Abstract
ABT-510 is a potent mimetic of an anti-angiogenic sequence from the second type 1 repeat of thrombospondin-1. ABT-510 and the original d-Ile mimetic from which it was derived, GDGV(dI)TRIR, are similarly active for inhibiting vascular outgrowth in a B16 melanoma explant assay. Because GDGV(dI)TRIR and thrombospondin-1 modulate nitric oxide signaling by inhibiting the fatty translocase activity of CD36, we examined the ability ABT-510 to modulate fatty acid uptake into vascular cells and downstream nitric oxide/cGMP signaling. Remarkably, ABT-510 is less active than GDGV(dI)TRIR for inhibiting myristic acid uptake into both endothelial and vascular smooth muscle cells. Correspondingly, ABT-510 is less potent than GDGV(dI)TRIR for blocking a myristate-stimulated increase in cell adhesion to collagen and nitric oxide-driven accumulation of cGMP. ABT-510 at concentrations sufficient to inhibit CD36 fatty acid translocase activity synergizes with thrombin in aggregating platelets and blunts the activity of NO to delay aggregation, but again less than GDGV(dI)TRIR. In contrast, ABT-510 is more potent than GDGV(dI)TRIR for inducing caspase activation in vascular cells. Thus, we propose that ABT-510 is a drug with at least two mechanisms of action, and its potent anti-tumor activity may be in part independent of CD36 fatty acid translocase inhibition.
1. Introduction
Tumor growth requires neo-vascularization that involves recruitment of surrounding vessels (angiogenesis) and endothelial precursors from bone marrow (reviewed in [1, 2]. This process is driven by regional loss of the balance between stimulating and inhibitory signals. To control cancer growth, efforts have been made to restore this balance in the tumor micro-environment [3]. Thrombospondin-1 (TSP1) is a major endogenous angiogenesis inhibitor. TSP1 expression is frequently suppressed during tumor progression and metastasis [4, 5]. The type 1 repeats of TSP1 block vessel formation in the presence of FGF2 by engaging the receptor CD36 [6]. Small molecules have been developed that mimic an active peptide sequence in the second type 1 repeat [7-9]. Of several derivatives tested, ABT-510 demonstrated the best pharmacological properties and efficacy [9, 10]. Growth of syngeneic and xenograft tumors in mice were delayed by continuous dosing with ABT-510 [8, 9, 11]. Some spontaneous tumors in dogs also responsed to ABT-510 [10]. In human cancer patients, ABT-510 has progressed to Phase I and II clinical trials as a single agent or in combination with chemotherapy [12-14]
ABT-510 is proposed to prevent tumor growth through binding to CD36 on tumor endothelial cells. Peptides related to ABT-510 inhibit TSP1 binding to melanoma cells over-expressing CD36 and to a CD36(93-155)-GST fusion protein [7]. Binding of ABT-510 to CD36 is inferred based on the activity of related TSP1-derived peptides to induce CD36-dependent activation of JNK, leading to endothelial cell apoptosis [15]. ABT-510, or a closely related peptide [16], induces both Fas and Fas-L expression in microvascular endothelial cells [11]. ABT-510 and related peptides also inhibit endothelial cell motility stimulated by several growth factors, which in the case of FGF2 was reversed in the presence of a CD36 antibody (FA6-152) that reverses the same inhibitory activity of TSP1 [7, 17]. Similarly, the pro-apoptotic activity of these peptides is reversed in the presence of FA6-152 [8]. ABT-510 also inhibits endothelial tube formation in a caspase-dependent manner, and this response was also reversed by a CD36 blocking antibody [18].
CD36 is considered necessary for the anti-angiogenic activities of TSP1 (and by inference ABT-510) because corneal neo-vascularization driven by FGF2 was not blocked by TSP1 in CD36 null mice [19]. However, we found that anti-angiogenic activity of TSP1 in the context of physiological nitric oxide (NO) is retained in vascular cells and tissue explants from CD36 null mice but not those from CD47 null mice [20]. Although picomolar concentrations of TSP1 signal exclusively via CD47, we also found a CD36-dependent response at low nanomolar concentrations [20]. The latter activity is associated with inhibition of the fatty acid translocase activity of CD36 [21]. TSP1 inhibits myristate uptake and inhibits downstream membrane translocation and activation of the Src kinase Fyn [21]. TSP1 also inhibits eNOS activation via CD36, identifying a second mechanism by which TSP1 can inhibit NO signaling.
A peptide derived from the second type 1 repeat of TSP1 that contains the same α-carbon inversion of Ile438 as ABT-510 potently inhibited fatty acid uptake through CD36 [21], suggesting that ABT-510 might also be a CD36 fatty acid translocase inhibitor. However, ABT-510 differs from other mimetics of this sequence in that it contains d-allo-Ile, which decreased its activity for inhibiting endothelial cell migration 30-fold but increased its activity for inhibiting tube formation 20-fold [9], which others have concluded to be an apoptotic response [18]. The divergent activities of these two derivatives suggested that they may inhibit angiogenesis through more than one receptor or signaling mechanism.
To determine whether inhibition of CD36 fatty acid translocase activity contributes to the anti-angiogenic activity of ABT-510, we examined its ability to modulate fatty acid uptake into vascular cells. We show that ABT-510 is less active than the original D-Ile mimetic from which it was derived for inhibiting myristic acid uptake and downstream NO/cGMP signaling. In contrast, ABT-510 more potently induces caspase activation, suggesting that its potent anti-tumor activity is independent of inhibiting CD36 translocase activity or NO/cGMP signaling.
2. Methods
2.1. Animals
C57BL/6 wild type and CD47 null mice [22] were housed in a pathogen free environment and allowed ad libitum access to food and water. Handling and care of animals was in compliance with the guidelines established by the Animal Care and Use Committees of the National Cancer Institute and the National Institutes of Health.
