Abstract
L1 is a cell adhesion molecule associated with axonal outgrowth and fasciculation during spinal cord development and may reiterate its developmental role in adults following injury; L1 is upregulated on certain sprouting and regenerating axons in adults, but it is unclear if L1 expression is necessary for, or contributes to, regrowth of axons. This study asks if L1 is required for small-diameter primary afferents to sprout by conducting unilateral dorsal rhizotomies (6 segments; T10-L2) on both wild-type and L1 mutant mice. First we determined that L1 co-localizes substantially with the peptidergic (calcitonin gene-related peptide; CGRP) but minimally with the nonpeptidergic (isolectin B4; IB4) primary afferents in intact wild-type and L1 mutant mice. However, we encountered a complication using IB4 to identify primary afferents post-rhizotomy; we detected extensive abnormal IB4 expression in the dorsal horn and dorsal columns. Much of this aberrant IB4 labeling is associated with fibrous astrocytes and microglia. Five days after dorsal rhizotomy a large decrease in peptidergic and nonpeptidergic afferents is evident on the deafferented side in both wild-type and L1 mutants. Three months after surgery the peptidergic primary afferents sprouted into the center of the denervated dorsal horn in both wild-type and mutant mice, and quantitative analyses confirmed a sprouting density of similar magnitude in both genotypes. In contrast, we did not detect sprouting in the nonpeptidergic primary afferents in either genotype. These results suggest that the absence of L1 neither diminishes nor enhances sprouting of peptidergic small-diameter primary afferent axons following a dorsal rhizotomy.
Keywords: cell adhesion molecule, denervated, IB4, CGRP, nociceptors, rhizotomy
The cell adhesion molecule L1 (L1 CAM) is an axonal glycoprotein and a member of the immunoglobulin superfamily (Kamiguchi et al., 1997). L1 plays a role in axonal outgrowth, fasciculation, and guidance during spinal cord development (Stallcup et al., 1985; Jessell, 1988; Stoeckli and Landmesser, 1995; Orlino et al., 2000; Tran and Phelps, 2000; Akopians et al., 2003) through homophilic and various heterophilic binding partners such as integrins (αVβ3 and α5β1) and the fibroblast growth factor receptor (Kamiguchi and Lemmon, 1997). L1 interacts with TAG-1 and DM-1 GRASP to promote neurite growth, whereas binding with neuropilin-1 and chondroitin sulfate proteoglycans results in inhibition of neurite growth (reviewed in Kamiguchi and Lemmon, 1997; Castellani et al., 2000).
Mutations in X-linked L1 in humans cause major developmental errors and result in a group of symptoms that include corpus callosum hypoplasia, spastic paraplegia and hydrocephalus (Fransen et al., 1995). Two different lines of mice lacking L1 have distinct developmental defects that display a reduced corticospinal tract and corpus callosum, exhibit less sensitivity to pain, enlarged ventricles, and errors in the topographical mapping of retinal axons in the superior colliculus (Cohen et al., 1997; Dahme et al., 1997; Demyanenko et al., 1999; Demyanenko and Maness et al., 2003).
While these mutant mice demonstrate the importance of L1 during development, the function of L1 in adults is less clear. L1 is among several growth-associated genes that are upregulated by neurons after nervous system injury (Daniloff, et al., 1986; Chaisuksunt et al., 2000; Kubasak et al., 2005), however its effects are contradictory. Some studies suggest that L1 CAM reiterates its developmental role following injury, as it is upregulated on sprouting and regenerating axons in many models (Daniloff et al., 1986; Martini and Schachner, 1988; Miragall et al., 1989; Styren et al., 1995; Chalmers et al., 1996; Brook et al., 2000; Kubasak et al., 2005; Chen et al., 2007). However, other studies conclude that L1 is not essential for axonal growth into the injury site (Jakeman et al., 2006) and that nerve growth factor-induced sprouting is even reduced by co-expression of L1 (Chaudhry et al., 2006).
Considering the conflicting reports on the function of L1 in sprouting and regeneration, we sought to better understand its role in a simple injury model of the adult wild-type and L1 mutant spinal cord. L1 expression in the superficial dorsal horn of adult mice co-localizes to different extents with markers of both the peptidergic (calcitonin gene-related peptide; CGRP) and nonpeptidergic (lectin IB4; P2X3) axons, which comprise the two major populations of small-diameter, unmyelinated primary afferents (Snider and McMahon, 1998; Runyan et al., 2005). The unilateral dorsal rhizotomy model, in which multiple dorsal roots are transected, established that intact axons from dorsal roots rostral and caudal to the rhizotomy can sprout into the partially denervated dorsal horn (Liu and Chambers, 1958; Goldberger and Murray, 1974; McNeill and Hulsebosch, 1987). Recently we used a unilateral rhizotomy model to demonstrate that L1 expression in laminae I-II is predominantly derived from the DRG and is expressed on sprouting axons that largely correlate with CGRP-labeled afferents (Runyan et al., 2005). To address the complex and sometimes contradictory role of L1 following injury we use a unilateral dorsal rhizotomy paradigm to determine if L1 is required for DRG axons to sprout by comparing the expression of peptidergic and nonpeptidergic primary afferent markers in both wild-type and L1 mutant mice.
EXPERIMENTAL PROCEDURES
Animals and surgical procedures
Wild-type (L1+/+) and L1 mutant (Y/-; B6;129S7-L1camtm1Sor; Cohen et al., 1997) mice originally obtained from both Dr. Vance Lemmon (University of Miami, Miami, Florida) and Jackson Labs were maintained as a breeding colony at UCLA and genotyped as reported in Demyanenko et al. (1999). Adult mice underwent unilateral, extradural dorsal rhizotomies as previously described in rats, although the deafferentation spanned from T10-L2 instead of the T12-L4 levels used previously (Runyan et al., 2005). Mice were anesthetized deeply with ketamine (133 mg/kg body weight) and xylazine (9 mg/kg body weight) administered intraperitoneally. Wild-type and L1 mutants were maintained for either 5–7 days (acute rhizotomy group; 3 wild-type/mutant pairs) or 3 months after surgery (chronic rhizotomy group; 3 pairs). Mice were perfused and spinal cords processed as described in Runyan et al. (2005). The Chancellor’s Animal Research Committee at UCLA approved all procedures.
Immunocytochemical procedures
Spinal cord sections throughout the six segment deafferentation were processed with L1 polyclonal antisera (1:30,000; rabbit anti-rat L1; Lemmon et al., 1989; gift from Dr. Vance Lemmon, University of Miami, Miami, Florida), CGRP (AB1971; 1:30,000; Chemicon; Temecula, CA), IB4 (1:400; Vector; Burlingame, CA), and an additional marker for a subgroup of nonpeptidergic axons, P2X3 (1:4,000; Chemicon), a receptor subunit in the P2X family of ATP-gated ion channels (Vulchanova et al., 1998). Immunocytochemical protocols for CGRP and IB4 are detailed in Runyan et al. (2005) and L1 labeling was performed according to the CGRP protocol. The P2X3 protocol was similar to that of CGRP with the following modifications. All steps in the P2X3 protocol used a phosphate-buffered saline containing 1% Triton (PBST), 3% normal horse serum for the blocking step, and the primary and secondary antibodies were diluted in PBST with 1% normal goat serum. To determine the source of the abnormal IB4 processes we used polyclonal antibodies to neuron specific class III β-tubulin (1:2500, Covance; Berkeley, CA) and two monoclonal antibodies to neurofilament proteins (RT97 1:1000; Wood and Anderton, 1981; 2H3 1:80; Dodd et al., 1988; Developmental Studies Hybridoma Bank, Department of Biological Sciences, University of Iowa, Iowa City, IA) to identify neuronal processes, anti-mouse F4/80 (1:25, gift from Dr. J. Tidball, UCLA, Los Angeles, CA; HB-198, ATCC; Manassas, VA; Austyn and Gordon, 1981) to label microglia, RC-2 to stain radial glia (1:20; Misson et al., 1988; Developmental Studies Hybridoma Bank), and monoclonal (1:10,000; BD Biosciences; San Jose, CA) and polyclonal (1:10,000, DAKO; Carpinteria, CA) GFAP antibodies to identify astrocytic cells and their processes.
