Abstract
In recent years there have been a number of reports that suggest the sub-physiological (<37 °C) temperature in vitro culturing of mammalian cells can result in enhanced heterologous protein production. Despite these reports, the mechanisms by which mammalian cells respond to such conditions are largely unknown. We therefore set out to use a model in vitro culture HeLa cell system to begin investigating the cold-shock response in mammalian cell systems. Sub-physiological temperature cultivation resulted in reduced growth and proliferation and a lower total cell protein content. Proteomic analysis confirmed that HeLa cells actively respond to sub-physiological temperature by up-regulating a number of proteins and immunoblot analysis confirmed that specific proteins are indeed up-regulated in a time and temperature dependent manner. Additional work is likely to improve our understanding of the cold-shock response in mammalian cells and identify candidate target proteins for cell engineering to further enhance heterologous protein production at sub-physiological temperatures.
Keywords: Cold-shock, HeLa cells, 2D-PAGE, Bioprocessing, Sub-physiological temperature culturing, Recombinant protein production
Introduction
In recent years there has been an ever-growing number of reports that the sub-physiological temperature in vitro culturing (<37 °C) of mammalian cells can lead to enhanced heterologous protein production (Beer et al. 2003; Ducommun et al. 2002; Fogolin et al. 2004; Fox et al. 2004; Fox et al. 2005b; Schatz et al. 2003; Yoon et al. 2003a; Yoon et al. 2003b). This effect is extremely variable however, and appears to be both cell line and expression system dependent (Al-Fageeh et al. 2006; Al-Fageeh and Smales, 2006). A further complication in understanding the response(s) underpinning enhanced heterologous protein production is that cold-stress results in cells being exposed to not one, but two stresses, temperature stress and stress due to changes in oxygen concentration at lower temperatures (Ohsaka et al. 2002). A better understanding of the mechanisms by which mammalian cell systems respond to sub-physiological temperature in vitro culturing (typically 27–34 °C), and the interactions between these responses, will therefore be required before systems and methodologies that routinely result in enhanced recombinant protein production under such conditions can be developed.
A number of investigations have now focussed upon furthering our understanding of the mechanisms underpinning enhanced recombinant protein production from mammalian cells cultured at sub-physiological temperatures. Such studies have shown that the culturing of CHO cells at 30 °C results in cell cycle arrest in the G1 phase of the cell cycle and a delay in the onset of apoptosis (Moore et al. 1997). More recently changes in the levels of target heterologous mRNA levels have been implicated as responsible for increases in recombinant protein production from CHO cells cultivated at reduced temperatures. For example, Yoon et al. showed that recombinant mRNA levels were increased at temperatures less than 37 °C (Yoon et al. 2003b) whilst others have shown that increased recombinant human interferon-gamma (IFN-gamma) production in CHO cells cultured at lower temperatures (32 °C) appears to be the result of increase mRNA levels (Fox et al. 2004; Fox et al. 2005a). Still others have utilized a proteomic approach to investigate global changes in protein expression in CHO cells cultured at sub-physiological temperatures (Baik et al. 2006; Kaufmann et al. 1999).
Despite these studies, to date the mechanisms by which mammalian cells respond to sub-physiological temperature culturing remain largely unknown. We have therefore utilised a proteomic approach to begin investigating the response of in vitro cultured HeLa cells to sub-physiological temperature culturing. Although HeLa cells are not used industrially, the advantage of using these as model system is the availability of the human genome, unlike the CHO genome, and the availability of well characterised 2D-PAGE maps of HeLa cells in the public domain (Fountoulakis et al. 2004). Further, we wished to investigate whether those responses observed in CHO cells to date were conserved in other mammalian cell lines. Here we present the preliminary findings from this study.
