Abstract
Hepatocellular carcinoma (HCC) is the fifth most common cause of cancer, and its incidence is increasing worldwide because of the dissemination of hepatitis B and C virus infection. Patients with cirrhosis are at the highest risk of developing HCC and should be monitored every 6 months to diagnose the tumour at an early, asymptomatic stage. Patients with early-stage HCC should be considered for any of the available curative therapies, including surgical resection, liver transplantation and percutaneous image-guided ablation. Liver transplantation is the only option that provides cure of both the tumour and the underlying chronic liver disease. However, the lack of sufficient liver donation greatly limits its applicability. Resection is the treatment of choice for HCC in non-cirrhotic patients, who account for about 5% of the cases in western countries. However, in patients with cirrhosis, candidates for resection have to be carefully selected to reduce the risk of postoperative liver failure. It has been shown that a normal bilirubin concentration and the absence of clinically significant portal hypertension are the best predictors of excellent outcomes after surgery. However, less than 5% of cirrhotic patients with HCC fit these criteria. Image-guided percutaneous ablation is the best therapeutic choice for non-surgical patients with early-stage HCC. While ethanol injection has been the seminal percutaneous technique, radiofrequency ablation has emerged as the most effective method for local tumour destruction and is currently used as the primary ablative modality at most institutions.
Keywords: Hepatocellular carcinoma, ablation, radiofrequency
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cause of cancer, and its incidence is increasing worldwide because of the dissemination of hepatitis B and C virus infection1–5. Patients with cirrhosis are at the highest risk of developing HCC and should be monitored every 6 months to diagnose the tumour at an asymptomatic stage6–8. In most solid malignancies, tumour stage at presentation determines prognosis and treatment management. Most patients with HCC, however, have two diseases – liver cirrhosis and HCC – and complex interactions between the two have major implications for prognosis and treatment choice9. The system that links staging with treatment modalities in HCC is the Barcelona Clinic Liver Cancer (BCLC) staging system4,5,10 (Table 1). It includes variables related to tumour stage, liver functional status (Child–Pugh classification, Table 2), physical status and cancer related symptoms (World Health Organization performance status, Table 3)11,12.
Table 1.
| Very early stage | PS 0, Child–Pugh A, single HCC <2 cm |
| Early stage | PS 0, Child–Pugh A–B, single HCC or 3 nodules <3 cm |
| Intermediate stage | PS 0, Child–Pugh A–B, multinodular HCC |
| Advanced stage | PS 1–2, Child–Pugh A–B, portal neoplastic invasion, nodal metastases, distant metastases |
| Terminal stage | PS >2, Child–Pugh C |
HCC, hepatocellular carcinoma; PS, performance status.
Table 2.
Child–Pugh classification of severity of liver disease11
| Parameter | Points assigned | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Ascites | Absent | Mild/moderate | Severe/refractory |
| Bilirubin (mg/dl) | ≤2 | 2–3 | >3 |
| Albumin (g/dl) | >3.5 | 2.8–3.5 | <2.8 |
| Prothrombin time | |||
| Seconds over control | <4 | 4–6 | >6 |
| INR | <1.7 | 1.7–2.3 | >2.3 |
| Encephalopathy | None | Mild | Severe |
A total score of 5–6 is considered grade A (well-compensated disease); 7–9 is grade B (significant functional compromise); and 10–15 is grade C (decompensated disease).
Table 3.
World Health Organization Performance Status grades 12
| Stage 0 | Fully active, normal life, no symptoms |
| Stage 1 | Minor symptoms, able to do light activity |
| Stage 2 | Capable of self-care but unable to carry out work activities; up for more than 50% waking hours |
| Stage 3 | Limited self care capacity; confined to bed or chair >50% waking hours |
| Stage 4 | Completely disabled; confined to bed or chair |
In the BCLC system, early-stage HCC includes patients with WHO performance status of 0, preserved liver function (Child–Pugh class A or B), and solitary tumour or up to 3 nodules each smaller than 3 cm in size, in the absence of macroscopic vascular invasion and extrahepatic spread. If the patient has Child–Pugh class A cirrhosis and a solitary tumour smaller than 2 cm in size, the stage may be defined as very early. Patients with multinodular HCC with neither vascular invasion nor extrahepatic spread are classified as intermediate-stage according to the BCLC staging system, provided that they have a performance status of 0 and Child–Pugh class A or B cirrhosis3–5. Patients with portal vein invasion or extrahepatic disease are classified as advanced stage. The terminal stage includes patients who have either severe hepatic decompensation (Child–Pugh class C) or performance status greater than 2 (Table 1).
Treatment options for early-stage hepatocellular carcinoma
Patients with early-stage HCC can benefit from curative therapies, including surgical resection, liver transplantation and percutaneous ablation, and have the possibility of long term cure, with 5-year survival figures ranging 50–75%5.