Photography and densitometry
We photographed sections with a Zeiss AxioCam digital camera and Openlab 5.0.1 software. Openlab files were converted to Adobe Photoshop 7.0 files. We compared CGRP expression levels between the intact and deafferented sides by densitometry to quantify the extent of axonal sprouting post-rhizotomy. We analyzed twelve nonadjacent sections at the center of the deafferented region (T12-T13) from three different animals in each of the four groups (wild-type acute and chronic, L1 mutant acute and chronic). Densitometry measurements of the superficial dorsal horn (laminae I-II) were obtained using Openlab 5.0.1 software. We then calculated the mathematical difference between density measurements of the intact and deafferented sides in three different animals from each experimental group and compared the mean differences in optical density between genotypes. Statistical significance (p < 0.05) was determined using a two-sided t-test. To best visualize the co-localization of L1 with CGRP and IB4 expression, we merged single z-plane confocal images captured by a Zeiss LSM510.
RESULTS
Distribution of small-diameter afferents in the dorsal horn of wild-type and L1 mutant mice
We compared L1 expression with markers of both the peptidergic (CGRP) and nonpeptidergic (lectin IB4; P2X3) axons which comprise the two major populations of small-diameter, unmyelinated primary afferents, or C-fibers (Snider and McMahon, 1998) in wild-type and L1 mutant mice. The expression patterns of L1, CGRP, IB4, and P2X3 immunoreactivity in the superficial dorsal horn of adult wild-type mice resemble those reported in adult rats (Fig. 1; Wiesenfeld-Hallin et al., 1984; Silverman and Kruger, 1990; Vulchanova et al., 1998; Runyan et al., 2005; Jakeman et al., 2006). L1 is concentrated in Lissauer’s Tract, laminae I-II, and the dorsolateral funiculus (Figs. 1A and 2A, E). CGRP immunopositive axons are located in Lissauer’s Tract, laminae I, II outer (IIo), and V (Figs. 1C–D and 2B), whereas IB4- and P2X3-positive fibers concentrate in Lissauer’s Tract and lamina II inner (IIi; Figs. 1E–H and 2F). L1 mutant mice exhibit a complete absence of the L1 protein (Fig. 1B), whereas the expression patterns of CGRP, IB4 and P2X3 are identical to wild-type controls (Fig. 1C–H).
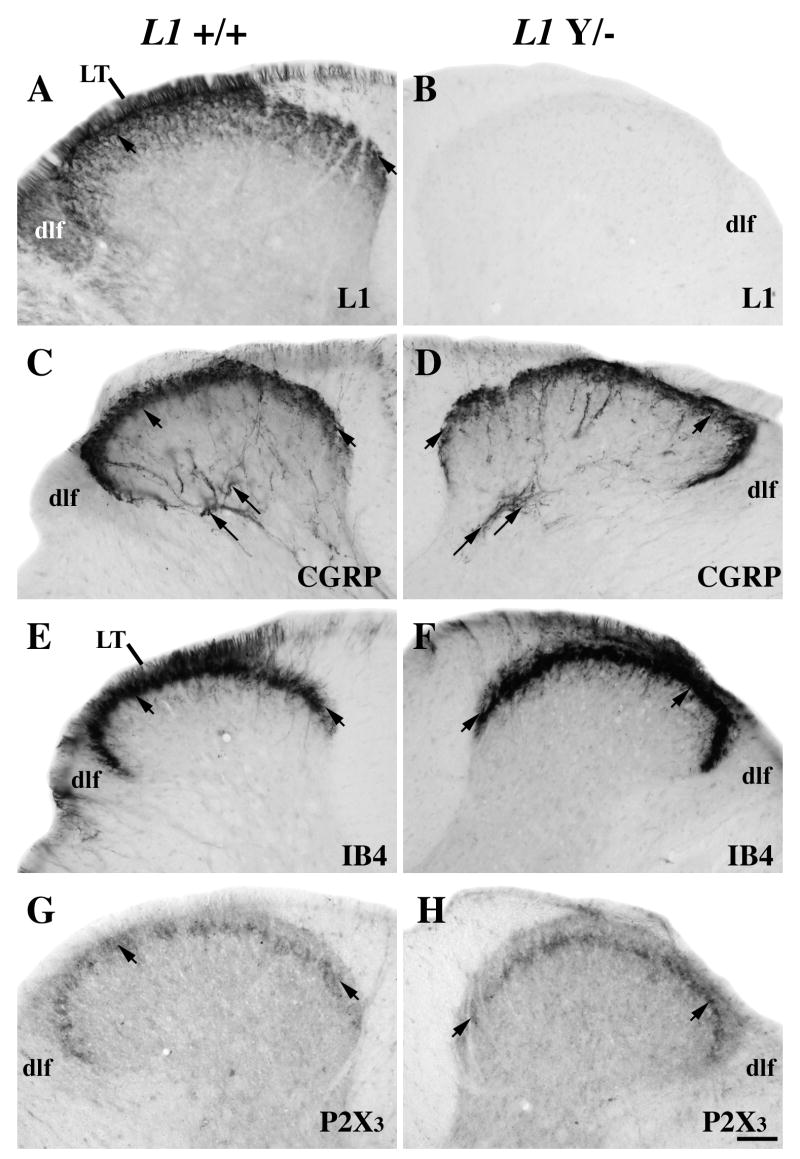
Figure 1.
Adult pattern of L1 (A-B), calcitonin gene-related peptide (CGRP; C-D), IB4 lectin (E-F), and P2X3 (G-H) expression in a mid-thoracic transverse dorsal hemisection from intact wild-type (A, C, E, G) and L1 mutant (B, D, F, H) mice.
A–B: L1-labeled axons are in Lissauer’s Tract (LT) and the dorsolateral funiculus (dlf). L1-positive axons are most concentrated in laminae I-II outer (I-IIo; short arrows), but also extend further ventrally. There are no labeled axons in the L1 mutant dorsal horn.
C–D: In both wild-type and L1 mutant mice CGRP expression is present in laminae I-IIo (short arrows) and in lamina V (long arrows), but is absent in the dlf.
E–F: IB4-positive axons are concentrated in LT and in lamina II (short arrows) in both wild-type and L1 mutant mice.
G–H: P2X3 immunoreactivity is in lamina II inner (short arrows) in both genotypes. Scale bar A-H = 50 μm.
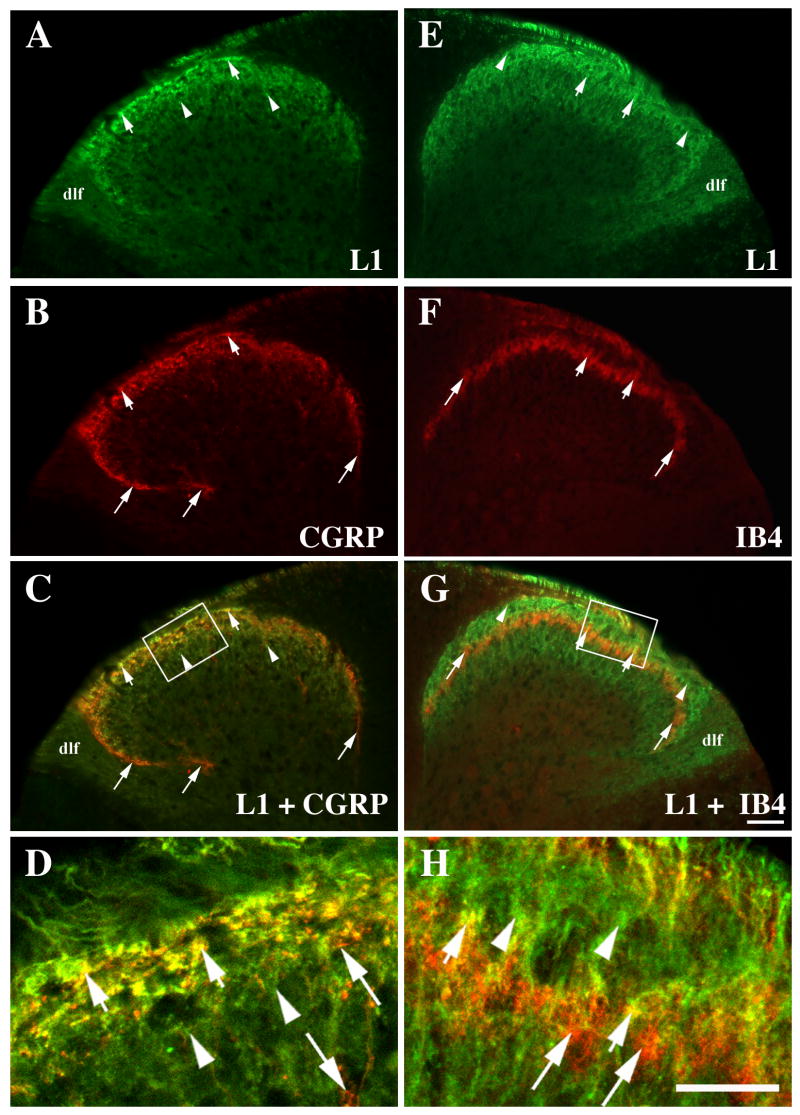
Figure 2.
