Abstract
Background
Antibodies produced by B-lymphocytes play a key role in the host defense against infection. The development, survival, and activation of B cell is regulated by multiple receptors including the B cell antigen receptor (BCR), which detects the presence of pathogens, CD40, which binds co-stimulatory molecules on activated T cells, and chemokines such as SDF-1 (CXCL12) that play key roles in B cell development and trafficking. Signaling by many receptors results in the generation of reactive oxygen species (ROS) that function as second messengers by regulating the activity of redox-sensitive kinases and phosphatases. We investigated the role of ROS in signaling by the BCR, CD40, and CXCR4, the receptor for SDF-1. We focused on activation of ERK, JNK, p38, and Akt, kinases that regulate multiple processes including cell survival, proliferation, and migration.
Results
Using the anti-oxidants N-acetyl L-cysteine (NAC) and ebselen to deplete intracellular ROS, we identified a differential requirement for ROS in the activation of ERK, JNK, p38, and Akt by these receptors. We found that CD40 activated JNK, p38, and Akt via redox-dependent pathways that were sensitive to ROS depletion by NAC and ebselen. In contrast, BCR-induced activation of ERK, JNK, p38, and Akt was not affected by ROS depletion. We also found that CXCR4-induced Akt activation was ROS-dependent even though activation of the ERK, JNK, and p38 MAP kinases by CXCR4 occurred via ROS-independent pathways.
Conclusion
The differential requirement for ROS in the activation of ERK, JNK, p38, and Akt by the BCR, CD40, and CXCR4 likely reflects the multiplicity of upstream activators for each of these kinases, only some of which may be regulated in a redox-dependent manner. These findings support the idea that ROS are important second messengers in B cells and suggest that oxidants or anti-oxidants could be used to modulate B cell activation.
Keywords: B-lymphocytes, Reactive oxygen species, CD40, BCR, CXCR4, MAP kinases, Akt
Background
B-lymphocytes play a key role in host defenses against infection by producing antibodies that help eliminate pathogens and neutralize secreted toxins. The development, selection, survival, activation, and proliferation of B-lymphocytes, as well as the differentiation of B cells into antibody-producing plasma cells, is regulated by antigens, T cell-derived co-stimulatory signals, and chemokines (Bishop et al. 2003). Antigen-induced signaling via the B cell antigen receptor (BCR) mediates the elimination or silencing of self-reactive B cells as well as the activation of B cells that recognize foreign antigens (Niiro and Clark 2002; Gold 2002). T cells deliver essential co-stimulatory signals to B cells via CD40, a tumor necrosis factor (TNF) family receptor that activates B cells and prevents BCR-induced tolerance (anergy) or apoptosis (Bishop and Hostager 2003; Santos-Argumedo et al. 1994). A variety of chemokines regulate B cell development and activation by directing the trafficking and adhesion of B cells. In particular, the chemokine stromal cell-derived factor-1 (SDF-1/CXCL12) is a survival factor for B cell progenitors, retains pro-B cells in the bone marrow where they develop (Nagasawa et al. 1996; Ma et al. 1998), contributes to the entry of mature B cells into lymphoid organs via high endothelial venules (Miyasaka and Tanaka 2004), and directs plasma cells to the bone marrow (Hargreaves et al. 2001), a niche in which they can survive and produce antibodies for long periods of time.
The ERK, JNK, and p38 mitogen-activated protein kinases (MAPKs) are key signaling intermediates by which many receptors regulate cell growth and survival, apoptosis, proliferation, and differentiation (Yoon and Seger 2006; Karin and Gallagher 2005; Zarubin and Han 2005). In addition to cytosolic proteins that regulate diverse processes, many MAPK substrates are either transcription factors or kinases that phosphorylate transcription factors. In B cells, the BCR and CD40 activate all three families of MAPKs, although to different extents (Sutherland et al. 1996; Purkerson and Parker 1998; Sakata et al. 1995; Berberich et al. 1996). For example, in the WEHI-231 B lymphoma cell line, the BCR activates ERK to a much greater extent than JNK or p38 while CD40 strongly activates JNK and p38 but causes only marginal ERK activation (Sutherland et al. 1996). CXCR4, the receptor for SDF-1, transiently activates both ERK and JNK in B cells (Ganju et al. 1998; McLeod et al. 2002; Ortolano et al. 2006) and JNK activation is important for SDF-1-induced B cell migration (Ortolano et al. 2006).
MAPK signaling plays an important role in BCR- and CD40-induced survival, activation and differentiation in both normal and malignant B cells. In murine splenic B cells, ERK activation is important for BCR-induced proliferation and for BCR-induced upregulation of the Egr-1 transcription factor, the CD44 adhesion molecule, and the CD69 activation marker (Richards et al. 2001). Activation of ERK by the BCR also promotes the phosphorylation and degradation of Bcl-6 (Niu et al. 1998), a transcriptional repressor whose elimination is required for B cells to differentiate into plasma cells. Both p38 and JNK mediate CD40-induced B cell activation. The p38 MAP kinases play a major role in CD40-induced gene expression in B cells (Craxton et al. 1998; Dadgostar et al. 2002) and are required for CD40-induced B cell proliferation (Craxton et al. 1998). JNK activation is required for CD40-mediated IgE class switching (Jabara and Geha 2005). In contrast, activation of JNK and p38 by the BCR promotes the apoptosis of B lymphoma cells lines (Graves et al. 1998; Takada et al. 2001; Swart and Chiles 2000).
Another important kinase that is activated in B cells by the BCR (Gold et al. 1999), CD40 (Andjelic et al. 2000), and CXCR4 (McLeod et al. 2002; Ortolano et al. 2006) is Akt/protein kinase B, a downstream effector of the phosphatidylinositol 3-kinase (PI3K) pathway. Akt is a central regulator of cell survival (Scheid and Woodgett 2001; Song et al. 2005) and Akt activation is required for the survival and proliferation of normal murine splenic B cells (Aiba et al. 2006), the WEHI-231 murine B cell lymphoma (Banerji et al. 2001), and many types of human B cell tumors including mantle cell lymphomas and multiple myelomas (Hsu et al. 2002; Rudelius et al. 2006). Akt promotes cell growth and survival by phosphorylating and inhibiting multiple proteins that promote cell cycle arrest and apoptosis including FKHRL-1, a transcription factor that induces the expression of cell cycle arrest proteins, p27kip1, a negative regulator of cell cycle progression, BAD, a pro-apoptotic member of the Bcl-2 family, and the GSK-3 kinase, a negative regulator of protein translation and cell cycle progression (Scheid and Woodgett 2001; Song et al. 2005). Akt also increases protein translation through the tuberin/Rheb/mTOR pathway (Manning and Cantley 2003), contributes to the activation of NF-κB (Kane et al. 1999), and plays a role in cell migration (Enomoto et al. 2005).