Materials and methods
Growth and maintenance of HeLa cells
HeLa Ohio cells (human cervical carcinoma cells) were obtained from the European collection of cell cultures (ECACC product number 93021013) and cultured in Dulbecco’s modified Eagle’s medium supplemented with 500 μM glutamic acid, 500 μM asparagine, 200 mM L-glutamine, 30 μM adenosine, 30 μM guanosine, 30 μM cytidine, 30 μM uridine, 10 μM thymidine, (100 x) non-essential amino acids (Invitrogen, Life Technologies, Scotland, UK), and 10% (v/v) dialyzed fetal calf serum (PAA laboratories, Kingston-upon-Thames, UK). Cells were maintained in 125 cm2 tissue culture flasks in a 5% CO2 incubator at 37 °C until 70–80% confluent. Cell numbers and viabilities were determined by counting after detachment with trypsin (0.25% (w/v), 1 mM EDTA; Invitrogen, Paisley, UK) using the trypan blue exclusion method.
For proteomic experiments exponentially growing cells were inoculated at 1 × 105 cell mL−1 into 75 cm2 tissue culture flasks containing 15 mL of culture medium. Initially, the cultures were cultured at 37 °C in a humidified 5% CO2 incubator for 24 h and then transferred to incubators at 37, 32 and 10 °C. After 24 h at the appropriate temperature flasks were removed from the incubator and protein extracts immediately prepared for analysis. All experiments were repeated in duplicated.
Protein extraction and 2D-PAGE analysis
Cell aliquots (1 × 107) were extracted into 400 μL of cell lysis buffer as described previously (Smales et al. 2004). Protein concentrations in HeLa cell extracts were determined using a modified Bradford Assay as described elsewhere (Ramagli 1999). HeLa proteins were resolved by large format (24 × 20 cm) 2D-PAGE using a non-linear pI range of 3–10 (24 cm immobilized pH gradient) as described previously (Smales et al. 2003). After 2D-PAGE, gels were fixed in a solution of 40% ethanol, 10% acetic acid overnight and protein spots were then detected using the silver stain plusone protocol (GE Healthcare, Little Chalfont, UK) as described by the manufacturer apart from the omission of glutaraldehyde in the sensitising step. The resulting gel images were captured at 200 dpi using a Powerlook III prepress colour scanner (GE Healthcare, Little Chalfont, UK).
2D-PAGE image analysis
Gel images were analysed using the commercially available Progenesis PG200 software package (Nonlinear Dynamics, Newcastle-upon-Tyne, UK). Spot detection was undertaken using the spot detection wizard with the parameters set as follows: minimum spot area 16; split factor 7; peak location use centre of mass as peak. Manual splitting of non-split spots and deletion of noise were then undertaken. Following spot detection, background subtraction was achieved using the mode of non-spot option with a margin of 45. For each time point one gel was selected as a reference gel and the other gel was matched after placement of user seeds across the gel to account for gel warping during running. Once the gels had been successfully matched, the reference gel was modified to include unmatched spots from gels being matched to it. An average gel was then created for each temperature and time point and the resulting average gels then subjected to pair-wise comparisons. Variability in normalised spot volumes between the different temperatures was tested by one-way analysis of variance (ANOVA) at p < 0.05 and the Students t-tests at p < 0.05.
Immunoblot analysis
Protein extract (2 mg mL−1) was diluted 1:1 with Laemmli sample buffer and 12 μL was subjected to 12.5% resolving SDS-PAGE analysis using standard procedures. Following electrophoresis, resolved proteins were transferred to PVDF membrane (Bio-Rad, Herts UK) at 25 V for 90 min in a semi-dry blotting apparatus using standard procedures. The membranes were then blocked by incubation with TBST buffer containing 20 mM Tris-HCl, 137 mM NaCl, 0.1% (v/v) Tween-20, pH 7.6 containing 5% (w/v) dried milk, overnight at 4 °C. After blocking the membranes were probed with antibodies raised against eIF2α (mouse monoclonal; kindly donated by Prof. C.G. Proud, University of British Columbia); phosphorylated eIF2α (rabbit polyclonal, Abcam, Cambridge, UK); Vimentin (mouse monoclonal; Sigma, Poole, UK); HSF-1 (rabbit polyclonal, Cambridge Bioscience, Cambridge, UK); PDI (rabbit polyclonal, kindly donated by Dr. P. Klappa, University of Kent, UK) and anti-β-actin (mouse monoclonal, Sigma, Poole, UK). The membranes were then incubated with the appropriate horseradish peroxidase conjugated secondary antibody (DAKO, Glostrup, Denmark) before detection using an Enhanced Chemiluminescence detection kit (GE Healthcare, Little Chalfont, UK) as per the manufacturer’s instructions.