Resection is the treatment of choice for HCC in non-cirrhotic patients, who account for about 5% of the cases in western countries. However, in patients with cirrhosis, candidates for resection have to be carefully selected to reduce the risk of postoperative liver failure. It has been shown that a normal bilirubin concentration and the absence of clinically significant portal hypertension are the best predictors of excellent outcomes after surgery13. In experienced hands, such patients – less than 5% of cirrhotic patients with HCC – have treatment-related mortality of less than 1–3% and may achieve a 5-year survival higher than 70%13–15. In contrast, survival drops to less than 50% at 5 years in patients with significant portal hypertension, and to less than 30% at 5 years in those with both adverse factors (portal hypertension and elevated bilirubin)13.
Liver transplantation is the only option that provides cure of both the tumour and the underlying chronic liver disease. It is recognized as the best treatment for patients with solitary HCC smaller than 5 cm in the setting of decompensated cirrhosis and for those with early multifocal disease (up to 3 lesions, none larger than 3 cm)1. The reported outcomes of patients who actually underwent transplantation are better than those of patients submitted to resection, especially if the substantially lower rates of tumour recurrence – less than 10–20% versus more than 70% at 5 years – are considered13,16. Overall survival, however, decreases on an intention-to-treat perspective13,16–18. In fact, because of the lack of sufficient liver donation, there is always a waiting period between listing and transplantation, during which the tumour may grow and develop contraindications to transplantation (vascular invasion, extrahepatic spread). The rate of dropouts may be as high as 25% if the waiting list is longer than 12 months19. This difficulty may be in part overcome by living donation, that, however, has inherent limitations and requires a highly skilled surgical team16.
Image-guided percutaneous ablation is currently accepted as the best therapeutic choice for non-surgical patients with early-stage HCC4,5. Over the past two decades, several methods for chemical ablation or thermal tumour destruction have been developed and clinically tested. These include the injection of ethanol or acetic acid and the administration of localized heating (radiofrequency, microwave, laser ablation) or freezing (cryoablation).
The seminal technique used for local ablation of HCC is percutaneous ethanol injection (PEI). Ethanol induces coagulation necrosis of the lesion as a result of cellular dehydration, protein denaturation, and chemical occlusion of small tumour vessels. PEI is a well-established technique for the treatment of nodular-type HCC. HCC nodules have a soft consistency and are surrounded by a firm cirrhotic liver. Consequently, injected ethanol diffuses within them easily and selectively, leading to complete necrosis of about 70% of small lesions20. Although there have not been any RCT comparing PEI and best supportive care or PEI and surgical resection, several retrospective studies have provided indirect evidence that PEI improves the natural history of HCC: the long-term outcomes of patients with small tumours who were treated with PEI were similar to those reported in surgical series, with 5-year survival rates ranging 41–60% in Child A patients20–25. Of importance, two cohort studies and one retrospective case–control study comparing surgical resection and PEI failed to identify any difference in survival, despite the fact that the patients in PEI groups had poorer liver function26–28.
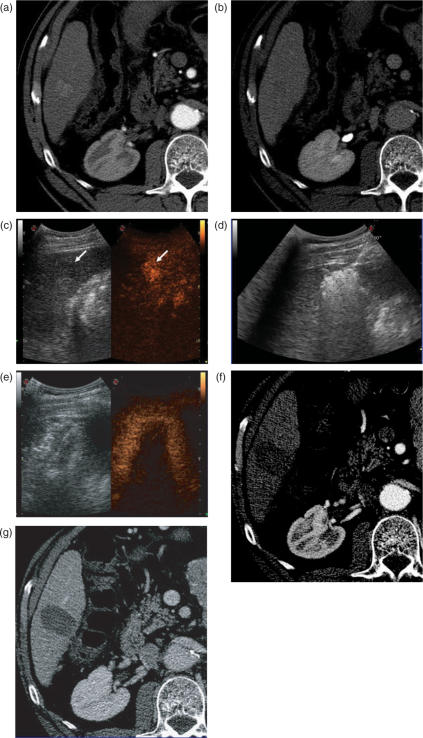
Figure 1.
Complete response of hepatocellular carcinoma treated with radiofrequency ablation. Pre-treatment CT obtained in the arterial (a) and the delayed phase (b) shows the lesion as a small hypervascular nodule (a). Injection of an ultrasound contrast agent confirms arterial-phase enhancing tumour (arrow, c). B-mode low-mechanical index image is shown in the left side of the image; the contrast-specific mode during the arterial phase is shown in the right side. The tumour is treated with RF ablation under US guidance. At the end of the procedure, a large hyperechoic cloud covering the tumour as well as a cuff of surrounding liver parenchyma is seen on US (d). Contrast-enhanced ultrasound study performed at the end of the procedure shows the ablation zone as an unenhanced area completely covering the tumour. Peri-ablation enhancement is also seen, representing reactive hyperaemia (e). On CT images obtained in the arterial (f) and the portal venous phase (g) 1 month after treatment, the tumour is replaced by a non-enhancing ablation zone. The findings are consistent with complete response.
