Abstract
In order to understand the basic mechanisms underlying the organismic aging process, considerable efforts have been devoted in the last half-century to biochemical (enzyme activity) alterations in specific tissues and organs of various organisms associated with aging. When a decline in enzyme activities with age has been found in a study, especially for key enzymes such as antioxidant enzymes, the results have often been interpreted as a cause for the aging of the entire body. Retrospectively, however, these changes turned out to be so variable—depending on species, strains and sexes of animals—that the interpretation of these results in general terms of aging became invalid. Further, unlike the prediction for the whole human body, many enzyme activities in a vital organ, such as the liver, remained unchanged, as long as the old subjects remained healthy. However, enzyme activities in old animals and humans are often more susceptible to morbidities and frailties, which themselves are often accompanied by infections and malnutrition. Despite the rather stable enzyme functions in the liver with age, a distinct and progressive decline in the lateral diffusion coefficient of proteins of hepatocyte plasma membranes has been demonstrated by fluorescence recovery after photobleaching (FRAP), which was implicated as the cause for the decline of hepatocyte functions such as ouabain (and taurocholate) hepatic uptake and their eventual biliary excretion. Since a similar decline in protein diffusion coefficients was observed in brain and muscle cells, it is likely that these changes are occurring in common with many cell types of the body, thus causing a delay in transmembrane transport of endogenous and exogenous substances whose transports are mediated by membrane proteins. In attempts to prolong the life spans of animals other than by calorie restriction, but instead using deprenyl or tetrahydrocurcumin, works by the author and coworkers are introduced and discussed. Despite limited success along these lines thus far, further attempts are encouraged, primarily to understand the mechanisms underlying organismic aging processes and to find a practical way to prolong the health span of the elderly.
Key words: aging rodents, altered enzymes, antioxidant enzymes, (-)deprenyl, hepatic microsomal P-450 functions, maximal seizure response, protein lateral diffusion coefficients of cell surface membranes, sex- and strain-related differences, tetrahydrocurcumin
It is a great but unexpected honour to receive the Hayflick award for 2006, which was something I never imagined. I thank all of the committee members who decided to honour me in this way. However, I am aware that it is not only an honour, but also more importantly a great responsibility, because the lecture I am going to give is expected to address the following three big questions as requested by Dr. Simin Meydani, the current president of our Association: (1) What are the unanswered questions in understanding how aging works? (2) Why are these questions important to an overall understanding of aging? and (3) How might we best approach those questions experimentally and clinically?
Obviously, it is beyond my capability to address these three important but difficult questions directly. Instead, what I am going to do today is to reproduce here my questions, and my own tentative answers which I have been repeating in my mind over the past 35 years, by reviewing mainly some of my own past research activities.
Everything is going down with age (Jon Ek)
When I started to work in the Tokyo Metropolitan Institute of Gerontology (TMIG), I was 36 years old. I had my English teacher, Mr. J. Ek, who was correcting the English of my manuscript. One day, he told me “I have become 50 years old today. I said “Happy birthday, but you look so young.” He said “No, at the age of 50, everything is going down.” He had just remarried a 22-year-old Japanese girl. My honest feeling at that time was “there is no wonder that everything is going down when one becomes 50 years old.” I think a majority of people around here will agree with Jon that everything is going down with aging, and that this is the natural and normal aging process.
What really declines with age?
However, when I started to get involved in aging studies, I began to wonder what is really going down with age. This question occurred to me immediately after I started to work on aging animals, and still remains essentially unresolved up to now. Before I started to work in TMIG, I had been working in the field of hepatology, the study of the liver. Accordingly, the first step I took was to examine whether and how liver functions are altered—namely decline—with age.
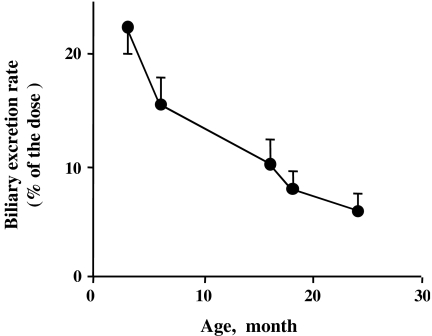
One of our early studies was to examine the biliary excretion of i.v. injected ouabain, a cardiac glycoside in rats of different ages. In this animal species, i.v. injected ouabain is very efficiently excreted into the bile. The biliary recovery of ouabain in the first 10 minutes was shown to decline in a linear fashion with rat age (Fig. 1), (Kitani et al. 1978). At first, I was not excited at all with this result, because I thought “Well, everything is going down with age. This may be one of everything.” Later, I realized that it was not true.
Fig. 1.
The biliary excretion rate (percent of the dose) for the first 10 minutes after i.v. injection of ouabain in male Wistar rats of different ages. All values are expressed as mean ± SD. (Created from the data reported in Kitani et al. 1978)
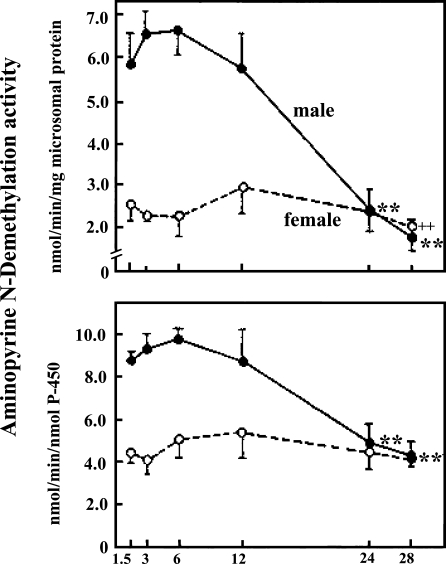
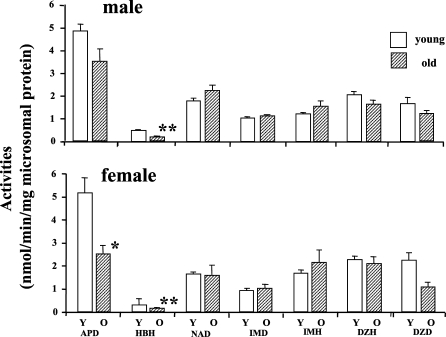
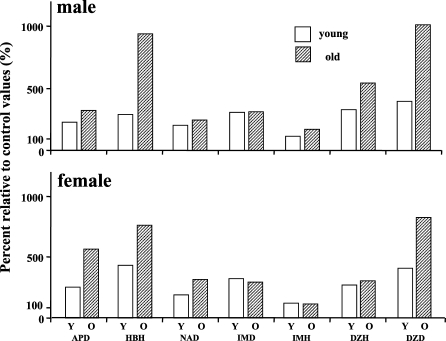
When we examined age-dependent changes in drug metabolizing enzyme activities mediated by the hepatic microsomal P-450 system in F344/Du rats, the P-450 concentrations as well as many enzyme activities dependent on P-450 all drastically declined with age in male rat livers. This may be another example of everything declining; however, in female rat livers, all drug metabolizing enzyme activities as well as P-450 concentration stayed totally unchanged with age, showing a plateau throughout their lives (Fig. 2) (Fujita et al. 1986; Kamataki et al. 1985). I thought maybe P-450 functions in female rat livers could be a rare exception. However, when we examined mouse livers, we found barely any decline with age in drug metabolizing enzyme activities in either sex (Fig. 3) (Fujita et al. 1986). Then I started to wonder: which is the exception, a decline or no change with age?