Upon ligand binding, a wide variety of receptors including receptor tyrosine kinases, antigen receptors, TNF receptor family members, Toll-like receptors, and G protein-coupled receptors induce the generation of intracellular reactive oxygen species (ROS) such as hydrogen peroxide and superoxide (Sundaresan et al. 1995; Fang et al. 1995; Devadas et al. 2002; Lo and Cruz 1995; Lee and Koretzky 1998; Ha and Lee 2004a; Matsuzawa et al. 2005; Griendling and Ushio-Fukai 2000). Importantly, a large body of work indicates that these ROS can act as second messengers. Many signaling molecules, particularly kinases and phosphatases, are sensitive to the redox state of their environment (Reth 2002; Tonks 2005; Adler et al. 1999). Treating cells with anti-oxidants that lower the levels of intracellular ROS can inhibit receptor-induced activation of JNK (Lee and Koretzky 1998; Lo et al. 1996; Viedt et al. 2000), p38 (Griendling and Ushio-Fukai 2000; Viedt et al. 2000; Asehnoune et al. 2004; Ushio-Fukai et al. 1998), ERK (Devadas et al. 2002; Asehnoune et al. 2004; Daou and Srivasta 2004; Tanaka et al. 2001; Hannken et al. 2000), and Akt (Asehnoune et al. 2004; Daou and Srivasta 2004; Ha et al. 2004b; Gorin et al. 2001; Ushio-Fukai et al. 1999). However, there are many examples in which receptor-induced activation of these kinases is unaffected by ROS scavengers (Ha et al. 2004b; Harfouche et al. 2005; Li and Malik 2005). Whether or not the MAP kinases and Akt are activated in a ROS-dependent manner depends on both the receptor and the cellular context. Activation of a kinase by a particular receptor can be ROS-dependent in one cell type but ROS-independent in another cell type. Also, within a single cell one receptor may activate a MAP kinase or Akt in a ROS-dependent manner while a different receptor activates the same kinase in a ROS-independent manner. This differential requirement for ROS likely reflects the large number of different upstream activators for each of the MAP kinases, as well as the multiple PI3K isoforms that can activate Akt. Only a subset of these upstream activators of the MAP kinases and Akt may be redox-dependent.
Although the BCR (Fang et al. 1995; Hamano et al. 2002; Singh et al. 2005) and CD40 (Lee and Koretzky 1998; Ha and Lee 2004a; Laxmanan et al. 2005) stimulate ROS generation when engaged, the role of ROS in B cell signaling and in B cell activation is not completely understood. Lee and colleagues have shown that ROS are required for CD40-induced activation of both JNK and p38 (Lee and Koretzky 1998; Ha and Lee 2004a). The role of ROS in CD40-induced activation of Akt has not been investigated. Although little is known about the role of ROS in the activation of specific signaling pathways by the BCR, the inhibition of protein tyrosine phosphatases by ROS plays an important role in the initiation and amplification of BCR signaling by Src family tyrosine kinases and the Syk tyrosine kinase (Singh et al. 2005; Rolli et al. 2002). Consistent with a role for ROS in BCR-induced B cell activation, treating murine splenic B cells with anti-oxidants inhibits BCR-induced proliferation (Fedyk and Phillips 1994). The role of ROS generation in CXCR4 signaling in B cells has not been investigated. In smooth muscle cells, SDF-1-induced production of tissue factor is blocked by anti-oxidants (Schecter et al. 2001), suggesting that CXCR4 might stimulate ROS production. Moreover, other G protein-coupled receptors that bind angiotensin II receptor and monocyte chemotactic protein-1 induce ROS formation (Griendling and Ushio-Fukai 2000; Lo et al. 2005).
To more fully understand the role of ROS in B cells, we investigated the requirement for ROS in the activation of MAPKs and Akt by the BCR, CD40, and CXCR4. By treating WEHI-231 B lymphoma cells with the membrane-permeable anti-oxidants N-acetyl L-cysteine (NAC) and ebselen, we reveal a differential role for ROS in coupling these receptors to the activation of JNK, p38, ERK, and Akt.
Results
Differential requirement for ROS in the activation of MAP kinases by CD40, the BCR, and CXCR4
ROS have been shown to be important for CD40-induced activation of the JNK and p38 MAP kinases in B cells (Lee and Koretzky 1998; Ha and Lee 2004a). Since signaling by the BCR and G protein-coupled receptors like CXCR4 also leads to the generation of intracellular ROS (Fang et al. 1995; Griendling and Ushio-Fukai 2000; Hamano et al. 2002; Singh et al. 2005; Lo et al. 2005), we investigated whether ROS are involved in the activation of JNK and p38 by the BCR and CXCR4. To test this, we pretreated WEHI-231 B lymphoma cells with two different compounds that reduce intracellular ROS levels, NAC and ebselen. NAC is a ROS scavenger (Aruoma et al. 1989) that also increases intracellular levels of reduced glutathione (Faruqi et al. 1997), the major endogenous anti-oxidant in cells. Ebselen is a glutathione peroxidase mimic that uses reduced glutathione to convert H2O2 to water and oxygen (Sies 1993). The WEHI-231 B lymphoma cell line was chosen as a model system since it has been extensively characterized in terms of the signaling and cellular responses induced by CD40, the BCR, and CXCR4 (Santos-Argumedo et al. 1994; Sutherland et al. 1996; McLeod et al. 2002; Richards et al. 2001; Gold et al. 1999; Lee and Koretzky 1998). Moreover, it resembles an immature/transitional B cell and has been widely used to study BCR-induced apoptosis, CD40-mediated survival, and SDF-1-induced migration. Finally, Lee et al. also used WEHI-231 cells to study the role of ROS in CD40 signaling (Lee and Koretzky 1998; Ha and Lee 2004a).
