Abstract
The basic tenet of several theories on aging is increasing genomic instability resulting from interactions with the environment. Chromosomal aberrations have been used as classic examples of increasing genomic instability since they demonstrate an increase in numerical and structural abnormalities with age in many species including humans. This accumulating damage may augment many aging processes and initiate age-related diseases, such as neoplasias. Calorie restriction (CR) is one of the most robust interventions for reducing the frequency of age-related diseases and for extending life span in many short-lived organisms. However, the mechanisms for the anti-aging effects of CR are not yet well understood. A study of rhesus monkeys was begun in 1987 to determine if CR is also effective in reducing the frequency of age-related diseases and retarding aging in a long-lived mammal. Male monkeys were begun on the diet in 1987, and females were added in 1992 to examine a possible difference in response to CR by sex. The CR monkeys have been maintained for over 10 years on a low-fat nutritional diet that provides a 30% calorie reduction compared to a control (CON) group. Because of the greater similarity of nonhuman primates to humans in life span and environmental responses to diet compared with those of rodents, the rhesus monkey provides an excellent model for the effects of CR in humans. This study examined the effects of CR on chromosomal instability with aging. Significant age effects were found in both CR and CON groups for the number of cells with aneuploidy: old animals had a higher loss and a higher gain than young animals. However, there was no effect of age on chromosomal breakage or structural aberrations in either diet group. Diet had only one significant effect: the CR group had a higher frequency of chromatid gaps than did the CON group. CR, implemented in adult rhesus monkeys, does not have a major effect on the reduction of numerical or structural aberrations related to aging.
Key words: aging, calorie restriction, chromosomal stability, diet, rhesus monkeys
Introduction
Many theories on aging propose that increasing genomic instability results from interactions with the environment (see review by Semsei 2000). Chromosomal aberrations have been used as classic examples of increasing genomic instability since they demonstrate an increase in numerical and structural abnormalities with age. This accumulating damage may augment many aging processes and initiate pathogenesis leading to age-related diseases, such as neoplasias (see reviews by Hirsch-Kauffmann and Schweiger 1999; Wojda and Witt 2003).
Stevenson and Curtis (1961) presented one of the first studies to demonstrate increasing genomic instability with advancing age manifested as increased chromosomal abnormalities. These investigators examined chromosomal aberrations in strains of mice with short and long life expectancies. Aberrations in the long-lived strain (C57BL/6J, life span approximately 600 days) increased more slowly than those in the short-lived strain (A/HEJ, life span approximately 395 days). Using the same technology, Curtis and Crowley (1963) showed that regenerating mouse liver parenchymal cells from old mice had significantly higher frequencies of chromosomal aberrations than cells from young mice. Martin et al. (1985) subsequently reported a five-fold increase in chromosomal aberrations in aging mouse kidney cells. These changes involved both numerical and structural abnormalities (see reviews by Vijg and Gossen 1993 and Wojda and Witt 2003).
Numerous studies of chromosome breakage in peripheral blood lymphocytes of human subjects have consistently reported an increase in numerical and structural chromosomal aberrations with age (Jacobs et al. 1961, 1963, 1964; Bender et al. 1988; Prieur et al. 1988; Bender et al. 1989; Tucker et al. 1994; Weirich-Schwaiger et al. 1994; Guttenbach et al. 1995; Ramsey et al. 1995; Bolognesi et al. 1997). This instability has long been considered a possible cause of accelerated aging and carcinogenesis (Solomon et al. 1991; Hagmar et al. 1994; Bonassi et al. 1995; DePinho 2000). Applying microarray analysis, Ly et al. (2000) suggested that altered expression of a number of genes involved in cell division occurs with advancing age and results in increased chromosomal instability and aberrations.
Underscoring the relationship of aging, maintenance of genomic stability and cancer, human progeroid syndromes, such as Werner syndrome and Cockayne syndromes, type A and B, have high somatic mutation rates and undergo changes that are indicative of premature aging, including early death from cancer or myocardial infarction as a result of coronary artery atherosclerosis (see review by Martin and Ohshima 2000). Increased numbers of chromosomal aberrations have also been found in homozygous DNA repair mutants, such as those underlying Bloom syndrome, Fanconi anemia, and ataxia telangiectasia, which have high frequencies of neoplasias (see reviews by Cohen and Levy 1989).
Using animal models, calorie restriction (CR) has consistently been shown to be one of the most effective ways to extend life span and reduce age-related diseases, including cancer (Weindruch and Walford 1988; Yu 1994). Numerous studies have clearly demonstrated that diet has a strong effect on the incidence of various cancers in different human populations (see review by Chessen and Collins 1997). However, defining the individual components of these diets that are effective in extending life span and prevention of age-related diseases including cancer has been more difficult.
Fenech (1998) reviewed studies on human diet related to chromosomal damage and aging. He summarized cross-sectional studies that determined baseline chromosomal damage rates in large populations of healthy individuals and related their variation to age and diet. However, most of these studies examined single components, such as vitamin supplements or deficiencies and moderate ingestion of red and white wines, but not overall diet (Xue et al. 1992; Fenech and Rinaldi 1994; Ortiz et al. 1994; Ames et al. 1995; Fenech et al. 1997; Fenech 1998, 2002; Crott and Fenech 1999).
Since the first study by McCay et al. (1935), an increase in life span and reduction of age-related diseases by CR has been demonstrated in a number of short-lived species, including yeast, nematodes, flies, and rodents (Weindruch and Walford 1988). To determine if CR would have the same effect in long-lived species, a study was begun in 1987 at the National Institute on Aging (NIA) in which rhesus monkeys were placed on a life-long calorie-restricted (30%), but balanced diet (Ingram et al. 1990). The history and selected results of this study have been reviewed elsewhere (Lane et al. 1997; Roth et al. 1999; Mattison et al. 2003). Monkeys on the restricted diet have a smaller body size, reduced body fat and a delay in sexual and skeletal development compared to controls (Lane et al. 1997; Mattison et al. 2003). Black et al. (2000) have recently shown that the CR group has a reduction in proliferative diseases, including neoplasias and endometriosis, compared to the control group.
Rhesus monkeys (Macaca mulatta) have 42 chromosomes that have many similarities to human chromosomes (Weinberg et al. 1992), e.g., the rhesus monkey chromosomal equivalents of human chromosomes 1, 8, 12, 19 and X show virtually identical banding patterns to those chromosomes in the human karyotype. Human whole chromosome painting probes (wcp) have been hybridized to macaque chromosomes to confirm karyotypic homologies and to follow evolutionary changes (Weinberg et al. 1992; Moore et al. 1999). Because of these similarities, chromosomal aberrations found in the rhesus monkey are easily compared to homologous regions of human chromosomes.
In the current study, we examined the effects of CR on chromosomal instability observed among monkeys in the NIA study of different age and diet groups. Using peripheral blood lymphocytes, we analyzed numerical and structural abnormalities in relation to age, sex, and dietary status. The standard analysis for chromosomal instability has been conventional Giemsa staining of the chromosomes without banding (see review by Maurer et al. 2003). However, chromosome painting, using wcp, is increasingly being used to detect subtle chromosomal translocations and insertions that could not easily be seen with conventional staining (see summaries by Ramsey et al. 1995 and Bonassi et al. 2005). We applied both types of analyses in this study to detect stable and unstable chromosomal abnormalities.