Fig. 2.
Aminopyrine N-demethylse activity in male and female F344 rats of different ages. All values are expressed as mean ± SEM. Reproduced with the permission of the publisher. (Fujita et al. 1982). Closed circles indicate male rats and open circles female rats. +, ++, **, Significantly different from respective 3-month values. (+P<0.05; ++, **P<0.01)
Fig. 3.
Drug-metabolizing enzyme activities dependent on hepatic microsomal P-450 in male and female C57BL mice. Y; young, O; old. Values are expressed as mean ± SEM (n = 4∼12). *, ** Significantly different from the corresponding values in the young mice: *P<0.05, **P<0.01. Abbreviations: APD; aminopyrine demethylase, HBH; hexobarbital hydroxylase, NAP; nitroanisole O-demethylae, IMD; imipramine N-demethylase, IMD; imipramine 2-hydroxylase, DZH; diazepam 3-hydroxylase, DZD; diazepam N-demethylase. (Created from Fujita et al. 1986)
And this question—what really declines with age?—has continued to stay in my mind up to now. In those days, it appears to have been a general consensus that all physiological and probably biochemical functions decline with aging. Early studies by the group of Dr. Nathan Shock in Baltimore strongly supported this thesis. In so-called healthy human volunteers, the cardiac output (Brandfonbrener et al. 1955), renal creatinine clearance (Rowe et al. 1976) and all other physiological parameters examined were demonstrated to steadily decline with age. However, later by the same group in Baltimore, in a longitudinal study, neither the cardiac output (Rodeheffer et al. 1984) nor renal creatinine clearance (Lindeman et al. 1985) was shown to decline with age. Instead, they found that only morbidities associated with aging down-regulated these functions.
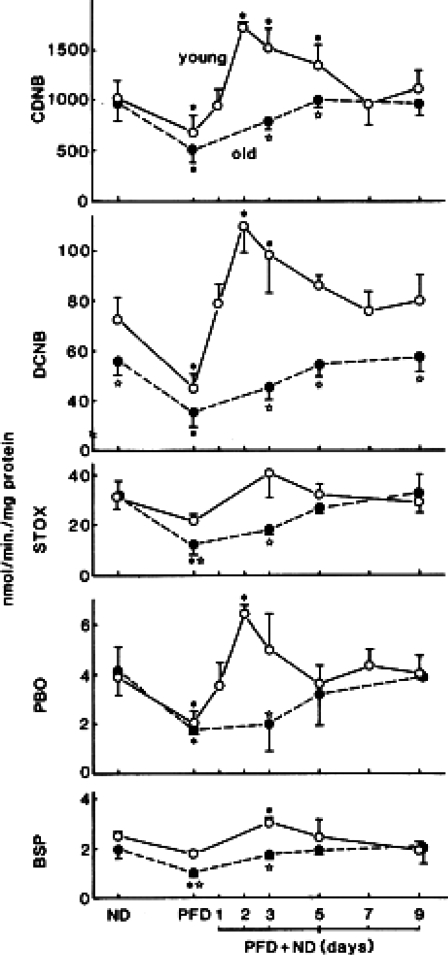
We continued to work on liver functions. We found that activities of glutathione S-transferase (GST), which is a cytosolic (and important detoxifying) enzyme, also remained unchanged with age towards 4 out of 5 substrates tested in female mouse livers (Carrillo et al. 1989). I finally came to a tentative conclusion that some liver functions may not decline with age. However, although physiological levels of enzyme activities stayed mostly unchanged with age, striking differences between young and old mice in the regulation of enzyme activities could be demonstrated when animals were fed a protein-free diet (PFD) and refed a normal diet. In young animal livers, after a normal diet refeeding, the GST activities immediately went up, up to far higher levels than physiological levels within 2 days, and then came down to their normal physiological levels, while in old mouse livers such an “overshooting” of enzyme activities never occurred, the values slowly coming back to physiological levels (Fig. 4) (Carrillo et al. 1989).
Fig. 4.
Changes in activities of glutathione S-transferases towards five different substrates in young (8-month old) and old (27-month old) female C57BL mice which were given different diets. All values are expressed as mean ± SD. Abbreviations: ND; normal diet for 2 weeks, PFD; protein free diet for 1 week, PFD + ND; normal diet refeeding after PFD for 1 week, CDNB; 1-chloro-2,4-dinitrobenzene, DCNB; 1,2-dichloro-4-nitrobenzene, STOX; styrene oxide, PBO; trans-4-phenyl-3-buten-2-one, BSP; sulfobromophthalein sodium tetrahydrate. All values are expressed as mean ± SD. Reproduced with permission (Carrillo et al. 1989) *Significantly different from corresponding basal values in control mice fed with normal diet only (P < 0.05). Open stars Significantly different from corresponding young values (P < 0.05)
Thus, a linear decline of GST enzyme activities with age was clearly observed only in the recovery phase after a normal diet refeeding, rather than at physiological levels or at bottom levels by PFD feeding (Carrillo et al. 2002). We concluded that young mice could recover their normal enzyme activities more quickly and more efficiently than old mice. We also confirmed this phenomenon in three other animal models—male mice and rats of both sexes (Carrillo et al. 1990, 1991, 1992b).
This phenomenon may look similar to what Adelman proposed many years ago as “a disrupted enzyme regulation” in old age (Adelman 1975). He classified different responses of enzyme activities in old animals to a variety of stimulations primarily into two prototypes, namely a lowered response and a delayed response (and their subtypes) of enzyme activities to stimuli in old animals (Adelman 1975). However, an impaired enzyme response is not as simple as his classification indicates.
When young and old mice were treated with phenobarbital for 7 days, so-called enzyme inductions were often greater in old mice than their young counterparts (Fig. 5) (Fujita et al. 1986). This type of an exaggerated response in old animals was cited by Adelman (1975); however, he did not specifically discuss it. Although the effect of enzyme inducers on drug metabolism in elderly humans is controversial in the literature (Kitani, 1988), a greater enzyme induction in old than in young humans has been reported (Bonde et al. 1985). It is questionable whether this type of response can be classified as one type of disrupted enzyme regulation in old age. To my mind, this can only be explained as due to a decrease in turnovers of enzyme molecules in old animal livers.
Fig. 5.
Percent increase in drug metabolizing enzyme activities in young and old mice of both sexes after 1 week of phenobarbital feeding. Values are expressed as a percent relative to corresponding basal values before treatment. Phenobarbital was administered by intubation at a dose od 20 mg/kg/day for the first 2 days followed by a 5-day treatment at a dose of 50 mg/kg/day. Abbreviations are the same as indicated in Fig. 3. (Created from the data reported in Fujita et al. 1986)
For many enzyme molecules, their synthesis may be decreased with age; however, their turnovers are also lowered, so that apparent enzyme unit numbers, and thus their activities, can be maintained marginally at young levels even in old age. A decrease in intracellular protein turnover is difficult to prove; however, the group of Goto in Japan has provided some evidence for this thesis by using a microinjection technique of proteins of different kinds into primary cultured hepatocytes of mice of different ages (Ishigami and Goto 1990, Goto et al. 2001).