The major limitation of PEI is the high local recurrence rate, which may reach 33% in lesions smaller than 3 cm and 43% in lesions exceeding 3 cm29,30. The injected ethanol does not always accomplish complete tumour necrosis because of its inhomogeneous distribution within the lesion – especially in the presence of intratumoural septa – and the limited effect on extracapsular cancerous spread. Moreover, PEI is unable to create a safety margin of ablation in the liver parenchyma surrounding the nodule, and therefore may not destroy tiny satellite lesions that – even in small tumours – may be located in close proximity to the main nodule.
Thermal ablation
Application of localized heating or freezing enables in situ destruction of malignant liver tumours preserving normal liver parenchyma. The thermal ablative therapies involved in clinical practice can be classified as either hepatic hyperthermic treatments – including radiofrequency (RF) ablation, microwave ablation, and laser ablation – or hepatic cryotherapy. Hepatic hyperthermic treatments are mostly performed via a percutaneous approach; until recently, an open or laparoscopic approach was widely adopted for hepatic cryotherapy.
The thermal damage caused by heating is dependent on both the tissue temperature achieved and the duration of heating. Heating of tissue at 50–55°C for 4–6 min produces irreversible cellular damage. At temperatures between 60°C and 100°C near immediate coagulation of tissue is induced, with irreversible damage to mitochondrial and cytosolic enzymes of the cells. At more than 100–110°C, tissue vaporizes and carbonizes31. For adequate destruction of tumour tissue, the entire target volume must be subjected to cytotoxic temperatures. Different physical mechanisms are involved in the hepatic hyperthermic treatments in order to generate a lethal temperature. A common important factor that affects the success of thermal ablation is the ability to ablate all viable tumour tissue and possibly an adequate tumour-free margin. Ideally, a 360°, 0.5–1-cm-thick ablative margin should be produced around the tumour31. This cuff would ensure that microscopic invasions around the periphery of a tumour have been eradicated. Thus, the target diameter of an ablation, or of overlapping ablations, must be larger than the diameter of the tumour undergoing treatment32.
Thermal ablation is usually performed under intravenous sedation with standard cardiac, pressure, and oxygen monitoring. Targeting of the lesion can be performed with ultrasound, computed tomography (CT), or magnetic resonance (MR) imaging. The guidance system is chosen largely on the basis of operator preference and local availability of dedicated equipment such as CT fluoroscopy or open MR systems. Real-time ultrasound/CT (or ultrasound/MR imaging) fusion imaging substantially improves the ability to guide and monitor liver tumour ablation procedures. Current virtual navigation systems can help to define the extent of the liver tumour burden, plan and simulate the insertion of the needle, and predict the amount of the induced necrosis. During the procedure, important aspects to be monitored include how well the tumour is being covered and whether any adjacent normal structures are being affected at the same time. While the transient hyperechoic zone that is seen at ultrasound within and surrounding a tumour during and immediately after RF ablation can be used only as a rough guide to the extent of tumour destruction, MR is currently the only imaging modality with validated techniques for real-time temperature monitoring. At the end of the procedure, the needle track can be ablated in most systems, aimed at preventing any tumour cell dissemination. Contrast-enhanced ultrasound performed at the end of the procedure may allow an initial evaluation of the effect of the treatment. However, contrast-enhanced CT and MR imaging are recognized as the standard modalities to assess treatment outcome. CT and MR images obtained after treatment show successful ablation as a non-enhancing area with or without a peripheral enhancing rim. The enhancing rim that may be observed along the periphery of the ablation zone appears a relatively concentric, symmetric area, with smooth inner margins. This is a transient finding that represents a benign physiologic response to thermal injury (initially, reactive hyperaemia; subsequently, fibrosis and giant cell reaction). Benign peri-ablational enhancement needs to be differentiated from irregular peripheral enhancement due to residual tumour that occurs at the treatment margin. In contrast to benign peri-ablational enhancement, residual unablated tumour often grows in scattered, nodular, or eccentric patterns33. Later follow-up imaging studies should be aimed at detecting the recurrence of the treated lesion (i.e. local tumour progression), the development of new hepatic lesions, or the emergence of extrahepatic disease.