Results and discussion
The effect of sub-physiological temperature on cell growth and total cell protein content
The culturing of mammalian cells at sub-physiological temperatures (<37 °C) is known to result in the arrest of growth and proliferation in the G1 phase of the cell cycle (Kaufmann et al. 1999). In agreement with this, 24 h post-temperature downshift at either 32 or 10 °C the number of viable HeLa cells in this study was reduced compared to the 37 °C control (Table 1), presumably as a result of cell cycle arrest. Indeed, after culturing at 32 °C for 24 h the viable cell concentration was reduced by approximately 25% compared to the 37 °C control whilst when cultured at 10 °C the reduction was more than 50% (Table 1). Despite this the number of viable cells at both 10 and 32 °C 24 h post-temperature downshift was increased relative to the number of cells before temperature shift (Table 1). At 32 °C the number of cells had actually doubled compared to that before temperature downshift, suggesting that cell proliferation had proceeded during the 24 h cold-shock period. This may be the result of cells progressing through the cell cycle to reach G1 phase before arresting, completing the current cycle. Others have also reported that mammalian cells can indeed grow and proliferate, albeit at a substantially reduced rate, at such temperatures (Al-Fageeh et al. 2006). On-the-other-hand, shifting the culture temperature of HeLa cells from 37–10 °C resulted in a more immediate and severe reduction in cell proliferation and only a small increase in viable cell numbers was observed (Table 1).
Table 1.
Viable cell concentrations and total cell protein content in HeLa cell extracts from control and sub-physiological temperature cultures 24 h post-temperature downshift
| Temperature/Time | Viable Cell Concentration (×105 cells mL−1) | Cellular Protein Content (pg cell−1) |
|---|---|---|
| 37 °C 0 h | 2.64 | 210 |
| 37 °C 24 h | 8.58 | 160 |
| 32 °C 24 h | 6.26 | 142 |
| 10 °C 24 h | 3.92 | 141 |
Determination of the total cell protein content of HeLa cell extracts for proteomic analysis showed that this changed during culture and upon reduced temperature cultivation (Table 1). The highest cellular protein content was observed in time zero samples and probably reflects the time at which cells are most active in terms of protein synthesis during proliferation and growth. Twenty-four hours later the control sample extracts had a reduced total cell protein content (Table 1). At this time the cells were almost confluent and the decrease in cell protein is most likely due to attenuation of cell growth and proliferation. Cells cultured for 24 h at either 32 or 10 °C had a lower total cellular protein content than the 37 °C control although there was no difference between either of these temperatures in terms of the total protein per cell.
Initial 2D-PAGE analysis of the HeLa cell cold-shock proteome
As described above, the responses of in vitro cultured mammalian cells to sub-physiological temperature are not well understood. We therefore set out to investigate the response in HeLa cells using a 2D-PAGE proteomic based approach to identify/confirm changes in protein expression under such conditions. To date only two cold-shock proteins have been described in detail in mammalian cells, cold inducible RNA binding protein (CIRP) and RNA binding motif protein 3 (Rbm3) (Al-Fageeh and Smales 2006). There is however a number of studies that have now utilised a proteomic approach to investigate the global protein response in mammalian cells upon sub-physiological temperature culturing. For example, Baik and colleagues have recently reported the findings from a proteomic investigation into the effect of culturing CHO cells at 33 °C (Baik et al. 2006). This report showed that under these conditions seven proteins were up regulated and two down regulated upon culturing at 33 °C.
