Abstract
Several factors can cause bone loss and fixation failure following total hip arthroplasty (THA), including polyethylene wear debris, implant micromotion and stress shielding. Various techniques have been used in an effort to detect bone density loss in vivo, all with varying success. Quantitative computed tomography (qCT)-assisted osteodensitometry has been shown to be useful in assessing the in vivo structural bone changes after THA. It has a high resolution, accuracy and reproducibility, thereby making it a useful tool for research purposes, and it is able to differentiate between cortical and cancellous bone structures and assess the bone/implant interface. This technique also provides valuable information about the pattern of stress shielding which occurs around the prosthesis and can show early bony changes, which may prove informative about the quality of implant fixation and surrounding bone adaptation. In conjunction with finite-element analysis, qCT is able to generate accurate patient-specific meshes on which to model implants and their effect on bone remodelling. This technology can be useful to predict bone remodelling and the quality of implant fixation using prostheses with different design and/or biomaterials. In the future, this tool could be used for pre-clinical validation of new implants before their introduction in the market-place.
Résumé
Plusieurs facteurs peuvent causer une perte osseuse et la faillite de la fixation après une arthroplastie totale de la hanche. Ils incluent les débris de polyéthylène, la micromobilité des implants et le transfert de contraintes. Plusieurs techniques ont été utilisées pour détecter la perte de densité osseuse, avec des succés variés. L’ostéodensitométrie quantitative par scanner s’est montrée utile dans l’étude in vivo des modifications structurales osseuses après arthroplastie totale de la hanche. Elle a une haute résolution, une précision et une reproductibilité qui en font un outil approprié pour la recherche. L’ostéodensitométrie quantitative peut différencier l’os cortical et l’os spongieux, étudier l’interface os-implant et donner des informations sur le modèle de déviation des contraintes qui surviennent autour d’une prothèse. Elle peut montrer précocement des modifications osseuses, ce qui renseigne sur la qualité de la fixation des implants et l’adaptation de l’os voisin. En conjonction avec l’analyse par éléments finis elle peut générer un maillage précis spécifique du patient permettant l’étude de modèles d’implants et leur effet sur le remodelage osseux. Cette technologie peut être utile pour prévoir le remodelage osseux et la qualité de la fixation pour des prothèses de différentes formes et/ou matériaux. Dans le future cet outil pourra être utilisé pour la validation pré-clinique de nouveaux implants avant leur introduction sur le marché.
Introduction
Periprosthetic bone remodelling following total hip arthroplasty (THA) is a well-recognised phenomenon [4, 6, 11]. Changes in bone density are likely to contribute to implant fixation failure, and the loss of bone stock makes subsequent revision surgery more difficult [8]. Bone densitometry provides useful information with regard to bone architecture around implants [2, 10], and various techniques have been employed to measure the degree of bone density change around implants – with varying degrees of accuracy. These techniques include radiographic absorptiometry, dual energy X-ray absorptiometry (DXA), and quantitative computed tomography (qCT) [3, 23]. Of these, qCT provides the most accurate method for in vivo evaluation of cortical and cancellous bone density [16].
Changes in both cortical and cancellous bone density following stress shielding of the bone may contribute to aseptic loosening, especially in conjunction with polyethylene wear [13, 15]. Stress shielding occurs as the physiological loads applied to bone are altered by the placement of an implant with a different stiffness to that of the host bone in which it is implanted. This shields the bone around the implant from normal stress transfer, and it subsquently remodels according to Wolff’s law: form follows the function [21]. The usual pattern of femoral remodelling is cortical hypertrophy distally with cortical and cancellous atrophy proximally [20]. The loss of bone mass adjacent to an implant leads to pain, increased fracture risk and implant instability. It also makes revision surgery more difficult.
Imaging modalities
In vivo assessment of bone density loss has been assessed using plain radiography, DXA and, more recently, by qCT. Radiographic absorptiometry, quantitative ultrasound and single energy X-ray absorptiometry are used in the peripheral skeleton to assess bone density as a screening tool for osteoporosis, but these modalities are unable to be used around prostheses or at deep sites such as the hip.
Plain radiography
It is difficult to make an assessment of periprosthetic bone resorption on plain radiographs, and to date reproducible results have not been shown. Too many factors can influence the results, including soft tissue artifacts, patient positioning and under- and overexposure of the films. Results of studies reported in the literature do not support the use of plain radiographs to assess bone density change following arthroplasty [3, 5].