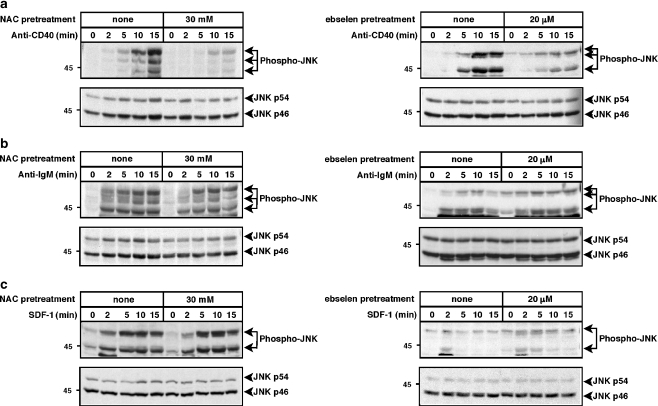
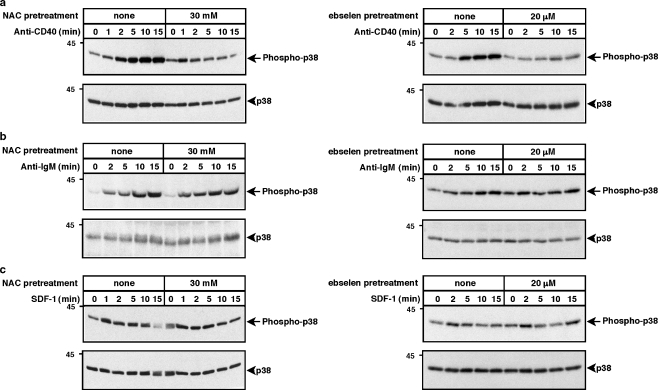
To assess the activation or JNK and p38, which are often activated in a coordinated manner, we probed cell lysates with antibodies that specifically detect the phosphorylation of these kinases on threonine-X-tyrosine motifs in their activation loops, modifications that are essential for their activation. Figures 1 and 2 show that CD40, the BCR, and CXCR4 all activated JNK and p38, with maximal responses usually occurring at 5–15 min. Reducing ROS levels by pre-treating WEHI-231 cells with either 30 mM NAC or 20 μM ebselen substantially reduced anti-CD40-induced phosphorylation of JNK and p38 (Figs. 1a, 2a), as had been shown previously by Lee et al. (Lee and Koretzky 1998; Ha and Lee 2004a). ROS depletion reduced CD40-induced JNK phosphorylation to a greater extent than p38 phosphorylation. In contrast to CD40-induced activation of JNK and p38, reducing ROS levels had very little effect on anti-IgM- or SDF-1-induced phosphorylation of JNK (Fig. 1b,c) or p38 (Fig. 2b,c). Thus, CD40 activates JNK and p38 via pathways that are largely ROS-dependent whereas the BCR and CXCR4 activate JNK and p38 via pathways that are relatively insensitive to ROS depletion. The inability of NAC and ebselen to suppress BCR-induced activation of JNK and p38 shows that these compounds were not toxic to the cells at the concentrations used and that they did not suppress all signaling reactions in a non-specific manner.
Fig. 1.
Depletion of ROS with NAC or ebselen inhibits anti-CD40-induced JNK activation but does not inhibit anti-IgM- or SDF-1-induced JNK activation. WEHI-231 cells were pretreated with 30 mM NAC for 2 h or with 20 μM ebselen for 1 h and then stimulated for the indicated times with a 10 μg/ml of the 1C10 anti-CD40 monoclonal antibody, b 30 μg/ml anti-IgM or c 100 ng/ml SDF-1. When cells were pretreated with ebselen, the control cells were incubated with an equivalent volume of DMSO. Cell extracts were separated by SDS-PAGE and analyzed by immunoblotting with a phospho-specific antibody that recognizes the activated form of JNK (upper panels). The blots were then stripped and reprobed with anti-JNK antibodies to show that equivalent amounts of JNK were present in each sample (lower panels). Molecular mass markers (in kDa) are shown to the left. For each panel, similar results were obtained in at least three independent experiments
Fig. 2.
Depletion of ROS with NAC or ebselen reduces anti-CD40-induced p38 activation but does not inhibit anti-IgM- or SDF-1-induced p38 activation. WEHI-231 cells were pretreated with 30 mM NAC for 2 h or with 20 μM ebselen for 1 h and then stimulated for the indicated times with a 10 μg/ml of the 1C10 anti-CD40 monoclonal antibody, b 30 μg/ml anti-IgM or c 100 ng/ml SDF-1. When cells were pretreated with ebselen, the control cells were incubated with an equivalent volume of DMSO. Cell extracts were separated by SDS-PAGE and analyzed by immunoblotting with a phospho-specific antibody that recognizes the activated form of p38 (upper panels). The blots were then stripped and reprobed with anti-p38 antibodies to show that equivalent amounts of p38 were present in each sample (lower panels). Molecular mass markers (in kDa) are shown to the left. For each panel, similar results were obtained in at least three independent experiments
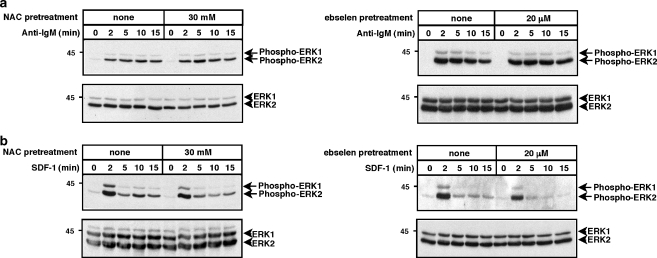
A similar approach was used to assess whether ROS play a role in activation of the ERK1 and ERK2 MAP kinases in WEHI-231 cells. Figure 3 shows that anti-IgM caused sustained ERK phosphorylation while SDF-1 caused strong initial ERK phosphorylation, followed by a rapid decrease. We have previously shown that CD40 causes little or no ERK activation in WEHI-231 cells (Sutherland et al. 1996). Neither NAC nor ebselen significantly reduced anti-IgM- or SDF-1-induced ERK phosphorylation (Fig. 3), indicating that the BCR and CXCR4 do not use ROS-dependent pathways to activate ERK. Thus, CD40-induced activation of JNK and p38 is ROS-dependent whereas BCR- and CXCR4-induced activation of ERK, JNK, and p38 are relatively insensitive to ROS depletion.
Fig. 3.
Depletion of ROS with NAC or ebselen does not inhibit anti-IgM- or SDF-1-induced ERK activation. WEHI-231 cells were pretreated with 30 mM NAC for 2 h or with 20 μM ebselen for 1 h and then stimulated for the indicated times with a 30 μg/ml anti-IgM or b 100 ng/ml SDF-1. When cells were pretreated with ebselen, the control cells were incubated with an equivalent volume of DMSO. Cell extracts were separated by SDS-PAGE and analyzed by immunoblotting with a phospho-specific antibody that recognizes the activated forms of ERK1 and ERK2 (upper panels). The blots were then stripped and reprobed with anti-ERK1/2 antibodies to show that equivalent amounts of ERK1/2 were present in each sample (lower panels). Molecular mass markers (in kDa) are shown to the left. For each panel, similar results were obtained in at least three independent experiments
Differential requirement for ROS in the activation of Akt by CD40, the BCR, and CXCR4
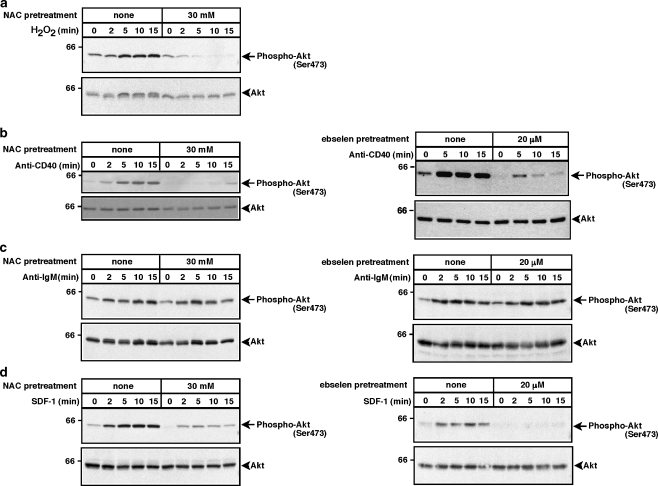
Akt is a critical regulator of B cell survival and has also been implicated in cell migration. Previous reports have indicated that Akt activation is dependent on ROS in osteoclasts and vascular smooth muscle cells (Daou and Srivasta 2004; Ha et al. 2004b; Gorin et al. 2001; Ushio-Fukai et al. 1999). Moreover, oxidative stress can induce Akt activation in the DT40 chicken B cell line (Qin and Chock 2003). Thus we investigated whether ROS are required for the BCR, CD40, and CXCR4 to activate Akt in B cells. As a readout for Akt activation we assessed the phosphorylation of Akt on serine 473, an essential step in Akt activation. Figure 4a shows that stimulating WEHI-231 cells with 300 μM H2O2 induced the phosphorylation of Akt on Ser473 and that this response to H2O2 could be completely blocked by pre-treating the cells with NAC. Thus activation of Akt in WEHI-231 cells can be regulated in a redox-dependent manner. Indeed, both SDF-1- and anti-CD40-induced activation of Akt was substantially reduced when ROS were depleted by pretreating the cells with either NAC or ebselen (Figs. 4b,d). In contrast, anti-IgM-stimulated Akt phosphorylation was unaffected by ROS depletion (Fig. 4c). Thus, CD40 and CXCR4 activate Akt via ROS-dependent pathways whereas the BCR activates Akt via a pathway that is largely ROS-independent.