2.2. Cells and Reagents
Human umbilical vein endothelial cells (HUVEC) and human aortic vascular smooth muscle cells (HAVSMC) (Cambrex, Walkersville, MD) were maintained in endothelial growth medium (EGM, Cambrex) or vascular smooth muscle growth medium (SMGM, Cambrex) respectively with 2% FCS in 5% CO2 at 37° C. Cells were utilized at passages 4-8. CD47-null VSMC where obtained from aortas harvested from 8 week old animals as described [23]. The nitric oxide donor diethylamine NONOate (DEA/NO) was kindly provided by Dr. Larry Keefer (NCI, Frederick, Maryland). TSP1 was purified from fresh human platelets as described [24]. Platelet rich plasma was provided by the blood bank of the Clinical Center of the National Institutes of Health. Type I collagen was purchased by Inamed (Fremont, CA). cGMP measurement was performed utilizing an immunoassay kit obtained from Amersham Bioscience (Piscataway, New Jersey). Fatty acid free (FAF) bovine serum albumin, myristic acid and [3H]-myristic acid were obtained from Sigma-Aldrich (St. Louis, MO). B16F10 melanoma cells were generously provided by Dr. Lubya Varticovski (NCI). GDGV(dI)TRIR (Gly-Asp-Gly-Val-(d-Ile)-Thr-Arg-Ile-Arg) was synthesized by Peptides International (Louisville, KY), and ABT-510 (NAc-Sar-Gly-Val-(d-allo-Ile)-Thr-Nva-Ile-Arg-ProNEt) was provided by Abbott Laboratories [9].
2.3. Explant Invasion Assay
C57BL/6 mice were injected subcutaneously with 106 B16F10 tumor cells to the lateral thigh. Animals were euthanized when tumors reached 1 cm. One mm3 tumor biopsies were harvested immediately following euthanasia and explanted into type I collagen matrix as described previously [25] and incubated in growth medium (EGM) with 1% FCS and 10 μM DETA/NO. Following seven days of incubation in the presence of treatment agents, maximum vascular cell outgrowth was measured and quantified as the maximum distance of vascular cell migration into the matrix in 4 quadrants. Results represent the mean of each treatment condition performed in triplicate and each experiment repeated three times.
2.4. Preparation of Human Platelets
Platelets were pelleted from platelet-rich plasma by centrifugation for 10 minutes at 200 g. They were then washed with acid citrate dextrose (85 mM citric acid, 65 mM sodium citrate, 100 mM glucose, pH 5.1) at a ratio of 1:7 at room temperature. After pelleting the platelets again and removing the supernatant, the platelets were resuspended in 5 mL of Tyrode’s buffer (137 mM NaCl, 3 mM KCl, 12 mM NaHCO3, 0.3 mM NaHPO4, 2 mM CaCl2, 1 mM MgCl2, 5.5 mM glucose, 5 mM HEPES, 3.5 mg/mL BSA, pH 7.4). The final platelet number was adjusted to 200 cells/μl in 500 μl of Tyrode’s buffer per cuvette.
2.5. Platelet Aggregation Assay
Aggregation of platelets under high shear conditions was assessed using standard optical aggregometer at 37 °C and 1200 rpm in a volume of 500 μl buffer with a final platelet concentration of 200 cells/μl over a 5 minute interval. Cells were pre-incubated for 15 minutes with the indicated doses of ABT-510. DEA/NO (10 μM) was added 30 seconds prior to activation with 0.2 U/ml human thrombin (Sigma/Aldrich, St Louis). Platelet aggregation under low shear conditions was assessed using a spectrophotometer (Beckman DU 640, Beckman Coulter, Fullerton, CA) and determined as a change in absorbance at 400 nm with continuous observation over a five minute interval. The cuvette was inverted once every 60 seconds. Pilot experiments had determined that 5 minutes was an optimum time interval for observation under low shear stress conditions. Pre-incubation with TSP1 and TSP1-based agents was for 15 minutes prior to addition of thrombin and/or the rapidly releasing nitric oxide donor DEA/NO to minimize the formation of thrombin-serpin-thrombospondin complexes [26], which interfere with thrombin-platelet interactions.
2.6. [3H]-Myristic Acid Uptake
The [3H]-myristic acid uptake assays were performed using sub-confluent HUVEC or HAVSMC monolayers (5 × 105 cells/well) in 24-well culture plates (Nunc, Denmark). Trace amounts of [3H]-myristic acid mixed (5 μCi/ml, 0.9 μM) with 9.1 μM non-radioactive myristic acid were dissolved in a FAF BSA solution at a ratio of 1:2 of the myristic acid/BSA solution. Cell monolayers were incubated in basal serum-free media with treatment agents for the indicated time interval at 37°C. The uptake was stopped by removal of the solution followed by the addition of chilled 0.9% NaCl with 0.5% BSA. The stop solution was discharged and cells washed again with stop solution. Cell lysis was achieved with 0.2 M NaOH (200 μl/well) for 2 h at 37° C. On completion of solubilization 0.2 M HCl in 1.5 M Tris-HCl 200 μl/well was added to each well. Radioactivity was determined after the addition of 10 ml of EcoscintA (National Diagnostics, Atlanta, GA) in a 1900CA liquid scintillation counter (Packard).
2.7. Intracellular cGMP Assay
Fresh human platelets at 200 cells/μl in Tyrode’s buffer were pre-incubated with the indicated agents for 15 minutes and then challenged with an NO-donor for 1 minute at room temperature and total cGMP determined via immunoassay (Amersham BioScience, New Brunswick, NJ).
2.8. Cell Adhesion
Cell adhesion was carried out in 96-well plates (Nunc, Denmark). After pre-coating wells with type I collagen (3 μg/ml) HAVSMC were plated at a density of 1 × 104 cells/well in SM-BM containing 0.1% FAF-BSA and treatment agents and incubated in 5% CO2 for 1 h. Wells were washed with PBS, and the cells were fixed with 1% glutaraldehyde for 10 min., washed and stained with 1% crystal violet for 20 min. Excess stain was rinsed away, the cells were extracted with 10% acetic acid, and the plates read at 570 nm.
2.9. Caspase Activation
HUVEC, HAVSMC and CD47 -/- VSMC were plated at a density of 1 × 104 cells/well in growth medium for 24 h, then treated in SM-BM + 0.5% FCS for 24 h. Activity of caspases 3 and 7 were measured as per the manufactures instructions with a fluorescent assay (Apo-One®, Promega Corp, Madison, WI) 18 h after substrate addition.