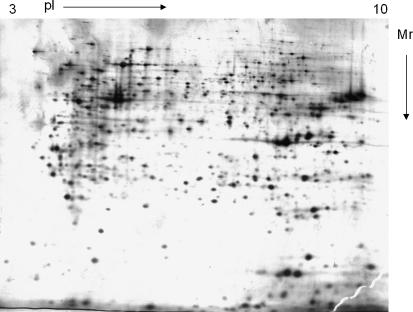
An example of a typical 2D-PAGE image from HeLa cell extracts is depicted in Fig. 1. Analysis of digital images of the 2D gels generated from the samples here 24 h post-temperature downshift was undertaken using the commercial Progenesis PG200 software as described in the methods section. Volumes were normalized on the basis of the total spot volume in gel images, to give each spot a normalized percentage of the whole gel. Average gels were then created from the duplicate samples for each temperature 24 h post cold-shock and then the spot volumes compared across the different average gels. Average gels at all temperatures were compared to each other in a pair-wise fashion and changes in abundance determined as outlined in the methods section. Table 2 details the number of changes in protein abundance as defined by our criteria for the pair-wise comparisons between samples and temperatures.
Fig. 1.
Representative 2D-PAGE map from a HeLa cell extract. The gel shown is that from HeLa cells cultured at 10 °C. For further details see text
Table 2.
Pair-wise comparisons of protein spots up- and down regulated 24 h post-temperature shift
| Up/Down | 37 °C 24 h |
|---|---|
| 37 °C 0 h | 5/5 |
| 32 °C 24 h | 12/9 |
| 10 °C 24 h | 27/1 |
Pair-wise comparison of the control average gel at 37 °C at 24 h relative to the 0 h 37 °C sample showed that very few proteins appeared to change in abundance over this time period (Table 2). This is somewhat surprising and we may have expected to see a larger number of proteins whose expression profiles changed, particularly as the growth and cellular protein data in Table 1 suggest a difference in the state of the cells at these times. On-the-other-hand, pair-wise comparison of the 37 °C 24 h control with the 32 °C sample identified 12 protein spots that were up-regulated and nine down-regulated, a similar number to that reported previously in CHO cells after culture at 33 °C (Baik et al. 2006). Comparison of the control with the 10 °C average gel showed more than twice as many spots up-regulated but fewer spots down-regulated (Table 2). Our initial analysis therefore suggests different responses at mild and harsher conditions of cold as might be expected. We were somewhat surprised that at ten degrees more protein spots were up regulated than at 32 °C as we expected protein synthesis and turnover to be severely hindered at the lower culture temperature. The reason for this discrepancy is not currently clear, however identification of those spots whose expression levels change may shed further light on this.
Targeted analysis of changes in the abundance of specific proteins upon sub-physiological culturing of HeLa cells
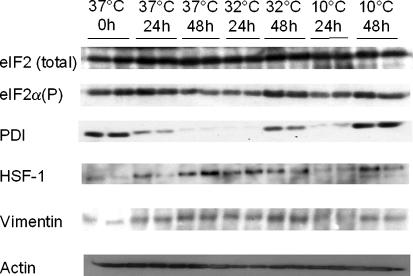
We utilised western blotting to investigate whether there were changes in the abundance of proteins previously identified as modulated under cold-shock conditions in the HeLa cell extracts. Specifically, we investigated PDI and vimentin, both reported by Baik et al. to be induced upon cold-shock in CHO cells (Baik et al. 2006). We also investigated the phosphorylation levels of the eukaryotic translation initiation factor eIF2α as attenuation of cap-dependent mRNA translation (such as that reported upon the perception of cold-shock) is regulated via the phosphorylation of the alpha subunit of eIF2 (Underhill et al. 2005). Finally, we investigated the levels of heat shock transcription factor 1 (HSF1) in order to determine if a heat-shock response might be initiated and the levels of β-actin.