Fig. 4.
Depletion of ROS with NAC or ebselen inhibits H2O2-, anti-CD40-, and SDF-1-induced Akt activation but does not inhibit anti-IgM-induced Akt activation. WEHI-231 cells were pretreated with 30 mM NAC for 2 h or with 20 μM ebselen for 1 h and then stimulated for the indicated times with a 300 μM H2O2, b 10 μg/ml of the 1C10 anti-CD40 monoclonal antibody, c 30 μg/ml anti-IgM or d 100 ng/ml SDF-1. When cells were pretreated with ebselen, the control cells were incubated with an equivalent volume of DMSO. Cell extracts were separated by SDS-PAGE and analyzed by immunoblotting with a phospho-specific antibody that recognizes the activated form of Akt, which is phosphorylated on serine 473 (upper panels). The blots were then stripped and reprobed with anti-Akt antibodies to show that equivalent amounts of Akt were present in each sample (lower panels). Molecular mass markers (in kDa) are shown to the left. For each panel, similar results were obtained in at least two independent experiments
Discussion
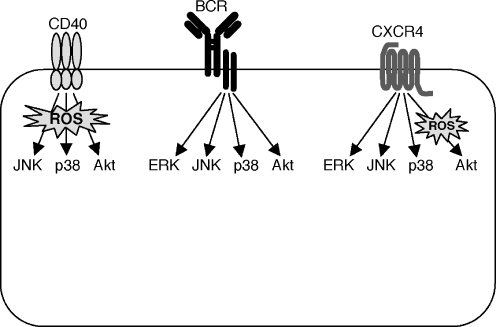
In this report we demonstrate a differential role for ROS in the activation of the MAP kinases and Akt by the BCR, CD40, and CXCR4 (Fig. 5). We found that activation of ERK, JNK, p38, and Akt by the BCR was relatively insensitive to ROS depletion and therefore involves signaling intermediates that are largely ROS-independent. In contrast, CD40 activates JNK, p38, and Akt for the most part via ROS-dependent signaling pathways that are sensitive to ROS depletion by either NAC or ebselen. Lee et al. had previously shown that the activation of JNK and p38 by CD40 is dependent on ROS (Lee and Koretzky 1998; Ha and Lee 2004a), but this is the first report that CD40-induced Akt activation is dependent on ROS. The role of ROS in CXCR4 signaling in B cells had not been investigated previously and we now show that CXCR4 activates the MAP kinases via ROS-independent pathways but that its ability to activate Akt is dependent on ROS.
Fig. 5.
Differential role of ROS in the activation of MAP kinases and Akt by the BCR, CD40, and CXCR4. Activation of ERK, JNK, p38, and Akt by the BCR is relatively insensitive to ROS depletion and involves signaling intermediates that are ROS-independent. In contrast, CD40 activates JNK, p38, and Akt via signaling pathways that are largely ROS-dependent. CXCR4 activates the MAP kinases via ROS-independent pathways but its ability to activate Akt is dependent on ROS
MAPK activation involves GTPase-regulated kinase cascades. For ERK, receptor-induced conversion of Ras to its active GTP-bound form promotes the activation of the Raf-1 kinase (a MAPK kinase kinase or MAP3K), which in turn phosphorylates the MEK1 and MEK2 kinases (MAPK kinases or MAP2Ks). MEK1 and MEK2 then phosphorylate and activate ERK1 and ERK2. Although ERK is activated in a ROS-dependent manner by some receptors (Daou and Srivasta 2004; Hannken et al. 2000), particularly the G protein-coupled receptor for angiotensin II (Hannken et al. 2000), we found that both CXCR4- and BCR-induced ERK activation were ROS-independent in WEHI-231 cells.
The kinase cascades that lead to activation of JNK and p38 are regulated by the Rac1 GTPase, which promotes the activation of MAP3Ks that coordinately activate the MKK4, MKK7, MKK3, and MKK6 MAP2Ks (Ichijo 1999). MKK4 and MKK7 activate JNK while MKK3 and MKK6 activate p38. A number of MAP3Ks including TAK1, the mixed lineage kinases (MLKs), MEKK1-4, and ASK1 can activate the JNK and p38 pathways. Each of these MAP3Ks may couple different classes of receptors or environmental stresses (e.g. oxidative stress) to the MAP2Ks that regulate JNK and p38. The possibility that some of these MAP3Ks are redox-sensitive while others are not could account for ROS-dependent versus ROS-independent activation of JNK and p38. While the redox dependence of each of the MAP3Ks upstream of JNK and p38 has not been investigated, activation of the ASK1 MAP3K is clearly dependent on ROS (Saitoh et al. 1998; Liu et al. 2000). In resting cells, thioredoxin binds to ASK1 and keeps it in an inhibited state. The generation of ROS causes thioredoxin to dissociate from ASK1, allowing ASK1 to dimerize and become activated by transphosphorylation. Once ASK1 is released from thioredoxin, it can also bind to the TRAF2, TRAF3, and TRAF6 adaptor proteins (Ha and Lee 2004a; Matsuzawa et al. 2005; Liu et al. 2000) that associate with TNF receptor family members such as CD40. Whether ASK1 is involved in the ROS-dependent activation of JNK and p38 by CD40 remains to be determined. Using a phospho-specific antibody directed against the activated form of ASK1, we were unable to detect CD40-induced ASK1 phosphorylation in WEHI-231 cells (data not shown).
While CD40 induced JNK and p38 activation in a largely ROS-dependent manner in WEHI-231 cells, we found that BCR- and CXCR4-induced activation of JNK and p38 was ROS-independent in these cells, indicating that the BCR and CXCR4 activate JNK and p38 via redox-insensitive MAP3Ks. Multiple scaffolding proteins that organize MAP3K/MAP2K/MAPK signaling modules have been identified including the JIP proteins and MEKK1 (Morrison and Davis 2003). One such scaffolding protein may couple CD40 to JNK and p38 signaling complexes that contain a redox-sensitive MAP3K such as ASK1 while different scaffolding proteins may connect the BCR and CXCR4 to JNK and p38 signaling complexes that contain a redox-insensitive MAP3K.
