Abstract
Vaccinia virus (VV) produces two antigenically and structurally distinct infectious virions, intracellular mature virus (IMV) and extracellular enveloped virus (EEV). Here we have investigated the resistance of EEV and IMV to neutralization by complement in the absence of immune antibodies. When EEV is challenged with complement from the same species as the cells used to grow the virus, EEV is resistant to neutralization by complement, whereas IMV is not. EEV resistance was not a result of EEV protein B5R, despite its similarity to proteins of the regulators of complement activation (RCA) family, or to any of the other EEV proteins tested (A34R, A36R, and A56R gene products). EEV was sensitive to complement when the virus was grown in one species and challenged with complement from a different species, suggesting that complement resistance might be mediated by host RCA incorporated into the EEV outer envelope. This hypothesis was confirmed by several observations: (i) immunoblot analysis revealed that cellular membrane proteins CD46, CD55, CD59, CD71, CD81, and major histocompatibility complex class I antigen were detected in purified EEV but not IMV; (ii) immunoelectron microscopy revealed cellular RCA on the surface of EEV retained on the cell surface; and (iii) EEV derived from rat cells expressing the human RCA CD55 or CD55 and CD59 were more resistant to human complement than EEV derived from control rat cells that expressed neither CD55 nor CD59. These data justify further analysis of the roles of these (and possible other) cellular proteins in EEV biology.
Complement is part of the innate immune system and is activated in a cascade manner through two main pathways known as classical and alternative. For review on the complement system and regulators of complement activation (RCA) see refs. 1 and 2. The classical pathway is activated by the recognition proteins C1q or mannose-binding lectin, which bind respectively to charge clusters or neutral sugars on targets. In contrast, activation of the alternative pathway is a default process that proceeds unless down-regulated by specific mechanisms. Complement activation results in cleavage and activation of C3 and deposition of opsonic C3 fragments on surfaces. Subsequent cleavage of C5 leads to assembly of the membrane attack complex (C5b, 6, 7, 8, 9), which disrupts lipid bilayers.
Complement activation on host cells is prevented by several membrane RCA, the activity of which is restricted predominantly to complement of the same species, a phenomenon called homologous restriction. These proteins down-regulate complement activity at two steps in the classical and the alternative pathways: complement receptor 1 (CD35) and decay-accelerating factor (CD55) inhibit the formation and accelerate the decay of the classical pathway and alternative pathway C3-activating enzymes (C3 convertases); complement receptor 1 and membrane cofactor protein (CD46) act as cofactors for Factor I (a serum protease), which catabolizes C4b and C3b, thereby inhibiting formation of the C3 convertases C4b2a and C3bBb; and, finally, at the end of the complement cascade, CD59 and possibly also homologous restriction factor (C8-binding protein) prevent the formation of the membrane attack complex.
In general, microorganisms lack mammalian RCA and thus cannot restrict complement deposition and amplification on their surfaces (3). However, the toxicity of the complement system has selected microorganisms that have evolved countermeasures; for reviews see refs. 4 and 5. Three main types of evasion strategies have been noted for enveloped viruses. Some viruses encode structural proteins that mimic the function of cellular RCA; for example, glycoprotein C of herpes simplex virus type 1 induces the dissociation of the alternative pathway C3 convertase (6). Other viruses, for instance, HIV, incorporate host RCA into their envelope by budding through the plasma membrane, and these protect the virion against host complement (7). Last, other viruses secrete a soluble protein that blocks complement activation; for instance, vaccinia virus (VV)-infected cells secrete an abundant 35-kDa soluble protein called VV complement control protein (VCP) (8). VCP shares amino acid similarity with mammalian RCA, which include CD46, CD55, CD35, factor H, and C4-binding protein, and like these proteins, VCP restricts complement activation (for review see ref. 9).
VV, the prototype of the poxvirus family, produces two morphologically distinct infectious forms of virions, termed intracellular mature virus (IMV) and extracellular enveloped virus (EEV) (10, 11). IMV represents the majority of infectious progeny and remains within the cytoplasm until cell lysis. However, a fraction of IMV acquires a double membrane derived from the trans-Golgi network (12) or early tubular endosomes (13) to form intracellular enveloped virus (IEV). EEV is formed when the outer IEV membrane fuses with the plasma membrane. EEV may also arise by IMV budding through the plasma membrane (14). EEV is important for virus dissemination both in vitro and in vivo (10, 15–17). With most strains of VV [International Health Department-J (IHD-J) strain is an exception], much of the EEV remains attached to the cell surface and is termed cell-associated enveloped virus (CEV) (18).
At least 10 proteins are associated with the outer envelope of EEV (19, 20) and 6 VV genes are known to encode EEV membrane proteins. These are A56R, encoding the virus hemagglutinin (HA) gp86 (21, 22); F13L, encoding a 37-kDa protein (37K protein), p37 (23); A34R, encoding a triplet of glycoproteins, gp22–24 (24); B5R, encoding a 42-kDa glycoprotein, gp42 (25, 26); A36R, encoding a 45- to 50-kDa protein, p45–50 (27); and A33R, encoding a 23- to 28-kDa glycoprotein, gp23–28 (28). The B5R protein is related to the RCA protein family and contains four copies of the complement control protein module (CCP) that are typical of this family (25, 26, 29). This similarity raised the possibility that B5R might protect EEV against complement, in a similar way as cellular RCA protect cells against complement.
