Abstract
Stability in cardiac indicators before birth and their utility in predicting variation in postnatal development were examined. Fetal heart rate and variability were measured longitudinally from 20 through 38 weeks gestation (n = 137) and again at age 2 (n = 79). Significant within-individual stability during the prenatal period and into childhood was demonstrated. Fetal heart rate variability at or after 28 weeks gestation and steeper developmental trajectories were significantly associated with mental and psychomotor development at 2 years (n = 82) and language ability at 2.5 years (n = 61). These data suggest that the foundations of individual differences in autonomic control originate during gestation and the developmental momentum of the fetal period continues after birth.
Speculation on the prenatal foundations of human development has witnessed burgeoning scientific interest as significant links between indicators of fetal growth and a broad range of health and developmental outcomes have been identified (Barker, 2006; O’Brien, Wheeler, & Barker, 1999; Young, 2002). However, the primary independent measure in such studies is birth weight, an exceedingly blunt instrument for distinguishing developmental course in utero. Absent from this discourse has been examination of less conspicuous but potentially more informative indicators of the developing fetus based on function. Technological advances afforded by the advent of real-time ultrasound have made clear that by the end of gestation, features of development that have been measured extensively in the neonate and infant and that are integral to theories of development, originate neither at term nor with birth. The late-term human fetus demonstrates virtually the same neurobehavioral repertoire as the newborn infant (Als, 1982; Comparetti, 1981; Prechtl, 1984).
The most prominent and accessible indicators of fetal function involve measures based on heart rate. Fetal heart rate and its patterning provide the basis for clinical evaluation of fetal well-being (Ware & Devoe, 1994). As gestation advances, fetal heart rate declines, but time-dependent (Dawes, Moulden, Sheil, & Red-man, 1992; Fleisher, DiPietro, Johnson, & Pincus, 1997; van Leeuwen, Lange, Bettermann, Gronemeyer, & Hatzmann, 1999) and time-independent (DiPietro et al., 2004; Nijhuis et al., 1998) indicators of variability increase. These trajectories have been attributed, in part, to increased parasympathetic innervation of the heart (Dalton, Dawes, & Patrick, 1983; Freeman, Garite, & Nageotte, 1991) and changes in autonomic control from the medulla oblongata to higher cortical processes commencing near 27 weeks gestation (Yoshizato et al., 1994). Alterations to typical development of fetal heart rate or its patterning have been reported in fetuses with prenatal conditions or exposures that have well-established postnatal developmental sequelae. These include conditions such as neural tube defects (Maeda et al., 2006; Yoshizato et al., 1994) and intrauterine growth restriction (Nijhuis et al., 2000; Snijders, Ribbert, Visser, & Mulder, 1992) as well as maternal factors that alter the intrauterine milieu, ranging from anemia (Nicolaides, Sadovsky, & Visser, 1989) to opioid drug use (Jansson, DiPietro, & Elko, 2005). Further consensus that features of fetal heart rate provide opportunities to indirectly assess the nervous system has come from numerous sources focused on typically developing fetuses (Dawes, 1986; DiPietro, Irizarry, Hawkins, Costigan, & Pressman, 2001; Hepper, 1995; James, Pillai, & Smoleniec, 1995; Nijhuis & ten Hof, 1999; Visser, 2004).
The degree to which individual differences in fetal heart rate and variation are conserved after birth is largely unknown. There is modest evidence for prenatal to infant stability for heart rate and variability through the first year of life (DiPietro, Costigan, Pressman, & Doussard-Roosevelt, 2000; Lewis, Wilson, Ban, & Baumel, 1970) and a small but significant relation has been shown between prenatal and postnatal heart rate at age 10 (Thomas, Haslum, MacGillivray, & Golding, 1989). Efforts to employ fetal measures as indicators of autonomic regulation associated with other aspects of function, such as temperament, have reported associations between higher fetal heart rate and lower threshold to novelty (Snidman, Kagan, Riordan, & Shannon, 1995) and emotional tone (DiPietro, Hodgson, Costigan, & Johnson, 1996) in early infancy. A single report links baseline fetal measures to developmental outcomes: Greater continuous and episodic indicators of fetal heart rate variability, as well as their developmental trajectories, were found to be significantly associated with child language competence and symbolic play during the third year of life (Bornstein et al., 2002).
Measurement of phasic or nonphasic cardiac variability has had a distinguished history in psychophysiological research (Bernston et al., 1997). Application within a developmental framework has focused on its use as a marker of the physiological regulation that corresponds to infant and child performance and behavior. Domains of function that have been linked to dynamic or static properties of heart rate variability include information processing (Bornstein & Suess, 2000b; Feldman, 2006; Richards, 1989), performance on standardized developmental or cognitive assessments (El-Sheikh & Buckhalt, 2005; Richards & Cameron, 1989), and focused examination during play (DiPietro, Porges, & Uhly, 1992). Variability in heart rate has also been shown to correspond to dimensions of temperament and behavioral regulation (Calkins & Dedmon, 2000; Fox, 1989; Snidman et al., 1995). Although a putative assumption is that neural maturation underlies these associations, few studies actually use developmental trajectory information as the independent measure. An exception is a series of longitudinal reports that show that the degree to which variability changes over a critical period of time in preterm infants measured prior to term predicts cognitive functioning at age 3 (Doussard-Roosevelt, Porges, Scanlon, Alemi, & Scanlon, 1997) and social competence in later childhood (Doussard-Roosevelt, McClenny, & Porges, 2001).
The primary goal of the current study was to provide a bridge from the fetal to the early childhood periods in application of cardiac patterning measurement within individuals. The specific objectives are twofold. The first is to ascertain stability, both within gestation and from the prenatal to the postnatal period, in heart rate and heart rate variability. Stability can be operationalized in a number of ways; here, we refer to preservation of order among individual values over time (Bornstein & Suess, 2000a), a prerequisite to establishing that a measure is suitable for consideration as an individual difference. The second is to investigate whether fetal heart rate and variability are useful predictors of child developmental outcome. The longitudinal design incorporated repeated assessment of heart rate and variability at six gestational ages and at 24 months after birth. Children were also evaluated at 24 months with a standard assessment of mental and motor development. A comprehensive language assessment battery was administered 6 months after the initial postnatal assessment to capture the explosive developmental trajectory in language ability during the third year of life. We expected fetal measures to show stability during the fetal period and be conserved from the prenatal period to the postnatal assessment. Based on the consensus that variability in physiological systems in general and heart rate in particular signifies a more optimal functional state in the perinatal period and beyond (Dunster, 1999), we hypothesized that higher levels of variability and steeper developmental gestational course would be reflected in more advanced developmental functioning in early childhood.