We also found that CD40- and CXCR4-induced Akt activation was dependent on ROS while BCR-induced activation of Akt was not inhibited by anti-oxidants. The activation of Akt depends on production of the membrane lipid phosphatidylinositol 3,4,5-trisphosphate (PIP3) by PI3K. PIP3 recruits Akt to the plasma membrane where it can be activated by being phosphorylated on threonine 308 and serine 473. There are multiple isoforms of the p110 catalytic subunit of PI3K and it is possible that there are redox-sensitive and redox-insensitive p110 isoforms that would mediate ROS-dependent versus ROS-independent Akt activation. H2O2-induced Akt phosphorylation in the DT40 chicken B cell line can be blocked wortmannin, a PI3K inhibitor that acts on the p110 catalytic subunit (Qin and Chock 2003). This is consistent with the idea that at least one of the PI3K p110 catalytic subunits can be activated in a redox-dependent manner. Knockout mouse studies, as well as the use of a p110δ-specific inhibitor have shown that BCR-induced Akt phosphorylation is largely dependent on the p110δ isoform of the PI3K catalytic subunit (Clayton et al. 2002; Bilancio et al. 2006). Thus our finding that the BCR activates Akt in a ROS-independent manner suggests that p110δ is not a redox-regulated enzyme. The p110 isoforms that mediate ROS-dependent activation of Akt by CD40 and CXCR4 have not been identified.
ROS generation appears to be a central event in CD40 signaling as it is required for CD40 to activate JNK, p38, NF-κB (Lee and Koretzky 1998; Ha and Lee 2004a), and Akt (Fig. 4) in B cells. NF-κB activation is essential for CD40 to promote B cell survival and to overcome BCR-induced apoptotic signals in WEHI-231 cells (Schauer et al. 1996). Andjelic et al. (2000) showed that CD40-induced NF-κB activation depends on PI3K and that the PI3K/Akt pathway also links CD40 to additional pro-survival events including downregulation of the p27kip cell cycle inhibitor and induction of Bcl-xL. Thus, by mediating CD40-induced activation of both NF-κB and the PI3K/Akt pathway, ROS may play a central role in the ability of CD40 to promote B cell survival.
The generation of ROS by CD40 involves the Rac1 GTPase and the NADPH oxidase complex (Ha and Lee 2004a). Activated Rac1 promotes the assembly of an NADPH oxidase complex at the plasma membrane, which then transfers electrons to molecular oxygen to generate superoxide. CD40 engagement has been shown to induce the binding of the p40phox subunit of NADPH oxidase to the CD40-associated adaptor protein TRAF3 (Ha and Lee 2004a). Consistent with the idea that the NADPH oxidase complex is involved in ROS-dependent signaling by CD40, pre-treating WEHI-231 cells with the NADPH oxidase inhibitor diphenyleneiodonium chloride inhibits CD40-induced activation of p38 (Ha and Lee 2004a).
The mechanisms by which the BCR and CXCR4 induce ROS generation in B cells are not known. Even though the BCR and CXCR4 activate Rac1 in B cells (McLeod et al. 2002; Grill and Schrader 2002), we found that these receptors activate JNK and p38 in a ROS-independent manner. Rac1 activation has been implicated in BCR-induced activation of JNK and p38 (Hashimoto et al. 1998) but the role of Rac1 in this process may be to activate a redox-insensitive MAP3K, rather than generating ROS.
Although BCR-induced activation of the MAP kinases and Akt was not inhibited by anti-oxidant concentrations (30 mM NAC) that effectively inhibited CD40-induced activation of these kinases, ROS have been reported to play a role in the initiation of BCR signaling (Singh et al. 2005; Rolli et al. 2002). Our results suggest that BCR signaling is more resistant to anti-oxidant-induced inhibition than CD40 signaling.
Conclusions
In this report we have defined the ROS dependency of several key signaling pathways activated by three important receptors on B cells, the BCR, CD40, and CXCR4 (Fig. 5). In addition to demonstrating differential requirements for ROS in the activation of JNK, p38, and Akt by these receptors, we show for the first time that both CD40- and CXCR4-induced activation of Akt is dependent on ROS. This suggests that ROS generation could contribute to the Akt-dependent pro-survival effects of CXCR4 signaling in B cell progenitors and CD40 signaling in mature B cells. A relationship between ROS generation and B cell survival also suggests that abnormally elevated levels of intracellular ROS could contribute to excessive B cell activation or to B cell malignancies. Indeed, in patients with diffuse large B cell lymphomas, the worst prognoses correlate with decreased expression of genes encoding anti-oxidant enzymes such as catalase (Tome et al. 2005).
Materials and methods
Antibodies and chemokines
The 1C10 anti-mouse CD40 monoclonal antibody (Santos-Argumedo et al. 1994) was purified from hybridoma culture supernatant using protein G-Sepharose. Goat anti-mouse IgM antibodies (μ-chain-specific) were obtained from Jackson Immunoresearch (West Grove, PA), the 4G10 anti-phosphotyrosine monoclonal antibody was from Upstate (Charlottesville, VA) and recombinant murine SDF-1α was from R&D Systems (Minneapolis, MN). NAC was purchased from Sigma-Aldrich (St. Louis, MO) and ebselen was obtained from Calbiochem (La Jolla, CA). Phospho-specific antibodies that recognize the activated forms of JNK, p38, ERK, and Akt, as well as antibodies against the non-phosphorylated forms of these kinases were purchased from Cell Signaling Technologies (Beverly, MA), with the exception of the anti-ERK antibody, which was from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell stimulation, preparation of cell extracts, and SDS-PAGE
WEHI-231 B lymphoma cells were obtained from ATCC (Manassas, VA). Early passage cells were grown in RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum, 2 mM L-glutamine, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol, 15 U/ml penicillin and 50 μg/ml streptomycin. To reduce basal signaling, the cells were cultured overnight in medium containing 1% fetal calf serum. Cells were washed once and resuspended to 107/ml in modified HEPES-buffered saline (25 mM sodium HEPES, pH 7.2, 125 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM Na2HPO4, 0.5 mM MgSO4, 1 mg/ml glucose, 2 mM glutamine, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol). The cells were pretreated with 30 mM NAC for 2 h at 37°C or with 20 μM ebselen or an equivalent volume of DMSO for 1 h. The cells were then stimulated at 37°C with 10 μg/ml of the 1C10 anti-CD40 monoclonal antibody, 30 μg/ml goat anti-mouse IgM antibodies, 100 ng/ml SDF-1, or 300 μM H2O2. Reactions were terminated by adding cold PBS, rapidly pelleting the cells, washing once with PBS, and then solubilizing the cells in RIPA buffer (30 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1% Igepal CA-630 (Sigma-Aldrich), 0.5% sodium deoxycholate, 0.1% SDS, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 1 mM Na3VO4, 25 mM β-glycerophosphate, 1 μg/ml microcystin-LR [Biomol, Plymouth Meeting, PA], 20 mM NaF, 1 mM Na3MoO4, 10 μg/ml leupeptin). After 10 min on ice, the samples were centrifuged at 14,000 rpm for 15 min to remove detergent-insoluble material. Protein concentrations were determined using the BCA Protein Assay (Pierce Chemical Company, Rockford, IL). Total cell extracts (35 μg protein for p38 and JNK; 20 μg protein for Akt and ERK) were separated by SDS-PAGE and transferred to nitrocellulose. Prior to immunoblotting, Ponceau S staining was used to show that equivalent amounts of cell proteins were loaded in each lane.