The presence of different proteins on the surface of IMV and EEV give these viruses different biological and immunological properties (10, 15, 30, 31) that are adapted to their different roles in VV pathogenesis. EEV and IMV bind to distinct cellular receptors (32) and penetrate cells by different mechanisms (33, 34). Moreover, EEV, in contrast to IMV, is resistant to antibody neutralization (34, 35). The EEV outer membrane is extremely fragile and is damaged by virus purification (32, 34, 35). Once the EEV outer membrane is ruptured, the particle retains infectivity as an IMV (36, 37). Consequently, fresh EEV with an intact outer envelope, rather than purified EEV with a damaged outer envelope, should be used for investigations of EEV biological properties.
In this study, the resistance of EEV and IMV to complement neutralization has been investigated. When the serum used as a source of complement and the cells used to grow the virus were from the same species, EEV is resistant to complement whereas IMV is not, and this resistance is not a result of EEV proteins B5R, A34R, A36R, and A56R. Resistance of EEV to complement is homologous-restricted, and cellular RCA are incorporated into the EEV outer membrane. These host proteins contribute to EEV complement resistance because EEV derived from rat endothelial cells expressing human CD55, or CD55 and CD59 exhibited a greater resistance to human complement than EEV grown in control cells that express neither human protein.
MATERIALS AND METHODS
Cells and Viruses.
RK13 cells were grown in minimum essential medium (GIBCO) supplemented with 10% heat-inactivated (56°C, 30 min) fetal bovine serum. HeLa cells were grown in DMEM (GIBCO) supplemented with 10% heat-inactivated fetal bovine serum. Simian virus 40-transformed aortic rat endothelial cells (SVAREC) stably transfected with the plasmid expression vector pDR2EF1 (Hygro) (control cells) or with pDR2EF1 constructs encoding human CD55 (CD55+) or human CD59 (CD59+) or both human CD55 and CD59 (CD55+/CD59+) were cultured in DMEM containing 10% heat-inactivated fetal bovine serum and hygromycin B (100 μg/ml) as described (38). VV strains IHD-J and Western Reserve (WR) and WR mutants lacking B5R (vΔB5R) (39), A36R (vΔA36R) (27), A34R (vΔA34R) (36), or A56R (vΔA56R) (G.L.S., unpublished material) were used. In addition, VV WR mutants lacking different combinations of B5R protein CCPs, previously called short consensus repeats, so that they contained CCP1 (vCCP1), CCPs 1 and 2 (vCCP1–2), or CCPs 1, 2, and 3 (vCCP1–3) linked to the other regions of the B5R protein (40), were also tested.
Virus Purification and Preparation of Fresh EEV.
IHD-J EEV and IMV were purified from HeLa cell cultures 48 h postinfection with 0.01 plaque-forming units (pfu)/cell. For EEV, the cell supernatant was clarified by centrifugation (1,000 × g, 20 min, 4°C) and the virus in the supernatant was pelleted by ultracentrifugation (35,000 × g, 90 min, 4°C). Pelleted virus was resuspended in 10 mM Tris⋅HCl, pH 9, and kept on ice until the next step of the purification. IMV was purified from dounce-homogenized, infected cell extracts from which nuclei and cell debris were removed by centrifugation (1,000 × g, 10 min, 4°C). Further steps of purification were identical for EEV and IMV. Both materials were sonicated and then sedimented (35,000 × g, 80 min, 4°C) through a sucrose cushion (36%, wt/vol, in 10 mM Tris⋅HCl, pH 9). The IMV and EEV pellets obtained were further purified by sucrose velocity sedimentation as described (41). Virus bands were collected, and the virus was recovered by centrifugation.
Fresh EEV was prepared from cells infected at 1 pfu/cell. The culture supernatant was harvested 18 h postinfection and centrifuged to remove detached cells and cell debris (1,000 × g, 20 min, 4°C). A fresh EEV preparation was produced before each experiment.
Inactivation of Infected Cell Supernatant.
The infectivity of the infected cell supernatant was removed by pelleting the virus by ultracentrifugation (35,000 × g, 90 min, 4°C) followed by treatment with 4,5′,8-trimethylpsoralen (Sigma) UV light as described (42).
Sources of Complement.
Rabbit and human complement (Sigma) were stored lyophilized at −70°C and reconstituted in cold deionized water immediately before use. All sera used were free of detectable antibodies against VV as demonstrated by immunoblotting and indirect immunofluorescent staining of fixed and permeabilized VV-infected cells.
Antibodies.
Antibodies used were murine mAbs AB1.1 (27) and 5B4/2F2 (43) raised against the D8L (α-D8L) and the A27L (α-A27L) IMV surface proteins, respectively; rabbit polyclonal serum raised against the B5R EEV surface protein (α-B5R) (25); murine mAbs J4–48, BRIC 110, MEM-43, and mAb JS64 (all from Serotec) raised against human CD46 (α-CD46), CD55 (α-CD55), CD59 (α-CD59), and CD81 (α-CD81), respectively; mAb HTR-H68.4 raised against human CD71, which was a gift of S. White (44); and mAb HCA2 (45) raised against the human major histocompatibility complex (MHC) class 1 antigen (α-MHC-I).