In a study designed to assess acetabular stress shielding in THA, Wright et al. found that although bone density had decreased by as much as 33%, plain radiographs failed to show any changes [24]. Engh et al. do not recommend using plain radiographs to assess this phenomenon due to the major inter-observer variations they found. They suggest that 70% of the bone mineral density (BMD) must be lost before it can be reliably shown on plain radiographs.
Dual energy X-ray absorptiometry
Dual energy X-ray absorptiometry DXA has been employed to measure bone density around implants. This technique measures the amount of attenuation of an X-ray beam caused by the bone region of interest [7, 9, 12, 14, 22, 25, 26]. The advantages of DXA are that it is widely available and can detect changes in bone-mineral content (BMC) with little exposure to radiation (0.001 mSV) and short examination times (2–5 min). It does, however, depend on exact positioning of the patient at each scan, with error introduced by patient movement. Conflicting results are reported in the literature with respect to the accuracy of using DXA to determine bone density following arthroplasty [2, 5, 10]. In vivo precision error quoted by Wilkinson et al. [23] varies from 1 to 5.3% depending on the regions of interest and the prosthesis. The difficulty arises in deep areas with asymmetric implant position, such as the acetabulum. Recently algorithms have been developed to detect bone to soft tissue and bone to implant interfaces to exclude the effects of attenuating implants although these require an increased radiation dose and scan time [23]. The major disadvantage of DXA is the inability to differentiate between cortical and cancellous bone. In addition, in the setting of cemented implants it is difficult to exclude the absolute effect on bone density arising from the cement. While cement density decay over time is small, the presence of cement has been shown to increase the apparent BMD [10, 23].
Quantitative CT-assisted osteodensitometry
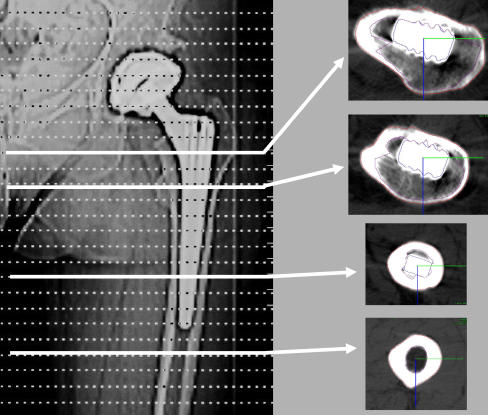
Quantitative CT-assisted osteodensitometry is the only technology to provide three-dimensional (3D) volumetric analysis [24]. Patients are evaluated with a standard CT scanner protocol, and specialised software is used to measure the relative density of the bone in Hounsfield units (HU) of each CT slice. Hounsfield units provide a density scale where the X-ray absorption of water is given the value of zero HU and air the value of −1000 HU [1]. Subsequent assessment of the bone architecture gives the surface area of the bone in each slice. At each session, a calibration phantom with a cylindrical section of known content of hydroxyl-apatite is also scanned, allowing conversion of radiological density measurements to true volumetric mineral density measurements (mg CaHA/ml) [16]. A range of CT slices can be taken at levels above, at and below the prosthesis to establish BMD change in different regions (Fig. 1).
Fig. 1.
(CT) showing regions of interest (ROI) of the femoral stem
The main advantage of CT is that it produces a cross-sectional image, which allows separate assessments of cortical and cancellous bone. This is unique to CT. As cancellous bone has a turnover rate of more than fivefold that of cortical bone, being able to distinguish between the two may allow for earlier detection of bone density change [5]. Quantitative CT has been shown to be accurate and reproducible [19]. The agreement and reproducibility of bone density measurements taken using qCT are very high, both for cemented and uncemented THA.
One of the disadvantages of qCT is the high radiation dose when compared to DXA. A typical dose for hip assessment using qCT is 0.5–1.0 mSv. Compare this with pelvic CT at 0.8–1.6 mSv and the background rate of radiation exposure in Europe per annum of 2.4 mSv. Typical doses for DXA are 0.001–0.1 mSv depending on the protocol used [16].
