Abstract
Experimentally restricting dietary calories, while maintaining adequate dietary nutrient content, extends lifespan in phylogenetically diverse species; thus suggesting the existence of conserved pathways which can modify lifespan in response to energy intake. However, in some cases the impact on longevity may depend on the quality of the energy source. In Drosophila, restriction of dietary yeast yields considerable lifespan extension whereas isocaloric restriction of dietary sugar yields only modest extension, indicating that other diet-responsive pathways can modify lifespan in this species. In rodents, restricting intake of a single amino acid – methionine – extends lifespan. Here we show that dietary methionine can modify lifespan in adult female, non-virgin Oregon-R strain Drosophila fed a chemically defined media. Compared to a diet containing 0.135% methionine and 15% glucose, high dietary methionine (0.405%) shortened maximum lifespan by 2.33% from 86 to 84 days and mean lifespan by 9.55% from 71.7 to 64.9 days. Further restriction of methionine to 0.045% did not extend maximum lifespan and shortened mean lifespan by 1.95% from 71.1 to 70.3 days. Restricting glucose from 15% to 5% while holding methionine at a concentration of 0.135%, modestly extended maximum lifespan by 5.8% from 86 to 91 days, without extending mean lifespan. All these diet-induced changes were highly significant (log-rank p < 0.0001). Notably, all four diets resulted in considerably longer life spans than those typically reported for flies fed conventional yeast and sugar based diets. Such defined diets can be used to identify lifespan-modifying pathways and specific gene-nutrient interactions in Drosophila.
Key words: aging, amino acid, caloric restriction, demography, dietary restriction, Drosophila, longevity, methionine, mortality, nutrition
Introduction
“Dietary restriction”, the experimental restriction of food and nutrient intake compared with ad libitum feeding, is a reliable means of extending lifespan in model organisms (Weindruch and Walford 1988). The repeated observation that dietary restriction retards aging in phylogenetically diverse species ranging from yeast to primates is the cornerstone of a fertile working hypothesis that diet regulates lifespan and aging through a universal mechanism that has been conserved throughout evolution. Commonalities between life-extending mutations in several species suggest a plausible account of how genes and diet might regulate growth and aging by converging on energy and nutrient-responsive pathways. Dietary restriction has not yet been demonstrated to act on a common pathway across different species (Houthoofd et al. 2003; Walker et al. 2005). However, in Drosophila specifically, candidates for the downstream effects of dietary restriction include the insulin-like growth factor 1 (IGF-1) signaling pathway (Sohal and Weindruch 1996; Gems and Partridge 2001; Britton et al. 2002; Clancy et al. 2002; Longo and Finch 2003), and the amino acid sensitive, nutrient-sensing, target of rapamycin (TOR) signaling pathway (Kapahi et al. 2004; Avruch et al. 2005).
The term “dietary restriction” is often used interchangeably with “caloric restriction”, implying that lifespan extension results specifically from reduction of the dietary energy content regardless of the source of the calories. This view, which emphasizes calorie quantity rather than quality, is based largely on the interpretation of studies in rats where isocaloric diets containing different macronutrient content resulted in similar extensions of lifespan (Masoro 1990). However, the evidence for this phenomenon is not unequivocal (Kritchevsky et al. 1984; Murtagh-Mark et al. 1995) and it may not be universally generalized across different species (Mair et al. 2005), not least because different nutrients have effects which are independent of energy metabolism (Iwasaki et al. 1988a, 1988b; Ni et al. 1998).
Distinguishing between the effects of specific nutrients independently of energy intake is technically challenging. For example, Drosophila are typically fed a mixture of yeast, sugar, cornstarch and other ingredients, dissolved in an agar gel. This conventional formulation limits the ability to control the dietary content of individual macro- and micronutrients. Moreover, it is difficult to determine how much food the flies actually consume. Nevertheless, early studies of dietary restriction and aging in Drosophila achieved dramatic lifespan extension. Diluting the concentration of both yeast and sugar from 15% to 5% in media that were fed to non-virgin female Dahomey strain flies achieved an 82% increase in mean lifespan from 25.4 to 46.2 days and a 66% increase in maximum lifespan from 47 to 78 days (Pletcher et al. 2002). Initially this finding was attributed to the restriction of dietary energy intake (caloric restriction). Recently however, yeast restriction, rather than either sugar or total calorie restriction has been shown to account for lifespan extension in Drosophila (Mair et al. 2005). The extension of lifespan through dietary protein restriction points to amino acid content as a key determinant (Min and Tatar 2006a); however, the factors in yeast which exert this profound effect on lifespan and the metabolic pathways through which they act have yet to be identified (Piper et al. 2005). The present study was designed to determine whether dietary methionine is one such factor.
Several lines of evidence implicate methionine intake and metabolism in modulating lifespan. The strongest evidence comes from studies in rats and mice in which amino acids are fed in lieu of protein, where restriction of dietary methionine alone can extend maximum lifespan by 10–45% (Orentreich et al. 1993; Richie et al. 1994; Zimmerman et al. 2003; Miller et al. 2005). Methionine metabolism is abnormal in long-lived mutant mice that are deficient in growth hormone, prolactin and thyroid stimulating hormone (Uthus and Brown-Borg 2003; Brown-Borg et al. 2005; Uthus and Brown-Borg 2006). Furthermore, methionine restriction specifically inhibits the induction of IGF-I expression by growth hormone in pig hepatocytes (Stubbs et al. 2002), indicating a potential regulatory interaction of methionine metabolism on the growth hormone—IGF-I signaling pathway. It is also conceivable that methionine restriction could impact on the amino acid (leucine-sensitive) TOR pathway, if only through indirect effects on branched chain amino acid metabolism (Long et al. 2005; Kimball and Jefferson 2006). Thus, it is theoretically possible that methionine intake and metabolism in mammals might interact with well established pathways to extend lifespan in response to caloric restriction.