Complement Neutralization Assay.
The resistance of purified IMV or fresh EEV to complement neutralization (in absence of specific antibodies) was investigated as follows. Fresh EEV or sonicated purified IMV were diluted in ice-cold minimal essential medium and mixed (1:1, vol/vol) with active or heat-inactivated (56°C, 30 min) (control) serum diluted in ice-cold minimal essential medium (final dilution of serum, 1/10, 1/20, or 1/30). After incubation for 75 min at 37°C, samples were cooled on ice and mAb 5B4/2F2 was added to fresh EEV samples to neutralize any contaminating IMV and ruptured EEV. Virions were then bound to RK13 cells for 1 h on ice, complement and unbound virions were washed away, and the number of plaques were counted 2 days later (32).
Immunoblotting.
Extracts from cells or purified virions were prepared as described (27). After electrophoresis on SDS-polyacrylamide gels, proteins were transferred to nitrocellulose membranes and identified with specific antibodies and a chemiluminescent detection system (NEN) (27). The primary antibodies were used at the following dilution or concentration: mAb AB1.1 (diluted 1/2,000), α-B5R (diluted 1/2,000), mAb J4–48 (0.5 μg/ml), mAb BRIC 110 (5 μg/ml), mAb MEM-43 (0.5 μg/ml), mAb JS64 (5 μg/ml), mAb HTR-H68.4 (10 μg/ml), and mAb HCA2 (10 μg/ml).
Preembedding Immunogold Labeling and Preparation of Cells for Electron Microscopy.
Cells were fixed in 250 mM Hepes buffer, pH 7.4, containing 4% paraformaldehyde (wt/vol) for 20 min on ice and 40 min at 20°C. After washing with PBS and PBS containing 10% heat-inactivated fetal bovine serum (PBSF), cells were incubated successively with murine mAbs (at 50× higher concentration than that used for immunoblotting), rabbit IgG anti-mouse IgG (10 μg/ml in PBSF) (Cappel), and finally with protein A-gold particles as described (46). All incubations were for 1 h at 37°C and were separated by extensive washing with PBSF. Finally, after washing with PBS, the samples were fixed for 10 min in 250 mM Hepes buffer, pH 7.4, containing 4% paraformaldehyde and prepared for electron microscopy as described (35).
Quantification of CD55 and CD59 Cell Surface Expression by Indirect Immunofluorescent Staining and Flow Cytometry.
Adherent cells were lifted into suspension by incubation in PBS/5 mM EGTA for 10 min at 20°C. After washing with PBSF, 106 cells were incubated for 1 h on ice in 0.2 ml PBSF containing either mAb BRIC 110 (anti-CD55, 5 μg/ml) or MEM-43 (α-CD59, 5 μg/ml). After washing with cold PBSF, the cells were incubated further with fluorescein isothiocyanate-conjugated F(ab′)2 goat anti-mouse IgG (8 μg/ml) (Sigma) for 30 min on ice. After washing with cold PBSF, the cells were fixed in PBS containing 4% formaldehyde (vol/vol) and analyzed by flow cytometry as described (32).
Statistical Analysis.
Student’s t test was used to test for the significance of the results (P < 0.05).
RESULTS
EEV Is Resistant to Neutralization by Complement.
EEV mediates virus spread within a host and therefore is exposed to the immune system. The goal of this study was to investigate the relative resistance of IMV and EEV to complement neutralization (in absence of specific antibodies) to determine whether EEV exhibits a resistant phenotype consistent with its role in virus dissemination within the host. Because resistance of EEV likely would be conferred by its outer membrane and because this fragile structure is ruptured by virus purification (32, 34), we used fresh EEV in which this membrane is intact (see Materials and Methods for details).
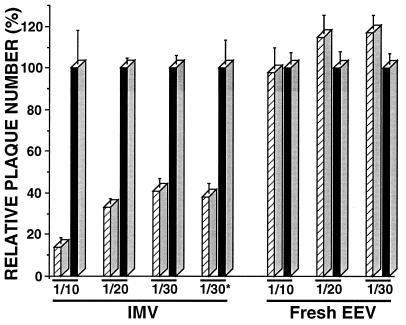
Fig. 1 shows that the infectivity of fresh EEV (diluted 10,000-fold from culture supernatant), in contrast to IMV (both derived from RK13 cells), is not affected by incubation with active complement. Fresh EEV generated a similar number of plaques after incubation with either active or heat-inactivated rabbit complement. In contrast, the infectivity of purified IMV was reduced by 59 and 86% when IMV was incubated with active complement at final serum dilution of 1/30 and 1/10, respectively (Fig. 1). The differences observed between purified IMV and fresh EEV cannot be attributed to the activity of diluted VCP in the EEV samples, because addition of inactivated cell supernatant to IMV (at concentrations 10 times higher than that present in the EEV samples) did not confer any protection (Fig. 1, compare IMV 1/30 with 1/30*). Nor is it possible that cellular complement proteins present in hypothetical vesicles in the supernatant were providing resistance to EEV in a bystander fashion. This was eliminated by two lines of evidence. First, addition of IMV-neutralizing antibody or complement reduced the infectivity of fresh EEV by the same amount (data not shown). Fresh EEV represents a mixture of IMV and EEV, and therefore under identical conditions only EEV was resistant to complement. Second, addition to IMV of infected cell supernatant prepared as described in Materials and Methods but with omission of the ultracentrifugation step, and at concentrations 10 times higher than that present in the EEV samples, did not confer any protection (data not shown).
