Abstract
Soft tissue sarcomas are uncommon malignancies that have a high risk of local recurrence despite adequate initial surgery. The aim of follow-up imaging with any malignancy is to detect recurrence promptly so that treatment can be instigated at the earliest possible opportunity. In this review article, we discuss the imaging modalities that can be employed to detect local recurrence following surgery for an extremity soft tissue sarcoma. The role of radiographs, computed tomography, magnetic resonance imaging and positron emission tomography is reviewed followed by a discussion on the imaging modalities useful in the detection of metastatic disease. Finally, we present a robust pathway that is suggested for the follow-up of patients with an extremity soft tissue sarcoma.
Keywords: Imaging, MR imaging, soft tissue tumours, recurrent sarcoma
Introduction
Soft tissue sarcomas are rare tumours that account for less than 1% of malignancy in adults1–4. Local recurrence following surgery for a soft tissue sarcoma will occur in between 5% and 35% of cases despite adequate initial treatment3,5–7. This typically occurs within 2 years of primary treatment3. The risk of developing a local recurrence is dependent on a number of variables including the lesion size, location, histological grade, adequacy of surgical resection and response to adjuvant therapies. In general, histologically high grade tumours have a higher risk of local recurrence and metastatic disease when compared to low grade tumours. If local recurrence occurs there is a higher risk of the development of metastatic disease and subsequent mortality from the tumour7. There is, however, no conclusive evidence that local recurrence in an extremity soft tissue sarcoma predisposes the patient to metastatic disease. It is rather that those tumours which recur locally are usually of higher grade and the biology of the tumour makes it more likely to lead to metastatic disease.
The main aim of post-operative follow-up imaging of soft tissue tumours is therefore to identify and characterize a clinically suspected recurrence. This will aid in both surgical planning if a recurrence is found and guide appropriate biopsy. Patient review and clinical examination is complimented by imaging using a number of modalities including ultrasound, computed tomography (CT), magnetic resonance (MR) imaging and positron emission tomography (PET) scanning. It is important to be aware of the imaging findings that may be observed in the normal post-operative phase so that inappropriate biopsy is not undertaken or unnecessary treatment instigated for incorrectly diagnosed tumour recurrence.
The aim of this article is to review the imaging interpretation of post-operative follow-up of soft tissue tumours. We discuss the relative values of different imaging modalities and the role they play in management. We also suggest a practical and robust pathway for the imaging follow-up of patients following surgery for a soft tissue tumour. We focus on soft tissue tumours involving the extremities. Those that originate within the abdomen, thorax or retroperitoneum are outside the remit of this article.
Imaging techniques for local recurrence
Radiographs
Radiographs have a limited role in the follow-up of recurrent soft tissue tumours. There are, however, a number of situations where they may have value. First, to identify the presence of matrix mineralization for example, synovial sarcoma may demonstrate soft tissue calcification in between 10 and 30% of cases8. Second, heterotopic ossification may be identified which may present as clinical recurrence. Finally, if the soft tissue lesion has a deep location, any adjacent involvement of bone may be visualized. In the post-operative phase, however, it is normal to lose the normal soft tissue planes and mass effect is a late radiographic sign.
Ultrasound
Ultrasound is readily available, cheap and rapid to perform and is an extremely useful modality in the monitoring of recurrent superficial masses9. Recurrent soft tissue sarcomas typically appear as low reflectivity nodules which are often round, oval or lobulated. Choi and co-workers compared MR imaging and ultrasound in the detection of local recurrence of soft tissue sarcoma and found both techniques to be equally useful10. Despite this, ultrasound has been underutilized in the evaluation of soft tissue masses11. Current high resolution transducers provide excellent depiction of soft tissues and can readily detect local recurrence. Furthermore, ultrasound also allows image guided fine needle aspiration or biopsy if a focal abnormality is identified. Alexander and co-workers evaluated the usefulness of ultrasound in the identification and sampling of recurrent malignancy. They were able to localize recurrent disease and accurately target the recurrence to obtain diagnostic material12.
Ultrasound is particularly helpful when compared to other imaging modalities in a number of circumstances. Fluid can be readily identified on ultrasound and the identification of posterior acoustic enhancement can be a useful imaging sign in evaluating post-operative seromas and may aid in the differentiation of cystic from solid masses which can be challenging on occasion on MR imaging13. In addition, the degree of neovascularity can be identified which can aid in the differentiation of fluid from a solid mass14. Finally, in the presence of metallic implants, ultrasound can provide improved assessment of the adjacent soft tissues when artifacts limit visualization. Previous workers have also found ultrasound useful in the differentiation of nodal from non-nodal soft tissue recurrence12. The drawback of ultrasound is that it is extremely difficult to review serial examinations and is insufficient when planning surgical re-excision of a recurrent sarcoma.
Computed tomography
The role of CT in imaging of extremity sarcoma is now limited as MR imaging with its superior soft tissue contrast allows better identification of recurrent tumour. CT may be used if MR imaging is contraindicated or the patient is unable to tolerate an MR examination. It is less accurate than MR imaging for small recurrent lesions (less than 15 cm3)15. It is recommended that when trying to identify recurrent masses on CT, reporting should be performed from a workstation using narrow window levels in order that small differences in attenuation values can be appreciated. Post-contrast imaging may also aid in the differentiation of tumour from adjacent soft tissues.
MR imaging
The inherent soft tissue contrast of MR imaging is ideally suited to the evaluation of soft tissue masses. It is widely accepted that MR imaging plays a vital role in the pre-operative evaluation and staging of soft tissue tumours. MR imaging also plays an important role in the post-operative period. During follow-up, differentiation has to be made between post-surgical soft tissue changes and tumour recurrence and this may be problematic. This is particularly true when the patient has undergone chemotherapy and radiotherapy in addition to surgery.
