone; T: tendon; H: IP-CHA; hematoxylin and eosin staining, original magnification ×200). (b) Collagen fibre continuity was observed between the tendon and fibrous tissue within some pores of the IP-CHA (Cf: collagen fibre; T: tendon; H: IP-CHA; azan staining, original magnification ×200)
Quantitative measurement of bone formation around tendon
The area of bone formation around the tendon was 0.03±0.05 mm2 at 3 weeks after the operation in BMSC(+); 0.03±0.03 mm2 at 3 weeks in BMSC(-); 0.27±0.18 mm2 at 6 weeks in BMSC(+); 0.08±0.04 mm2 at 6 weeks in BMSC(-). Three weeks after the operation, there was no significant difference between the BMSC(+) group and the BMSC(-) group (P=0.58). However, 6 weeks after the operation, bone formation was significantly greater in the BMSC(+) group than in the BMSC(-) group (P=0.04).
Biomechanical measurement
The maximum pull-out load of the tendon from the IP-CHA was 9.22±6.86 N in the BMSC(+) group and 6.50±4.73 N in the BMSC(-) group. The difference between the two groups was significant (P=0.03). In all models, tendons were pulled out from the hole in the IP-CHA, and no mid-substance rupture was observed.
Discussion
Abundant fibrous tissue with vessels was conducted into the pores of the IP-CHA around the tendon, even in the BMSC(-) group 3 weeks after the operation, suggesting that a bioactive interface between the tendon and IP-CHA had been achieved 3 weeks after the operation. The quantitative result revealed BMSC-enhanced bone formation around the tendon 6 weeks after the operation. The increasing volume of bone in contact with the tendon indicated firm adhesion of the tendon to IP-CHA via bone within the pores. The biomechanical data supported the histological data. This showed that BMSCs cultured in IP-CHA could augment the tendon attachment to IP-CHA. This also indicated that a bone defect including tendon attachment could be repaired using a tissue-engineering technique with IP-CHA as the scaffold and BMSCs as the cell source.
IP-CHA is a useful scaffold in bone regeneration. Hydroxyapatite ceramics are already widely used as an artificial bone substitute because they have good biocompatibility and can bind chemically to the host bone. Porous hydroxyapatite ceramics with an interconnected pore structure have been developed for their superior osteoconductivity in various methods. The IP-CHA used in this study was made by the “foam-gel” technique, and according to our analysis of its porous structure, the pore diameter was 100–250 μm, the total porosity was 63.6%, and most of the interpore connections ranged from 10 to 100 μm, with a maximum peak at 40 μm. Tamai et al. reported the superior osteoconductivity of the same IP-CHA in an animal model [11]. The same IP-CHA has been used in studies of bone and cartilage regeneration because its interconnected pores can work as a scaffold for cultured cells and growth factors [1, 3, 4, 6, 12]. Ito et al. reported good bone formation using the same IP-CHA hybridized with cultured BMSCs derived from a transgenic green fluorescent rat [3]. Akita et al. reported that vascular bundle insertion into the same IP-CHA produced a vascular network within the porous structure and that vascular bundle insertion enhanced new bone formation in tissue-engineered bone using recombinant human bone morphogenetic protein-2 (rhBMP-2) and IP-CHA [1]. For cartilage regeneration, Tamai et al. reported that the triple composite of rhBMP-2, poly-D,L-lactic acid/polyethylene glycol (PLA-PEG) and the same IP-CHA promoted the repair of full-thickness articular defects [12]. Thus, we decided to evaluate the regeneration of a bone defect including tendon attachment in tissue engineering using IP-CHA as a scaffold and BMSCs as a cell source. For tendon attachment to IP-CHA, abundant bone formation within the pores of IP-CHA in contact with the grafted tendon was supposed to be required. Therefore, in this study, BMSCs were subcultured in IP-CHA for the enhancement of bone formation around the tendon. Many previous studies on the reattachment of tendon to bone have been reported; however, there have been no studies on the regeneration of a bone defect including tendon attachment to a porous scaffold using cultured cells. In this study, the cultured BMSCs increased bone formation around the tendon and strengthened the mechanical adhesion of the tendon to IP-CHA. This indicates that a bone defect including tendon attachment could be repaired by the tissue-engineering technique using IP-CHA as a scaffold and BMSCs as a cell source.
There are three weak points to this study. (1) A fibrocartilagenous layer and collagen fibres penetrating the tendon from bone were not observed at the interface between the tendon and the bone in pores of IP-CHA. (2) We did not investigate the effect of BMSCs cultured in IP-CHA on bone formation using cell markers. (3) The maximum pull-out load of the tendon measured in this study was smaller than that in the studies of tendon reattachment to bone [2, 8, 9, 10, 13], and even smaller than that in our previous study [7]. In previous reports evaluating tendon healing in a bone tunnel, a fibrocartilagenous layer and/or collagen fibres penetrating the tendon from the bone were observed at the interface [2, 5, 8, 9, 10, 13]. In this study, abundant bone formation around the tendon was observed in the BMSC(+) group 6 weeks after the operation, and collagen continuity between the tendon and fibrous tissue in the pores and direct apposition of tendon to bone were observed in some pores; however, a fibrocartilagenous layer and penetrating collagen fibres were not observed in the interface. Yamakado et al. reported in their animal study that tensile stress enhanced the healing process at the tendon-bone junction [14]. In our model, the tendon was pulled medially through the hole in the IP-CHA and the bone tunnel and sutured to the medial collateral ligament, but the tensile stress in the joint motion was not obtained to the tendon. This may have been the reason for the lack of a fibrocartilagenous layer and penetrating collagen fibres in this study. Abundant bone formation around the tendon in the BMSC(+) group indicated that a fibrocartilagenous layer and penetrating collagen fibres would be observed when tensile stress was obtained to the grafted tendon. We did not investigate the effect of BMSCs cultured in IP-CHA on bone formation using cell markers. Ito et al. investigated the effect of cultured BMSCs derived from a transgenic green fluorescent rat with the same IP-CHA and reported that BMSCs could survive and differentiate into osteoblasts within the pores of IP-CHA [3]. Therefore, in this study, BMSCs were likely to survive and contribute to bone formation. The average maximum pull-out load was 9.2 N even in the BMSC(+) group, which seemed to be weak at the tendon insertion. In the biomechanical analysis, to eliminate the effects of the area where the tendon passed through the bone tunnel of the femur, the bone and tendon were cut at the medial margin of the IP-CHA. Furthermore, the tendon was detached from the lateral wall of the IP-CHA up to the area where the tendon came in contact with the hole of the IP-CHA for the exact measurement of adhesive strength between the tendon and the hole of the IP-CHA, so the load measured in this study might be smaller.