Double labeling of L1 (green; A, E) and CGRP (red; B) or IB4 (red; F) in the mid-thoracic, wild-type dorsal horn. Co-localization is marked by short arrows (yellow; C, D, G, H), unique L1 expression by arrowheads (A, C, D, E, G, H), and unique CGRP or IB4 labeling by long arrows (B, C, D, F, G, H). High magnification confocal images in D and H represent single merged z-planes.
A–D: Many L1 and CGRP double-labeled axons are seen in laminae I-II outer (short arrows). Only L1 expression is seen in lamina II inner (IIi; arrowheads) and farther ventrally, and in the dorsolateral funiculus (dlf). Only CGRP-positive axons (B, long arrows) course into lamina V.
E–H: IB4-positive only axons terminate in lamina IIi (long arrows) and are interspersed among L1-positive immunoreactivity (arrowheads). Occasional double-labeled yellow axons are designated by short arrows. Scale bar A-C, E-G = 50 μm. Scale bar D, H = 25 μm.
Similar to findings in adult rats (Runyan et al., 2005), L1 expression does not completely co-localize with either the peptidergic (CGRP) or nonpeptidergic (lectin IB4) axons in mice. L1-and CGRP-positive axons are double-labeled in laminae I-IIo (Fig. 2C, D, short arrows) but the CGRP-labeled fibers that course into lamina V are L1-negative (Fig. 2B, C, long arrows). Additionally, L1 expression expands ventrally, beyond the CGRP lamination boundary (Fig. 2C, D, arrowheads). Most IB4 immunoreactive axons that concentrate in lamina IIi appear single-labeled and interspersed within a band of L1 (Fig. 2G, H). However, the more dorsally positioned IB4 axons are co-labeled with L1 (Fig. 2G, H; short arrows) as well as axons within Lissauer’s Tract.
Do peptidergic primary afferents sprout in L1 mutant mice?
Previously we identified a population of L1-positive primary afferents that sprout into the center of the denervated area following a dorsal rhizotomy in rats (Runyan et al., 2005). Here we ask if L1 CAM is required for sprouting of these CGRP/L1-positive primary afferents by examining the effects of unilateral dorsal rhizotomy at the center of the deafferented region (T12-T13) in mice lacking the L1 gene. Five days after unilateral deafferentation, CGRP immunoreactivity in L1 wild-type mice greatly declines in laminae I-IIo in contrast to the robust labeling on the intact side (Figs. 3A, B). These results confirm previous studies in rats in which peptidergic afferents that largely coincide with L1-positive axons in laminae I-IIo originate primarily from the small-diameter DRG nociceptors (Chung et al., 1988; Traub et al., 1989; Runyan et al., 2005). Three months after deafferentation, substantially more CGRP immunoreactive axons are present in the superficial dorsal horn at the center of the denervated region than observed in acutely deafferented wild-type mice (Fig. 3A, E). In our mouse model the CGRP-positive axons that sprout are most dense in lamina I and do not expand beyond their normal lamination boundaries (Fig. 3E), in contrast to findings from similar experiments in rats that show CGRP-labeled axons extending into laminae IIi-III (Runyan et al., 2005).
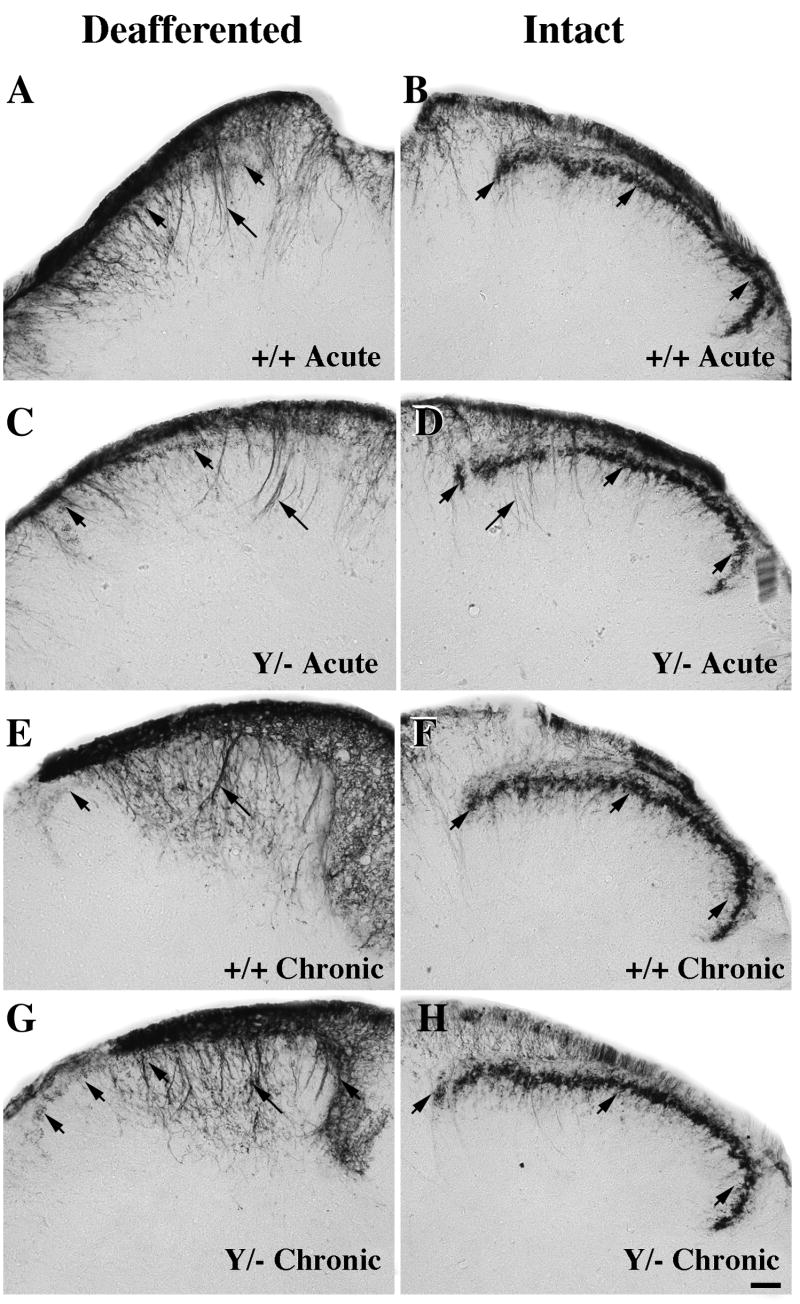
Figure 3.
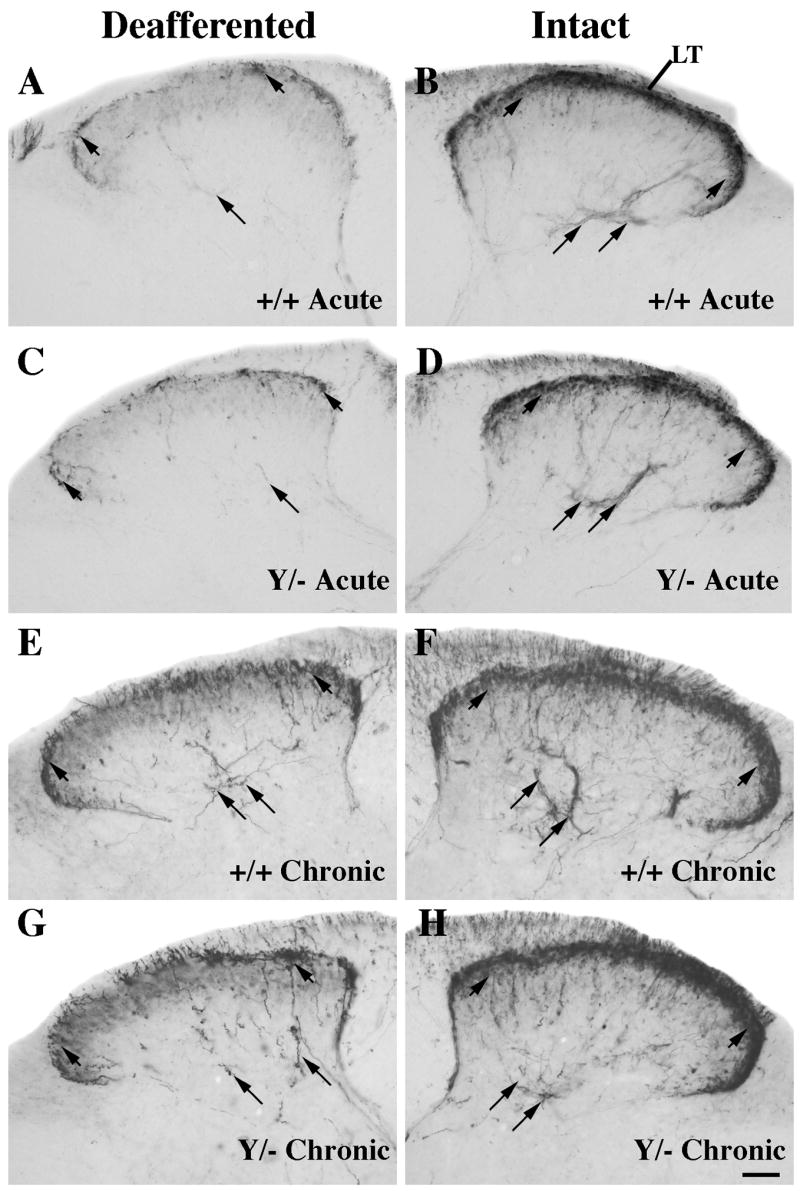
Comparison of CGRP expression at the center of the denervated region (~T12-T13) 5–7 days (acute; A-D) or 3 months (chronic; E-H) post-rhizotomy in wild-type (A-B, E-F) or L1 mutant (C-D, G-H) mice. Intact and denervated dorsal horn images are photographed from the same section.