Figure 1.
IMV and EEV sensitivity to neutralization by complement. Purified IMV and fresh EEV (final dilution of 104) were derived from RK13 cells infected with VV strain IHD-J and were assayed for their sensitivity to neutralization by rabbit complement (final serum concentrations of 1/10, 1/20, and 1/30) as described in Materials and Methods. For IMV, 1/30* represents purified IMV assayed in the presence of inactivated supernatant from VV-infected cells (final dilution of 103). The number of plaques obtained with active complement (hatched bars) are expressed as the percentage of the number of plaques obtained with heat-inactivated complement (control, solid bars). Data represent the average ± SD for triplicate measures. The average number of plaques obtained for each of the controls was about 200.
The Resistance of EEV to Complement Neutralization Is Not Conferred by the EEV Outer Membrane Protein B5R.
Because the B5R protein of the EEV outer membrane is related to members of the RCA superfamily, we investigated whether B5R mediates the resistance of EEV to complement. Fresh EEV from WR and from the three B5R mutants, vCCP1, vCCP1–2, and vCCP1–3 (40), were analyzed for their sensitivity to neutralization by complement. No differences were observed between the WR and mutants tested (data not shown); all exhibited a resistant phenotype as described for the IHD-J strain (Fig. 1), indicating that the majority of the B5R CCPs are not responsible for the phenotype observed. This conclusion was confirmed by the demonstration that the EEV produced by vΔB5R (39) also exhibited a resistant phenotype (data not shown).
In addition to B5R, there are five other known EEV-specific proteins, four of which (A33R, A34R, A36R, and A56R) are exposed on the surface of EEV. With the exception of A33R, deletion mutants of each have been described. EEV from mutants vΔA34R, vΔA36R, and vΔA56R (grown in RK13 cells) was found to be resistant to rabbit complement (data not shown), just as for IHD-J and WR.
EEV Is Resistant to Neutralization by Complement Only when Its Cellular Origin and the Challenging Complement Are from the Same Species.
The observation that none of the EEV proteins tested is required for the complement-resistant phenotype suggested that this resistance is mediated by another viral protein and/or by host proteins. If the latter hypothesis were true, and because cellular RCA generally are more active against homologous complement, one would expect EEV derived from cells of one species to be resistant to complement from the same species. To test this hypothesis, fresh EEV derived from rabbit and human cells were challenged with human complement (Fig. 2).
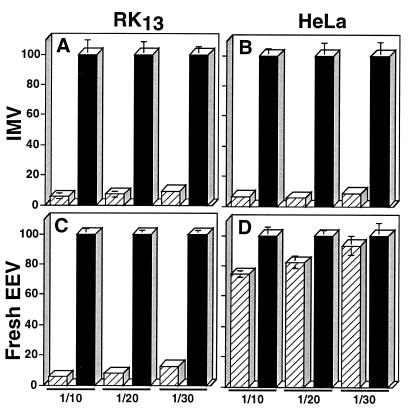
Figure 2.
Effect of host cell species on the resistance of EEV to complement toxicity. Purified IMV (IMV, A and B) and fresh EEV (final dilution of 104 for RK13-derived EEV and 2 × 103 for HeLa-derived EEV) (C and D) derived from RK13 (A and C) or from HeLa (B and D) cells infected with VV IHD-J strain, were assayed for their resistance to neutralization by human complement (final serum concentrations of 1/10, 1/20, and 1/30) as described in Materials and Methods. The number of plaques obtained with active complement (hatched bars) is expressed as percentage of the number of plaques obtained with heat-inactivated complement (control, solid bars). Data represent the average ± SD for triplicate measures. The average number of plaques for the controls was about 200.
Fig. 2 shows that EEV derived from RK13 and HeLa cells differed greatly in their sensitivity to human complement. At a 1/30 dilution of serum there was no significant effect on HeLa cell-derived EEV, but 87% of RK13 cell-derived EEV was neutralized (Fig. 2 C and D). In contrast to EEV, IMV derived from RK13 and HeLa cells exhibited similar sensitivity (Fig. 2 A and B).
Cellular Proteins Are Incorporated into EEV Outer Membrane.
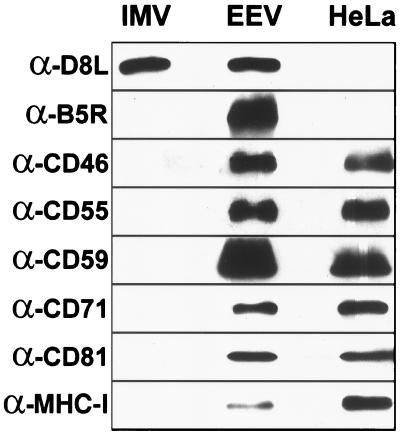
The homologous restriction of EEV resistance to complement suggested that the resistance might be mediated by cellular complement regulators incorporated into the EEV outer membrane. To test this hypothesis, purified IMV and EEV were analyzed by immunoblotting alongside a lysate from uninfected HeLa cells. Blots were probed with antibodies raised against six different surface antigens expressed by HeLa cells: CD71, CD81, MHC class I, and three complement regulators—CD46, CD55, and CD59 (Fig. 3).