2.10. Statistics
All assays were repeated at least in triplicate and are presented as the mean ± SD with significance being determined by the Students t test for a p > 0.05.
3. Results
3.1. ABT-510 inhibits vascular outgrowth from B16 melanoma explants
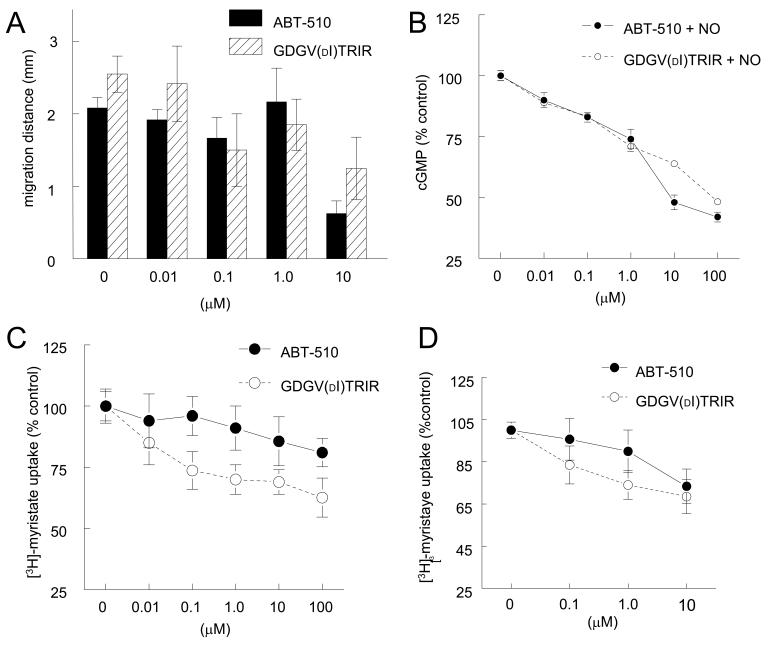
ABT-510 has shown inhibitory activities in murine and dog models of tumor growth and Matrigel plug assays of growth factor driven angiogenesis [10, 17, 18, 27-29]. We used our recently developed ex vivo assay of tumor driven angiogenesis [25] to compare the activities of ABT-510 and GDGV(dI)TRIR. Using tissue biopsies of B16F10 melanoma tumors grown in C57BL/6 mice and explanted into three-dimensional type I collagen gels, we found that ABT-510 and GDGV(dI)TRIR similarly inhibited NO-stimulated vascular cell outgrowth into and invasion through extracellular matrix (Fig. 1A). Thus, the reported enhanced potency of ABT-510 relative to GDGV(dI)TRIR in vitro [9] is not evident when an angiogenic response is driven by NO.
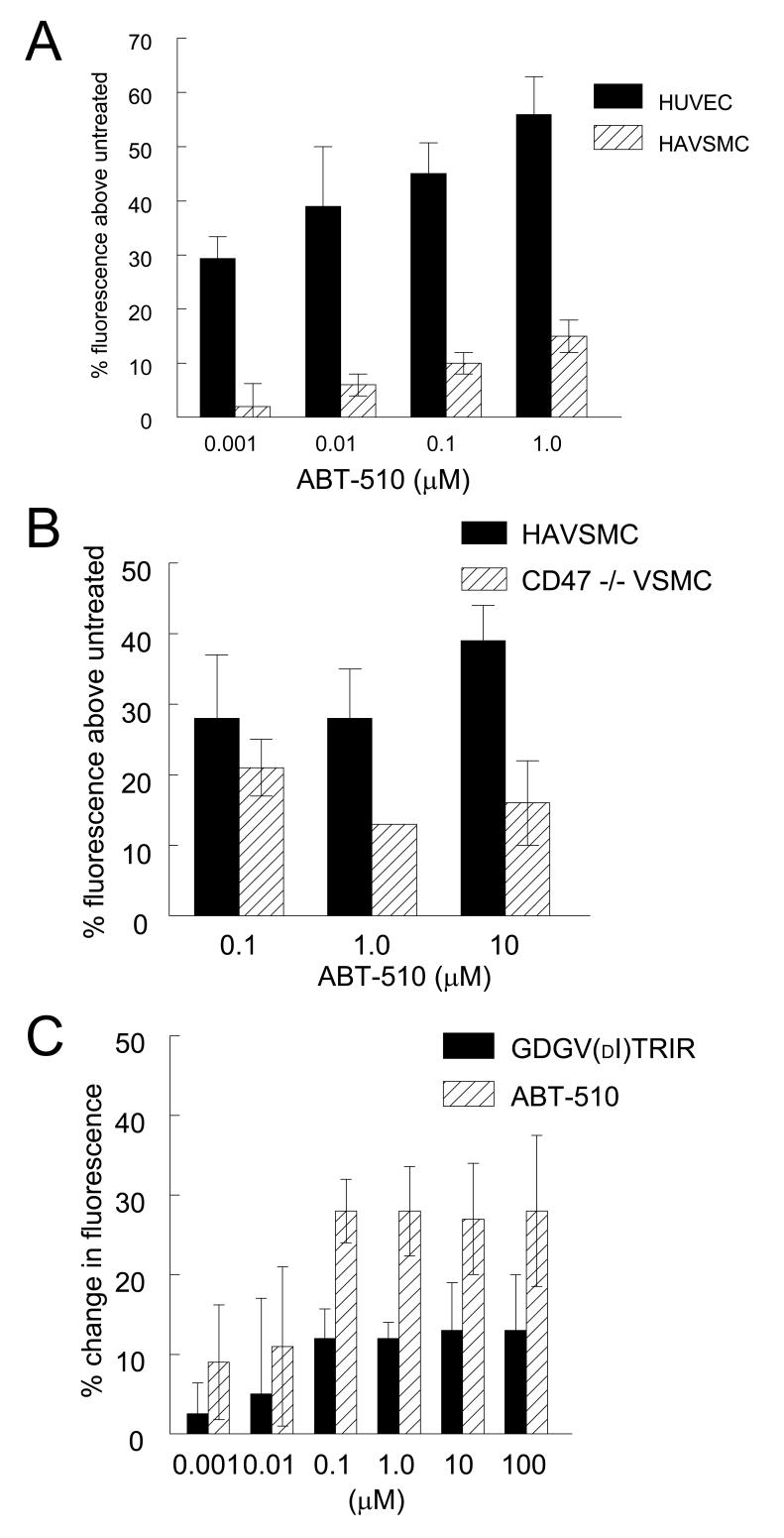
Figure 1. ABT-510 blocks tumor-driven vascular cell outgrowth, NO-driven cGMP flux, and CD36 mediated fatty acid uptake.