Other indirect but strong evidence supports this thesis. There is the formation of lipofuscin-like lipopigments in rat hepatocytes and other internal organs, such as kidneys, following an intraperitoneal administration of protease inhibitors, such as leupeptin (Ivy et al. 1990, 1991a,b). This observation strongly supports the idea that the perturbation of protein degradation and its intracellular disposition in secondary lysosomes is a fundamental mechanism for lipofuscinogenesis, as Ivy initially proposed for brain cells 20 years ago (Ivy et al. 1984). At the same time, this observation strongly suggests that a decrease in proteolysis during aging leads to progressive decreases in turnovers of intracellular proteins, including enzyme molecules.
The marginally balanced protein synthesis and degradation which maintains enzyme activities at young levels in old age can be supported indirectly by results of clinical studies. A group in the UK examined the conjugation capacity of acetaminophen in human livers by measuring urinary clearance of its conjugates, a glucuronide and a sulphate. They found that if clearance values were corrected for liver volume, there was no significant difference in clearance values between the fit young and fit elderly; however, in the frail elderly, values were significantly lower than in even the fit elderly (Wynne et al. 1990). This suggests that not only a morbidity but also even a frailty decreases liver functions considerably in elderly humans. Could this be an exception? I collected many pharmacokinetic studies which examined the difference between young and old human subjects (Kitani 1988).
All these drugs are metabolized by the liver and, accordingly, their clearances depend on liver drug metabolism. Other than benzodiazepines, 28 drugs have been reported to have clearance values lowered with age in 54 studies. In contrast, clearance values for 26 drugs have been reported to stay unaltered with age in 35 studies (Kitani 1988). Among these drugs, 14 drugs were listed in both categories namely, having both lowered and unchanged clearances with age (Kitani 1988). Similarly, 11 different benzodiazepines were reported to have a decrease in their clearance values, while 9 benzodiazepines were reported to have no significant decline in their clearance values in the elderly. Two drugs were listed in both categories (Kitani 1988). As such, the results are quite contradictory among different studies. Similar to findings in early studies by the Baltimore group, I found that the criteria for the definition of healthy elderly profoundly affected the results in many of these studies.
A drastic decline in P-450 functions with age reported in male rats as early as in the sixties (Kato et al. 1964) has long been taken as an experimental basis for the possible decline in hepatic drug metabolism in the elderly (Kitani 1988). However, it is my tentative conclusion that for a majority of drugs metabolized by the liver, their clearance values are not altered with age per se, especially when clearance values are corrected for liver volume. It means that enzyme activities per unit liver volume are not lowered in old age. However, morbidities and even a subtle frailty, which is accompanied by infections that are known to impair enzyme activities of Phase I reactions (Au et al. 1985, Sonne et al. 1985), and (protein) malnutrition, which is known to lower activities of Phase II reactions such as GST, can lower these liver functions considerably in the elderly (Anderson et al. 1982), as has been clearly shown for actaminophen by the UK group (Wynne et al. 1990).
An altered enzyme hypothesis for SOD
Is a drug-metabolizing enzyme system in the liver an exception for the rule of J. Ek? Gershon in Israel found an age-dependent decline in superoxide dismutase (SOD) activities in male rat livers, and attempted to explain his observation by postulating a so-called alteration of the SOD molecule (Reiss and Gershon 1978). He insisted that a subtle alteration of SOD chemical structure yields a molecule more susceptible to high temperatures in terms of its enzyme activity, but stable in terms of its antigenicity as a protein. A similar alteration was reported for other enzymes (Wulf and Cutler 1975). This alteration causes an age-dependent decline in SOD enzyme activity and consequently a reduction in radical scavenging capacity in tissues, leading to a further decline in functions of the liver by means of “a vicious circle” (Reiss and Gershon 1978). Further, a group in San Antonio reported that catalase (CAT) and SOD activities and their respective gene expressions declined with age in the liver (Rao et al. 1990) as well as in the brain (Rao et al. 1990, Semsei et al. 1991), and postulated the hypothesis that this is a basic mechanism regulating an age-associated decline in many functions in the liver and brain through—again—“a vicious circle”. These were and still are attractive theories. The primary problem with these hypotheses, however, is that they are based on an assumption that the SOD enzyme activity in the liver declines in general terms with age.
When our group examined CAT and SOD enzyme activities in livers of young and old F344/DuCrj rats, we found no decline in SOD enzyme activities with age (Carrillo et al. 1992a). CAT activities declined with age in male rat livers; however, we found a significant increase with age in female rat livers (Carrillo et al. 1992a).
Rikans et al. (1991) also previously reported that there is no decline in SOD enzyme activities with age in rat livers. Interestingly, they also reported an age-dependent decline in CAT activities in male rat livers, and conversely an age-dependent increase in female rat livers (Rikans et al. 1991), as we ourselves found (Carrillo et al. 1992a). I think when Gershon found a decrease in SOD activities in his rat liver, he never doubted that it was not true in other animal models, probably because he was also biased by the idea of J. Ek that everything is going down with age.
How about the brain? We found in male F344/Du rats, in most brain regions examined, that Mn-SOD activities were significantly (3- to 5-fold) higher rather than lower in old rats than in their young counterparts, which contributed to a significantly higher total SOD activity in the brains of old animals (Carrillo et al. 1992a). These findings correspond to the observation by Williams et al. (1995), who found that messenger RNA levels for Mn-SOD are significantly elevated in most brain regions in old male rats—which, however, was at variance with observations made by the San Antonio group (Rao et al. 1990, Semsei et al. 1991). In females, the differences between young and old rats were very minor, showing that enzyme activities were affected only modestly by aging, but that they certainly show no decline with age (Carrillo et al. 1992a). It is obvious that the hypotheses raised by Gershon and the group in San Antonio do not hold up even in the same species of animals in general.
One of my messages in this talk is that in order to make a general theory of aging, our observations must be reproducible in a diversity of animal species, strains and sexes, that is, they must be “a public observation” as defined by Martin (1997). The second message is, of course, we must be very careful about our own bias that everything is going down with age, when we deal with our own experimental results. This reminds me of my own experience a long time ago. When I submitted our manuscript stating that P-450 functions in mouse livers stay basically unchanged with age, the paper was quickly turned down with the comment “a drug metabolism in mouse livers does not decline with age, WHY?” I think this reviewer was also totally trapped by the idea that everything is going down with age, as Mr. Ek felt at his age of 50.
After spending more than 10 years on liver and aging (Kitani 1978, 1982, 1986, 1991b), I realized and now am convinced that many if not all liver functions, especially enzyme activities, stay unchanged with age, as long as the subjects remain healthy. Finally, I came to the conclusion that the change in P-450 function(s) in male rat livers is (are) a rare exception, which was nothing but the feminization of P-450 functions in male rat livers (Fujita et al. 1986, Kamataki et al. 1985). Then, I came back to our initial observations of ouabain excretion.