Materials and methods
Animals
Monkeys used in the present study were part of an ongoing study of aging and CR at the NIA. At the time of sampling in the current study, there were a total of 89 monkeys, 41 CR and 48 controls (CON). Monkeys ranged in age from 1 to 23 years at the time CR was initiated, for males in 1987 or 1988 and females in 1992. Thus, the CR regime has been maintained for 11–15 years (Lane et al. 1997, 2002; Roth et al. 1999). The diet was developed by primate nutritionists to be low in fat, high in fiber and supplemented with vitamins, minerals and trace elements to provide adequate nutrition with a 30% reduction in calories. Diet and husbandry have been described in detail elsewhere (Ingram et al. 1990). Briefly, monkeys were housed individually indoors in a temperature-controlled environment. Monkeys were fed two meals a day (0700 and 1400 hours) and allotments for the CON monkeys were based on National Research Council requirements for monkeys for a given age and weight and approximated ad libitum amounts. CR monkeys received approximately 30% less than control animals of the same sex and similar age and weight. Diet composition did not differ between the two groups and thus the experimental manipulation was a reduction in total caloric intake.
Rhesus monkeys have a median life span in captivity of 25 years (Roth et al. 1999; Bodkin et al. 2003). During the extent of this project, monkeys in this study ranged in age from 12–39 years. Monkeys that were 12–18 years old at the time of sampling are referred to as “young” because they entered the NIA study when they were 1–3 years of age, while monkeys that were 27–39 years old at time of sampling were classified as “old” as they entered the NIA study when they were 15–23 years of age. In the CR group, the youngest males and females were 2 years of age when they were placed on CR. For the “old” animals, the youngest males were 16 years of age when they were placed on CR, while the only “old” female that was available for our study was 19 years old when she was placed on CR. From the total colony, we examined all of the available “old” monkeys (17) and 23 “young” monkeys to comprise a total of 40 monkeys studied (Table 1).
Table 1.
Animals selected from the National Institute on Aging (NIA) colony. CR Calorie restriction, CON control.
| Sex | Young | Old | ||||||
|---|---|---|---|---|---|---|---|---|
| CON | CR | CON | CR | |||||
| n | Ages | n | Ages | n | Ages | n | Ages | |
| Male | 6 | 17–18 | 7 | 17–18 | 7 | 30–35 | 6 | 31–39 |
| Female | 5 | 12–14 | 5 | 13–14 | 3 | 27–30 | 1 | 30 |
| Total | 11 | 12–18 | 12 | 13–18 | 10 | 27–35 | 7 | 30–39 |
For collection of heparinized whole blood, monkeys were anesthetized with ketamine (7–10 mg/kg, i.m.) or Telazol (3–5 mg/kg, i.m.) after an overnight fast. Blood samples were obtained by venipuncture of the femoral vein using a vacutainer and vacuum tubes containing sodium heparin. Samples were sent by overnight delivery at room temperature to the University of Texas Health Science Center at San Antonio where cultures were initiated. Each of the 40 monkeys was sampled two to three times within a 6–12 month period.
Analysis of chromosomal instability
Following a preliminary study that determined the optimal time to obtain first division metaphases in order to identify all stable and unstable aberrations, lymphocyte cultures were incubated for 42 h and then harvested using standard cytogenetic protocols for preparing well-spread metaphase chromosomes. Metaphase spreads were then either hybridized to wcp probes or stained conventionally for chromosomal breakage studies.
Three wcp probes to human chromosomes 1, 2, and 4 and labeled in Spectrum Green, Spectrum Orange and Spectrum Aqua, respectively, were obtained from Vysis (Des Plaines, IL). These human chromosomes are homologous to rhesus chromosomes 1, 6, 9 and 15. Human chromosomes 1 and 4 are homologous to rhesus chromosomes 1 and 6, respectively. Human chromosome 2 is homologous to two rhesus monkey chromosomes, 9 and 15, as a result of an evolutionary fusion of two chromosomes in the lineage that led to humans (Weinberg et al. 1992). The three human chromosomes represent 22% of the human genome and detect approximately 34% of all chromosomal exchanges (Tucker et al. 1994).
Slides were prepared according to the Vysis protocol, and stringency was altered to produce a strong signal without loss of specificity (Weinberg et al. 1992). Slides were visualized with a Zeiss Axiophot II fluorescent microscope, using an Applied Imaging Multi-FISH System for capture and analysis. Additional slides were stained conventionally with Giemsa without banding for analysis of chromosome and chromatid breaks and gaps.
Chromosomal analysis was performed in a blinded fashion. The sex of the animal could be determined from the metaphase by the presence or absence of a Y chromosome, but age and dietary status were unknown to the individual scoring for aberrations. One technologist analyzed all the samples. Abnormalities were photographed and confirmed by one of the authors (B.G.D.), so that inter-observer variation was virtually eliminated. Where appropriate, the fluorescent studies were combined with those of the conventionally stained chromosomes.
We examined 100 cells from each of 28 animals using wcp probes. Only one aberration was observed (a dicentric chromosome with an acentric fragment). This aberration could also have been detected in an unbanded study. Therefore, due to the low aberration rate detectable with these probes, only the results from conventional analyses were analyzed (Figure 1). This allowed for specific identification of the Y chromosome (Y) from other chromosomes by size, but not of the X chromosome (X) or the autosomes (A). The average number of metaphases analyzed was 300 or 370 per animal, depending on the type of aberration detected (total number of cells analyzed was 12,473; Table 2). The specific aberrations analyzed included chromatid gaps (CTG) and breaks (CTB), chromosome gaps (CSG) and breaks (CSB), exchanges such as translocations and insertions, ring chromosomes formed by the union of the ends of the short and long arms, dicentric chromosomes containing two centromeres and acentric fragments lacking a centromere. Total breakage included all chromatid and chromosome gaps and breaks. Total structural aberrations included all exchanges, rings, dicentrics and acentric fragments.
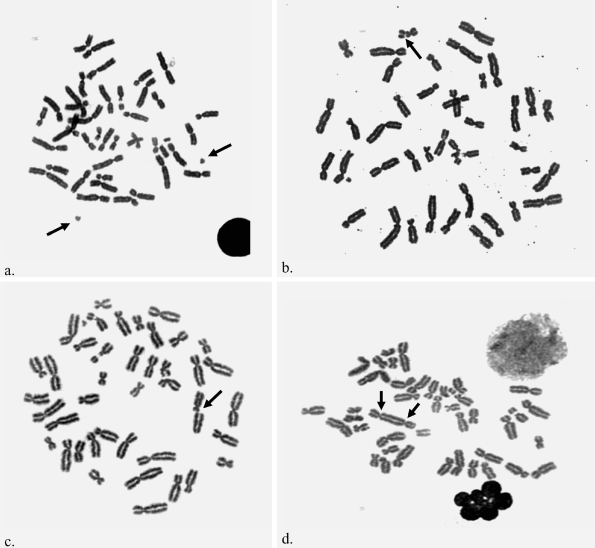
Figure 1a–d.