A–D: On the intact side CGRP-labeled axons are seen in Lissauer’s Tract (LT), laminae I-IIo (short arrows), and on axons coursing into lamina V (long arrows) in both genotypes. After acute rhizotomy, the deafferented side in both genotypes exhibits relatively few CGRP-labeled axons (short arrows) compared to the intact side.
E–H: Wild-type and L1 mutant mice contain more numerous CGRP axons on the denervated side (E, G) in laminae I (short arrows) and V (long arrows) following chronic, compared to acute, rhizotomy (A, C). Scale bar A-H = 50 μm.
Next we examined L1 mutant mice to determine if the L1 protein is required for the peptidergic/CGRP-labeled axon population to sprout after rhizotomy. Five days post-rhizotomy CGRP immunoreactivity greatly declines in the denervated dorsal horn at the center of the deafferented region (T12-T13) in L1 mutant mice (Fig. 3C). Three months later, both wild-type and L1 mutant mice exhibit higher levels of CGRP expression in lamina I of the denervated dorsal horn (compare Fig. 3E, G) than seen five days post deafferentation.
To quantify the changes in CGRP expression over time, we performed densitometric analyses of laminae I-II on sections from the central segments of the deafferented area in mice of both genotypes. The difference in mean density between the intact and deafferented sides indicates loss of CGRP expression; thus higher numbers represent a larger loss in CGRP expression relative to the intact side. Measurements of optical density revealed no significant difference in the loss of CGRP between wild-type and L1 mutants following either acute or chronic deafferentation (Fig. 4). However, the difference in density of CGRP expression was significantly greater between the deafferented and intact sides after acute rhizotomy in wild-type mice compared to both wild-type (p ≤ 0.01) and mutant (p ≤ 0.04) mice at the chronic time point. These results imply that the higher CGRP intensity detected at three months compared to 5–7 days post-rhizotomy in both genotypes represents peptidergic axons from adjacent uninjured segments sprouting into the superficial dorsal horn and that this sprouting does not require L1 expression.
Figure 4.
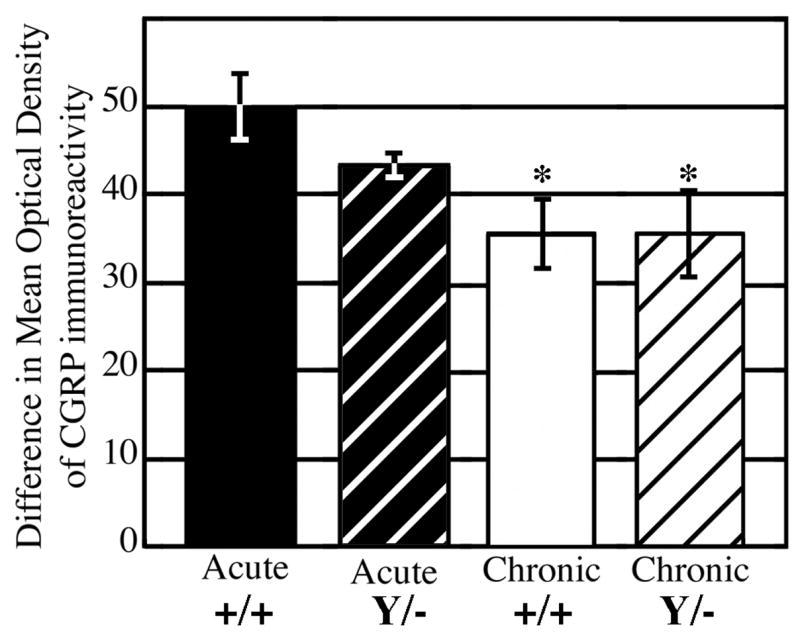
Densitometric analyses of CGRP immunoreactivity in laminae I-II at the center of the unilateral deafferentation (~T12-T13). The mathematical difference between optical density measurements of the intact and denervated dorsal horns was calculated from three animals in each experimental group. The values are mean ± SEM. * significantly different from wild-type mice following acute rhizotomy (p < 0.04).
Absence of nonpeptidergic sprouting post-rhizotomy
To determine if the lack of L1 affects sprouting of the nonpeptidergic population of primary afferents, we compared the distribution of the IB4-positive afferents in wild-type and L1 mutant mice. IB4-positive axons are barely detectable in lamina II (T12-T13) on the deafferented side following acute rhizotomy in wild-type (Fig. 5A, short arrows) and L1 mutant (Fig. 5C, short arrows) mice. Furthermore, lamina II is nearly devoid of IB4-immunoreactivity even three months after deafferentation in both genotypes (Fig. 5E, G; short arrows). While the characteristic pattern of IB4-labeled primary afferents is greatly decreased on the denervated side, abnormal immunoreactive fiber-like processes are present in both dorsal horns after acute and chronic deafferentation (Fig. 5A, C, E, G; long arrows) preventing a clear analysis of the nonpeptidergic population. Due to the extensive IB4 processes that form post-rhizotomy (see following section), we used an alternate marker of nonpeptidergic primary afferents, P2X3 (Vulchanova et al., 1998), to determine if these axons sprout in L1 mutants. The percentage of IB4 neurons that express P2X3 ranges from 67–87% whereas 98% of P2X3-positive fibers express IB4; thus P2X3 labels a subset of the IB4-positive primary afferents (Bradbury et al., 1998; Zwick et al., 2002). P2X3-immunoreactive axons terminate in lamina IIi in both genotypes (Fig. 6B, D, F, H; between short arrows). Following short-term rhizotomy these axons are missing in both wild-type (Fig. 6A) and L1 mutant (Fig. 6C) mice, and remain absent up to three months later (Fig. 6E, G). Thus the P2X3-positive primary afferents do not sprout into the center of the denervated region even after chronic rhizotomy.
Figure 5.
Comparison of IB4 distribution following acute (A-D) and chronic (E-H) unilateral rhizotomy in wild-type (A-B, E-F) or L1 mutant (C-D, G-H) mice. Sections were incubated in the same experiment to allow comparison of staining intensities between groups. Photographs of the denervated and intact dorsal horns are from the same section (~T12-T13).
A–D: IB4-labeled primary afferents on the intact side are concentrated in lamina II inner (IIi; B, D; short arrows). Five days post-rhizotomy, a large decrease is detected on the deafferented side in mice of both genotypes (A, C). Additionally, there is an increase in IB4-positive fiber-like processes coursing into the deep dorsal horn, primarily, but not exclusively, on the deafferented side (A, C, D; long arrows).
E–H: Three months post-rhizotomy, IB4-labeled primary afferents in lamina IIi are barely detectable on the denervated side in both genotypes (E, G; short arrows). There is an extensive infiltration of IB4-immunoreactive processes (E, G; long arrows) into the dorsal horn and intense IB4 reaction product in the dorsal columns in mice 3 months post-rhizotomy. Scale bar A-H = 50 μm.
Figure 6.