Hepatic uptake and biliary excretion of ouabain and hepatocyte surface membrane quality alterations with age
In 1982, one young and bright Hungarian guy, Imre Zs.-Nagy, came to our laboratory from Debrecen to construct and start to use an instrument called “fluorescence recovery after photobleaching”, or “FRAP”. There is not sufficient space to describe this instrument in detail, but with this home-made machine we are able to measure the protein lateral diffusion coefficient of cellular surface membranes (for details, see Zs.-Nagy 1994, Zs-Nagy et al. 1984, 1986).
When we examined hepatocyte surface membranes in rats, we found a linear decline with age of this parameter (Zs.-Nagy et al. 1986). We then compared the ouabain excretion into the bile with protein lateral diffusion coefficients of hepatocyte surface membranes in rats of different ages, and found a significant correlation between these two parameters (Fig. 6) (Kitani et al. 1988). Interestingly, spironolactone feeding for 4 days, which had been known to considerably increase the biliary ouabain excretion, caused an increase of protein diffusion coefficient, showing again a significant correlation between these two parameters in spironolactone-fed rats (Fig. 6) (Kitani et al. 1988).
Fig. 6.
Comparison of the lateral diffusion constants of hepatocyte plasma membrane proteins and ouabain excretion into the bile (first 10-min value) in male Wistar rats of different ages with and without spironolactone pretreatment. All values are expressed as mean ± SD. Reproduced with permission. (Kitani et al. 1988)
Then we examined the hepatic uptake velocity of ouabain by using isolated hepatocyte preparations, and again we observed a linear decline with age of hepatic uptake velocity, and a positive correlation between the hepatic uptake velocity and the other two parameters, protein diffusion coefficient and biliary excretion of ouabain (Ohta et al. 1988). We have also shown that the hepatic uptake velocity of taurocholate, an organic anion unlike a neutral steroid, ouabain, also declines with age in a very similar manner to that of ouabain (Ohta and Kitani 1990). These two compounds have totally different transport proteins in hepatic surface membranes. Accordingly, we postulated that an age-dependent decrease in protein mobility in hepatocyte surface membranes caused a decline in the hepatocyte uptake velocity of ouabain as well as of taurocholate, leading to a decrease in the biliary recovery of ouabain in the first 10 minutes in particular (Kitani et al. 1988).
As of that moment, I had found one parameter in the liver which steadily declines with aging, namely the protein lateral diffusion coefficient of hepatocyte surface membranes. This observation was confirmed in five different rat models (F344 and BN/Bi rats of both sexes and male Wistar rats), two mouse models (C57BL/6 mice of both sexes), and short-lived (Mus musculus) and long-lived (Peromiscus) wild mouse strains of both sexes (Zs.-Nagy et al. 1993) (for review see Kitani 1999). This finding also turned out to be true in brain cells (Zs.-Nagy et al. 1999) as well as in skeletal muscle cells (Zs-Nagy et al. 1998). It is likely that this change in cell surface membranes has a profound impact on physiological functions in these organs, especially for transmembrane transport functions for various (endogenous and exogenous) substances mediated by membrane proteins (Kitani 1991a, 1999, Kitani et al. 1991).
Membrane hypothesis of aging (I. Zs.-Nagy)
After introducing the FRAP studies of Zs.-Nagy, I need to discuss what I think of his hypothesis, namely “The membrane hypothesis of aging” (Zs.-Nagy 1991, 1994, 2001). Dr. Zs.-Nagy insists that the surface membranes of all cell types in animals become increasingly rigid with age, which causes intracellular dehydration leading to a general decline in enzyme activities with age, since the mobility of intracellular enzyme molecules is more and more restricted with aging (Zs.-Nagy 1991, 1994, 2001). The first tenet of his theory, the qualitative alteration of cell surface membranes with age, has been unequivocally supported by our collaborative works using FRAP (Zs.-Nagy 1991, Kitani 1999). However, I totally disagree with his final conclusion that all intracellular enzyme activities decline with age, as I have discussed already in this talk using my own studies as well as many in vivo clinical pharmacokinetic studies reported in the past (Kitani 1988). This issue has been discussed previously (Kitani 1991a, 1999). His response to my view was published also (Zs.-Nagy 2001). Readers are advised to form their own views on this issue by examining views of both sides.
A decline in seizure response capability with ageA decline in a function in a whole organism
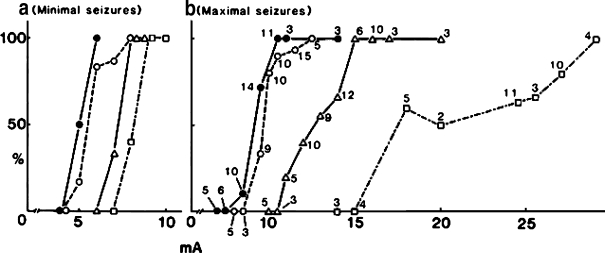
While I have had a hard time identifying a liver function which definitively declines with age, we incidentally found one parameter which shows a definitive decline with age. In the mid-1980s, we were working to examine pharmacodynamic alterations affected by aging, which are quite important in pharmacotherapy for the elderly but are very poorly elucidated even now. We found that as animals age, they become more sensitive to all anticonvulsants tested, such as phenytoin (Kitani et al. 1984), phenobarbital (Kitani et al. 1985b), oxazepam (Kitani et al. 1989) and AD810 (Kitani et al. 1987), regardless of their receptor differences. That is, a lower concentration of drugs in serum and the brain is enough to block the maximal seizure induced by chemical or electrical shock as animals get older as shown in Fig. 7. However, we finally concluded that these changes of apparent drug sensitivity to central nervous system depressants are most likely due to the general decline in seizure response capability with age, rather than to changes in receptors for individual drugs. This was clearly demonstrated as an age-related increase in stimulatory intensity of an electric shock (Kitani et al. 1985a) required for inducing a maximal seizure as shown in Fig. 8.
Fig. 7.
Minimal effective concentration (MEC) of phenytoin in the brain to completely abolish the tonic hind-limb extension component a maximal seizure induced by a corneal electroshock (a 50 Hz alternating current, 55 mA for 0.2 sec) in male and female BDF1- mice of different ages. All values are expressed as mean ± SD. *Significantly different from corresponding values in 6-month-old mice (P<0.01). (Created from the data reported in Kitani et al. 1984)
Fig. 8.
The incidence of minimal (a) and maximal (b) seizures and electroshock (a 50 Hz alternating current with varying current intensity for 0.2 sec.) intensity in female BDF1 mice of different ages. Closed circles indicate 6-month-old values, open circles 12-month-old values, triangles 24-month-old values and squares 30-month-old values. The number near symbols in panel B indicates the number of BDF1 mice tested. The number of mice of each group for minimal seizure (panel a) ranges from 3 to 7. Reproduced with permission. (Kitani et al. 1985a)
This is a very clear age-dependent alteration, a “decline” of physiological function of an organ in the body. But it is a very complex polysynaptic sequence in the whole body, starting from current spread to the cornea, through to the brain stem, then to the spinal cord and ending in an extensor tonic convulsion of the hind limb. I think what Jon Ek said still holds, because he was feeling something as a whole organism and not as a liver homogenate or a tissue slice or a cell in culture. However, as I have shown you, if we look at sophisticated biochemical or molecular genetic parameters, we really have difficulty in finding a definitive decline with age for many parameters.