Examples of aneuploidy, breakage and structural aberrations. a Additional Y chromosome. Arrows identify two Y chromosomes. Note small size of Y compared to other chromosomes. b Chromatid gap. Arrow identifies gap. c Chromatid break. Arrow identifies break. d Dicentric chromosome. Arrows identify two centromeres
Table 2.
Descriptive statistics. Results are expressed as a fraction (percent) of the number of metaphases analyzed. CTG chromatid gaps, CTB chromatid breaks, CSG chromosome gaps, CSB chromosome breaks.
| Variable | Number of animals | Average metaphase analyzed/ animal | Mean | Standard deviation | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Aneuploidy | ||||||
| Loss (A or X or Y) | 40 | 370 | 4.92 | 3.32 | 0.5 | 12.0 |
| Gain (A or X or Y) | 40 | 370 | 0.34 | 0.38 | 0 | 1.50 |
| Total breakage | 40 | 300 | 10.67 | 3.01 | 7.33 | 21.00 |
| CTG | 40 | 300 | 3.19 | 1.16 | 1.00 | 6.00 |
| CTB | 40 | 300 | 1.58 | 0.79 | 0 | 4.00 |
| CSG | 40 | 300 | 2.83 | 1.61 | 0.67 | 7.00 |
| CSB | 40 | 300 | 3.07 | 1.53 | 0.67 | 9.00 |
| Total structural | 40 | 370 | 0.88 | 0.57 | 0 | 2.50 |
| Exchange | 40 | 370 | 0.07 | 0.13 | 0 | 0.50 |
| Ring | 40 | 370 | 0.02 | 0.07 | 0 | 0.25 |
| Dicentric | 40 | 370 | 0.05 | 0.12 | 0 | 0.50 |
| Acentric | 40 | 370 | 0.74 | 0.52 | 0 | 2.25 |
Statistical analyses
The data for all samples from a single animal were combined. The number of defects was analyzed for effects of age group, diet, and sex using a generalized linear model analysis (McCullagh and Nelder 1989). The logarithm was used as the link function and the distribution was negative binomial. If the estimated dispersion parameter was less than its standard error, the Poisson distribution was used. All calculations were carried out using PROC GENMOD of SAS v9.1. The results in Tables 2, 3, 4, 5, 6, 7 and 8 are expressed as a fraction (percent) of the number of metaphases analyzed. Statistical significance was P≤ 0.05.
Table 3.
Descriptive statistics for aneuploidy, by sex.
| Variable | Number of animals | Average metaphase analyzed/ animal | Mean | Standard deviation | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Aneuploidy—females only | ||||||
| Loss (A or X) | 14 | 370 | 2.33 | 1.27 | 0.5 | 5.5 |
| Gain (A or X) | 14 | 370 | 0.44 | 0.44 | 0 | 1.25 |
| Aneuploidy—males only | ||||||
| Loss (A or X) | 26 | 370 | 1.49 | 0.89 | 0.25 | 4.5 |
| Gain (A or X) | 26 | 370 | 0.22 | 0.34 | 0 | 1.5 |
| Loss (Y) | 26 | 370 | 4.83 | 3.06 | 1.00 | 11.00 |
| Gain (Y) | 26 | 370 | 0.05 | 0.12 | 0 | 0.33 |
Table 4.
Percentages by sex and diet, young animals only.
| Variable | Diet | Significance (sex) | Significance (diet) | Significance (sex by diet) | |||
|---|---|---|---|---|---|---|---|
| CON | CR | ||||||
| Female | Male | Female | Male | ||||
| n =5 | n =6 | n =5 | n =7 | ||||
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ||||
| Aneuploidy | |||||||
| Loss (A or X or Y) | 1.72 (1.11, 2.67) | 3.42 (2.46, 4.76) | 2.33(1.56, 3.48) | 5.37 (4.06, 7.10) | P = 0.0004* | P = 0.0538 | P = 0.6932 |
| Gain (A or X or Y) | 0.17 (0.05, 0.52) | 0.18 (0.07, 0.48) | 0.44(0.22, 0.89) | 0.20 (0.08, 0.48) | P = 0.4588 | P = 0.2550 | P = 0.3503 |
| Total breakagea | 10.00 (8.09, 12.37) | 10.83 (8.96, 13.10) | 11.67 (9.52, 14.30) | 10.10 (8.44, 12.08) | P = 0.7476 | P = 0.6775 | P = 0.2686 |
| CTGa | 3.20 (2.29, 4.48) | 2.67 (1.92, 3.70) | 3.60 (2.61, 4.97) | 3.29 (2.48, 4.35) | P = 0.4026 | P = 0.3189 | P = 0.7791 |
| CTBa | 1.60 (1.07, 2.39) | 1.72 (1.21, 2.45) | 1.47 (0.97, 2.23) | 1.81 (1.32, 2.49) | P = 0.4555 | P = 0.9216 | P = 0.7205 |
| CSGa | 2.47 (1.64, 3.71) | 3.00 (2.11, 4.26) | 3.00 (2.04, 4.40) | 2.24 (1.57, 3.19) | P = 0.7994 | P = 0.7994 | P = 0.2083 |
| CSBa | 2.73 (1.90, 3.94) | 3.44 (2.53, 4.69) | 3.60 (2.58, 5.02) | 2.76 (2.03, 3.76) | P = 0.9200 | P = 0.8710 | P = 0.1489 |
| Total structural | 1.11 (0.72, 1.72) | 0.86 (0.55, 1.35) | 0.72 (0.42, 1.24) | 0.92 (0.61, 1.38) | P = 0.9832 | P = 0.4353 | P = 0.2927 |
| Exchange | 0.11 (0.03, 0.44) | 0.05 (0.01, 0.32) | 0.06 (0.01, 0.39) | 0.08 (0.02, 0.32) | P = 0.7604 | P = 0.9412 | P = 0.4551 |
| Ring | 0.00 (0.00, −) | 0.05 (0.01, 0.32) | 0.06 (0.01, 0.39) | 0.00 (0.00, −) | P = 1.0000 | P = 1.0000 | P = 0.0884 |
| Dicentric | 0.06 (0.01, 0.39) | 0.05 (0.01, 0.32) | 0.00 (0.00, −) | 0.16 (0.06, 0.43) | P = 0.1592 | P = 0.5036 | P = 0.1163 |
| Acentric | 0.94 (0.58, 1.52) | 0.73 (0.45, 1.19) | 0.61 (0.34, 1.10) | 0.68 (0.42, 1.09) | P = 0.7671 | P = 0.3327 | P = 0.4779 |
*Indicates significance at P ≤ 0.05
a300 Metaphases analyzed per animal
Table 5.