A capacity of methionine to modify lifespan would be consistent with the observation that mammals are exquisitely sensitive to dietary methionine intake. Methionine is an essential sulfur amino acid and inadequate dietary methionine intake will slow the growth of young mammals. However, excess intake of methionine is highly toxic to both young and adult mammals, and this toxicity far exceeds that produced by the excessive intake of any other amino acid (Harper et al. 1970). Merely doubling the normal methionine intake has been shown to result in a variety of adverse outcomes including growth retardation (Benevenga et al. 1976), anemia (Yokota et al. 1978), vascular damage (Troen et al. 2003), kidney damage and specific hypertrophy of the tubules (Kumagai et al. 2002), changes in acinar pancreatic cells and iron accumulation in liver and spleen (Ekperigin and Vohra 1981). Similar changes in liver, kidney and spleen have also been observed in human patients with methioninemia (Goldfischer et al. 1981). Some of these harmful effects have been attributed to methionine’s product—homocysteine. However, there is considerable evidence (Harper et al. 1970) including a recent study in our laboratory (Troen et al. 2003), suggesting that at least some of these toxic effects are not mediated by homocysteine but are the direct result of an imbalance in methionine metabolism. It is significant in this regard that homocysteine levels increase with age (Selhub et al. 1993) along with altered activity of some enzymes in the methionine pathway (Stramentinoli et al. 1977; Finkelstein and Benevenga 1984), indicating an important interaction between aging and altered methionine metabolism (Russo et al. 2003).
This sensitivity to methionine may result from impairment of one or more aspects of eukaryotic methionine metabolism. These include the synthesis of polyamines, cysteine and glutathione, and the methylation of DNA, lipid, hormones and enzyme substrates (Finkelstein 1990). Moreover, some sulfur containing intermediates of methionine metabolism such as homocysteine are reactive and may be toxic. Finally, the regeneration of methionine from homocysteine is intimately linked to activity of the folate pathway that synthesizes thymidine and purines (Selhub 2002). Healthy aging could be compromised by the effect of suboptimal or excessive methionine intake on any of these pathways.
In light of the above, we hypothesized that if methionine modifies lifespan through a phylogenetically conserved pathway, then methionine restriction should prolong life not only in rodents but also in Drosophila. Furthermore, comparing the effects of sugar restriction and methionine restriction might provide insight into generalizable pathways that modify longevity across species. To test this hypothesis we developed a chemically defined medium containing purified amino acids, carbohydrates, vitamins, minerals and other essential nutrients. This diet enabled us to vary dietary glucose or methionine content while holding all other nutrients constant so as to determine the effect of dietary methionine or glucose restriction on lifespan in non-virgin female adult Oregon-R strain of Drosophila melanogaster.
Methods
Diets
Coordinately decreasing dietary yeast and sugar concentrations from 15% to 5% in conventional fly medium has been shown to extend mean lifespan by 82% in adult, non-virgin female, Dahomey strain Drosophila (Pletcher et al. 2002). Using this diet as a reference, we adapted the chemically defined diet described by Hinton et al. (1951) to study the separate effects of dietary methionine and sugar on life-span. This allowed us to vary the methionine or sugar concentration in the medium while maintaining constant concentrations of all other nutrients (Table 1). We compared life-span and survival in flies fed chemically defined media containing either:
15% glucose and 0.135% methionine; corresponding to the 15% yeast-sugar control diet used by Pletcher et al. (Pletcher et al. 2002)
5% glucose and 0.135% methionine; in which calories from sugar were restricted to 1/3 the content of the reference diet without restricting methionine
15% glucose and 0.045% methionine; in which methionine was restricted to 1/3 the content of the reference diet without restricting calories
15% glucose and 0.405% methionine; in which methionine was increased by three times the content of the reference diet without restricting calories
Table 1.
Nutrient content of chemically defined medium.
| Diet | Units per liter |
|---|---|
| Reference | 1.35 g L-methionine/150 g glucose |
| Sugar restricted | 1.35 g L-methionine/50 g glucose |
| High methionine | 4.05 g L-methionine/150 g glucose |
| Low methionine | 0.45 g L-methionine/150 g glucose |
| Other nutrients (constant for all media) | |
| L-aspartic acida | 2.5 g |
| L-glutamic acida | 5.6 g |
| L-serinea | 2.3 g |
| Glycinea | 1.0 g |
| L-histidine HCl-H2Oa | 1.1 g |
| L-arginine HCla | 3.9 g |
| L-threoninea | 2.1 g |
| L-alaninea | 2.6 g |
| L-prolinea | 2.1 g |
| L-tyrosinea | 1.9 g |
| L-valinea | 3.0 g |
| L-cystinea | 1.0 g |
| L-isoleucinea | 1.9 g |
| L-leucinea | 3.1 g |
| L-phenylalaninea | 2.2 g |
| L-lysine HCla | 6.5 g |
| L-tryptophanb | 17.4 g |
| Vitamin B12b | 18.8 μg |
| Biotinc | 0.015 μg |
| P-aminobenzoic acid (PABA)c | 2 mg |
| Inositolc | 42 mg |
| Niacinc | 10 mg |
| Pantothenic acidc | 6 mg |
| Folic acidc | 6 mg |
| Pyridoxine HClc | 3 mg |
| Riboflavinc | 2.4 mg |
| Thiamin HClc | 1.5 mg |
| Cholineb | 16 mg |
| Vitamin Ad | 1350 IU |
| Vitamin Ed | 16.5 IU |
| Vitamin Dd | 335 IU |
| Vitamin K (MSBC)d | 0.5 mg |
| Zince | 11.284 mg |
| Coppere | 4.675 mg |
| Chromiume | 0.5616 mg |
| Potassiumc | 446.1372 mg |
| Phosphorusc | 245.6724 mg |
| Calciumc | 4.6569 mg |
| Chloridec | 16.0476 mg |
| Ironc | 2.58 mg |
| Magnesiumc | 24.2556 mg |
| Manganesec | 3.185 mg |
| Sodiumc | 5.0826 mg |
| Cholesterolc | 10 mg |
| Lecithinc,f | 0.1 g |
| Ribonucleic acid, gc,f | 1.0 g |
a Based on empirical determination of amino acid content in 15% yeast/sugar medium. Analysis conducted by Nutritional & Environmental Analytical Services Laboratory, Cornell University (Ithaca, NY).
