Abstract
Historically, the proteomic investigation of filamentous fungi has been restrained by difficulties associated with efficient protein extraction and the lack of extensive fungal genome sequence databases. The advent of robust protein extraction and separation technologies, combined with protein mass spectrometry and emerging genome sequence data, is leading to the emergence of extensive new knowledge on the nature of these organisms. In this review, we discuss some recent technological advances and their role in exploring the proteome of Aspergillus spp., along with other biotechnologically relevant fungi.
Keywords: Aspergillus fumigatus, Hypothetical protein identification, Invasive aspergillosis, MALDI-ToF, Mass spectrometry, Proteomics, Fungal proteomics
Pathogenic fungi
The main fungal pathogens of humans are Candida albicans and Aspergillus fumigatus. C. albicans is a commonly occurring pathogen in the human population, and in particular in patients undergoing cancer chemotherapy. A recent review has described the application of proteomics to study diamorphism, drug-induced changes in the Candida proteome, host-pathogen interactions and immunoproteomics (Rupp 2004). A. fumigatus is an opportunistic fungal pathogen of immunocompromised patients, causes approximately 4% of all hospital-based deaths in Europe and is the most common Aspergillus species associated with invasive aspergillosis (IA) (Brookman and Denning 2000; Brakhage and Langfelder 2002). The mortality rate associated with IA can be as high as 60–90%. In particular, IA causes severe morbidity and mortality in organ transplant (bone marrow and solid organ) and leukaemia patients. Moreover, it has been estimated that over 3,500 deaths per annum in the USA result from aspergillosis (Kontoyiannis and Bodey 2002). A growing, though limited antifungal drug repertoire is available to control IA and includes agents such as voriconazole, amphotericin B and the echinocandins (Enoch et al. 2006). The challenge for the research community is to exploit many emerging technologies, such as gene disruption strategies, microarray analysis and functional proteomics, to further our understanding of the biology of Aspergilli in general, and A. fumigatus in particular with view to identification of new antifungal drug targets, in addition to identifying enzymes with biotechnological potential. The purpose of this article is to outline general proteomic concepts and to provide an update on fungal proteomic studies, with an emphasis on those which have been carried out on A. fumigatus.
Proteomic technologies
Several studies have shown that mRNA levels do not correlate well with protein expression levels, hence the study of the whole dynamic proteome has gained elevated significance (Griffin et al. 2002; Gygi et al. 1999). Proteomic studies to date have used a wide range of techniques, with the majority of studies following the conventional approach of two-dimensional electrophoresis (2-DE) followed by Matrix Assisted Laser Desorption Ionization—Time of Flight (MALDI-ToF) mass spectrometry (MS). Although still a useful technique, Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) has several inescapable limitations such as the presence of several proteins in a single stained band, which can lead to misidentified proteins and a difficulty in quantifying differential regulation responses.
2-DE, which facilitates resolution of complex protein mixtures based on both charge (pI) and molecular mass, and peptide MS have been the two key enabling technologies behind the proteomics revolution. A variety of pre- and post- 2-DE staining methods are available including colloidal Coomassie blue dyes, silver and fluorescent stains (Patton 2002; Miller et al. 2006; Wu et al. 2006). Silver staining is more sensitive than Coomassie based stains and recently an MS compatible silver stain was introduced (Sinha 2001). Although fluorescence based stains have a greater dynamic range and sensitivity than either of the others, cost and questions over suitability for MS (Lanne and Panfilov 2004) mean that colloidal Coomassie staining remains a favourite for subsequent MS analysis.
Following 2-DE, protein spots are identified, excised and subject to digestion with proteolytic enzymes (almost always trypsin). These peptide mixtures are then subjected to MS separation and the resultant peptide mass fingerprints compared to gene/protein sequence databases to facilitate protein identification (Resing and Ahn 2005). MS instruments comprise an ionisation source, a time of flight tube and an ion detector with various types of peptide ionization employed including MALDI or electrospray ionization (ESI). Peptide sequence information can be obtained by so-called tandem MS (i.e., ESI Q-ToF or ion trap MS/MS) and used for database interrogation to enable protein identification as noted above.