Immunoblotting
Filters were blocked with TBS (10 mM Tris–HCl, pH 8, 150 mM NaCl) containing 0.1% Tween-20 and 5% (w/v) milk powder. The blots were then probed with phospho-specific antibodies for the active forms of ERK, JNK, p38, or Akt. Blots were stripped using TBS, pH 2 and then reprobed with the corresponding antibodies to ERK, JNK, p38, or Akt. Antibodies were diluted in TBS plus 1% (w/v) bovine serum albumin. Immunoreactive bands were visualized using ECL (Amersham Pharmacia Biotech, Baie d’Urfe, Quebec, Canada).
Acknowledgment
This work was supported by a grant (to M.R.G.) from the Canadian Institutes of Health Research.
Competing financial interests The authors declare that they have no competing interests.
Authors’ contributions RLL performed the majority of the experiments and contributed to the writing of the manuscript. JW carried out the experiments shown in Fig. 4b and confirmed other results. MRG conceived the study and prepared the final version of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Rosaline L. Lee, Email: rosalinelee@gmail.com
Jens Westendorf, Email: westendorf@biochem.mpg.de.
Michael R. Gold, Phone: +1-604-8224070, FAX: +1-604-8226041, Email: mgold@interchange.ubc.ca
References
- Adler V, Yin Z, Tew KD, Ronai Z (1999) Role of redox potential and reactive oxygen species in stress signaling. Oncogene 18:6104–6111 [DOI] [PubMed]
- Aiba Y, Yamazaki T, Okada T, Gotoh K, Sanjo H, Ogata M, Kurosaki T (2006) BANK negatively regulates Akt activation and subsequent B cell responses. Immunity 24:259–268 [DOI] [PubMed]
- Andjelic S, Hsia C, Suzuki H, Kadowaki T, Koyasu S, Liou HC (2000) Phosphatidylinositol 3-kinase and NF-kappa B/Rel are at the divergence of CD40-mediated proliferation and survival pathways. J Immunol 165:3860–3867 [DOI] [PubMed]
- Aruoma OI, Halliwell B, Hoey BM, Butler J (1989) The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med 6:593–597 [DOI] [PubMed]
- Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E (2004) Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol 172:2522–2529. [DOI] [PubMed]
- Banerji L, Glassford J, Lea NC, Thomas NS, Klaus GG, Lam EW (2001) BCR signals target p27(Kip1) and cyclin D2 via the PI3-K signalling pathway to mediate cell cycle arrest and apoptosis of WEHI 231 B cells. Oncogene 20:7352–7367 [DOI] [PubMed]
- Berberich I, Shu G, Siebelt F, Woodgett JR, Kyriakis JM, Clark EA (1996) Cross-linking CD40 on B cells preferentially induces stress-activated protein kinases rather than mitogen-activated protein kinases. EMBO J 15:90–92 [PMC free article] [PubMed]
- Bilancio A, Okkenhaug K, Camps M, Emery JL, Ruckle T, Rommel C, Vanhaesebroeck B (2006) Key role of the p110delta isoform of PI3K in B-cell antigen and IL-4 receptor signaling: comparative analysis of genetic and pharmacologic interference with p110delta function in B cells. Blood 107:642–650 [DOI] [PubMed]
- Bishop GA, Haxhinasto SA, Stunz LL, Hostager BS (2003) Antigen-specific B-lymphocyte activation. Crit Rev Immunol 23:149–197 [DOI] [PubMed]
- Bishop GA, Hostager BS (2003) The CD40-CD154 interaction in B cell-T cell liaisons. Cytokine Growth Factor Rev 14:297–309 [DOI] [PubMed]
- Clayton E, Bardi G, Bell SE, Chantry D, Downes CP, Gray A, Humphries LA, Rawlings D, Reynolds H, Vigorito E, Turner M (2002) A crucial role for the p110delta subunit of phosphatidyl-Inositol 3-kinase in B cell development and activation. J Exp Med 196:753–763 [DOI] [PMC free article] [PubMed]
- Craxton A, Shu G, Graves JD, Saklatvala J, Krebs EG, Clark EA (1998) p38 MAPK is required for CD40-induced gene expression and proliferation in B lymphocytes. J Immunol 161:3225–3236 [PubMed]
- Dadgostar H, Zarnegar B, Hoffmann A, Qin XF, Truong U, Rao G, Baltimore D, Cheng G (2002) Cooperation of multiple signaling pathways in CD40-regulated gene expression in B lymphocytes. Proc Natl Acad Sci U S A 99:1497–1502 [DOI] [PMC free article] [PubMed]
- Daou GB, Srivastava AK (2004) Reactive oxygen species mediate Endothelin-1-induced activation of ERK1/2, PKB, and Pyk2 signaling, as well as protein synthesis, in vascular smooth muscle cells. Free Radic Biol Med 37:208–215 [DOI] [PubMed]
- Devadas S, Zaritskaya L, Rhee SG, Oberley L, Williams MS (2002) Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: selective regulation of mitogen-activated protein kinase activation and fas ligand expression. J Exp Med 195:59–70 [DOI] [PMC free article] [PubMed]
- Enomoto A, Murakami H, Asai N, Morone N, Watanabe T, Kawai K, Murakumo Y, Usukura J, Kaibuchi K, Takahashi M (2005) Akt/PKB regulates actin organization and cell motility via Girdin/APE. Dev Cell 9:389–402 [DOI] [PubMed]
- Fang W, Rivard JJ, Ganser JA, LeBien TW, Nath KA, Mueller DL, Behrens TW (1995) Bcl-xL rescues WEHI 231 B lymphocytes from oxidant-mediated death following diverse apoptotic stimuli. J Immunol 155:66–75 [PubMed]
- Faruqi RM, Poptic EJ, Faruqi TR, De La Motte C, DiCorleto PE (1997) Distinct mechanisms for N-acetylcysteine inhibition of cytokine-induced E-selectin and VCAM-1 expression. Am J Physiol 273:H817–H826 [DOI] [PubMed]
- Fedyk ER, Phipps RP (1994) Reactive oxygen species and not lipoxygenase products are required for mouse B-lymphocyte activation and differentiation. Int J Immunopharmacol 16:533–546 [DOI] [PubMed]
- Ganju RK, Brubaker SA, Meyer J, Dutt P, Yang Y, Qin S, Newman W, Groopman JE (1998) The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem 273:23169–23175 [DOI] [PubMed]
- Gold MR (2002) To make antibodies or not: signaling by the B-cell antigen receptor. Trends Pharmacol Sci 23:316–324 [DOI] [PubMed]