My next message is that it is of paramount importance to realize that aging is a phenomenon occurring in the whole organism. If we look at functions of the whole body, we can clearly and easily demonstrate a definitive and reproducible decline in functions of the body. The hepatic GST response as discussed earlier is also one example of a phenomenon occurring in the whole body, and the integration of all functions in the body may be most important in keeping animals alive. In contrast, it is amazing to realize how little is changing with age in a single vital organ like the liver, where many biochemical parameters are maintained at their young levels even in old age. When we think of the biological aging of animals, in my opinion, we always need to come back to the whole body functions of organisms.
Pharmacological and nutritional interventions in aging
Finally, I will introduce the manipulation of animal life spans. Obviously, it is still the general consensus in experimental gerontology that the only means to reproducibly prolong the life spans of animals is calorie restriction. Personally, however, I belong to a minority who believes that the calorie restriction paradigm does not work so effectively in humans, in contrast to what has been successfully demonstrated in all other animal species tested. I will refrain from my current discussion of this issue (see Kitani and Goto 2005). I just cite two studies, one theoretical (Demetrius 2004), the other practical (Dirks and Leeuwenburgh 2006), which have raised some doubt or a caution on the practice of calorie restriction in humans. Here, I will show results of our own attempts, with methods other than calorie restriction, to modify or intervene in life spans of animals.
In 1993, we reported our initial study which demonstrated a significant prolongation of average life spans of male F344/Du rats by s.c. deprenyl injections (Kitani et al. 1993). This was the third study of this kind, following the initial study by Knoll (1988) and another study by a Canadian group (Milgram et al. 1990).
Not only in rats, but also in three other species—specifically dogs, hamsters and mice—a positive effect of deprenyl on life spans has been reported (reviewed in Kitani et al. 2002a,b). In contrast to three rat studies in the past which demonstrated a significantly positive effect of deprenyl on life spans of rats (Knoll 1988, Milgram et al. 1990, Kitani et al. 1993), one study reported that the effect was insignificant (Bickford et al. 1997). Most intriguingly, another study from the UK reported an adverse effect, that is a shortening of life spans of rats (Gallagher et al. 1998). We speculated that this effect of the drug may have an upper limit, and that above this range the effect will become less effective and finally adversely effective. With our most recent data, a predicted inverse U-shaped dose–response relationship in terms of the effect of deprenyl on life spans in F-344 rats has emerged (Kitani et al. 2005, 2006). A similar inverse U-shaped response was also previously demonstrated by our group for another effect of the drug, that is the increase of SOD and CAT activities (Carrillo et al. 2000, discussed in Kitani et al. 2002a,b, 2006). We have demonstrated that the effective dose ranges for these two effects of the drug are overlapping with each other, which is compatible with our long-standing contention that these two effects are causally interrelated (Kitani et al. 2005, 2006). Now we can easily explain the negative effect of the drug reported by the UK group (Gallagher et al. 1998) as due to an overdose of the drug to the rat strain they used.
If the above hypothesis is true, however, it is rather unique, since almost all past studies genetically up-regulating SOD and/or CAT enzymes have failed to increase life spans of rodents. Only recently, the group in Seattle has reported a significant prolongation of life span in mice overexpressed for their mitochondrial CAT (Schriner et al. 2005). Another recent preliminary study reported a successful prolongation of the life span of male F344 rats that were overexpressed for SOD1 (Ikeno et al. 2005).
We have not reached the final conclusion on the precise mechanisms for the prolongation of life span by deprenyl.
We have found that deprenyl induces SOD and CAT activities not only in brain dopaminergic regions but also in some internal organs such as the heart, kidneys and adrenal glands, which were accompanied by increases of their mRNA levels (Kitani et al. 2002a,b). Deprenyl also mobilizes a variety of humoral factors such as interleukines, neuronal factors (including neurotrophins), hormonal factors etc. (Kitani et al. 2002a). Some of these appear to be related to the up-regulation of antioxidant enzyme activities induced by this drug. It is possible that mobilization of some of these humoral factors is at least partially involved in the effects of deprenyl on life span in animals.
Finally, based on the free radical theory of aging by Harman (1956, 1994), we selected a micronutrient contained in Indian curry, namely curcumin. Tetrahydrocurcumin (TC) is a biotransformed product of curcumin which is a more potent antioxidant. We fed male mice with a diet containing purified TC and found the survival curve shifting towards the right. The average life span as well as the 10% longest life span of mice was significantly prolonged (by more than 10%) by feeding a diet containg 0.2% (but not 0.1%) TC. The prolongation of average life expectancy after 24 months was more than two-fold (Kitani and Osawa 2005). We have also observed that the feeding of green-tea polyphenols in drinking water could significantly extend the average life spans of male mice (Kitani and Yokozawa 2003).
Much work remains to be done on the mechanisms of modification of life spans of animals by deprenyl as well as by TC and many other substances, especially antioxidant micronutrients. I do believe that it is worthwhile to examine the effects of such substances on life spans of animals. It is my belief that life spans of animals are the best parameter for animal aging, and that the manipulation of or interventions into life spans by various means may help us further our understanding of aging mechanisms. Furthermore, studies using micronutrients may prove a rational basis for nutritional strategies for interventions in aging and age-associated disorders, which at the moment are mostly based on epidemiological studies in the past (Kitani 2005).
In the present talk, I have emphasized that a definitive functional decline with age can be observed when we look at a whole organism. However, it is possible that I am not correct if I say that all functions of a whole body decline with age. According to Jeanne Wei at Harvard University (at that time), there are three functions which actually can increase with age, that is (1) creativity, (2) wisdom, and (3) spiritual strength. It is hard to prove this thesis, but we tend to believe it when we look at some special persons, for example Dr. and Mrs. Denham Harman, who really demonstrate evidence for the thesis of Wei. If she is correct, our gerontology should aim at an augmentation of function in the elderly, rather than just looking for the deficits or declines in old age, in order to promote a longer, healthier and more productive and creative life span.
Acknowledgements
The author truly expresses his deep appreciation to all coworkers over the past 35 years who contributed to the progress in aging research in his laboratory. Special thanks are due to Dr. G.O.Ivy who carefully read the manuscript, Ms. S. Kanai and Ms. M. Ratnam for their technical help and Ms. T. Ohara for her efficient secretarial work to complete this manuscript.
