Abstract
Purpose
Determine if the response of human head and neck cancer xenografts to cisplatin (CIS) could be enhanced with 2-deoxyglucose (2DG) and determine if 2-[F-18]-fluoro-2-deoxy-D-glucose (FDG) uptake correlated with responses to this drug combination. Determine if 2DG would enhance CIS-induced radiosensitization.
Experimental Design
Clonogenic survival responses to CIS + 2DG were determined in FaDu and Cal-27 cells and GSH/GSSG levels were monitored as parameters indicative of oxidative stress. The efficacy of CIS + 2DG was determined in FaDu and Cal-27 xenografts and FDG uptake was determined with Positron Emission Tomography (PET).
Results
CIS + 2DG enhanced cell killing of FaDu and Cal-27 cells, compared to either drug alone, while increasing %GSSG in vitro. CIS + 2DG inhibited FaDu and Cal-27 tumor growth and increased disease free survival, compared to either drug alone. Cal-27 tumors demonstrated greater pretreatment FDG uptake and increased disease free survival when treated with 2DG + CIS, relative to FaDu tumors. 2DG treatment enhanced CIS-induced radiosensitization in FaDu tumor cells grown in vitro and in vivo and resulted in apparent cures in 50% of tumors.
Conclusions
These results demonstrate the enhanced therapeutic efficacy of CIS + 2DG in human head and neck cancer cells in vitro and in vivo when compared to either drug alone as well as demonstrating the potential for FDG uptake to predict tumor sensitivity to 2DG + CIS. These findings provide a strong rationale for evaluating 2DG + CIS in combined modality head and neck cancer therapy with radiation in a clinical setting.
Keywords: cisplatin, 2-deoxyglucose, HNSCC, FDG-PET, oxidative stress, radiation
INTRODUCTION
Squamous cell carcinoma of the head and neck (HNSCC) comprises 3–5 % of all cancers in the United States and 40,000 patients are diagnosed annually with a 5 year survival rate of 56% (1). Management of locally advanced or recurrent HNSCC usually involves treatment with cisplatin (CIS) alone or in combination with other chemotherapeutic agents, radiotherapy and/or surgery (2). The concurrent use of CIS and radiotherapy results in improved survival relative to radiotherapy alone both in unresectable cases (3) and when used as adjuvant therapy after resection (4,5). However, the concurrent use of CIS based chemotherapy leads to significant toxicity including myelosuppression, nephrotoxicity, enhanced toxicities of radiotherapy including dysphagia and voice dysfunction (6,7), decreased quality of life (8) and increased treatment related deaths (4,5). Therefore, combining cisplatin with agents selectively toxic to cancer cells has potential advantages, including utilization of lower doses of CIS, fewer side effects, loss of chemoresistance and enhanced therapeutic response.
One common abnormal biochemical characteristic associated with cancer cells including head and neck malignancies is increased intracellular utilization of glucose (9). We have previously reported that glucose deprivation induces selective cytotoxicity and oxidative stress in transformed human cells vs. normal cells (10). Additionally, increased prooxidant production and disruptions in thiol metabolism consistent with metabolic oxidative stress were noted in cancer cells during glucose deprivation or when treated with the glycolytic inhibitor 2-deoxy-D-glucose (2DG) (11). 2DG-induced cytotoxicity and increases in parameters indicative of oxidative stress were inhibited by the thiol antioxidant, N-acetylcysteine (NAC, 12, 13), as well as the over expression of enzymes that scavenge reactive oxygen species (ROS) such as superoxide and hydrogen peroxide (13). These results have led to the hypothesis that glucose deprivation in cancer cells causes metabolic oxidative stress by limiting hydroperoxide metabolism (10, 11). Although the mechanisms responsible for increased glucose metabolism in cancer cells are not fully understood, this phenomenon has proven useful in locating metabolically active cancer cells based on preferential uptake of 18F-2-deoxy-D-glucose coupled with Positron Emission Tomography (FDG-PET) imaging (14,15). FDG-PET is now a standard test in HNSCC for staging pretreatment (16), and surveillance or restaging post-treatment (17). FDG-PET uptake also has prognostic value, as several groups have reported poor survival for patients with tumors showing high pretreatment FDG uptake (18,19).
We propose that inhibition of glucose metabolism with 2DG would cause increased oxidative stress and cytotoxicity, thereby sensitizing cancer cells (relative to normal cells) to conventional cancer therapies that increase oxidative stress (i.e., radiation and some chemotherapies). Following the same logic, we propose that the relative increase of pretreatment FDG uptake will be proportional to the degree of 2DG-induced chemosensitization.
The current study identifies 2DG + CIS ± radiation as an effective antitumor combination in vivo in FaDu and Cal-27 human xenograft tumors in mice. Pretreatment FDG uptake in FaDu and Cal-27 xenograft tumors was also determined and increased uptake correlated with improved tumor responses to 2DG + CIS.
METHODS AND MATERIALS
Cells and culture conditions
FaDu and Cal-27 human head and neck squamous cell carcinoma cells were obtained from the American Type Culture Collection (Manassas, VA) and maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 4.5 g/L glucose, 4 mM L-glutamine, and 1 mM sodium pyruvate (FBS; Hyclone, Logan, UT). Cultures were maintained in 5% CO2 and humidified in a 37°C incubator. Experiments were performed with cells from passage 3–20.
In vitro drug treatment and clonogenic cell survival experiments
2-deoxy-D-glucose (2DG) was obtained from Sigma Chemical Co. (St. Louis, MO). Cis-diamminedichloroplatinum(II) (cisplatin, CIS) was obtained from Bedford Laboratories (Bedford, OH). Drugs were added to cells at 20 mM 2DG and 0.5 μM CIS. Cells were placed in a 37°C incubator and harvested at time points indicated. Clonogenic cell survival experiments were performed as described previously (13).
In vitro radiation treatment
Radiation was delivered using a JC Shepherd cesium irradiator with a dose rate of 0.805 Gy/min. Cells were irradiated at 2 Gy at room temperature at the end of 2DG and/or CIS drug treatment. The cells were plated for clonogenic survival immediately following radiation.