b After Hinton et al. (1951) Medium 4.
c After Villee and Bissell (1948).
d Requirements for Drosophila uncertain. Added at 1/3 concentration recommended in American Institute of Nutrition guidelines for rodent diets (AIN-93M).
e Requirements for Drosophila uncertain. Calculated from theoretical nutrient content in 15 grams Brewers yeast.
f Diet does not contain Iodine, Selenium and other trace minerals present in mammalian diets
Diet b where dietary glucose is reduced from 15% to 5% significantly restricts dietary calories. In contrast, the caloric difference between diets a, c and d that range from 0.045% to 0.405% methionine is negligible.
We composed the media to approximate amino acid concentrations corresponding to a 15% yeast sugar diet. Amino acid concentrations in a 15% yeast sugar diet were determined by HPLC at the Nutritional & Environmental Analytical Services Laboratory at Cornell University (Ithaca, NY), except for methionine and tryptophan, which could not be reliably measured. The theoretical methionine content of the reference diet was derived from the typical methionine content of dried Brewer’s yeast (150 g yeast/liter medium × 0.9% methionine/1 g yeast = 1.35 g methionine/liter medium = 0.135% methionine. Genuine Grain Grown Brewer’s Yeast; Twin Lab, Hauppauge, NY). Tryptophan concentrations were chosen according to Hinton et al. (1951).
Since the specific vitamin, mineral and other nutrient intakes that are required for maximal longevity in adult Drosophila are largely unknown, we derived the medium nutrient concentrations from requirements for optimal larval development for those nutrients for which data are available (Villee and Bissell 1948; Hinton et al. 1951; Sang 1955). Where nutrient requirements are uncertain, amounts in the defined medium correspond to the theoretical content of the 15% yeast-sugar reference diet. A basal diet mix was prepared by Harlan Teklad according to our specifications (Diet TD.04310, Harlan Teklad, Madison, WI). Media were prepared by boiling 62.08 g basal mix, 500 mg nucleic acids, 100 mg lecithin, 20 g agar and the appropriate amounts of L-methionine and glucose in 1 liter water. Propionic and phosphoric acids were added to inhibit microbial growth at final concentrations of 0.285% and 0.0255% (v/v), respectively. The complete nutritional content of these media is given in Table 1.
Fly husbandry
Flies of the Oregon-R Drosophila strain were used for this study. We studied longevity in non-virgin female flies because of known sex-related influences on lifespan in Drosophila (Magwere et al. 2004). Cohorts were established by allowing flies of the same ages to mate at a density of 10 males and 20 females per bottle. Flies were provided with a conventional yeast based food and removed after three days, leaving their eggs to develop into adult flies. Newly emerged flies were transferred to fresh bottles with conventional food and allowed to mate for 1–2 days. Three-day-old mated female flies were collected over light CO2 and systematically randomized into standard 50 mL vials containing the four chemically defined diets at a starting density of 40 flies per vial. Flies were maintained on a 12:12 hr light:dark cycle at 25°C and 68% humidity. Food was replaced every Monday, Wednesday and Friday. Survival was determined by recording dead and censored flies before replacing food. Initial cohort sizes were calculated from the sum of censored and dead flies observed at all ages. Three cohorts were established by this method (Table 2). In addition, flies were sampled at ages 10, 31, 50, and 75 days. Sampled flies were collected under light CO2 anesthesia, immediately frozen in liquid nitrogen and stored at −80°C or fixed in 4% phosphate buffered formalin for future analysis.
Table 2.
Survivorship demographic parameters.
| Medium | No. deaths | Lifespan (days) | ||||||
|---|---|---|---|---|---|---|---|---|
| Maximum | % Change | Mean ± SE | % Change | Percentiles ± SE | ||||
| 25th | Median | 75th | ||||||
| 5% Glu : 0.135% Met | 2,300 | 91 | +5.81% | 71.91 ± 0.29a | 0% | 82 ± 0.08a | 75 ± 0.17a | 68 ± 0.41a |
| 15% Glu : 0.135% Met | 1,686 | 86 | Reference | 71.72 ± 0.26a | Reference | 79 ± 0.16b | 75 ± 0.20a | 68 ± 0.28a |
| 15% Glu : 0.045% Met | 2,217 | 86 | 0% | 70.32 ± 0.27b | −1.95% | 77 ± 0.19c | 72 ± 0.20b | 68 ± 0.28a |
| 15% Glu : 0.405% Met | 1,746 | 84 | −2.33% | 64.87 ± 0.27c | −9.55% | 72 ± 0.19d | 68 ± 0.22c | 61 ± 0.34b |
Table gives total number of observed deaths and demographic values pooled across three replicate experiments. Different superscripts in each column for mean and percentile values indicate significant differences between diets.
Food intake assessment
Food intake was assessed according to Min and Tatar (2006b). Briefly, flies were habituated to their assigned experimental diet for 6 days, after which they were provided with fresh food containing 0.5% food coloring FD&C Blue No.1. Twenty four hours later, 140–160 flies per diet were collected and homogenized 20 flies at a time in 1 mL PBS. After centrifugation, absorbance of the supernatant at 625 nm (OD625nm) provides an estimate of the amount of ingested dye. Intake of a conventional yeast based diet was also assessed at the same time.