References
- Adelman RC. Disruption in enzyme regulation during aging. In: Parke DV, editor. Enzyme Induction. London: Plenum Press; 1975. pp. 303–311. [Google Scholar]
- Anderson KE, Conney AH, Kappas A. Nutritional influences on chemical biotransformations in humans. Nutr Rev. 1982;40(6):161–171. doi: 10.1111/j.1753-4887.1982.tb05298.x. [DOI] [PubMed] [Google Scholar]
- Au WY, Dutt AK, DeSoyza N. Theophylline kinetics in chronic obstructive airway disease in the elderly. Clin Pharmacol Ther. 1985;37(4):472–478. doi: 10.1038/clpt.1985.74. [DOI] [PubMed] [Google Scholar]
- Bickford PC, Adams CE, Boyson SJ, Curella P, Gerhardt GA, Hero C, et al. Long-term treatment of male F344 rats with deprenyl: Assessment of effects on longevity, behavior, and brain function. Neurobiol Aging. 1997;18:309–318. doi: 10.1016/S0197-4580(97)80313-2. [DOI] [PubMed] [Google Scholar]
- Bonde J, Pedersen LE, Bodtker S, Angelo HR, Svendsen TL, Kampmann JP. The influence of age and smoking on the elimination of disopyramide. Br J Clin Pharmacol. 1985;20:453–458. doi: 10.1111/j.1365-2125.1985.tb05096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandfonbrener M, Landowne M, Shock NW. Changes in cardiac output with age. Circulation. 1955;12:557–566. doi: 10.1161/01.cir.12.4.557. [DOI] [PubMed] [Google Scholar]
- Carrillo MC, Kanai S, Kitani K, Ivy GO. A high dose of long term treatment with deprenyl loses its effect on antioxidant enzyme activities as well as on survivals of Fischer-344 rats. Life Sci. 2000;67:2539–2548. doi: 10.1016/S0024-3205(00)00838-9. [DOI] [PubMed] [Google Scholar]
- Carrillo MC, Kanai S, Miyasaka K, Kitai K. A protein free diet uncovers the potential age-difference in the hepatic detoxifying system, glutathione S-transferase in female mice. Mech Ageing Dev. 2002;123:1617–1623. doi: 10.1016/S0047-6374(02)00097-0. [DOI] [PubMed] [Google Scholar]
- Carrillo MC, Kanai S, Sato Y, Kitani K. Agerelated changes in antioxidant enzyme activities are region and organ selective as well as sex in the rat. Mech Ageing Dev. 1992a;65:187–198. doi: 10.1016/0047-6374(92)90035-C. [DOI] [PubMed] [Google Scholar]
- Carrillo MC, Kitani K, Kanai S, Sato Y, Nokubo M, Ohta M, Otsubo K. Differences in the influence of diet on hepatic glutathione S-transferase activity and glutathione content between young and old C57 black female mice. Mech Ageing Dev. 1989;47:1–15. doi: 10.1016/0047-6374(89)90002-X. [DOI] [PubMed] [Google Scholar]
- Carrillo MC, Nokubo M, Kitani K, Kanai S, Sato Y, Ohta M. Different responses of subunit concentrations and enzyme activities of glutathione S-transferases (GSTs) to protein-free diet (PFD) between young and old rodent livers. In: Kitani K, editor. Liver and Aging-1990. Amsterdam: Elsevier Science; 1991. pp. 75–86. [Google Scholar]
- Carrillo MC, Sato Y, Kanai S, Nokubo M, Kitani K. Difference in response of hepatic glutathione S-transferase activities to protein free diet between young and old C57 male mice. Mech Ageing Dev. 1992b;65:301–311. doi: 10.1016/0047-6374(92)90043-D. [DOI] [PubMed] [Google Scholar]
- Carrillo MC, Nokubo M, Sato Y, Kanai S, Ohta M, Kitani K. Effect of protein-free diet on activities and subunits of glutathione S-transferase in livers of young and aged female rats. Mech Ageing Dev. 1990;56:237–251. doi: 10.1016/0047-6374(90)90085-T. [DOI] [PubMed] [Google Scholar]
- Demetrius L. Caloric restriction, metabolic rate, and entropy. J Gerontol Biol Sci. 2004;59A:902–915. doi: 10.1093/gerona/59.9.b902. [DOI] [PubMed] [Google Scholar]
- Dirks AJ, Leeuwenburgh CL. Calorie restriction in humans: Potential pitfalls and health concerns. Mech Ageing Dev. 2006;127:1–7. doi: 10.1016/j.mad.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Fujita S, Chiba M, Susuki M, Kitani K. Effect of senescence on the hepatic metabolism of drugs affecting the central nervous system in rats and mice. In: Kitani K, editor. Liver and Aging-1986, Liver and Brain. Amsterdam: Elsevier Science; 1986. pp. 115–126. [Google Scholar]
- Fujita S, Uesugi T, Kitagawa H, Suzuki T, Kitani K. Hepatic microsomal monooxygenase and azoreductase activities in aging Fischer-344 rats. Importance of sex difference for aging study. In: Kitani K, editor. Liver and Aging-1982, Liver and Drugs. Amsterdam, Oxford, New York: Elsevier/North-Holland Biomedical Press; 1982. pp. 55–71. [Google Scholar]
- Gallagher IM, Clow A, Glover V. Long term administration of (-)deprenyl increases mortality in male Wistar rats. J Neural Transm. 1998;52:315–320. doi: 10.1007/978-3-7091-6499-0_32. [DOI] [PubMed] [Google Scholar]
- Goto S, Takahashi R, Kumiyama A, Radak Z, Hayashi T, Takenouchi M, et al. Implications of protein degradation in aging. Ann NY Acad Sci. 2001;928:54–64. doi: 10.1111/j.1749-6632.2001.tb05635.x. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;12:257–263. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Harman D. Free-radical theory of aging. Increasing the functional life span. In: Zs.-Nagy I, Harman D, Kitani K, editors. Pharmacology of Aging Processes. Methods of Assessment and Potential Interventions. New York: Ann NY Acad Sci; 1986. pp. 1–15. [Google Scholar]
- Ikeno Y, Cortez L, Lew C, Qi W, Chaudhur A, Richardson A (2005) Cu/Zn SOD transgenic rats show reduced oxidative damage and increased survival. Abstract, 34th Annual Meeting of the American Aging Association, Oakland, P32
- Ishigami A, Goto S. Age-related change in the degradation rate of ovalbumin microinjected into mouse liver parenchymal cells. Arch Biochem Biophys. 1990;277:189–195. doi: 10.1016/0003-9861(90)90568-J. [DOI] [PubMed] [Google Scholar]
- Ivy GO, Kanai S, Ohta M, Ihara Y, Kitani K. Leupeptin causes an accumulation of lipofuscin-like substances and ubiquitin immunoreactivity in liver cells of young rats: a possible model for hepatocellular aging. In: Kitani K, editor. Liver and Aging-1990. Amsterdam: Elsevier Science; 1991a. pp. 109–122. [Google Scholar]