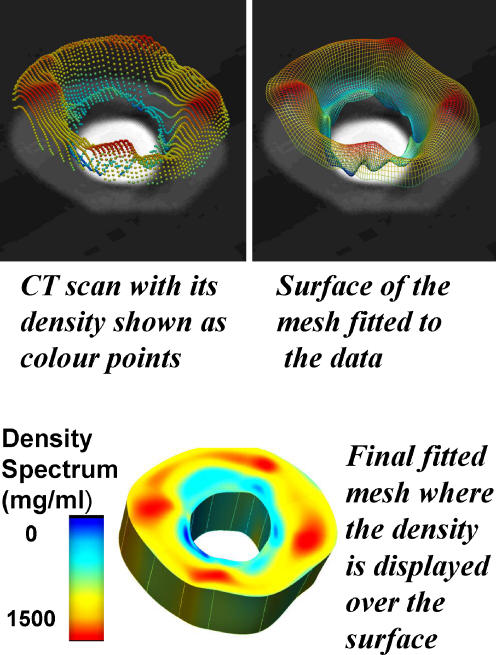
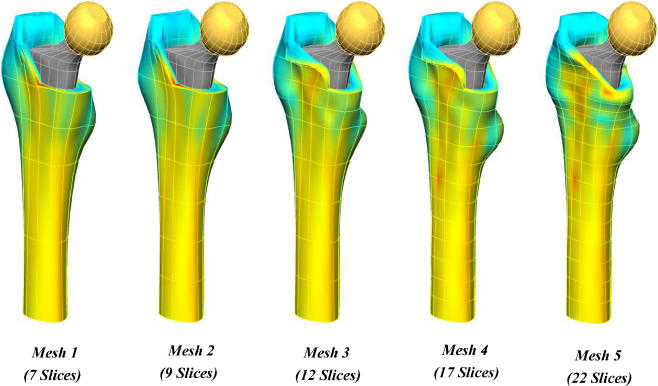
Based on mathematical equations, Shim et al. have demonstrated a technique using restricted numbers of CT slices to generate accurate computer models predicting BMD in areas around implants [20]. The use of existing data with multiple slices allows selection of critical slices to build a model of the regional density in the anatomical site of interest (Fig. 2). This requires as few as a quarter of the scans usually obtained with a reciprocal radiation dose reduction.
Fig. 2.
Application of finite-element (FE) technology to qCT imaging
Consequently, modern technological applications of qCT-assisted volumetric analysis provides an accurate, reproducible method for in vivo evaluation of cortical and cancellous bone structure around cemented and cementless implants with limited radiation exposure [20].
Quantitative CT-assisted osteodensitometry following total hip arthroplasty
Periprosthetic bone changes have been studied using qCT-assisted bone densitometry in the femur and acetabulum [16, 24]. The degree of bone loss is, in part, a function of the stresses applied to the different areas of bone which are fundamentally altered by implants. Implant design and biomaterials may alter the bone remodelling response through, for example, the stiffness of bulky femoral implants and the use of metal-backed acetabular components [20]. Although it has been shown that qCT-assisted densitometry can detect early bone loss surrounding implants, this technology is still used exclusively as a research tool [1].
Femoral remodelling in vivo
The bone adjacent to the proximal part of the femoral prosthesis has been shown to suffer the greatest amount of loss, both cortical and cancellous. Schmidt et al. [17, 18] used qCT to examine the changes occurring between 2 weeks and 1 year following THA. These researchers showed that the bone lateral to the proximal part of the prosthetic stem was the most affected, with a BMD loss of up to 22.1%, while the medial side suffered a loss of 16.2%. The mean femoral cortical density decreased by as much as 15.5% at the level of the medial femoral diaphysis (no significant difference between the medial and lateral sides at this level); however, bone density at the distal tip of the stem bone seemed to be relatively preserved, with a BMD loss of only 1.9–2.3%. There was a relatively greater reduction in cancellous BMD than in the BMD of the cortical bone, and this may reflect how the different areas are affected by the presence of an implant. This differentiation is not possible with the more commonly used DXA.