Radiofrequency ablation
The goal of RF ablation is to induce thermal injury to the tissue through electromagnetic energy deposition. The patient is part of a closed-loop circuit, that includes an RF generator, an electrode needle, and a large dispersive electrode (ground pads). An alternating electric field is created within the tissue of the patient. Because of the relatively high electrical resistance of tissue in comparison with the metal electrodes, there is marked agitation of the ions present in the target tissue that surrounds the electrode, since the tissue ions attempt to follow the changes in direction of alternating electric current. The agitation results in frictional heat around the electrode. The discrepancy between the small surface area of the needle electrode and the large area of the ground pads causes the generated heat to be focused and concentrated around the needle electrode. Several electrode types are available for clinical RF ablation, including internally cooled electrodes and multitined expandable electrodes with or without perfusion33.
RF ablation has been the most widely assessed alternative to PEI for local ablation of HCC34. Histologic data from explanted liver specimens in patients who underwent RF ablation showed that tumour size and the presence of large (3 mm or more) abutting vessels significantly affect the local treatment effect. Complete tumour necrosis was pathologically shown in 83% of tumours less than 3 cm and 88% of tumours in a non-perivascular location35. Three RCTs compared RF ablation versus PEI for the treatment of early-stage HCC36–38. The first trial, performed in European centres, failed to show a statistically significant difference in overall survival between patients who received RF ablation and those treated with PEI36. However, survival advantages were identified in a subgroup analysis of a trial coming from Taiwan37 and in a Japanese study, although in the latter the survival benefit was not confirmed in the subgroup analysis of patients with solitary tumours38. All three investigations showed that RF ablation had a higher local anticancer effect than PEI, leading to better local control of the disease. Therefore, RF ablation appears as the preferred percutaneous treatment for patients with early-stage HCC on the basis of more consistent local tumour control.
Recently, the long-term survival outcomes of RF ablation-treated patients were reported (Table 4). In the first published report, 206 patients with early-stage HCC who were not candidates for resection or transplantation were enrolled in a prospective, intention-to-treat clinical trial39. RF ablation was considered as the first-line non-surgical treatment and was actually performed in 187 (91%) of 206 patients. Nineteen (9%) of 206 patients had to be excluded from RF treatment because of the unfavourable location of the tumour. In patients who underwent RF ablation, survival depended on the severity of the underlying cirrhosis and the tumour multiplicity. Patients in Child class A with solitary HCC had a 5-year survival rate of 61%. Three other studies confirmed that the survival of naive patients (i.e. patients who received radiofrequency ablation as primary treatment) with well-compensated cirrhosis bearing early-stage HCC ranged from 43% to 64%40–42 (Table 4). Of interest, in a randomized trial of RF ablation versus surgical resection in patients with solitary HCC less than 5 cm in diameter, no differences in overall survival rates and cumulative recurrence-free survival rates were observed43.
Table 4.
Studies reporting long-term survival outcomes of patients with early-stage hepatocellular carcinoma who underwent percutaneous radiofrequency ablation
| Author and year | No. patients | Survival rates (%) | |||
|---|---|---|---|---|---|
| 1 year | 3 years | 5 years | |||
| Lencioni et al., 200539 | Child A, 1 HCC <5 cm or 3 <3 cm | 144 | 100 | 76 | 51 |
| 1 HCC <5 cm | 116 | 100 | 89 | 61 | |
| Child B, 1 HCC <5 cm or 3 <3 cm | 43 | 89 | 46 | 31 | |
| Tateishi et al., 200540 | Naive patientsa | 319 | 95 | 78 | 54 |
| Non-naive patientsb | 345 | 92 | 62 | 38 | |
| Cabassa et al., 200641 | 59 | 94 | 65 | 43 | |
| Choi et al., 200742 | Child A, 1 HCC <5 cm or 3 <3 cm | 359 | NA | 78 | 64 |
| Child B, 1 HCC <5 cm or 3 <3 cm | 160 | NA | 49 | 38 | |
HCC, hepatocellular carcinoma.
aPatients who received radiofrequency ablation as primary treatment.
bPatients who received radiofrequency ablation for recurrent tumour after previous treatment including resection, ethanol injection, microwave ablation, and transarterial embolization.
RF ablation of HCC is associated with very low mortality rates and acceptable morbidity. Recently, three separate multicentre surveys have reported mortality rates ranging from 0.1% to 0.5%, major complication rates ranging from 2.2% to 3.1%, and minor complication rates ranging from 5% to 8.9%44. The most common causes of death were sepsis, hepatic failure, colon perforation, and portal vein thrombosis, while the most common complications were intraperitoneal bleeding, hepatic abscess, bile duct injury, hepatic decompensation, and grounding pad burns45–47. Minor complications and side effects were usually transient and self-limiting. An uncommon late complication of RF ablation is tumour seeding along the needle track. In patients with HCC, tumour seeding occurred in 8 (0.5%) of 1610 cases in a multicentre survey45 and in 1 (0.5%) of 187 cases in a single-institution series39. Lesions with subcapsular location and an invasive tumoural pattern, as shown by a poor degree of differentiation, seem to be at higher risk for such a complication48. While these data indicate that RF ablation is a relatively safe procedure, a careful assessment of the risks and benefits associated with the treatment has to be made in each individual patient by a multidisciplinary team.