Life span and mortality analysis
Analyses were performed on pooled data across the three cohorts for each of the four diets. Survivorship was compared and tested for significance with log-rank tests using SPSS 12.0 statistical software. Survivorship is a cumulative function where differences between diets at any age are carried forward to subsequent age intervals. In some cases, this can inflate the effects of log-rank tests. In contrast, mortality rates estimate the age-specific risk of death. For this reason, we examined the effect of diet on age-specific mortality rates and parameters. We used the freely available WinModest Demographic Analysis Tool, version 1.0.2 (Pletcher et al. 2000), to fit the observed data to a class of mathematical models that assume an exponential rise in the mortality rate with increasing age, while allowing for age-independent and senescent changes in mortality rates. The program determines the model that best fits the observed data using a maximum likelihood procedure and facilitates hypothesis testing of whether the fitted models differ by experimental treatment (Pletcher 1999; Pletcher et al. 2000). Mortality models fitted with the WinModest program established that the Gompertz-Makeham function provided the best fit model for all diets. In this model, mortality at age x (μx) is given as 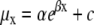 , where α is the baseline mortality rate (intercept), β is the age-dependent increase in mortality (slope) and c is the age-independent mortality constant (Table 3).
, where α is the baseline mortality rate (intercept), β is the age-dependent increase in mortality (slope) and c is the age-independent mortality constant (Table 3).
Table 3.
Mortality parameters derived from maximum likelihood estimated best-fit models.
| Medium | Gompertz-Makeham mortality parameters | ||||||
|---|---|---|---|---|---|---|---|
| α | 95% CI | β | 95% CI | c | 95% CI | Likelihood | |
| 5% Glu : 0.135% Met | 1.10 (10−6) | [6.83 (10−7), 1.79 (10−6)] | 0.150 | [0.144, 0.157] | 0.00126 | [0.00106, 0.00151] | −8816.42 |
| 15% Glu : 0.135% Met | 3.21 (10−7) | [1.88 (10−7), 5.49 (10−7)] | 0.173 | [0.166, 0.180] | 0.00075 | [0.00058, 0.00096] | −6065.03 |
| 15% Glu : 0.045% Met | 8.99 (10−7) | [5.83 (10−7), 1.39 10−6)] | 0.159 | [0.153, 0.165] | 0.00094 | [0.00077, 0.00115] | −8231.28 |
| 15% Glu : 0.405% Met | 2.54 (10−6) | [1.69 (10−6), 3.83 (10−6)] | 0.159 | [0.153, 0.165] | 0.00081 | [0.00064, 0.00104] | −6278.36 |
Mortality parameters for the best-fit Gompertz-Makeham models, where mortality at age x (μx) is given as  , where α is the baseline mortality rate (intercept), β is the age-dependent increase in mortality (slope) and c is the age-independent mortality.
, where α is the baseline mortality rate (intercept), β is the age-dependent increase in mortality (slope) and c is the age-independent mortality.
Results
All of the chemically defined diets were well tolerated and did not adversely affect lifespan as described below. Estimated food intake was similar for flies fed the reference and low methionine diets (OD625nm = 0.06 (0.01) and 0.07 (0.01), respectively. Values are given as mean (SD)). In comparison to flies fed the reference and low methionine diets, flies fed the high methionine and sugar restricted diets had increased intake (OD625nm = 0.10 (0.01) and 0.11 (0.03) , respectively. p<0.05 by one-way analysis of variance after Bonferroni adjustment for multiple comparisons); however, the intakes of the high methionine and sugar restricted flies were not different from each other. Estimated intake of the conventional yeast diet (OD625nm = 0.11 (0.03)) was similar to intake of the high methionine and sugar restricted diets and significantly higher than intake of the reference and low methionine diets.
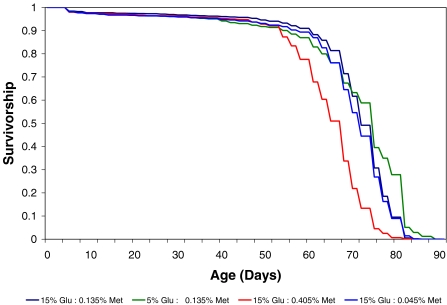
Dietary methionine and sugar concentrations in chemically defined media independently modified longevity in non-virgin female adult Oregon-R strain Drosophila melanogaster flies. Survivorship curves in Figure 1 plot the proportion of living flies for each diet as a function of age. Survivorship curves for each diet differed significantly from each other by log-rank test (statistic = 1162.74, p < 0.0001). Maximum, median, top (25%) and bottom (75%) lifespan quartiles for each diet are given in Table 2.
Figure 1.
Cumulative survival distributions by diet. The survivorship curves show the proportion of living flies for each diet as a function of age. The median, top and bottom quartile life spans are the ages at which the curves intersect with 50, 25 and 75 percent survivorship, respectively. Survivorship curves for each diet were significantly different from the other diets by log-rank test (statistic = 1162.74, p < 0.0001)
Sugar restriction extended lifespan only among older flies, with maximum lifespan extended by 5.8% from 86 to 91 days compared to flies fed the reference diet. Sugar restriction extended lifespan for flies in the top quartile by 3.8% but failed to extend the mean, median and bottom quartile lifespan (Table 2).
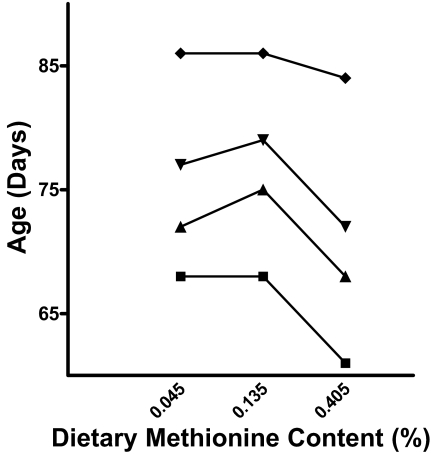
Dietary methionine was related to lifespan by an inverse U-shaped curve (Figure 2). The reference concentration of 0.135% methionine yielded the longest lived flies. Restricting methionine intake to one third this amount (0.045%) decreased mean lifespan by 1.95%, top quartile lifespan by 2.53% and median lifespan by 4.0%, with no effect on the bottom quartile. Increasing methionine intake to three times this amount (0.405%) was more harmful, limiting maximal lifespan by 2.33% compared to the reference diet and curtailing longevity across all ages. High methionine decreased maximum lifespan by only 2.33%, however it decreased mean lifespan by 9.55% from 71.72 to 64.87 days, compared to flies fed the reference diet. Furthermore, high methionine decreased lifespan by 8.86% for flies in the top quartile, 9.33% for flies with median lifespan and by 10.29% for flies in the bottom quartile. All changes were statistically significant (Table 2).