Several groups have published annotated “reference maps” for many species with the idea of using them as standard comparisons for further 2-DE analysis. However, this has been attempted for only a very few fungal species (Wildgruber et al. 2002; COMPLUYEAST-2DPAGE database (http://babbage.csc.ucm.es/2d/); Weeks et al. 2006). However as with most methods employed in proteome research, the 2-DE approach has limitations and is complemented by alternative strategies. Protein fractionation by chromatography usually involves pre-fractionation of a protein extract prior to trypsin digestion of each fraction. Peptides from each fraction are then separated on a strong cation-exchange (SCX) column and passed directly onto a reversed-phase high performance liquid chromatography (HPLC) column from which peptides are directly eluted for tandem MS sequence analysis. This approach has been termed Multidimensional protein identification technology (MudPIT) and has the potential to identify protein–protein interactions in yeast (Graumann et al. 2004) and separate and identify over 1,480 proteins (Washburn et al. 2001). An improvement in this method was described by Wei et al. (2005) and increased the number of yeast proteins identified by MudPIT analysis to 3109 by adding an extra RP-HPLC step prior to SCX fractionation, leading to increased resolving power and desalting of the peptides. Many of the limitations of 2-DE can be overcome with these LC-MS techniques as shown by studies of membrane bound proteins of Neurospora crassa (Schmitt et al. 2006), however a combination of both techniques, as shown by Breci et al. (2005), demonstrated that each approach complements the other, increasing the overall coverage and significance of the data.
Challenges to functional proteomics in A. fumigatus
A. fumigatus presents a number of significant barriers to the execution of rigorous proteomic studies. Firstly, the rigid cell wall means that protein isolation, prior to 2-DE, requires the application of more extensive extraction technologies than other eukaryotic systems. Secondly, the differential expression of many proteins, which is dependent on environmental conditions, allied to the presence of low abundance and high molecular mass proteins, means that full proteome elucidation will require extensive analysis. The identification of post-translational modifications in holo-enzymes also represents a considerable challenge, although one not unique to A. fumigatus. Fortunately, the A. fumigatus genome (30 Mb encoding approximately 10,000 open reading frames) has been sequenced and is now available at ‘CADRE’ (http://www.cadre-genomes.org.uk) (Mabey et al. 2004; Nierman et al. 2005). However, although in silico annotation of the A. fumigatus genome has been carried out, experimental data to support gene identification is limited and many genes (approximately 5% of total) are identified as encoding ‘hypothetical proteins’. In addition, although many genes have been identified based on homology analyses, the actual functions of the cognate proteins in A. fumigatus remain to be elucidated.
Proteomic strategies to overcome limitations
Until recently, strategies for A. fumigatus 2-DE have not been forthcoming. However, Kniemeyer et al. 2006 and Carberry et al. 2006 have presented comparable protocols for the efficient extraction of proteins from A. fumigatus mycelia prior to 2-DE (Fig. 1). Both publications have noted the importance of mycelial disruption in liquid N2 and the presence of thiourea in extraction buffers, while Kniemeyer et al. (2006) observed that sulfobetaine improved 2-DE resolution. Moreover, differential expression of enzymes (identified by MALDI and tandem MS) involved in the glyoxylate cycle, gluconeogenesis and ethanol degradation pathways was observed during growth on glucose and ethanol, respectively. Using MALDI MS detection, Carberry et al. (2006) noted the identification of a number of previously ‘hypothetical proteins’, now more accurately described as unknown function proteins. Shimizu and Wariishi (2005) have demonstrated that protein extraction and subsequent 2-DE from protoplasts from the basidiomycete, Tyromyces palustris, gave superior results to mycelial protein extraction.