Method
Participants
Participants were initially enrolled in a study of normative fetal neurobehavioral development. Recruitment was restricted to low-risk, nonsmoking women at least 20 years of age with singleton pregnancies and consistent pregnancy dating validated by early first-trimester pregnancy testing, examination, and/or ultrasound. Enrollment eligibility for the follow-up was limited to the 137 healthy offspring born at term of women with uncomplicated pregnancies who completed the prenatal portion of the protocol. Developmental evaluations were conducted when children were approximately 2 and 2.5 years old. A total of 105 of the eligible children (76.7%) participated in one or both the visits; 94 (68.4%) participated in the first assessment (mean age = 24.8 months, SD = 0.9) and 81 (59.1%) in the second (mean age = 30.7 months, SD = 0.5). Sociodemographic characteristics of the original full sample reflect a sample of mature (mean maternal age = 32.0, SD = 3.6), well-educated (mean years of maternal education = 17.0, SD = 2.0), married (95.7%), and nonminority (European American, 85.1%; African American, 11.7%; and Asian American 3.2%) families. Newborns were of appropriate birth weight (M = 3,478.5 g; SD = 428) and term gestational age (M = 39.4 weeks, SD = 1.2), with good Apgar scores (M = 9.0, SD = 0.4). Fifty-one percent were girls.
Loss to follow-up was principally attributable to families moving out of the area and family time demands that made daytime testing impracticable. Mothers of children who participated at each follow-up period were significantly older than those who did not, t(135) = 2.42, p < .05 and t(135) = 4.81, p < .001; age differences = 1.8 and 3.1 years at 24 and 30 months, respectively; and more educated, t(135) = 2.42, p < .05 and t(135) = 2.36, p <.05, mean education differences = 0.9 and 0.8 years. There were no differences between followed and not followed children in terms of maternal race/ethnicity, parity, or marital status. Child characteristics, including birth weight, gestational age, and sex ratio, did not differ between tested and untested children at either visit, nor did mean values of fetal heart rate and variability.
Design and Procedure
Prenatal Period
Fetal monitoring commenced at 20 weeks gestation and was repeated at 24, 28, 32, 36, and 38 weeks. To control for potential diurnal and prandial effects, fetuses were tested at the same time each visit (1:00 or 3:00 p.m.), and women were instructed to eat 1.5 hr prior to testing but not again before testing. Monitoring proceeded for 50 min, with the mother resting in a semirecumbent, left-lateral position. Fetal data were collected using a Toitu MT320 fetal actocardiograph (Toitu Co., Ltd., Tokyo, Japan), which detects fetal heart rate using a single wide-array Doppler transducer positioned on the maternal abdomen. Data were collected from the output port of the monitor, digitized via an external A/D board using streaming software, and analyzed offline using software developed in our laboratory (GESTATE; James Long Company, Caroga Lake, NY). Digitized heart rate data were processed using error rejection procedures based on moving averages of acceptable values as needed. Fetal heart rate and variability (SD of heart rate per minute epochs) were computed in 1-min epochs and averaged over the 50-min period.
Postnatal Period
Two-year follow-up
Child development was evaluated shortly after children reached their second birthday using the Mental Development Index (MDI) and Psychomotor Development Index (PDI) of the Bayley Scales of Infant Development II (BSID-II) (Bayley, 1993), a widely used, psychometrically validated assessment of general functioning for children from 1 to 42 months of age. The BSID-II was administered by a licensed clinical psychologist who was blind to the fetal data and hypotheses of the study. Testing occurred in a child development laboratory environment.
Prior to the evaluation, cardiac activity was recorded in children using a three-lead electrocardiogram (ECG) while seated quietly on their mothers’ laps and distracted with a toy or a book. Three disposable Ag – AgCL electrodes were triangulated on the toddler’s trunk, and the signal was amplified (PhysioControl, Model Lifepak 5, Plainview, NY), digitized, and recorded on a computer. R-wave detection, editing for artifact, and timing of sequential heart periods proceeded offline using MXedit software (Delta-Biometrics, Inc., Bethesda, MD). Heart period and heart period variability (SD) were computed in 30-s epochs and averaged over the recording duration. In addition, a measure of vagal tone was computed using the analytic method developed by Porges (1985). Briefly, this procedure uses a 21-point polynomial to detrend sequential heart period data and a band-pass filter to extract the variance within the frequency band consistent with respiration within this age-group (i.e., 0.24 – 1.04 Hz). The estimate of V, which corresponds to the respiratory sinus arrhythmia, is calculated as the natural log of the extracted variance. Mean values of the 30-s epochs were calculated.
Follow-up of 2.5 years
Evaluation of language proficiency was based on a multidimensional, three-pronged approach. One week prior to the home visit, mothers completed the MacArthur Communicative Development Inventory (MCDI): toddlers (Fenson et al., 1993) based on their general knowledge of the child. The total number of words that the mother reported her child produced was calculated. Children were administered the Comprehension Scale ‘‘A’’ and the Expressive Language Scale of the Reynell Developmental Language Scales (RDLS), Second Revision (Reynell & Gruber, 1990) during the home visit. Children’s standard language scores for comprehension and production were calculated. The Vineland Adaptive Behavior Scales (VABS): Interview Edition Survey Form (Sparrow, Balla, & Cicchetti, 1984) Communication Domain, obtained by interview of the mother after the home visit, was used to assess children’s communication skills. The score for the Communication Domain is the sum of raw scores for the Expressive and Receptive Sub-domains converted to a standard score. A total of 61 participants generated data from all three sources. As expected, the language assessments were interrelated, rs(59) = .50 to .68, ps < .001; their mean standard score was used as a comprehensive language aggregate score. The Peabody Picture Vocabulary Test—Revised (PPVT-R) (Dunn & Dunn, 1981), a reliable and valid measure of adult verbal – perceptual intelligence, was administered to the mother at the end of the home visit to determine whether maternal intellectual level provided a potential confound to child language development.
Data Analysis
Pearson correlations were used to compute the within-fetal stability of fetal heart rate and variability during gestation and to determine potential linear relations between fetal and child measures. One-way analysis of variance was used to examine the associations between a composite fetal heart rate/variability categorical variable, constructed with median dichotomization, and outcomes. Post hoc contrasts relied on the Tukey Honestly Significant Difference (HSD) test. To examine the association between fetal cardiac and developmental outcomes beyond simple linear associations captured by correlation coefficients, a cubic smoothing spline with 3 degrees of freedom (df) was fitted to the raw values of fetal heart rate variability at each gestational age to estimate the 36-week value. Cubic smoothing spline is a commonly used nonparametric noise reduction method and takes advantage of longitudinal data to model a specific data point that may be influenced by known or unknown elements (Hastie & Tibshirani, 1990); 3 df were used to permit nonlinearity of the parameter. Following this, a two-dimensional kernel smoother was used to estimate the joint density between each of the three developmental outcomes with significant fetal predictors. Smoothing parameters were robustly set by cross-validation using 1,000 bootstrap samples resulting in very stable density estimates (Bowman & Azzalini, 1997).