Figure 3.
Detection of cellular proteins in VV virions by immunoblotting. Purified IMV (3 μg/lane), purified EEV (3 μg/lane), and mock-infected HeLa cell (HeLa) (1.2 × 104 cells per lane) extracts were immunoblotted as described in Materials and Methods.
All six host proteins were detected in the HeLa cell lysate and EEV samples, but not in IMV (Fig. 3). The surprising presence of host proteins in the EEV preparation is unlikely to be the result of cellular contamination, because IMV and EEV were purified concurrently by using an identical procedure. Moreover, because IMV was purified from lysed cells rather then clarified cell supernatant, it was much more likely to be contaminated with cellular membranes than EEV. To show that equal amounts of IMV and EEV had been loaded, the blots were reprobed with mAb α-D8L (to an antigen common to both forms of the virus) (Fig. 3, α-D8L). Probing with an EEV-specific antibody, α-B5R, confirmed that the IMV sample was not contaminated with EEV (Fig. 3, α-B5R).
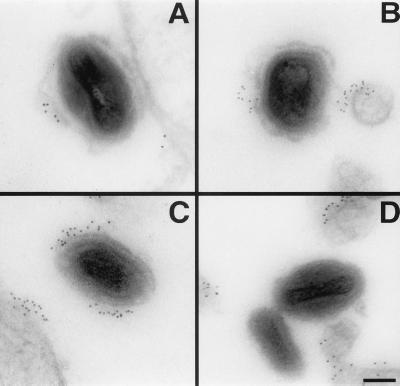
The presence of host complement regulators in the EEV outer envelope was confirmed by an independent approach. HeLa cells were infected with WR (which forms large amounts of CEV) and 14-h postinfection treated for preembedding immunogold staining with antibodies against CD46, CD55, and CD59, the three complement regulators known to be expressed by HeLa cells (CD35 is not expressed by HeLa cells, data not shown, and homologous restriction factor was not tested). The three antibodies generated a patchy staining of the plasma membrane as well as staining the surface of CEV (Fig. 4). The strongest staining was observed with mAb α-CD59 (Fig. 4C), but CD46 and CD55 also were clearly positive (Fig. 4 A and B). Only a single representative virion is shown, but all CEVs were positive for these human proteins. Nonuniform distribution of CD55 has been described previously and was artifactual because of the fixation and staining conditions (47). The outer CEV membrane appeared intact only rarely (Fig. 4C) and was mostly ruptured and partially detached from the particle (Fig. 4 A and B). The specificity of the staining of the three antibodies was controlled by staining cells infected on ice with purified IMV. None of the antibodies stained IMV particles (Fig. 4D and data not shown); for example, Fig. 4D illustrates the staining with mAb α-CD59. Confocal microscopy confirmed that all CEV particles stained with antibodies to human complement proteins and VV antigens (data not shown).
Figure 4.
Detection of CD46 (A), CD55 (B), and CD59 (C and D) on VV virion surface by immunogold labeling. HeLa cells infected with WR (5 pfu/cell) for 14 h (A–C) or HeLa cells inoculated on ice with purified WR IMV (20 pfu/cell) (D) were fixed and treated as described in Materials and Methods for preembedding immunogold labeling. (Bar = 100 nm.)
The Incorporation of Cellular Proteins into the EEV Outer Envelope Confers Protection Against Neutralization by Complement.
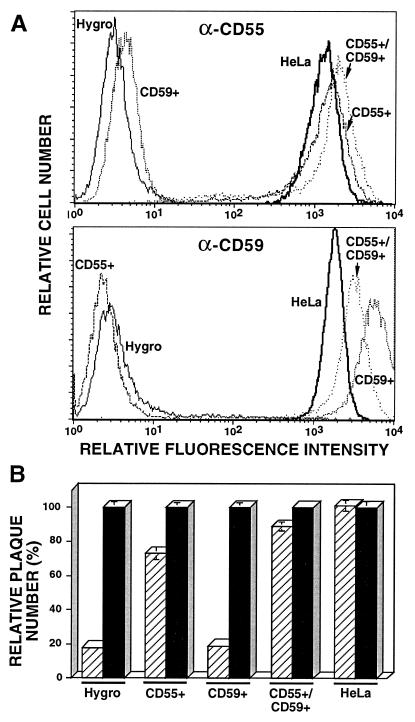
Figs. 3 and 4 showed that cellular complement regulators are incorporated into the EEV outer membrane. To determine whether these proteins contributed to the resistance of EEV to complement, the resistance of EEV derived from SVAREC expressing no human protein (Hygro) or human CD55 and/or CD59 to human complement was investigated. The level of expression of human CD55 and/or CD59 by SVAREC was analyzed and found to be similar to or higher than the level of expression by HeLa cells (Fig. 5A), indicating the suitability of these cells for the proposed experiment.
Figure 5.
