Abstract
Neurons in the superior paraolivary nucleus (SPON) respond to the offset of pure tones with a brief burst of spikes. Medial nucleus of the trapezoid body (MNTB) neurons, which inhibit the SPON, produce a sustained pure tone response followed by an offset response characterized by a period of suppressed spontaneous activity. This MNTB offset response is duration dependent and critical to the formation of SPON offset spikes (Kadner et al., 2006; Kulesza, Jr. et al., 2007). Here we examine the temporal resolution of the MNTB/SPON circuit by assessing its capability to i) detect gaps in tones, and ii) synchronize to sinusoidally amplitude modulated (SAM) tones. Gap detection was tested by presenting two identical pure tone markers interrupted by gaps ranging from 0–25 ms duration. SPON neurons responded to the offset of the leading marker even when the two markers were separated only by their ramps (i.e., a 0 ms gap); longer gap durations elicited progressively larger responses. MNTB neurons produced an offset response at gap durations of 2 ms or longer, with a subset of neurons responding to 0 ms gaps. SAM tone stimuli used the unit’s characteristic frequency as a carrier, and modulation rates ranged from 40–1160 Hz. MNTB neurons synchronized to modulation rates up to ~1 KHz, whereas spiking of SPON neurons decreased sharply at modulation rates ≥ 400 Hz. Modulation transfer functions based on spike count were all-pass for MNTB neurons and low-pass for SPON neurons; the modulation transfer functions based on vector strength were low-pass for both nuclei, with a steeper cut-off for SPON neurons. Thus, the MNTB/SPON circuit encodes episodes of low stimulus energy, such as gaps in pure tones and troughs in amplitude modulated tones. The output of this circuit consists of brief SPON spiking episodes; their potential effects on the auditory midbrain and forebrain are discussed.
Keywords: superior olivary complex, brainstem, gap detection, amplitude modulation, sound envelopes
INTRODUCTION
The ability to resolve the fine temporal structure of auditory stimuli is a critical aspect of hearing, and deficits in temporal processing are reportedly related to language impairment (Tallal et al., 1985a, 1985b). Two paradigms commonly used to assess the temporal acuity of auditory circuits are gap detection (reviewed by Phillips, 1999) and coding of sound envelopes (for reviews see Langner, 1992; Frisina, 2001; Joris et al., 2004).
The acuity with which gaps, or silent periods, are detected between closely spaced sounds or components of sounds plays an important role in speech perception (Tyler et al., 1982; Irwin and McAuley, 1987; Glasberg and Moore, 1989; Snell and Frisina, 2000; Snell et al., 2002). Most often, gap detection tasks involve presenting two sounds of equal duration, referred to as leading and trailing markers, with a gap of variable length between them. In humans, the perception in this stimulus paradigm is of two stimuli separated in time when the gap is relatively long. For short gap durations, the perception changes to that of a single discontinuity in an otherwise homogeneous sound (Moore, 1993). Human gap detection thresholds in this paradigm, using noise bursts well above threshold as gap markers, are 2–3 ms (Moore, 1993).
Neural correlates of gap detection have been demonstrated in the frog auditory system (Feng et al., 1994) and various mammalian species including chinchilla (Giraudi et al., 1980, Giraudi-Perry et al., 1982; Salvi and Arehole, 1985), rat (Syka et al., 2002; Rybalko and Syka, 2005), mouse (Barsz et al., 2002; Walton et al., 2002; Allen et al., 2003), gerbil (Wagner et al., 2003), ferret (Kelly et al., 1996), and cat (Eggermont, 1999, 2000) using a variety of behavioral and electrophysiological paradigms. A study comparing behavioral and auditory brainstem response (ABR) gap detection thresholds showed the two thresholds to be similar, with the ABR thresholds tending to be lower (Werner et al., 2001). Thus, the information necessary to explain psychophysical performance in a gap detection paradigm appears to be present at the level of the auditory brainstem. To our knowledge however, the neuronal basis for gap detection has not been previously examined in the brainstem.
The envelope of speech signals contains amplitude modulations over a wide range of modulation rates (MRs), ranging from 3–4 Hz up to several hundred Hz, and considerable speech information is contained in these amplitude modulations (reviewed by Joris et al., 2004). Auditory neuropathy and aging can degrade temporal processing mechanisms in the auditory system and impair speech comprehension even when hearing thresholds are unchanged (Frisina and Frisina, 1997; Zeng et al., 1999), suggesting that the ability to encode envelope fluctuations is critical to speech processing. Most studies of the responses of superior olivary nuclei to sinusoidally amplitude modulated (SAM) stimuli have focused on mechanisms of directional hearing, describing the interplay of contralateral inhibition and ipsilateral excitation in the lateral superior olive (Joris and Yin, 1995, 1998), and excitatory inputs from both ears in the medial superior olive (Joris and Yin, 1998). Recordings from awake rabbits suggest that while distinct mechanisms underlie the detection of interaural delays between SAM stimuli in the lateral and medial superior olives, both are ultimately based on coincidence detection (Batra et al., 1997a, 1997b).
The Medial Nucleus of the Trapezoid Body / Superior Paraolivary Nucleus circuit
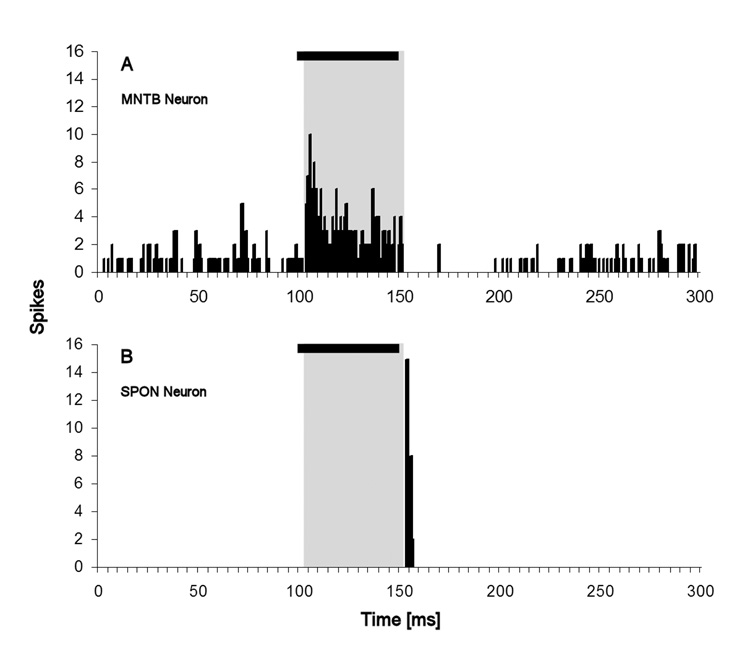
Single unit responses from the medial nucleus of the trapezoid body (MNTB) and superior paraolivary nucleus (SPON) of the rat demonstrate that these two brainstem nuclei form a neural circuit producing duration dependent responses to the offset of pure tones and phase locked responses to SAM stimuli (Kulesza, Jr. et al., 2003, 2007; Kadner et al., 2006). MNTB neurons display high rates of spontaneous activity and primary-like responses to characteristic frequency tones (cats: (Guinan, Jr. et al., 1972a, 1972b; Smith et al., 1998), gerbils: (Kopp-Scheinpflug et al., 2002), mice: (Kopp-Scheinpflug et al., 2003), rats: (Sommer et al., 1993; Paolini et al., 2001; Kulesza, Jr. et al., 2003; Kadner et al., 2006)). The sustained excitatory response of MNTB units is followed by a period of suppressed spontaneous activity, sometimes lasting several tens of milliseconds, depending on stimulus parameters, that we refer to as the MNTB offset response (Fig. 1A) (Kadner et al., 2006). The MNTB provides a strong glycinergic input to the nearby SPON (Helfert et al., 1989; Banks and Smith, 1992), whose neurons also display offset responses to pure tones; these however take the form of single or brief bursts of action potentials that occur after the stimulus offset (Fig 1B) (Kulesza, Jr. et al., 2003, 2007; Kadner et al., 2006). Release from MNTB-derived glycinergic inhibition is critical to the formation of SPON offset responses, as demonstrated by reversible pharmacological blockade of glycine receptors in the SPON (Kulesza, Jr. et al., 2007). SPON neurons utilize GABA as their neurotransmitter (Kulesza, Jr. and Berrebi, 2000) and their projection targets include the inferior colliculus and thalamus (Saldaña and Berrebi, 2000; Jin and Berrebi, 2006). There is also evidence for an extensive system of recurrent axon collaterals within SPON that provide a means for intrinsic GABAergic inhibition of SPON neurons as well as self-inhibition via autapses (Saldaña and Berrebi, 2000; Kulesza, Jr. et al., 2000).
Fig. 1. PSTHs display typical response characteristics of MNTB and SPON neurons to CF tones.
A: MNTB units display high rates of spontaneous activity, and in response to CF tones (20 dB above threshold) display prominent onset and sustained firing during the stimulus presentation. Note the suppression of spontaneous rate following the termination of the stimulus. B: SPON units have little or no spontaneous activity; in response to CF tones they discharge briefly at the stimulus offset. The black bar at the top of each panel represents the stimulus. The gray shading in both panels denotes the time interval of the excitatory component of the MNTB response.
In contrast to the medial and lateral superior olives, neurons in the rat SPON are monaurally driven and show no evidence of binaural interactions (Kulesza, Jr. et al., 2003, 2007; Kadner et al., 2006), suggesting that the MNTB/SPON circuit encodes a stimulus feature other than sound source location. Based on current knowledge of its response properties, we hypothesized that this brainstem circuit may be particularly well suited for encoding stimulus features associated with episodes of low or rapidly decreasing sound energy, such as tone offsets and the falling flanks and troughs in SAM stimuli.
Hypotheses and experimental paradigms
Because they respond to the stimulus offset, we predicted that SPON neurons would respond to the offset of both the leading and trailing markers in a gap detection paradigm, with the response to the leading marker indicating the presence of a gap. Thus, we expected the SPON to encode gaps by firing brief bursts of actions potentials at or near the beginning of the gap. Since the SPON offset response critically depends on the MNTB offset response, we expected that MNTB offset responses would display gap detection thresholds similar to those of SPON units. Another indication that MNTB neurons process the leading and trailing markers as separate stimuli would be the appearance of an onset response component to the trailing gap marker. The experimental paradigm used to test these hypotheses was based on the commonly used psychophysical approach described above; we recorded responses of SPON and MNTB neurons to a sequence of two identical characteristic frequency (CF) tones separated by a silent gap of variable duration.
SPON neurons also respond to SAM stimuli with precise synchronization to the stimulus envelope. Our original characterization of SPON neurons suggested that at low MRs their firing coincides with the periodically occurring gaps in MNTB responses to SAM stimuli (Kulesza, Jr. et al., 2003). Therefore, another aim of the present study was to shed light on the neuronal mechanisms underlying SAM processing by examining how neurons of the MNTB/SPON circuit encode envelope periodicities. Under the assumption that responses of SPON units to SAM stimuli are generated by the same rebound mechanism underlying their pure tone responses, we hypothesized that the occurrence of SPON spikes would closely follow the timing of silent periods in the SAM response of MNTB neurons.
EXPERIMENTAL PROCEDURES
Animals and surgery
Fifty-seven female Sprague-Dawley rats (Hilltop Lab Animals, Scottsdale, PA or Harlan Sprague Dawley, Indianapolis, IN) weighing 190–250 grams were used in this study. These animals were housed in the West Virginia University Health Sciences Center vivarium, an AAALAC-approved animal facility. All procedures were approved by the University’s Animal Care and Use Committee and conformed to all applicable federal guidelines regarding the care and use of laboratory animals.
Anesthesia was administered by intramuscular injection of a mixture of ketamine (70 mg/kg) and xylazine (5 mg/kg). The head was then shaved and the animal mounted in a stereotaxic frame using blunt hollow earbars to avoid injury to the tympanic membrane. A scalp incision was made and the connective tissue covering the skull removed. A custom-fabricated head post was secured to the skull at bregma by drilling into the skull and applying screws and dental cement.
To access both sides of the brainstem, a craniotomy (approximately 3 × 7 mm) was performed with the rostral edge of the bone defect extending to the posterior aspect of the transverse sinus. The dura mater was opened and the exposed cerebellum aspirated to uncover the floor of the fourth ventricle, whose midline was used as a landmark for electrode penetrations. The rat was then transferred to a sound-attenuated recording booth and placed on a heating blanket that maintained body temperature at approximately 37° C. When deemed necessary, subcutaneous injections of 1 ml physiological saline were given at hourly intervals to compensate for loss of body fluids. Supplementary injections of 24 mg/kg ketamine were also administered during the recording sessions as needed to maintain anesthesia.