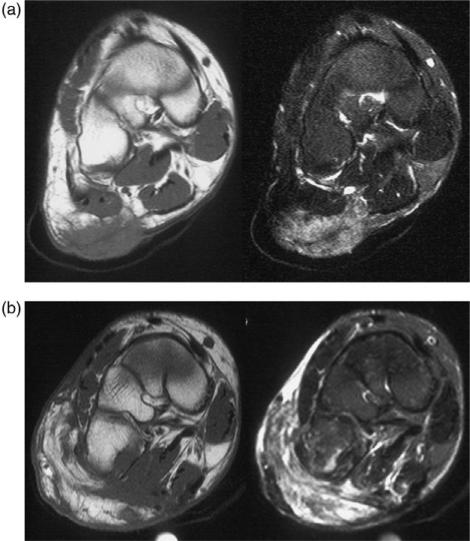
When diagnosing tumour recurrence it is important to identify a focal mass that is typically of high signal on T2-weighted and short time inversion recovery (STIR) sequences (Fig. 1)16. In the post-operative situation, however, there are a number of entities which can demonstrate increased signal intensity on T2-weighted sequences which are not tumour recurrence. These include seroma formation, fatty atrophy and post-irradiation inflammatory change (Fig. 2)17–19. Fat-suppressed T2-weighted images are more sensitive than conventional T2-weighted fast spin echo images in the detection of subtle fluid hyperintensity. A number of studies have identified imaging characteristics that can aid in differentiation of post-operative change and tumour recurrence. Vanel and co-workers found that the presence of low signal on T2-weighted sequences had 96% sensitivity for exclusion of recurrent or residual tumour17. However, this signal characteristic is rarely identified in the early post-operative period. Biondetti described the ‘muscle texture sign’ on T1-weighted sequences. Normal muscle demonstrates a distinct imaging pattern where muscle fibres are interspersed between fat and connective tissue (Fig. 2). If an abnormal signal is identified in the post-operative period on T2-weighted sequences, comparison with a T1-weighted sequence obtained in an orthogonal plane to the long axis of the muscle allows further assessment. If this ‘muscle texture sign’ is preserved, it is much less likely that the finding is secondary to tumour recurrence20.
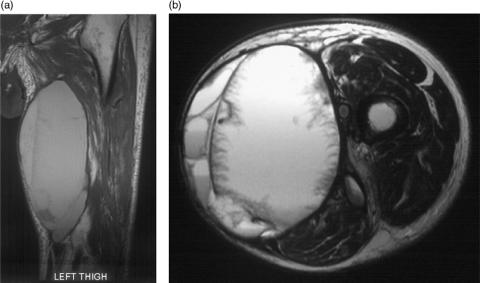
Figure 1.
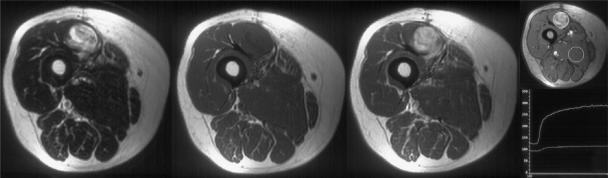
Recurrent soft tissue sarcoma in the biceps femoris muscle. Axial T2-weighted fast spin echo, T1-weighted pre- and post-intravenous gadolinium and dynamic sequence showing regions of interest and a time/signal intensity curve. The recurrence is revealed as hyperintense with mass effect on the T2-weighted image which enhances with gadolinium. The time/signal intensity curve shows rapid uptake in the region of interest (ROI) positioned over the tumour.
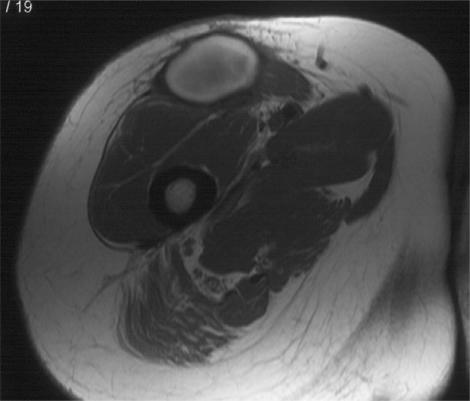
Figure 2.
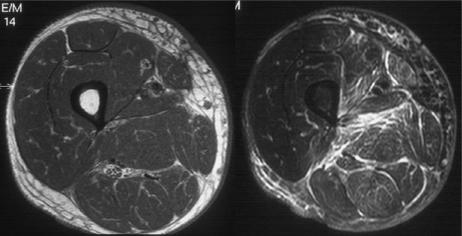
Post-radiotherapy changes. Axial T1-weighted and T2-weighted fat suppressed fast spin echo images of the thigh in a patient following excision of a soft tissue sarcoma and post-operative radiotherapy. There is diffuse oedema throughout the soft tissues within the radiation field. Note the muscle texture sign on the T1-weighted image and the absence of hyperintensity with mass effect.
The original signal characteristics of the tumour need to be considered and pre-operative imaging should be available when assessing for post-operative recurrence. In the case of some myxoid tumours, their signal characteristics can be difficult to differentiate from fluid and care should be taken post-operatively in diagnosing a seroma. In this situation, gadolinium enhanced imaging will show enhancement of the myxoid tumour whereas seromas will not enhance or will show minimal rim enhancement21,22. Similarly, if there are areas of tumour mineralization or a predominantly fibrous lesion this may show low signal intensity on T1- and T2-weighted sequences so differentiating recurrent tumour from scar tissue may be problematic.