Despite the many published reports, some questions concerning image-guided RF ablation in HCC treatment are still unanswered. Some authors have reported that RF ablation may be a safe and effective bridge to liver transplantation49–52. However, randomized studies would be needed to determine the advantages and disadvantages of RF ablation with respect to transarterial chemoembolization (TACE) for HCC patients awaiting transplantation. Recent studies have reported encouraging initial results in the treatment of intermediate-size HCC lesions with a combination of RF ablation and balloon catheter occlusion of the tumour arterial supply or prior TACE53–57. However, further clinical trials are warranted to determine the survival benefit associated with this approach.
Microwave ablation
Microwave ablation is the term used for all electromagnetic methods of inducing tumour destruction by using devices with frequencies greater than or equal to 900 kHz. The passage of microwaves into cells or other materials containing water results in the rotation of individual molecules. This rapid molecular rotation generates and uniformly distributes heat, which is instantaneous and continuous until the radiation is stopped. Microwave irradiation creates an ablation area around the needle in a column or round shape, depending on the type of needle used and the generating power58.
The local effect of treatment in HCC was assessed by examining the histological changes of the tumour after microwave ablation59,60. In one study, 89% of 18 small tumours were ablated completely59. Coagulative necrosis with faded nuclei and eosinophilic cytoplasm were the predominant findings in the ablated areas. There were also areas in which the tumours maintained their native morphological features as if the area was fixed, but their cellular activity was destroyed as demonstrated by succinic dehydrogenase stain. One study compared microwave ablation and PEI in a retrospective evaluation of 90 patients with small HCC61. The overall 5-year survival rates for patients with well-differentiated HCC treated with microwave ablation and PEI were not significantly different. However, among the patients with moderately or poorly differentiated HCC, overall survival with microwave ablation was significantly better than with PEI. In a large series including 234 patients, the 3- and 5-year survival rates were 73% and 57%, respectively62. At a multivariate analysis, tumour size, number of nodules, and Child–Pugh classification had a significant effect on survival63. Only one randomized trial compared the effectiveness of microwave ablation with that of RF ablation64. Seventy-two patients with 94 HCC nodules were randomly assigned to RF ablation and microwave ablation groups. Unfortunately, in this study the data were analyzed with respect to lesions and not to patients. Although no statistically significant differences were observed with respect to the efficacy of the two procedures, a tendency favouring RF ablation was recognized with respect to local recurrences and complications rates.
Laser ablation
The term laser ablation should be used for ablation with light energy applied via fibres directly inserted into the tissue. A great variety of laser sources and wavelengths are available. In addition, different types of laser fibres, modified tips, and single or multiple laser applicators can be used. From a single, bare 400-μm laser fibre, a spherical volume of coagulative necrosis up to 2 cm in diameter can be produced. Use of higher power results in charring and vaporization around the fibre tip. Two methods have been developed for producing larger volumes of necrosis. The first consists of firing multiple bare fibres arrayed at 2-cm spacing throughout a target lesion; the second uses cooled-tip diffuser fibres that can deposit up to 30 W over a large surface area, thus diminishing local overheating65.
To date, few data are available concerning the clinical efficacy of laser ablation. No randomized trials to compare laser ablation with any other treatment have been published thus far. In one study including 74 patients with early-stage HCC, overall survival rates were 68% at 3 years and 15% at 5 years, respectively66. Laser ablation appears to be relatively safe, with a major complication rate less than 2%67. The major drawback of current laser technology appears to be the small volume of ablation that can be created with a single-probe insertion. Insertion of multiple fibres is technically cumbersome and may not be feasible in lesions that are not conveniently located. New devices could overcome this limitation.
Conclusions
Several image-guided ablation techniques have been developed to treat non-surgical patients with HCC. These minimally invasive procedures can achieve effective and reproducible tumour destruction with low morbidity. Percutaneous ablation is accepted as the best therapeutic choice for patients with early-stage HCC when resection or transplantation are precluded. On the basis of the current evidence, RF ablation seems to offer higher cumulative survival and recurrence-free survival rates compared with other image-guided treatments and is accepted as the primary ablative modality at most institutions. Further trials are needed to establish the clinical value of image-guided ablation in combination with intra-arterial treatments68.