Figure 2.
Effect of dietary methoinine concentrations on lifespan parameters. Age of maximum (♦), top quartile (▾), median (▴) and bottom quartile (▪) lifespan showing an inverse U-shaped relationship to dietary methionine concentration
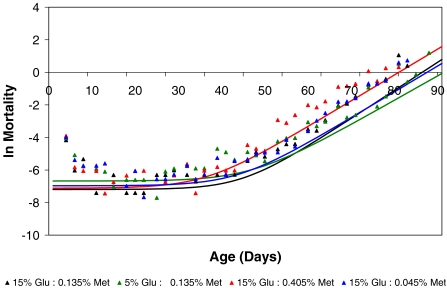
Observed mortality rates were modified by changing dietary sugar and methionine concentrations (estimated as –ln(px) where px is the proportion alive at age x (Figure 3). The estimated Gompertz-Makeham mortality parameters for the reference diet were significantly different from the parameters for each of the other experimental diets (Table 3). Although the sugar restricted diet had a higher baseline mortality rate than the reference diet (α=1.10 × 10−6 vs. 3.21 × 10−7), the rate of increase in mortality with age (β) was significantly lower than for the reference diet (0.150 vs. 0.173) and the age-independent mortality constant was higher (c = 0.00126 vs. 0.00075), yielding a longer overall lifespan. Using these models, the projected reference and sugar restricted mortality curves intersect by age 65 days, when the mortality rate for the sugar restricted diet falls below that of the reference diet (Figure 3). Similarly, both the high-methionine and methionine-restricted diets have a lower slope than the reference diet (β = 0.159 for both high- and methionine-restricted diets). However, the age-independent risk parameters c are not significantly different from the reference diet, while the high-methionine diet has a much higher baseline mortality rate (α = 2.54 × 10−6) and the methionine-restricted diet has a slightly higher baseline mortality rate (α = 8.99 × 10−7) than the reference diet (Table 3). When these estimates are factored into the model, the projected mortality rate of the high methionine diet exceeds that of all other diets at as early as 40 days of age. In contrast, the projected mortality rate for the methionine-restricted diet only drops below the rate of the reference diet at an age greater than 80 days, which is beyond the lifespan of most flies (Figure 3).
Figure 3.
Observed and projected mortality rates by diet. Scatter plot of observed mortality rates –lnμx (triangles) and projected mortality curves (lines) as a function of age. The projected mortality curves are fitted according to the parameters derived from the Gompertz-Makeham models given in Table 3
Discussion
In this study we hypothesized that, as in rodents where selectively restricting methionine intake can extend lifespan (Orentreich et al. 1993; Richie et al. 1994; Zimmerman et al. 2003; Miller et al. 2005), the restriction of methionine might also extend lifespan in Drosophila. If true, this would help to explain the observed extension of lifespan in this species by selective yeast (Mair et al. 2005) and protein restriction (Min and Tatar 2006a).
We found that the lifespan of Drosophila can be modified by varying the dietary content of a single essential amino acid—methionine. However, of the three methionine concentrations that we used, the intermediate concentration of 0.135% (w/v) was optimal with regard to lifespan when all other amino acids and nutrients were provided at constant concentrations (Table 1). This concentration is the theoretical concentration of methionine in the conventional 15% yeast and sugar diet, which has been shown in mated female Dahomey strain flies to yield a considerably shorter lifespan than in flies fed a dilute 5% yeast and sugar diet (Pletcher et al. 2002) or yeast restricted diet (Mair et al. 2005). Selectively restricting methionine three-fold to 0.045% did not increase lifespan, as would be expected if methionine overfeeding was solely responsible for the lifespan extension produced by the dilute 5% diet. Increasing methionine three-fold above this concentration to 0.405%, decreased longevity, revealing a harmful effect of methionine overfeeding. Thus, even though methionine intake can modify lifespan in Drosophila, methionine restriction alone is unlikely to account for the lifespan extension that is achieved by selective yeast or protein restriction in other studies (Mair et al. 2005).
We also found that restricting calories solely by limiting sugar intake had only modest benefit for longevity. Our finding of a ∼6% maximal lifespan extension by sugar restriction is consistent with the modest ∼8% lifespan extension by sugar restriction when yeast is held constant (Mair et al. 2005). This finding lends support to the hypothesis that it is the quality of the macronutrient from which calories are derived rather than the quantity of restricted calories which may be essential for lifespan extension in Drosophila.
Differences in survivorship are not explained by changes in estimated food ingestion. Flies fed the reference and low methionine diets had comparable food ingestion, whereas flies fed the high methionine and sugar restricted diets ingested relatively more food. An increased ingestion of the sugar restricted diet did not abrogate the modest lifespan extending effect of sugar restriction. Similarly, increased ingestion of the high methionine diet would be expected to exacerbate any toxicity due to excess food intake and would be consistent with the observed shorter lifespan. Nevertheless, the colorimetric food ingestion assay is not strictly quantitative and is limited by potential confounders such as diet-related changes in fly growth and gut capacity, changes in feeding and gastrointestinal food transit rates. Until better assays become available, outcome measures in dietary studies in Drosophila will be more readily attributable to dietary nutrient density than to estimated food intake.