Percentages by diet and age group, data combined over sex.
| Variable | Age | Significance (age) | Significance (diet) | Significance (age by diet) | |||
|---|---|---|---|---|---|---|---|
| Young | Old | ||||||
| Diet=CON | Diet=CR | Diet=CON | Diet=CR | ||||
| n = 11 | n = 12 | n = 10 | n = 7 | ||||
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ||||
| Aneuploidy | |||||||
| Loss (A or X or Y) | 2.65 (1.88, 3.75) | 4.08 (2.98, 5.57) | 6.89 (4.97, 9.55) | 7.18 (4.88, 10.56) | P = 0.0001* | P = 0.1838 | P = 0.2726 |
| Gain (A or X or Y) | 0.18 (0.08, 0.39) | 0.30 (0.16, 0.56) | 0.43 (0.24, 0.77) | 0.54 (0.28, 1.01) | P = 0.0334* | P = 0.2650 | P = 0.6479 |
| Total breakagea | 10.46 (8.94, 12.23) | 10.75 (9.26, 12.48) | 10.77 (9.14, 12.68) | 10.71 (8.81, 13.03) | P = 0.8785 | P = 0.8929 | P = 0.8479 |
| CTGa | 2.91 (2.38, 3.55) | 3.42 (2.86, 4.08) | 2.80 (2.26, 3.47) | 3.81 (3.06, 4.74) | P = 0.7335 | P = 0.0237* | P = 0.4783 |
| CTBa | 1.67 (1.28, 2.17) | 1.67 (1.29, 2.15) | 1.67 (1.26, 2.20) | 1.14 (0.77, 1.71) | P = 0.2202 | P = 0.2210 | P = 0.2207 |
| CSGa | 2.76 (2.00, 3.81) | 2.56 (1.87, 3.50) | 3.03 (2.18, 4.23) | 3.14 (2.12, 4.67) | P = 0.3896 | P = 0.9076 | P = 0.7500 |
| CSBa | 3.12 (2.38, 4.10) | 3.11 (2.40, 4.04) | 3.27 (2.46, 4.34) | 2.62 (1.83, 3.75) | P = 0.6756 | P = 0.4596 | P = 0.4726 |
| Total structural | 0.98 (0.71, 1.33) | 0.84 (0.60, 1.16) | 1.03 (0.75, 1.41) | 0.64 (0.41, 1.02) | P = 0.5624 | P = 0.0876 | P = 0.3872 |
| Exchange | 0.08 (0.02, 0.23) | 0.07 (0.02, 0.22) | 0.08 (0.03, 0.25) | 0.07 (0.02, 0.29) | P = 0.9340 | P = 0.8708 | P = 0.9645 |
| Ring | 0.03 (0.00, 0.18) | 0.02 (0.00, 0.17) | 0.03 (0.00, 0.19) | 0.00 (0.00, −) | P = 0.3878 | P = 0.3387 | P = 0.3621 |
| Dicentric | 0.05 (0.01, 0.20) | 0.09 (0.04, 0.25) | 0.03 (0.00, 0.19) | 0.04 (0.01, 0.25) | P = 0.3201 | P = 0.5888 | P = 0.8365 |
| Acentric | 0.83 (0.59, 1.16) | 0.65 (0.45, 0.94) | 0.89 (0.63, 1.26) | 0.54 (0.32, 0.89) | P = 0.7711 | P = 0.0606 | P = 0.4966 |
*Indicates significance at P ≤ 0.05
a300 Metaphases analyzed per animal
Table 6.
Percentages by diet and age group, females only.
| Variable | Age | Significance (age) | Significance (diet) | Significance (age by diet) | |||
|---|---|---|---|---|---|---|---|
| Young | Old | ||||||
| Diet=CON | Diet=CR | Diet=CON | Diet=CR | ||||
| n = 5 | n = 5 | n = 3 | n = 1 | ||||
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ||||
| Aneuploidy | |||||||
| Loss (A or X) | 1.72 (1.10, 2.68) | 2.33 (1.55, 3.49) | 3.17 (1.98, 5.07) | 3.00 (1.31, 6.85) | P = 0.1478 | P = 0.6673 | P = 0.5398 |
| Gain (A or X) | 0.17 (0.05, 0.52) | 0.44 (0.22, 0.89) | 0.83 (0.45, 1.55) | 0.75 (0.24, 2.33) | P = 0.0296* | P = 0.3576 | P = 0.2310 |
*Indicates significance at P ≤ 0.05
Table 7.
Percentages by diet and age group, males only.
| Variable | Age | Significance (age) | Significance (diet) | Significance (age by diet) | |||
|---|---|---|---|---|---|---|---|
| Young | Old | ||||||
| Diet=CON | Diet=CR | Diet=CON | Diet=CR | ||||
| n = 6 | n = 7 | n = 7 | n = 6 | ||||
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ||||
| Aneuploidy | |||||||
| Loss (A or X) | 1.19 (0.76, 1.84) | 1.63 (1.13, 2.36) | 2.00 (1.42, 2.82) | 1.13 (0.73, 1.74) | P = 0.7135 | P = 0.5362 | P = 0.0355* |
| Gain (A or X) | 0.14 (0.04, 0.46) | 0.12 (0.04, 0.40) | 0.16 (0.06, 0.47) | 0.50 (0.24, 1.03) | P = 0.1423 | P = 0.3602 | P = 0.2498 |
| Loss (Y) | 2.23 (1.46, 3.42) | 3.73 (2.61, 5.34) | 6.59 (4.71, 9.21) | 6.75 (4.73, 9.64) | P = 0.0001* | P = 0.1633 | P = 0.2047 |
| Gain (Y) | 0.05 (0.01, 0.32) | 0.08 (0.02, 0.32) | 0.08 (0.02, 0.32) | 0.00 (0.00, −) | P = 0.2624 | P = 0.2624 | P = 0.1036 |
*Indicates significance at P ≤ 0.05
Table 8.
Percentages by diet and age group, combined over sex.
| Variable | Age | Significance (age) | Significance (diet) | Significance (age by diet) | |||
|---|---|---|---|---|---|---|---|
| Young | Old | ||||||
| Diet=CON | Diet=CR | Diet=CON | Diet=CR | ||||
| n = 5 | n = 5 | n = 3 | n = 1 | ||||
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ||||
| Total Aberrations | |||||||
| Sum of CTG, CTB, CSG, CSB, exchange, ring, dicentric, acentric a | 11.49 (9.94, 13.27) | 11.64 (10.14, 13.36) | 12.03 (10.36, 13.98) | 11.48 (9.58, 13.75) | P = 0.8359 | P = 0.8284 | P = 0.6995 |
a300 Metaphases analyzed per animal
Results
Descriptive statistics are given in Table 2 for all animals combined and in Table 3 for aneuploidy (loss or gain of a chromosome) separately for females and males. The young age group averaged 15.8 years old (12–18 years) and the old age group averaged 31.8 years of age (27–39 years).
Because there was only one old CR female, we conducted an investigation of the effects of sex and diet in the young animals only. The means are given in Table 4 along with significance levels for the main (average) effects of sex and diet, and the sex by diet interaction. There were no significant interactions of sex and diet. We did find a significant effect of sex on loss of a chromosome (either an autosome or a sex chromosome). Because of this, we analyzed the categories of loss and gain for males and females both together and separately. However, in other categories there was a general lack of differences between the sexes and, therefore, in subsequent analyses, we combined the results for males and females for breakage and structural abnormalities.