- Ivy GO, Kanai S, Ohta M, Sato Y, Otsubo K, Kitani K. Leupeptin causes an accumulation of lipofuscin-like substances in liver cells of young rats. Mech Ageing Dev. 1991b;57:213–231. doi: 10.1016/0047-6374(91)90048-5. [DOI] [PubMed] [Google Scholar]
- Ivy GO, Kanai S, Ohta M, Smith G, Sato Y, Kobayashi M, et al. Lipofuscin-like substances accumulate rapidly in brain, retina and internal organs with cysteine protease inhibition. In: Porta EA, et al., editors. Lipofuscin and ceroid pigments. New York: Plenum Press; 1990. pp. 31–47. [DOI] [PubMed] [Google Scholar]
- Ivy GO, Schottler F, Wenzel J, Baudry M, Lynch G. Inhibitors of lysosomal enzymes: accumulation of lipofuscin-like dense bodies in the brain. Science. 1984;226:985–987. doi: 10.1126/science.6505679. [DOI] [PubMed] [Google Scholar]
- Kamataki T, Maeda K, Shimada M, Kitani K, Nagai T, Kato R. Age-related alteration in the activities of drug-metabolizing enzymes and contents of sex-specific forms of cytochrome P-450 in liver microsomes from male and female rats. J Pharmacol Exp Ther. 1985;233:222–228. [PubMed] [Google Scholar]
- Kato R, Vassanelli P, Frontino G, Chiesara E. Variation in the activity of liver misrosomal drug-metabolizing enzymes in rats in relation to the age. Biochem Pharmacol. 1964;13:1037–1051. doi: 10.1016/0006-2952(64)90100-5. [DOI] [PubMed] [Google Scholar]
- Kitani K. Drugs and the ageing liver. Life Chem Rep. 1988;6:143–230. [Google Scholar]
- Kitani K. Aging of the liver: facts and theories. Arch Gerontol Geriatr. 1991a;12:133–154. doi: 10.1016/0167-4943(91)90024-K. [DOI] [PubMed] [Google Scholar]
- Kitani K. Lateral mobility of proteins and lipids of cell surface membranes during aging: do the data support “The Membrane Hypothesis of Aging”? Mech Ageing Dev. 1999;107:299–322. doi: 10.1016/S0047-6374(98)00139-0. [DOI] [PubMed] [Google Scholar]
- Kitani K (ed) (1978) Liver and Aging-1978. Elsevier/North-Holland Biomedical Press, Amsterdam, Oxford, New York
- Kitani K (ed) (1982) Liver and Aging-1982. Liver and Drugs, Elsevier Biochemical Press, Amsterdam, Oxford, New York
- Kitani K (ed) (1986) Liver and Aging-1986. Liver and Brain, Elsevier Science Publishers, B.V., Amsterdam, Oxford, New York
- Kitani K (ed.) (1991b) Liver and Aging-1990. Elsevier Science Publishers, B.V., Amsterdam, Oxford, New York
- Kitani K. Nutritional interventions in aging and age-associated disorders. In: Rattan SIS, editor. Aging Interventions and Therapies. Singapore: World Scientific Publishers; 2005. pp. 219–245. [Google Scholar]
- Kitani K, Goto S. Interview, "My involvement in aging research was just a series of coincidences". An interview with Kenichi Kitani. Bipogerontology. 2005;6:211–221. doi: 10.1007/s10522-005-7949-2. [DOI] [PubMed] [Google Scholar]
- Kitani K, Osawa T (2005) Tetrahydrocurcumin (TC) feeding increases the life span of genetically contaminated C57BL male mice. Abtract, 34th Annual Meeting of the American Aging association, Oakland, USA, p34
- Kitani K, Yokozawa T (2003) Green tea polyphenol (sunphenon) prolongs the average life span of male C57/BL mice. Abstract, 32nd Annual Meeting of American Aging Association, P8
- Kitani K, Kanai S, Miura R, Morita Y, Kasahara M. The effect of aging on the biliary excretion of ouabain in the rat. Exp Gerontol. 1978;13:9–17. doi: 10.1016/0531-5565(78)90024-4. [DOI] [PubMed] [Google Scholar]
- Kitani K, Kanai S, Miyasaka K, Carrillo MC, Ivy GO. Dose-dependency of life span prolongation of F344/DuCrj rats injected with (-)deprenyl. Biogerontology. 2005;6:1–6. doi: 10.1007/s10522-005-4804-4. [DOI] [PubMed] [Google Scholar]
- Kitani K, Kanai S, Miyasaka K, Carrillo MC, Ivy GO. The necessity of having a proper dose of (-)deprenyl (D) to prolong the life spans of rats explains discrepancies among different studies in the past. Ann New York Acad Sci. 2006;1067:375–382. doi: 10.1196/annals.1354.053. [DOI] [PubMed] [Google Scholar]
- Kitani K, Kanai S, Sato Y, Ohta M, Ivy GO, Carrillo MC. Chronic treatment of (-)deprenyl prolongs the life span of male Fischer 344 rats. Further evidence. Life Sci. 1993;52:281–288. doi: 10.1016/0024-3205(93)90219-S. [DOI] [PubMed] [Google Scholar]
- Kitani K, Klotz U, Kanai S, Sato Y, Ohta M, Nokubo M. Age-related differences in the coordination disturbance and anticonvulsant effect of oxazepam in mice. Arch Gerontol Geriatr. 1989;9:31–43. doi: 10.1016/0167-4943(89)90022-8. [DOI] [PubMed] [Google Scholar]
- Kitani K, Masuda Y, Sato Y, Kanai S, Ohta M, Nokubo M. Increased anticonvulsant effect of phenytoin in aging BDF1 mice. J Pharmacol Exp Ther. 1984;229:231–236. [PubMed] [Google Scholar]
- Kitani K, Minami C, Isobe K, Maehara K, Kanai S, Ivy GO, et al. Why(-)deprenyl prolongs survivals of experimental animals: Increase of anti-oxidant enzymes in brain and other body tissues as well as mobilization of various humoral factors may lead to systemic anti aging effects. Mech Ageing Dev. 2002a;123:1087–1100. doi: 10.1016/S0047-6374(01)00392-X. [DOI] [PubMed] [Google Scholar]
- Kitani K, Minami C, Yamamoto T, Kanai S, Ivy GO, Carrillo MC. Pharmacological interventions in aging and age-associated disorders. Potentials of propargylamines for human use. Ann New York Acad Sci. 2002b;959:295–307. doi: 10.1111/j.1749-6632.2002.tb02101.x. [DOI] [PubMed] [Google Scholar]
- Kitani K, Ohta M, Kanai S, Sato Y, Nokubo M, Zs.-Nagy I. Age-induced restricted mobility of proteins in hepatocyte surface membranes: A possible determinant of membrane transport function. In: Kitani K, editor. Liver and Aging-1990. Amsterdam: Elsevier Science; 1991. pp. 305–318. [Google Scholar]
- Kitani K, Sato Y, Kanai S, Nokubo M, Ohta M, Masuda Y. Age related increased threshold for electroshock seizure in BDF1 mice. Life Sci. 1985a;36:657–662. doi: 10.1016/0024-3205(85)90170-5. [DOI] [PubMed] [Google Scholar]
- Kitani K, Sato Y, Kanai S, Nokubo M, Ohta M, Masuda Y. Increased anticonvulsant effect of phenobarbital with age in mice - A possible pharmacological index for brain aging. Life Sci. 1985b;37:1451–1460. doi: 10.1016/0024-3205(85)90085-2. [DOI] [PubMed] [Google Scholar]