Acoustic stimuli and sound delivery
The acoustic stimuli were created as digital waveforms using BATLAB control software (created by Dr. Donald P. Gans, Northeastern Ohio Universities College of Medicine, Rootstown OH). Digital signals were converted to analog by a Microstar DAP5216a data acquisition processor (Microstar Laboratories, Bellevue, WA) and passed through an anti-aliasing filter (FT6-2, Tucker Davis Technologies (TDT), Alachua, FL). Attenuation was controlled by PA-5 programmable attenuators (TDT). The signals were then routed to a TDT ED1 speaker driver and presented through ES1 free-field speakers mounted in the stereotaxic frame approximately 5 mm from the opening of the ear canal. To avoid spectral contamination of acoustic stimuli by on and offset clicks, all stimuli were phased in and out using cos2 ramps of 2 ms duration. This duration was selected to extend over two full periods of a 1 KHz tone, the lowest characteristic frequency we expected to encounter (Kulesza, Jr. et al., 2003; Kadner et al., 2006). The output of the speakers was calibrated and converted to dB SPL offline using a B&K Type 4939 microphone connected to a Type 2610 measuring amplifier (Brüel and Kjaer North America, Norcross, GA).
Electrophysiological recordings
The MNTB and SPON were approached using stereotaxic coordinates provided in the atlas of the rat brain (Paxinos and Watson, 1986). Electrode penetrations were initially placed 1.5 mm on either side of the midline. Single units in each nucleus were found using a repetitive 50 ms broadband noise burst, presented at 80–100 dB SPL, as a search stimulus. Recorded units were presumed to be from the MNTB if they were located 3.5–4.5 mm beneath the floor of the fourth ventricle and displayed a contralaterally driven sustained response to noise bursts and tones with first spike latencies >3 ms from the stimulus onset. The absence of binaural interactions was verified by presenting 50 ms noise bursts at 5–10 dB above threshold monaurally to each ear and then binaurally. Multiple electrode penetrations were made along the lateral-medial axis of the brainstem of each animal, and the CF and position of each presumed MNTB unit was cross-checked against the well-established tonotopic gradient of characteristic frequencies in this structure (Paolini et al., 2001).
Contralaterally driven units encountered at a depth of 2.8–3.5 mm from the floor of the fourth ventricle were presumed to represent SPON neurons; in virtually all cases these units displayed brief bursts of action potentials with first spike latencies of 6–8 ms from the stimulus offset. The limited medial-lateral extent of the SPON made it difficult to confirm the existence of a gradient of CFs in the nucleus. However, in accord with previously published tonotopic maps of this nucleus, we verified that recorded SPON units were superficial to, and had higher CFs than, MNTB units recorded in the same vertical electrode penetrations (Kulesza, Jr. et al., 2003).
Single unit responses were recorded using glass pipette electrodes (tip diameters of 2–4 µm) filled with 2.5% Biocytin (Sigma Chemical, St. Louis, MO) dissolved in physiological saline. Electrode signals were amplified and bandpass filtered between 200 and 3000 Hz with a Krohn-Hite Model 3364 Filter (Krohn-Hite, Brockton, MA). The overall signal gain was set so that the amplitude of an action potential at the end of the amplifier chain was between 2 and 3 V. The waveforms were digitized at a rate of 42 KHz by the Microstar DAP5216a data acquisition processor. A unit was considered well isolated if the auditory evoked spike waveforms were homogeneous and could be reliably separated from the background noise by a trigger window. The isolation of the units was later verified by offline examination of digital records of the spike waveforms. Characteristic frequency and threshold were determined for all units by presenting numerous frequency-intensity combinations of pure tones, each 50 ms in duration.
Histological localization of recording sites
When well-isolated units were recorded, Biocytin was deposited at several locations along the electrode track by applying an anodic current of 1.5 µA for 5 min., and recording sites were subsequently verified histologically as described previously (Kulesza, Jr. et al., 2003). Briefly, upon completion of the recording session each animal was perfused through the ascending aorta with saline followed by 4% paraformaldehyde in 0.12 M sodium phosphate buffer, pH 7.2. Brains were then removed from the cranium, cryoprotected overnight, and frozen sectioned in the coronal plane. Free-floating sections were processed according to the ABC method (Vector Laboratories) using diaminobenzidine with cobalt chloride and nickel ammonium sulfate as the chromogen. These sections were then mounted onto glass slides and counterstained with cresyl violet or neutral red. Boundaries of the rat MNTB and SPON were determined relative to the midline and ventral surface of the brainstem, other SOC nuclei, and prominent bundles of fibers that demarcate their borders (Fig. 2) (Kulesza, Jr. et al., 2002).
Fig. 2. Localization of superior paraolivary nucleus (SPON) and medial nucleus of the trapezoid body (MNTB) recording sites.
A: Transverse sections of the brainstem were counterstained with neutral red to facilitate accurate identification of the boundaries of superior olivary nuclei. B: Higher magnification photomicrograph reveals small biocytin deposit in SPON (arrow). In most cases, such deposits caused the retrograde labeling of neighboring neurons in SPON, as well as neurons in MNTB neurons that project to the injection site (lower right corner). C: Photomicrograph of a recorded MNTB neuron labeled by biocytin deposit. D: dorsal; M, medial; LNTB, lateral nucleus of the trapezoid body; LSO, lateral superior olive; MSO, medial superior olive; tb, midline trapezoid body; VNTB, ventral nucleus of the trapezoid body.
Experimental paradigms
To determine a unit’s gap detection threshold, we employed a multiple step recording protocol. First, a no stimulation condition was used to determine the unit’s spontaneous activity rate. Next, we recorded the unit’s response to a continuous 100 ms CF tone, presented at 20 dB above threshold. This continuous stimulus was then replaced by two separate, but identical, 50 ms markers. For control purposes, the cell’s responses to the two markers were recorded separately (hereafter called the leading and trailing marker only conditions, respectively). These leading and trailing markers were then presented in succession as the duration of the gap between them was varied from 0 to 25 ms. Note that in the 0 ms condition the downward ramp of the leading marker and the upward ramp of the trailing marker abutted each other, so that no true silent period existed between the stimuli. To create the gap, the interval between the beginning of the recording epoch and the onset of the leading marker was varied, whereas the onset of the trailing marker relative to the recording epoch was held constant at 150 ms. Each of the stimulus conditions was presented 20 times.
SAM stimuli used each unit’s CF as the carrier frequency, and the MR was varied from 40 to 1160 Hz in 40 Hz steps. All stimuli had durations of 500 ms and were presented eight times. A subset of neurons was presented with 20 repetitions of SAM tones with MRs between 40 and 360 Hz.
Definition of temporal analysis windows
Detection of a gap requires processing of the leading and trailing markers as separate stimuli. If the markers are processed separately, response components associated with the offset of the leading and/or the onset of the trailing marker should be present. SPON neurons may signal the presence of a gap by producing an offset response to the leading marker, whereas in MNTB units the presence of a gap may evoke an offset response to the leading marker, an onset response to the trailing marker, or both. Therefore, to assess whether or not a gap was detected, unit responses in the presence of a gap were compared with the response to the continuous stimulus condition. In the case of the MNTB offset response to the leading marker, a comparison to the spontaneous discharge rate was also necessary to ascertain if a deviation from the response to the continuous stimulus constituted an actual MNTB offset response, i.e., a suppression of spiking activity to below spontaneous levels.
Defining the analysis window for the SPON offset responses was straightforward, since SPON neurons showed very little or no spontaneous activity and virtually all spikes present in the recordings belonged to the offset responses (Fig. 1B). Therefore, the analysis window could be of fixed duration and in a fixed position relative to the markers without incurring large errors. Thus, the defined analysis windows for SPON offset responses started at the leading marker offset and lasted 35 ms.
Responses of MNTB neurons, however, are characterized by extremely rapid changes of discharge rate. One such change occurs at the beginning of the response where the spike rate changes instantaneously from the neuron’s spontaneous rate to the elevated rate in the onset component of the response. A change in the opposite direction occurs at the end of the sustained response component, where the elevated spike rate is succeeded by a spike rate near zero during the neuron’s offset response. When two markers are presented in rapid succession, the offset response to the leading marker, with its near zero spike rate, continues until the onset component of the response to the trailing marker occurs, and with it another instantaneous change of spike rate. To accurately quantify the offset responses of MNTB neurons, during which spiking activity is suppressed, we needed to define temporal analysis windows that excluded spikes from the adjacent excitatory response components. Therefore, we used the leading marker only condition to determine, for each MNTB unit, the first spike latency (FSL), the duration of the continued excitation (i.e. the period after the end of the stimulus during which the sustained part of the MNTB response persisted), and the offset response duration, defined as the interval between the end of the continued excitation and the resumption of the unit’s spontaneous activity (Fig. 3). We then defined the analysis window for each MNTB unit’s offset response to the leading marker as beginning at the end of the continued excitation and lasting either until the end of the FSL of the response to the trailing marker, or until the end of the previously determined offset response, whichever occurred first. Note that the timing of this analysis window varied between gap durations and also between recordings of different neurons when gap durations were the same. Moreover, if the continued excitation was of equal duration or longer than the FSL, analysis windows could only be defined for stimulus conditions where the gap duration was longer than the duration difference between the continued excitation and FSL. The rules defining the time windows for measuring the MNTB offset response are provided in Table 1. The analysis window for the MNTB onset response to the trailing marker began at the end of the FSL and lasted for 15 ms.
Fig. 3. Timing of MNTB response components and gap detection analysis windows.
A: Response of an MNTB neuron in the leading marker only condition. The black bar at the top of the diagram represents the stimulus. The light gray shaded areas represent the first spike latency (FSL) and continued excitation (CE), in this example 4 and 3 ms, respectively; the dark gray shading marks the MNTB offset response duration (ORD) (43 ms in this example). B: Response of the same MNTB neuron to leading and trailing markers (black bars) separated by a 10 ms gap. The continued excitation (CE) to the leading marker and the first spike latency (FSL) of the response to the trailing marker are denoted by dotted vertical lines; these parameters were used to define the temporal analysis window for the MNTB offset response to the leading marker (shaded in light gray). The analysis window in this example started at 143 ms and ended at 154 ms.
Table 1.
Analysis windows defining the MNTB offset response to the leading marker
| Condition | Begin | End |
|---|---|---|
| No stimulation | 0 ms | 300 ms |
| Leading marker only | 150 ms + CE | 150 ms + CE + ORD |
| Trailing marker only | 150 ms + CE | 150 ms + CE + ORD |
| Continuous stimulus | 150 ms + CE | 150 ms + CE + 30 ms |
| Markers with gaps | 150 ms + CE − Gap | 150 ms + CE + ORD |
| or | ||
| 150 ms + FSL |
CE, continued excitation; ORD, offset response duration; FSL, first spike latency.
For analyzing SAM responses we defined a window starting 15 ms after the onset and ending at the offset of the stimulus. This minimized the influence of unsynchronized spikes associated with stimulus onset on the calculation of vector strength (see below).
Data analysis and statistical methods
Gap Detection
Because quantification of the MNTB offset response to the leading marker required analysis windows of variable duration, spike counts had to be normalized for window duration, converting them into spike rates. To facilitate comparison with the peristimulus time histograms (PSTHs) shown in Figure 5–Figure 7, rates are reported in spikes/ms units and refer to 20 presentations of the stimulus. Therefore, the reported spike rates must be multiplied by 50 to yield the more conventional measure of spikes/s per presentation. To arrive at the best possible estimate of spontaneous rate, this measure was calculated from the entire 300 ms recording duration of the no stimulation condition.