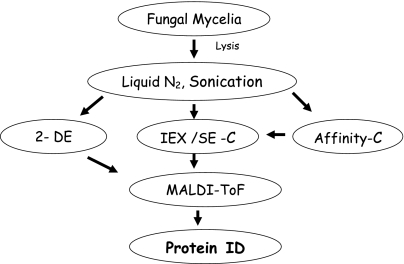
Fig. 1.
A general strategy for protein extraction and identification from filamentous fungi of biomedical and commercial importance and for which extensive genome sequence data is available. Following mycelial lysis, protein extracts can either be fractionated by 2-DE or a combination of ion-exchange (IEX), size-exclusion (SE) and affinity chromatography. Following trypsinisation, MALDI-ToF MS facilitates peptide mass fingerprinting and database interrogation leading to protein identification (ID)
The aim of most proteomic analyses is the generation of quantitative data on differential protein expression in response to environmental alterations. Difference Gel Electrophoresis (DIGE) was developed by Unlu et al. (1997) and uses fluorescent cyanide dyes to pre-label the protein samples prior to IEF. Currently three different dyes are available, which means that three differently labeled protein extracts can be electrophoresed together on the same IEF strip, thereby preventing inter-gel variation. Also due to the high sensitivity of the dyes, only 50 μg of each protein mixture is required for labeling, giving a total of 150 μg protein loaded onto each IEF strip so high protein concentrations are not required. The general approach is to label two separate protein extracts with a separate dye and then label a pooled preparation of both unlabeled extracts with the third dye; therefore each gel has an internal control. After electrophoresis of all three labeled protein extracts on the same gel, images are scanned using a fluorescent scanner and quantitative results are based on the total fluorescence intensity of each spot. This technique has been used in fungi to identify stress-related responses whereby the DIGE identification of 260 differentially expressed protein isoforms from 2-DE via MALDI MS revealed the complexity of the cellular response to oxidative stress (Weeks et al. 2006).
Sub-proteomic strategies
Many researchers have opted to use sub-proteomic approaches to study proteins of interest due to the complexity of whole cell proteomic analysis (Fig. 1). As with LC-MS/MS analysis, pre-fractionation of proteins prior to 2-DE analysis is common, with many studies employing prior protein purification. In A. fumigatus, glutathione (GSH)-Sepharose affinity chromatography was used to selectively detect and purify glutathione binding proteins, resulting in more than ten proteins resolved on 2-DE and the identification of a putative translation elongation factor with GST activity (Carberry et al. 2006). Bruneau et al. (2001) used an octyl-Sepharose fractionation followed by 2-DE and MALDI MS to identify nine glycosylphosphatidylinositol-anchored proteins in A. fumigatus, five of which were homologs of putatively GPI-anchored yeast proteins. Reiber et al. (2005) used Q-Sepharose separation followed by gel permeation chromatography to partially purify two proteins whose expression was up-regulated under iron-free conditions from A. fumigatus. Subsequent MALDI and tandem-MS analysis identified both proteins as nonribosomal peptide synthetases, SidD and SidC (Fig. 1).
Sub-proteomics is also exemplified by the analysis of proteins secreted by many of the industrially important strains of fungi. Filamentous fungi in particular have the ability to secrete various proteins, peptides and enzymes, and to this end secretome analysis has been studied by several groups. Taka-amylase, glucoamylase and aspergillopepsin were identified by Zhu et al. (2004) as major enzymes produced during conidial germination by Aspergillus oryzae strain RIB40, an important industrial fungus. Another study of A. oryzae compared the extracellular proteins produced under submerged and solid-state culture conditions (Oda et al. 2006). Extensive secretome analysis has also been performed on A. flavus, which degrades the flavonoid rutin as the only source of carbon via an extracellular enzyme system. 2-DE analysis identified only 20 proteins in un-induced cultures in comparison to 70 proteins that were detected when A. flavus was cultured in the presence of rutin (Medina et al. 2004). In a follow up study, 51 unique A. flavus secreted proteins from the three growth conditions whereby ten proteins were unique to rutin-, five to glucose- and one to potato dextrose-grown A. flavus, with sixteen secreted proteins common to growth on all three media. Fourteen hypothetical proteins or proteins of unknown function were detected (Medina et al. 2005). Similar studies have also been conducted on plant pathogenic fungi and wood degrading fungi (Belen Suarez et al. 2005; Abbas et al. 2005).