Table 5 presents means by age group and diet, combined across sex. There were no significant interactions of age and diet. There were significant effects of age observed for loss or gain of a chromosome (either an autosome or a sex chromosome). There was a significant effect of diet on CTG with the CR group being higher than the CON group. Breakage, however, did not increase with age, either totally or in any specific category.
Table 6 shows the percentages by age group and diet in females only. We noted a significant effect of age on gain of a chromosome (X and autosomes combined) with old females having a higher percentage than young females. The gain of a chromosome, however, was not affected by diet.
Table 7 provides the percentages by age group and diet in males only. Loss of a Y chromosome in old animals was significantly different from loss of a Y in young animals. There was not a significant loss or gain of an autosome or X chromosome in old animals, nor a significant gain of the Y chromosome. However, there was an interaction of age and diet on loss of a chromosome when autosomes and the X were combined. In the young animals, the CR group had a higher percentage than the CON group, while in the old animals, the CON group had a higher percentage than the CR group.
Table 8 gives the sum of all breakage and structural abnormalities by diet and age, combined across sex. There were no significant effects of age or diet, nor was there an interaction of the two.
Discussion
Total aneuploidy and aberrations
The percent of aneuploid cells ranged from 0.5% to 12.0% for cells with loss of a chromosome and from 0 to 1.50% for cells with gain of a chromosome (Table 2). Total chromosomal aberrations ranged from 9.58% to 13.98% (Table 8). Total structural aberrations were much lower in frequency (0–2.50%) than chromosome or chromatid breaks (7.33–21.00%) (Table 2). These frequencies are similar to those found by Bender et al. (1988, 1989) in human lymphocytes. In contrast, the chromosomal exchange rate in the monkeys detected by wcp was quite low, when compared to the findings of Tucker et al. (1994) of translocations or stable insertions in lymphocytes from a normal human population.
Age
When all animals were examined, significant effects of age were found for the number of lymphocytes with aneuploidy: the old animals had a higher loss and a higher gain than the young animals (Table 5). This is compatible with human studies in which loss and gain of a chromosome has been shown to increase with age. Jacobs et al. (1961) were the first to demonstrate increasing percentages of aneuploidy in human lymphocytes with aging. Cells that had a loss of chromosomes were in higher proportions than cells that had a gain of chromosomes, in approximately a 10:1 ratio. Subsequent studies have uniformly reported much higher percentages of cells with chromosomal loss than those with chromosomal gain (see review by Maurer et al. 2003). Our results are consistent with these earlier studies, with a loss-to-gain ratio of approximately 14:1 (Table 2). In humans, chromosomal loss and gain in aneuploid lymphocytes most often involve the sex chromosomes (Jacobs et al. 1961, 1963, 1964; Pierre and Hoagland 1972; Mattevi and Salzano 1975; Fitzgerald and McEwan 1977; Galloway and Buckton 1978; Schneider 1978; Ford and Russell 1985; Nowinski et al. 1990; Richard et al. 1993; Guttenbach et al. 1995; Catalan et al. 2000). In hypodiploid cells, primarily the X chromosome is lost in females, but this loss does not become significant until after menopause. Loss in male cells primarily involves the Y chromosome, which becomes significant at puberty and increases steadily afterwards. This was also found in our study (Table 7) where rhesus males showed a significant increase in loss of the Y related to age. Loss of autosomes, however, is not correlated with age in humans, but remains constant throughout life. In human hyperdiploid cells, gain of a chromosome generally involves addition of an extra X in females and an X or Y in males. In the current study, loss of a chromosome increased with age in the female monkeys (Table 6), but the increase was not statistically significant. In contrast, gain of a chromosome was significant, with an increase in the frequency of hyperdiploid cells in the old female monkeys—comparable to findings in the human studies.
There was no effect of age on chromosomal breakage or structural aberrations in either diet group (Table 5). This is not consistent with the majority of chromosomal studies reported for other species. In human lymphocytes, structural aberrations, including chromatid and chromosome breaks, increase with age (Jacobs et al. 1963, 1964; Bochkov 1972; Galloway and Buckton 1978; Obe and Herha 1978; Evans 1979; Galloway et al. 1986; Marlhens et al. 1986; Prieur et al. 1988; Nowinski et al. 1990; Richard et al. 1993; Guttenbach et al. 1994: Hando et al. 1994; Guttenbach et al. 1995; Nath et al. 1995; Stone and Sandberg 1995). Stable aberrations such as translocations and insertions generally demonstrate up to a 10-fold increase with age with a smaller increase (3-fold) in dicentrics and acentric fragments (Ramsey et al. 1995; Tucker et al. 1994; Lucas et al. 1999). Tucker et al. (1999) studied mice that had served as unexposed controls in experiments. They showed an increase in translocations and insertions with age in peripheral blood lymphocytes (in C57BL/6 animals, but not in three different F1 hybrids) but no increase in dicentrics or acentrics, emphasizing an increase with age in stable, but not in unstable, aberrations. This led the authors to hypothesize that increases in stable aberrations with age are due to altered biological processes rather than being due to environmental exposures. It has also been suggested that this finding could be related to species differences in telomere dynamics (DePinho 2000). Some investigators using human lymphocytes (Bochkov 1972; Obe and Herha 1978; Evans 1979; Galloway et al. 1986) did not see an age effect on dicentrics but did find an increase in overall aberration frequency with age. In contrast, Bender et al. (1989) saw an age effect only on the frequency of dicentric chromosomes and not on any other chromosomal aberration. The difference in these studies may be related to the techniques used to detect the chromosomal aberrations and to the different populations or species and strains studied. We were unable to evaluate translocations and insertions in our study due to a very low exchange rate. Our findings for unstable rearrangements were similar to those in other studies (e.g., Bender et al. 1988, 1989; Tucker et al. 1999), except for lower frequencies of dicentrics and acentrics. These results may reflect a more stable repair process in the rhesus monkey than in humans or some strains of mice. Another possible explanation for the lack of an age-related increase in aberrations may be the advanced age of the “young” group. The “young” animals (ages 18–23) were older adults. A study of pre-pubertal animals may identify an age-related response corresponding to those seen in other species.
Sex
Only one significant effect was found for sex on chromosomal instability: sex was significant when only the young animals were examined (Table 4). Young males had a higher loss of a chromosome than young females. However, in humans, Guttenbach et al. (1995) reported that although both the X and Y chromosomes show an age-dependent loss, the frequency of X chromosome loss in females is greater than the loss of the Y in males. Male rhesus monkeys had a higher loss of the Y chromosome (1.46–9.64%, Table 7) than that reported in humans (0.05–1.34%, Guttenbach et al. 1995). This variation could be due to the difference in size of the Y chromosome in the two species, the rhesus Y being much smaller than the human Y and perhaps, therefore, more prone to loss. The loss of the X or autosome in female monkeys (1.10–6.85%, Table 6) was more comparable to the frequency of X chromosome loss in human females (1.5–5%, Guttenbach et al. 1995). As noted above, the loss of the X in human females increases significantly with age only beyond menopause (>51 years), while the loss of the Y in human males is low until puberty (15 years) and then steadily increases with age (Guttenbach et al. 1995). The young female rhesus monkeys were not menopausal, and the young males were well past puberty. Even though the X chromosomes were not specifically identified in the hypodiploid cells, this finding in young monkeys can be explained by loss of the Y in young males being greater than loss of the X in young females.