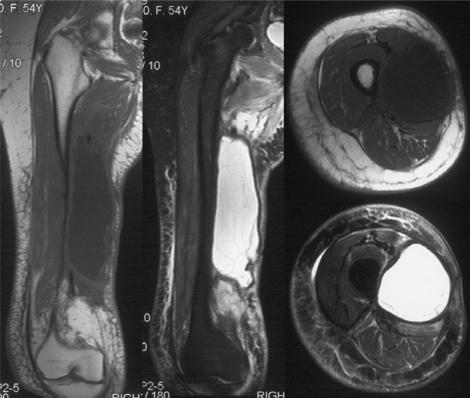
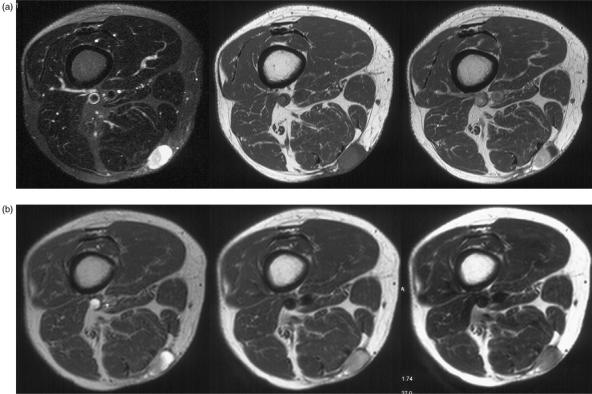
Post-operative seromas occur in approximately 17–19% of patients following surgery for soft tissue sarcoma and are more common in the lower extremity23,24. The typical appearances are of a homogeneous, fairly well-defined area of low to intermediate signal relative to muscle on T1- and high signal on T2-weighted sequences and rim enhancement on contrast enhanced images (Figs. 3 and 4)25. However, a spectrum of imaging findings may be identified secondary to organizing haematoma or granulation tissue. In the presence of blood products, areas of high signal may be identified on T1-weighted sequences and the seroma may demonstrate a heterogeneous signal on T2-weighted sequences (Fig. 5a). In addition, a feathery appearance may be identified on the inner margin of the seroma in approximately 10% of cases (Fig. 5b). These atypical seromas may occur in up to 25% of cases26. The seroma may have a smooth or irregular border and may be surrounded by mild soft tissue oedema23. The border of the seroma may demonstrate a low signal intensity margin on T2-weighted sequences due to the deposition of haemosiderin (Fig. 5b). The shape can be variable including round, oval, or angular in nature in the transverse plane and oval, elliptical or flame-shaped in the longitudinal plane22. Typically, the longitudinal extent of the seroma will exceed its transverse dimension (Figs. 3 and 5a). Follow-up studies demonstrate that these fluid collections may remain unchanged for considerable periods of time, although resolution usually occurs within 3–18 months23. Similarly, post-operative haematomas may be identified in a subacute phase in the weeks following surgery. An intermediate signal mass with a high signal rim is demonstrated on T1-weighted sequences due to the presence of extracellular methaemoglobin (Fig. 6)22.
Figure 3.
Post-operative seroma in a patient following excision of a soft tissue sarcoma from the thigh. Coronal T1-weighted, STIR and axial T1-weighted and T2-weighted fat suppressed fast spin echo images showing typical features with a homogeneous mass hypointense on T1-weighted and hyperintense on the T2-weighted and STIR images. There are florid post-radiotherapy changes in the surrounding soft tissues.
Figure 4.
Post-operative seroma. Axial STIR, T1-weighted spin echo and contrast enhanced T1-weighted images with and without fat suppression. The STIR image shows a small focus of hyperintense signal with mass effect at the site of previous excision of a soft tissue sarcoma deep to the fascia lata. The contrast enhanced images show minor rim enhancement consistent with a small seroma best shown on the fat suppressed image.
Figure 5.
Post-operative seroma. Coronal T1-weighted and axial T2-weighted fat suppressed fast spin echo images. The seroma appears hyperintense on the T1-weighted image due to methaemoglobin. The axial image shows a low signal intensity rim due to haemosiderin deposition and a feathery internal pattern seen in approximately 10% of cases.
Figure 6.
Axial T1-weighted image 5 weeks after excision of a sarcoma from the anterior thigh. Typical appearances of a subacute haematoma. The hyperintensity is due to the paramagnetic effect of methaemoglobin.
Post-radiation reactive changes demonstrate a high signal on T2-weighted sequences but are not usually associated with a mass lesion (Figs. 2 and 7). The oedema may be demonstrated in the subcutaneous tissue or tracking along the normal myofibrillar planes with a typical feathery pattern (Fig. 7). When this is identified, comparison with the T1-weighted sequences should demonstrate the ‘muscle texture sign’ to still be present. There are wide variations in the length of time it takes for this soft tissue oedema to resolve, however, it usually peaks at between 6 and 18 months following treatment and persists for between 2 and 4 years. The oedema tends to persist for longer around the intramuscular septa than in the fat or muscle itself and is more prominent for patients treated with neutron radiation than photon radiation. STIR sequences will identify this imaging finding for longer periods that T2-weighted sequences. Following radiation therapy, the thickness of the subcutaneous fat increases whereas the muscle size tends to decrease27. Post-operative fibrosis typically demonstrates low signal intensity on both T1- and T2-weighted sequences (similar to muscle) and shows little or no enhancement with gadolinium (Fig. 8)28.
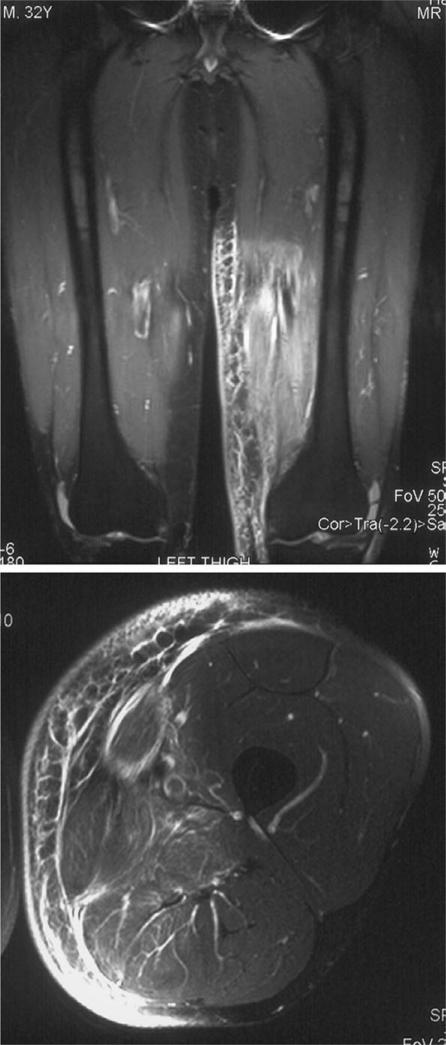
Figure 7.