Hierarchical lineal modeling (HLM) 5 (Raudenbush, Byrk, & Congdon, 2000) was used to model the rate of change in fetal cardiac measures and generate independent measures of growth rate. Previously, a change in slope at 28 weeks gestation for the fetal heart rate variability, but not heart rate, was detected in this sample (DiPietro et al., 2004), such that there was a significant reduction in slope after 28 weeks. Accordingly, a piecewise linear growth function that included two slopes (20 – 28 weeks and 28 – 38 weeks) was used in the Level 1 analysis. In the second level, all coefficients were unconditionally predicted by the mean across fetuses and a random effect representing the unique increment/decrement for each fetus, thereby allowing the growth trajectory coefficients to vary. The fetal heart rate HLM was similar, with the exception that only a single overall slope was used in the Level 1 analysis.
Results
Stability of Fetal Measures
Table 1 presents the within-subject prenatal correlations for the original sample. Missing data for the 137 participants were minimal prior to term, involving only two cases at 28 weeks gestation. However, 33% of women delivered prior to their final scheduled 38-week visit; thus, 38-week coefficients are based on 91 participants. Fetal heart rate (upper diagonal) was significantly correlated (ps < .001) between each pair of gestational ages, and the magnitudes of these associations did not vary much across gestation. Fetal heart rate variability (lower diagonal) exhibited a similarly consistent pattern of correlations, with the exception of an absent association between the first and the last data points. The means of each pair of correlations for both measures over gestation were r = .58 for fetal heart rate and r = .40 for fetal heart rate variability.
Table 1.
Intercorrelations of Fetal Heart Rate and Variability During Gestation (n = 137)
| GA
|
||||||
|---|---|---|---|---|---|---|
| GA | 20 | 24 | 28 | 32 | 36 | 38a |
| 20 | — | .85 | .72 | .52 | .54 | .43 |
| 24 | .59 | — | .74 | .61 | .54 | .51 |
| 28 | .40 | .59 | — | .65 | .57 | .56 |
| 32 | .20 | .27 | .46 | — | .50 | .44 |
| 36 | .31 | .42 | .63 | .51 | — | .47 |
| 38 | .01 | .28 | .44 | .44 | .46 | — |
Note. The rs above the diagonal are for fetal heart rate and those below are for fetal heart rate variability. All correlations for fetal heart rate are significant at p < .0001 and those for fetal heart rate variability are significant at p <.001, with the exception of pair 20 – 38 weeks, ns, and 20 – 32 weeks, p < .05. GA = gestational age.
Based on 91 participants.
Child Measures
Cardiac Monitoring
Seven of the 94 toddlers refused application of ECG electrodes, and a total of 79 provided at least 5 min of data (mean recording duration = 7.8 min, SD = 1.2). Values were as follows: heart period, M = 496.9, SD = 39.3, and heart period variability, M = 6.27, SD = 0.68, V = 4.43, SD = 1.09. Given the stability of the correlations over gestation and to reduce the number of analyses, mean values were computed from 20 to 36 weeks gestation for each cardiac measure, thereby providing a more stable prenatal estimate of each. The final 38-week visit was not included in this value because its inclusion would have reduced the sample for this analysis by a third. Pearson correlations between prenatal and postnatal variables were as follows: fetal heart rate/child heart period, r(77) = −.35, p < .01, and fetal heart rate variability/child heart period variability, r(77) = .25, p <.05. Individual correlations for the 38-week visit were comparable: r(52) = −.28, p <.05 and r(52) = .22, p < .10. Note that the negative relation for the first pair of measures results from the use of ECG-derived, interbeat interval data in the postnatal period, which approximate the inverse of heart rate. Child V was not significantly associated with either averaged fetal cardiac measure, rs(77) = −.03 and .16 for fetal heart rate and variability, respectively. However, three of the six gestational age – specific correlations between child vagal tone and fetal heart rate variability were or neared significance: r(78) = .23, p < .05 at 24 weeks; r(77) = .20, p <.05 at 28 weeks; and r(52) = .24, p <.10 at 38 weeks.
Developmental Assessment
Bayley testing was incomplete for a subset of children who were too shy (n = 2) or noncompliant (n = 8). Two exams were not completed due to examiner unavailability. Two children generated either an MDI or a PDI score that was 2 standard deviations lower than the mean. Both records indicated that child irritability and uncooperativeness threatened validity of the scores; each was removed from the analyses to circumvent outlier influence. One participant was referred for subsequent developmental evaluation due to low scores and was ultimately diagnosed with autism. This individual is excluded from these analyses, resulting in a total of 80 participants with MDI and PDI scores. Mean values and variability, which approach population norms (i.e., M = 100, SD = 15), are presented in Table 2.
Table 2.
Child Developmental Values at Two Follow-Up Visits
| M | SD | Range | |
|---|---|---|---|
| 2-year measures (n = 80) | |||
| Mental Development Indexa | 102.90 | 12.08 | 74 – 132 |
| Psychomotor Development Indexa | 97.99 | 10.42 | 72 – 121 |
| 2.5-year measures (n = 61) | |||
| Language aggregate scoreb | 0.00 | 0.81 | −2.13 − 1.54 |
| MacArthurc—Vocabulary Production | 517.03 | 123.03 | 176 – 676 |
| Reynella—Comprehension | 107.74 | 15.46 | 71 – 136 |
| Reynella—Expressive | 99.30 | 11.19 | 71 – 129 |
| Vinelanda—Communication | 112.84 | 11.63 | 79 – 134 |
Standard score.
Mean Z-score.
Raw score.
Language
Complete language data from all three sources included in the composite were available for 61 children at 30 months. Missing values are a result of incomplete data on one or more measures; no particular measure was more likely to be incomplete than any other. Mean RDLS Comprehension and Expressive scores were both within 1 standard deviation of the normed average (Reynell & Gruber, 1990). The mean number of words that mothers reported their children used was consistent with the 30-month normed average on the MCDI (Fenson et al., 1993). The VABS Communicative Domain score fell less than 1 standard deviation above the mean reported in a standardized sample of 30- to 32-month-olds (Sparrow et al., 1984). Maternal PPVT-R scores were not significantly related to the child language composite, r(53) = .13, thus were not included in any analyses.