We found no significant effects of sex on breakage or structural rearrangements in the young animals (Table 4). Frequencies of stable translocations and inversions were particularly low compared to human studies (Tucker et al. 1994). Only 1 out of 2,800 cells showed a rearrangement using wcp. One important factor to consider is the small number of old females in the colony past menopause and especially the presence of only a single old CR female available for study.
Diet
A 30% CR diet affected only one type of aberration, the frequency of chromatid gaps (CTG), which was higher in the CR monkeys than in the CON group (Table 5). This very modest diet effect could be related to a protective effect of the manufactured diet that these monkeys consumed, which was well-balanced and fortified with a 40% increase in vitamins and minerals above the Recommended Dietary Allowance (RDA), so that the CR monkeys also received 100% of the RDA. Fenech (1998, 2002) suggests that diet is a major component in genomic stability, and a diet marginally deficient in micronutrients such as folate, vitamin B12, niacin and zinc can significantly affect the rate of spontaneous chromosome damage. Alternatively, a well-fortified diet may protect against this damage. The diet may have protected against age-related increases in aberrations, and the 40% additional micronutrients in the CON diet may also have played a role in lowering the CTG frequency in the CON group.
Interactions between age and diet
There was an interaction of age and diet in males with loss of an autosome or X chromosome. The CON group showed an increasing age-related loss, while the CR group showed an actual decrease in loss of an autosome or X with age (Table 7). This loss is most probably related to the X chromosome, as many studies in humans have shown that gain and loss of chromosomes primarily involves the sex chromosomes, while loss of an autosome is not correlated with age (Ford and Russell 1985; Nowinski et al. 1990; Richard et al. 1993; Guttenbach et al. 1995). The decrease in loss of an autosome or X with age in the CR group could represent a protective CR effect, or a random statistical finding related to the level of statistical significance. Additional studies would be necessary to distinguish between these possibilities.
Future studies
Similar studies in animals in which CR is begun at a pre-pubertal age may show significant effects, especially by examining subsets of lymphocytes or cultured fibroblasts. Investigating chromosomal instability at a different level, such as examining double-strand breakage in control cells and X-ray treated cells from the same individual, may also be informative.
Conclusion
CR in rhesus monkeys, implemented in adult males and females, does not have a major effect on the reduction of numerical or structural chromosomal aberrations related to aging.
Acknowledgements
This study was supported in part by a grant from the National Institute on Aging. We acknowledge the excellent technical assistance of Catherine D. Weaver, B.S., and the staff at NIH, Poolesville, in particular April Hobbs and Edward Tilmont and veterinarians Drs. Doug Powell and Rick Herbert.
References
- Ames BN, Gold L, Willett WC. The causes and prevention of cancer. Proc Natl Acad Sci USA. 1995;92:5258–5265. doi: 10.1073/pnas.92.12.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender MA, Preston RJ, Leonard RC, Pyatt BE, Gooch PC, Shelby MD. Chromosomal aberration and sister-chromatid exchange frequencies in peripheral blood lymphocytes of a large human population sample. Mutat Res. 1988;204:421–433. doi: 10.1016/0165-1218(88)90038-9. [DOI] [PubMed] [Google Scholar]
- Bender MA, Preston RJ, Leonard RC, Gooch PC. Chromosomal aberration and sister-chromatid exchange frequencies in peripheral blood lymphocytes of a large human population sample. II. Extension of age range. Mutat Res. 1989;212:149–154. doi: 10.1016/0027-5107(89)90065-1. [DOI] [PubMed] [Google Scholar]
- Black A, Tilmont EM, Handy AM, Ingram DK, Roth GS, Lane MA (2000) Calorie restriction reduces the incidence of proliferative disease: preliminary data from the NIA CR in nonhuman primate study. Gerontologist 40, Special Issue II, p 5
- Bochkov NP. Spontaneous chromosome aberrations in human somatic cells. Humangenetik. 1972;16:159–164. doi: 10.1007/BF00394003. [DOI] [PubMed] [Google Scholar]
- Bodkin NL, Alexander TM, Ortmeyer HK, Johnson E, Hansen BD. Morbidity and mortality in laboratory-maintained rhesus monkeys and effects of long-term dietary restriction. J Gerontol Biol Sci. 2003;58A:212–219. doi: 10.1093/gerona/58.3.b212. [DOI] [PubMed] [Google Scholar]
- Bolognesi C, Abbondandolo A, Barale R, Casalone R, Dalpra L, Ferrari M, Degrassi F, Forni A, Lamberti L, Lando C, Migliore L, Padovani P, Pasquini R, Puntoni R, Sbrana I, Stella M, Bonassi S. Age-related increase of base-line frequencies of sister chromatid exchanges, chromosomal aberrations and micronuclei in human lymphocytes. Cancer Epidemiol Biomark Prev. 1997;6:249–256. [PubMed] [Google Scholar]
- Bonassi S, Abbondandolo A, Camurri L, Dalpra L, Ferrari M, Degrassi F, Forni A, Lamberti L, Lando C, Padovani P, Sbrana I, Vecchio D, Puntoni R. Are chromosome aberrations in circulating lymphocytes predictive of future cancer onset in humans? Cancer Genet Cytogenet. 1995;79:133–135. doi: 10.1016/0165-4608(94)00131-T. [DOI] [PubMed] [Google Scholar]
- Bonassi S, Ugolini D, Strömberg U, Vermeulen R, Tucker JD. Human population studies with cytogenetic biomarkers: review of the literature and future prospectives. Environ Mol Mutagen. 2005;45:258–270. doi: 10.1002/em.20115. [DOI] [PubMed] [Google Scholar]
- Catalan J, Surralles J, Falck GCM, Autio K, Norppa H. Segregation of sex chromosomes in human lymphocytes. Mutagenesis. 2000;15:251–255. doi: 10.1093/mutage/15.3.251. [DOI] [PubMed] [Google Scholar]
- Chessen A, Collins A. Assessment of the role of diet in cancer prevention. Cancer Lett. 1997;114:237–245. doi: 10.1016/S0304-3835(97)04673-9. [DOI] [PubMed] [Google Scholar]
- Cohen MM, Levy HP. Chromosome instability syndromes. Adv Hum Genet. 1989;18:42–149. doi: 10.1007/978-1-4613-0785-3_2. [DOI] [PubMed] [Google Scholar]