Post-radiotherapy change. Coronal STIR and axial T2-weighted fat suppressed images showing diffuse oedema affecting the medial aspect of the distal thigh corresponding exactly with the radiation field. Note that although there is a hyperintense signal it does not have true mass effect.
Figure 8.
Post-operative scar tissue. Axial STIR and T1-weighted images pre- and post-gadolinium. There is a small irregular mass at the site of previous surgery over the posterior aspect of the proximal femur but it is hypointense on the STIR image and does not show significant enhancement with gadolinium.
If amputation is required for the treatment of soft tissue sarcoma, follow-up imaging of the amputation stump may be required. MR imaging is ideally suited for this purpose and can readily identify complications and recurrence in an amputation stump. In the post-operative period, it is important to be aware of the wide variety of problems that may be encountered other than tumour recurrence including local bursitis, cellulitis, osteomyelitis, neuroma formation, haematoma or heterotopic ossification29.
The use of myocutaneous flaps for complex reconstructive surgery is increasing and these are often encountered when reporting follow-up studies for excised sarcomas. The MR signal characteristics are initially of increased signal on T2-weighted sequences with some contrast enhancement following gadolinium administration. Subsequently, there is often atrophic change in the flap itself and a relatively high signal on T1-weighted sequences (Fig. 9)30. If unaware of the nature of the surgery the oedematous muscle flap can appear as a hyperintense mass on T2-weighted images mimicking tumour recurrence (Fig. 9).
Figure 9.
Coronal T1-weighted and T2-weighted fat suppressed images pre- and post-surgery. The initial images show an infiltrative soft tissue sarcoma arising in the subcutaneous tissues over the sole of the foot. The post-operative images show an irregular mass lesion at the site of the excised tumour with fatty atrophy on the T1- and oedema on the T2-weighted images. This mass is due to a muscle flap and not recurrent sarcoma.
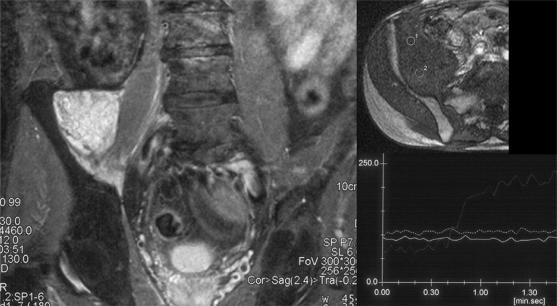
Dynamic gadolinium enhanced MR imaging can help in differentiation of recurrent tumour from an inflammatory pseudotumour (Fig. 1)31. This latter entity is unusual and demonstrates later enhancement, at approximately 2 min following a gadolinium bolus (Fig. 10). Recurrent tumour will tend to enhance at an earlier stage, usually within a few seconds19. A number of other imaging characteristics of malignant tissue have been described including a rapid initial enhancement curve and non-peripheral enhancement. The sensitivity of this technique has been reported as 87% with a positive predictive value of 70%32.
Figure 10.
Inflammatory pseudotumour. Coronal STIR and dynamic sequence with regions of interest (ROI) and time/signal intensity curve. The STIR image shows a hyperintense mass at the site of a previously excised soft tissue sarcoma in the right iliacus muscle. This mass shows no appreciable enhancement on the time/signal intensity curve compared with the ROI over the iliac vessels.
Diffusion weighted MR imaging has also been evaluated in the detection of tumour recurrence and post-treatment soft tissue changes33. This technique enables the quantification of water molecule motion in terms of signal loss. Malignant lesions are often highly cellular and tend to inhibit diffusion, therefore benign lesions tend to show low signal versus malignant lesions demonstrating high signal. Baur and co-workers suggest that this technique and the presence of signal loss can be used to characterize different tissue types when monitoring for sarcoma recurrence. They found that they were able to differentiate muscle oedema from post-operative seroma and tumour recurrence33. Recently, Kolla and co-workers have also found diffusion-weighted imaging useful to aid in characterization of simple fluid and soft tissue oedema (Fig. 11)34.
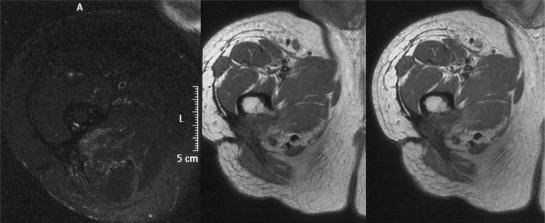
Figure 11.
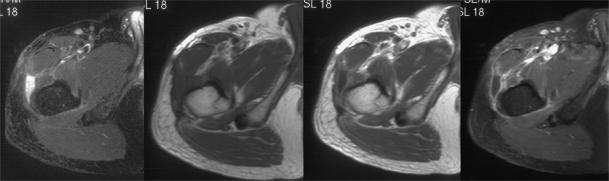
Recurrent soft tissue sarcoma in the subcutaneous tissues. Axial T2-weighted fat suppressed fast spin echo image showing a hyperintense mass. The pre- and post-contrast T1-weighted images show a posterior solid component and an anterior cystic component. The three axial images all obtained at the same position with increasing diffusion weighting show decreasing signal from the cystic component but little change in the solid. Diffusion weighting can be used to distinguish fluid from solid but in this case is unable to differentiate a benign fluid collection from a cystic area in a recurrent sarcoma.
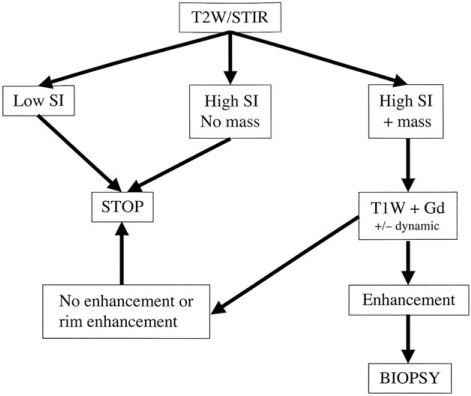
Fig. 12 shows an algorithm for the MR imaging approach to the patient with a suspected locally recurrent soft tissue sarcoma. This relies on identifying a hyperintense signal on T2-weighted or STIR images with or without mass effect. If the latter, consideration should be given to administer gadolinium to distinguish tumour recurrence from a post-operative seroma.