Associations Among Developmental Measures
Concurrent child heart period, variability, and V were unrelated to MDI, PDI, or the language composite (rs range from −.07 to .17). MDI and PDI scores were correlated, r(77) = .40, p < .001, and, in turn, related to language scores at 30 months, r(47) = .71, p <.001, with MDI and r(47) =.37, p <.001, with PDI.
Cross-Sectional Associations Between Fetal Measures and Developmental Outcomes
Correlations did not reveal any significant associations between fetal heart rate at any gestational age with MDI, PDI, or the language composite scores. In contrast, significant associations with child developmental measures emerged for fetal heart rate variability; these coefficients are presented in Table 3. One influential outlier was identified for more than one correlation between heart rate variability and both developmental scores at 24 months and was thus removed from the analyses, reducing the ns for each outcome by one case (Cook’s D ranged from 0.13 to 0.44). Fetal heart rate variability was significantly correlated with MDI and PDI scores from 28 through 38 weeks gestation. The ages at which significant associations were displayed with language were compressed to 32 – 36 weeks, but both growth trajectories revealed significant associations.
Table 3.
Associations Between Fetal Heart Rate Variability and Infant Developmental Outcome Measures
| Fetal heart
rate variability |
MDI
(n = 79) |
PDI
(n = 79) |
Language
(n = 61) |
|---|---|---|---|
| 20 weeks | .13 | .05 | .05 |
| 24 weeks | .20† | .09 | .04 |
| 28 weeks | .28* | .29* | .15 |
| 32 weeks | .34** | .33** | .27* |
| 36 weeks | .30** | .27* | .33** |
| 38 weeksa | .30* | .33* | .07 |
| Growth trajectory at 20 – 28 weeks | .39*** | .37*** | .27* |
| Growth trajectory at 28 – 38 weeks | .34** | .37*** | .25* |
Note. MDI = Mental Development Index; PDI = Psychomotor Development Index.
Based on n = 54 for MDI and PDI and n = 43 for language.
p < .10.
p < .05.
p < .01.
p < .001. Significance values based on two-tailed tests.
To incorporate both fetal heart rate and variability into a single analysis, categorical variables were constructed based on median splits of the averaged scores across gestation for each measure. Scores were distributed into low and high categories at the closest percentile to the 50th, which included the next integer for fetal heart rate (i.e., 144 beats per minute [bpm]; 52.4 percentile) and next decimal place for variability (i.e., 4.7; 47.2 percentile). A composite measure yielded the following heart rate categories: slow and stable, slow and variable, fast and stable, and fast and variable. One-way analysis of variance conducted with MDI, PDI, and language scores revealed no overall association with PDI scores but significant effects with MDI, F(3, 72) = 4.62, p < .01, and the language composite, F(3, 53) = 2.99, p < .05. The values were .16 and .14 for the MDI and language results, respectively, both indicating large effect sizes (Cohen, 1988). Results are presented in Figures 1 and 2. Slow and variable fetal heart rate was associated with the highest MDI and language scores, and fast and stable was associated with the lowest. For MDI scores, Tukey HSD contrasts indicate significant differences between the slow and variable group from both the slow and the stable group, 95% confidence interval for mean difference = 1.73 – 20.04, p < .05, and the fast and stable group, 95% confidence interval for mean difference = 1.90 – 22.13, p < .05. For language, only the contrast between the slow and variable and the fast and stable groups reached significance, 95% confidence interval for mean difference = 0.08 – 1.61, p < .05.
Figure 1.
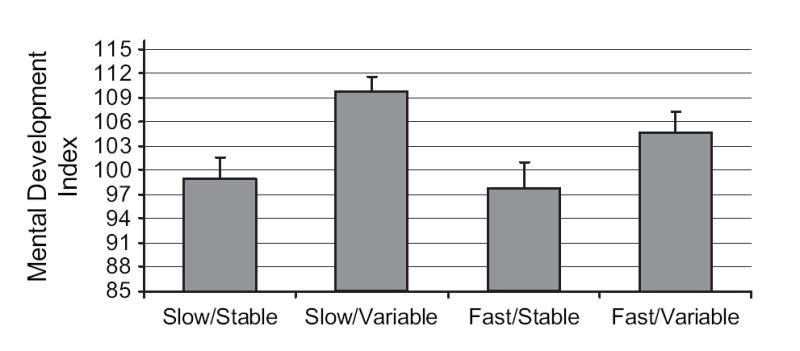
Mental Development Index scores at 24 months distributed by fetal heart rate/variability categories, F(3, 72) = 4.62, p < .01. Tukey HSD post hoc contrasts indicated significant differences between the slow and variable group and the slow and stable and fast and stable groups, ps < .05. The ns per group = 22, 20, 15, and 19, respectively, based on order of presentation.
Figure 2.
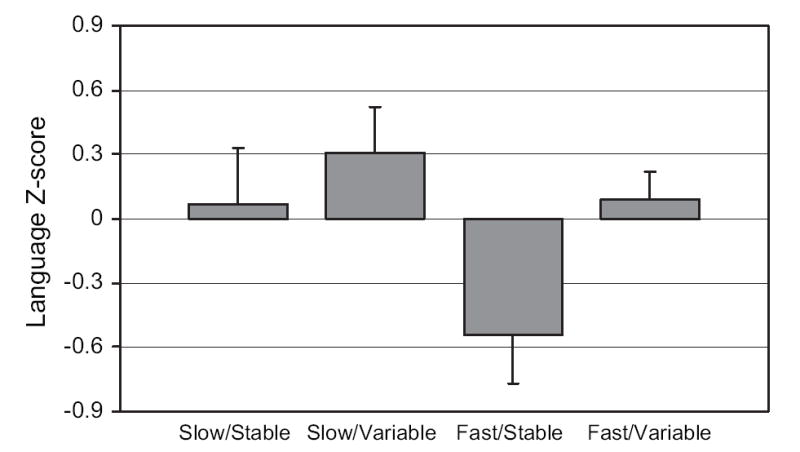
Language composite Z-score at 30 months distributed by fetal heart rate/variability categories, F(3, 53) = 2.99, p <.05. Tukey HSD contrasts indicated a significant difference between the slow and variable and the fast and stable groups, p < .05. The ns per group = 12, 17, 13, and 15, respectively.
Nonparametric Display of Associations at 36 Weeks
Gestational age – specific correlation coefficients (e.g., Table 3) or use of mean values over gestation (e.g., Figures 1 and 2) fail to take full advantage of the longitudinal data generated in this study. Nonparametric noise reduction techniques capitalize on longitudinal data using prior and subsequent data for individual fetuses to model data that may be subject to influence by uncontrollable situational factors at any given gestational age point. Prominent among these for late gestation fetuses, as with infants, is the role of behavioral state in influencing cardiac patterns during the window of observation. Thus, we examined these associations using a nonparametric method to plot the relation between the dependent and the independent measures. Although the magnitude of the significant Pearson correlation coefficients varied somewhat from 28 weeks and beyond, the 36-week period was selected for this analysis because it reflects the most advanced period of fetal maturity just prior to term and is the gestational age commonly selected for many cross-sectional fetal neurobehavioral studies.