Demographic modeling has suggested that dietary restriction in Drosophila acts by lowering age independent mortality rather than by slowing the accumulation of senescent damage (Mair et al. 2003; Magwere et al. 2004; Partridge et al. 2005). In the present study, we did not observe such an effect. Compared with the reference diet sugar restriction, low methionine and high methionine all resulted in significantly increased baseline mortality and in decreased slope coefficients (Table 3). However, the net effect of these changes on mortality trajectories was minor (Figure 3), reflecting the modest effects of methionine and glucose on survivorship (Figure 1).
Notably, flies fed any of the defined media display remarkable longevity. Virgin, female, Oregon-R strain flies, fed conventional diets and raised under comparable conditions of temperature and humidity have a 50 day median and 75 day maximum lifespan (Yui et al. 2003). Although we did not compare defined and conventional diets directly, median lifespans of 68–75 days for flies fed the defined diets were 36–50% longer than expected for flies fed conventional diets, and maximum lifespans of 84–91 days for flies fed the defined diets were 12–21% longer than expected for flies fed conventional diets, regardless of methionine or sugar concentration. This observed longevity is particularly impressive because non-virgin females of the present study would be expected to have shorter lifespans than those reported previously for virgin females, due to the cost of reproduction (Chapman et al. 1998). The full impact of these defined media on longevity in relation to fecundity in both female and males remains to be explored (Good and Tatar 2001; Magwere et al. 2004).
The ratio of median to maximum lifespan in the present study was also high. The median lifespans reached by flies fed any diet were at least 75% of the maximal lifespan of 91 days obtained by flies fed the sugar-restricted diet. In other words, over half the flies lived up to 75% or more of the maximum observed lifespan, regardless of dietary methionine and sugar. This is a higher ratio than that which is typical of other dietary restriction studies. For example, the mean lifespan of control Dahomey flies reported by Mair et al. (2005) is only 47% of the maximum lifespan reached by the longest lived yeast and sugar restricted flies. Such extension of median lifespan towards maximum lifespan, which is sometimes referred to as “squaring” of the survivorship curve, indicates successful aging in which the onset of age-related morbidity is delayed and its duration is shortened (Figure 1). This effect is unlikely to be explained by a general restriction of food intake in response to the chemically defined media, since intakes of the shortest-lived high-methionine and longest-lived reference diets were equivalent to the intake of flies fed a conventional yeast-based diet.
This apparent quality of the defined media may explain why non-optimal methionine and glucose concentrations exerted only a modest effect on lifespan in this study. In many aging studies, the magnitude of obtainable lifespan extension is a function of the control group’s lifespan, where shorter-lived strains are more amenable to lifespan extension than those that are longer-lived (Orr et al. 2003). For example, 82% mean and 66% maximum lifespan extensions resulting from dietary restriction in Dahomey flies, were obtained in comparison to short mean and maximum lifespans of 25.4 and 47 days (Pletcher et al. 2002). Regression analysis of data from several Drosophila aging studies shows a linear relationship between control lifespan and experimental lifespan extension, predicting that lifespan extension would be limited to a 10% increase where control lifespans were 60 to 70 days (Orr et al. 2003). Hence, the fact that the reference group was long-lived in the present study may account for the relatively small differences in lifespan induced by modifying dietary methionine and sugar and suggests that the defined diets were close to optimal with respect to longevity. In other words, the overall nutritional balance of the defined diet might have mitigated the individual effects of sub-optimal methionine and sugar intake.
The difficulty of controlling the dietary content of individual macro and micronutrients in conventional yeast and sugar based fly food has impeded efforts to identify the factors responsible for lifespan extension by dietary restriction in Drosophila. These chemically defined media now make it possible to do so, and provide an essential starting point for studies of nutrition and aging in Drosophila. Further studies on the relation of specific nutrients to lifespan will help to identify those nutrients that can modify aging and to reveal the mechanisms by which dietary restriction operates.
Acknowledgements
This research was supported by the U.S. Department of Agriculture cooperative research agreement 58-1950-4-401 and by pilot grant DAX601 from the Jean Mayer USDA Human Nutrition Research Center on Aging. Thanks to Nathalie Dupin, Fabienne LeRoy, Susan Hiller Troen, and Christine Schueller for technical assistance and to Mary Roberts, Isabelle Draper and Robert Jackson for their advice throughout.