Sub-proteomics of fungal species has also involved separation and analysis of constituent proteins of fungal cell walls, thought to be a major factor in virulent strains of fungi, and organelles such as mitochondria. Cell wall and membrane bound proteins are difficult to analyse via 2-DE techniques as they are hydrophobic and poorly represented, remain insoluble in most IEF buffers and require solubilisation by detergents that generally are not IEF compatible. The use of novel sulfobetaine detergents suitable for IEF has been used to increase the solubility of such proteins (Grinyer et al. 2004a; Kniemeyer et al. 2006). Additionally, conidial surface associated proteins of A. fumigatus, extracted at pH 8.5 in the presence of a 1,3-beta-glucanase, were analysed using a 2-DE / LC-tandem MS approach by Asif et al. (2006). In total, 26 separate conidial surface proteins were identified and although many identified proteins contained secretion signal sequences, one protein, the allergen Aspf3, was present without a secretion signal and was postulated to have a possible role in triggering allergic responses due to A. fumigatus. Significantly, Ito et al. (2006) have used a combined immunoproteomics/MS approach to demonstrate that antibodies from immunocompromised mice, previously immunised with A. fumigatus conidia, are primarily directed against allergen Asp f3 and further demonstrated that vaccination with recombinant Asp f3 was protective.
The fully sequenced and annotated model fungus Neurospora crassa and the unsequenced biocontrol agent T. harzianum have both been used to dissect the proteome of the fungal mitochondria (Schmitt et al. 2006; Grinyer et al. 2004b). Both studies used a combined 2-DE and LC-MS/MS approach of selected trypsinised proteins, resulting in the identification of 249 proteins by Schmitt et al. (2006), highlighting the success of the sub-proteomic and 2-DE approaches in functional proteomics.
Conclusion
The availability of genome sequence availability and protein MS technologies are beginning to reveal the complex and dynamic nature of fungal proteomes. Significant biotechnological and biomedical advances have already been made and many more await the exploitation of the above strategies.
Acknowledgements
All funding for this work was obtained from the Irish Higher Education Authority—Programme for Research in Third Level Institutions (PRTLI) Cycle 3.
References
- Abbas A, Koc H, Liu F, Tien M (2005) Fungal degradation of wood: initial proteomic analysis of extracellular proteins of Phanerochaete chrysosporium grown in oak substrate. Curr Genet 47:49–56 [DOI] [PubMed]
- Asif AR, Oellerich M, Amstrong VW, Riemenschneider B, Monod M, Reichard U (2006) Proteome of conidial surface associated proteins of Aspergillus fumigatus reflecting potential vaccine candidates and allergens. J Proteome Res 4:954–962 [DOI] [PubMed]
- Belen Suarez M, Sanz L, Chanorro MI, Rey M, Gonzalez FJ, Llobell A, Monte E (2005) Proteomic analysis of secreted proteins from Trichoderma harzianum. Identification of a fungal cell wall-induced aspartic protease. Fungal Genet Biol 11:924–934 [DOI] [PubMed]
- Brakhage AA, Langfelder K (2002) Menacing mold: the molecular biology of Aspergillus fumigatus. Ann Rev Microbiol 56:433–455 [DOI] [PubMed]