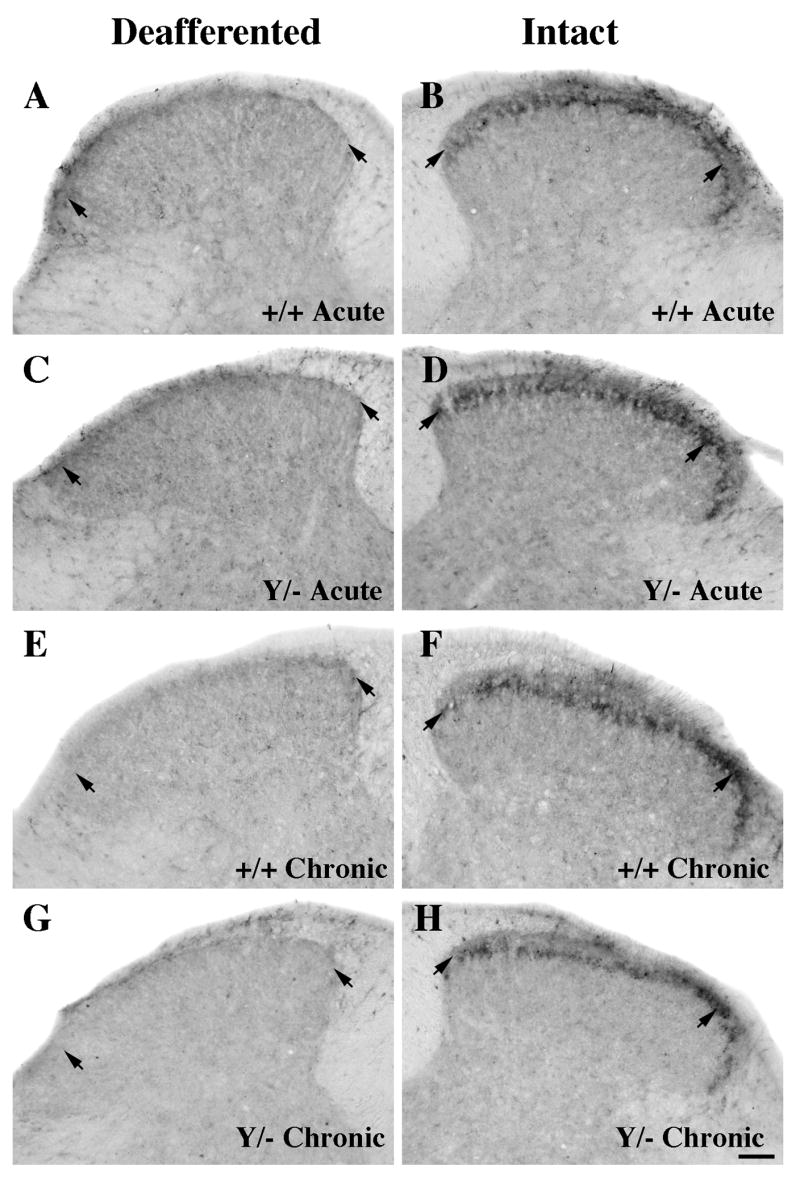
Comparison of P2X3 immunoreactivity at the center of the deafferented region (~T12-T13) following acute (A-D) and chronic (E-H) unilateral rhizotomy in wild-type (A-B, E-F) or L1 mutant (C-D, G-H) mice. The intact and deafferented dorsal horn images are from the same section, and all sections were processed together in the same experiment.
A–D: P2X3 expression on the intact side is concentrated in lamina II inner (short arrows) in both wild-type (B) and L1 mutant (D) mice. Five to seven days after rhizotomy, a large decrease is detected on the deafferented side in mice of both genotypes (A, C).
E–H: Three months post-rhizotomy, primary afferents labeled with P2X3 are still absent in both genotypes (E, G; short arrows). Scale bar A-H = 50 μm.
Identification of the aberrant IB4-labeled processes following rhizotomy
We sought to identify the source of the abnormal IB4-stained processes in the mouse dorsal horn and the dense reaction product in the dorsal columns. We tested the specificity of the IB4 labeling by conducting a serial dilution experiment (Supplemental Fig. 1). The nonpeptidergic primary afferent labeling in lamina IIi disappeared while the fiber-like processes and funicular labeling remained evident following a 10-fold dilution of the IB4 lectin (Supplemental Fig. 1). Therefore, these processes express the galactose moieties recognized by the IB4 lectin (Goldstein and Winter, 1999) but the fiber-like processes clearly are not primary afferents as they persist after acute rhizotomy and, in fact, appear more extensive on the deafferented than on the intact side in both genotypes (compare Fig. 5A, B and 5C, D). Furthermore, following chronic deafferentation the dorsal funiculus contains extensive IB4 labeling and abundant immunoreactive fiber-like processes primarily on the deafferented side (Fig. 5 E, G).
To eliminate axons other than primary afferents as a source of the IB4-labeled fiber-like processes we examined three neuronal markers (neuron-specific β-tubulin, and two neurofilament antigens) and found that none of them recognized the extra IB4-positive fiber-like processes (data not shown). In some sections, IB4-positive fibers penetrated through the lateral funiculus, resembling the orientation of radial glia, but we found no specific staining with an anti-radial glial antibody (RC2; data not shown). Next we localized microglia (F4/80 antibody) and found many microglia in the area of the abnormal IB4 distribution, particularly in the dorsal columns and dorsal horn of the denervated side (Fig. 7J, L). Finally, antibodies to glial fibrillary acidic protein (GFAP, Fig. 7A, C) co-labeled with IB4 (Fig. 7D, F) in most of the fiber-like projections in the dorsal horn and in the dorsal funiculi (Fig. 7G, I). Together these findings suggest that the majority of aberrant IB4-labeled processes originate from astrocytes, but that microglia also contribute to the abnormal pattern of IB4 immunoreactivity in these mice.
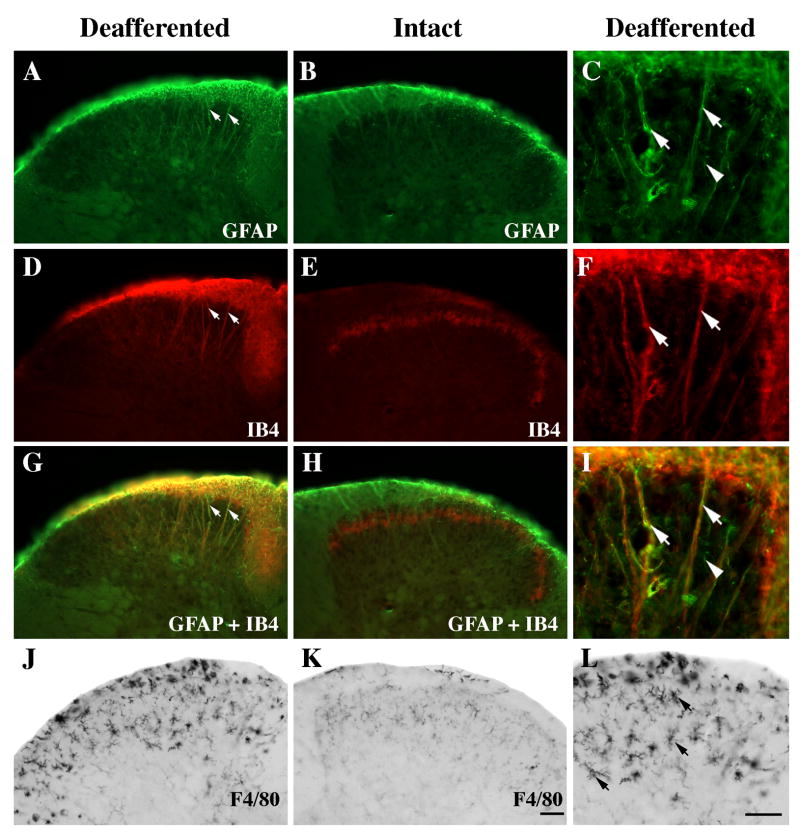
Figure 7.
Identification of abnormal IB4-positive funicular and fibrous labeling using antibodies that identify glial fibrillary acidic protein (GFAP; A-C) and microglia (F4/80; J-L) in a wild-type mouse following chronic unilateral rhizotomy (A, D, G, J; ~T12-T13)).
A–I: The denervated dorsal horn exhibits GFAP- (A) and IB4-positive (D) immunoreactivity that co-localizes substantially in the dorsal funiculus as well as on the long fibrous processes that extend deep into the dorsal horn (A, D, G; arrows). On the intact side, IB4 expression is primarily limited to lamina II inner (E) and a few fiber-like GFAP processes are found along the edge of the dorsal funiculus (B). When high magnification images of GFAP (C) and IB4 (F) are merged many fiber-like processes (I; arrows) and much of the funicular labeling is co-localized; a few thin fibers express GFAP only (C, I; arrowheads).