(A) Surface expression of CD55 and CD59 by SVAREC. Hygro (negative control), CD55+, CD59+, CD55+/CD59+ SVAREC, and HeLa cells were analyzed for expression of CD55 (α-CD55) or CD59 (α-CD59) by fluorescence-activated cell sorter analysis as described in Materials and Methods. (B) Resistance of EEV derived from SVAREC to human complement. Fresh EEV (final dilution of 5 × 103) derived from Hygro, CD55+, CD59+, CD55+/CD59+ SVAREC, or HeLa cells infected with IHD-J were assayed for their resistance to human complement (final serum concentration of 1/30) neutralization as described in Materials and Methods. The number of plaques obtained with active complement (hatched bars) is expressed as the percentage of the number of plaques obtained with heat-inactivated complement (control, solid bars). Data represent the average ± SD for triplicate measures. The average number of plaques for the controls was about 200.
The infectivity of EEV derived from SVAREC expressing neither human CD55 nor CD59 was reduced by 81% by human complement (Fig. 5B, Hygro). In contrast, the infectivity of EEV harvested from SVAREC cells expressing either CD55 or CD55 and CD59 was reduced by only 27 and 12%, respectively (Fig. 5B, CD55+ and CD55+/CD59+). EEV derived from SVAREC expressing CD59 alone exhibited sensitivity to complement similar to EEV derived from SVAREC Hygro (Fig. 5B, CD59+).
DISCUSSION
The two infectious forms of VV have different structures and surface proteins that provide IMV and EEV with biological properties. EEV mediates virus spread in cell culture and within an infected host (17). Consistent with this role, EEV is resistant to neutralization by antibody (34, 35) and complement (this work). This envelope, however, is very fragile to physical forces. In contrast, IMV is sensitive to antibody and complement, but is resistant to physical forces. IMV released from lysed cells or ruptured EEV seems better suited to transmit infection between hosts.
EEV resistance to complement is conferred by incorporation of host complement regulators into the EEV envelope. This is particularly advantageous for viruses having a broad host range, such as VV, because by this mechanism the progeny virus will always exhibit surface complement regulators adapted to the complement of their host. The resistance of EEV to complement is demonstrated here only in the absence of anti-VV antibody. In the presence of anti-VV antibody, and absence of VCP, EEV infectivity was destroyed by complement (data not shown). However, VCP derived from the undiluted supernatant of VV-infected cells protected EEV from neutralization by antibody plus complement (data not shown) because of its ability to block activation of the classical complement pathway. This demonstrates that VV has multiple defenses against complement.
The incorporation of cellular plasma membrane proteins into the EEV outer membrane has implications for EEV biology and for the origin of the EEV outer membrane. Three origins have been proposed that are not mutually exclusive: the plasma membrane when IMV particles bud directly from the cell (14), or the early tubular endosomes (13) and the trans-Golgi network (12) when IMVs are wrapped intracellularly. If the EEV outer envelope were derived from the early tubular endosomes, the presence of cellular plasma membrane proteins in EEV would be consistent with the roles of these vesicles in recycling endocytosed membrane proteins to the plasma membrane, or acting as an intracellular store of plasma membrane (13, 48). If the EEV outer membrane were derived from the trans-Golgi membrane the presence of plasma membrane proteins within this compartment would be more surprising. Late during infection, when wrapping of IMV occurs, host cellular protein synthesis is inhibited so that host membrane proteins in the trans-Golgi network can be explained only by their recycling from the plasma membrane or from a pool of plasma membrane. However, VV infection does greatly increase the recycling pathway from endosomes to the trans-Golgi network (12, 13). Because all the host plasma membrane proteins examined were found in EEV, it is very likely that others will also be present. If other proteins from intracellular membrane compartments are also present, these may be informative about the origin of the wrapping membrane.
VV can render tumor cells susceptible to homologous complement by activation of the alternative pathway, even though complement activation is strictly regulated by membrane complement regulators on the surface of noninfected cells (49). This phenomenon was explained by the decrease of RCA expression on VV-infected cells (50). Interestingly, RCA expression was stable during the first 12 h of postinfection and then decreased quickly (50). This led Baranyi et al. (50) to suggest that the decrease in expression was not a result of the inhibition of host protein synthesis induced by VV infection, but rather the consequence of depletion of cellular RCA because of their incorporation into the envelope of released EEV (50). Our results are consistent with this hypothesis.
The host proteins incorporated into the EEV outer membrane are not restricted to complement regulators, and three other proteins (CD71, CD81, and MHC-I) were detected. These cellular proteins have a variety of membrane topologies: type I, CD46, CD81, and MHC class I; type II, CD71; and GPI-anchored, CD55 and CD59; indicating that membrane topology does not influence recruitment. That all host proteins tested were found in EEV makes it likely that further cell proteins might be present, such as, for instance, host proteins that promote the polymerization of actin on intracellular enveloped virus (51). Cellular proteins may confer biological properties on EEV other than resistance to complement. This possibility is supported by studies with HIV, where cellular proteins incorporated into the virus envelope protect the virus against complement (7) and also modify virus binding to cells and, consequently, virus tropism (52). Similarly, cellular proteins inserted into the EEV outer membrane may contribute to EEV binding, which may broaden EEV cell tropism and possibly contribute to the observed EEV resistance to antibody neutralization (33–35).