Figure 12.
Algorithm for the MR approach to a patient with a suspected local recurrence of a soft tissue sarcoma modified after Vanel et al.16. SI, signal intensity.
Positron emission tomography (PET)
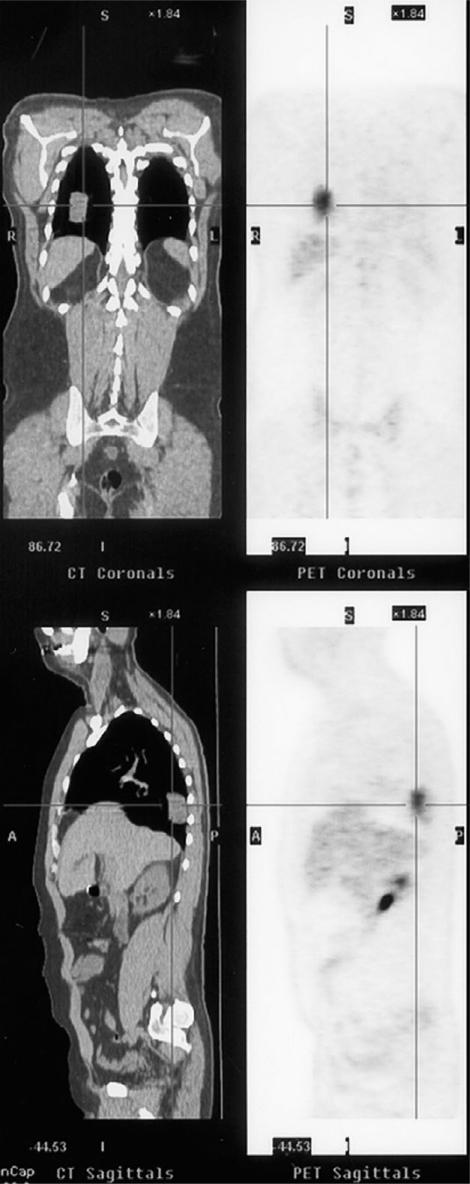
PET is typically performed using fluorine-18-deoxyglucose and the uptake of this radiopharmaceutical in general is greater in malignant tumours than in benign tumours. Similarly there is variation in the degree of uptake with less uptake being seen in low versus high grade lesions. The expansion in availability of both the pharmaceutical and the development of PET-CT has increased interest in the use of this technique in oncological imaging35,36. Johnson and co-workers found PET to be more sensitive than CT and MR imaging in the detection of recurrent disease from sarcomas37. It may also be used for the detection and localization of metastatic disease (Fig. 13).
Figure 13.
PET-CT showing a right lower lobe metastasis from a low grade soft tissue sarcoma excised 10 years before.
Imaging techniques for metastatic disease
Between 10 and 15% of patients with an extremity soft tissue sarcoma will have metastatic disease at the time of presentation, most commonly to the lungs. Following treatment, approximately 70% of patients with extremity sarcomas will recur with isolated lung metastases as the manifestation of recurrent disease3. Metastases to lymph nodes are uncommon, occurring in approximately 5% of cases. The most common tumours to spread via this route are epithelioid sarcoma, synovial sarcoma, rhabdomyosarcoma and clear cell sarcoma25.
Radiographs
The role of routine chest radiography in the post-operative period is controversial. Lord and co-workers found that only 27% of cases of pulmonary metastatic disease were identified at routine 6 monthly examination as compared with 42% during non-routine radiography and 32% during staging studies for local recurrence38.
Radiographic assessment of bone metastases in the post-operative phase is typically performed for painful lesions. Yoshikawa and co-workers reported the incidence, imaging findings and sites of involvement in soft tissue sarcomas that were metastatic to bone. They found a 9% incidence of skeletal metastases with alveolar soft part sarcoma, dedifferentiated liposarcoma, angiosarcoma and rhabdomyosarcoma having a predilection for metastatic spread via this route39. The majority of metastases from soft tissue sarcomas will be osteolytic in nature and most commonly involve the axial skeleton.
Computed tomography
The most common site for patients with an extremity soft tissue sarcoma to develop metastatic disease is the lungs40. Typically, metastatic lung disease occurs within 3 years of diagnosis41. Non-contrast CT can adequately assess for the presence of pulmonary metastatic disease. Multislice CT is extremely accurate in the identification of pulmonary metastases, however, a number of workers have questioned the routine use of this technique in terms of cost-effectiveness42,43. Furthermore, up to 80% of pulmonary nodules identified on CT, which are not identifiable on a chest radiograph in patients with known extrapulmonary malignancy are of benign cause44.
It is well recognized that the majority of soft tissue sarcomas metastasize to the lung, however, intra-abdominal metastatic spread may occur. Ogose and co-workers studied 505 patients with musculoskeletal sarcomas and found intra-abdominal spread occurred to the liver in 4%, the bowel in 1.2%, the pancreas in 0.8% and the peritoneal surface in 0.8%. The most frequent soft tissue tumours to metastasize to the abdominal cavity in this series were liposarcoma (4/58) and malignant fibrous histiocytoma (3/76)45. Other workers have also reported myxoid liposarcoma to preferentially spread to extra-pulmonary sites46,47 including the abdominal cavity48. This has prompted some workers to suggest that in higher grade liposarcoma, routine follow-up should include chest and abdominal CT45.