Glutathione assay
Total glutathione content was determined by the method of Anderson (20). GSH and GSSG were distinguished as described previously (21). All glutathione determinations were normalized to the protein content of whole homogenates using the method of Lowry et al. (22).
Tumor cell implantation
Eighty female 4–5 week old athymic-nu/nu nude mice were purchased from Harlan Laboratories (Indianapolis, IN). All mice were housed in a pathogen-free barrier room in the Animal Care Facility at the University of Iowa and handled using aseptic procedures. All procedures were approved by the IACUC committee of the University of Iowa and conformed to the guidelines established by NIH. Mice were allowed 3 days to acclimate prior to beginning experimentation, and food and water were made freely available. Tumor cells were inoculated into mice by subcutaneous (s.c.) injection of 0.1 mL aliquots of sterile saline containing 4 × 106 FaDu cells or 8 × 106 Cal-27 cells into the right flank using 26-gauge needles.
Tumor measurements
The first measurable tumor appearance was considered when individual tumor volumes measured 0.01 cm3. Mice were evaluated daily and tumor measurements taken three times per week using Vernier calipers. Tumor volumes were calculated using the formula: tumor volume = (length × width2)/2 where the length was the longest dimension, and width was the dimension perpendicular to length. Mice were euthanized via CO2 gas asphyxiation or lethal overdose of sodium pentobarbital (100 mg/kg) when tumor length exceeded 1.5 cm in any dimension.
In vivo drugs administration
The above-mentioned nude mice were divided into five groups (n = 6–12 mice/group). 2DG group: 2DG was dissolved in saline and administered intraperitoneally (i.p.) 0.5 g/kg every other day for 6 total doses during 2 weeks (days 1, 3, 5, 8, 10, 12). CIS group: CIS was dissolved in saline and administered i.p. 2 mg/kg every other day for 6 total doses during 2 weeks. 2DG+CIS group: mice were administered i.p. 0.5 g/kg 2DG plus 2 mg/kg CIS every other day for 6 total doses during 2 weeks. 2DG+CIS* group: mice were administered i.p. 0.5 g/kg 2DG every day (weekends off) plus 6 mg/kg CIS on days 2 and 9 of treatment for a total of ten 2DG doses and two CIS doses during 2 weeks. Control group: mice were administered i.p. saline every other day. Treatment began ≈ 2 weeks after tumor inoculation.
Radiation treatment
FaDu tumor cells (4 × 106) were inoculated into 32 mice by s.c. injection into the right flank. Mice were divided into five groups (n = 5–6 mice/group) and treated with 2DG and/or CIS as mentioned above. Mice were anaesthetized with ketamine and xylazine mix i.p. containing 87.5 mg ketamine and 12.5 mg xylazine per kg bodyweight. The tumor tissue was exposed to fractionized ionizing radiation (FIR) in 2 Gy fractions (250 kVp x-rays filtered with 0.25 mm Cu, 0.25 mm aluminum, from a Pantak Therapax DXT 300 x-ray machine) after drug treatment twice a week for 2 weeks (day 3, 5, 10 and 12) for a total dose of 8 Gy (4 × 2 Gy fractions). Dosimetry was confirmed with a Victoreen R meter. The remainder of the body was shielded in a lead box to reduce exposure of normal tissues.
FDG-PET Imaging of FaDu xenografts
On the day of imaging, food was removed 4 h prior to injection of FDG. Blood glucose levels were checked before injection of FDG using a Therasense Freestyle Glucometer and were determined to be within normal limits. Conscious mice (mildly sedated with Midazolam 5mg/kg i.p.) were injected via tail vein with 23 ±6.7 MBq FDG in 0.2 ml and returned to their cage for a 30 min uptake period with access to drinking water. Following the labeled FDG uptake period, the mouse was placed in a temperature controlled imaging holder. The mouse was imaged in a darkened and quiet environment. Mouse body temperature was maintained and warmed oxygen was administered during acquisition. The imaging holder was affixed and remotely translated into the center of the axial field-of-view (FOV) of the Philips MOSAIC animal PET scanner (12 cm). The entire mouse was positioned within the sensitive region of the scanner. Images (3 frames at 5 min each) from one bed position were acquired since the MOSAIC scanner has an axial FOV larger than the typical nude mouse longitudinal body dimension. A total of 240, 0.5 mm trans-axial slices were reconstructed spanning the total body of the mouse.
Quantitative Image Analysis
Transaxial slices were reconstructed using a 3D algorithm using projection data corrected for random coincidences, scatter and dead time. Three orthogonal views (transverse, sagittal, and coronal) as well as rotating projection images were displayed post-reconstruction. A commercial software package MIMVISTA (Cleveland, OH) was used to obtain standardized uptake values (SUVs) from regions drawn on the coronal slices through the tumor and normal muscle on the contralateral side of the animal. The SUV of the tumor was determined from the maximum voxel within the tumor volume. This was compared with the SUV from the normal muscle region using the average activity concentration in the muscle region.
Data analysis
To determine differences between 3 or more means, one-way ANOVA with Bonferroni post-tests were performed. Two-way ANOVA was used to determine differences and interaction effects between cell lines and treatment groups in the in vitro experiments. Individual tumor measurements were serially recorded and corresponding volumes calculated for each mouse. Mean tumor volumes for all treatment groups were calculated by averaging together all individual tumor volumes per measurement date. Survival curves were estimated with the method of Kaplan-Meier. Disease free survival was defined as no evidence of tumor at the time of sacrifice and an apparent cure of their disease. Cox proportional hazards regression was used to estimate and compare survival across treatment regimen and cell lines. To determine statistical differences in tumor volumes over time, a generalized linear model was fit to the data. Model estimates were obtained via the method of Generalized Estimating Equations (GEE) in order to account for the repeated tumor measurements for each subject (23). An autoregressive structure was specified for the within-subject correlation in the GEE analysis. All statistical tests were two-sided and carried out at the 5% level of significance with the SAS statistical software package (Cary, NC).