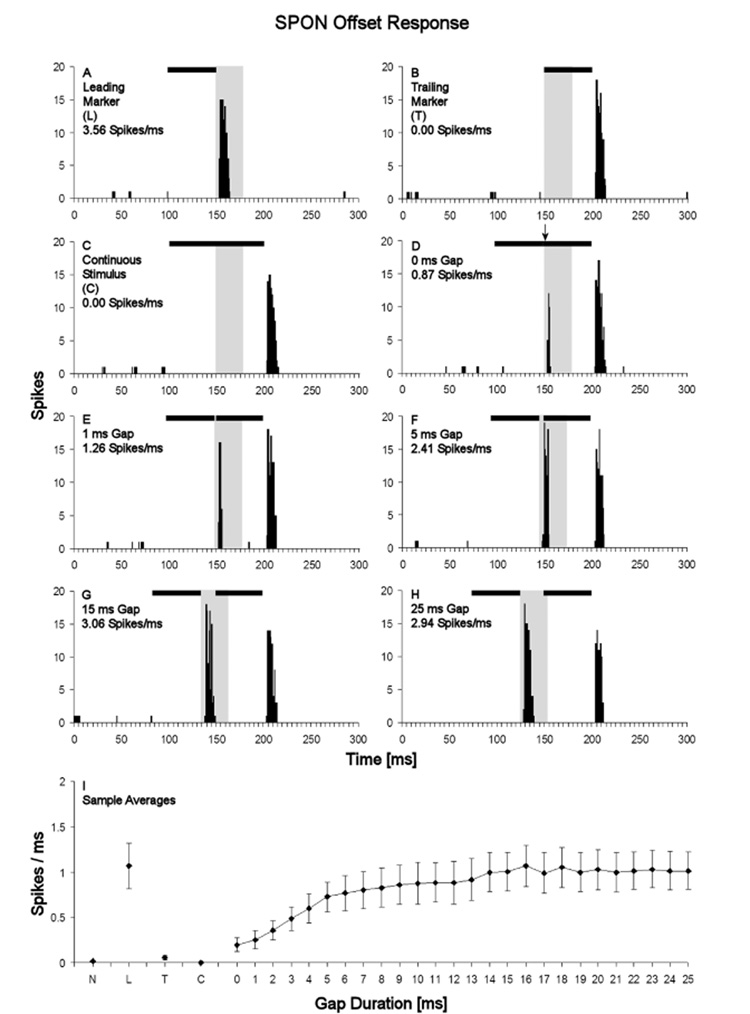
Fig. 5. Gap sensitivity of the SPON offset response to the leading gap marker.
Peri-stimulus time histograms show the responses of an SPON neuron in the leading marker only condition (A), the trailing marker only condition (B), and the continuous stimulus control condition (C). D–H: Responses of this neuron to gaps of 0, 1, 5, 15 and 25 ms duration, respectively. Black bars at the top of the each PSTH denotes the stimulus; the arrow above the bar in panel D marks the point at which the leading marker ends and the trailing marker begins. The analysis window used for quantifying the neuron’s offset responses to the leading marker are shaded in gray. I: Sample averages of the magnitude of SPON offset responses to the leading marker plotted over gap duration. Note that response magnitudes in panels A–H are given as rates, expressed in spikes/ms. Error bars represent standard errors of the means. Abbreviations on the horizontal axis are: N, no stimulation condition; L, leading marker only condition; T, trailing marker only condition; C, continuous stimulus control condition.
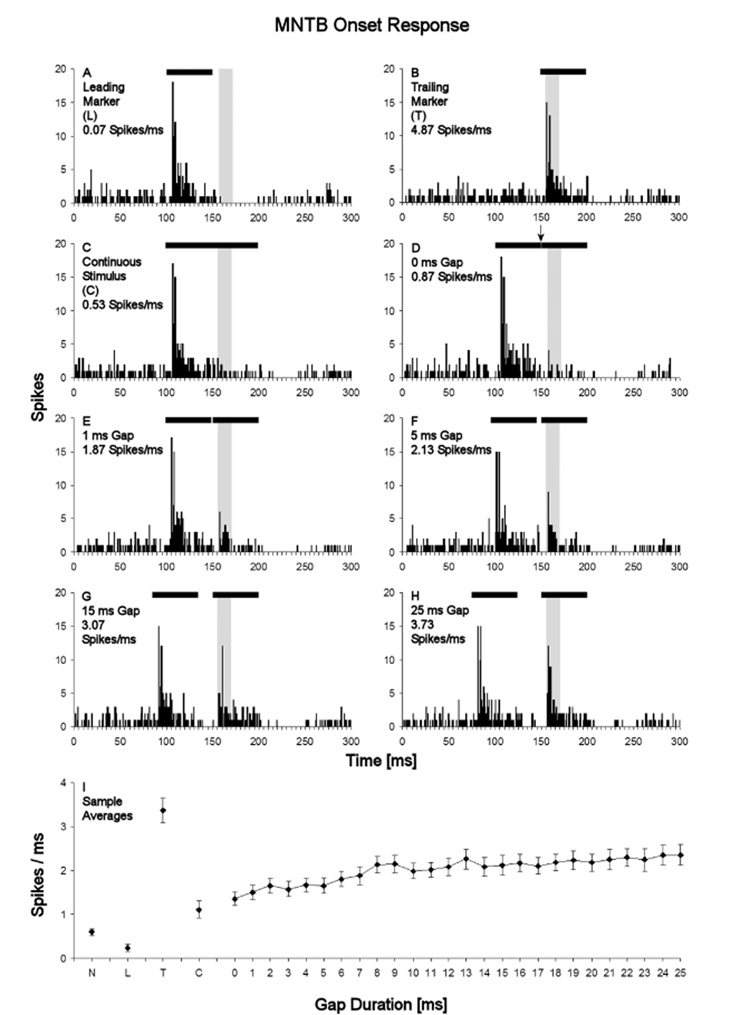
Fig. 7. Gap sensitivity of the MNTB onset response to the trailing marker.
Peristimulus time histograms show the responses of the same MNTB neuron as in Figure 4, in the leading marker only condition (A), the trailing marker only condition (B), and the continuous stimulus control condition (C). D–H: Responses of this neuron to gaps of 0, 1, 5, 15 and 25 ms duration, respectively. Black bars at the top of each PSTH denotes the stimulus, and the arrow above the bar in panel D marks the point at which the leading marker ends and the trailing marker begins. The analysis window used for quantifying the neuron’s onset responses to the trailing marker are shaded in gray. I: Sample averages of the magnitude of MNTB onset responses to the trailing marker plotted over gap duration. Note that response magnitudes in panels A–H are given as rates, expressed in spikes/ms. Error bars represent standard errors of the mean. Other abbreviations are as in Figure 4.
Statistical analyses were based on within-subjects comparisons of spike rates between the various stimulus conditions. Visual inspection of the error bars in Figure 5–Figure 7 revealed that the variance of spike rates within our samples varied widely between the stimulus conditions. Therefore, the non-parametric Friedman test, which makes no assumptions about variance, was used for the within-subject comparisons. Following a significant result of the Friedman test, responses to individual stimulus conditions were compared using a post-hoc Wilcoxon matched-pairs signed-rank test.
Synchronization to SAM stimuli
At each MR, discharge rates were determined, and the synchronization of responses to the MR of the stimulus was quantified by calculating the vector strength r (Goldberg and Brown, 1969) using the formula
| (1) |
where ai is the phase angle of spike i relative to the modulation cycle of the stimulus, and n is the total number of spikes in the analysis window. To minimize the influence of isolated spikes that were time-locked to the stimulus onset (and therefore occurred at a constant phase angle), the vector strength was set to 0 when the spike count fell to fewer than 2 spikes per stimulus presentation. A vector strength of 1 indicates perfect entrainment between the neuronal response and the modulation phase, while a vector strength of 0 indicates no correlation. The Rayleigh test (Stephens, 1969; Batschelet, 1981; Wilkie, 1983) was used to assess whether the distribution of spikes relative to the phase angle of the stimulus significantly differed from randomness, i.e., whether the stimulus imparted a temporal structure on the response. It is noteworthy that the Rayleigh test can return significant results from very few spikes that might not constitute actual phase locking. This can occur if spikes are time locked to the stimulus onset and therefore occur at near constant phase angles, making them appear phase locked. Therefore, as with vector strengths, units that displayed fewer than 2 spikes per stimulus were not considered phase-locked.
Period histograms were constructed from the subset of recordings containing 20 stimulus presentations by plotting the phase angle of each spike relative to the amplitude modulation of the stimulus. The phase angles at which spiking began and ended were measured in all recordings where phase locking was found. To visualize the relative timing of MNTB and SPON spiking, sample averages and standard errors of the mean were calculated. Because the phase angle is a circular variable, formulae for the mean phase angle and circular standard deviation were taken from (Batschelet, 1981). The mean phase angle ā is given by
| (2) |
and the standard deviation Stdevcirc of a circular variable is given by
| (3) |
where r is the vector strength from formula (1). Finally, the standard error of the mean was calculated as
| (4) |
RESULTS
GAP DETECTION PARADIGM
Gap sensitivity of the SPON offset response to the leading marker
We recorded from 15 SPON units with CFs ranging from 3 to 42 KHz and whose spontaneous rates averaged 0.016 ± 0.01 spikes/ms (equivalent to 0.812 ± 0.52 spikes/s per presentation). The thresholds and CFs of these units are plotted in Figure 4. The typical pure tone response of SPON neurons consisted of a single spike or a short burst of spikes following the stimulus offset (Fig. 1B; Fig. 5A–C). Peri-stimulus time histograms from a representative SPON unit and the rate-gap function calculated for our SPON sample are provided in Figure 5.
Fig. 4. Characteristic frequencies and thresholds of recorded MNTB and SPON neurons.
We recorded from a total of 52 MNTB units (×) and 35 SPON units (Δ). The MNTB sample displayed CFs ranging from 2.1-56 kHz and thresholds ranging from 6.5-79 dB SPL. The SPON sample displayed CFs ranging from 0.9-50 kHz and thresholds ranging from 8.5-77 dB SPL. On average, thresholds of MNTB units were 17.3 dB lower than those of SPON units.
To establish a reference point for the assessment of gap responses, the spiking activity in response to the continuous stimulus condition was measured in the same time window as that defined to quantify the leading marker offset response in the 0 ms gap condition (grey shaded boxes in Figs. 5A–H). For our sample of units, the spike rate in this continuous stimulus control condition was 0.000 ± 0.00 spikes/ms, i.e., none of the neurons fired any spikes during this period (Fig. 5I). The introduction of even a 0 ms gap elicited an offset response to the leading marker with an average spike rate of 0.197 ± 0.07 spikes/ms, and longer gaps elicited progressively higher spike rates until a maximal spike rate of 1.066 ± 0.22 spikes/ms was reached at a gap duration of 16 ms (Fig. 5I). At this point the spike rate had reached the same value as that obtained in the leading marker only condition, which was 1.067 ± 0.25 spikes/ms. Further lengthening of the gap did not lead to increased spike rates; the value observed for the 25 ms gap was 1.016 ± 0.21 spikes/ms (Fig. 5I). Statistical analysis of spike rates in the temporal analysis windows defined for the SPON offset response to the leading marker showed that this offset response was significantly dependent on stimulus condition (Friedman test, p<0.001).
The presence of an offset response to the leading marker was considered evidence of gap detection. The relevant post-hoc comparisons were between the gap conditions and the continuous stimulus control condition, where responses were never observed in the analysis window (Figs. 5C, I). Therefore, this comparison revealed a significantly elevated spike rate, i.e., the presence of an offset response to the leading marker, for all gap durations tested (Wilcoxon matched-pairs signed-rank test, p<0.05 for 0 and 1 ms gap durations; p<0.01 for all longer gap durations).
First spike latencies of SPON responses
Our sample of SPON units responded to the isolated leading marker with average first spike latencies (FSLs) of 5.3 ± 0.5 ms. Six neurons (40%) responded to 0 ms gaps with an average FSL of 4.8 ± 0.6 ms; these latencies were statistically equivalent (p=0.7, Wilcoxon Signed Rank test for paired samples), indicating that the temporal relationship between the stimulus and the response is the same for isolated pure tones as for leading gap markers. Consequently, we can assume that the temporal relationship between MNTB and SPON offset responses is also unchanged, which supports our selection of the temporal analysis window for measuring the MNTB offset response to the leading marker (see Definition of temporal analysis windows). Moreover, the average FSLs of responses to the isolated leading marker was significantly shorter in units that responded to the 0 ms gap (n = 6; mean FSL = 4.2 ± 0.4 ms) than in those that did not (n = 9; mean FSL = 6.3 ± 0.6 ms; p>0.05, Mann-Whitney U test). Thus, it appears that an SPON neuron can only respond to a gap when the FSL of its response to the offset of the leading marker is shorter than the time required for inhibition generated by the trailing marker to arrive.