- Crott JW, Fenech M. Effect of vitamin C supplementation on chromosome damage, apoptosis and necrosis ex vivo. Carcinogenesis. 1999;20:1035–1041. doi: 10.1093/carcin/20.6.1035. [DOI] [PubMed] [Google Scholar]
- Curtis H, Crowley C. Chromosome aberrations in liver cells in relation to the somatic mutation theory of aging. Radiat Res. 1963;19:337–344. doi: 10.2307/3571455. [DOI] [PubMed] [Google Scholar]
- DePinho RA. The age of cancer. Nature. 2000;408:248–254. doi: 10.1038/35041694. [DOI] [PubMed] [Google Scholar]
- Evans HJ. The induction of aberrations in human chromosomes following exposure to mutagens/carcinogens. In: Emmelot P, Kriek E, editors. Environmental carcinogens. Amsterdam: Elsevier; 1979. pp. 57–74. [Google Scholar]
- Fenech M. Chromosomal damage rate, aging and diet. Ann N Y Acad Sci. 1998;854:23–36. doi: 10.1111/j.1749-6632.1998.tb09889.x. [DOI] [PubMed] [Google Scholar]
- Fenech M. Micronutrients and genomic stability: a new paradigm for recommended dietary allowances (RDAs) Food Chem Toxicol. 2002;40:1113–1117. doi: 10.1016/S0278-6915(02)00028-5. [DOI] [PubMed] [Google Scholar]
- Fenech M, Rinaldi J. The relationship between micronuclei in human lymphocytes and plasma levels of vitamin C, vitamin E, vitamin B12 and folic acid. Carcinogenesis. 1994;15:1405–1411. doi: 10.1093/carcin/15.7.1405. [DOI] [PubMed] [Google Scholar]
- Fenech M, Stockley C, Aitken C. Moderate wine consumption protects against hydrogen peroxide-induced DNA damage. Mutagenesis. 1997;12:289–296. doi: 10.1093/mutage/12.4.289. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PH, McEwan CM. Total aneuploidy and age-related sex chromosome aneuploidy in cultured lymphocytes of normal men and women. Hum Genet. 1977;39:329–337. doi: 10.1007/BF00295428. [DOI] [PubMed] [Google Scholar]
- Ford JH, Russell JA. Differences in the error mechanisms affecting sex and autosomal chromosomes in women of different ages within the reproductive age group. Am J Hum Genet. 1985;37:973–983. [PMC free article] [PubMed] [Google Scholar]
- Galloway S, Buckton K. Aneuploidy and aging: chromosome studies on a random sample of the population using G-banding. Cytogenet Cell Genet. 1978;20:78–95. doi: 10.1159/000130842. [DOI] [PubMed] [Google Scholar]
- Galloway SM, Berry PK, Nichols WW, Wolman SR, Soper KA, Stolley PD, Archer P. Chromosome aberrations in individuals occupationally exposed to ethylene oxide, and in a large control population. Mutat Res. 1986;170:55–74. doi: 10.1016/0165-1218(86)90082-0. [DOI] [PubMed] [Google Scholar]
- Guttenbach M, Schakowski R, Schmid M. Aneuploidy and ageing: sex chromosome exclusion into micronuclei. Hum Genet. 1994;94:295–298. doi: 10.1007/BF00208287. [DOI] [PubMed] [Google Scholar]
- Guttenbach M, Koschorz B, Bernthaler U, Grimm T, Schmid M. Sex chromosome loss and aging: in situ hybridization studies on human interphase nuclei. Am J Hum Genet. 1995;57:1143–1150. [PMC free article] [PubMed] [Google Scholar]
- Hagmar L, Brogger A, Hansteen I, Heim S, Hogstedt B, Knudsen L, Lambert B, Linnainmaa K, Mitelman F, Nordenson I, Reuterwall C, Salomaa S, Skerfving S, Sorsa M. Cancer risk in humans predicted by increased levels of chromosomal aberrations in lymphocytes: Nordic Study Group on the health risk of chromosome damage. Cancer Res. 1994;54:2919–2922. [PubMed] [Google Scholar]
- Hando JC, Nath J, Tucker JD. Sex chromosomes, micronuclei and aging in women. Chromosoma. 1994;103:186–192. doi: 10.1007/BF00368011. [DOI] [PubMed] [Google Scholar]
- Hirsch-Kauffmann M, Schweiger M. Aging and chromosomal instability. Rev Physiol Biochem Pharmacol. 1999;139:141–174. doi: 10.1007/BFb0033651. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Cutler RG, Weindruch R, Renquist DM, Knapka JJ, April M, Belcher CT, Clark MA, Hatcherson CD, Marriott BM, Roth GS. Dietary restriction and aging: the initiation of a primate study. J Gerontol. 1990;45:B148–B163. doi: 10.1093/geronj/45.5.b148. [DOI] [PubMed] [Google Scholar]
- Jacobs PA, Court-Brown WM, Doll R. Distribution of human chromosome counts in relation to age. Nature. 1961;191:1178–1180. doi: 10.1038/1911178a0. [DOI] [PubMed] [Google Scholar]
- Jacobs PA, Brunton M, Court-Brown WM, Doll R, Goldstein H. Change in human chromosome count distributions with age: evidence for a sex difference. Nature. 1963;197:1080–1081. doi: 10.1038/1971080a0. [DOI] [PubMed] [Google Scholar]
- Jacobs PA, Brunton M, Court-Brown WM. Cytogenetic studies in leucocytes on the general population: subjects 65 years or more. Ann Hum Genet. 1964;27:353–365. doi: 10.1111/j.1469-1809.1963.tb01532.x. [DOI] [PubMed] [Google Scholar]
- Lane MA, Ingram DK, Roth GS. Beyond the rodent model: calorie restriction in rhesus monkeys. AGE. 1997;20:45–56. doi: 10.1007/s11357-997-0004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Mattison J, Ingram DK, Roth GS. Caloric restriction and aging in primates: relevance to humans and possible CR mimetics. Microsc Res Tech. 2002;59:335–338. doi: 10.1002/jemt.10214. [DOI] [PubMed] [Google Scholar]
- Lucas JN, Deng W, Moore D, Hill F, Wade M, Lewis A, Sailes F, Kramer C, Hsieh A, Galvan N. Background ionizing radiation plays a minor role in the production of chromosome translocations in a control population. Int J Radiat Biol. 1999;75:819–827. doi: 10.1080/095530099139872. [DOI] [PubMed] [Google Scholar]
- Ly DH, Lockhart DJ, Lerner RA, Schultz PG. Mitotic misregulation and human aging. Science. 2000;287:2486–2492. doi: 10.1126/science.287.5462.2486. [DOI] [PubMed] [Google Scholar]
- Marlhens F, Achkar W, Aurias A, Couturier J, Dutrillaux AM, Gerbault-Seureau M, Hoffschir F, Lamoliatte E, Lefrancois D, Lombard M, Muleris M, Prieur M, Prod’homme M, Sabatier L, Viegas-Péquignot E, Volobouev V, Dutrillaux B. The rate of chromosome breakage is age dependent in lymphocytes of adult controls. Hum Genet. 1986;73:290–297. doi: 10.1007/BF00279088. [DOI] [PubMed] [Google Scholar]
- Martin GM, Ohshima J. Lessons from human progeroid syndromes. Nature. 2000;408:263–266. doi: 10.1038/35041705. [DOI] [PubMed] [Google Scholar]
- Martin GM, Smith AC, Ketterer DJ, Ogburn CE, Disteche CM. Increased chromosomal aberrations in first metaphases of cells isolated form the kidneys of aged mice. Isr J Med. 1985;21:296–301. [PubMed] [Google Scholar]