Conclusions
BMSCs cultured in IP-CHA could enhance bone formation around a grafted tendon, and a more mature interface was achieved histologically and biomechanically. BMSCs cultured in IP-CHA were able to augment tendon attachment to IP-CHA.
Acknowledgements
This study was supported in part by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (16209045, 16659409).
References
- 1.Akita S, Tamai N, Myoui A, Nishikawa M, Kaito T, Takaoka K, Yoshikawa H (2004) Capillary vessel network integration by inserting a vascular pedicle enhances bone formation in tissue-engineered bone using interconnected porous hydroxyapatite ceramics. Tissue Eng 10:789–795 [DOI] [PubMed]
- 2.Goradia VK, Rochat MC, Grana WA, Rohrer MD, Prasad HS (2000) Tendon-to-bone healing of a semitendinosus tendon autograft used for ACL reconstruction in a sheep model. Am J Knee Surg 13:143–151 [PubMed]
- 3.Ito Y, Tanaka N, Fujimoto Y, Yasunaga Y, Ishida O, Agung M, Ochi M (2004) Bone formation using novel interconnected porous calcium hydroxyapatite ceramic hybridized with cultured marrow stromal stem cells derived from Green rat. J Biomed Mater Res A 69:454–461 [DOI] [PubMed]
- 4.Kaito T, Myoui A, Takaoka K, Saito N, Nishikawa M, Tamai N, Ohgushi H, Yoshikawa H (2005) Potentiation of the activity of bone morphogenetic protein-2 in bone regeneration by a PLA-PEG/hydroxyapatite composite. Biomaterials 26:73–79 [DOI] [PubMed]
- 5.Liu SH, Panossian V, al-Shaikh R, Tomin E, Shepherd E, Finerman GA, Lane JM (1997) Morphology and matrix composition during early tendon to bone healing. Clin Orthop Relat Res 339:253–260 [DOI] [PubMed]
- 6.Nishikawa M, Myoui A, Ohgushi H, Ikeuchi M, Tamai N, Yoshikawa H (2004) Bone tissue engineering using novel interconnected porous hydroxyapatite ceramics combined with marrow mesenchymal cells: quantitative and three-dimensional image analysis. Cell Transplant 13:367–376 [DOI] [PubMed]
- 7.Omae H, Mochizuki Y, Yokoya S, Adachi N, Ochi M (in press) Effects of interconnecting porous structure of hydroxyapatite ceramics on interface between grafted tendon and ceramics. J Biomed Mater Res A [DOI] [PubMed]
- 8.Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF (1993) Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am 75:1795–1803 [DOI] [PubMed]
- 9.Shaieb MD, Singer DI, Grimes J, Namiki H (2000) Evaluation of tendon-to-bone reattachment: a rabbit model. Am J Orthop 29:537–542 [PubMed]
- 10.Soda Y, Sumen Y, Murakami Y, Ikuta Y, Ochi M (2003) Attachment of autogenous tendon graft to cortical bone is better than to cancellous bone: a mechanical and histological study of MCL reconstruction in rabbits. Acta Orthop Scand 74:322–326 [DOI] [PubMed]
- 11.Tamai N, Myoui A, Tomita T, Nakase T, Tanaka J, Ochi T, Yoshikawa H (2002) Novel hydroxyapatite ceramics with an interconnective porous structure exhibit superior osteoconduction in vivo. J Biomed Mater Res 59:110–117 [DOI] [PubMed]
- 12.Tamai N, Myoui A, Hirao M, Kaito T, Ochi T, Tanaka J, Takaoka K, Yoshikawa H (2005) A new biotechnology for articular cartilage repair: subchondral implantation of a composite of interconnected porous hydroxyapatite, synthetic polymer (PLA-PEG), and bone morphogenetic protein-2 (rhBMP-2). Osteoarthr Cartil 13:405–417 [DOI] [PubMed]
- 13.Tomita F, Yasuda K, Mikami S, Sakai T, Yamazaki S, Tohyama H (2001) Comparisons of intraosseous graft healing between the doubled flexor tendon graft and the bone-patellar tendon-bone graft in anterior cruciate ligament reconstruction. Arthroscopy 17:461–476 [DOI] [PubMed]
- 14.Yamakado K, Kitaoka K, Yamada H, Hashiba K, Nakamura R, Tomita K (2002) The influence of mechanical stress on graft healing in a bone tunnel. Arthroscopy 18:82–90 [DOI] [PubMed]