Gap sensitivity of the MNTB offset response to the leading marker
We recorded from 19 MNTB units whose CFs ranged from 3 to 47 KHz. These units displayed vigorous spontaneous activity (averaging 0.596 ± 0.08 spikes/ms, equivalent to 29.78 ± 3.90 spikes/s per presentation); their thresholds and CFs are shown in Figure 4. FSLs averaged 4.7 ± 0.3 ms from the onset of the leading marker. Offline inspection of digitally recorded waveforms showed no evidence of pre-potentials as described in gerbil MNTB recordings (Kopp-Scheinpflug et al., 2002). Peri-stimulus time histograms from a representative MNTB neuron and the rate-gap functions calculated for the offset response of our sample of MNTB cells are provided in Figure 6. The pure tone response of MNTB units was characterized by a strong onset component, followed by a period of sustained firing during the stimulus and the suppression of spiking following the stimulus offset (Fig. 6B). An offset response to the leading marker was identified by a diminished spike rate compared to the no stimulation and continuous stimulus control conditions. In individual unit PSTHs, the offset response to the leading marker was usually clearly discernible at gap durations of 5 ms or longer (Figs. 6D–H). Knowledge of the timing of the analysis window was required to visually identify the offset response at shorter gap durations. In our sample of MNTB neurons, the spike rate recorded in the defined offset response window during the continuous stimulus control condition was 1.072 ± 0.19 spikes/ms (Figs. 6C, I). When a 0 ms gap was introduced, the spike rate fell to 0.549 ± 0.12 spikes/ms; for a 1 ms gap a spike rate of 0.350 ± 0.11 spikes/ms was recorded. At longer gap durations the spike rates remained nearly constant, reaching a value of 0.222 ± 0.06 spikes/ms for a 25 ms gap (Fig. 6I). Statistical analysis of the spike rates in the temporal analysis window defined for the offset response to the leading marker revealed that spike rates varied significantly between stimulus conditions (Friedman test, p<0.001).
Fig. 6. Gap sensitivity of the MNTB offset response to the leading gap marker.
Peri-stimulus time histograms show the responses of an MNTB neuron in the no stimulation condition (A) from which its spontaneous rate was determined, the leading marker only condition (B), and the continuous stimulus condition (C). D–H: Responses of this neuron to gaps of 0, 1, 5, 15 and 25 ms duration, respectively. Black bars at the top of each PSTH denotes the stimulus; the arrow above the bar in panel D marks the point at which the leading marker ends and the trailing marker begins. The analysis window used for quantifying the neuron’s offset responses to the leading marker are shaded in gray. I: Sample averages of the magnitude of MNTB offset responses to the leading marker plotted over gap duration. The dashed horizontal line indicates the sample’s average rate of spontaneous activity. Note that response magnitudes in panels A–H are given as rates, expressed in spikes/ms. Error bars represent standard errors of the means. Other abbreviations are as in Figure 3.
The presence of an offset response to the leading marker was considered evidence of gap detection. Since the MNTB offset response manifests itself as a suppression of spiking activity, in gap conditions we expected spike rates lower than those observed in the no stimulation and continuous stimulus control conditions. Therefore, the relevant post-hoc comparisons were between the gap conditions and the continuous stimulus control condition, and between the gap conditions and the no stimulation control condition (Fig. 6I). The spike counts in the offset response analysis window were significantly lower than in the continuous stimulus condition for gaps of 1 ms or longer (p<0.05 for the 1 ms gap; p<0.01 for all longer gaps, Wilcoxon matched-pairs signed-rank test). Moreover, the spike rates in the offset response analysis window were also significantly lower than the spontaneous activity for gap durations of 2 ms or longer (p<0.05 for gaps between 2 and 8 ms; p <0.001 for gap durations above 8 ms, Wilcoxon matched-pairs signed-rank test). Significant outcomes of both statistical comparisons indicated the presence of an MNTB offset response to the leading marker.
Gap sensitivity of the MNTB onset response to the trailing marker
Another indication that markers are processed as separate stimuli is the emergence of an onset response to the trailing marker. PSTHs from the same MNTB neuron presented above, and the rate-gap functions calculated for the onset response to the trailing marker in our MNTB sample are provided in Figure 7. For this sample, the average spike rate recorded for the continuous stimulus condition within the temporal analysis window defined to quantify the trailing marker onset response was 1.102 ± 0.19 spikes/ms (Figs. 7I). When gaps were introduced, the spike rate in the trailing marker onset response window was elevated relative to the continuous stimulus control; a 0 ms gap produced an average spike rate of 1.351 ± 0.15 spikes/ms, rising in nearly monotonic fashion to 2.356 ± 0.25 spikes/ms for a 25 ms gap (Fig. 7I). However, the spike rate of the trailing marker onset response following a gap never approximated the value observed in the trailing marker only condition, which averaged 3.509 ± 0.30 spikes/ms (Fig. 7I). An onset response component to the trailing marker usually could be identified by an elevated spike rate relative to the continuous stimulus control in its analysis window when the gap duration was 0 ms, and the onset component was usually clearly discernible at gap durations of 1 ms or longer. These qualitative observations were confirmed by within-subjects comparison of spike rates in the onset response analysis window, which showed the responses to be significantly different between the various stimulus conditions (Friedman test, p<0.001). Post-hoc comparisons showed a significantly elevated spike rate for all gap durations compared to the continuous stimulus control condition (Wilcoxon matched-pairs signed-rank test, p<0.05). However, the spike rates of the onset responses to the trailing marker were significantly lower than those of the onset responses in the trailing marker only condition for all gap durations tested (Wilcoxon matched-pairs signed-rank test, p<0.01). This result indicates that MNTB neurons do not process the leading and trailing markers as fully separate stimuli, even at the longest gap durations tested.
SINUSOIDALLY AMPLITUDE MODULATED TONES
Synchronization of MNTB neurons to fluctuations in stimulus envelope
We recorded the SAM responses of 50 MNTB neurons, with CFs ranging from 2.1 to 56 KHz (Fig. 4; 17 of these were also studied in the gap detection paradigm). A subset of 27 of these neurons were presented with 20 repetitions of SAM tones with MRs between 40 and 360 Hz. PSTHs and period histograms from a representative MNTB neuron are provided in Figure 8. The responses of MNTB neurons to SAM stimuli are characterized by a pronounced onset component followed by a sustained component that is interrupted by regular silent periods. At low MRs these silent periods can be resolved in the PSTHs (Fig. 8, left panels), and period histograms (Fig. 8, right panels) reveal that this pattern persists at higher MRs, where the resolution of the normal PSTH is not sufficient to reveal the temporal fine structure of the response. For the range of MRs tested in this study, MNTB spiking is largely confined to about two-thirds of the modulation cycle.
Fig. 8. Typical responses of an MNTB neuron to sinusoidally amplitude modulated tones.
Left panels show PSTHs of responses to sinusoidally amplitude modulated tones; the analysis windows are shaded in gray. Right panels show period histograms generated from the spikes contained in the analysis window. MR, modulation rate; r, vector strength; p, p-value from the Rayleigh-test.
Synchronization of SPON neurons to fluctuations in stimulus envelope
We recorded the SAM responses of 30 SPON neurons, with CFs ranging from 0.9 to 50 KHz (Fig. 4; 10 of these were also studied in the gap detection paradigm). A subset of 13 SPON neurons were presented with 20 repetitions of SAM tones with MRs between 40 and 360 Hz. PSTHs and period histograms from a representative SPON neuron are provided in Figure 9. SPON responses to SAM stimuli contain fewer spikes than MNTB responses (Fig. 9, left panels; Fig. 10B). Spikes time-locked to the stimulus onset occur rarely and are less pronounced that seen in MNTB responses. At low MRs it is evident from the PSTHs that SPON spikes are much more tightly clustered than MNTB spikes, and period histograms (Fig. 9, right panels) show that this tight clustering of spikes into about one-third of the modulation cycle persists at all MRs that elicit regular spiking. At higher MRs, the spiking during the stimulus presentation is replaced by an offset response following the stimulus offset.
Fig. 9. Typical responses of an SPON neuron to sinusoidally amplitude modulated tones.
Left panels show PSTHs of responses to sinusoidally amplitude modulated tones; the analysis windows are shaded in grey. Right panels show period histograms generated from the spikes contained in the analysis window. MR, modulation rate; r, vector strength; p, p-value from the Rayleigh test.
Fig. 10. Response properties of MNTB and SPON neurons as a function of modulation rate.
A: Percentage of neurons demonstrating phase locking to SAM stimuli, as indicated by a significant result of the Rayleigh test, as a function of modulation rate. B: Mean number of spikes per presentation as a function of modulation rate. C: Mean vector strength as a function of modulation rate. Error bars in B and C represent the standard errors of the means.
Quantitative comparisons of MNTB and SPON responses to SAM stimuli
The vast majority of SPON neurons (29 of 30 96.7%) and all MNTB neurons in our sample met our criteria for phase locking for at least one MR. However, the percentage of phase locked neurons decreased as MR increased (Fig. 10A). For MRs up to ~ 700 Hz, between 70 and 100% of MNTB neurons demonstrated phase locking; this percentage decreased gradually until reaching 0% at an MR of 1160 Hz. By comparison, about 80% of SPON neurons synchronized to an MR of 40 Hz, and this percentage dropped sharply at 400 Hz MR; phase locking in SPON neurons ceased at an MR of 600 Hz. MNTB neurons produced ~ 20 to 25 spikes per stimulus presentation and this value remained largely constant across MRs (Fig. 10B). In contrast, SPON neurons produced only about five spikes per stimulus presentation at 40 Hz MR; the number of spikes dropped sharply at 400 Hz modulation and spiking ceased at an MR of 600 Hz. MNTB neurons reached their highest vector strength of 0.59 ± 0.02 at an MR of 80 Hz (Fig. 10C). At higher MRs the vector strengths gradually decreased, reaching a value of 0.1 ± 0.02 at 1160 Hz. The sample of SPON neurons reached its highest vector strength of 0.68 ± 0.07 at an MR of 40 Hz. From there the vector strengths decreased steeply, reaching 0 at 600 Hz modulation.
In summary, MNTB neurons phase locked to relatively high MRs and lost their phase locking because their spike timing ceased to follow the modulation of the stimulus. In contrast, SPON neurons phase locked only to relatively low MRs and their loss of synchronization at higher MRs occurred because they ceased to produce spikes. The modulation transfer functions based on spike count can be characterized as all-pass for MNTB neurons and low-pass for SPON neurons, whereas the modulation transfer functions based on vector strength can be characterized as low-pass for both nuclei, with a steeper cut-off for SPON neurons.
Relative timing of MNTB and SPON spikes during responses to SAM stimuli
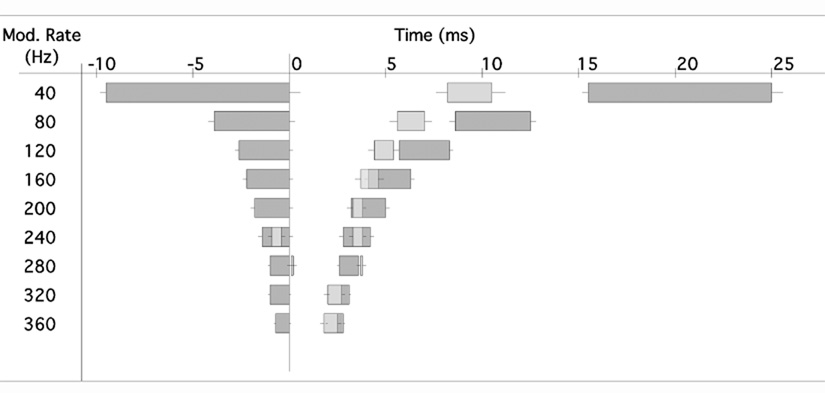
To understand the temporal relationship between the SAM responses of MNTB and SPON neurons, we plotted the average time intervals in which our sample of units produced their spikes (Fig. 11). Because we expected the cessation of MNTB spiking to trigger phase locked SPON spiking, we assigned a phase angle of zero to the end of the MNTB spiking episode and all other events were plotted in relation to it. Phase angles were converted to time in order to visualize the absolute time intervals between MNTB and SPON spiking episodes. At MRs between 40 and 120 Hz, SPON spiking was confined to the silent intervals between MNTB spiking episodes. We noted, however, an unexpectedly long delay between the offset of MNTB spiking and the onset of SPON spiking, which measured 8.1 ms at 40 Hz MR and 4.2 ms at 120 Hz MR. When the MR was increased to 160 Hz, SPON spiking overlapped MNTB spiking by 0.4 ms. The delay between the onset of MNTB firing and the cessation of SPON firing lengthened systematically until at 240 Hz MR the SPON spiking was entirely contained in the MNTB spiking interval. At 280 Hz MR, the delay between the end of MNTB spiking and the onset of SPON spiking exceeded one stimulus cycle, with the onset of the SPON response now lagging 0.1 ms behind the MNTB spiking period immediately preceding it, and 3.6 ms behind the MNTB spiking period assumed to be the trigger. At higher MRs, the overlap between SPON firing and MNTB spiking was similar to that observed for MRs between 160 and 200 Hz.