- Mattevi MS, Salzano FM. Senescence and human chromosome changes. Humangenetik. 1975;27:1–8. doi: 10.1007/BF00283497. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Lane MA, Roth GS, Ingram DK. Calorie restriction in rhesus monkeys. Exp Gerontol. 2003;38:35–46. doi: 10.1016/S0531-5565(02)00146-8. [DOI] [PubMed] [Google Scholar]
- Maurer B, Guttenbach M, Schmid M (2003) Chromosomal instability in normative aging. In: Hisama FM, Weissman SM, Martin GM (eds) Chromosomal instability and aging. basic science and clinical implications. pp 125–147
- McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of the lifespan and upon ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- McCullagh P, Nelder JA. Generalized linear models. New York: Chapman and Hall; 1989. [Google Scholar]
- Moore CM, Janish C, Eddy CA, Hubbard GB, Leland MM, Rogers J. Cytogenetic and fertility studies of a rheboon, rhesus macaque (Macaca mulatta) x baboon (Papio hamadryas) cross: further support for a single karyotype nomenclature. Am J Phys Anthropol. 1999;110:119–127. doi: 10.1002/(SICI)1096-8644(199910)110:2<119::AID-AJPA1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Nath J, Tucker JD, Hando JC. Y chromosome aneuploidy, micronuclei, kinetochores and aging in men. Chromosoma. 1995;103:725–731. doi: 10.1007/BF00344234. [DOI] [PubMed] [Google Scholar]
- Nowinski GP, Dyke DL, Tilley BC, Jacobsen G, Babu VR, Worsham MJ, Wilson GN, Weiss L. The frequency of aneuploidy in cultured lymphocytes is correlated with age and gender but not with reproductive history. Am J Hum Genet. 1990;46:1101–1111. [PMC free article] [PubMed] [Google Scholar]
- Obe G, Herha J. Chromosomal aberrations in heavy smokers. Hum Genet. 1978;41:259–263. doi: 10.1007/BF00284759. [DOI] [PubMed] [Google Scholar]
- Ortiz R, Campos C, Gómez JL, Espinoza M, Ramos-Motilla M, Betancourt M. Sister-chromatid exchange (SCE) and cell proliferation in lymphocytes from infected and non-infected children with severe protein calorie malnutrition (PCM) Mutat Res. 1994;312:33–37. doi: 10.1016/0165-1161(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Pierre R, Hoagland H. Age-associated aneuploidy: loss of the Y chromosome from human bone marrow cells with aging. Cancer. 1972;30:889–894. doi: 10.1002/1097-0142(197210)30:4<889::AID-CNCR2820300405>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Prieur M, Achkar A, Aurias A, Couturier J, Dutrillaux A, Dutrillaux B, Flury-Herard A, Gerbault-Seureau M, Hoffschir F, Lamoliatte E, Lefrancois D, Lombard M, Muleris M, Ricoul M, Sabatier L, Viegas-Pequinot E. Acquired chromosome rearrangements in human lymphocytes: effects of aging. Hum Genet. 1988;79:147–150. doi: 10.1007/BF00280554. [DOI] [PubMed] [Google Scholar]
- Ramsey MJ, Moore DH, Briner JF, Lee DA, Olsen L, Senft JR, Tucker JD. The effects of age and life-style factors on the accumulation of cytogenetic damage as measured by chromosomal painting. Mutat Res. 1995;338:95–106. doi: 10.1016/0921-8734(95)00015-x. [DOI] [PubMed] [Google Scholar]
- Richard F, Aurias A, Couturier J, Dutrillaux AM, Flüry-Hérard A, Gerbault-Seureau M, Hoffschir F, Lamoliatte E, Lefrancois D, Lombard M, Muleris M, Prieur M, Richoul M, Sabatier L, Viegas-Péquignot E, Volobouev V, Dutrillaux B. Aneuploidy in human lymphocytes: an extensive study of eight individuals of various ages. Mutat Res. 1993;295:71–80. doi: 10.1016/0921-8734(93)90003-l. [DOI] [PubMed] [Google Scholar]
- Roth GS, Ingram DK, Lane MA. Calorie restriction in primates: will it work and how will we know? J Am Geriatr Soc. 1999;47:896–903. doi: 10.1111/j.1532-5415.1999.tb03851.x. [DOI] [PubMed] [Google Scholar]
- Schneider EL. Cytogenetics of aging. In: Schneider EL, editor. The genetics of aging. New York: Plenum; 1978. pp. 27–52. [Google Scholar]
- Semsei I. On the nature of aging. Mech Ageing Dev. 2000;117:93–108. doi: 10.1016/S0047-6374(00)00147-0. [DOI] [PubMed] [Google Scholar]
- Solomon E, Borrow J, Goddard AD. Chromosomal aberrations and cancer. Science. 1991;254:1153–1160. doi: 10.1126/science.1957167. [DOI] [PubMed] [Google Scholar]
- Stevenson KG, Curtis HJ. Chromosomal aberrations in irradiated and nitrogen mustard-treated mice. Radiat Res. 1961;15:774–785. doi: 10.2307/3571114. [DOI] [PubMed] [Google Scholar]
- Stone JF, Sandberg AA. Sex chromosome aneuploidy and aging. Mutat Res. 1995;338:107–113. doi: 10.1016/0921-8734(95)00016-y. [DOI] [PubMed] [Google Scholar]
- Tucker JD, Lee DA, Ramsey MJ, Briner J, Olsen L, Moore DH., II On the frequency of chromosome exchanges in a control population measured by chromosome painting. Mutat Res. 1994;313:193–202. doi: 10.1016/0165-1161(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Tucker JD, Spruill MD, Ramsey MJ, Director AD, Nath J. Frequency of spontaneous chromosome aberrations in mice: effects of age. Mutat Res. 1999;425:135–141. doi: 10.1016/s0027-5107(99)00036-6. [DOI] [PubMed] [Google Scholar]
- Vijg J, Gossen JA. Somatic mutations and cellular aging. Comp Biochem Physiol. 1993;104B:429–437. doi: 10.1016/0305-0491(93)90264-6. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Stanyon R, Jauch A, Cremer T. Homologies in human and Macaca fuscata chromosomes revealed by in situ suppression hybridization with human chromosome specific libraries. Chromosoma. 1992;101:265–270. doi: 10.1007/BF00346004. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford R. The retardation of aging and disease by dietary restriction. Springfield, IL: Thomas; 1988. [Google Scholar]
- Weirich-Schwaiger H, Weirich HG, Gruber B, Schweiger M, Hirsch-Kauffmann M. Correlation between senescence and DNA repair in cells from young and old individuals and in premature aging syndromes. Mutat Res. 1994;316:37–48. doi: 10.1016/0921-8734(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Wojda A, Witt M. Manifestations of ageing at the cytogenetic level. J Appl Genet. 2003;44:383–399. [PubMed] [Google Scholar]
- Xue K, Wang S, Zhou P, Wu P, Zhang R, Xu Z, Chen W, Wang Y. Micronucleus formation in peripheral blood lymphocytes from smokers and the influence of alcohol- and tea-drinking habits. Int J Cancer. 1992;50:702–705. doi: 10.1002/ijc.2910500506. [DOI] [PubMed] [Google Scholar]
- Yu BP. Modulation of aging processes by dietary restriction. Boca Raton: CRC; 1994. [Google Scholar]