References
- 1.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–17. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 2.Sherman M. Hepatocellular carcinoma: epidemiology, risk factors, and screening. Semin Liver Dis. 2005;25:143–54. doi: 10.1055/s-2005-871194. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Boix L, Sala M, Llovet JM. Focus on hepatocellular carcinoma. Cancer Cell. 2004;5:15–219. doi: 10.1016/s1535-6108(04)00058-3. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M, Llovet JM, et al. EASL Panel of Experts on HCC. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–30. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 6.Lencioni R, Cioni D, Della Pina C, et al. Imaging diagnosis. Semin Liver Dis. 2005;25:162–70. doi: 10.1055/s-2005-871196. [DOI] [PubMed] [Google Scholar]
- 7.Kojiro M, Roskams T. Early hepatocellular carcinoma and dysplastic nodules. Semin Liver Dis. 2005;25:133–42. doi: 10.1055/s-2005-871193. [DOI] [PubMed] [Google Scholar]
- 8.Levy I, Greig PD, Gallinger S, et al. Resection of hepatocellular carcinoma without preoperative tumor biopsy. Ann Surg. 2001;234:206–9. doi: 10.1097/00000658-200108000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson PJ. Hepatocellular carcinoma: is current therapy really altering outcome? Gut. 2002;51:459–62. doi: 10.1136/gut.51.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–38. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 11.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–9. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 12.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 13.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–40. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 14.Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229:790–800. doi: 10.1097/00000658-199906000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wayne JD, Lauwers GY, Ikai I, et al. Preoperative predictors of survival after resection of small hepatocellular carcinomas. Ann Surg. 2002;235:722–731. doi: 10.1097/00000658-200205000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 17.Jonas S, Beckstein WO, Steinmuller T, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantion for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080–6. doi: 10.1053/jhep.2001.23561. [DOI] [PubMed] [Google Scholar]
- 18.Yao FY, Ferrel L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 19.Yao FY, Bass NM, Nikolai B, et al. Liver transplantation for hepatocellular carcinoma: analysis of survival according to the intention-to-treat principle and dropout from the waiting list. Liver Transpl. 2002;8:873–83. doi: 10.1053/jlts.2002.34923. [DOI] [PubMed] [Google Scholar]
- 20.Lencioni R, Bartolozzi C, Caramella D, et al. Treatment of small hepatocellular carcinoma with percutaneous ethanol injection. Analysis of prognostic factors in 105 Western patients. Cancer. 1995;76:1737–46. doi: 10.1002/1097-0142(19951115)76:10<1737::aid-cncr2820761010>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 21.Livraghi T, Giorgio A, Marin G, et al. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology. 1995;197:101–8. doi: 10.1148/radiology.197.1.7568806. [DOI] [PubMed] [Google Scholar]
- 22.Ryu M, Shimamura Y, Kinoshita T, et al. Therapeutic results of resection, transcatheter arterial embolization and percutaneous transhepatic ethanol injection in 3225 patients with hepatocellular carcinoma: a retrospective multicenter study. Jpn J Clin Oncol. 1997;27:251–7. doi: 10.1093/jjco/27.4.251. [DOI] [PubMed] [Google Scholar]
- 23.Lencioni R, Pinto F, Armillotta N, et al. Long-term results of percutaneous ethanol injection therapy for hepatocellular carcinoma in cirrhosis: a European experience. Eur Radiol. 1997;7:514–19. doi: 10.1007/s003300050194. [DOI] [PubMed] [Google Scholar]
- 24.Teratani T, Ishikawa T, Shiratori Y, et al. Hepatocellular carcinoma in elderly patients: beneficial therapeutic efficacy using percutaneous ethanol injection therapy. Cancer. 2002;95:816–23. doi: 10.1002/cncr.10735. [DOI] [PubMed] [Google Scholar]
- 25.Ebara M, Okabe S, Kita K, et al. Percutaneous ethanol injection for small hepatocellular carcinoma: therapeutic efficacy based on 20-year observation. J Hepatol. 2005;43:458–464. doi: 10.1016/j.jhep.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 26.Castells A, Bruix J, Bru C, et al. Treatment of small hepatocellular carcinoma in cirrhotic patients: a cohort study comparing surgical resection and percutaneous ethanol injection. Hepatology. 1993;18:1121–6. [PubMed] [Google Scholar]