Abbreviations
- IGF-1
Insulin-like growth factor 1
- TOR
Target of rapamycin
Footnotes
An erratum to this article can be found online at 10.1007/s11357-010-9133-0
References
- Avruch J, Lin Y, Long X, Murthy S, Ortiz-Vega S. Recent advances in the regulation of the TOR pathway by insulin and nutrients. Curr Opin Clin Nutr Metab Care. 2005;8:67–72. doi: 10.1097/00075197-200501000-00010. [DOI] [PubMed] [Google Scholar]
- Benevenga NJ, Yeh MH, Lalich JJ. Growth depression and tissue reaction to the consumption of excess dietary methionine and S-methyl-L-cysteine. J Nutr. 1976;106:1714–1720. doi: 10.1093/jn/106.12.1715. [DOI] [PubMed] [Google Scholar]
- Britton JS, Lockwood WK, Li L, Cohen SM, Edgar BA. Drosophila’s insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev Cell. 2002;2:239–249. doi: 10.1016/S1534-5807(02)00117-X. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG, Uthus EO. Growth hormone alters methionine and glutathione metabolism in Ames dwarf mice. Mech Ageing Dev. 2005;126:389–398. doi: 10.1016/j.mad.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Chapman T, Miyatake T, Smith HK, Partridge L. Interactions of mating, egg production and death rates in females of the Mediterranean fruit fly, Ceratitis capitata. Proc Biol Sci. 1998;265:1879–1894. doi: 10.1098/rspb.1998.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Hafen E, Leevers SJ, Partridge L. Dietary restriction in long-lived dwarf flies. Science. 2002;296:319. doi: 10.1126/science.1069366. [DOI] [PubMed] [Google Scholar]
- Ekperigin HE, Vohra P. Histopathological and biochemical effects of feeding excess dietary methionine to broiler chicks. Avian Dis. 1981;25:82–95. doi: 10.2307/1589829. [DOI] [PubMed] [Google Scholar]
- Finkelstein JD. Methionine metabolism in mammals. J Nutr Biochem. 1990;1:228–237. doi: 10.1016/0955-2863(90)90070-2. [DOI] [PubMed] [Google Scholar]
- Finkelstein A, Benevenga NJ. Developmental changes in the metabolism of 3-methylthiopropionate in the rat. J Nutr. 1984;114:1622–1629. doi: 10.1093/jn/114.9.1622. [DOI] [PubMed] [Google Scholar]
- Gems D, Partridge L. Insulin/IGF signalling and ageing: seeing the bigger picture. Curr Opin Genet Dev. 2001;11:287–292. doi: 10.1016/S0959-437X(00)00192-1. [DOI] [PubMed] [Google Scholar]
- Goldfischer S, Grotsky HW, Chang CH, Berman EL, Richert RR, Karmarkar SD, Roskamp JO, Morecki R. Idiopathic neonatal iron storage involving the liver, pancreas, heart, and endocrine and exocrine glands. Hepatology. 1981;1:58–64. doi: 10.1002/hep.1840010110. [DOI] [PubMed] [Google Scholar]
- Good TP, Tatar M. Age-specific mortality and reproduction respond to adult dietary restriction in Drosophila melanogaster. J Insect Physiol. 2001;47:1467–1473. doi: 10.1016/S0022-1910(01)00138-X. [DOI] [PubMed] [Google Scholar]
- Harper AE, Benevenga NJ, Wohlhueter RM. Effects of ingestion of disproportionate amounts of amino acids. Physiol Rev. 1970;50:428–558. doi: 10.1152/physrev.1970.50.3.428. [DOI] [PubMed] [Google Scholar]
- Hinton T, Ellis J, Noyes DT. Amino acids and growth factors in a chemically defined medium for Drosophila. Physiol Zool. 1951;24:335. [Google Scholar]
- Houthoofd K, Braeckman BP, Johnson TE, Vanfleteren JR. Life extension via dietary restriction is independent of the Ins/IGF-1 signalling pathway in Caenorhabditis elegans. Exp Gerontol. 2003;38:947–954. doi: 10.1016/S0531-5565(03)00161-X. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Gleiser CA, Masoro EJ, McMahan CA, Seo EJ, Yu BP. The influence of dietary protein source on longevity and age-related disease processes of Fischer rats. J Gerontol. 1988;43:B5–B12. doi: 10.1093/geronj/43.1.b5. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Gleiser CA, Masoro EJ, McMahan CA, Seo EJ, Yu BP. Influence of the restriction of individual dietary components on longevity and age-related disease of Fischer rats: the fat component and the mineral component. J Gerontol. 1988;43:B13–B21. doi: 10.1093/geronj/43.1.b13. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball S, Jefferson L. New functions for amino acids: effects on gene transcription and translation. Am J Clin Nutr. 2006;83:500S–507S. doi: 10.1093/ajcn/83.2.500S. [DOI] [PubMed] [Google Scholar]
- Kritchevsky D, Weber MM, Klurfeld DM. Dietary fat versus caloric content in initiation and promotion of 7,12-dimethylbenz(a)anthracene-induced mammary tumorigenesis in rats. Cancer Res. 1984;44:3174–3177. [PubMed] [Google Scholar]
- Kumagai H, Katoh S, Hirosawa K, Kimura M, Hishida A, Ikegaya N. Renal tubulointerstitial injury in weanling rats with hyperhomocysteinemia. Kidney Int. 2002;62:1219–1228. doi: 10.1111/j.1523-1755.2002.kid558.x. [DOI] [PubMed] [Google Scholar]
- Long X, Ortiz-Vega S, Lin Y, Avruch J. Rheb binding to mammalian target of rapamycin (mTOR) is regulated by amino acid sufficiency. J Biol Chem. 2005;280:23433–23436. doi: 10.1074/jbc.C500169200. [DOI] [PubMed] [Google Scholar]
- Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299:1342–1346. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- Magwere T, Chapman T, Partridge L. Sex differences in the effect of dietary restriction on life span and mortality rates in female and male Drosophila melanogaster. J Gerontol Ser A Biol Sci Med Sci. 2004;59:3–9. doi: 10.1093/gerona/59.1.b3. [DOI] [PubMed] [Google Scholar]
- Mair W, Goymer P, Pletcher SD, Partridge L. Demography of dietary restriction and death in Drosophila. Science. 2003;301:1731–1733. doi: 10.1126/science.1086016. [DOI] [PubMed] [Google Scholar]
- Mair W, Piper MD, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ. Assessment of nutritional components in prolongation of life and health by diet. Proc Soc Exp Biol Med. 1990;193:31–34. doi: 10.3181/00379727-193-42985. [DOI] [PubMed] [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KJ, Tatar M (2006a) Restriction of amino acids extends lifespan in Drosophila melanogaster. Mech Ageing Dev 127(7) [DOI] [PubMed]