B16F10 melanoma tumor biopsies from C57BL/6 wild type mice were explanted in type I collagen matrices and incubated in growth medium plus 10 μM DETA/NO with the indicated treatment agents for seven days and vascular outgrowth quantified (A). Fresh washed human platelets (200/μl) were incubated in Tyrode’s buffer and the indicated treatment agents for 15 min and exogenous NO (10 μM DEA/NO) added. After 60 sec cells were lysed and cGMP levels determined via immunoassay (B). HUVEC (C) or HAVSMC (D) were plated at 1 × 105 cells/well in either EBM or SM-BM + 0.1% FAF BSA and incubated in the presence of the indicated dosages of ABT-510 or GDGV(dI)TRIR for 15 min and then treated with [3H]-myristic acid complexed to FAF-BSA for 5 min, and uptake into the cells was determined following lysis by scintillation counting.
3.2. ABT-510 blocks NO-driven cGMP flux in platelets
TSP1 and both CD36 and CD47-binding peptides derived from TSP1 block NO driven cGMP accumulation in endothelial cells and platelets [20](manuscript submitted). Based on the rapid cGMP response to NO in platelets, we used these cells to examine the ability of ABT-510 to modulate cGMP signaling. ABT-510 and GDGV(dI)TRIR inhibited an NO-stimulated nucleotide flux in platelets with similar dose responses (Fig. 1B). However, the IC50 for ABT-510 in this assay (>1 μM) is much higher than that previously reported for ABT-510 to inhibit endothelial cell migration (IC50 = 0.9 nM) [9]. Therefore, the latter activity probably can not be explained by antagonism of NO signaling at the level of cGMP.
3.3. ABT-510 weakly inhibits myristate uptake in vascular cells
CD36 transports free fatty acids across cell membranes, and TSP1 blocks the ∼50% of myristate uptake into vascular endothelial and smooth muscle cells that is mediated by CD36 [21], suggesting that ABT-510 might also modulate fatty acid uptake via CD36. Under fatty acid free conditions, ABT-510 moderately inhibited myristate uptake into HUVEC (Fig. 1C) and HAVSMC (Fig. 1D). In both cell types, ABT-510 was weaker than its precursor peptide GDGV(dI)TRIR. Similar results were obtained for myristate uptake into CD47 null VSMC, indicating that this TSP1 receptor is not required for activity of either peptide (results not shown). Therefore, the moderate activity of ABT-510 for inhibiting myristate uptake can not account for its enhanced anti-angiogenic activity relative to GDGV(dI)TRIR [9].
3.4. ABT-510 modestly blocks NO- and myristate-stimulated VSMC adhesion to collagen
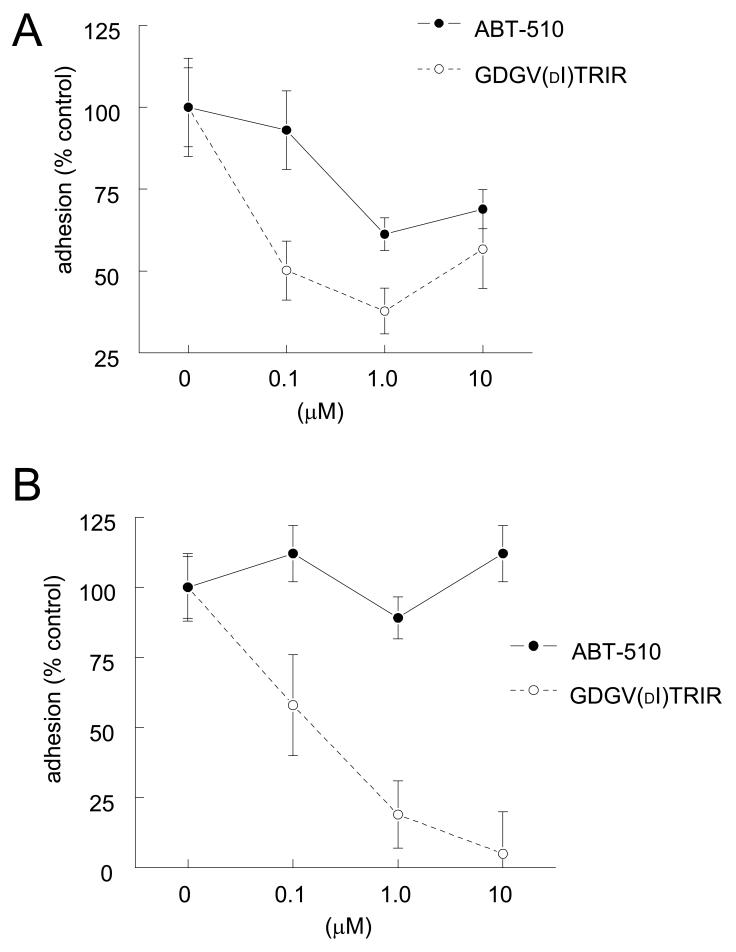
We previously reported that myristate stimulated vascular cell adhesion to type I collagen and that this could be blocked by anti-angiogenic agents that target CD36 [21]. Using HAVSMC, which express large amounts of CD36, we found that myristate-stimulated adhesion to collagen was inhibited by ABT-510, but only at dose ≥1 μM (Fig. 2A). Consistent with its more potent activity for inhibiting myristate uptake in these cells (Fig. 1), GDGV(dI)TRIR was more active than ABT-510 for inhibiting myristate-stimulated HAVSMC adhesion on collagen (Fig. 2A).