In conclusion, this study demonstrated that several cellular proteins are incorporated into the EEV outer envelope and that inserted cellular RCA function to protect EEV from complement-mediated destruction. These data justify a more complete analysis of the other putative biological properties that virally incorporated cellular proteins may confer to EEV.
Acknowledgments
SVAREC were kindly provided by Dr. I. Anegon (Institut National de la Santé et de la Recherche Médicale U211, Nantes, France). mAb 5B4/2F2 was a gift from Dr. Claus P. Czerny (Institute of Medical Microbiology, Infectious and Epidemic Diseases, Ludwig-Maximilians-University Munich). A.V. is a senior research assistant of the Fonds National Belge de la Recherche Scientifique and was the laureate of NATO and Huyen research fellowships. This work was supported by a Programme Grant from the United Kingdom Medical Research Council (PG8901790) and by an equipment grant from The Wellcome Trust.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: VV, vaccinia virus; IMV, intracellular mature virus; EEV, extracellular enveloped virus; RCA, regulators of complement activation; CCP, complement control protein module; MHC, major histocompatibility complex; SVAREC, SV40-transformed aortic rat endothelial cells; IHD-J, International Health Department-J; WR, Western Reserve; PBSF, PBS containing 10% heat-inactivated fetal bovine serum; VCP, VV complement control protein.
References
- 1.Sim R B, Dodds A W. In: Complement. A Practical Approach. Dodds A W, Sim R B, editors. Oxford: IRL; 1997. pp. 1–18. [Google Scholar]
- 2.Law S K A, Reid K B M. Complement. Oxford: IRL; 1995. [Google Scholar]
- 3.Cooper N R, Nemerow G R. Springer Semin Immunopathol. 1983;6:327–337. doi: 10.1007/BF02116278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horstmann R D. Infect Immunol. 1992;60:721–727. doi: 10.1128/iai.60.3.721-727.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper N R. Immunol Today. 1991;12:327–331. doi: 10.1016/0167-5699(91)90010-Q. [DOI] [PubMed] [Google Scholar]
- 6.Friedman H M, Cohen G J, Eisenberg R J, Seidel C A, Cines D B. Nature (London) 1984;309:633–635. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- 7.Saifuddin M, Parker C J, Peeples M E, Gorny M K, Zolla-Pazner S, Ghassemi M, Rooney I A, Atkinson J P, Spear G T. J Exp Med. 1995;182:501–509. doi: 10.1084/jem.182.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isaacs S N, Kotwal G J, Moss B. Proc Natl Acad Sci USA. 1992;89:628–632. doi: 10.1073/pnas.89.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isaacs S N, Moss B. In: Viroceptors, Virokines and Related Immune Modulators Encoded by DNA Viruses. McFadden G, editor. Austin, TX: R. G. Landes; 1994. pp. 55–66. [Google Scholar]
- 10.Appleyard G, Hapel A J, Boulter E A. J Gen Virol. 1971;13:9–17. doi: 10.1099/0022-1317-13-1-9. [DOI] [PubMed] [Google Scholar]
- 11.Ichihashi Y, Matsumoto S, Dales S. Virology. 1971;46:507–532. doi: 10.1016/0042-6822(71)90056-0. [DOI] [PubMed] [Google Scholar]
- 12.Schmelz M, Sodeik B, Ericsson M, Wolffe E J, Shida H, Hiller G, Griffiths G. J Virol. 1994;68:130–147. doi: 10.1128/jvi.68.1.130-147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tooze J, Hollinshead M, Reis B, Radsak K, Kern H. Eur J Cell Biol. 1993;60:163–178. [PubMed] [Google Scholar]
- 14.Tsutsui K. J Electron Microsc. 1983;32:125–140. [PubMed] [Google Scholar]
- 15.Boulter E A, Appleyard G. Prog Med Virol. 1973;16:86–108. [PubMed] [Google Scholar]
- 16.Payne L G, Kristensson K. J Gen Virol. 1985;66:643–646. doi: 10.1099/0022-1317-66-3-643. [DOI] [PubMed] [Google Scholar]
- 17.Payne L G. J Gen Virol. 1980;50:89–100. doi: 10.1099/0022-1317-50-1-89. [DOI] [PubMed] [Google Scholar]
- 18.Blasco R, Moss B. J Virol. 1992;66:4170–4179. doi: 10.1128/jvi.66.7.4170-4179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Payne L G. J Virol. 1978;27:28–37. doi: 10.1128/jvi.27.1.28-37.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Payne L G. J Virol. 1979;31:147–155. doi: 10.1128/jvi.31.1.147-155.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Payne L G, Norrby E. J Gen Virol. 1976;32:63–72. doi: 10.1099/0022-1317-32-1-63. [DOI] [PubMed] [Google Scholar]
- 22.Shida H. Virology. 1986;150:451–462. doi: 10.1016/0042-6822(86)90309-0. [DOI] [PubMed] [Google Scholar]