Fig. 11. Relative timing of MNTB and SPON spiking in response to amplitude modulated tones.
In order to show the temporal relationship between spiking episodes in the MNTB and SPON, the end of MNTB spiking was plotted as the beginning of the cycle (see results section). The average timing of MNTB spiking episodes (dark bars) and SPON spiking episodes (light bars) during one full modulation cycle of the stimulus is shown on the right side of the diagram, with positive time values. All events following the onset of MNTB spiking are also shown on the left side of the diagram with negative time values, representing the preceding phase. Whiskers represent the standard errors of the means.
The surprisingly long delay between the offset of MNTB spiking and the onset of SPON spiking in this stimulus paradigm is difficult to explain solely by a glycinergic post-inhibitory rebound mechanism, because pure tone data indicate that the SPON offset response should follow the offset of MNTB spiking within about 1 ms. Therefore, we considered whether SPON-derived GABAergic inhibition influenced SPON spike timing, which might account for the long response latencies observed. To test this notion, we compared the latency of SPON spikes following modulation cycles of the SAM stimulus that evoked SPON discharges to those occurring after modulation cycles that failed to evoke spikes (Fig. 12). Specifically, for this analysis we grouped the FSLs of SPON neurons according to the number of modulation cycles that had elapsed since the neurons’ last preceding spike. To establish a reference latency for comparison, we pooled the FSLs of each neuron’s first response to every presentation of the SAM stimulus (reasoning that this was appropriate since SPON neurons display very low rates of spontaneous activity) with the FSLs observed following interspike intervals >50 ms (reasoning that this interval was sufficiently long to outlast any possible lingering influence of previous GABAergic discharges). Because we were not aware of tests for multiple groups for circular variables, statistical tests were limited to comparing the FSLs of SPON spikes following a modulation cycle that also evoked a spike to those that followed one modulation cycle without a spike. At 40 Hz MR, FSLs following a modulation cycle that also evoked a spike (i.e., an inter-spike interval of up to 25 ms, or zero cycles skipped) exceeded the FSLs of the reference spikes by 3.4±1.2 ms and were significantly longer than the FSLs following one modulation cycle that did not evoke a spike (i.e., with a preceding inter-spike interval of 25–50 ms; Fig. 11; p<0.05; Rank-sum test for circular variables). Similar latency shifts were observed for MRs of 80, 120 and 160 Hz, but did not reach statistical significance. At MRs ≥ 200 Hz, all spikes were preceded by at least one modulation cycle without a discharge.
Fig. 12. In response to amplitude modulated tones, SPON latency shifts are dependent on discharges to previous modulation cycles.
Latencies of SPON unit responses to individual modulation cycles of SAM tones were grouped according to the number of modulation cycles that had elapsed since the neurons’ last preceding spike, and plotted relative to the timing of reference spikes (those evoked by the first response to the stimulus and those preceded by an interspike interval of > 50 ms; see text). At MRs between 40 and 160 Hz, latencies were increased when the preceding modulation cycle also evoked a discharge from the unit (i.e., 0 modulation cycles skipped; p < .05 at 40 Hz MR). At modulation rates ≥ 200 Hz, all spikes were preceded by at least one modulation cycle without a discharge. *= p<.05.
DISCUSSION
Neural mechanisms of gap detection in the MNTB/SPON circuit
The present results show that neurons comprising the MNTB/SPON circuit detect and signal the presence of a gap in an otherwise continuous tone in at least three different ways. First, SPON neurons produce an offset response to the leading marker. Second, MNTB neurons produce an offset response to the leading marker. Finally, MNTB neurons can signal the presence of a gap by forming an onset response to the trailing marker. Each of these mechanisms of gap detection will be discussed in turn.
SPON neurons are highly sensitive to gaps, producing clear offset responses at 0 ms gap duration. At gap durations of 10 ms or longer, SPON neurons responded with the same magnitude as observed in the leading marker only condition. This response represents the final output of the neural computation in the MNTB/ SPON circuit that is transmitted to higher levels of the auditory pathway. Clues to the potential role of SPON in the context of gap detection are suggested by previous demonstrations that i) the SPON offset response is transient, ii) SPON neurons are GABAergic, and iii) the synaptic targets of SPON neurons are cells of the ipsilateral inferior colliculus (IC) (Kulesza, Jr. et al., 2003; Kulesza, Jr. and Berrebi, 2000; reviewed in Saldaña and Berrebi, 2000, and see below). Taken together, these findings support a role for SPON in sharpening the separation of IC neuron responses to the stimuli surrounding the gap (see also the discussion of neural circuitry below). Behavioral experiments have shown that rats are capable of detecting gaps as short as 2 ms in noise stimuli (Leitner et al., 1993). Given the well-developed offset responses observed in the present study even at 0 ms gap duration, it appears that the MNTB/SPON circuit is capable of generating the information required for the rat’s behavioral gap detection performance.
In the MNTB, when the markers were separated by a 25 ms gap, neurons clearly produced responses containing onset, sustained and offset response components to both the leading and trailing markers. When presented with short gaps, between 0 and 5 ms, the offset responses to the leading markers and the onset responses to trailing markers were more difficult to discern. Nonetheless, we observed statistically significant MNTB offset responses with gaps of 2 ms duration, and a subset of neurons displayed offset responses to gaps of 0 ms duration. However, at short gap durations these offset responses are not always readily apparent from the individual PSTHs because the response duration is similar to that of the gap, and because the PSTHs contain other brief periods of silence unrelated to the gap. Therefore, in order to properly quantify the MNTB offset response, precise knowledge of its timing relative to the stimulus was required. This difficulty in identifying the MNTB offset response gives rise to some speculation about the nature of the projection from MNTB to SPON. While short gaps clearly evoke brief MNTB offset responses to the leading marker, SPON neurons must somehow distinguish between these offset responses and other silent periods during the MNTB onset and sustained response components. One possible mechanism underlying this discrimination is that the projection from MNTB to SPON may display a relatively high degree of convergence. If so, multiple MNTB neurons projecting to a single SPON neuron together provide sufficient glycinergic inhibition during a continuous stimulus to suppress SPON spiking, even if individual MNTB neurons are briefly silent. However, in the presence of a gap, all the MNTB neurons projecting to a given SPON neuron would be expected to suppress their spiking activity synchronously in producing their offset response, thereby sufficiently releasing the SPON neuron from glycinergic inhibition to trigger an SPON offset response.
The presence of a gap may also be signaled by an MNTB onset response to the trailing marker. At short gap durations the onset response to the trailing marker was much less pronounced than in the trailing marker only control condition. At longer gap durations this onset response became progressively larger, but with the gap durations tested in the present study, it never matched the magnitude of the control response. This suppression of the MNTB onset response to the trailing marker may be accounted for by a persistent inhibitory response to the leading marker that serves to counteract the stimulus-driven excitation elicited by the trailing marker. Our observation that the onset component of the MNTB response to the trailing marker gradually re-emerges at longer gap durations parallels an ABR study of gaps in noise conducted in gerbils (Boettcher et al., 1996). In that study, the amplitudes of the compound action potential, wave II and wave III in response to the noise burst following the gap were reduced, but at gap durations of about 30 ms recovered approximately to the values observed in responses to the leading marker. Since the onset component of the MNTB unit response likely contributes to the ABR, this parallel result is not surprising.
The MNTB offset response is the critical response component for triggering SPON offset spikes (Kulesza, Jr. et al., 2007). However, it should be noted that the onset and sustained response components of MNTB neurons play a well-defined role in coding sound source location (Park et al., 1997; Tollin and Yin, 2002; Pollak et al., 2003) by inhibiting lateral superior olive neurons, their main projection targets (Spangler et al., 1985; Banks and Smith, 1992). In view of the extremely sensitive gap detection displayed by MNTB and SPON offset responses, it is possible that the gradual recovery of MNTB onset response components to progressively longer gaps represents a generic pattern of auditory brainstem circuitry that may not be specifically related to the gap detection processes underlying perception and behavior.
Since the MNTB/SPON circuit is located in the auditory brainstem, information about the temporal structure of stimuli extracted by MNTB and SPON neurons must be relayed to the auditory midbrain and forebrain in order to contribute to gap detection phenomena observed in rat behavioral (Leitner et al., 1993, 1997) and human psychophysical experiments (reviewed by Phillips, 1999). The main projection target of the SPON is the ipsilateral IC (Beyerl, 1978; Zook and Casseday, 1982; Adams, 1983; Nordeen et al., 1983; Willard and Ryugo, 1983; Saint Marie and Baker, 1990; Schofield, 1991; Gonzalez-Hernandez et al., 1996; Saldaña and Berrebi, 2000), and neuronal correlates of gap detection attributable to the inhibitory input from the SPON might be expected there. Gap detection has been studied extensively in the mouse, and behavioral gap detection thresholds (GDTs) have been compared to GDTs recorded in the mouse IC (Walton et al. 1977). IC units were found with minimum GDTs of 1–2 ms which were in good agreement with the measured behavioral thresholds of 2 ms. However, mean GDTs depended on the neuron’s response type, with primary-like and sustained IC units producing the shortest mean GDTs of 2 ms and 4 ms, respectively, and IC units showing the longest mean GDTs of 14 ms displayed inhibitory responses. Primary-like and sustained units responded to gaps by a brief interruption of their ongoing response, and the primary-like units also produced an onset response to the trailing marker. Onset units produced an onset response to the trailing marker, whereas inhibitory units resumed their spontaneous activity between the inhibitory responses. Based on the timing of these responses to gaps, and considering the latency expected due to neuronal conduction times and synaptic delays, the reduction in discharge rate shown by the primary-like and sustained units in the IC is consistent with a contribution of GABAergic inputs arising from the SPON. Although GDTs observed by (Walton et al., 1997) and in the present study are not directly comparable because different stimuli were used, it appears that rat SPON neurons and mouse IC neurons show similar sensitivity to gaps. This would be expected if SPON-derived inhibition mediates or at least contributes to gap detection in the IC across rodent species.
Gap information generated by the MNTB/SPON circuit may also reach the auditory forebrain via the recently discovered direct projection from SPON to the medial geniculate body of the thalamus (Jin and Berrebi, 2006). Such direct transmission of gap information would have the advantage of preserving the temporal acuity of gap information without this information being altered in any way by processing in the IC. Furthermore, a direct GABAergic inhibitory input from the SPON might be more effective in segmenting an ongoing response in the thalamus than would be accomplished by the brief interruption of an excitatory input from the IC.
Responses to SAM tones in the MNTB/SPON circuit
At low MRs the responses of MNTB and SPON neurons to individual cycles of amplitude modulated tones are reminiscent of their responses to pure tones. MNTB neurons respond to each cycle of the modulation with a burst of spikes followed by a period of silence, whereas SPON neurons discharge single spikes at very narrowly defined phase angles relative to the stimulus modulation. As MRs are raised, however, both nuclei lose the ability to respond to the SAM stimulus on a cycle-by-cycle basis, and respond to it as a whole. In MNTB, this takes the shape of a loss of synchronization as evidenced by the non-significant Rayleigh tests and decreased vector strengths, whereas in SPON, spiking during the stimulus is abolished and a response emerges after the stimulus offset. This transition from cycle-by-cycle responses to responses to the overall stimulus at higher MRs is driven in the MNTB by a loss of phase locking in the presence of largely unchanged spike counts. Conversely, in the SPON spikes occurring during the stimulus presentation maintain their phase locking, but fewer and fewer spikes are produced so that this transition is driven by the decreasing spike count. In view of the low spike counts and small proportion of neurons synchronizing at MRs above 360 Hz, SPON neurons apparently transmit physiologically meaningful stimulus information only at relatively low modulation rates.
Our previous pharmacological study of SPON responses to pure tones (Kulesza, Jr. et al., 2007) led us to hypothesize that release from MNTB-derived glycinergic inhibition would trigger phase locked SPON spiking in response to SAM stimuli. We confirmed in the present study that SPON spiking at low MRs indeed occurs during the period when the MNTB is silent. However, unlike the SPON offset response to pure tones, which occurs ~1 ms after the cessation of MNTB spiking, responses to the SAM stimuli revealed a substantial delay between the cessation of MNTB spiking and the onset of SPON spiking. Previous immunocytochemical and tract-tracing data have shown that SPON neurons utilize GABA as their neurotransmitter and give rise to extensive axonal collaterals within the nucleus (Kulesza, Jr. and Berrebi, 2000; Saldaña and Berrebi, 2000), and preliminary evidence suggests that individual SPON neurons form autapses (Kulesza, Jr. et al., 2000). We therefore questioned whether the delay of SPON spiking relative to MNTB spiking in this stimulus paradigm might be mediated by GABAergic inhibition originating in the SPON itself. Our analysis of latencies showed that at 40 Hz MR, SPON spikes occurred significantly later if the preceding modulation cycle also evoked a spike, consistent with the notion that GABAergic self-inhibition delays subsequent SPON spiking. Since this GABA-mediated latency shift only occurs when SPON neurons produce spike trains with comparatively short interspike intervals, shorter latencies would be expected with pure tone stimuli where the offset response is preceded by an interspike interval of many tens of milliseconds.