- Gold MR, Scheid MP, Santos L, Dang-Lawson M, Roth RA, Matsuuchi L, Duronio V, Krebs DL (1999) The B cell antigen receptor activates the Akt (protein kinase B)/glycogen synthase kinase-3 signaling pathway via phosphatidylinositol 3-kinase. J Immunol 163:1894–1905 [PubMed]
- Gorin Y, Kim NH, Feliers D, Bhandari B, Choudhury GG, Abboud HE (2001) Angiotensin II activates Akt/protein kinase B by an arachidonic acid/redox-dependent pathway and independent of phosphoinositide 3-kinase. FASEB J 15:1909–1920 [DOI] [PubMed]
- Graves JD, Draves KE, Craxton A, Krebs EG, Clark EA (1998) A comparison of signaling requirements for apoptosis of human B lymphocytes induced by the B cell receptor and CD95/Fas. J Immunol 161:168–174 [PubMed]
- Griendling KK, Ushio-Fukai M (2000) Reactive oxygen species as mediators of angiotensin II signaling. Regulatory Pept 91:21–27 [DOI] [PubMed]
- Grill B, Schrader JW (2002) Activation of Rac-1, Rac-2, and Cdc42 by hemopoietic growth factors or cross-linking of the B-lymphocyte receptor for antigen. Blood 100:3183–3192 [DOI] [PubMed]
- Ha YJ, Lee JR (2004a) Role of TNF receptor-associated factor 3 in the CD40 signaling by production of reactive oxygen species through association with p40phox, a cytosolic subunit of nicotinamide adenine dinucleotide phosphate oxidase. J Immunol 172:231–239 [DOI] [PubMed]
- Ha H, Kwak HB, Lee SW, Jin HM, Kim HM, Kim HH, Lee ZH (2004b) Reactive oxygen species mediate RANK signaling in osteoclasts. Exp Cell Res 301:119–127 [DOI] [PubMed]
- Hamano T, Iwasaki T, Ogata A, Hashimoto N, Kakishita E (2002) The molecular mechanism in activation-induced cell death of an Ag-reactive B cell clone. Clin Exp Immunol 128:436–443 [DOI] [PMC free article] [PubMed]
- Hannken T, Schroeder R, Zahner G, Stahl RA, Wolf G (2000) Reactive oxygen species stimulate p44/42 mitogen-activated protein kinase and induce p27(Kip1): role in angiotensin II-mediated hypertrophy of proximal tubular cells. J Am Soc Nephrol 11:1387–1397 [DOI] [PubMed]
- Harfouche R, Malak NA, Brandes RP, Karsan A, Irani K, Hussain SN (2005) Roles of reactive oxygen species in angiopoietin-1/tie-2 receptor signaling. FASEB J 19:1728–1730 [DOI] [PubMed]
- Hargreaves DC, Hyman PL, Lu TT, Ngo VN, Bidgol A, Suzuki G, Zou YR, Littman DR, Cyster JG (2001) A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med 194:45–56 [DOI] [PMC free article] [PubMed]
- Hashimoto A, Okada H, Jiang A, Kurosaki M, Greenberg S, Clark EA, Kurosaki T (1998) Involvement of guanosine triphosphatases and phospholipase C-gamma2 in extracellular signal-regulated kinase, c-Jun NH2-terminal kinase, and p38 mitogen-activated protein kinase activation by the B cell antigen receptor. J Exp Med 188:1287–1295 [DOI] [PMC free article] [PubMed]
- Hsu JH, Shi Y, Hu L, Fisher M, Franke TF, Lichtenstein A (2002) Role of the AKT kinase in expansion of multiple myeloma clones: effects on cytokine-dependent proliferative and survival responses. Oncogene 21:1391–1400 [DOI] [PubMed]
- Ichijo H (1999) From receptors to stress-activated MAP kinases. Oncogene 18:6087–6093 [DOI] [PubMed]
- Jabara HH, Geha RS (2005) Jun N-terminal kinase is essential for CD40-mediated IgE class switching in B cells. J Allergy Clin Immunol 115:856–863 [DOI] [PubMed]
- Kane LP, Shapiro VS, Stokoe D, Weiss A (1999) Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol 9:601–604 [DOI] [PubMed]
- Karin M, Gallagher E (2005) From JNK to pay dirt: jun kinases, their biochemistry, physiology and clinical importance. IUBMB Life 57:283–295 [DOI] [PubMed]
- Laxmanan S, Datta D, Geehan C, Briscoe DM, Pal S (2005) CD40: a mediator of pro- and anti-inflammatory signals in renal tubular epithelial cells. J Am Soc Nephrol 16:2714–2723 [DOI] [PubMed]
- Lee JR, Koretzky GA (1998) Production of reactive oxygen intermediates following CD40 ligation correlates with c-Jun N-terminal kinase activation and IL-6 secretion in murine B lymphocytes. Eur J Immunol 28:4188–4197 [DOI] [PubMed]
- Li F, Malik KU (2005) Angiotensin II-induced Akt activation is mediated by metabolites of arachidonic acid generated by CaMKII-stimulated Ca2(+)-dependent phospholipase A2. Am J Physiol Heart Circ Physiol 288:H2306–H2316 [DOI] [PubMed]
- Liu H, Nishitoh H, Ichijo H, Kyriakis JM (2000) Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol Cell Biol 20:2198–2208 [DOI] [PMC free article] [PubMed]
- Lo YY, Cruz TF (1995) Involvement of reactive oxygen species in cytokine and growth factor induction of c-fos expression in chondrocytes. J Biol Chem 270:11727–11730 [DOI] [PubMed]
- Lo YY, Wong JM, Cruz TF (1996) Reactive oxygen species mediate cytokine activation of c-Jun NH2-terminal kinases. J Biol Chem 271:15703–15707 [DOI] [PubMed]
- Lo IC, Shih JM, Jiang MJ (2005) Reactive oxygen species and ERK 1/2 mediate monocyte chemotactic protein-1-stimulated smooth muscle cell migration. J Biomed Sci 12:377–388 [DOI] [PubMed]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA (1998) Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A 95:9448–9453 [DOI] [PMC free article] [PubMed]
- Manning BD, Cantley LC (2003) Rheb fills a GAP between TSC and TOR. Trends Biochem Sci 28:573–576 [DOI] [PubMed]
- Matsuzawa A, Saegusa K, Noguchi T, Sadamitsu C, Nishitoh H, Nagai S, Koyasu S, Matsumoto K, Takeda K, Ichijo H (2005) ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol 6:587–592 [DOI] [PubMed]
- McLeod SJ, Li AH, Lee RL, Burgess AE, Gold MR (2002) The Rap GTPases regulate B cell migration toward the chemokine stromal cell-derived factor-1 (CXCL12): potential role for Rap2 in promoting B cell migration. J Immunol 169:1365–1371 [DOI] [PubMed]
- Miyasaka M, Tanaka T (2004) Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat Rev Immunol 4:360–370 [DOI] [PubMed]
- Morrison DK, Davis RJ (2003) Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol 19:91–118 [DOI] [PubMed]