Figure 3 presents scatter plots between the modeled 36-week fetal heart rate variability and (a) the MDI, (b) PDI, and (c) language composite. To allow application of the full longitudinal model, only those fetuses that completed the 38-week visit are included in these figures. The dotted line represents the fitted cubic smoothing spline with 3 df. To assess the strength of the relation suggested by the smoothing spline, the estimated 95% coverage region of the joint distribution of outcome and predictor is presented by the solid line surrounding most of the data points. Assuming that the data set is representative of the general population, these boundaries indicate that there is a 95% likelihood that a fetus born at or after 38 weeks of gestation will fall within the area enclosed by the ovoid-like regions. Figure 3a indicates that, consistent with Table 3, higher values of fetal heart rate variability at 36 weeks are associated with higher MDI values. However, the relation is not perfectly linear, such that the association is stronger (i.e., steeper slope) for values of variability above 6 bpm. Positive, and somewhat more linear, associations are also indicated for both PDI and language scores. Inspection of these plots further reveals that the correlation findings are not attributable to the influence of outliers.
Figure 3.
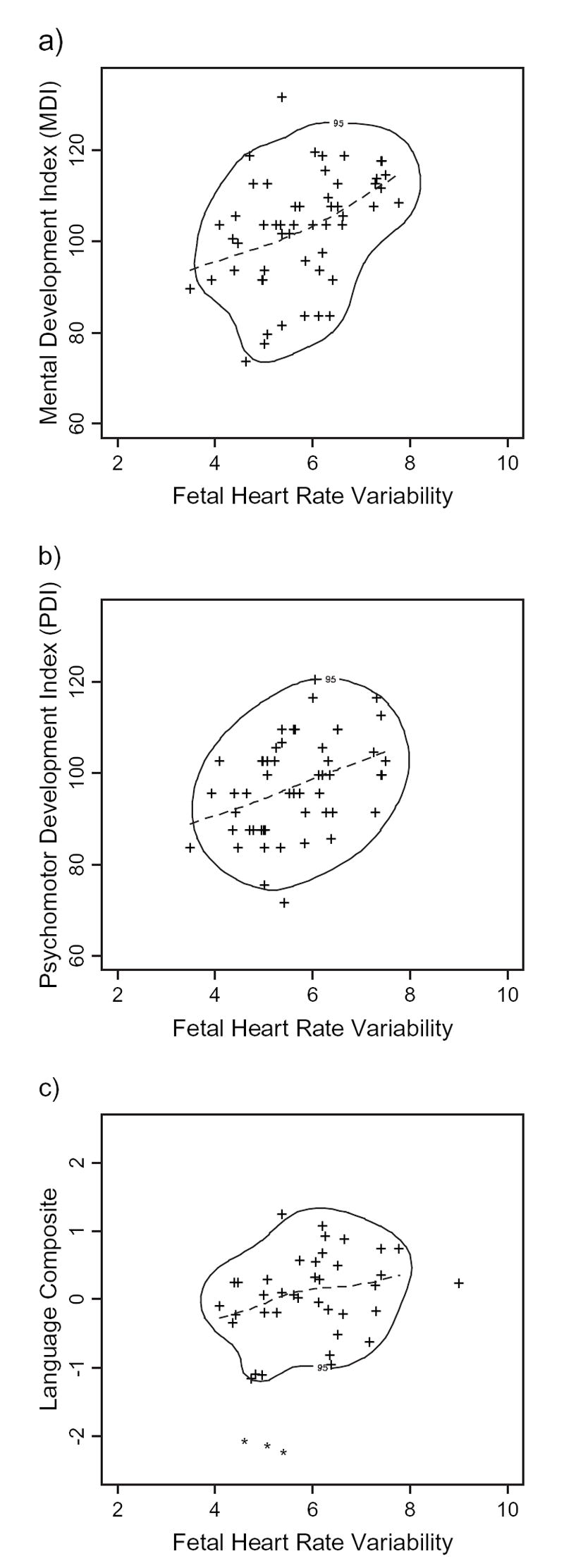
Nonparametric modeling of associations between fetal heart rate variability at 36 weeks gestation and child (a) Mental Development Index, (b) Psychomotor Development Index, and (c) language composite scores. Individual data points represent the estimated values generated by the fitted cubic smoothing spline across each individual fetal trajectory from 20 through 38 weeks gestation. Dotted lines represent fitted cubic smoothing spline, with 3 df for the sample as a group. The solid line surrounding most data points is the estimated 95% coverage region of the joint distribution of fetal predictor and child outcomes.
Fetal Longitudinal Trajectory Associations With Developmental Outcomes
Confirmational HLM analyses on the subsets of postnatal participants in this study affirmed that the 20- to 28-week fetal heart rate variability slopes were significantly different from the 28- to 38-week slopes and that there were no significant discontinuities in slope during gestation for fetal heart rate. Fetal heart rate growth rate from 20 to 38 weeks was not associated with MDI, PDI, or language scores. However, both early and late growth rates for fetal heart rate variability were significantly associated with each developmental measure. These correlation coefficients, included in Table 3, indicate that steeper slopes during each period were associated with better developmental outcomes. There was little variation within individual growth trajectories from 20 to 28 weeks and from 28 to 38 weeks, r(135) = .91; thus, individuals who displayed faster or slower than average growth rate of fetal heart rate variability in the initial period almost always continued to do so after the point of discontinuity.
Discussion
These results support the premise that individual differences in spontaneous heart rate and variability are established prior to birth, show stability from the prenatal to the postnatal period, and provide prediction to developmental outcomes in early childhood. As such, cardiac measures serve as indicators of individuality in developing neural control during gestation and have prognostic value for postnatal functioning in young children.
Within-individual stability in heart rate, variability, and/or respiratory sinus arrhythmia had been previously demonstrated in a small sample of fetuses (DiPietro et al., 1996), preterm infants prior to term (DiPietro, Caughy, Cusson, & Fox, 1994), and full-term infants during infancy and early childhood (Bar-Haim, Marshall, & Fox, 2000; Bornstein & Suess, 2000a; Fox, 1989; Izard et al., 1991; Snidman et al., 1995). The current results establish that the origins of this stability reach back to at least the 20th gestational week. Detection of significant prenatal to postnatal associations of both rate and variability confirms an earlier report on a small sample with fewer and later fetal data points (DiPietro et al., 2000) and extends that finding from 12 to 24 months postnatal age. Given the less precise method of heart rate detection necessary for the fetus (i.e., Doppler-based detection of fetal heart motions) compared to the child (i.e., ECG), it is possible that actual prenatal to postnatal stability in rate and variability may be larger than detected.