Inadequate primary resection
The inadequate excision of a soft tissue tumour at initial operation is a problem that is unfortunately encountered all too often. This may occur because the diagnosis of a sarcoma has not been considered and imaging may not have been performed prior to surgery. Second, sampling error may have occurred during biopsy leading to false reassurance as to the nature of the mass. Often, the tumour is then treated with local excision, but without wide margins leading to a potential local recurrence rate of 70–90%49. In this circumstance further imaging to localize the lesion is often unrewarding as the primary mass has usually been excised. Kaste and co-workers found that MR imaging was unreliable in detecting the need for additional resection when incomplete resection had been performed for a soft tissue sarcoma50. Siebenrock and co-workers reported that MR imaging has a poor negative predictive value for the identification of residual tumour following unexpected resection of a soft tissue sarcoma51. In the immediate post-operative situation, there is often extensive signal abnormality in the soft tissues and unless there is a large residual mass, it is impossible to differentiate inflammatory tissue from residual tumour. Surgical resection in these cases may yield residual tumour in 45–59% of cases so imaging has a limited role in guiding any planned repeat procedure52,53. In these cases of inadequate initial excision, repeat surgery can be challenging as it is difficult to locate the original tumour borders and the required margins are therefore hard to define. A number of workers have identified the relatively high prevalence of tumour positive margins at repeat excision52,54. In the authors’ experience, MR imaging may be helpful in detecting the relationship of any significant residual sarcoma to adjacent neurovascular structures, but it has to be stressed that it cannot detect microscopic areas of residual tumour55.
Follow-up strategy
Baseline MR imaging is useful following surgical resection of a soft tissue sarcoma as this aids in interpretation of subsequent follow-up studies. However, this should not be performed until at least 6–8 weeks following the initial surgery so that the inflammatory change in the immediate post-operative period will have subsided. There is no standardized protocol for the post-operative imaging for extremity soft tissue sarcomas, with few studies looking at the cost-effectiveness of such regimes40,42,43. Varma and co-workers suggest a baseline MR imaging followed by 6-monthly MR imaging for the first 2 years following primary excision as most recurrences will recur in that time period25. Whooley and co-workers found that clinical assessment of symptomatology, examination and chest X-ray (CXR) were the most effective methods of detection of disease recurrence. Chest CT was best reserved for patients with an abnormal CXR and routine MR imaging on an annual basis was ineffective at detecting recurrence40. They also found that CXR was a cost effective method of detecting pulmonary metastatic disease compared with routine chest CT42,43. Similarly, a recent study on follow-up of patients with soft tissue sarcoma concluded that intensive regular surveillance of the surgical field with MR imaging did not add to clinical assessment in detecting local recurrence56. In the author's own institution, routine follow-up with MR imaging is reserved for those cases considered at particularly high risk of developing a local recurrence. These are histologically high grade tumours where the surgical margin is considered at best marginal.
Conclusion
Soft tissue sarcomas are rare. However, when they are encountered they have a relatively high incidence of local recurrence and metastatic disease. Monitoring these patients in the post-operative period is challenging in view of the spectrum of imaging findings that can be observed. MR imaging is the modality of choice for follow-up of the surgical field, however, a number of other modalities can be useful in specific instances to confirm or exclude tumour recurrence. The imaging modalities for the detection and diagnosis of local recurrence and metastatic disease have been discussed and a practical follow-up strategy for these patients suggested.
References
- 1.Westbury G. The management of soft tissue sarcomas. J Bone Joint Surg (Br) 1989;71:2–3. doi: 10.1302/0301-620X.71B1.2914997. [DOI] [PubMed] [Google Scholar]
- 2.Landis SH, Wurray T, Bolden S, et al. Cancer statistics 1999. Cancer J Clin. 1999;49:8–31. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Potter DA, Glenn J, Kinsella T, et al. Patterns of recurrence in patients with high-grade soft tissue sarcomas. J Clin Oncol. 1985;3:353–66. doi: 10.1200/JCO.1985.3.3.353. [DOI] [PubMed] [Google Scholar]
- 4.Rosenthal D. Imaging modalities for sarcomas of bone and soft tissues. Curr Opin Orthop. 2004;15:468–72. [Google Scholar]
- 5.Cormier JN, Pollock RE. Soft tissue sarcomas. Cancer J Clin. 2004;54:94–109. doi: 10.3322/canjclin.54.2.94. [DOI] [PubMed] [Google Scholar]
- 6.Trovik CS, Bauer HC, Alvegard TA. Surgical margins, local recurrence and metastasis in soft tissue sarcomas: 559 surgically treated patients from the Scandinavian Sarcoma Group Register. Eur J Cancer. 2000;36:710–6. doi: 10.1016/s0959-8049(99)00287-7. [DOI] [PubMed] [Google Scholar]
- 7.Lewis JJ, Leung D, Heslin M, et al. Association of local recurrence with subsequent survival in extremity soft tissue sarcoma. J Clin Oncol. 1997;15:646–52. doi: 10.1200/JCO.1997.15.2.646. [DOI] [PubMed] [Google Scholar]