Acetabular remodelling in vivo
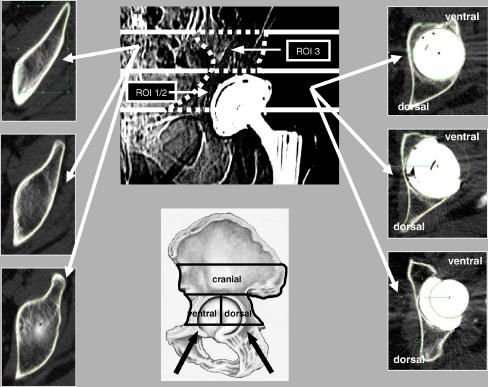
In the acetabulum, the BMD is significantly affected immediately adjacent to the implant [16, 24]. Schmidt et al. showed that while cortical density proximal to the cup increased slightly (3.4%), BMD in the cancellous region had decreased by up to 30% at the 3-year follow-up when compared to measurements taken at the index operation [16]. The decrease in retro-acetabular BMD can be explained by stress shielding created by the prosthesis (Fig. 3). The distribution of BMD change suggests that the peripheral cortical bone is exposed to a higher level of stress transfer than cancellous bone [20, 24]. An important consequence of BMD loss occurs in revision surgery. If the peripheral rim should be deficient, the cancellous floor may not be able to support the acetabular component. Likewise, failures in rim support may alter the BMD of this region, predisposing the patient to intra-operative fracture or the early failure of components.
Fig. 3.
Computerised tomography (CT) showing ROI of the acetabulum
Mechanical and histological correlation
Rosenbaum et al. [13] investigated the relationship between physical activity, BMD and cross-sectional cortical bone area following THA. They studied cadaveric femurs from patients who had undergone THA during their lifetime. The control group consisted of the contra-lateral, native femurs from the study patients. A mechanical usage score (modified from the Harris Hip Score) was recorded from all the patients at the time of the final-follow-up after surgery. All femurs were measured for BMC, using DXA at pre-determined levels around the implant (25, 45, 65 and 85% of the implant length), and cross-sectional geometry, by cutting the femurs at these same levels. The cortical bone was then defined in these slices and measured. These measurements were repeated and compared to those taken on the contra-lateral control side. The study found that lower mechanical usage scores (MUS) correlated well with a greater loss of cortical bone cross-sectional area as measured directly. MUS, however, was not found to correlate with BMC as measured by DXA. This is the first study to show how physical activity is related to bone architecture following THA. It also illustrates the deficiency of DXA technology for detecting changes in bone volume, which is an important component in BMD. Changes in cross-sectional area detectable by CT may give an earlier, more accurate indication of true BMD change.
Finite-element analysis
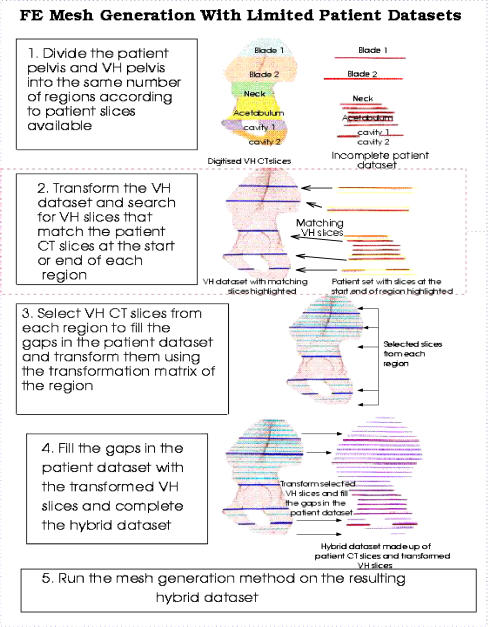
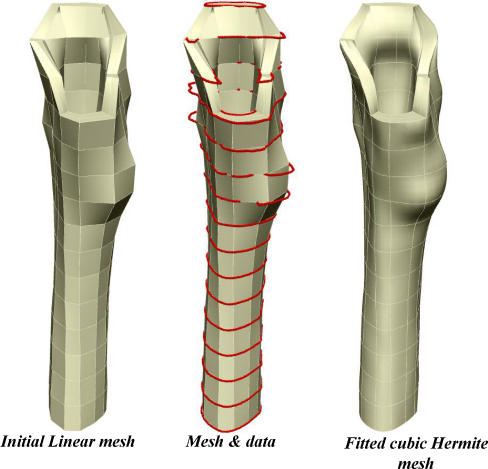
It has been shown that is possible to generate a patient-specific finite-element (FE) model of BMD distribution using a mathematical model with limited numbers of CT slices. Limited data sets in conjunction with known complete data sets, such as that of the Visible Human [20], can be used to generate an anatomically accurate, patient-specific FE element mesh. The clinical applications of this include its use in preoperative planning, follow-up investigations and clinical studies. The process of FE modelling is outlined in Figs. 4, 5 and 6.