RESULTS
2DG enhanced CIS-induced cytotoxicity in vitro
Treatment with 2DG caused 32 and 30 % cell killing in FaDu and Cal-27 cells respectively relative to untreated control cells (p<0.001, Fig. 1A), while CIS caused 62 and 54 % cell killing respectively (p<0.01, Fig. 1A). The combination of 2DG and CIS caused a significant increase in cell killing in both cell lines (88 and 86 % respectively, p<0.001) showing an additive effect of 2DG and CIS (Fig. 1A).
Figure 1.
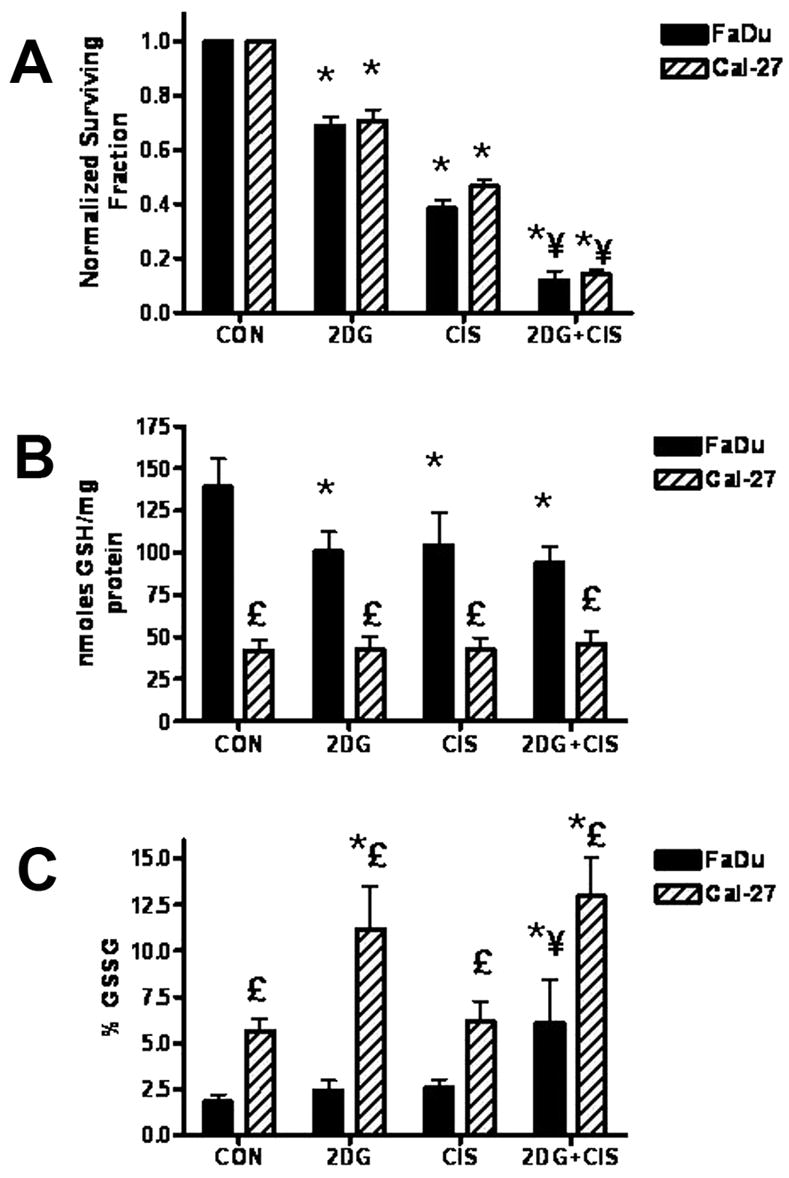
Effect of 2-deoxy-D-glucose (2DG) and cisplatin (CIS) on cytotoxicity (A), total glutathione (B) and percentage oxidized glutathione (% GSSG) levels (C) in FaDu and Cal-27 cells. Clonogenic cell survival data were normalized to control (CON). Error bars represent the SEM of N=3. *, p< 0.001 versus control. ¥, p<0.001 versus 2DG and CIS alone. £, p<0.001 FaDu versus Cal-27.
2DG in combination with CIS induced disruptions in glutathione metabolism
FaDu cells demonstrated a decrease in total GSH when treated with 2DG and CIS alone and in combination (p<0.01, Fig. 1B) and a significant increase in % GSSG when treated with the combination of 2DG and CIS (Fig. 1C) compared to untreated cells (p<0.01). Cal-27 cells showed no changes in total GSH with drug treatment (Fig. 1B) but showed significant increases in % GSSG with 2DG alone (p<0.001) and the combination of 2DG and CIS (p<0.001, Fig. 1C). These results suggest that the toxicity of 2DG in combination with CIS was mediated by disruptions in thiol metabolism consistent with oxidative stress.