These investigations showed that actin levels appeared to be more or less constant at all temperatures and time points investigated (Fig. 2). Further, vimentin levels seemed relatively unchanged (Fig. 2) in direct contrast to those results previously observed in CHO cells as described above. On-the-other-hand, PDI levels were elevated at both 32 and 10 °C 48 h after temperature downshift in agreement with those findings in CHO cells. The reason for an increase in PDI levels at reduced temperature with time is currently unknown, however it may be a response to protein aggregation or misfolding in the endoplasmic reticulum. It is interesting to note that PDI levels monitor the levels of eIF2α phosphorylation which were also elevated at sub-physiological temperatures relative to 37 °C controls 48 h post-temperature downshift (Fig. 2). Although phosphorylation of eIF2α can occur via four kinases in mammalian cells, one of these is an ER resident protein kinase (PERK) that is known to phosphorylate eIF2α in response to ER stress. It is therefore tempting to speculate that prolonged cold temperature stress (48 h) results in ER stress and hence activates a classical response to this. Further investigation is required to confirm this. The absolute levels of eIF2α did not appear to change with temperature (Fig. 2).
Fig. 2.
Expression of specific target proteins during the in vitro culturing of HeLa cells at 37, 32 and 10 °C over a 48 h period as determined by immunoblotting. For further details see text
Finally we investigated whether the transcription factor HSF1 was up regulated upon cold temperature stress. HSF1 is a transactivator of heat shock proteins in response to heat stress (Inouye et al. 2004). Previous studies in HeLa cells have shown that the heat shock proteins HSP70 and HSP90 are actually down regulated in response to cryopreservation. Our results show that HSF1 expression levels were lower at sub-physiological temperatures relative to those observed at 37 °C at all time points investigated (Fig. 2). This suggests that a general heat shock protein response is not initiated in response to cold-stress in HeLa cells. However, it would be interesting to investigate whether such a response was observed upon recovery of cold stressed cells back to 37 °C.
In conclusion, the present study has confirmed that in vitro cultured HeLa cells actively respond to cold stress. Reduced temperature culture results in the induction and down regulation of a small set of proteins. Further studies based upon these initial findings are likely to improve our understanding of the cold-shock response in mammalian cells and identify candidate target proteins for cell engineering to enhance heterologous protein production from mammalian cells at sub-physiological temperatures.
Acknowledgements
This work was partially supported by Grant BB/C006569/1 from the Biotechnology and Biological Sciences Research Council, UK.
Footnotes
A manuscript submitted from the Global mRNA and Protein Expression Analysis, Research Applications in Cancer and other Diseases and in Biopharmaceutical Production conference, Dublin City University, Dublin 9, Ireland, September 7–8th 2006
References
- Al-Fageeh MB, Marchant RJ, Carden MJ, Smales CM (2006) The cold-shock response in cultured mammalian cells: harnessing the response for the improvement of recombinant protein production. Biotechnol Bioeng 93:829–835 [DOI] [PubMed]
- Al-Fageeh MB, Smales CM (2006) Control and regulation of the cellular responses to cold shock: the responses in yeast and mammalian systems. Biochem J 397:247–259 [DOI] [PMC free article] [PubMed]
- Baik JY, Lee MS, An SR, Yoon SK, Joo EJ, Kim YH, Park HW, Lee GM (2006) Initial transcriptome and proteome analyses of low culture temperature-induced expression in CHO cells producing erythropoietin. Biotechnol Bioeng 93:361–371 [DOI] [PubMed]