Fig. 4.
Process of generating a hybrid data set consisting of patient CT slices and Visible Human (VH)CT slices
Fig. 5.
Finite-element analysis model based on CT imaging
Fig. 6.
Mesh generation using limited numbers of CT slices
Conclusion
Multiple factors are involved in the aetiology of bone loss and fixation failure following THA [13]. These include stress shielding, age, wear debris, osteolysis and micromotion of the implant. Various techniques have been used in the past in an effort to detect bony changes in vivo – all with varying success [5, 23]. Currently, DXA is a widely used modality, and several validation studies have demonstrated its level of accuracy [9, 10]. However, even the best results only allow for 2-D measurements to be made, and these cannot distinguish between cortical and cancellous bone.
Quantitative CT-assisted osteodensitometry has been shown to be useful in assessing the in vivo structural bone changes after THA [16, 24]. It has high resolution, accuracy and reproducibility, making it a useful tool for research purposes. Computerised tomography imaging is able to differentiate between cortical bone, cancellous bone, metal and cement. It provides information about the pattern of stress shielding which occurs around THA, can show early bony changes, which may prove informative about the quality of implant fixation and subsequent loosening and should also be able to provide information on the different implant designs and on the effect of using different materials on the load transfer to the adjacent bone. Quantitative CT-assisted osteodensitometry in conjunction with FE analysis is able to generate accurate patient-specific meshes on which to model implants and their effect on bone remodelling [20]. This technology can be useful to predict bone remodelling and the quality of implant fixation using prostheses with different design and/or biomaterials. In the future, this tool could be used for pre-clinical validation of new implants before their introduction into the market-place.
References
- 1.Aamodt A, Kvistad KA, Andersen E (1999) Determination of the Hounsfield value for CT-based design of custom femoral stems. J Bone Joint Surg Br 81:143–147 [DOI] [PubMed]
- 2.Cohen B, Rushton N (1995) Accuracy of DEXA measurement on bone mineral density after total hip arthroplasty. J Bone Joint Surg Br 77:479–483 [PubMed]
- 3.Engh CA Jr, McAuley J, Sychterz CJ, Sacco ME, Engh CA Sr (2000) The accuracy and reproducibility of radiographic assessment of stress-shielding. J Bone Joint Surg Am 82:1414–1420 [DOI] [PubMed]
- 4.Gibbons CE, Davies AJ, Amis AA, Olearnik H, Parker BC, Scott JE (2001) Periprosthetic bone mineral density changes with femoral components of differing design philosophy. Int Orthop 25:89–92 [DOI] [PMC free article] [PubMed]
- 5.Kroger H, Venesmaa P, Jurvelin J, Miettinen H, Suomalainen O, Alhava E (1998) Bone density at the proximal femur after total hip arthroplasty. Clin Orthop Rel Res 352:66–74 [PubMed]
- 6.Laine HJ, Puolakka TJ, Moilanen T, Pajamaki KJ, Wirta J, Lehto MU (2000) The effects of cementless femoral stem shape and proximal surface texture on ‘fit-and-fill’ characteristics and on bone remodeling. Int Orthop 24:184–190 [DOI] [PMC free article] [PubMed]
- 7.Leali A, Fetto JF (2004) Preservation of femoral bone mass after total hip replacements with a lateral flare stem. Int Orthop 28:151–154 [DOI] [PMC free article] [PubMed]
- 8.Lindgren JU, Svensson O, Mathieson EB (1996) Remodelling and pain after uncemented total hip replacement. Int Orthop 20:7–11 [DOI] [PubMed]
- 9.Martini F, Sell S, Kremling E, Kusswetter W (1996) Determination of periprosthetic bone density with the DEXA method after implantation of custom-made uncemented femoral stems. Int Orthop 20:218–221 [DOI] [PubMed]
- 10.Mirsky EC, Einhorn TA (1998) Bone densitometry in orthopaedic practice. J Bone Joint Surg Am 80:1687–1698 [DOI] [PubMed]
- 11.Mow VC, Flatow EL, Foster RJ (1994) Biomechanics. In: Simon SR (ed) Orthopaedic basic science. American Academy of Orthopaedic Surgeons, Rosemont, Ill.