2DG in combination with CIS inhibited growth of HNSCC xenografts
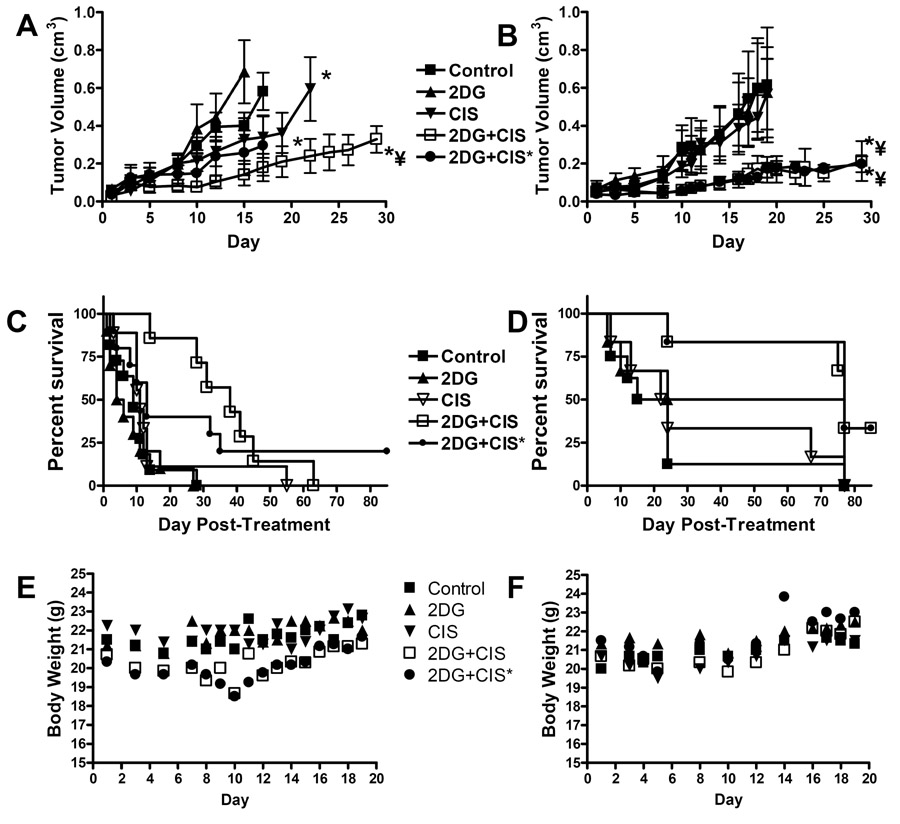
We examined in vivo activity of 2DG and CIS in FaDu and Cal-27 tumor bearing athymic nude mice. The control and 2DG groups demonstrated no differences in tumor growth for both FaDu and Cal-27 tumors (p>0.05, Fig. 2A,B). Additionally, the Cal-27 tumors did not demonstrate any growth delay when treated with CIS (p>0.05, Fig. 2B) whereas the FaDu tumors showed a slight but significant growth delay (p=0.01, Fig. 2A) when treated with CIS. The combination of 2DG and CIS demonstrated a pronounced inhibition of growth in both FaDu and Cal-27 tumors for both dosing schedules when compared to control and 2DG alone (p<0.01, Fig. 2A,B). However, the combination of 2DG+CIS* where CIS was administered as 2 bolus doses of 6 mg/kg once per week, exhibited a significant growth delay in only Cal-27 tumors compared to CIS alone (p=0.02, Fig. 2B). When the CIS was administered in 6 × 2 mg/kg doses over the course of two weeks in combination with 2DG, both FaDu and Cal-27 demonstrated significant inhibition of growth compared to CIS alone (p<0.01, Fig. 2A,B). These results show that 2DG in combination with CIS cause significant inhibition of growth in HNSCC tumors in vivo compared to either agent, alone which is consistent with the in vitro data shown in Figure 1A.
Figure 2.
Tumor growth curves for athymic (nu/nu) mice with FaDu (A) and Cal-27 (B) tumors treated with 2-deoxy-D-glucose (2DG) and cisplatin (CIS). *, p< 0.001 versus control. ¥, p<0.001 versus 2DG and CIS alone. Data points represent the average values for 7–12 mice. Survival analysis of athymic nu/nu mice bearing FaDu (C) and Cal-27 (D). Body weight measurements of mice bearing FaDu (E) and Cal-27 (F) tumors.
2DG in combination with CIS increased disease free survival of mice bearing HNSCC tumors
There was no significant increase in overall disease free survival of mice treated with 2DG or CIS alone compared to control mice regardless of tumor type (p>0.05, Fig. 2C,D, Table 1). Mice bearing FaDu tumors treated with 2DG in combination with CIS had significantly longer median survival times than control (p<0.001), 2DG alone (p<0.001) and CIS alone (p=0.012) when administered in the low daily dosing schedule (2DG+CIS, Fig. 2B, Table 1). Mice administered 2DG and CIS in the high weekly dosing schedule (2DG+CIS*) had significantly longer median survival times than control (p=0.002) and 2DG alone (p<0.00001, Fig. 2C, Table 1). Interestingly, 2DG and CIS administered to FaDu tumor bearing mice in the high weekly dosing schedule appeared to produce more disease free survivors yet lead to shorter median survival. (p=0.058, Fig. 2C, Table 1). In contrast, mice bearing Cal-27 tumors that were treated with 2DG and the combination of 2DG and CIS had significantly longer median survival times compared to control mice regardless of the dosing schedule (p<0.05, Fig. 2D, Table 1). The overall disease free surviving fractions of the FaDu and Cal-27 tumor bearing mice at 150 days post-treatment are shown in Table 1. There were no surviving mice at day 150 post-treatment in the control, 2DG or CIS alone groups for both tumor types (Table 1). However, 33% of the Cal-27 tumor bearing mice treated with 2DG in combination with CIS in both dosing schedules were disease free and alive at day 150 post-treatment, while 22% of the FaDu tumor bearing mice were disease free and alive at 150 days when administered 2DG and CIS in the high weekly CIS dosing schedule despite the poor median survival observed for this dosing schedule (Table 1). There were no FaDu animals surviving at 150 days in the daily CIS dosing schedule (Table 1). When the surviving fraction and median survival times were compared between the two tumor types in Table 1, mice with Cal-27 tumors showed greater disease free surviving fractions when treated with the combination of 2DG + CIS at both dosing schedules at the termination of the experiment, and demonstrated longer median survival times when treated with 2DG and the combination of 2DG and CIS (Table 1). There were no significant changes in the body weight of mice receiving 2DG and CIS alone or in combination during or after treatment (Figure 2E,F). Collectively all of the above data (Fig. 2 and Table 1) indicate that 2DG and CIS increased overall survival for both tumor types and that mice bearing Cal-27 tumors appeared to respond better after 2DG-based chemotherapy than mice bearing FaDu tumors.
Table 1.
Survival analysis of athymic nu/nu mice bearing FaDu and Cal-27 tumors treated with 2-deoxy-D-glucose (2DG) and cisplatin (CIS) at 150 days post-treatment.
| Disease Free Surviving Fraction | Median survival (days) | |||
|---|---|---|---|---|
| FaDu | Cal-27 | FaDu | Cal-27 | |
| Control | 0 (0 of 12) | 0 (0 of 6) | 9 | 19.5 |
| 2DG | 0 (0 of 11) | 0 (0 of 6) | 5 | 50.5*† |
| CIS | 0 (0 of 11) | 0 (0 of 6) | 11 | 23 |
| 2DG+CIS | 0 (0 of 7) | 0.33 (2 of 6) | 38** | 77** |
| 2DG+CIS* | 0.22 (2 of 9) | 0.33 (2 of 6) | 13* | 77**† |
, p<0.01versus control;
, p<0.05 versus 2DG and CIS alone;
, p<0.05 versus FaDu; numbers in parenthesis indicate disease free animals relative to total animals/group.