J–L: The F4/80 antibody identifies many more microglia in the dorsal gray and white matter on the denervated (G) than intact side (H). Microglial cells (L, arrows) have short, branched processes. Scale bar A, B, D, E, G, H, J, K = 50 μm. Scale bar C, F, I, L = 50 μm.
DISCUSSION
We found that both peptidergic and nonpeptidergic populations of small-diameter primary afferents normally innervate the superficial dorsal horn in both wild-type and L1 mutant mice. Three months after unilateral dorsal rhizotomy of six adjacent dorsal roots, wild-type and L1 mutant mice both exhibit sprouting of peptidergic, but not nonpeptidergic, afferents into the center of the denervated dorsal horn. In spite of the temporal expression pattern of L1 that is highly expressed during embryonic axon pathfinding, down-regulated in postnatal development, and upregulated during axonal regeneration, we found that L1 is not required for axonal sprouting into the superficial dorsal horn post-rhizotomy.
What is the source of the aberrant IB4 immunoreactivity?
Following a dorsal rhizotomy in L1 wild-type and mutant mice, many fiber-like processes extend along a pathway similar to that of primary afferents and express IB4. However, such extensive regrowth of IB4-labeled primary afferents into the center of the deafferented region five days post-rhizotomy is unlikely, especially since the other nonpeptidergic marker, P2X3, is absent. Although IB4 is a common marker of nonpeptidergic afferents, the galactose recognized by the IB4 lectin also is expressed on microglia (Streit and Kreutzberg, 1987) and these microglia contribute to the dense IB4 expression we observed after rhizotomy. Additionally, the GFAP- and IB4-positive fiber-like processes we detected in the dorsal horn are most likely the long processes of fibrous astrocytes, i.e., fibers that contribute to gliotic scar formation in injured brain tissue (Hájos and Kálmán, 1989; Hantaz-Ambroise et al., 1994; Latov et al., 1979). Furthermore, the IB4-positive fibrous astrocytes are also present in control mice and other uninjured mouse models such as wild-type and reeler mice (see Fig. 4G, H in Villeda et al., 2006). These additional targets clearly limit the use of IB4 as a marker of the nonpeptidergic afferents in the mouse spinal cord following injury and, to a lesser degree, in the intact mouse spinal cord.
Peptidergic and nonpeptidergic axonal sprouting in wild-type mice
As in previous acute and chronic rhizotomy studies, we found that CGRP labels sprouting primary afferents in adult mice (McNeill and Hulsebosch, 1987; McNeill et al., 1990), but in a somewhat different pattern than detected in rats (Ondarza et al., 2003; Runyan et al., 2005). In the current study, CGRP-positive fibers that sprout into the center of the deafferented region remain within the normal lamination boundary, in contrast to reports of sprouting C-fibers expanding into novel laminae in rats (Ondarza et al., 2003; Runyan et al., 2005). These findings suggest that signals regulating the pattern and extent of primary afferent sprouting may differ between species.
In contrast to the peptidergic sprouting, the P2X3 subpopulation of primary afferents is completely absent at the middle of the denervated area in adult mice. This finding is consistent with the limited or absence of nonpeptidergic sprouting post-rhizotomy in rats (Belyantseva and Lewin, 1999; Runyan et al. 2005). Although Li and Zhou (2001) demonstrate sprouting IB4-positive fibers in the DRG after a peripheral nerve injury, they note that the onset for this sprouting was slow compared to that of sympathetic and peptidergic neurons. Thus, more than three months may be needed for the nonpeptidergic population to sprout into the middle of the deafferented region. Alternatively, the rostrocaudal extent of this subpopulation may be shorter than that of the peptidergic neurons and, therefore, unable to reach the center of the denervated area (Belyantseva and Lewin, 1999).
L1 is not required for CGRP-positive afferent sprouting
L1 is expressed on sprouting primary afferents after dorsal rhizotomy in rats (Runyan et al., 2005) and is implicated as a possible factor for axonal growth following injury to the nervous system (Styren et al., 1995; Aubert et al., 1998; Brook et al., 2000; Roonprapunt et al., 2003; Kubasak et al., 2005; Chen et al., 2007). If L1 is necessary for primary afferent sprouting, we would expect to see little or no sprouting in its absence. However, our data reveal equivalent levels of CGRP-positive axons in the denervated superficial dorsal horn in both wild-type and mutant genotypes. Thus L1 is not required for the CGRP-labeled axons to sprout, a finding similar to that of a recent study reporting that L1 expression is not essential for sprouting of the corticospinal tract following a spinal cord contusion in the same L1 mutant model (Jakeman et al., 2006). If L1 has a role in CGRP-positive afferent sprouting after injury, the present results indicate that alternate factors must compensate for its absence (Adcock et al., 2004; Jakeman et al., 2006).
Although L1 promotes axonal outgrowth in some models (Kobayashi et al., 1995; Webb et al., 2001; Adcock et al., 2004; Zhang et al., 2005; Chen et al., 2007), it also can have inhibitory effects depending on its binding partners (for review see Kamiguchi and Lemmon, 1997; Castellani et al., 2000). For example, Chaudhry et al. (2006) found that co-expression of L1 with nerve growth factor reduced CGRP-positive sprouting within the spinal cord, and that this reduction did not occur with other cell adhesion molecules. Furthermore, this finding (Chaudhry et al., 2006) is supported by the growth-cone collapsing interaction of L1 with neuropilin-1 and sema3A (Castellani et al., 2000) and the neurite growth inhibition due to interactions with the proteoglycans neurocan and phosphocan (Friedlander et al., 1994; Grumet et al., 1996). If L1 is playing a role in growth inhibition after rhizotomy, we would expect to see an increase in the CGRP-positive sprouting into the center of the denervated dorsal horn in the L1 mutant compared to wild-type mice. However, densitometry measurements of CGRP are similar in the two genotypes, both in the absence and presence of L1. In addition, we might expect to see P2X3-positive axons sprouting in the absence of L1, but this did not occur.
Although the addition of L1 by soluble, viral, or genetic means produces conflicting evidence that L1 either can enhance or inhibit axonal outgrowth (Kobayashi et al., 1995; Webb et al., 2001; Adcock et al., 2004; Zhang et al., 2005; Chen et al., 2007), results from the adult L1 mutant model provide clear-cut negative evidence that L1 is not required for C-fiber primary afferents (present study) or corticospinal tract axons (Jakeman et al., 2006) to sprout. Furthermore, we can conclude from the present data that the absence of L1 does not permit enhanced outgrowth of peptidergic sensory neurons after an injury.
Supplementary Material
Serial dilution of the IB4 lectin (routinely used at 1:400) on sections from a wild-type mouse three months after dorsal rhizotomy. These thoracic sections taken from the edge of the deafferented region were incubated together in the same experiment to allow direct comparison of staining intensities. Intact (B, D, F) and denervated (A, C, E) dorsal horn images are photographed from the same section.
A–B: A 1:1000 dilution detects IB4 immunoreactivity on the intact side (B) and high levels of aberrant IB4 signal on the deafferented side (A).
C–D: With a 1:5000 dilution of IB4 the primary afferent labeling in the intact superficial dorsal horn is reduced (D), but not the fiber-like processes and dorsal funicular labeling on the deafferented side (C).
E–F: After reducing the concentration to 1:10,000, the IB4 immunoreactivity on the intact side is nearly abolished (F) yet the aberrant labeling on the deafferented side is still detected (E). Scale bar = 50 μm.
Acknowledgments
The authors are grateful to Dr. Vance Lemmon for generously providing L1 breeding pairs and the L1 polyclonal antisera, and to Tiffany Yap for animal care and genotyping.
Grant sponsors: National Institute of Neurological Disorders and Stroke R21 NS42000 and Christopher Reeves Paralysis Foundation PA 1-0101-2, PAC1-0102-2.