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T (1996) Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 382:635–638 [DOI] [PubMed]
- Niiro H, Clark EA (2002) Regulation of B-cell fate by antigen-receptor signals. Nat Rev Immunol 2:945–956 [DOI] [PubMed]
- Niu H, Ye BH, Della-Favera R (1998) Antigen receptor signaling induces MAP kinase-mediated phosphorylation and degradation of the BCL-6 transcription factor. Genes Dev 12:1953–1961 [DOI] [PMC free article] [PubMed]
- Ortolano S, Hwang IY, Han SB, Kehrl JH (2006) Roles for phosphoinositide 3-kinases, Bruton’s tyrosine kinase, and Jun kinases in B lymphocyte chemotaxis and homing. Eur J Immunol 36:1285–1295 [DOI] [PubMed]
- Purkerson JM, Parker DC (1998) Differential coupling of membrane Ig and CD40 to the extracellularly regulated kinase signaling pathway. J Immunol 160:2121–2129 [PubMed]
- Qin S, Chock PB (2003) Implication of phosphatidylinositol 3-kinase membrane recruitment in hydrogen peroxide-induced activation of PI3K and Akt. Biochemistry 42:2995–3003 [DOI] [PubMed]
- Reth M (2002) Hydrogen peroxide as second messenger in lymphocyte activation. Nat Immunol 3:1129–1134 [DOI] [PubMed]
- Richards JD, Dave SH, Chou CH, Mamchak AA, DeFranco AL (2001) Inhibition of the MEK/ERK signaling pathway blocks a subset of B cell responses to antigen. J Immunol 166:3855–3864 [DOI] [PubMed]
- Rolli V, Gallwitz M, Wossning T, Flemming A, Schamel WW, Zurn C, Reth M (2002) Amplification of B cell antigen receptor signaling by a Syk/ITAM positive feedback loop. Mol Cell 10:1057–1069 [DOI] [PubMed]
- Rudelius M, Pittaluga S, Nishizuka S, Pham TH, Fend F, Jaffe ES, Quintanilla-Martinez L, Raffeld M (2006) Constitutive activation of Akt contributes to the pathogenesis and survival of mantle cell lymphoma. Blood 108:1668–1676 [DOI] [PMC free article] [PubMed]
- Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H (1998) Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 17:2596–2606 [DOI] [PMC free article] [PubMed]
- Sakata N, Patel HR, Terada N, Aruffo A, Johnson GL, Gelfand EW (1995) Selective activation of c-Jun kinase mitogen-activated protein kinase by CD40 on human B cells. J Biol Chem 270:30823–30828 [DOI] [PubMed]
- Santos-Argumedo L, Gordon J, Heath AW, Howard M (1994) Antibodies to murine CD40 protect normal and malignant B cells from induced growth arrest. Cell Immunol 156:272–285 [DOI] [PubMed]
- Schauer SL, Wang Z, Sonenshein GE, Rothstein TL (1996) Maintenance of nuclear factor-kB/Rel and c-myc expression during CD40 ligand rescue of WEHI-231 early B cells from receptor-mediated apoptosis through modulation of IkB proteins. J Immunol 157:81–86 [PubMed]
- Schecter AD, Berman AB, Yi L, Mosoian A, McManus CM, Berman JW, Klotman ME, Taubman MB (2001) HIV envelope gp120 activates human arterial smooth muscle cells. Proc Natl Acad Sci U S A 98:10142–10147 [DOI] [PMC free article] [PubMed]
- Scheid MP, Woodgett JR (2001) PKB/AKT: functional insights from genetic models. Nat Rev Mol Cell Biol 2:760–768 [DOI] [PubMed]
- Sies H (1993) Ebselen, a selenoorganic compound as glutathione peroxidase mimic. Free Radic Biol Med 14:313–323 [DOI] [PubMed]
- Singh DK, Kumar D, Siddiqui Z, Basu SK, Kumar V, Rao KV (2005) The strength of receptor signaling is centrally controlled through a cooperative loop between Ca2+ and an oxidant signal. Cell 121:281–293 [DOI] [PubMed]
- Song G, Ouyang G, Bao S (2005) The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med 9:59–71 [DOI] [PMC free article] [PubMed]
- Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T (1995) Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270:296–299 [DOI] [PubMed]
- Sutherland CL, Heath AW, Pelech SL, Young PR, Gold MR (1996) Differential activation of the ERK, JNK, and p38 mitogen-activated protein kinases by CD40 and the B cell antigen receptor. J Immunol 157:3381–3390 [PubMed]
- Swart JM, Chiles TC (2000) Rescue of CH31 B cells from antigen receptor-induced apoptosis by inhibition of p38 MAPK. Biochem Biophys Res Commun 276:417–421 [DOI] [PubMed]
- Takada E, Toyota H, Suzuki J, Mizuguchi J (2001) Prevention of anti-IgM-induced apoptosis accompanying G1 arrest in B lymphoma cells overexpressing dominant-negative mutant form of c-Jun N-terminal kinase 1. J Immunol 166:1641–1649 [DOI] [PubMed]
- Tanaka K, Honda M, Takabatake T (2001) Redox regulation of MAPK pathways and cardiac hypertrophy in adult rat cardiac myocyte. J Am Coll Cardiol 37:676–685 [DOI] [PubMed]
- Tome ME, Johnson DB, Rimsza LM, Roberts RA, Grogan TM, Miller TP, Oberley LW, Briehl MM (2005) A redox signature score identifies diffuse large B-cell lymphoma patients with a poor prognosis. Blood 106:3594–3601 [DOI] [PMC free article] [PubMed]
- Tonks NK (2005) Redox redux: revisiting PTPs and the control of cell signaling. Cell 121:667–670 [DOI] [PubMed]
- Ushio-Fukai M, Alexander RW, Akers M, Griendling KK (1998) p38 Mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. J Biol Chem 273:15022–15029 [DOI] [PubMed]
- Ushio-Fukai M, Alexander RW, Akers M, Yin Q, Fujio Y, Walsh K, Griendling KK (1999) Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J Biol Chem 274:22699–22704 [DOI] [PubMed]
- Viedt C, Soto U, Krieger-Brauer HI, Fei J, Elsing C, Kubler W, Kreuzer J (2000) Differential activation of mitogen-activated protein kinases in smooth muscle cells by angiotensin II: involvement of p22phox and reactive oxygen species. Arterioscler Thromb Vasc Biol 20:940–948 [DOI] [PubMed]
- Yoon S, Seger R (2006) The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors 24:21–44 [DOI] [PubMed]
- Zarubin T, Han J (2005) Activation and signaling of the p38 MAP kinase pathway. Cell Res 15:11–18 [DOI] [PubMed]