Comparison of pretreatment FDG-PET scans in FaDu and Cal-27 xenografts in vivo
Figure 3 shows representative PET images from mice bearing FaDu (A) and Cal-27 (B) tumors following injection of FDG. The images demonstrate that the tumors were clearly identified from normal tissues such as the bladder and other organs (Figure 3A,B) in order to identify adequate regions of interest (ROIs) for quantitative analysis. The FDG distribution across normal tissues was consistent with the expected rates of glucose uptake and label excretion (high in brain, brown fat, and bladder) and was similar in mice bearing FaDu and Cal-27 tumors. SUVs for FaDu and Cal-27 tumor types were determined for each tumor. When all FaDu and Cal-27 tumor SUV values were compared and matched according to smaller (0.01 – 0.1 cm3, Fig. 3C) and larger (0.2 – 0.4 cm3, Fig. 3D) sized tumors, it was clear that Cal-27 tumors demonstrated greater average SUVs, relative to FaDu tumors regardless of tumor size (p=0.015). These data demonstrate that pretreatment uptake of FDG was greater in Cal-27 tumors relative to FaDu tumors and that differences in FDG uptake can be quantified by FDG-PET imaging in these model systems.
Figure 3.
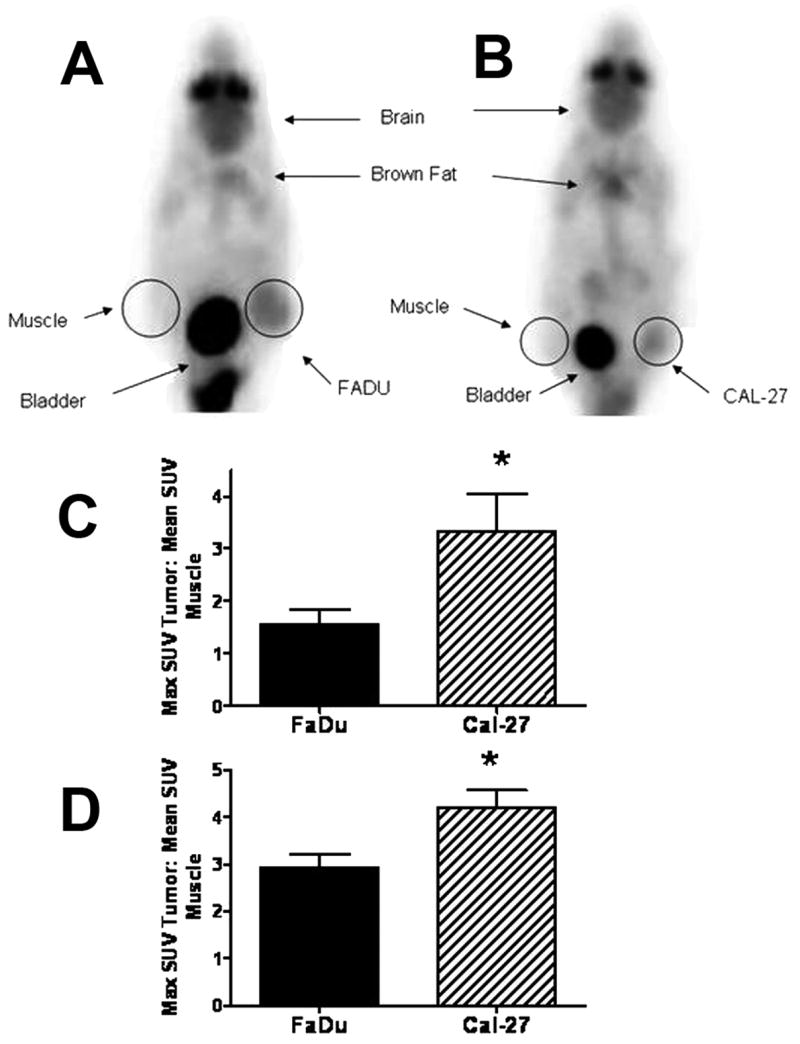
Comparison of glucose uptake as determined by FDG-PET inaging of FaDu and Cal-27 xenografts. FaDu (A) and Cal-27 (B) tumor bearing mice were imaged from each tumor type. Three tumor bearing animals of each tumor type with average volumes of 0.04±0.03 (C) and 0.27±0.05 (D) cm3 were imaged with FDG-PET and SUVs were determined from regions overlaid on tumor (right flank) and contra-lateral muscle (left flank). Errors are ± 1SD. *, p< 0.01 versus FaDu.
2DG and CIS-induced radiosensitization in vitro
To determine the effect of 2DG on CIS-induced radiosensitization in the FaDu cells, 2 Gy was found to cause 55 % killing and 2DG + 2Gy caused 67 % cell killing (Fig. 4A). CIS + 2 Gy caused 78 % cell killing and 2DG + CIS + 2 Gy significantly increased cell killing to 93 % in FaDu cells, compared to all other treatments (Fig. 4A). These results show that CIS-induced radiosensitization could be further enhanced by 2DG in FaDu cells, in at least an additive manner in vitro.
Figure 4.

2-deoxy-D-glucose (2DG) and cisplatin (CIS)-induced radiosensitization in FaDu cells in vitro (A) and in vivo (B). Clonogenic cell survival data were normalized to control (CON). Error bars represent ± 1SD of N=3 experiments. Data points on tumor growth graphs represent the average values for 5–6 mice. *, p<0.001 versus respective treatment without 2GY, ¥, p<0.01 versus CIS+2GY. C: Survival analysis of athymic nu/nu mice bearing FaDu tumors. D: Body weight measurements of mice bearing FaDu tumors treated with FIR.