ABBREVIATIONS
- CAM
cell adhesion molecule
- CGRP
calcitonin gene-related peptide
- IB4
Griffonia (bandeiraea) simplicifolia lectin I
- TAG-1
transiently expressed axonal glycoprotein
- DM-1 GRASP
immunoglobulin-like restricted axonal surface protein
- DRG
Dorsal root ganglia
- PBST
phosphate-buffered saline containing 1% Triton
- GFAP
glial fibrillary acidic protein
- Lamina IIo
lamina II outer
- Lamina IIi
lamina II inner
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adcock KH, Brown DJ, Shearer MC, Shewan D, Schachner M, Smith GM, Geller HM, Fawcett JW. Axon behaviour at Schwann cell - astrocyte boundaries: manipulation of axon signalling pathways and the neural adhesion molecule L1 can enable axons to cross. Eur J Neurosci. 2004;20:1425–1435. doi: 10.1111/j.1460-9568.2004.03573.x. [DOI] [PubMed] [Google Scholar]
- Akopians A, Runyan SA, Phelps PE. Expression of L1 decreases during postnatal development of rat spinal cord. J Comp Neurol. 2003;467:375–388. doi: 10.1002/cne.10956. [DOI] [PubMed] [Google Scholar]
- Aubert I, Ridet JL, Schachner M, Rougon G, Gage FH. Expression of L1 and PSA during sprouting and regeneration in the adult hippocampal formation. J Comp Neurol. 1998;399:1–19. [PubMed] [Google Scholar]
- Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981;11:805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Belyantseva IA, Lewin GR. Stability and plasticity of primary afferent projections following nerve regeneration and central degeneration. Eur J Neurosci. 1999;11:457–468. doi: 10.1046/j.1460-9568.1999.00458.x. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci. 1998;12:256–268. doi: 10.1006/mcne.1998.0719. [DOI] [PubMed] [Google Scholar]
- Brook GA, Houweling DA, Gieling RG, Hermanns T, Joosten EAJ, Bär DPR, Gipsen WH, Schmitt AB, Leprince P, Noth J, Nacimiento W. Attempted endogenous tissue repair following experimental spinal cord injury in the rat: Involvement of cell adhesion molecules L1 and NCAM? Eur J Neurosci. 2000;12:3224–3238. doi: 10.1046/j.1460-9568.2000.00228.x. [DOI] [PubMed] [Google Scholar]
- Castellani V, Chédotal A, Schachner M, Faivre-Sarrailh C, Rougon G. Analysis of the L1-deficient mouse phenotype reveals cross-talk between Sema3A and L1 signaling pathways in axonal guidance. Neuron. 2000;27:237–249. doi: 10.1016/s0896-6273(00)00033-7. [DOI] [PubMed] [Google Scholar]
- Chaisuksunt V, Zhang Y, Anderson PN, Campbell G, Vaudano E, Schachner M, Lieberman AR. Axonal regeneration from CNS neurons in the cerebellum and brainstem of adult rats: correlation with the patterns of expression and distribution of messenger RNAs for L1, CHL1, c-jun and growth-associated protein-43. Neurosci. 2000;100:87–108. doi: 10.1016/s0306-4522(00)00254-2. [DOI] [PubMed] [Google Scholar]
- Chalmers GR, Peterson DA, Gage FH. Sprouting adult CNS cholinergic axons express NILE and associate with astrocytic surfaces expressing neural cell adhesion molecule. J Comp Neurol. 1996;371:287–299. doi: 10.1002/(SICI)1096-9861(19960722)371:2<287::AID-CNE8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Chaudhry N, de Silva U, Smith GM. Cell adhesion molecule L1 modulates nerve-growth-factor-induced CGRP-IR fiber sprouting. Exp Neurol. 2006;202:238–249. doi: 10.1016/j.expneurol.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Chen J, Wu J, Apostolova I, Skup M, Irintchev A, Kügler S, Schachner M. Adeno-associated virus-mediated L1 expression promotes functional recovery after spinal cord injury. Brain. 2007;130:954–969. doi: 10.1093/brain/awm049. [DOI] [PubMed] [Google Scholar]
- Chung K, Lee WT, Carlton SM. The effects of dorsal rhizotomy and spinal cord isolation on calcitonin gene-related peptide-labeled terminals in the rat lumbar dorsal horn. Neurosci Letters. 1988;90:27–32. doi: 10.1016/0304-3940(88)90781-1. [DOI] [PubMed] [Google Scholar]
- Cohen NR, Taylor JSH, Scott LB, Guillery RW, Soriano P, Furley AJW. Errors in corticospinal axon guidance in mice lacking the neural cell adhesion molecule L1. Curr Biol. 1997;8:26–33. doi: 10.1016/s0960-9822(98)70017-x. [DOI] [PubMed] [Google Scholar]
- Dahme M, Bartsch U, Martini R, Anliker B, Schachner M, Mantei N. Disruption of the mouse L1 gene leads to malformations of the nervous system. Nature Genet. 1997;17:346–349. doi: 10.1038/ng1197-346. [DOI] [PubMed] [Google Scholar]
- Daniloff JK, Levi G, Grumet M, Rieger F, Edelman GM. Altered expression of neuronal cell adhesion molecules induced by nerve injury and repair. J Cell Biol. 1986;103:929–945. doi: 10.1083/jcb.103.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyanenko GP, Tsai AY, Maness PF. Abnormalities in neuronal process extension, hippocampal development, and the ventricular system of L1 knockout mice. J Neurosci. 1999;19:4907–4920. doi: 10.1523/JNEUROSCI.19-12-04907.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyanenko GP, Maness PF. The L1 cell adhesion molecule is essential for topographic mapping of retinal axons. J Neurosci. 2003;23:530–538. doi: 10.1523/JNEUROSCI.23-02-00530.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J, Morton SB, Karagogeos D, Yamamoto M, Jessell TM. Spatial regulation of axonal glycoprotein expression on subsets of embryonic spinal neurons. Neuron. 1988;1:105–116. doi: 10.1016/0896-6273(88)90194-8. [DOI] [PubMed] [Google Scholar]
- Fransen E, Lemmon V, Van Camp G, Vits L, Coucke P, Willems PJ. CRASH syndrome: clinical spectrum of corpus callosum hypoplasia, retardation, adducted thumbs, spastic paraparesis and hydrocephalus due to mutations in one single gene, L1. Eur J Hum Genet. 1995;3:273–284. doi: 10.1159/000472311. [DOI] [PubMed] [Google Scholar]
- Friedlander DR, Milev P, Karthikeyan L, Margolis RK, Margolis RU, Grumet M. The neuronal chondroitin sulfate proteoglycan neurocan binds to the neural cell adhesion molecules Ng-CAM/L1/NILE and N-CAM, and inhibits neuronal adhesion and neurite outgrowth. J Cell Biol. 1994;125:669–680. doi: 10.1083/jcb.125.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger ME, Murray M. Restitution of function and collateral sprouting in the cat spinal cord: the deafferented animal. J Comp Neurol. 1974;158:37–54. doi: 10.1002/cne.901580104. [DOI] [PubMed] [Google Scholar]
- Goldstein IJ, Winter HG. The Griffonia simplicifolia I-B4 isolectin. A probe for alpha-D-galactosyl end groups. Subcell Biochem. 1999;32:127–141. [PubMed] [Google Scholar]
- Grumet M, Friedlander DR, Sakurai T. Functions of brain chondroitin sulfate proteoglycans during developments: interactions with adhesion molecules. Perspect Dev Neurobiol. 1996;3:319–330. [PubMed] [Google Scholar]
- Hajós F, Kálmán M. Distribution of glial fibrillary acidic protein (GFAP)-immunoreactive astrocytes in the rat brain. Exp Brain Res. 1989;78:164–173. doi: 10.1007/BF00230695. [DOI] [PubMed] [Google Scholar]
- Hantaz-Ambroise D, Blondet B, Murawsky M, Rieger F. Abnormal astrocyte differentiation and defective cellular interactions in wobbler mouse spinal cord. J Neurocytol. 1994;23:179–192. doi: 10.1007/BF01181559. [DOI] [PubMed] [Google Scholar]
- Jakeman LB, Chen Y, Lucin KM, McTigue DM. Mice lacking L1 cell adhesion molecule have deficits in locomotion and exhibit enhanced corticospinal tract sprouting following mild contusion injury to the spinal cord. Eur J Neurosci. 2006;23:1997–2011. doi: 10.1111/j.1460-9568.2006.04721.x. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Adhesion molecules and the hierarchy of neural development. Neuron. 1988;1:3–13. doi: 10.1016/0896-6273(88)90204-8. [DOI] [PubMed] [Google Scholar]
- Kamiguchi H, Lemmon V. Neural cell adhesion molecule L1: signaling pathways and growth cone motility. J Neurosci Res. 1997;49:1–8. doi: 10.1002/(sici)1097-4547(19970701)49:1<1::aid-jnr1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Miura M, Asou H, Inoue HK, Ohye C, Uyemura K. Grafts of genetically modified fibroblasts expressing neural cell adhesion molecule L1 into transected spinal cord of adult rats. Neurosci Lett. 1995;188:191–194. doi: 10.1016/0304-3940(95)11429-z. [DOI] [PubMed] [Google Scholar]