- Beer C, Buhr P, Hahn H, Laubner D, Wirth M (2003) Gene expression analysis of murine cells producing amphotropic mouse leukaemia virus at a cultivation temperature of 32 and 37 degrees C. J Gen Virol 84:1677–1686 [DOI] [PubMed]
- Ducommun P, Ruffieux PA, Kadouri A, von Stockar U, Marison I W (2002) Monitoring of temperature effects on animal cell metabolism in a packed bed process. Biotechnol Bioeng 77:838–842 [DOI] [PubMed]
- Fogolin MB, Wagner R, Etcheverrigaray M, Kratje R (2004) Impact of temperature reduction and expression of yeast pyruvate carboxylase on hGM-CSF-producing CHO cells. J Biotechnol 109:179–191 [DOI] [PubMed]
- Fountoulakis M, Tsangaris G, Oh JE, Maris A, Lubec G (2004) Protein profile of the HeLa cell line. J Chromatogr A 1038:247–265 [DOI] [PubMed]
- Fox SR, Patel UA, Yap MG, Wang DIC (2004) Maximizing interferon-gamma production by Chinese hamster ovary cells through temperature shift optimization: experimental and modeling. Biotechnol Bioeng 85:177–184 [DOI] [PubMed]
- Fox SR, Tan HK, Tan MC, Wong S, Yap MGS, Wang DIC (2005a) A detailed understanding of the enhanced hypothermic productivity of interferon-gamma by Chinese-hamster ovary cells. Biotechnol Appl Biochem 41:255–264 [DOI] [PubMed]
- Fox SR, Yap MX, Yap MG, Wang DIC (2005b) Active hypothermic growth: a novel means for increasing total interferon-gamma production by Chinese-hamster ovary cells. Biotechnol Appl Biochem 41:265–272 [DOI] [PubMed]
- Inouye S, Izu H, Takaki E, Suzuki H, Shirai M, Yokota Y, Ichikawa H, Fujimoto M, Nakai A (2004) Impaired IgG production in mice deficient for heat shock transcription factor 1. J Biol Chem 279:38701–38709 [DOI] [PubMed]
- Kaufmann H, Mazur X, Fussenegger M, Bailey JE (1999) Influence of low temperature on productivity, proteome and protein phosphorylation of CHO cells. Biotechnol Bioeng 63:573–582 [DOI] [PubMed]
- Moore A, Mercer J, Dutina G, Donahue CJ, Bauer KD, Mather JP, Etcheverry T, Ryll T (1997) Effects of temperature shift on cell cycle, apoptosis and nucleotide pools in CHO cell batch cultues. Cytotechnology 23:47–54 [DOI] [PMC free article] [PubMed]
- Ohsaka Y, Ohgiya S, Hoshino T, Ishizaki K (2002) Phosphorylation of c-Jun N-terminal kinase in human hepatoblastoma cells is transiently increased by cold exposure and further enhanced by subsequent warm incubation of the cells. Cell Physiol Biochem 12:111–118 [DOI] [PubMed]
- Ramagli LS (1999) Quantifying protein in 2-D PAGE solubilization buffers. In: Link AJ (ed) Methods in molecular biology. 2-D proteome analysis protocols. Humana Press Inc, Totowa, NJ pp. 99–103 [DOI] [PubMed]
- Schatz SM, Kerschbaumer RJ, Gerstenbauer G, Kral M, Dorner F, Scheiflinger F (2003) Higher expression of Fab antibody fragments in a CHO cell line at reduced temperature. Biotechnol Bioeng 84:433–438 [DOI] [PubMed]
- Smales CM, Birch JR, Racher AJ, Marshall CT, James DC (2003) Evaluation of individual protein errors in silver-stained two-dimensional gels. Biochem Biophys Res Commun 306:1050–1055 [DOI] [PubMed]
- Smales CM, Dinnis DM, Stansfield SH, Alete DE, Sage EA, Birch JR, Racher AJ, Marshall CT, James DC (2004) Comparative proteomic analysis of GS-NS0 murine myeloma cell lines with varying recombinant monoclonal antibody production rate. Biotechnol Bioeng 88:474–488 [DOI] [PubMed]
- Underhill MF, Birch JR, Smales CM, Naylor LH (2005) eIF2alpha phosphorylation, stress perception, and the shutdown of global protein synthesis in cultured CHO cells. Biotechnol Bioeng 89:805–814 [DOI] [PubMed]
- Yoon SK, Kim SH, Lee GM (2003a) Effect of low culture temperature on specific productivity and transcription level of anti-4–1BB antibody in recombinant Chinese hamster ovary cells. Biotechnol Prog 19:1383–1386 [DOI] [PubMed]
- Yoon SK, Song JY, Lee GM (2003b) Effect of low culture temperature on specific productivity, transcription level, and heterogeneity of erythropoietin in Chinese hamster ovary cells. Biotechnol Bioeng 82:289–298 [DOI] [PubMed]