One should note that the latency shift analysis takes into account only the autapse mediated GABAergic self-inhibition caused by preceding spikes from the individual neuron under study. It is likely that other SPON neurons provide collateral GABAergic input that may or may not coincide with the neuron’s self inhibition. At low MRs, when the probability that SPON neurons will respond to a given modulation cycle is high, the contribution from other neurons must be relatively large. Conversely, the cycle-by-cycle probability that SPON neurons will respond decreases at higher MRs, and the resulting inhibition from other SPON neurons during a given modulation cycle will be relatively small. Moreover, the large GABAergic input from other SPON neurons at low MRs would be expected to cause large inhibitory postsynaptic potentials with long decay times. Decay time constants between 15.8 and 54.8 ms have been reported for IPSPs mediated by the GABAA receptor (Nusser et al., 1999; Russier et al., 2002; Bacci et al., 2003), with time constants varying depending on the receptor’s subunit composition. Given the slow kinetics of the GABAA receptor, we can readily detect the inhibitory influence of SPON-derived inhibition at interspike intervals up to 25 ms (i.e., the duration of one modulation cycle at 40 Hz MR), whereas at higher MRs the presumed effects of such inhibition are much more difficult to discern. In contrast, much shorter decay time constants, ranging from 4.5 to 7.5 ms, were reported for glycinergic IPSPs (Singer et al., 1998; Russier et al., 2002). These faster kinetics are in keeping with our observation that the SPON response to pure tones follows the release from glycinergic inhibition very closely.
Based on these considerations, we propose a framework to explain the output of the MNTB/SPON circuit based on the timing of glycinergic and GABAergic inhibition acting upon SPON neurons. The two key considerations are: i) a rebound from MNTB-derived glycinergic inhibition is sufficient to induce SPON spiking in the absence of GABAergic inhibition, as demonstrated with pure tones in the present gap detection study; and ii) SPON spiking episodes in response to SAM stimuli can only occur after the lingering GABAergic inhibition derived from responses to preceding modulation cycles has subsided. At low MRs, the interval between MNTB spiking episodes is sufficiently long to allow newly generated GABAergic inhibition to limit the duration of SPON offset responses; subsequently, due to the relatively long decay time for GABAergic IPSPs, the latency of offset responses to subsequent cycles of the stimulus is lengthened. On a cycle-by-cycle basis the inhibitory influences acting upon SPON become difficult to estimate at higher MRs (> 120 Hz), with the relative timing of SPON spiking episodes being controlled by a complex interplay of inhibitory strength and timing. However, beginning at around 400 Hz MR, the cessation of SPON spiking during the stimulus presentation must be attributed to glycinergic inhibition alone.
Comparison of SPON responses to gap and SAM stimuli
SPON responses to gap stimuli are directly predictable from their responses to pure tones. The response to the leading gap marker is identical to the response to an isolated pure tone, except that it may be truncated by the arrival of the inhibitory input from the MNTB triggered by the trailing marker; the SPON response to the trailing gap marker is virtually identical to the response to an isolated pure tone. In contrast, responses of SPON neurons to SAM stimuli differ substantially from those to pure tones. The temporal relationship between the offset response of MNTB neurons (corresponding to periods of silence in their responses to SAM tones) and the SPON spiking episodes is changed by the appearance of a delay that is not observed in responses to pure tones or gap stimuli. As discussed above, we presume that this delay is caused by GABAergic inhibition intrinsic to the SPON. This intrinsic inhibition does not play a role in shaping responses to pure tones or gap stimuli, since the interspike intervals evoked by these stimuli are too long for the intrinsic inhibition to have an effect. The fact that responses to SAM stimuli are affected by the intrinsic inhibition in SPON, whereas responses to gap stimuli are not, suggests that the MNTB/SPON circuit is more sensitive to short stimulus discontinuities occurring in isolation than to those embedded in ongoing amplitude modulations. However, amplitude modulations are encoded quite precisely if they occur at relatively low rates, where intrinsic GABAergic inhibition limits the time window during which the SPON is fully disinhibited and can fire action potentials. Thus, GABAergic inhibition serves to ensure that SPON responses to low MRs are highly synchronized, but at higher MRs it prevents the SPON from spiking reliably.
To assess the difference in sensitivity, we made a rough quantitative comparison between the temporal resolution of SPON responses to gap and SAM stimuli, by calculating the firing probabilities in both cases. In the gap experiments the highest temporal resolution was displayed by responses to stimuli containing a 0 ms gap. Since the leading and trailing markers each had 2 ms ramps, the total time of diminished stimulus energy in this stimulus was 4 ms. This corresponds roughly to the duration of one modulation cycle in a 240 Hz MR SAM stimulus, which is 4.17 ms long. We found that the firing probability for one presentation of a stimulus containing a 0 ms gap was 0.35, whereas the firing probability for a single cycle of a 240 Hz SAM stimulus is only 0.05. In other words, even though the periods of low stimulus energy were of nearly identical duration, SPON neurons were seven times more likely to respond to a 0 ms gap stimulus than to any given cycle of a SAM stimulus with 240 Hz MR.
Previous studies of temporal processing in the SPON
Kuwada and Batra (1999) compared the SAM responses of “off” responding neurons in the SOC of the unanesthetized rabbit to those of neurons producing sustained responses to pure tones. Some characteristics of off responding neurons, namely their contralateral drive and location medial to the LSO, are shared by SPON neurons, leading the authors to conclude that the dorsal medial periolivary nucleus (DMPO, the presumed rabbit homolog of the rodent SPON) is the source of the off responding neurons. As in the rat, offset responses in the rabbit are presumably driven by post-inhibitory rebound mechanisms. However, whereas offset responses of rat SPON neurons are transient, frequently containing only a single spike per stimulus presentation, offset responses from the rabbit SOC continue for 30 ms or longer (see their Figure 1). Moreover, Kuwada and Batra (1999) deduced that the high synchronization observed in off neurons resulted from inhibition driven by the rising flank of the modulation cycle following the previous modulation cycle that caused the inhibitory rebound. Our analysis of relative MNTB and SPON spike times in the rat indicates that this may be the case at higher MRs, but SPON spiking at lower MRs terminates well before the arrival of the next cycle of MNTB-derived inhibition. We conclude that the strong synchronization of SPON responses at low MRs in the rat is caused by a mechanism that may not be present in rabbits, namely GABAergic inhibition, presumably originating in the SPON itself, that permits a very brief window for SPON spiking during each modulation cycle. This conclusion is consistent with previous data showing that SPON offset responses to pure tones are truncated by GABAergic inhibition (Kulesza, Jr. et al., 2007).
The results of the present study also closely parallel our own preliminary assessment of SPON neurons in unanesthetized CBA mice (Felix and Berrebi, 2007). All neurons in the mouse SPON are contralaterally driven, and the vast majority produce offset responses to pure tones that closely resemble those of rat SPON neurons with regard to their latency, threshold and tuning properties. Moreover, as observed in the present study, responses to SAM tones show high vector strengths and display low pass filter characteristics with regard to spike count.
The similarities in many response features of SPON neurons between rats, rabbits and mice do not extend to the Mongolian gerbil. Behrend and colleagues (2002) recorded several pure tone discharge patterns in the gerbil SPON, including sustained, phasic-on and phasic-off responses, with the off responses accounting for a very small proportion (< 10 %) of the total. Moreover, unlike the rat SPON, whose neurons are driven exclusively by contralateral stimulation and uniformly show very low rates of spontaneous activity, about 50% of SPON neurons in the gerbil showed binaural interactions with many units displaying spontaneous rates of more than 10 spikes/s. Finally, although two-thirds of gerbil SPON neurons responded to SAM stimuli with ongoing phase locked discharges, none of the units with phasic-off discharges produced an ongoing response to SAM stimuli. In another study of gerbil SPON, more than half of recorded neurons produced off-chopper, periodic-off or tonic-off responses, with two-thirds of units responding only to contralateral stimulation (Dehmel et al., 2002). Whereas both studies indicated that the distribution of recorded CFs covered most of the gerbil’s audiogram, Behrend et al., (2002) noted a clear bias to frequencies below 6 kHz.
Despite the apparent discrepancies between these two reports on the gerbil SPON, both provide evidence for binaural interactions and response types not found in the rat. These disparities suggest that the synaptic inputs impinging on the nucleus may differ between these species. The connectivity of the gerbil SPON has not been studied in detail, but it has been shown to contain neurons that send descending projections to the cochlear nuclei, whereas the rat SPON does not (Faye-Lund, 1986; Helfert et al., 1988). It seems plausible, therefore, that differences in ascending inputs and, perhaps, the presence of different cell types underlie the discrepant physiological properties of SPON neurons in the two species. Perhaps these differences resulted from evolutionary pressures engendered by the specialized requirements for low-frequency hearing in gerbils and other desert rodents (Rosowski et al., 1999); indeed, nuclei of the superior olivary complex, and particularly the SPON and DMPO, display considerable interspecies variability in their constituent neuronal morphologies and certain aspects of their connectivity (Covey and Casseday, 1995; Grothe, 2000; reviewed in Saldaña and Berrebi, 2000).
Functional Implications
The processing of SAM stimuli in the central auditory system is characterized by the transition from a synchronization code present in the auditory nerve to a rate code that dominates in the auditory midbrain and forebrain (Paolini et al., 2001; Joris et al., 2004). The synchronization of responses to SAM stimuli in the IC, the SPON’s main projection target, is limited to MRs of ~ 200 Hz in all mammalian species tested, including rats (Rees and Moller, 1987), guinea pigs (Rees and Palmer, 1989), gerbils (Krishna and Semple, 2000), squirrel monkeys (Muller-Preuss et al., 1994), and mice (Walton et al., 2002), and is similarly limited in the medial geniculate body (Creutzfeldt et al., 1980; Preuss and Muller-Preuss, 1990), another recently identified projection target of the SPON (Jin and Berrebi, 2006). Therefore, by providing a highly synchronized inhibitory input, the SPON may serve to shape responses in its target nuclei by segmenting their ongoing responses. We assume that this inhibitory input would be most effective over a range of MRs where SPON neurons maintain phase locking with high vector strengths and relatively high spike counts. These criteria are met for MRs up to ~ 200 Hz, which coincides with the limited range of response synchronization observed in the inferior colliculus and medial geniculate body. It should be noted, however, that the SPON is not the only nucleus providing precisely synchronized inhibition to the IC, as neurons in the ventral nucleus of the lateral lemniscus of rabbits (Batra, 2006) and rats (Zhang and Kelly, 2006) also respond to SAM stimuli with high vector strengths
Human psychophysical studies show that SAM stimuli evoke three different perceptions depending on MR (Zwicker and Fastl, 1990). At MRs below 20 Hz, every modulation cycle is perceived as a separate stimulus and the overall perception is of rhythm; at MRs between 20 and 200 Hz, the rhythm can no longer be fully resolved and the perception is of roughness. Finally, at MRs >200 Hz, the perception changes to periodicity-driven pitch (Langner, 1997, 2005). Interestingly, these psychophysical findings coincide with response properties of SPON neurons to SAM tones, since at the lowest MRs nearly every modulation cycle evokes SPON spikes. This reliable spiking reflects processing of each modulation cycle as a separate stimulus and might contribute to a perception of rhythm. At higher MRs the SPON spiking skips progressively larger numbers of modulation cycles, suggesting that the resulting perception might be somewhat segmented, but not sufficiently to constitute a rhythm; this is consistent with a roughness perception. At the highest MRs, the cycle-by-cycle response of the SPON becomes unreliable and eventually ceases completely. Thus, while pitch perception is likely mediated by neural circuits not involving the SPON, it is plausible that this nucleus may participate in generating the percepts of rhythm and roughness.