Variability in fetal heart rate was significantly and positively associated with child developmental performance during the third year of life. Significant positive associations between fetal heart rate variability and developmental outcomes began at 28 weeks gestation for the developmental assessment scores and at 32 weeks for the language composite. The latter finding replicates that of a preliminary report on a smaller sample (Bornstein et al., 2002). In addition, nonparametric modeling techniques, which provided a portrait of the relation between fetal heart rate variability and developmental outcomes, confirmed the cross-sectional correlational results. The observed emergence and stabilization of significant correlations between heart rate variability and outcomes after 28 weeks gestation is coincident with the gestational point of discontinuity in development of fetal heart rate variability. A number of other aspects of fetal neurobehavior also transition during the period between 28 and 32 weeks (DiPietro et al., 2001; Groome, Gotlieb, Neely, & Waters, 1993; Kisilevsky, Muir, & Low, 1992; Kozuma, Nemoto, Okai, & Mizuno, 1991; Pillai & James, 1990), suggesting a consistent ontogenic shift coincident with a period of rapid increase in neural development and myelination, including cortical and vagal processes (Kinney, Karthigasan, Borenshteyn, Flax, & Kirschner, 1994; Sachis, Armstrong, Becker, & Bryan, 1982; Yoshizato et al., 1994). Such observations suggest a period of consolidation of neural function and highlight the importance of gestational age selection in studies that seek to establish associations between fetal function and infant outcomes.
Spontaneous fetal heart rate was not correlated with any developmental outcome, although the combination of rate and variability yielded a significant pattern of results. Fetuses exhibiting slower and more variable heart rates had significantly higher MDI scores, although the contrasts were significantly different only from the two lower variability categories. However, fetuses with slow and variable heart rates had significantly better language development than those with faster and more stable heart rates. The results indicate that a pattern of relatively faster heart rate coupled with lower variability may signify less optimal autonomic regulation prior to birth.
The significant predictive associations between fetal heart rate variability and child outcomes reported here are of similar magnitude to those detected in cross-sectional postnatal studies, despite the time elapse between measurement as well as the considerably different circumstances under which fetal and child data were collected. Examples of previously demonstrated concurrent associations include newborn vagal tone and neonatal attentional orientation (r = .32) (Feldman, 2006) and habituation efficiency at 2 and 5 months (rs = −.26 and −.28) (Bornstein & Suess, 2000b), heart rate variability and MDI scores at 1 year (r = .31) (Richards, 1989), and respiratory sinus arrhythmia and standardized cognitive test scores (r = .31) during middle childhood (El-Sheikh & Buckhalt, 2005). Unexpectedly, we failed to detect significant concurrent associations and have no ready explanation for this, other than the observation that most studies reporting concurrent associations rely on performance measures that assess information-processing capabilities rather than more global measures of developmental status.
The observed trajectories of development during both the earlier (20 – 28 weeks) and the later (28 – 38 weeks) gestational periods were also significantly and positively associated with outcomes. The developmental outcomes used in the current study may be best characterized as indicators of the rate at which children attained a series of developmental milestones in relation to their peers and, as such, reflect the trajectory of postnatal development. These findings may be interpreted as evidence for conservation of developmental rate from the prenatal to the postnatal period. The current results echo those reported on preterm infants who were serially monitored after birth during an interval corresponding roughly to the second fetal trajectory (i.e., 32 – 37 weeks postconceptional age). In that study, significant associations were reported between vagal tone trajectory and mental processing scores on a standardized scale (r =.38) and gross motor development (r = .37) 3 years later (Doussard-Roosevelt et al., 1997). These associations are comparable in magnitude to those for fetal heart rate variability trajectories and MDI/PDI scores in this study, thereby underscoring the value of examining developmental trajectories in conjunction with age-specific levels.
The current findings were generated from a sample of relatively mature, well-educated women with healthy pregnancies. As such, replication on a more diverse spectrum of participants is necessary. However, limiting the sample in this way excluded potential confounders associated with medical or socioeconomic risk factors that may have independently affected both cardiac patterns and developmental outcomes. Heart rate patterning has been associated with a broad band of developmental acquisitions and temperamental characteristics in the postnatal period. The results of this study confirm the emergence of individual differences during gestation that portend developmental functioning after birth, both within and across domain. Such longitudinal associations may be the result of perpetuation of developmental momentum from the prenatal to postnatal period as well as continuity in one or more aspects of autonomic regulation. Opening a window on the developing fetus illuminates the contribution and continuity of the period before birth in the development of the child.
Acknowledgments
The prenatal portion of this research was supported by R01 HD 27592, NICHD, awarded to the first author. The postnatal components were supported by R40 MC00181, Maternal and Child Health Bureau, HRSA, and the Intramural Research Program of the NIH, NICHD. As always, we thank the generous and diligent participation of our study families, without which this work would not be possible.
Contributor Information
Janet A. DiPietro, Johns Hopkins University
Marc H. Bornstein, National Institutes of Health
Chun-Shin Hahn, National Institutes of Health.
Kathleen Costigan, Johns Hopkins Medical Institutions.
Aristide Achy-Brou, Johns Hopkins University.