2DG and CIS-induced radiosensitization in vivo
Animals were treated with 2DG and Cisplatin in protocols that were the same as Figure 2. Tumors treated with 4 × 2 Gy fractions (FIR) showed very little inhibition of growth compared to control tumors (Figure 4B) in the first 30 days and only one animal demonstrated disease free survival at 125 days when treated with this radiation dose (Figure 4C and Table 2). Using this modest radiation dosing schedule, 2DG + FIR had no pronounced antitumor activity, and was not significantly different from control or FIR alone (Fig. 4B, C, Table 2). CIS + FIR significantly inhibited tumor growth and median survival (but not disease free survival) compared to control (p<0.01, Fig. 4B, C, Table 2). The combination of 2DG+CIS+FIR administered in the lower daily dose CIS treatment schedule significantly enhanced tumor growth inhibition, median survival, and overall disease free survival (50%) when compared to any other treatment group (p<0.05, Fig. 4B, C, Table 2) without showing any significant changes in body weight (Figure 4D). Interestingly, 2DG+CIS*+FIR administered in the higher weekly CIS dosing schedule was not as effective at inhibiting tumor growth, median survival, or disease free survival (data not shown) as was the lower dose CIS schedule combined with FIR which is consistent with the findings for FaDu tumors treated with 2DG+CIS without radiotherapy in Figure 2C. These data show that 2DG is able to significantly enhance CIS-induced radiosensitivity and overall disease free survival when administered in the low daily CIS dosing schedule without showing any overt signs of morbidity and mortality.
Table 2.
Survival analysis of athymic nu/nu mice bearing FaDu tumors treated with 2-deoxy-D-glucose (2DG), cisplatin (CIS) and X-ray radiation (2GY) at 125 days post-treatment.
| Disease Free Surviving Fraction | Median Survival (days) | |
|---|---|---|
| Control | 0 (0 of 5) | 10.5 |
| FIR | 0.2 (1 of 5) | 14 |
| 2DG+FIR | 0 (0 of 5) | 19 |
| CIS+FIR | 0 (0 of 5) | 35* |
| 2DG+CIS | 0 (0 of 6) | 38* |
| 2DG+CIS+FIR | 0.50** (3 of 6) | 123.5** |
, p<0.001 versus control;
, p<0.001 versus CIS+2GY; numbers in parenthesis indicate disease free animals relative to total animals/group.
DISCUSSION
Clinical trials have shown that concomitant cisplatin/radiation therapy is effective in many HNSCC disease sites (3,4). Concomitant chemoradiotherapy with cisplatin has emerged as the standard of care for locally advanced unresectable tumors, for laryngeal preservation in appropriately selected patients (24). Currently the most widely used standard regimen is 100 mg/m2 cisplatin every 3 weeks combined with radiation (5,24). A common alternative is lower daily CIS dosing during radiotherapy for those unable to tolerate high dose therapy (25,26). Although this is an accepted standard, consistently high locoregional failure rates of 30–40% have been reported and this regimen causes severe toxic side effects, which include nephro-toxicity, oto-toxicity, nausea, weight loss, and vomiting (5,27). Consequently, multiagent chemoradiotherapy has been difficult to use because of the incidence of adverse effects. Cisplatin has been combined with 5-FU (28), paclitaxel (29) and cetuximab (30) in various chemoradiotherapy regimens, which have shown favorable results in overall patient survival but have frequently caused increased morbidity.
Several studies have shown that 2DG enhances the cytotoxic effects of therapeutic agents in vitro such as TNF-α in lymphoma cells (31) as well as topoisomerase inhibitors such as etoposide, camptothecin and hoechst 3342 in cerebral glioma cells (32) and BSO in breast cancer cells (11). Consistent with these results we show that 2DG significantly enhanced the cytotoxicity of CIS in HNSCC cell lines in vitro (Fig. 1A). In addition to the in vitro data, we found similarly enhanced anti-tumor responses with HNSCC xenografts in nude mice where the combination of 2DG and CIS significantly inhibited FaDu and Cal-27 tumor growth and increased overall disease free survival compared to either agent alone (Fig. 2A–D).
Efforts to decrease the toxicity of CIS based regimens have typically employed more frequent dosing with lower doses to avoid high peak serum levels (29,33). In an attempt to mimic these different types of CIS dosing strategies, we employed two CIS dosing regimens; one with frequent small doses and another with less frequent but higher doses. 2DG in combination with CIS was administered in low daily doses and high weekly dosing schedules. The lower daily dosing schedule was chosen because preliminary studies had shown these doses to be well tolerated, while the high weekly dosing schedule was chosen for comparison to a common clinically relevant schedule. Although 2DG in combination with CIS in both dosing schedules resulted in significant tumor growth inhibition in both tumor types compared to control or 2DG only treated tumors (Fig. 2A,B), 2DG+CIS in the high weekly dosing schedule (2DG+CIS*) did not inhibit growth as effectively in the FaDu tumors as in the Cal-27 tumors (Fig. 2A,B). The reason for this is not known at this time but we speculate that CIS may be more bioavailable in the lower daily dosing schedule. Overall, these data strongly suggest that 2DG may be able to enhance the cytotoxic effects of chemotherapeutic agents currently used in the clinic that have been suggested to exert their toxicity via oxidative stress.
Because CIS based chemoradiotherapy is the standard of care for many patients with head and neck cancer, we determined if the combination of 2DG with CIS+radiation would improve anti-cancer responses in vitro and in vivo. Figure 4A shows in FaDu human head and neck cancer cells that 2DG+CIS+FIR significantly enhanced tumor growth inhibition in vitro as well as increasing overall disease free survival in vivo (Fig. 4B,C Table 2), as compared to CIS+FIR. These results provide strong justification for pursuing the potential of 2DG to serve as a relatively non-toxic adjuvant to the standard of care (CIS + FIR) for head and neck cancer patients.
Integration of 2DG into the therapy of head and neck cancer exploits a basic difference between normal and cancer cells and has the advantage of being linked to imaging assessment where treatment and imaging can be seamlessly integrated. Fundamental differences in glucose metabolism between transformed and normal cells are used clinically for imaging cancerous tissues using tracer amounts of FDG with PET (15). FDG uptake measurements using PET imaging have suggested that glucose metabolism may correlate directly with the degree of malignancy and resistance to treatment (16). Although the precise mechanisms responsible for these relationships have not been determined, there do seem to be strong correlations between glucose uptake, glycolysis and treatment resistance in tumors (34). More specifically, tumors with lower FDG uptake tend to respond better to standard treatments than those with higher FDG uptake (18,35). These results suggest that new adjuvants to chemoradiotherapy are needed to treat head and neck tumors with high FDG uptake.