- Kitani K, Sato Y, Kanai M, Nokubo M, Ohta M, Masuda Y. Increasing anticonvulsant effect of AD-810 (Zonisamide) in aging BDF1 mice. Life Sci. 1987;41:1339–1344. doi: 10.1016/0024-3205(87)90607-2. [DOI] [PubMed] [Google Scholar]
- Kitani K., Zs.-Nagy I., Kanai S., Sato Y., Ohta M. Correlation between the biliary excretion of ouabain and the lateral mobility of hepatocyte plasma membrane proteins in the rat - The effects of age and spironolactone pretreatment. Hepatology. 1988;8:125–131. doi: 10.1002/hep.1840080124. [DOI] [PubMed] [Google Scholar]
- Knoll J. The striatal dopamine dependency of life span in male rats: longevity study with (-)deprenyl. Mech Ageing Dev. 1988;46:237–262. doi: 10.1016/0047-6374(88)90128-5. [DOI] [PubMed] [Google Scholar]
- Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33(4):276–285. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- Martin GM. The Werner mutation: does it lead to a “public” or “private” mechanism of aging? Mol Med. 1997;3:356–358. [PMC free article] [PubMed] [Google Scholar]
- Milgram NW, Racine RJ, Nellis P, Mendonca A, Ivy GO. Maintenance of L-deprenyl prolongs life in aged male rats. Life Sci. 1990;47:415–420. doi: 10.1016/0024-3205(90)90299-7. [DOI] [PubMed] [Google Scholar]
- Ohta M, Kanai S, Sato Y, Kitani K. Age-dependent decrease in the hepatic uptake and biliary excretion of ouabain in rats. Biochem Pharmacol. 1988;37:935–942. doi: 10.1016/0006-2952(88)90184-0. [DOI] [PubMed] [Google Scholar]
- Ohta M, Kitani K. Age-dependent decrease in the hepatic uptake of taurocholic acid resembles that for ouabain. Biochem Pharmacol. 1990;39:1223–1228. doi: 10.1016/0006-2952(90)90266-N. [DOI] [PubMed] [Google Scholar]
- Rao G, Xia E, Richardson A. Effect of age on the expression of antioxidant enzymes in male Fischer 344 rats. Mech Ageing Dev. 1990;53:49–60. doi: 10.1016/0047-6374(90)90033-C. [DOI] [PubMed] [Google Scholar]
- Reiss U, Gershon D. Methionine sulfoxide reductase: A novel protective enzyme in liver and its potentially significant role in aging. In: Kitani K, editor. Liver and Aging-1978. Amsterdam: Elsevier North-Holland; 1978. pp. 55–61. [Google Scholar]
- Rikans LE, Snowden CD, Moore DR. Sex-dependent differences in the effect of aging on antioxidant defense mechanisms of rat liver. Biochim Biophys Acta. 1991;1074:195–200. doi: 10.1016/0304-4165(91)90061-k. [DOI] [PubMed] [Google Scholar]
- Rodeheffer RJ, Gerstenblith G, Becker LC, Fleg JL, Weisfeidt ML, Lakatta EG. Exercise cardiac output is maintained with advancing age in healthy human subjects: cardiac dilatation and increased stroke volume compensate for a dimished heart rate. Circulation. 1984;69:203–213. doi: 10.1161/01.cir.69.2.203. [DOI] [PubMed] [Google Scholar]
- Rowe JW, Andres R, Tobin JD, Norris AH, Shock NW. The effect of age on creatinine clearance in men: a cross-sectional and longitudinal study. J Gerontol. 1976;31(2):155–163. doi: 10.1093/geronj/31.2.155. [DOI] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Semsei I, Rao G, Richardson A. Expression of superoxide dismutase and catalase in rat brain as a function of age. Mech Ageing Dev. 1991;58:13–19. doi: 10.1016/0047-6374(91)90116-H. [DOI] [PubMed] [Google Scholar]
- Sonne J, Dossing M, Loft S, Andreasen PB. Antipyrine clearance in pneumonia. Clin Pharmacol Ther. 1985;37(6):701–704. doi: 10.1038/clpt.1985.117. [DOI] [PubMed] [Google Scholar]
- Williams LR, Carter DB, Dunn E, Conner JR. Indicators of oxidative stress in aged Fischer 344 rats: potential for neurotrophic treatment. In: Hannin I, Fischer A, Yoshida M, editors. Alzheimer’s and Parkinson’s Disease: Recent Advances. New York: Plenum Pub Corp; 1995. pp. 641–646. [Google Scholar]
- Wulf J, Cutler RG. Altered protein hypothesis of mammalian aging process I. Thermal stability of glucose-6-phosphate dehydroenase in C57BL/6J mouse tissues. Exp Gerontol. 1975;10:101–117. doi: 10.1016/0531-5565(75)90040-6. [DOI] [PubMed] [Google Scholar]
- Wynne HA, Cope LH, Herd B, Rawlins MD, James OF, Woodhouse KW. The association of age and frailty with paracetamol conjugation in man. Age Ageing. 1990;19:419–424. doi: 10.1093/ageing/19.6.419. [DOI] [PubMed] [Google Scholar]
- Zs.-Nagy I. The horizons of interdisciplinary synthesis in experimental gerontology. A review. Arch Gerontol Geriatr. 1991;12:329–349. doi: 10.1016/0167-4943(91)90038-R. [DOI] [PubMed] [Google Scholar]
- Zs.-Nagy I. The membrane hypothesis of aging. Boca Raton, F.L: C.R.C, Press; 1994. [Google Scholar]
- Zs.-Nagy I. Enzyme activities in the light of membrane hypothesis of aging [Answer to K. Kitani, Mech Ageing Dev. 107 (1999) 299–322] Mech Ageing Dev. 2001;122:811–821. doi: 10.1016/S0047-6374(01)00237-8. [DOI] [PubMed] [Google Scholar]
- Zs.-Nagy I., Cutler R.G., Kitani K., Ohta M. Comparison of the lateral diffusion constant of hepatocyte membrane proteins in two wild mouse strains of considerably different longevity: FRAP studies on liver smears. J Gerontol Biological Sci. 1993;48:B86–B92. doi: 10.1093/geronj/48.3.b86. [DOI] [PubMed] [Google Scholar]
- Zs.-Nagy I., Kitani K., Ohta M., Imahori K. Age-dependent decrease of the lateral diffusion constant of proteins in the plasma membrane of hepatocytes as revealed by fluorescence recovery after photo bleaching in tissue smears. Arch Gerontol Geriatr. 1986;5:131–146. doi: 10.1016/0167-4943(86)90016-6. [DOI] [PubMed] [Google Scholar]
- Zs.-Nagy I., Ohta M., Kitani K., Imahori K. An automated method for measuring lateral mobility of proteins in the plasma membrane of cells. Microskopie. 1984;41:12–25. [Google Scholar]
- Zs.-Nagy I., Tanaka S., Kitani K. Age-dependence of the lateral diffusion coefficient of Con-A-receptor protein in the skeletal muscle membrane of C57BL/6J mice. Mech Ageing Dev. 1998;101:257–268. doi: 10.1016/S0047-6374(97)00179-6. [DOI] [PubMed] [Google Scholar]
- Zs.-Nagy I., Tanaka S., Kitani K. Age-dependence of the lateral diffusion coefficient of Con-A-receptor protein in the plasma membrane of ex vivo prepared brain cortical cells of BN/BiRijHsd rats. Exp Brain Res. 1999;124:233–240. doi: 10.1007/s002210050618. [DOI] [PubMed] [Google Scholar]