- 23.Hirt P, Hiller G, Wittek R. J Virol. 1986;58:757–764. doi: 10.1128/jvi.58.3.757-764.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duncan S A, Smith G L. J Virol. 1992;66:1610–1621. doi: 10.1128/jvi.66.3.1610-1621.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engelstad M, Howard S T, Smith G L. Virology. 1992;188:801–810. doi: 10.1016/0042-6822(92)90535-w. [DOI] [PubMed] [Google Scholar]
- 26.Isaacs S N, Wolffe E J, Payne L G, Moss B. J Virol. 1992;66:7217–7224. doi: 10.1128/jvi.66.12.7217-7224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parkinson J E, Smith G L. Virology. 1994;204:376–390. doi: 10.1006/viro.1994.1542. [DOI] [PubMed] [Google Scholar]
- 28.Roper R L, Payne L G, Moss B. J Virol. 1996;70:3753–3762. doi: 10.1128/jvi.70.6.3753-3762.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi-Nishimaki F, Funahashi S, Miki K, Hashizume S, Sugimoto M. Virology. 1991;181:158–164. doi: 10.1016/0042-6822(91)90480-y. [DOI] [PubMed] [Google Scholar]
- 30.Boulter E A. Proc R Soc Med. 1969;62:295–297. doi: 10.1177/003591576906200349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turner G S, Squires E J. J Gen Virol. 1971;13:19–25. doi: 10.1099/0022-1317-13-1-19. [DOI] [PubMed] [Google Scholar]
- 32.Vanderplasschen A, Smith G L. J Virol. 1997;71:4032–4041. doi: 10.1128/jvi.71.5.4032-4041.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanderplasschen A, Hollinshead M, Smith G L. J Gen Virol. 1998;79:877–887. doi: 10.1099/0022-1317-79-4-877. [DOI] [PubMed] [Google Scholar]
- 34.Ichihashi Y. Virology. 1996;217:478–485. doi: 10.1006/viro.1996.0142. [DOI] [PubMed] [Google Scholar]
- 35.Vanderplasschen A, Hollinshead M, Smith G L. J Gen Virol. 1997;78:2041–2048. doi: 10.1099/0022-1317-78-8-2041. [DOI] [PubMed] [Google Scholar]
- 36.McIntosh A A G, Smith G L. J Virol. 1996;70:272–281. doi: 10.1128/jvi.70.1.272-281.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolffe E J, Katz E, Weisberg A, Moss B. J Virol. 1997;71:3904–3915. doi: 10.1128/jvi.71.5.3904-3915.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charreau B, Cassard A, Tesson L, Le Mauff B, Navenot J-M, Blanchard D, Lublin D, Soulillou J-P, Anegon I. Transplantation. 1994;58:1222–1229. [PubMed] [Google Scholar]
- 39.Engelstad M, Smith G L. Virology. 1993;194:627–637. doi: 10.1006/viro.1993.1302. [DOI] [PubMed] [Google Scholar]
- 40.Mathew E, Sanderson C M, Hollinshead M, Smith G L. J Virol. 1998;72:2429–2438. doi: 10.1128/jvi.72.3.2429-2438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doms R W, Blumenthal R, Moss B. J Virol. 1990;64:4884–4892. doi: 10.1128/jvi.64.10.4884-4892.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanson C V, Riggs J L, Lennette E H. J Gen Virol. 1978;40:345–358. doi: 10.1099/0022-1317-40-2-345. [DOI] [PubMed] [Google Scholar]
- 43.Czerny C P, Mahnel H. J Gen Virol. 1990;71:2341–2352. doi: 10.1099/0022-1317-71-10-2341. [DOI] [PubMed] [Google Scholar]
- 44.White S, Miller K, Hopkins C, Trowbridge I S. Biochem Biophys Acta. 1992;1136:28–34. doi: 10.1016/0167-4889(92)90081-l. [DOI] [PubMed] [Google Scholar]
- 45.Stam N J, Vroom T M, Peters P J, Pastoors E B, Ploegh H L. Intern Immunol. 1990;2:113–125. doi: 10.1093/intimm/2.2.113. [DOI] [PubMed] [Google Scholar]
- 46.Slot J W, Geuze H J. Eur J Cell Biol. 1985;38:87–93. [PubMed] [Google Scholar]
- 47.Mayor S, Rothberg K G, Maxfield F R. Science. 1994;264:1948–1951. doi: 10.1126/science.7516582. [DOI] [PubMed] [Google Scholar]
- 48.Tooze J, Hollinshead M. J Cell Biol. 1991;115:635–653. doi: 10.1083/jcb.115.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okada H, Wakamiya N, Okada N, Kato S. Cancer Immunol Immunother. 1987;25:7–9. doi: 10.1007/BF00199294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baranyi L, Okada N, Baranji K, Takizawa H, Okada H. Clin Exp Immunol. 1994;98:134–139. doi: 10.1111/j.1365-2249.1994.tb06619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cudmore S, Cossart P, Griffiths G, Way M. Nature (London) 1995;378:636–638. doi: 10.1038/378636a0. [DOI] [PubMed] [Google Scholar]
- 52.Castilletti C, Capobianchi M R, Fais S, Abbate I, Ficociello B, Ameglio F, Cordiali Fei P, Santini S M, Dianzani F. AIDS Res Hum Retroviruses. 1995;11:547–553. doi: 10.1089/aid.1995.11.547. [DOI] [PubMed] [Google Scholar]