- 27.Yamamoto J, Okada S, Shimada K, et al. Treatment strategy for small hepatocellular carcinoma: comparison of long-term results after percutaneous ethanol injection therapy and surgical resection. Hepatology. 2001;34:707–13. doi: 10.1053/jhep.2001.27950. [DOI] [PubMed] [Google Scholar]
- 28.Daniele B, De Sio I, Izzo F, et al. CLIP Investigators. Hepatic resection and percutaneous ethanol injection as treatments of small hepatocellular carcinoma: a Cancer of the Liver Italian Program (CLIP 08) retrospective case-control study. J Clin Gastroenterol. 2003;36:63–7. doi: 10.1097/00004836-200301000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Khan KN, Yatsuhashi H, Yamasaki K, et al. Prospective analysis of risk factors for early intrahepatic recurrence of hepatocellular carcinoma following ethanol injection. J Hepatol. 2000;32:269–78. doi: 10.1016/s0168-8278(00)80072-0. [DOI] [PubMed] [Google Scholar]
- 30.Koda M, Murawaki Y, Mitsuda A, et al. Predictive factors for intrahepatic recurrence after percutaneous ethanol injection therapy for small hepatocellular carcinoma. Cancer. 2000;88:529–37. [PubMed] [Google Scholar]
- 31.Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancies: a unfied approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol. 2000;174:323–31. doi: 10.2214/ajr.174.2.1740323. [DOI] [PubMed] [Google Scholar]
- 32.Dodd GD, 3rd, , Frank MS, Aribandi M, et al. Radiofrequency thermal ablation: computer analysis of the size of the thermal injury created by overlapping ablations. AJR Am J Roentgenol. 2001;177:777–82. doi: 10.2214/ajr.177.4.1770777. [DOI] [PubMed] [Google Scholar]
- 33.Goldberg SN, Charboneau JW, Dodd GD, 3rd, et al. International Working Group on Image-Guided Tumor Ablation. Image-guided tumor ablation: proposal for standardization of terms and reporting criteria. Radiology. 2003;228:335–45. doi: 10.1148/radiol.2282021787. [DOI] [PubMed] [Google Scholar]
- 34.Lencioni R, Crocetti L. A critical appraisal of the literature on local ablative therapies for hepatocellular carcinoma. Clin Liver Dis. 2005;9:301–14. doi: 10.1016/j.cld.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Lu DS, Yu NC, Raman SS, et al. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005;234:954–60. doi: 10.1148/radiol.2343040153. [DOI] [PubMed] [Google Scholar]
- 36.Lencioni R, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radiofrequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235–40. doi: 10.1148/radiol.2281020718. [DOI] [PubMed] [Google Scholar]
- 37.Lin SM, Lin CJ, Lin CC, et al. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or = 4 cm. Gastroenterology. 2004;127:1714–23. doi: 10.1053/j.gastro.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation versus ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122–30. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Lencioni R, Cioni D, Crocetti L, et al. Early-stage hepatocellular carcinoma in cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234:961–7. doi: 10.1148/radiol.2343040350. [DOI] [PubMed] [Google Scholar]
- 40.Tateishi R, Shiina S, Teratani T, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. Cancer. 2005;103:1201–9. doi: 10.1002/cncr.20892. [DOI] [PubMed] [Google Scholar]
- 41.Cabassa P, Donato F, Simeone F, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term experience with expandable needle electrodes. AJR Am J Roentgenol. 2006;185:S316–21. doi: 10.2214/AJR.05.0243. [DOI] [PubMed] [Google Scholar]
- 42.Choi D, Lim HK, Rhim H, et al. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol. 2007;17:684–92. doi: 10.1007/s00330-006-0461-5. [DOI] [PubMed] [Google Scholar]
- 43.Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–8. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rhim H. Complications of radiofrequency ablation in hepatocellular carcinoma. Abdom Imaging. 2005;30:409–18. doi: 10.1007/s00261-004-0255-7. [DOI] [PubMed] [Google Scholar]
- 45.Livraghi T, Solbiati L, Meloni MF, et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;26:441–51. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 46.De Baere T, Risse O, Kuoch V, et al. Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol. 2003;181:695–700. doi: 10.2214/ajr.181.3.1810695. [DOI] [PubMed] [Google Scholar]
- 47.Bleicher RJ, Allegra DP, Nora DT, et al. Radiofrequency ablation in 447 complex unresectable liver tumors: lessons learned. Ann Surg Oncol. 2003;10:52–58. doi: 10.1245/aso.2003.03.018. [DOI] [PubMed] [Google Scholar]
- 48.Llovet JM, Vilana R, Bru C, et al. Barcelona Clinic Liver Cancer (BCLC) Group. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124–9. doi: 10.1053/jhep.2001.24233. [DOI] [PubMed] [Google Scholar]