- 8.Paul AS, Charalambous C, Maltby, et al. The management of soft-tissue sarcomas of the extremities. Curr Orthop. 2003;17:124–33. [Google Scholar]
- 9.Briccoli A, Galletti S, Salone M, Morganti A, Pelotti P, Rocca M. Ultrasonography is superior to computed tomography and magnetic resonance imaging in determining superficial resection margins of malignant chest wall tumours. J Ultrasound Med. 2007;26:157–62. doi: 10.7863/jum.2007.26.2.157. [DOI] [PubMed] [Google Scholar]
- 10.Choi H, Varma D, Fornage B, et al. Soft tissue sarcoma: MR imaging vs sonography for detection of local recurrence after surgery. AJR. 1991;157:353–8. doi: 10.2214/ajr.157.2.1853821. [DOI] [PubMed] [Google Scholar]
- 11.Fornage B. Soft tissue masses: the underutilization of sonography. Semin Musculoskelet Radiol. 1999;3:115–33. doi: 10.1055/s-2008-1080056. [DOI] [PubMed] [Google Scholar]
- 12.Alexander AA, Nazarian LN, Feld RI. Superficial soft-tissue masses suggestive of recurrent malignancy: sonographic localization and biopsy. AJR. 1997;169:1449–51. doi: 10.2214/ajr.169.5.9353478. [DOI] [PubMed] [Google Scholar]
- 13.Ma LD, McCarthy EF, Bluemke DA, et al. Differentiation of benign from malignant musculoskeletal lesions using MR imaging: pitfalls in MR evaluation of lesions with a cystic appearance. AJR Am J Roentgenol. 1998;170:1251–8. doi: 10.2214/ajr.170.5.9574596. [DOI] [PubMed] [Google Scholar]
- 14.Fornage BD. Role of color Doppler imaging in differentiating between pseudocystic malignant tumours and fluid collections. J Ultrasound Med. 1995;14:125–8. doi: 10.7863/jum.1995.14.2.125. [DOI] [PubMed] [Google Scholar]
- 15.Reuther G, Mutschler W. Detection of local recurrent disease in musculoskeletal tumors: magnetic resonance imaging versus computed tomography. Skeletal Radiol. 1990;19:85–90. doi: 10.1007/BF00197611. [DOI] [PubMed] [Google Scholar]
- 16.Vanel D, Shapeero LG, De Baere T, et al. MR imaging in the follow up of malignant and aggressive soft tissue tumors: results of 511 examinations. Radiology. 1994;190:263–68. doi: 10.1148/radiology.190.1.8259417. [DOI] [PubMed] [Google Scholar]
- 17.Vanel D, Lacombe MJ, Couanet D, et al. Musculoskeletal tumors: follow up with MR imaging after treatment with surgery and radiation therapy. Radiology. 1987;164:243. doi: 10.1148/radiology.164.1.3588913. [DOI] [PubMed] [Google Scholar]
- 18.Panicek DM, Schwartz LH, Heelan RT, et al. Non-neoplastic causes of high signal intensity at T2-weighted MR imaging after treatment for musculoskeletal neoplasm. Skeletal Radiol. 1995;24:185–90. doi: 10.1007/BF00228920. [DOI] [PubMed] [Google Scholar]
- 19.Shapeero LG, Vanel D. MR imaging in the follow-up evaluation of aggressive soft tissue tumors. Semin Musculoskelet Radiol. 1999;3:197–205. doi: 10.1055/s-2008-1080063. [DOI] [PubMed] [Google Scholar]
- 20.Biondetti PR, Ehman RL. Soft tissue sarcomas: use of textural patterns in skeletal muscle as a diagnostic feature in postoperative MR imaging. Radiology. 1992;183:845–8. doi: 10.1148/radiology.183.3.1584945. [DOI] [PubMed] [Google Scholar]
- 21.Peterson KK, Renfrew DL, Feddersen RM, et al. Magnetic resonance imaging of myxoid containing tumors. Skeletal Radiol. 1991;20:245–50. doi: 10.1007/BF02341657. [DOI] [PubMed] [Google Scholar]
- 22.Davies AM, Vanel D. Follow-up of musculoskeletal tumors. Eur Radiol. 1998;8:791–9. doi: 10.1007/s003300050474. [DOI] [PubMed] [Google Scholar]
- 23.Poon-Chue A, Menendez L, Gerstner MM, Colletti P, Terk M. MRI evaluation of post-operative seromas in extremity soft tissue sarcomas. Skeletal Radiol. 1999;28:279–82. doi: 10.1007/s002560050516. [DOI] [PubMed] [Google Scholar]
- 24.Skibber JM, Lotze MT, Seipp CA, et al. Limb-sparing surgery for soft tissue sarcomas: wound related morbidity in patients undergoing wide local excision. Surgery. 1987;102:447. [PubMed] [Google Scholar]
- 25.Varma DGK, Jackson EF, Pollock RE, Benjamin RS. Soft tissue sarcoma of the extremities. Magn Reson Imaging Clin N Am. 1995;3:695–712. [PubMed] [Google Scholar]
- 26.Davies AM, Hall AD, Strouhall PD, et al. The MR imaging appearances and natural history of seromas following excision of soft tissue tumours. Eur Radiol. 2004;14:1196–202. doi: 10.1007/s00330-004-2255-y. [DOI] [PubMed] [Google Scholar]
- 27.Richardson ML, Zink-Brody GC, Patten RM, et al. MR characterization of post-irradiation soft tissue edema. Skeletal Radiol. 1996;25:537–43. doi: 10.1007/s002560050131. [DOI] [PubMed] [Google Scholar]
- 28.Glazer HS, Lee JKT, Levitt RG, et al. Radiation fibrosis: differentiation from recurrent tumor by MR imaging. Radiology. 1985;156:721–6. doi: 10.1148/radiology.156.3.4023233. [DOI] [PubMed] [Google Scholar]
- 29.Boutin RD, Pathria MN, Resnick D. Disorders in the stumps of amputee patients: MR imaging. Am J Roentgenol. 1998;171:497–501. doi: 10.2214/ajr.171.2.9694483. [DOI] [PubMed] [Google Scholar]
- 30.Fox MG, Bancroft LW, Peterson JJ, et al. MRI appearance of myocutaneous flaps commonly used in orthopedic reconstructive surgery. AJR Am J Roentgenol. 2006;187:800–6. doi: 10.2214/AJR.05.0484. [DOI] [PubMed] [Google Scholar]
- 31.Shapeero LG, Vanel D, Verstraete KL, et al. Dynamic contrast enhanced MR imaging for soft tissue sarcomas. Semin Musculoskelet Radiol. 1999;3:101–13. doi: 10.1055/s-2008-1080055. [DOI] [PubMed] [Google Scholar]
- 32.Einarsdottir H, Soderlund V, Skoog L, et al. Dynamic MRI and fine needle aspiration cytology in the evaluation of soft tissue lesions. Skeletal Radiol. 2003;32:695–700. doi: 10.1007/s00256-003-0656-7. [DOI] [PubMed] [Google Scholar]