- Breci L, Hattrup E, Keeler M, Letarte J, Johnson R, Haynes PA (2005) Comprehensive proteomics in yeast using chromatographic fractionation, gas phase fractionation, protein gel electrophoresis, and isoelectric focusing. Proteomics 5:2018–2028 [DOI] [PubMed]
- Brookman JL, Denning DW (2000) Molecular genetics in Aspergillus fumigatus. Curr Opin Microbiol 3:468–474 [DOI] [PubMed]
- Bruneau JM, Magnin T, Tagat E, Legrand R, Bernard M, Diaquin M, Fudali C, Latge JP (2001) Proteome analysis of Aspergillus fumigatus identifies glycosylphosphatidylinositol-anchored proteins associated to the cell wall biosynthesis. Electrophoresis 13:2812–2823 [DOI] [PubMed]
- Carberry S, Neville CM, Kavanagh KA, Doyle S (2006) Analysis of major intracellular proteins of Aspergillus fumigatus by MALDI mass spectrometry: identification and characterisation of an elongation factor 1B protein with glutathione transferase activity. Biochem Biophys Res Commun 24:1096–1104 [DOI] [PubMed]
- Enoch DA, Ludlam HA, Brown NM (2006) Invasive fungal infections: a review of epidemiology and management options. J Med Microbiol 55:809–818 [DOI] [PubMed]
- Graumann J, Dunipace LA, Seol JH, McDonald WH, Yates JR 3rd, Wold BJ, Deshaies RJ (2004) Applicability of tandem affinity purification MudPIT to pathway proteomics in yeast. Mol Cell Proteomics 3:226–237 [DOI] [PubMed]
- Griffin TJ, Gygi SP, Ideker T, Rist B, Eng J, Hood L, Aebersold R (2002) Complementary profiling of gene expression at the transcriptome and proteome levels in Saccharomyces cerevisiae. Mol Cell Proteomics 1:323–333 [DOI] [PubMed]
- Grinyer J, McKay M, Nevalainen H, Herbert BR (2004a) Fungal proteomics: initial mapping if biological control strain Trichoderma harzianum. Curr Genet 45:163–169 [DOI] [PubMed]
- Grinyer J, McKay M, Herbert B, Nevalainen H (2004b) Fungal proteomics: mapping the mitochondrial proteins of a Trichoderma harzianum strain applied for biological control. Curr Genet 3:170–175 [DOI] [PubMed]
- Gygi SP, Rochon Y, Franza BR, Aebersold R (1999) Correlation between protein and mRNA abundance in yeast. Mol Cell Biol 19: 1720–1730 [DOI] [PMC free article] [PubMed]
- Kontoyiannis DP, Bodey GP (2002) Invasive aspergillosis in 2002: an update. Eur J Clin Microbiol Infect Dis 21:161–172 [DOI] [PubMed]
- Ito JI, Lyons JM, Hong TB, Tamae D, Liu YK, Wilczynski SP, Kalkum M (2006) Vaccinations with recombinant variants of Aspergillus fumigatus allergen Asp f 3 protect mice against invasive aspergillosis. Infect Immun 74:5075–5084 [DOI] [PMC free article] [PubMed]
- Kniemeyer O, Lessing F, Scheibner O, Hertweck C, Brakhage AA (2006) Optimisation of a 2-D gel electrophoresis protocol for the human-pathogenic fungus Aspergillus fumigatus. Curr Genet 49:178–189 [DOI] [PubMed]
- Lanne B, Panfilov O (2004) Protein Staining Influences the Quality of Mass Spectra Obtained by Peptide Mass Fingerprinting after Separation on 2-D Gels. A Comparison of Staining with Coomassie Brilliant Blue and Sypro Ruby. J Proteome Res 4:175–179 [DOI] [PubMed]
- Mabey JE, Anderson MJ, Giles PF et al (2004). CADRE: the Central Aspergillus Data REpository. Nucleic Acids Res 32:401–405 [DOI] [PMC free article] [PubMed]