References
- Als H. Toward a synactive theory of development: Promise for the assessment and support of infant individuality. Infant Mental Health Journal. 1982;3:229–243. [Google Scholar]
- Bar-Haim Y, Marshall PJ, Fox NA. Developmental changes in heart period and high frequency heart period variability from 4 months to 4 years of age. Developmental Psychobiology. 2000;37:44–56. doi: 10.1002/1098-2302(200007)37:1<44::aid-dev6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Barker DJP. Adult consequences of fetal growth restriction. Clinical Obstetrics and Gynecology. 2006;49:270–283. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley scales of infant development. 2. San Antonio TX: Harcourt Assessment; 1993. [Google Scholar]
- Bernston G, Bigger J, Eckberg D, Grossman P, Kaufmann P, Malik M, et al. Heart rate variability: Origins, methods, and interpretative caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, DiPietro JA, Hahn CS, Painter K, Haynes OM, Costigan KA. Prenatal cardiac function and postnatal cognitive development: An exploratory study. Infancy. 2002;3:475–494. [Google Scholar]
- Bornstein MH, Suess PE. Child and mother cardiac vagal tone: Continuity, stability, and concordance across the first 5 years. Developmental Psychology. 2000a;36:54–65. [PubMed] [Google Scholar]
- Bornstein MH, Suess PE. Physiological self-regulation and information processing in infancy: Cardiac vagal tone and habituation. Child Development. 2000b;71:273–287. doi: 10.1111/1467-8624.00143. [DOI] [PubMed] [Google Scholar]
- Bowman AW, Azzalini A. Applied smoothing techniques for data analysis: The kernel approach with s-plus illustration. Oxford, England: Oxford University Press; 1997. [Google Scholar]
- Calkins S, Dedmon S. Physiological and behavioral regulation in two-year-old children with aggressive/destructive behavior problems. Journal of Abnormal Child Psychology. 2000;28:103–118. doi: 10.1023/a:1005112912906. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Comparetti AM. The neurophysiologic and clinical implications of studies on fetal motor behavior. Seminars in Perinatology. 1981;5:183–189. [PubMed] [Google Scholar]
- Dalton K, Dawes GS, Patrick JE. The autonomic nervous system and fetal heart rate variability. American Journal of Obstetrics and Gynecology. 1983;146:456–462. doi: 10.1016/0002-9378(83)90828-1. [DOI] [PubMed] [Google Scholar]
- Dawes GS. The central nervous control of fetal behaviour. European Journal of Obstetrics Gynecology and Reproductive Biology. 1986;21:341–346. doi: 10.1016/0028-2243(86)90013-4. [DOI] [PubMed] [Google Scholar]
- Dawes GS, Moulden M, Sheil O, Redman CWG. Approximate entropy, a statistic of regularity, applied to fetal heart rate data before and during labor. Obstetrics and Gynecology. 1992;80:763–768. [PubMed] [Google Scholar]
- DiPietro JA, Caughy M, Cusson R, Fox NA. Cardiorespiratory functioning of preterm infants: Stability and risk associations for measures of heart rate variability and oxygen saturation. Developmental Psychobiology. 1994;27:137–152. doi: 10.1002/dev.420270302. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Caulfield LE, Costigan KA, Merialdi M, Nguyen RHN, Zavaleta N, et al. Fetal neurobehavioral development: A tale of two cities. Developmental Psychology. 2004;40:445–456. doi: 10.1037/0012-1649.40.3.445. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Costigan KA, Pressman EK, Doussard-Roosevelt J. Antenatal origins of individual differences in heart rate. Developmental Psychobiology. 2000;37:221–228. doi: 10.1002/1098-2302(2000)37:4<221::aid-dev2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Hodgson DM, Costigan KA, Johnson TRB. Fetal antecedents of infant temperament. Child Development. 1996;67:2568–2583. [PubMed] [Google Scholar]
- DiPietro JA, Irizarry RA, Hawkins M, Costigan KA, Pressman EK. Cross-correlation of fetal cardiac and somatic activity as an indicator of antenatal neural development. American Journal of Obstetrics and Gynecology. 2001;185:1421–1428. doi: 10.1067/mob.2001.119108. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Porges SW, Uhly B. Reactivity and developmental competence in preterm and full-term infants. Developmental Psychology. 1992;28:831–841. [Google Scholar]
- Doussard-Roosevelt JA, McClenny B, Porges SW. Neonatal cardiac vagal tone and school-age developmental outcome in very low birth weight infants. Developmental Psychobiology. 2001;38:56–66. [PubMed] [Google Scholar]
- Doussard-Roosevelt JA, Porges SW, Scanlon JW, Alemi B, Scanlon KB. Vagal regulation of heart rate in the prediction of developmental outcome for very low birth weight preterm infants. Child Development. 1997;68:173–186. [PubMed] [Google Scholar]
- Dunn LM, Dunn L. Peabody Picture Vocabulary Test—Revised manual. Circle Pines, MN: American Guidance Service; 1981. [Google Scholar]
- Dunster KR. Physiologic variability in the perinatal period. Clinics in Perinatology. 1999;26:801–809. [PubMed] [Google Scholar]
- El-Sheikh M, Buckhalt JA. Vagal regulation and emotional intensity predict children’s sleep problems. Developmental Psychobiology. 2005;46:307–317. doi: 10.1002/dev.20066. [DOI] [PubMed] [Google Scholar]
- Feldman R. From biological rhythms to social rhythms: Physiological precursors of mother-infant synchrony. Developmental Psychology. 2006;42:175–188. doi: 10.1037/0012-1649.42.1.175. [DOI] [PubMed] [Google Scholar]
- Fenson L, Dale P, Reznick J, Thal D, Bates E, Harters J, et al. The Macarthur Communication Development Inventories: User’s guide and technical manual. San Diego, CA: Singular Publishing Group; 1993. [Google Scholar]
- Fleisher LA, DiPietro JA, Johnson TRB, Pincus S. Complementary and non-coincident increases in heart rate variability and irregularity during fetal development. Clinical Science. 1997;92:345–349. doi: 10.1042/cs0920345. [DOI] [PubMed] [Google Scholar]
- Fox NA. Psychophysiological correlates of emotional reactivity during the first year of life. Developmental Psychology. 1989;25:364–372. [Google Scholar]
- Freeman RK, Garite TJ, Nageotte MP. Physiologic basis of fetal monitoring. In: Freeman RK, Garite TJ, Nageotte MP, editors. Fetal heart rate monitoring. 2. Baltimore: Williams & Wilkins.; 1991. pp. 7–20. [Google Scholar]
- Groome LJ, Gotlieb SJ, Neely CL, Waters MD. Developmental trends in fetal habituation to vibroacoustic stimulation. American Journal of Perinatology. 1993;10:46–49. doi: 10.1055/s-2007-994700. [DOI] [PubMed] [Google Scholar]
- Hastie T, Tibshirani R. Generalized additive models. London: Chapman & Hall/CRC; 1990. [DOI] [PubMed] [Google Scholar]