Figure 2. ABT-510 minimally inhibits vascular cell adhesion to collagen.
HAVSMC (104 cells/well) were plated in 96-well plates pre-coated with type I collagen (3 μg/ml) in SM-BM + 0.1% FAF-BSA and the indicated concentrations of and ABT-510 or GDGV(dI)TRIR ± 10 μM myristate (A). Adherent cells were quantified after staining with crystal violet at 570 nm. Results are expressed as the mean ± SD of the difference between myristate treated and untreated cells and are representative of at least three experiments. HAVSMC (104 cells/well) were plated in 96-well plates pre-coated with type I collagen (3 μg/ml) in SM-BM + 0.1% FAF-BSA and the indicated concentrations of and ABT-510 or GDGV(dI)TRIR ± 10 μM DEA/NO (B). Adherent cells were quantified after staining with crystal violet at 570 nm. Results are expressed as the mean ± SD of the difference between NO treated and untreated cells and are representative of at least three experiments.
TSP1, recombinant type 1 repeats, modified type 1 repeat peptides, and CD36 antibodies also inhibit NO-stimulated endothelial and VSMC adhesion on a collagen substrate [23, 30]. Consistent with our previous data, GDGV(dI)TRIR effectively inhibited NO-stimulated HAVSMC adhesion on collagen, but ABT-510 had minimal activity in this assay (Fig. 2B).
3.5. ABT-510 blocks NO-stimulated delay in platelet aggregation
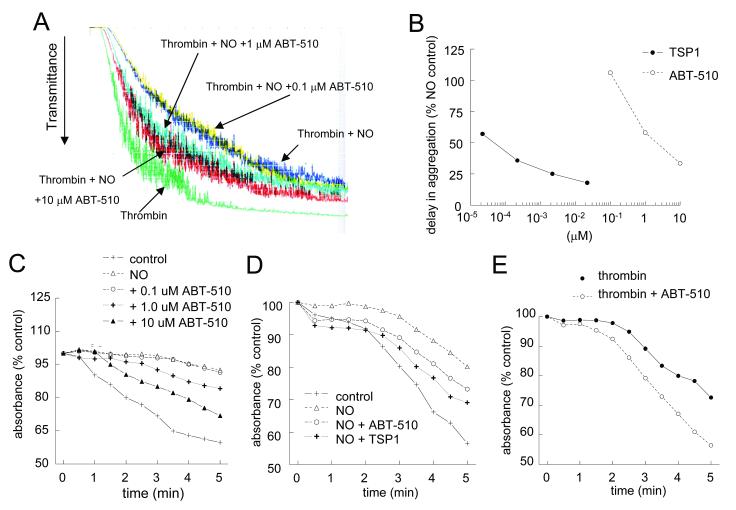
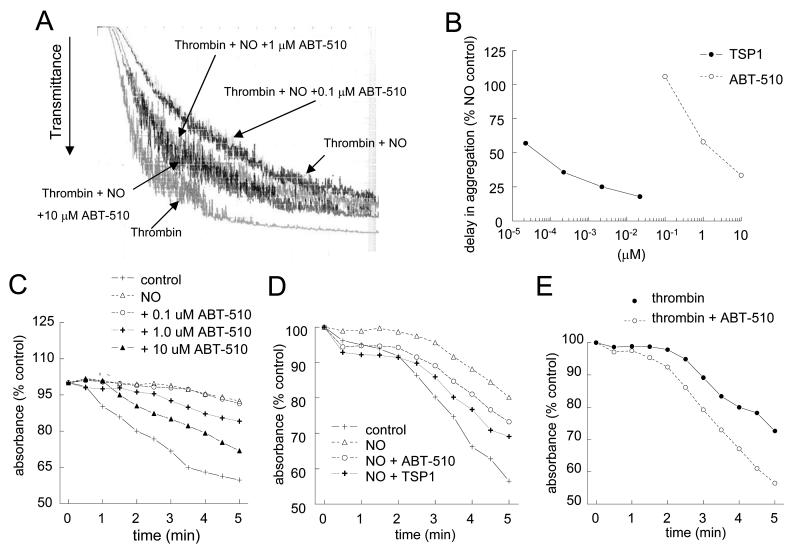
NO is known to delay platelet activation and aggregation [31]. Recently we demonstrated that TSP1 plays a critical role in the regulation of an NO-stimulated delay in platelet aggregation, with picomolar concentrations reversing the inhibitory effect of NO [32]. In contrast ABT-510 was several log units less potent for blocking an NO-stimulated delay in aggregation (Fig. 3A). At doses of 1-10 μM, ABT-510 was able to reverse an NO-stimulated delay in platelet aggregation. This was true for both high shear conditions (Fig. 3A) and low shear conditions (Fig. 3C). Dose dependence analysis of ABT-510 as compared to TSP1 under high shear conditions demonstrated TSP1 to be approximately 10,000-fold more potent at blocking an NO-driven delay in platelet aggregation (Fig. 3B). Even under low shear conditions TSP1, at all doses tested, was more potent than ABT-510 (Fig. 3D). In the absence of NO, ABT-510 was also found to synergize with thrombin and moderately accelerate aggregation (Fig. 3E).
Figure 3. NO-stimulated delay in platelet aggregation is partially prevented by ABT-510.
Washed human platelets (200 cells/μl) were incubated in the presence of thrombin (0.5 U/ml) and exogenous NO (DEA/NO 10 μM) for 5 minutes. Cells were pre-incubated in buffer and the indicated doses of ABT-510 prior to initiating aggregation under high shear (A) or low shear (C) conditions. Dose response curves for TSP1 and ABT-510 to reverse an NO delay in platelet aggregation under high shear conditions (B). In some experiments washed platelets were treated with ABT-510 (1 μM) or TSP1 (2.2 nM) ± NO (DEA/NO 10 μM) (D) or thrombin (0.5 U/ml) and ABT-510 (100 nM) (E) for 15 minutes, and aggregation was determined. Results are representative of at least three experiments.