CONCLUSIONS
We have characterized an auditory brainstem circuit that shows high sensitivity to gaps in auditory stimuli and responds to SAM signals with precisely synchronized discharges. The outputs of this circuit include a direct GABAergic projection to the inferior colliculus and medial geniculate body. Overall, the MNTB/SPON circuit is capable of encoding episodes of diminished stimulus energy, such as gaps and the falling flanks of an amplitude modulation, by producing short and precisely timed bursts of inhibition that presumably serve to impose a temporal structure on neural activity in the midbrain and thalamus.
ACKNOWLEDGMENTS
We gratefully acknowledge advice and feedback on this work from our colleagues in the WVU Sensory Neuroscience Research Center. Special thanks to Drs. Aric Agmon, Kevin Daly and Ronald Millechia and Mr. Richard Felix II for valuable pre-submission comments on the manuscript. We are also grateful to the anonymous reviewers of this manuscript for their insightful critiques. Mr. Dennis Cole provided technical support.
GRANT SPONSOR National Institute on Deafness and Other Communication Disorders; RO1 DC-02266 to A. S. B.
ABBREVIATIONS
- ABR
auditory brainstem response
- CF
characteristic frequency
- FSL
first spike latency
- GDT
gap detection threshold
- IC
inferior colliculus
- MNTB
medial nucleus of the trapezoid body
- MR
modulation rate
- PSTH
peristimulus spike time histogram
- SAM
sinusoidally amplitude modulated
- SPON
superior paraolivary nucleus
- TDT
Tucker-Davis Technologies
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams JC. Cytology of periolivary cells and the organization of their projections in the cat. J. Comp. Neurol. 1983;215:275–289. doi: 10.1002/cne.902150304. [DOI] [PubMed] [Google Scholar]
- Allen PD, Burkard RF, Ison JR, Walton JP. Impaired gap encoding in aged mouse inferior colliculus at moderate but not high stimulus levels. Hear. Res. 2003;186:17–29. doi: 10.1016/s0378-5955(03)00300-9. [DOI] [PubMed] [Google Scholar]
- Bacci A, Rudolph U, Huguenard JR, Prince DA. Major differences in inhibitory synaptic transmission onto two neocortical interneuron subclasses. J. Neurosci. 2003;23:9664–9674. doi: 10.1523/JNEUROSCI.23-29-09664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks MI, Smith PH. Intracellular recordings from neurobiotin-labeled cells in brain slices of the rat medial nucleus of the trapezoid body. J. Neurosci. 1992;12:2819–2837. doi: 10.1523/JNEUROSCI.12-07-02819.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsz K, Ison JR, Snell KB, Walton JP. Behavioral and neural measures of auditory temporal acuity in aging humans and mice. Neurobiol. Aging. 2002;23:565–578. doi: 10.1016/s0197-4580(02)00008-8. [DOI] [PubMed] [Google Scholar]
- Batra R. Responses of neurons in the ventral nucleus of the lateral lemniscus to sinusoidally amplitude modulated tones. J. Neurophysiol. 2006;96:2388–2398. doi: 10.1152/jn.00442.2006. [DOI] [PubMed] [Google Scholar]
- Batra R, Kuwada S, Fitzpatrick DC. Sensitivity to interaural temporal disparities of low- and high-frequency neurons in the superior olivary complex. I. Heterogeneity of responses. J. Neurophysiol. 1997a;78:1222–1236. doi: 10.1152/jn.1997.78.3.1222. [DOI] [PubMed] [Google Scholar]
- Batra R, Kuwada S, Fitzpatrick DC. Sensitivity to interaural temporal disparities of low- and high-frequency neurons in the superior olivary complex. II. Coincidence detection. J. Neurophysiol. 1997b;78:1237–1247. doi: 10.1152/jn.1997.78.3.1237. [DOI] [PubMed] [Google Scholar]
- Batschelet E. Circular Statistics in Biology. London, New York, Toronto, Sydney, San Francisco: Academic Press; 1981. [Google Scholar]
- Behrend O, Brand A, Kapfer C, Grothe B. Auditory response properties in the superior paraolivary nucleus of the gerbil. J. Neurophysiol. 2002;87:2915–2928. doi: 10.1152/jn.2002.87.6.2915. [DOI] [PubMed] [Google Scholar]
- Beyerl BD. Afferent projections to the central nucleus of the inferior colliculus in the rat. Brain Res. 1978;145:209–223. doi: 10.1016/0006-8993(78)90858-2. [DOI] [PubMed] [Google Scholar]
- Boettcher FA, Mills JH, Swerdloff JL, Holley BL. Auditory evoked potentials in aged gerbils: responses elicited by noises separated by a silent gap. Hear. Res. 1996;102:167–178. doi: 10.1016/s0378-5955(96)90016-7. [DOI] [PubMed] [Google Scholar]
- Covey E, Casseday JH. The lower brain stem auditory pathway. In: Popper A, Fay RR, editors. Hearing by Bats. New York: Springer Verlag; 1995. pp. 235–295. [Google Scholar]
- Creutzfeldt O, Hellweg FC, Schreiner C. Thalamocortical transformation of responses to complex auditory stimuli. Exp. Brain Res. 1980;39:87–104. doi: 10.1007/BF00237072. [DOI] [PubMed] [Google Scholar]
- Dehmel S, Doerrscheidt G, Reubsamen R. Electrophysiological characterization of neurons in the superior paraolivary nucleus of the gerbil (Meriones unguiculatus) Hear. Res. 2002;172:18–36. doi: 10.1016/s0378-5955(02)00353-2. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Neural correlates of gap detection in three auditory cortical fields in the Cat. J. Neurophysiol. 1999;81:2570–2581. doi: 10.1152/jn.1999.81.5.2570. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Neural responses in primary auditory cortex mimic psychophysical, across-frequency-channel, gap-detection thresholds. J. Neurophysiol. 2000;84:1453–1463. doi: 10.1152/jn.2000.84.3.1453. [DOI] [PubMed] [Google Scholar]
- Faye-Lund H. Projection from the inferior colliculus to the superior olivary complex in the albino rat. Anat. Embryol. (Berl) 1986;175:35–52. doi: 10.1007/BF00315454. [DOI] [PubMed] [Google Scholar]
- Felix RA, Berrebi AS. Characterization of the Superior Paraolivary Nucleus in the Unanesthetized Mouse. Assoc. Res. Otolaryngol. Abstr. 2007;30:496. [Google Scholar]
- Feng AS, Lin WY, Sun L. Detection of gaps in sinusoids by frog auditory nerve fibers: importance in AM coding. J. Comp. Physiol. [A] 1994;175:531–546. doi: 10.1007/BF00199475. [DOI] [PubMed] [Google Scholar]
- Frisina DR, Frisina RD. Speech recognition in noise and presbycusis: relations to possible neural mechanisms. Hear. Res. 1997;106:95–104. doi: 10.1016/s0378-5955(97)00006-3. [DOI] [PubMed] [Google Scholar]
- Frisina RD. Subcortical neural coding mechanisms for auditory temporal processing. Hear. Res. 2001;158:1–27. doi: 10.1016/s0378-5955(01)00296-9. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Brown PB. Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: some physiological mechanisms of sound localization. J. Neurophysiol. 1969;32(4):613–636. doi: 10.1152/jn.1969.32.4.613. [DOI] [PubMed] [Google Scholar]
- Giraudi D, Salvi R, Henderson D, Hamernik R. Gap detection by the chinchilla. J. Acoust. Soc. Am. 1980;68:802–806. doi: 10.1121/1.384818. [DOI] [PubMed] [Google Scholar]
- Giraudi-Perry DM, Salvi RJ, Henderson D. Gap detection in hearing-impaired chinchillas. J. Acoust. Soc. Am. 1982;72:1387–1393. doi: 10.1121/1.388444. [DOI] [PubMed] [Google Scholar]
- Glasberg BR, Moore BC. Psychoacoustic abilities of subjects with unilateral and bilateral cochlear hearing impairments and their relationship to the ability to understand speech. Scand. Audiol. 1989;32 Suppl.:1–25. [PubMed] [Google Scholar]
- Gonzalez-Hernandez T, Mantolan-Sarmiento B, Gonzalez-Gonzalez B, Perez-Gonzalez H. Sources of GABAergic input to the inferior colliculus of the rat. J. Comp. Neurol. 1996;372:309–326. doi: 10.1002/(SICI)1096-9861(19960819)372:2<309::AID-CNE11>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Grothe B. The evolution of temporal processing in the medial superior olive, an auditory brainstem structure. Prog. Neurobiol. 2000;61:581–610. doi: 10.1016/s0301-0082(99)00068-4. [DOI] [PubMed] [Google Scholar]
- Guinan JJ, Jr, Guinan SS, Norris BE. Single units in the superior olivary complex I: responses to sounds and classification based on physiological properties. Int. J. Neurosci. 1972a;4:101–120. [Google Scholar]
- Guinan JJ, Jr, Norris BE, Guinan SS. Single units in the superior olivary complex II: localizations of unit categories and tonotopic organization. Int. J. Neurosci. 1972b;4:147–166. [Google Scholar]
- Helfert RH, Bonneau JM, Wenthold RJ, Altschuler RA. GABA and glycine immunoreactivity in the guinea pig superior olivary complex. Brain Res. 1989;501:269–286. doi: 10.1016/0006-8993(89)90644-6. [DOI] [PubMed] [Google Scholar]
- Helfert RH, Schwartz IR, Ryan AF. Ultrastructural characterization of gerbil olivocochlear neurons based on differential uptake of 3H-D-aspartic acid and a wheatgerm agglutinin-horseradish peroxidase conjugate from the cochlea. J. Neurosci. 1988;8:3111–3123. doi: 10.1523/JNEUROSCI.08-09-03111.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RJ, McAuley SF. Relations among temporal acuity, hearing loss, and the perception of speech distorted by noise and reverberation. J. Acoust. Soc. Am. 1987;81:1557–1565. doi: 10.1121/1.394508. [DOI] [PubMed] [Google Scholar]
- Jin Y, Berrebi AS. Direct projections from the superior olivary complex to the medial geniculate body in rats. Assoc. Res. Otolaryngol. Abstr. 2006;29:136. [Google Scholar]
- Joris PX, Schreiner CE, Rees A. Neural processing of amplitude-modulated sounds. Physiol. Rev. 2004;84:541–577. doi: 10.1152/physrev.00029.2003. [DOI] [PubMed] [Google Scholar]
- Joris PX, Yin TC. Envelope coding in the lateral superior olive. I. Sensitivity to interaural time differences. J. Neurophysiol. 1995;73:1043–1062. doi: 10.1152/jn.1995.73.3.1043. [DOI] [PubMed] [Google Scholar]
- Joris PX, Yin TC. Envelope coding in the lateral superior olive. III. Comparison with afferent pathways. J. Neurophysiol. 1998;79:253–269. doi: 10.1152/jn.1998.79.1.253. [DOI] [PubMed] [Google Scholar]
- Kadner A, Kulesza RJ, Jr, Berrebi AS. Neurons in the medial nucleus of the trapezoid body and superior paraolivary nucleus of the rat may play a role in sound duration coding. J. Neurophysiol. 2006;95:1499–1508. doi: 10.1152/jn.00902.2005. [DOI] [PubMed] [Google Scholar]
- Kelly JB, Rooney BJ, Phillips DP. Effects of bilateral auditory cortical lesions on gap-detection thresholds in the ferret (Mustela putorius) Behav. Neurosci. 1996;110:542–550. doi: 10.1037//0735-7044.110.3.542. [DOI] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Fuchs K, Lippe WR, Tempel BL, Rubsamen R. Decreased temporal precision of auditory signaling in Kcna1-null mice: an electrophysiological study in vivo. J. Neurosci. 2003;23:9199–9207. doi: 10.1523/JNEUROSCI.23-27-09199.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Lippe WR, Dorrscheidt GJ, Rubsamen R. The medial nucleus of the trapezoid body in the gerbil is more than a relay: comparison of pre- and postsynaptic activity. J. Assoc. Res. Otolaryngol. 2002;4:1–23. doi: 10.1007/s10162-002-2010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna BS, Semple MN. Auditory temporal processing: responses to sinusoidally amplitude-modulated tones in the inferior colliculus. J. Neurophysiol. 2000;84:255–273. doi: 10.1152/jn.2000.84.1.255. [DOI] [PubMed] [Google Scholar]