- Hepper PG. Fetal behavior and neural functioning. In: Lecanuet JP, Fifer WP, Krasnegor NA, Smotherman WP, editors. Fetal development: A psychobiological perspective. Hillsdale, NJ: Lawrence Erlbaum Associates; 1995. pp. 405–417. [Google Scholar]
- Izard C, Porges SW, Simons R, Haynes O, Hyde C, Parisi M, et al. Infant cardiac activity: Developmental change and relations with attachment. Developmental Psychology. 1991;27:432–439. [Google Scholar]
- James D, Pillai M, Smoleniec J. Neurobehavioral development in the human fetus. In: Lecanuet JP, Fifer WP, Krasnegor NA, Smotherman WP, editors. Fetal development: A psychobiological perspective. Hillsdale, NJ: Lawrence Erlbaum Associates; 1995. pp. 101–128. [Google Scholar]
- Jansson LM, DiPietro JA, Elko A. Fetal response to maternal methadone administration. American Journal of Obstetrics and Gynecology. 2005;193:611–617. doi: 10.1016/j.ajog.2005.02.075. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Karthigasan J, Borenshteyn N, Flax J, Kirschner D. Myelination in the developing brain: Biochemical correlates. Neurochemical Research. 1994;19:983–996. doi: 10.1007/BF00968708. [DOI] [PubMed] [Google Scholar]
- Kisilevsky BS, Muir DW, Low JA. Maturation of human fetal responses to vibroacoustic stimulation. Child Development. 1992;63:1497–1508. [PubMed] [Google Scholar]
- Kozuma S, Nemoto A, Okai T, Mizuno M. Maturational sequence of fetal breathing movements. Biology of the Neonate. 1991;60:36–40. doi: 10.1159/000251015. [DOI] [PubMed] [Google Scholar]
- Lewis M, Wilson C, Ban P, Baumel M. An exploratory study of resting cardiac rate and variability from the last trimester of prenatal life through the first year of postnatal life. Child Development. 1970;41:799–811. [Google Scholar]
- Maeda K, Morokuma S, Yoshida S, Ito T, Pooh R, Serizawa M. Fetal behavior analyzed by ultrasonic actocardiogram in cases with central nervous system lesions. Journal of Perinatal Medicine. 2006;34:398–403. doi: 10.1515/JPM.2006.079. [DOI] [PubMed] [Google Scholar]
- Nicolaides KH, Sadovsky G, Visser GHA. Heart rate patterns in normoxemic, hypoxemic, and anemic second-trimester fetuses. American Journal of Obstetrics and Gynecology. 1989;160:1034–1037. doi: 10.1016/0002-9378(89)90154-3. [DOI] [PubMed] [Google Scholar]
- Nijhuis IJM, ten Hof J. Development of fetal heart rate and behavior: Indirect measures to assess the fetal nervous system. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 1999;87:1–2. doi: 10.1016/s0301-2115(99)00143-8. [DOI] [PubMed] [Google Scholar]
- Nijhuis IJM, ten Hof J, Mulder EJ, Nijhuis JG, Narayan H, Taylor D, et al. Numerical fetal heart rate analysis: Nomograms, minimal duration of recording, and intrafetal consistency. Prenatal and Neonatal Medicine. 1998;3:314–322. [Google Scholar]
- Nijhuis IJM, ten Hof J, Mulder EJ, Nijhuis JG, Narayan H, Taylor D, et al. Fetal heart rate in relation to its variation in normal and growth retarded fetuses. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2000;89:27–33. doi: 10.1016/s0301-2115(99)00162-1. [DOI] [PubMed] [Google Scholar]
- O’Brien P, Wheeler T, Barker D, editors. Fetal programming: Influences on development and disease in later life. London: RCOG Press; 1999. [Google Scholar]
- Pillai M, James D. Hiccups and breathing in human fetuses. Archives of Disease in Childhood. 1990;65:1072–1075. doi: 10.1136/adc.65.10_spec_no.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. Method and apparatus for evaluating rhythmic oscillations in aperiodic physiological response systems. Washington, DC: US Patent Office; 1985. [Google Scholar]
- Prechtl HFR. Continuity and change in early neural development. In: Prechtl H, editor. Continuity in neural functions from prenatal to postnatal life. Philadelphia: J.B. Lippincott; 1984. pp. 1–15. Clinics in Developmental Medicine No 94. [Google Scholar]
- Raudenbush SW, Byrk AS, Congdon R. HLM5: Hierarchical linear and non-linear modeling. Lincolnwood, IL: Scientific Software International; 2000. [Google Scholar]
- Reynell J, Gruber C. Developmental Language Scales U.S. Edition. Los Angeles: Western Psychological Services; 1990. [Google Scholar]
- Richards JE. Development and stability in visual sustained attention in 14, 20, and 26 week infants. Psychophysiology. 1989;26:422–430. doi: 10.1111/j.1469-8986.1989.tb01944.x. [DOI] [PubMed] [Google Scholar]
- Richards JE, Cameron D. Infant heart rate variability and behavioral developmental status. Infant Behavior and Development. 1989;12:42–58. [Google Scholar]
- Sachis PN, Armstrong D, Becker L, Bryan A. Myelination of the human vagus nerve from 24 weeks postconceptional age to adolescence. Journal of Neuropathology and Experimental Neurology. 1982;41:466–472. doi: 10.1097/00005072-198207000-00009. [DOI] [PubMed] [Google Scholar]
- Snidman N, Kagan J, Riordan L, Shannon D. Cardiac function and behavioral reactivity during infancy. Psychophysiology. 1995;32:199–207. doi: 10.1111/j.1469-8986.1995.tb02949.x. [DOI] [PubMed] [Google Scholar]
- Snijders RJM, Ribbert LSM, Visser GHA, Mulder EJH. Numeric analysis of heart rate variation in intrauterine growth-retarded fetuses. American Journal of Obstetrics and Gynecology. 1992;166:22–27. doi: 10.1016/0002-9378(92)91821-q. [DOI] [PubMed] [Google Scholar]
- Sparrow S, Balla D, Cicchetti D. Vineland Adaptive Behavior Scales—Interview Edition, Survey Form. Circle Pine, MN: American Guidance Service; 1984. [Google Scholar]
- Thomas PW, Haslum MN, MacGillivray I, Golding MJ. Does fetal heart rate predict subsequent heart rate in childhood? Early Human Development. 1989;19:147–152. doi: 10.1016/0378-3782(89)90125-4. [DOI] [PubMed] [Google Scholar]
- van Leeuwen P, Lange S, Bettermann H, Gronemeyer D, Hatzmann W. Fetal heart rate variability and complexity in the course of pregnancy. Early Human Development. 1999;54:259–269. doi: 10.1016/s0378-3782(98)00102-9. [DOI] [PubMed] [Google Scholar]
- Visser GHA. Fetal behaviour: A commentary. Neurobiology of Aging. 2004;24:S47–S49. [Google Scholar]
- Ware DJ, Devoe LD. The nonstress test: Reassessment of the “gold standard”. Clinics in Perinatology. 1994;21:779–796. [PubMed] [Google Scholar]
- Yoshizato T, Koyanagi T, Takashima T, Satoh S, Akazawa K, Nakano H. The relationship between age-related heart rate changes and developing brain function: A model of anencephalic human fetuses in utero. Early Human Development. 1994;36:101–112. doi: 10.1016/0378-3782(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Young J. Programming of sympathoadrenal function. Trends in Endocrinology and Metabolism. 2002;13:381–385. doi: 10.1016/s1043-2760(02)00661-6. [DOI] [PubMed] [Google Scholar]