- 49.Fontana RJ, Hamidullah H, Nghiem H, et al. Percutaneous radiofrequency thermal ablation of hepatocellular carcinoma: a safe and effective bridge to liver transplantation. Liver Transpl. 2002;8:1165–74. doi: 10.1053/jlts.2002.36394. [DOI] [PubMed] [Google Scholar]
- 50.Wong LL, Tanaka K, Lau L, Komura S. Pre-transplant treatment of hepatocellular carcinoma: assessment of tumor necrosis in explanted livers. Clin Transplant. 2004;18:227–34. doi: 10.1111/j.1399-0012.2004.00164.x. [DOI] [PubMed] [Google Scholar]
- 51.Lencioni R, Cioni D, Crocetti L, Bartolozzi C. Percutaneous ablation of hepatocellular carcinoma: state-of-the-art. Liver Transpl. 2004;10:S91–7. doi: 10.1002/lt.20043. [DOI] [PubMed] [Google Scholar]
- 52.Lu DS, Yu NC, Raman SS, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology. 2005;41:1130–37. doi: 10.1002/hep.20688. [DOI] [PubMed] [Google Scholar]
- 53.Rossi S, Garbagnati F, Lencioni R, et al. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000;217:119–26. doi: 10.1148/radiology.217.1.r00se02119. [DOI] [PubMed] [Google Scholar]
- 54.Yamasaki T, Kurokawa F, Shirahashi H, et al. Percutaneous radiofrequency ablation therapy for patients with hepatocellular carcinoma during occlusion of hepatic blood flow. Comparison with standard percutaneous radiofrequency ablation therapy. Cancer. 2002;95:2353–60. doi: 10.1002/cncr.10966. [DOI] [PubMed] [Google Scholar]
- 55.Kitamoto M, Imagawa M, Yamada H, et al. Radiofrequency ablation in the treatment of small hepatocellular carcinomas: comparison of the radiofrequency effect with and without chemoembolization. AJR Am J Roentgenol. 2003;181:997–1003. doi: 10.2214/ajr.181.4.1810997. [DOI] [PubMed] [Google Scholar]
- 56.Lencioni R, Della Pina C, Bartolozzi C. Percutaneous image-guided radiofrequency ablation in the therapeutic management of hepatocellular carcinoma. Abdom Imaging. 2005;30:401–8. doi: 10.1007/s00261-004-0254-8. [DOI] [PubMed] [Google Scholar]
- 57.Veltri A, Moretto P, Doriguzzi A, et al. Radiofrequency thermal ablation (RFA) after transarterial chemoembolization (TACE) as a combined therapy for unresectable non-early hepatocellular carcinoma (HCC) Eur Radiol. 2006;16:661–9. doi: 10.1007/s00330-005-0029-9. [DOI] [PubMed] [Google Scholar]
- 58.Lu MD, Chen JW, Xie XY, et al. Hepatocellular carcinoma: US-guided percutaneous microwave coagulation therapy. Radiology. 2001;221:167–72. doi: 10.1148/radiol.2211001783. [DOI] [PubMed] [Google Scholar]
- 59.Yamashiki N, Kato T, Bejarano PA, et al. Histopathological changes after microwave coagulation therapy for patients with hepatocellular carcinoma: review of 15 explanted livers. Am J Gastroenterol. 2003;98:2052–9. doi: 10.1111/j.1572-0241.2003.07642.x. [DOI] [PubMed] [Google Scholar]
- 60.Yu NC, Lu DS, Raman SS, et al. Hepatocellular carcinoma: microwave ablation with multiple straight and loop antenna clusters – pilot comparison with pathologic findings. Radiology. 2006;239:269–75. doi: 10.1148/radiol.2383041592. [DOI] [PubMed] [Google Scholar]
- 61.Seki T, Wakabayashi M, Nakagawa T, et al. Percutaneous microwave coagulation therapy for patients with small hepatocellular carcinoma: comparison with percutaneous ethanol injection therapy. Cancer. 1999;85:1694–702. [PubMed] [Google Scholar]
- 62.Dong B, Liang P, Yu X, et al. Percutaneous sonographically guided microwave coagulation therapy for hepatocellular carcinoma: results in 234 patients. AJR Am J Roentgenol. 2003;180:1547–41. doi: 10.2214/ajr.180.6.1801547. [DOI] [PubMed] [Google Scholar]
- 63.Liang P, Dong B, Yu X, et al. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology. 2005;235:299–307. doi: 10.1148/radiol.2351031944. [DOI] [PubMed] [Google Scholar]
- 64.Shibata T, Iimuro Y, Yamamoto Y, et al. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223:331–7. doi: 10.1148/radiol.2232010775. [DOI] [PubMed] [Google Scholar]
- 65.Vogl TJ, Muller PK, Hammerstingl R, et al. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: technique and prospective results. Radiology. 1995;196:257–65. doi: 10.1148/radiology.196.1.7540310. [DOI] [PubMed] [Google Scholar]
- 66.Pacella CM, Bizzarri G, Magnolfi F, et al. Laser thermal ablation in the treatment of small hepatocellular carcinoma: results in 74 patients. Radiology. 2001;221:712–20. doi: 10.1148/radiol.2213001501. [DOI] [PubMed] [Google Scholar]
- 67.Vogl TJ, Straub R, Eichler K, et al. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: experience with complications in 899 patients (2520 lesions) Radiology. 2002;225:367–77. doi: 10.1148/radiol.2252011171. [DOI] [PubMed] [Google Scholar]
- 68.Lencioni R, Della Pina C, Crocetti L, Cioni D. Percutaneous ablation of hepatocellular carcinoma. Recent Results Cancer Res. 2006;167:91–105. doi: 10.1007/3-540-28137-1_7. [DOI] [PubMed] [Google Scholar]