- 33.Baur A, Huber A, Arbogast S, et al. Diffusion-weighted imaging of tumor recurrencies and posttherapeutical soft-tissue changes in humans. Eur Radiol. 2001;11:828–33. doi: 10.1007/s003300000761. [DOI] [PubMed] [Google Scholar]
- 34.Kolla S, Motamedi K, Binesh N, et al. Post-treatment assessment of soft tissue sarcomas with diffusion weighted MRI. Society of Skeletal Radiology, Annual Meeting Scientific Session 18–21 March 2007. Skeletal Radiol. 2007;36:361. [Google Scholar]
- 35.Schuetze SM. Utility of positron emission tomography in sarcomas. Curr Opin Oncol. 2006;18:369–73. doi: 10.1097/01.cco.0000228744.49294.12. [DOI] [PubMed] [Google Scholar]
- 36.Weyl Ben Arush M, Israel O, Postovsky S, et al. Positron emission tomography/computed tomography with 18fluoro-deoxyglucose in the detection of local recurrence and distant metastases of paediatric sarcoma. Pediatr Blood Cancer. 2007;49:901–5. doi: 10.1002/pbc.21150. [DOI] [PubMed] [Google Scholar]
- 37.Johnson GR, Zhuang H, Khan J, et al. Roles of positron emission tomography with fluorine-18-deoxyglucose in the detection of local recurrent and distant metastatic sarcoma. Clin Nucl Med. 2003;28:815–20. doi: 10.1097/01.rlu.0000089523.00672.2b. [DOI] [PubMed] [Google Scholar]
- 38.Lord HK, Salter DM, MacDougall RH, et al. Is routine chest radiography a useful test in the follow up of all adult patients with soft tissue sarcoma. Br J Radiol. 2006;79:799–800. doi: 10.1259/bjr/69175634. [DOI] [PubMed] [Google Scholar]
- 39.Yoshikawa H, Myoui A, Ochi T, et al. Bone metastases from soft tissue sarcomas. Semin Musculoskelet Radiol. 1999;3:183–9. doi: 10.1055/s-2008-1080061. [DOI] [PubMed] [Google Scholar]
- 40.Whooley BP, Mooney MM, Giggs JF, et al. Effective follow-up strategies in soft tissue sarcoma. Semin Surg Oncol. 1999;17:83–7. doi: 10.1002/(sici)1098-2388(199907/08)17:1<83::aid-ssu11>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 41.Bearcroft PWP, Davies AM. Follow-up of musculoskeletal tumours. Eur Radiol. 1999;9:192–200. doi: 10.1007/s003300050654. [DOI] [PubMed] [Google Scholar]
- 42.Kane JM. Surveillance strategies for patients following surgical resection of soft tissue sarcomas. Curr Opin Oncol. 2004;16:238–321. doi: 10.1097/01.cco.0000127879.62254.d3. [DOI] [PubMed] [Google Scholar]
- 43.Chang AE, Schaner EG, Conkle DM, et al. Evaluation of computed tomography in the detection of pulmonary metastases: a prospective study. Cancer. 1979;43:913–6. doi: 10.1002/1097-0142(197903)43:3<913::aid-cncr2820430319>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 44.Chalmers N, Best JJ. The significance of pulmonary nodules detected by CT but not by chest radiography in tumour staging. Clin Radiol. 1991;44:410–12. doi: 10.1016/s0009-9260(05)80661-0. [DOI] [PubMed] [Google Scholar]
- 45.Ogose A, Morita T, Hotta T, et al. Intra-abdominal metastases in musculoskeletal sarcomas. J Orthop Sci. 2000;5:463–9. doi: 10.1007/s007760070024. [DOI] [PubMed] [Google Scholar]
- 46.Pearlstone DB, Pisters PWT, Bold RJ, et al. Patterns of recurrence in extremity liposarcoma. Implications for staging and follow up. Cancer. 1999;85:85–92. doi: 10.1002/(sici)1097-0142(19990101)85:1<85::aid-cncr12>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 47.Cheng EY, Springfield DS, Mankin HJ. Frequent incidence of extrapulmonary sites of initial metastasis in patients with liposarcoma. Cancer. 1995;75:1120–7. doi: 10.1002/1097-0142(19950301)75:5<1120::aid-cncr2820750511>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe H, Ohmori K, Kanamori M, Araki N, Yoshikawa H, Kimura T. A myxoid liposarcoma in the lower leg, with a large intra-abdominal metastasis. J Orthop Sci. 2001;6:95–7. doi: 10.1007/s007760170032. [DOI] [PubMed] [Google Scholar]
- 49.Shiu MH, Castro EB, Hajdu SI, et al. Surgical treatment of 297 soft tissue sarcomas of the extremity. Ann Surg. 1975;182:597–602. doi: 10.1097/00000658-197511000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaste SC, Hill A, Conley L, et al. Magnetic resonance imaging after incomplete resection of soft tissue sarcoma. Clin Orthop. 2002;397:204–11. doi: 10.1097/00003086-200204000-00025. [DOI] [PubMed] [Google Scholar]
- 51.Siebenrock KA, Hertel R, Ganz R. Unexpected resection of soft-tissue sarcoma. Arch Orthop Trauma Surg. 2000;120:65–9. [PubMed] [Google Scholar]
- 52.Goodland JR, Fletcher CDM, Smith MA. Surgical resection of primary soft-tissue sarcoma. J Bone Joint Surg (Br) 1996;78:658–61. [PubMed] [Google Scholar]
- 53.Zornig C, Peiper M, Schroder S. Re-excision of soft tissue sarcoma after inadequate initial operation. Br J Surg. 1995;82:278–79. doi: 10.1002/bjs.1800820247. [DOI] [PubMed] [Google Scholar]
- 54.Noria S, Davis A, Kandel R, et al. Residual disease following unplanned excision of a soft-tissue sarcoma of an extremity. J Bone Joint Surg (Am) 1996;78:650–5. doi: 10.2106/00004623-199605000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Davies AM, Mehr A, Parsonage S, Evans N, Grimer RJ, Pynsent PB. MR imaging in the assessment of residual tumour following inadequate primary excision of soft tissue sarcomas. Eur Radiol. 2004;14:506–13. doi: 10.1007/s00330-003-2023-4. [DOI] [PubMed] [Google Scholar]
- 56.V Rijswijk CSP, Geirnaerdt MJA, Taminiau AHM, van Coevorden F, Hogendoorn PCW, Bloem JL. Surveillance imaging of soft tissue sarcoma and aggressive fibromatosis. Abstract 14th Annual Meeting of the European Society of Musculoskeletal Radiology. Skeletal Radiol. 2007;36:561–604. [Google Scholar]