- Kulesza RJ, Jr, Berrebi AS. The superior paraolivary nucleus of the rat is a GABAergic nucleus. J. Assoc. Res. Otolaryngol. 2000;1:255–269. doi: 10.1007/s101620010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesza RJ, Jr, Holt GA, Spirou G, Berrebi AS. Intracellular labeling of axonal collaterals of SPON neurons. Assoc. Res. Otolaryngol. Abstr. 2000;23:37. [Google Scholar]
- Kulesza RJ, Jr, Kadner A, Berrebi AS. Distinct roles for glycine and GABA in shaping the response properties of neurons in the superior paraolivary nucleus of the rat. J. Neurophysiol. 2007;97:1610–1620. doi: 10.1152/jn.00613.2006. [DOI] [PubMed] [Google Scholar]
- Kulesza RJ, Jr, Spirou GA, Berrebi AS. Physiological response properties of neurons in the superior paraolivary nucleus of the rat. J. Neurophysiol. 2003;89:2299–2312. doi: 10.1152/jn.00547.2002. [DOI] [PubMed] [Google Scholar]
- Kulesza RJ, Jr, Viñuela A, Saldaña E, Berrebi AS. Unbiased stereological estimates of neuron number in subcortical auditory nuclei of the rat. Hear. Res. 2002;168:12–24. doi: 10.1016/s0378-5955(02)00374-x. [DOI] [PubMed] [Google Scholar]
- Kuwada S, Batra R. Coding of sound envelopes by inhibitory rebound in neurons of the superior olivary complex in the unanesthetized rabbit. J. Neurosci. 1999;19:2273–2287. doi: 10.1523/JNEUROSCI.19-06-02273.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner G. Periodicity coding in the auditory system. Hear. Res. 1992;60:115–142. doi: 10.1016/0378-5955(92)90015-f. [DOI] [PubMed] [Google Scholar]
- Langner G. Neural processing and representation of periodicity pitch. Acta. Otolaryngol. 1997;532 Suppl.:68–76. doi: 10.3109/00016489709126147. [DOI] [PubMed] [Google Scholar]
- Langner G. Neuronal mechanisms underlying the perception of pitch and harmony. Ann. N. Y. Acad. Sci. 2005;1060:50–52. doi: 10.1196/annals.1360.043. [DOI] [PubMed] [Google Scholar]
- Leitner DS, Carmody DP, Girten EM. A signal detection theory analysis of gap detection in the rat. Percept. Psychophys. 1997;59:774–782. doi: 10.3758/bf03206023. [DOI] [PubMed] [Google Scholar]
- Leitner DS, Hammond GR, Springer CP, Ingham KM, Mekilo AM, Bodison PR, Aranda MT, Shawaryn MA. Parameters affecting gap detection in the rat. Percept. Psychophys. 1993;54:395–405. doi: 10.3758/bf03205275. [DOI] [PubMed] [Google Scholar]
- Moore BC. Temporal analysis in normal and impaired hearing. Ann. N. Y. Acad. Sci. 1993;682:119–136. doi: 10.1111/j.1749-6632.1993.tb22964.x. [DOI] [PubMed] [Google Scholar]
- Muller-Preuss P, Flachskamm C, Bieser A. Neural encoding of amplitude modulation within the auditory midbrain of squirrel monkeys. Hear. Res. 1994;80:197–208. doi: 10.1016/0378-5955(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Nordeen KW, Killackey HP, Kitzes LM. Ascending auditory projections to the inferior colliculus in the adult gerbil, Meriones unguiculatus. J. Comp. Neurol. 1983;214:131–143. doi: 10.1002/cne.902140203. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Mody I. Differential regulation of synaptic GABAA receptors by cAMP-dependent protein kinase in mouse cerebellar and olfactory bulb neurones. J. Physiol. 1999;521(2):421–435. doi: 10.1111/j.1469-7793.1999.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini AG, FitzGerald JV, Burkitt AN, Clark GM. Temporal processing from the auditory nerve to the medial nucleus of the trapezoid body in the rat. Hear. Res. 2001;159:101–116. doi: 10.1016/s0378-5955(01)00327-6. [DOI] [PubMed] [Google Scholar]
- Park TJ, Monsivais P, Pollak GD. Processing of interaural intensity differences in the LSO: role of interaural threshold differences. J. Neurophysiol. 1997;77:2863–2878. doi: 10.1152/jn.1997.77.6.2863. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, New York, Boston, London, Sydney, Tokyo, Toronto: Academic Press; 1986. [Google Scholar]
- Phillips DP. Auditory gap detection, perceptual channels, and temporal resolution in speech perception. J. Am. Acad. Audiol. 1999;10:343–354. [PubMed] [Google Scholar]
- Pollak GD, Burger RM, Klug A. Dissecting the circuitry of the auditory system. Trends Neurosci. 2003;26:33–39. doi: 10.1016/s0166-2236(02)00009-7. [DOI] [PubMed] [Google Scholar]
- Preuss A, Muller-Preuss P. Processing of amplitude modulated sounds in the medial geniculate body of squirrel monkeys. Exp. Brain Res. 1990;79:207–211. doi: 10.1007/BF00228890. [DOI] [PubMed] [Google Scholar]
- Rees A, Moller AR. Stimulus properties influencing the responses of inferior colliculus neurons to amplitude-modulated sounds. Hear. Res. 1987;27:129–143. doi: 10.1016/0378-5955(87)90014-1. [DOI] [PubMed] [Google Scholar]
- Rees A, Palmer AR. Neuronal responses to amplitude-modulated and pure-tone stimuli in the guinea pig inferior colliculus, and their modification by broadband noise. J. Acoust. Soc. Am. 1989;85:1978–1994. doi: 10.1121/1.397851. [DOI] [PubMed] [Google Scholar]
- Rosowski JJ, Ravicz ME, Teoh SW, Flandermeyer D. Measurements of middle-ear function in the Mongolian gerbil, a specialized mammalian ear. Audiol. Neurootol. 1999;4:129–136. doi: 10.1159/000013831. [DOI] [PubMed] [Google Scholar]
- Russier M, Kopysova IL, Ankri N, Ferrand N, Debanne D. GABA and glycine corelease optimizes functional inhibition in rat brainstem motoneurons in vitro. J. Physiol. 2002;541:123–137. doi: 10.1113/jphysiol.2001.016063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybalko N, Syka J. Effect of noise exposure on gap detection in rats. Hear. Res. 2005;200:63–72. doi: 10.1016/j.heares.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Baker RA. Neurotransmitter-specific uptake and retrograde transport of [3H]glycine from the inferior colliculus by ipsilateral projections of the superior olivary complex and nuclei of the lateral lemniscus. Brain Res. 1990;524:244–253. doi: 10.1016/0006-8993(90)90698-b. [DOI] [PubMed] [Google Scholar]
- Saldaña E, Berrebi AS. Anisotropic organization of the rat superior paraolivary nucleus. Anat. Embryol. (Berl) 2000;202:265–279. doi: 10.1007/s004290000109. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Arehole S. Gap detection in chinchillas with temporary high-frequency hearing loss. J. Acoust. Soc. Am. 1985;77:1173–1177. doi: 10.1121/1.392181. [DOI] [PubMed] [Google Scholar]
- Schofield BR. Superior paraolivary nucleus in the pigmented guinea pig: separate classes of neurons project to the inferior colliculus and the cochlear nucleus. J. Comp. Neurol. 1991;312:68–76. doi: 10.1002/cne.903120106. [DOI] [PubMed] [Google Scholar]
- Singer JH, Talley EM, Bayliss DA, Berger AJ. Development of glycinergic synaptic transmission to rat brain stem motoneurons. J. Neurophysiol. 1998;80:2608–2620. doi: 10.1152/jn.1998.80.5.2608. [DOI] [PubMed] [Google Scholar]
- Smith PH, Joris PX, Yin TC. Anatomy and physiology of principal cells of the medial nucleus of the trapezoid body (MNTB) of the cat. J. Neurophysiol. 1998;79:3127–3142. doi: 10.1152/jn.1998.79.6.3127. [DOI] [PubMed] [Google Scholar]
- Snell KB, Frisina DR. Relationships among age-related differences in gap detection and word recognition. J. Acoust. Soc. Am. 2000;107:1615–1626. doi: 10.1121/1.428446. [DOI] [PubMed] [Google Scholar]
- Snell KB, Mapes FM, Hickman ED, Frisina DR. Word recognition in competing babble and the effects of age, temporal processing, and absolute sensitivity. J. Acoust. Soc. Am. 2002;112:720–727. doi: 10.1121/1.1487841. [DOI] [PubMed] [Google Scholar]
- Sommer I, Lingenhohl K, Friauf E. Principal cells of the rat medial nucleus of the trapezoid body: an intracellular in vivo study of their physiology and morphology. Exp. Brain Res. 1993;95:223–239. doi: 10.1007/BF00229781. [DOI] [PubMed] [Google Scholar]
- Spangler KM, Warr WB, Henkel CK. The projections of principal cells of the medial nucleus of the trapezoid body in the cat. J. Comp. Neurol. 1985;238:249–262. doi: 10.1002/cne.902380302. [DOI] [PubMed] [Google Scholar]
- Stephens MA. Tests for Randomness of Directions Against two Circular Alternatives. Am. Stat. Assoc. J. 1969;64:280–289. [Google Scholar]
- Syka J, Rybalko N, Mazelova J, Druga R. Gap detection threshold in the rat before and after auditory cortex ablation. Hear. Res. 2002;172:151–159. doi: 10.1016/s0378-5955(02)00578-6. [DOI] [PubMed] [Google Scholar]
- Tallal P, Stark RE, Mellits D. The relationship between auditory temporal analysis and receptive language development: evidence from studies of developmental language disorder. Neuropsychologia. 1985a;23:527–534. doi: 10.1016/0028-3932(85)90006-5. [DOI] [PubMed] [Google Scholar]
- Tallal P, Stark RE, Mellits ED. Identification of language-impaired children on the basis of rapid perception and production skills. Brain Lang. 1985b;25:314–322. doi: 10.1016/0093-934x(85)90087-2. [DOI] [PubMed] [Google Scholar]
- Tollin DJ, Yin TC. The coding of spatial location by single units in the lateral superior olive of the cat. I. Spatial receptive fields in azimuth. J. Neurosci. 2002;22:1454–1467. doi: 10.1523/JNEUROSCI.22-04-01454.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler RS, Summerfield Q, Wood EJ, Fernandes MA. Psychoacoustic and phonetic temporal processing in normal and hearing-impaired listeners. J. Acoust. Soc. Am. 1982;72:740–752. doi: 10.1121/1.388254. [DOI] [PubMed] [Google Scholar]
- Wagner E, Klump GM, Hamann I. Gap detection in Mongolian gerbils (Meriones unguiculatus) Hear. Res. 2003;176:11–16. doi: 10.1016/s0378-5955(02)00643-3. [DOI] [PubMed] [Google Scholar]
- Walton JP, Frisina RD, Ison JR, O'Neill WE. Neural correlates of behavioral gap detection in the inferior colliculus of the young CBA mouse. J. Comp. Physiol. [A] 1997;181:161–176. doi: 10.1007/s003590050103. [DOI] [PubMed] [Google Scholar]
- Walton JP, Simon H, Frisina RD. Age-related alterations in the neural coding of envelope periodicities. J. Neurophysiol. 2002;88:565–578. doi: 10.1152/jn.2002.88.2.565. [DOI] [PubMed] [Google Scholar]
- Werner LA, Folsom RC, Mancl LR, Syapin CL. Human auditory brainstem response to temporal gaps in noise. J. Speech Lang. Hear. Res. 2001;44:737–750. doi: 10.1044/1092-4388(2001/058). [DOI] [PubMed] [Google Scholar]
- Wilkie D. Rayleigh Test for Randomness of Circular Data. Appl. Statist. 1983;32:311–312. [Google Scholar]
- Willard FH, Ryugo DK. The Auditory Psychobiology of the Mouse. Springfield: Thomas; 1983. Anatomy of the central auditory system; pp. 201–304. [Google Scholar]
- Zeng FG, Oba S, Garde S, Sininger Y, Starr A. Temporal and speech processing deficits in auditory neuropathy. Neuroreport. 1999;10:3429–3435. doi: 10.1097/00001756-199911080-00031. [DOI] [PubMed] [Google Scholar]
- Zhang H, Kelly JB. Responses of neurons in the rat's ventral nucleus of the lateral lemniscus to amplitude-modulated tones. J. Neurophysiol. 2006;96:2905–2914. doi: 10.1152/jn.00481.2006. [DOI] [PubMed] [Google Scholar]
- Zook JM, Casseday JH. Origin of ascending projections to inferior colliculus in the mustache bat, Pteronotus parnellii. J. Comp. Neurol. 1982;207:14–28. doi: 10.1002/cne.902070103. [DOI] [PubMed] [Google Scholar]
- Zwicker H, Fastl H. Psychoacoustics. Facts and Models. Berlin: Springer Verlag; 1990. [Google Scholar]