- Min KJ, Tatar M. Drosophila diet restriction in practice: do flies consume fewer nutrients? Mech Ageing Dev. 2006;127:93–96. doi: 10.1016/j.mad.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Murtagh-Mark CM, Reiser KM, Harris R, Jr, McDonald RB. Source of dietary carbohydrate affects life span of Fischer 344 rats independent of caloric restriction. J Gerontol Ser A Biol Sci Med Sci. 1995;50:B148–154. doi: 10.1093/gerona/50a.3.b148. [DOI] [PubMed] [Google Scholar]
- Ni W, Tsuda Y, Sakono M, Imaizumi K. Dietary soy protein isolate, compared with casein, reduces atherosclerotic lesion area in apolipoprotein E-deficient mice. J Nutr. 1998;128:1884–1889. doi: 10.1093/jn/128.11.1884. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. J Nutr. 1993;123:269–274. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- Orr WC, Mockett RJ, Benes JJ, Sohal RS. Effects of overexpression of copper-zinc and manganese superoxide dismutases, catalase, and thioredoxin reductase genes on longevity in Drosophila melanogaster. J Biol Chem. 2003;278:26418–26422. doi: 10.1074/jbc.M303095200. [DOI] [PubMed] [Google Scholar]
- Partridge L, Pletcher SD, Mair W. Dietary restriction, mortality trajectories, risk and damage. Mech Ageing Dev. 2005;126:35–41. doi: 10.1016/j.mad.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Piper MD, Mair W, Partridge L. Counting the calories: the role of specific nutrients in extension of life span by food restriction. J Gerontol Ser A Biol Sci Med Sci. 2005;60:549–555. doi: 10.1093/gerona/60.5.549. [DOI] [PubMed] [Google Scholar]
- Pletcher SD. Model fitting and hypothesis testing for age-specific mortality data. J Evol Biol. 1999;12:430–439. doi: 10.1046/j.1420-9101.1999.00058.x. [DOI] [Google Scholar]
- Pletcher SD, Khazaeli AA, Curtsinger JW. Why do life spans differ? Partitioning mean longevity differences in terms of age-specific mortality parameters. J Gerontol Ser A Biol Sci Med Sci. 2000;55:B381–B389. doi: 10.1093/gerona/55.8.b381. [DOI] [PubMed] [Google Scholar]
- Pletcher SD, Macdonald SJ, Marguerie R, Certa U, Stearns SC, Goldstein DB, Partridge L. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Curr Biol. 2002;12:712–723. doi: 10.1016/S0960-9822(02)00808-4. [DOI] [PubMed] [Google Scholar]
- Richie JP, Jr, Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N, Zimmerman JA. Methionine restriction increases blood glutathione and longevity in F344 rats. Faseb J. 1994;8:1302–1307. doi: 10.1096/fasebj.8.15.8001743. [DOI] [PubMed] [Google Scholar]
- Russo GT, Friso S, Jacques PF, Rogers G, Cucinotta D, Wilson PW, Ordovas JM, Rosenberg IH, Selhub J. Age and gender affect the relation between methylenetetrahydrofolate reductase C677T genotype and fasting plasma homocysteine concentrations in the Framingham offspring study cohort. J Nutr. 2003;133:3416–3421. doi: 10.1093/jn/133.11.3416. [DOI] [PubMed] [Google Scholar]
- Sang JH. The quantitative nutritional requirements of Drosophila melanogaster. J Exp Biol. 1955;33:45. doi: 10.1038/icb.1955.6. [DOI] [Google Scholar]
- Selhub J. Folate, vitamin B12 and vitamin B6 and one carbon metabolism. J Nutr Health Aging. 2002;6:39–42. [PubMed] [Google Scholar]
- Selhub J, Jacques PF, Wilson PW, Rush D, Rosenberg IH. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. Jama. 1993;270:2693–2698. doi: 10.1001/jama.270.22.2693. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stramentinoli G, Gualano M, Catto E, Algeri S. Tissue levels of S-adenosylmethionine in aging rats. J Gerontol. 1977;32:392–394. doi: 10.1093/geronj/32.4.392. [DOI] [PubMed] [Google Scholar]
- Stubbs AK, Wheelhouse NM, Lomax MA, Hazlerigg DG. Nutrient-hormone interaction in the ovine liver: methionine supply selectively modulates growth hormone-induced IGF-I gene expression. J Endocrinol. 2002;174:335–341. doi: 10.1677/joe.0.1740335. [DOI] [PubMed] [Google Scholar]
- Troen AM, Lutgens E, Smith DE, Rosenberg IH, Selhub J. The atherogenic effect of excess methionine intake. Proc Natl Acad Sci USA. 2003;100:15089–15094. doi: 10.1073/pnas.2436385100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uthus EO, Brown-Borg HM. Altered methionine metabolism in long living Ames dwarf mice. Exp Gerontol. 2003;38:491–498. doi: 10.1016/S0531-5565(03)00008-1. [DOI] [PubMed] [Google Scholar]
- Uthus EO, Brown-Borg HM. Methionine flux to transsulfuration is enhanced in the long living Ames dwarf mouse. Mech Ageing Dev. 2006;127:444–450. doi: 10.1016/j.mad.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villee CA, Bissell HB. Nucleic acids as growth factors in Drosophila. JBC. 1948;172(1):59–66. [PubMed] [Google Scholar]
- Walker G, Houthoofd K, Vanfleteren JR, Gems D. Dietary restriction in C. elegans: from rate-of-living effects to nutrient sensing pathways. Mech Ageing Dev. 2005;126:929–937. doi: 10.1016/j.mad.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford R. The retardation of aging and disease by dietary restriction. Springfield, Illinois: Thomas; 1988. [Google Scholar]
- Yokota F, Esashi T, Suzue R. Nutritional anemia induced by excess methionine in rat and the alleviative effects of glycine on it. J Nutr Sci Vitaminol. 1978;24:527–533. doi: 10.3177/jnsv.24.527. [DOI] [PubMed] [Google Scholar]
- Yui R, Ohno Y, Matsuura ET. Accumulation of deleted mitochondrial DNA in aging Drosophila melanogaster. Genes Genet Syst. 2003;78:245–251. doi: 10.1266/ggs.78.245. [DOI] [PubMed] [Google Scholar]
- Zimmerman JA, Malloy V, Krajcik R, Orentreich N. Nutritional control of aging. Exp Gerontol. 2003;38:47–52. doi: 10.1016/S0531-5565(02)00149-3. [DOI] [PubMed] [Google Scholar]