- Medina ML, Kiernan UA, Francisci WA (2004) Proteomic analysis of rutin-induced secreted proteins from Aspergillus flavus. Fungal Genet Biol 41:327–335 [DOI] [PubMed]
- Medina ML, Haynes PA, Breci L, Francisco WA (2005) Analysis of secreted proteins from Aspergillus flavus. Proteomics 5:3153–161 [DOI] [PubMed]
- Miller I, Crawford J, Gianazza E (2006) Protein stains for proteomic applications: Which, when, why? Proteomics 6:5385–5408 [DOI] [PubMed]
- Nierman WC, Pain A, Anderson MJ et al (2005) Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 438:1151–1156 [DOI] [PubMed]
- Oda K, Kakizono D, Yamada O et al (2006) Proteomic analysis of extracellular proteins from Aspergillus oryzae grown under submerged and solid-state culture conditions. Appl Environ Microbiol 72:3448–457 [DOI] [PMC free article] [PubMed]
- Patton WF (2002) Detection technologies in proteome analysis. J Chromatogr B 771:3–31 [DOI] [PubMed]
- Resing KA, Ahn NG (2005) Proteomics strategies for protein identification. FEBS Lett 579:885–889 [DOI] [PubMed]
- Reiber K, Reeves EP, Neville CM et al (2005) The expression of selected non-ribosomal peptide synthetases in Aspergillus fumigatus is controlled by the availability of free iron. FEMS Microbiol Lett 248:83–91 [DOI] [PubMed]
- Rupp S (2004) Proteomics on its way to study host-pathogen interaction in Candida albicans. Curr Opin Microbiol 7:330–335 [DOI] [PubMed]
- Schmitt S, Prokisch H, Schlunck T et al (2006) Proteome analysis of mitochondrial outer membrane from Neurospora crassa. Proteomics 6:72–80 [DOI] [PubMed]
- Shimizu M, Wariishi H (2005) Development of a sample preparation method for fungal proteomics. FEMS Microbiol Lett 247:17–22 [DOI] [PubMed]
- Sinha P, Poland J, Schnolzer M, Rabilloud T (2001) A new silver staining apparatus and procedure for matrix-assisted laser desorption/ionization-time of flight analysis of proteins after two-dimensional electrophoresis. Proteomics 1:835–840 [DOI] [PubMed]
- Unlu M, Morgan ME, Minden JS (1997) Difference gel electrophoresis: a single gel method for detecting changes in protein extracts. Electrophoresis 18:2071–2077 [DOI] [PubMed]
- Washburn MP, Wolters D, Yates JR 3rd (2001) Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol 19:242–247 [DOI] [PubMed]
- Weeks ME, Sinclair J, Butt A et al (2006) A parallel proteomic and metabolomic analysis of the hydrogen peroxide- and Sty1p-dependent stress response in Schizosaccharomyces pombe. Proteomics 6:2772–2796 [DOI] [PubMed]
- Wei J, Sun J, Yu W et al (2005) Global proteome discovery using an online three-dimensional LC-MS/MS. J Proteome Res 4:801–8 [DOI] [PubMed]
- Wildgruber R, Reil G, Drews O, Parlar H, Gorg A (2002) Web-based two-dimensional database of Saccharomyces cerevisiae proteins using immobilized pH gradients from pH 6 to pH 12 and matrix-assisted laser desorption/ionization-time of flight mass spectrometry. Proteomics 6:727–732 [DOI] [PubMed]
- Wu WW, Wang G, Baek SJ, Shen RF (2006) Comparative Study of Three Proteomic Quantitative Methods, DIGE, cICAT, and iTRAQ, Using 2D Gel- or LC-MALDI TOF/TOF. J Proteome Res 5:651–658 [DOI] [PubMed]
- Zhu L, Nguyen C.H, Sato T, Takeuchi M (2004) Analysis of Secreted Proteins during Conidial Germination of Aspergillus oryzae RIB40. Biosci Biotechnol Biochem 68:2607–2612 [DOI] [PubMed]