Tumors with high FDG uptake may represent tumors with high metabolic production of hydroperoxides and thus increased susceptibility to 2DG-induced radio-/chemo-sensitization. Using this logic we predicted that 2DG should sensitize tumors with greater FDG uptake to a greater extent to agents that further increase hydroperoxide production and metabolic oxidative stress, relative to low FDG-uptake tumors. At all tumor sizes, Cal-27 tumors had significantly greater SUV values than FaDu tumors (Fig. 3A,B). Additionally, Cal-27 responded very well to 2DG+CIS in both dosing schedules while FaDu only responded well to 2DG+CIS at the low dosing schedule with respect to tumor growth. (Fig. 2A,B). In addition, more apparent differences between the tumor types were noted in overall median survival times (Table 1). The differences were pronounced in the 2DG treatment group where the Cal-27 tumor bearing mice had over 10 times the median survival time as compared to the FaDu tumor bearing mice (Table 1). Additionally Cal-27 tumor bearing animals had significantly longer median survival for 2DG+CIS in both dosing schedules (Table 1), relative to mice with FaDu tumors. Furthermore, overall disease free survival in the Cal-27 tumor bearing animals with 2DG+CIS was greater than that seen in animals with FaDu tumors treated in a similar fashion. This suggests that the increased FDG uptake in Cal-27 tumors correlated positively with greater sensitivity to 2DG and 2DG+CIS compared to FaDu tumors, which demonstrated lower FDG uptake. These data support our hypothesis that HNSCC tumors with higher FDG uptake (as determine in vivo by PET imaging) are more susceptible to 2DG and 2DG+CIS.
The present studies support the hypothesis that 2DG in combination with CIS could be useful in enhancing the efficacy of the standard of care (CIS + radiotherapy) for HNSCC without any obvious signs of enhanced normal tissue toxicity. Additionally FDG uptake using PET imaging may be useful as a predictor of responses to 2DG + CIS in human head and neck cancers. Overall, these data provide a rationale for initiating clinical trials to study the efficacy of 2DG in combination with CIS chemo/radiotherapy.
Acknowledgments
This work was supported by: NIH RO1-CA100045 (DRS), NIH P01-CA66081 (ALS), NIH P30-CA086862 (BJS, MMG, RDH, DRS), T32 CA078586-08 (MAF, ALS), RSNA HPSD0602 (DMM), and the Department of Radiation Oncology at the University of Iowa (DMM, KJD, JMB). The authors thank Amanda Kalen, Mitchell Coleman and Ling Li in the Radiation and Free Radical Research Core Lab in the Holden Comprehensive Cancer Center at the University of Iowa for technical assistance and advice and Julie A. Riggert, Christine A. Mundt, and Dr. Laurie L. Boles-Ponto from the UIHC PET Imaging Center for data analysis assistance.
Footnotes
CONFLICT OF INTEREST
The authors do not have a conflict of interest, either direct, potential, or implied.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Caravalho AL, Nishimoto IN, Caifano JA, et al. Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. Int J Cancer. 2005;114:806–816. doi: 10.1002/ijc.20740. [DOI] [PubMed] [Google Scholar]
- 2.Forastiere AA. Overview of platinum chemotherapy in head and neck cancer. Semin Oncol. 1994;21:20–27. [PubMed] [Google Scholar]
- 3.Bourhis J, Le Maitre A, Baujat B, et al. Individual patients’ data meta-analyses in head and neck cancer. Curr Opin Oncol. 2007;19(3):188–194. doi: 10.1097/CCO.0b013e3280f01010. [DOI] [PubMed] [Google Scholar]
- 4.Marcu L, van Doorn T, Olver I. Cisplatin and radiotherapy in the treatment of locally advanced head and neck cancer. Acta Oncologica. 2003;42(4):315–325. doi: 10.1080/02841860310004364. [DOI] [PubMed] [Google Scholar]
- 5.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 6.Zackrisson B, Mercke C, Strander H, et al. A systematic overview of radiation therapy effects in head and neck cancer. Acta Oncol. 2003;42(5–6):443–461. doi: 10.1080/02841860310014886. [DOI] [PubMed] [Google Scholar]
- 7.Dornfeld K, Simmons JR, Karnell L, et al. Radiation doses to structures within and adjacent to the larynx are correlated with long-term diet- and speech-related quality of life. Int J Radiat Oncol Biol Phys. 2007 Apr 6; doi: 10.1016/j.ijrobp.2007.01.047. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.El-Deiry M, Funk GF, Nalwa S, et al. Long-term quality of life for surgical and nonsurgical treatment of head and neck cancer. Arch Otolaryngol Head and Neck Surg. 2005;131:879–885. doi: 10.1001/archotol.131.10.879. [DOI] [PubMed] [Google Scholar]
- 9.Warburg O. On the origin of cancer cells. Science. 1956;132:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 10.Spitz DR, Sim JE, Ridnour LA, et al. Glucose deprivation-induced oxidative stress in human tumor cells: a fundamental defect in metabolism? Ann NY Acad Sci. 2000;899:349–62. doi: 10.1111/j.1749-6632.2000.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 11.Andringa KK, Coleman MC, Aykin-Burns N, et al. Inhibition of glutamate cysteine ligase (GCL) activity sensitizes human breast cancer cells to the toxicity of 2-deoxy-D-glucose. Cancer Res. 2006;66:1605–1610. doi: 10.1158/0008-5472.CAN-05-3462. [DOI] [PubMed] [Google Scholar]
- 12.Lin X, Zhang F, Bradbury CM, et al. 2-Deoxy-D-glucose-induced cytotoxicity and radiosensitization in tumor cells is mediated via disruptions in thiol metabolism . Cancer Res. 2003;63:3413–3417. [PubMed] [Google Scholar]