3.6. ABT-510 selectively induces caspase activation
ABT-510 is reported to induce apoptosis in umbilical artery endothelial cells at nM concentrations [9], and in brain microvascular endothelial cells. This correlates with activation of caspases 3 and 7 and PARP cleavage [18]. We used a fluorimetric caspase 3/7 assay to compare the pro-apoptotic activities of GDGV(dI)TRIR and ABT-510 in HUVEC and HAVSMC (Fig. 4). Initial studies showed that ABT-510 activated caspases 3/7 in HAVSMC as well as in HUVEC. In HAVSMC, the response was time dependent and optimal at 18 h in medium containing 0.5% FCS (data not shown). ABT-510 induced dose-dependent caspase 3/7 activation in both cell types at nM concentrations (Fig. 4A). Although HAVSMC express higher levels of CD36, caspase activation was higher in HUVEC at all levels of ABT-510 (Fig. 4A). Remarkably, the ability of ABT-510 to induce caspase activation was diminished in CD47-null VSMC (Fig. 4B). Consistent with the previously published structure/activity data [9], GDGV(dI)TRIR was less active than ABT-510 for inducing caspase activation in HAVSMC (Fig. 4C).
Figure 4. Differential caspase 3/7 activation by ABT-510 and GDGV(dI)TRIR.
HUVEC and HAVSMC were plated at a density of 1 × 104 cells/well in growth medium for 24 h, then treated in endothelial or smooth muscle cell growth medium + 0.5% FCS for 24 h. Activity of caspases 3 and 7 were measured as per the manufactures instructions at 18 h after substrate addition with an excitation wavelength of 499 nm and an emission wavelength of 521 nm (A). HAVSMC and CD47 -/- VSMC were plated at a density of 1 × 104 cells/well in smooth muscle cell growth medium for 24 h, then treated in the same + 0.5% FCS for 24 h. Activity of caspases 3 and 7 were measured 18 h after substrate addition (B). HAVSMC were plated at a density of 1 × 104 cells/well in growth medium for 24 h, then treated in the same + 0.5% FCS for 24 h. Activity of caspases 3 and 7 were measured 18 h after substrate addition (C).
4. Discussion
These studies demonstrate that some activities of ABT-510 differ from those of its parent peptide sequence GDGV(dI)TRIR and TSP1. The latter peptide resembles native TSP1 in its activities to inhibit NO/cGMP signaling in vascular cells and platelets and to inhibit myristate uptake via CD36. ABT-510 shares these activities with GDGV(dI)TRIR but is generally less active. Conversely, ABT-510 is a potent inducer of caspase 3/7 activation and is more active than GDGV(dI)TRIR for this endpoint. Thus, the chemical modifications leading to ABT-510 appear to have greatly enhanced a weak pro-apoptotic activity of this TSP1 sequence while diminishing the activity of the same TSP1 sequence to antagonize NO signaling and CD36 fatty acid translocase activities.
Our data show that VSMC, platelets, and endothelial cells are all targets of ABT-510. Previously, ABT-510 was assumed to be an endothelial cell targeted drug, but VSMC and platelets also express CD36, and we show that ABT-510 regulates CD36-mediated responses in both cell types. Furthermore, as discussed below, other receptors may mediate ABT-510 activities and be expressed in other cell types. Relevant to its clinical application, ABT-510 seems to have some pro-thrombotic activity for platelets. These three vascular cell targets should be considered in interpreting the reported hematological side effects of ABT-520 in Phase I trials (reviewed in [16])
The decreased sensitivity of CD47 null VSMC cells to ABT-510-induced caspase activation may be important for understanding the mechanism of action of this drug. CD47 is known to mediate apoptotic responses to TSP1 in several cell types [33-37]. Furthermore, we found that the ability to GDGV(dI)TRIR to inhibit NO/cGMP signaling was lost in CD47 null cells [20]. Thus, CD47 plays an important role in signaling responses initiated by ligating CD36. Further work is needed to determine whether ABT-510 can modulate signaling downstream of CD47.
Is CD36 the receptor that mediates both of these activities of ABT-510? While it is likely that ABT-510 is acting as an analog of GDGV(dI)TRIR in its weak antagonism of myristate uptake and NO/cGMP signaling via CD36, it is less clear that the potent pro-apoptotic activity of ABT-510 is mediated by CD36. TSP1 can induce endothelial cell apoptosis by either CD36-dependent or CD36-independent pathways [19, 38, 39]. TSP1-induced apoptosis in dermal microvascular endothelial cells is associated with an increased ratio of Bax/Bcl-2 and activation of caspase-3 [19, 39]. ABT-510 similarly induces apoptosis in umbilical artery and brain microvascular endothelial cells [9, 18]. In the latter cells, 50-100 nM ABT-510 was sufficient to activate caspase-3 and -7. Here we found that HAVSMC expressing a higher level of CD36 are less sensitive to ABT-510-induced caspase 3/7 activation than are HUVEC, which express only low levels of CD36 [21].
Previously we found that GDGV(dI)TRIR retained some activity to inhibit NO/cGMP signaling in CD36 null VSMC [20], suggesting that this peptide may also signal through a different undefined receptor. Further work is needed to determine whether this also applies to ABT-510 and to clarify the basis for its more potent pro-apoptotic activity relative to GDGV(dI)TRIR. This could involve ABT-510 binding more potently to a site on CD36 distinct from that where TSP1 and GDGV(dI)TRIR bind to inhibit fatty acid uptake or could be mediated by ABT-510 binding to a different receptor that is expressed on both endothelial and VSMC. As ABT-510 demonstrates an increasing range of anti-tumor activities in mice, dogs, and humans, it is important to further define these distinct modes of action that may contribute to its clinical efficacy. It is also important for basic research studies to recognize that ABT-510 may have activities distinct from those of TSP1 and the TSP1 peptide sequence from which it was derived. Thus, in some situations ABT-510 may not function as a surrogate for TSP1. Further efforts should be made to identify receptors for ABT-510 that mediate its potent pro-apoptotic activity and to determine whether these receptors contribute to the same activity of TSP1.