- Kubasak MD, Hedlund E, Roy RR, Carpenter EM, Edgerton VR, Phelps PE. L1 CAM expression is increased surrounding the lesion site in rats with complete spinal cord transection as neonates. Exp Neurol. 2005;194:363–375. doi: 10.1016/j.expneurol.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Latov N, Nilaver G, Zimmerman EA, Johnson WG, Siverman AJ, Defendini R, Cote L. Fibrillary astrocytes proliferate in response to brain injury. Dev Biol. 1979;72:381–384. doi: 10.1016/0012-1606(79)90127-1. [DOI] [PubMed] [Google Scholar]
- Lemmon V, Farr KL, Lagenaur C. L1-mediated axon outgrowth occurs via a homophilic binding mechanism. Neuron. 1989;2:1597–1603. doi: 10.1016/0896-6273(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Li L, Zhou XF. Pericellular Griffonia simplicifolia I isolectin B4-binding ring structures in the dorsal root ganglia following peripheral nerve injury in rats. J Comp Neurol. 2001;439:259–274. doi: 10.1002/cne.1349. [DOI] [PubMed] [Google Scholar]
- Liu CN, Chambers WW. Intraspinal sprouting of dorsal root axons: development of new collaterals and preterminals following partial denervation of the spinal cord in the cat. Arch Neurol Psychiat. 1958;79:46–61. [PubMed] [Google Scholar]
- Martini R, Schachner M. Immunoelectron microscopic localization of neural cell adhesion molecules (L1, N-CAM, and myelin-associated glycoprotein) in regenerating adult mouse sciatic nerve. J Cell Biol. 1988;106:1735–1746. doi: 10.1083/jcb.106.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill DL, Hulsebosch CE. Intraspinal sprouting of rat primary afferents after deafferentation. Neurosci Lett. 1987;81:57–62. doi: 10.1016/0304-3940(87)90340-5. [DOI] [PubMed] [Google Scholar]
- McNeill DL, Carlton SM, Coggeshall RE, Hulsebosch CE. Denervation-induced intraspinal synaptogenesis of calcitonin gene-related peptide containing primary afferent terminals. J Comp Neurol. 1990;296:263–268. doi: 10.1002/cne.902960206. [DOI] [PubMed] [Google Scholar]
- Miragall F, Kadmon G, Schachner M. Expression of L1 and N-CAM cell adhesion molecules during development of the mouse olfactory system. Dev Biol. 1989;135:272–286. doi: 10.1016/0012-1606(89)90179-6. [DOI] [PubMed] [Google Scholar]
- Misson JP, Edwards MA, Yamamoto M, Caviness VS., Jr Identification of radial glial cells within the developing murine central nervous system: studies based upon a new immunohistochemical marker. Dev Brain Res. 1988;44:95–108. doi: 10.1016/0165-3806(88)90121-6. [DOI] [PubMed] [Google Scholar]
- Ondarza AB, Ye Z, Hulsebosch CE. Direct evidence of primary afferent sprouting in distant segments following spinal cord injury in the rat: colocalization of GAP-43 and CGRP. Exp Neurol. 2003;184:373–380. doi: 10.1016/j.expneurol.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Orlino EN, Wong CM, Phelps PE. L1 and GAD65 are expressed on dorsal commissural axons in embryonic rat spinal cord. Dev Brain Res. 2000;125:117–130. doi: 10.1016/s0165-3806(00)00087-0. [DOI] [PubMed] [Google Scholar]
- Roonprapunt C, Huang W, Grill R, Friedlander D, Grumet M, Chen S, Schachner M, Young W. Soluble cell adhesion molecule L1-Fc promotes locomotor recovery in rats after spinal cord injury. J Neurotrauma. 2003;20:871–82. doi: 10.1089/089771503322385809. [DOI] [PubMed] [Google Scholar]
- Runyan SA, Roy R, Zhong H, Phelps PE. L1 CAM expression in the superficial dorsal horn is derived from the dorsal root ganglion. J Comp Neurol. 2005;485:267–279. doi: 10.1002/cne.20479. [DOI] [PubMed] [Google Scholar]
- Silverman JD, Kruger L. Selective neuronal glycoconjugate expression in sensory and autonomic ganglia: Relation of lectin reactivity to peptide and enzyme markers. J Neurocytol. 1990;19:789–801. doi: 10.1007/BF01188046. [DOI] [PubMed] [Google Scholar]
- Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20:629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- Stallcup WB, Beasley LL, Levine JM. Antibody against nerve growth factor-inducible large external (NILE) glycoprotein labels nerve fiber tracts in the developing rat nervous system. J Neurosci. 1985;5:1090–1101. doi: 10.1523/JNEUROSCI.05-04-01090.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckli ET, Landmesser LT. Axonin-1, Nr-CAM, and Ng-CAM play different roles in the in vivo guidance of chick commissural neurons. Neuron. 1995;14:1165–1179. doi: 10.1016/0896-6273(95)90264-3. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Kreutzberg GW. Lectin binding by resting and reactive microglia. J Neurocytol. 1987;16:249–260. doi: 10.1007/BF01795308. [DOI] [PubMed] [Google Scholar]
- Styren SD, Miller PD, Lagenaur CF, DeKosky ST. Alternate strategies in lesion-induced reactive synaptogenesis: differential expression of L1 in two populations of sprouting axons. Exp Neurol. 1995;131:165–173. doi: 10.1016/0014-4886(95)90038-1. [DOI] [PubMed] [Google Scholar]
- Tran TS, Phelps PE. Axons crossing in the ventral commissure express L1 and GAD65 in the developing rat spinal cord. Dev Neurosci. 2000;22:228–236. doi: 10.1159/000017445. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Solodkin A, Ruda MA. Calcitonin gene-related peptide immunoreactivity in the cat lumbosacral spinal cord and the effects of multiple dorsal rhizotomies. J Comp Neurol. 1989;287:225–237. doi: 10.1002/cne.902870206. [DOI] [PubMed] [Google Scholar]
- Villeda SA, Akopians AL, Babayan AH, Basbaum AI, Phelps PE. Absence of Reelin results in altered nociception and aberrant neuronal positioning in the dorsal spinal cord. Neuroscience. 2006;139:1385–1396. doi: 10.1016/j.neuroscience.2006.01.042. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Riedl MS, Shuster SJ, Stone LS, Hargreaves KM, Buell G, Surprenant A, North RA, Elde R. P2X3 is expressed by DRG neurons that terminate in inner lamina II. Eur J Neurosci. 1998;10:3470–3478. doi: 10.1046/j.1460-9568.1998.00355.x. [DOI] [PubMed] [Google Scholar]
- Webb K, Budko E, Neuberger TJ, Chen S, Schachner M, Tresco PA. Substrate-bound human recombinant L1 selectively promotes neuronal attachment and outgrowth in the presence of astrocytes and fibroblasts. Biomaterials. 2001;22:1017–1028. doi: 10.1016/s0142-9612(00)00353-7. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z, Hokfelt T, Lundberg JM, Forssmann WG, Reinecke M, Tschopp FA, Fischer JA. Immunoreactive calcitonin gene-related peptide and substance P coexist in sensory neurons to the spinal cord and interact in spinal behavioral responses of the rat. Neurosci Lett. 1984;52:199–204. doi: 10.1016/0304-3940(84)90374-4. [DOI] [PubMed] [Google Scholar]
- Wood JN, Anderton BH. Monoclonal antibodies to mammalian neurofilaments. Biosci Rep. 1981;1:263–268. doi: 10.1007/BF01114913. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bo X, Schoepfer R, Holtmaat AJ, Verhaagen J, Emson PC, Lieberman AR, Anderson PN. Growth-associated protein GAP-43 and L1 act synergistically to promote regenerative growth of Purkinje cell axons in vivo. Proc Natl Acad Sci. 2005;102:14883–14888. doi: 10.1073/pnas.0505164102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick M, Davis BM, Woodbury CJ, Burkett JN, Koerber HR, Simpson JF, Albers KM. Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J Neurosci. 2002;22:4057–4065. doi: 10.1523/JNEUROSCI.22-10-04057.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Serial dilution of the IB4 lectin (routinely used at 1:400) on sections from a wild-type mouse three months after dorsal rhizotomy. These thoracic sections taken from the edge of the deafferented region were incubated together in the same experiment to allow direct comparison of staining intensities. Intact (B, D, F) and denervated (A, C, E) dorsal horn images are photographed from the same section.
A–B: A 1:1000 dilution detects IB4 immunoreactivity on the intact side (B) and high levels of aberrant IB4 signal on the deafferented side (A).
C–D: With a 1:5000 dilution of IB4 the primary afferent labeling in the intact superficial dorsal horn is reduced (D), but not the fiber-like processes and dorsal funicular labeling on the deafferented side (C).
E–F: After reducing the concentration to 1:10,000, the IB4 immunoreactivity on the intact side is nearly abolished (F) yet the aberrant labeling on the deafferented side is still detected (E). Scale bar = 50 μm.