- 12.Niinimaki T, Junila J, Jalovaara P (2001) A proximal fixed anatomic femoral stem reduces stress shielding. Int Orthop 25:85–88 [DOI] [PMC free article] [PubMed]
- 13.Rosenbaum TG, Bloebaum RD, Ashrafi S, Lester K (2006) Ambulatory activities maintain cortical bone after total hip arthroplasty. Clin Orthop Rel Res (in press) [DOI] [PubMed]
- 14.Rosenthall L, Bobyn JD, Tanzer M (1999) Bone densitometry: influence of prosthetic design and hydroxyapatite coating on regional adaptive bone remodelling. Int Orthop 23:325–329 [DOI] [PMC free article] [PubMed]
- 15.Sanchez-Sotelo J, Lewallen DG, Harmsen WS, Harrington J, Cabanela ME (2004) Comparison of wear and osteolysis in hip replacement using two different coatings of the femoral stem. Int Orthop 28:206–210 [DOI] [PMC free article] [PubMed]
- 16.Schmidt R, Muller L, Kress A, Hirschfelder H, Aplas A, Pitto RP (2002) A computed tomography assessment of femoral and acetabular bone changes after total hip arthroplasty. Int Orthop 26:299–302 [DOI] [PMC free article] [PubMed]
- 17.Schmidt R, Mueller L, Nowak TE, Pitto RP (2003) Clinical outcome and periprosthetic bone remodelling of an uncemented femoral component with taper design. Int Orthop 27:204–207 [DOI] [PMC free article] [PubMed]
- 18.Schmidt R, Nowak TE, Mueller L, Pitto RP (2004) Osteodensitometry after total hip replacement with uncemented taper-design stem. Int Orthop 28:74–77 [DOI] [PMC free article] [PubMed]
- 19.Schmidt R, Pitto RP, Kress A et al (2005) Inter- and Intraobserver assessment of periacetabular osteodensitometry after cemented and uncemented total hip arthroplasty using computed tomography. Arch Ortho Trauma Surg 125:291–297 [DOI] [PubMed]
- 20.Shim V, Pitto RP, Streicher RM, Hunter PJ, Anderson IA (2006) The use of sparse CT datasets for auto-generating FE models of the femur and pelvis. J Biomech (in press; Epub: Jan 19) [DOI] [PubMed]
- 21.Sumner DR, Galante JO (1992) Determinants of stress shielding: design versus materials versus interface. Clin Orthop Rel Res 274:202–212 [PubMed]
- 22.Weber D, Pomeroy DL, Brown R, Schaper LA, Badenhausen WE Jr, Smith W, Curry JI, Suthers KE (2000) Proximally porous coated femoral stem in total hip replacement–5- to 13-year follow-up report. Int Orthop 24:97–100 [DOI] [PMC free article] [PubMed]
- 23.Wilkinson JM, Peel NFA, Elson RA, Stockley I, Eastell R (2001) Measuring bone mineral density of the pelvis and proximal femur after total hip arthroplasty. J Bone Joint Surg Br 83:283–288 [DOI] [PubMed]
- 24.Wright JM, Pellicci PM, Salvati EA, Ghelman B, Roberts MM, Koh JL (2001) Bone density adjacent to press-fit acetabular components: a prospective analysis with quantitative computed tomography. J Bone Joint Surg Am 83:529–533 [DOI] [PubMed]
- 25.Zerahn B, Storgaard M, Johansen T, Olsen C, Lausten G, Kanstrup IL (1998) Changes in bone mineral density adjacent to two biomechanically different types of cementless femoral stems in total hip arthroplasty. Int Orthop 22:225–229 [DOI] [PMC free article] [PubMed]
- 26.Zerahn B, Lausten GS, Kanstrup IL (2004) Prospective comparison of differences in bone mineral density adjacent to two biomechanically different types of cementless femoral stems. Int Orthop 28:146–150 [DOI] [PMC free article] [PubMed]