Acknowledgements
We thank Larry Keefer for providing NO donors, Abbott Laboratories for providing ABT-510, Lubya Varticovski for providing B16 melanoma cells, and Bill Frazier for providing CD47 null mice. This work was supported by the Intramural Research Program of the NIH, NCI, Center for Cancer Research (D.D.R.)
Nonstandard Abbreviations
- DEA/NO
diethylamine NONOate
- DETA/NO
diethyltriamine NONOate
- eNOS
endothelial nitric oxide synthase
- FAF-BSA
fatty acid-free bovine serum albumin
- FGF2
fibroblast growth factor-2
- HAVSMC
human aortic vascular smooth muscle cells
- HUVEC
human umbilical vein endothelial cells
- TSP1
thrombospondin-1
- VSMC
vascular smooth muscle cells
Footnotes
Recommended section: Antibiotics and Chemotherapeutics
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].De Palma M, Naldini L. Role of haematopoietic cells and endothelial progenitors in tumour angiogenesis. Biochim Biophys Acta. 2006;1766:159–66. doi: 10.1016/j.bbcan.2006.06.003. [DOI] [PubMed] [Google Scholar]
- [2].Sata M. Role of circulating vascular progenitors in angiogenesis, vascular healing, and pulmonary hypertension: lessons from animal models. Arterioscler Thromb Vasc Biol. 2006;26:1008–14. doi: 10.1161/01.ATV.0000206123.94140.f3. [DOI] [PubMed] [Google Scholar]
- [3].Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- [4].Roberts DD. Regulation of tumor growth and metastasis by thrombospondin-1. Faseb J. 1996;10:1183–91. [PubMed] [Google Scholar]
- [5].Lawler J, Detmar M. Tumor progression: the effects of thrombospondin-1 and -2. Int J Biochem Cell Biol. 2004;36:1038–45. doi: 10.1016/j.biocel.2004.01.008. [DOI] [PubMed] [Google Scholar]
- [6].Dawson DW, Pearce SF, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the In vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol. 1997;138:707–17. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dawson DW, Volpert OV, Pearce SF, Schneider AJ, Silverstein RL, Henkin J, et al. Three distinct D-amino acid substitutions confer potent antiangiogenic activity on an inactive peptide derived from a thrombospondin-1 type 1 repeat. Mol Pharmacol. 1999;55:332–8. doi: 10.1124/mol.55.2.332. [DOI] [PubMed] [Google Scholar]
- [8].Reiher FK, Volpert OV, Jimenez B, Crawford SE, Dinney CP, Henkin J, et al. Inhibition of tumor growth by systemic treatment with thrombospondin-1 peptide mimetics. Int J Cancer. 2002;98:682–9. doi: 10.1002/ijc.10247. [DOI] [PubMed] [Google Scholar]
- [9].Haviv F, Bradley MF, Kalvin DM, Schneider AJ, Davidson DJ, Majest SM, et al. Thrombospondin-1 mimetic peptide inhibitors of angiogenesis and tumor growth: design, synthesis, and optimization of pharmacokinetics and biological activities. J Med Chem. 2005;48:2838–46. doi: 10.1021/jm0401560. [DOI] [PubMed] [Google Scholar]
- [10].Rusk A, Cozzi E, Stebbins M, Vail D, Graham J, Valli V, et al. Cooperative activity of cytotoxic chemotherapy with antiangiogenic thrombospondin-I peptides, ABT-526 in pet dogs with relapsed lymphoma. Clin Cancer Res. 2006;12:7456–64. doi: 10.1158/1078-0432.CCR-06-0110. [DOI] [PubMed] [Google Scholar]
- [11].Quesada AJ, Nelius T, Yap R, Zaichuk TA, Alfranca A, Filleur S, et al. In vivo upregulation of CD95 and CD95L causes synergistic inhibition of angiogenesis by TSP1 peptide and metronomic doxorubicin treatment. Cell Death Differ. 2005;12:649–58. doi: 10.1038/sj.cdd.4401615. [DOI] [PubMed] [Google Scholar]
- [12].Markovic SN, Suman VJ, Rao RA, Ingle JN, Kaur JS, Erickson LA, et al. A phase II study of ABT-510 (thrombospondin-1 analog) for the treatment of metastatic melanoma. Am J Clin Oncol. 2007;30:303–9. doi: 10.1097/01.coc.0000256104.80089.35. [DOI] [PubMed] [Google Scholar]
- [13].Hoekstra R, de Vos FY, Eskens FA, de Vries EG, Uges DR, Knight R, et al. Phase I study of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 with 5-fluorouracil and leucovorin: a safe combination. Eur J Cancer. 2006;42:467–72. doi: 10.1016/j.ejca.2005.08.040. [DOI] [PubMed] [Google Scholar]
- [14].Gietema JA, Hoekstra R, de Vos FY, Uges DR, van der Gaast A, Groen HJ, et al. A phase I study assessing the safety and pharmacokinetics of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 with gemcitabine and cisplatin in patients with solid tumors. Ann Oncol. 2006;17:1320–7. doi: 10.1093/annonc/mdl102. [DOI] [PubMed] [Google Scholar]
- [15].Jimenez B, Volpert OV, Reiher F, Chang L, Munoz A, Karin M, et al. c-Jun N-terminal kinase activation is required for the inhibition of neovascularization by thrombospondin-1. Oncogene. 2001;20:3443–8. doi: 10.1038/sj.onc.1204464. [DOI] [PubMed] [Google Scholar]
- [16].Westphal JR. Technology evaluation: ABT-510, Abbott. Curr Opin Mol Ther. 2004;6:451–7. [PubMed] [Google Scholar]
- [17].Huang H, Campbell SC, Bedford DF, Nelius T, Veliceasa D, Shroff EH, et al. Peroxisome proliferator-activated receptor gamma ligands improve the antitumor efficacy of thrombospondin peptide ABT510. Mol Cancer Res. 2004;2:541–50. [PubMed] [Google Scholar]
- [18].Anderson JC, Grammer JR, Wang W, Nabors LB, Henkin J, Stewart JE, Jr., et al. ABT-510, a Modified Type 1 Repeat Peptide of Thrombospondin, Inhibits Malignant Glioma Growth In Vivo by Inhibiting Angiogenesis. Cancer Biol Ther. 2007;6:454–62. doi: 10.4161/cbt.6.3.3630. [DOI] [PubMed] [Google Scholar]