- 13.Simons AL, Ahmad IM, Mattson DM, et al. 2-Deoxy-D-glucose (2DG) combined with cisplatin enhances cytotoxicity via metabolic oxidative stress in human head and neck cancer cells. Cancer Res. 2007;67(7):3364–3370. doi: 10.1158/0008-5472.CAN-06-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wahl RL, Cody RL, Hutchins GD, et al. Primary and metastatic breast carcinoma: initial clinical evaluation with PET with the radiolabeled glucose analogue 2-[F-18]-fluoro-2-deoxy-D-glucose. Radiology. 1991;179(3):765–770. doi: 10.1148/radiology.179.3.2027989. [DOI] [PubMed] [Google Scholar]
- 15.Hannah A, Scott AM, Tochon-Danguy H, et al. Evaluation of 18 F-fluorodeoxyglucose positron emission tomography and computed tomography with histopathologic correlation in the initial staging of head and neck cancer. Ann Surg. 2002;236(2):208–217. doi: 10.1097/00000658-200208000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz DL, Rajendran J, Yueh B, et al. Staging of head and neck squamous cell cancer with extended-field FDG-PET. Arch Otolaryngol Head Neck Surg. 2003;129:1173–1178. doi: 10.1001/archotol.129.11.1173. [DOI] [PubMed] [Google Scholar]
- 17.Yao M, Graham M, Smith R, et al. Value of FDG PET in assessment of treatment response and surveillance in head-and-neck cancer patients after intensity modulated radiation treatment: A preliminary report. Int J Radiation Oncology Biol Phys. 2004;60(5):1410–1418. doi: 10.1016/j.ijrobp.2004.05.058. [DOI] [PubMed] [Google Scholar]
- 18.Dobert N, Kovacs AF, Menzel C, et al. The prognostic value of FDG PET in head and neck cancer. Correlation with histopathology. J Nucl Med Mol Imaging. 2005;49(3):253–257. [PubMed] [Google Scholar]
- 19.Schwartz DL, Rajendran J, Yueh B, et al. FDG-PET prediction of head and neck squamous cell cancer outcomes. Arch Otolaryngol Head Neck Surg. 2004;130(12):1361–1367. doi: 10.1001/archotol.130.12.1361. [DOI] [PubMed] [Google Scholar]
- 20.Anderson ME. Handbook of Methods for Oxygen Radical Research. Florida: CRC Press Inc; 1985. pp. 317–23. [Google Scholar]
- 21.Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 22.Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 23.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 24.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 25.Jeremic B, Shibamoto Y, Stanisavljevic B, et al. Radiation therapy alone or with concurrent low-dose daily either cisplatin or carboplatin in locally advanced unresectable squamous cell carcinoma of the head and neck: A prospective randomized trial. Radiother Oncol. 1997;43:29–37. doi: 10.1016/s0167-8140(97)00048-0. [DOI] [PubMed] [Google Scholar]
- 26.Jeremic B, Shibamoto Y, Milicic B, et al. Hyperfractionated radiation therapy with or without concurrent low-dose cisplatin in locally advanced squamous cell carcinoma of the head and neck: A prospective randomized trial. J Clin Oncol. 2000;18:1458–1464. doi: 10.1200/JCO.2000.18.7.1458. [DOI] [PubMed] [Google Scholar]
- 27.Bachaud JM, Cohen-Jonathan E, Alzieu C, et al. Combined postoperative radiotherapy and weekly cisplatin infusion for locally advanced head and neck carcinoma: final report of a randomized trial. Int J Radiat Oncol Biol Phys. 1996;36(5):999–1004. doi: 10.1016/s0360-3016(96)00430-0. [DOI] [PubMed] [Google Scholar]
- 28.Taylor SG, Murthy AK, Griem KL, et al. Concomitant cisplatin/5-FU infusion and radiotherapy in advanced head and neck cancer: 8-year analysis of results. Head Neck. 1997;19:684–691. doi: 10.1002/(sici)1097-0347(199712)19:8<684::aid-hed6>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Garden AS, Harris J, Vokes EE, et al. Preliminary results of Radiation Therapy Oncology Group 97-03: a randomized phase II trial of concurrent radiation and chemotherapy for advanced squamous cell carcinomas of the head and neck. J Clin Oncol. 2004;22:2856–2864. doi: 10.1200/JCO.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Pfister DG, Su YB, Kraus DH, et al. Concurrent cetuximab, cisplatin, and concomitant boost radiotherapy for locoregionally advanced, squamous cell head and neck cancer: a pilot phase II study of a new combined-modality paradigm. J Clin Oncol. 2006;24:1072–1078. doi: 10.1200/JCO.2004.00.1792. [DOI] [PubMed] [Google Scholar]
- 31.Halicka HD, Ardelt B, Li X, et al. 2-Deoxy-D-glucose enhances sensitivity of human histiocytic lymphoma U937 cells to apoptosis induced by tumor necrosis factor. Cancer Res. 1995;55(2):444–449. [PubMed] [Google Scholar]
- 32.Dwarakanath BS, Khaitan D, Ravindranath T. 2-deoxy-D-glucose enhances the cytotoxicity of topoisomerase inhibitors in human tumor cell lines. Cancer Biol Ther. 2004;3(9):864–870. doi: 10.4161/cbt.3.9.1040. [DOI] [PubMed] [Google Scholar]
- 33.Jeremic B, Milicic B, Dagovic A, et al. Radiation therapy with or without concurrent low-dose daily chemotherapy in locally advanced, nonmetastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2004;22(17):3540–3548. doi: 10.1200/JCO.2004.10.076. [DOI] [PubMed] [Google Scholar]
- 34.Stokkel MPM, ten Broek FW, van Rijk PP. The role of FDG PET in the clinical management of head and neck cancer. Oral Oncology. 1998;34:466–471. doi: 10.1016/s1368-8375(98)00050-5. [DOI] [PubMed] [Google Scholar]
- 35.Kitagawa Y, Nishizawa S, Sano K, et al. FDG-PET for the prediction of tumor aggressiveness and response to intra-arterial chemotherapy and radiotherapy in head and neck cancer. Eur J Nucl Med. 2003;30:63–71. doi: 10.1007/s00259-002-0978-z. [DOI] [PubMed] [Google Scholar]