- [19].Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–8. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- [20].Isenberg JS, Ridnour LA, Dimitry J, Frazier WA, Wink DA, Roberts DD. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem. 2006;281:26069–80. doi: 10.1074/jbc.M605040200. [DOI] [PubMed] [Google Scholar]
- [21].Isenberg JS, Jia Y, Fukuyama J, Switzer CH, Wink DA, Roberts DD. Thrombospondin-1 inhibits nitric oxide signaling via CD36 by inhibiting myristic acid uptake. J Biol Chem. 2007;282:15404–15. doi: 10.1074/jbc.M701638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lindberg FP, Bullard DC, Caver TE, Gresham HD, Beaudet AL, Brown EJ. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science. 1996;274:795–8. doi: 10.1126/science.274.5288.795. [DOI] [PubMed] [Google Scholar]
- [23].Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, Roberts DD. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci U S A. 2005;102:13141–6. doi: 10.1073/pnas.0502977102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Roberts DD, Cashel J, Guo N. Purification of thrombospondin from human platelets. J Tissue Cult Methods. 1994;16:217–22. [Google Scholar]
- [25].Isenberg JS, Jia Y, Field L, Ridnour LA, Sparatore A, Del Soldato P, et al. Modulation of angiogenesis by dithiolethione-modified NSAIDs and valproic acid. Br J Pharmacol. 2007;151:63–72. doi: 10.1038/sj.bjp.0707194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chang AC, Detwiler TC. Reactions of thrombin-serpin complexes with thrombospondin. Arch Biochem Biophys. 1992;299:100–4. doi: 10.1016/0003-9861(92)90249-v. [DOI] [PubMed] [Google Scholar]
- [27].Shaked Y, Bertolini F, Man S, Rogers MS, Cervi D, Foutz T, et al. Genetic heterogeneity of the vasculogenic phenotype parallels angiogenesis; Implications for cellular surrogate marker analysis of antiangiogenesis. Cancer Cell. 2005;7:101–11. doi: 10.1016/j.ccr.2004.11.023. [DOI] [PubMed] [Google Scholar]
- [28].Yap R, Veliceasa D, Emmenegger U, Kerbel RS, McKay LM, Henkin J, et al. Metronomic low-dose chemotherapy boosts CD95-dependent antiangiogenic effect of the thrombospondin peptide ABT-510: a complementation antiangiogenic strategy. Clin Cancer Res. 2005;11:6678–85. doi: 10.1158/1078-0432.CCR-05-0621. [DOI] [PubMed] [Google Scholar]
- [29].Yang Q, Tian Y, Liu S, Zeine R, Chlenski A, Salwen HR, et al. Thrombospondin-1 peptide ABT-510 combined with valproic acid is an effective antiangiogenesis strategy in neuroblastoma. Cancer Res. 2007;67:1716–24. doi: 10.1158/0008-5472.CAN-06-2595. [DOI] [PubMed] [Google Scholar]
- [30].Isenberg JS, Wink DA, Roberts DD. Thrombospondin-1 antagonizes nitric oxide-stimulated vascular smooth muscle cell responses. Cardiovasc Res. 2006;71:785–93. doi: 10.1016/j.cardiores.2006.05.024. [DOI] [PubMed] [Google Scholar]
- [31].Radomski MW, Palmer RM, Moncada S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc Natl Acad Sci U S A. 1990;87:5193–7. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Isenberg JS, Hyodo F, Matsumoto K, Romeo MJ, Abu-Asab M, Tsokos M, et al. Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood. 2007;109:1945–52. doi: 10.1182/blood-2006-08-041368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lamy L, Foussat A, Brown EJ, Bornstein P, Ticchioni M, Bernard A. Interactions between CD47 and thrombospondin reduce inflammation. J Immunol. 2007;178:5930–9. doi: 10.4049/jimmunol.178.9.5930. [DOI] [PubMed] [Google Scholar]
- [34].Rath GM, Schneider C, Dedieu S, Sartelet H, Morjani H, Martiny L, et al. Thrombospondin-1 C-terminal-derived peptide protects thyroid cells from ceramide-induced apoptosis through the adenylyl cyclase pathway. Int J Biochem Cell Biol. 2006;38:2219–28. doi: 10.1016/j.biocel.2006.07.004. [DOI] [PubMed] [Google Scholar]
- [35].Johansson U, Higginbottom K, Londei M. CD47 ligation induces a rapid caspase-independent apoptosis-like cell death in human monocytes and dendritic cells. Scand J Immunol. 2004;59:40–9. doi: 10.1111/j.0300-9475.2004.01355.x. [DOI] [PubMed] [Google Scholar]
- [36].Roue G, Bitton N, Yuste VJ, Montange T, Rubio M, Dessauge F, et al. Mitochondrial dysfunction in CD47-mediated caspase-independent cell death: ROS production in the absence of cytochrome c and AIF release. Biochimie. 2003;85:741–6. doi: 10.1016/s0300-9084(03)00129-9. [DOI] [PubMed] [Google Scholar]
- [37].Manna PP, Frazier WA. CD47 mediates killing of breast tumor cells via Gi-dependent inhibition of protein kinase A. Cancer Res. 2004;64:1026–36. doi: 10.1158/0008-5472.can-03-1708. [DOI] [PubMed] [Google Scholar]
- [38].Guo N, Krutzsch HC, Inman JK, Roberts DD. Thrombospondin 1 and type I repeat peptides of thrombospondin 1 specifically induce apoptosis of endothelial cells. Cancer Res. 1997;57:1735–42. [PubMed] [Google Scholar]
- [39].Nor JE, Mitra RS, Sutorik MM, Mooney DJ, Castle VP, Polverini PJ. Thrombospondin-1 induces endothelial cell apoptosis and inhibits angiogenesis by activating the caspase death pathway. J Vasc Res. 2000;37:209–18. doi: 10.1159/000025733. [DOI] [PubMed] [Google Scholar]