Abstract
The role of adipokinetic hormones (AKHs) in the regulation of carbohydrate and lipid metabolism and flight performance was evaluated for females of the African malaria mosquito, Anopheles gambiae. Injection of various dosages of synthetic Anoga-AKH-I increased carbohydrate levels in the haemolymph and reduced glycogen reserves in sugar-fed females but did not affect lipid levels. Anoga-AKH-I enhanced the flight performance of both intact and decapitated sugar-fed females, during a 4 hour flight period. Anoga-AKH-II had no effect on carbohydrate or lipid levels or flight performance, thus its function remains unknown. Targeted RNA-interference lowered Anoga-AKH receptor expression in sugar-fed females, consequently injections of Anoga-AKH-I failed to mobilize glycogen reserves. Taken together, these results show that a primary role for the neurohormone, Anoga-AKH-I, is to elevate trehalose levels in the haemolymph of female mosquitoes.
Keywords: Diptera, adipokinetic hormone, neuropeptide, metabolism, flight
Introduction
Energy expenditure while flying, running, and swimming requires the mobilization of metabolites in insects, and the storage and release of carbohydrates, lipids, proteins, and amino acids are strongly under endocrine control (Gäde, 2004). Neuropeptides in the adipokinetic hormone family are important regulators of energy metabolism, and more than 40 peptide isoforms in this family – herein called AKHs – have been extensively characterized across the insect orders (Gäde et al., 1997; van der Horst, 2003; Gäde, 2004). The number of AKHs known to exist in a particular insect species varies from one in the lower orders, such as Odonata, to as many as four in Blattodea and Orthoptera, and in the higher order of Diptera, only one or two AKHs are known. AKHs occur as octa-, nona-, or decapeptides and are characterised by a pyroglutamate at the amino-terminus, two conserved aromatic amino acid residues at position 4 (in most cases a phenylalanine) and a tryptophan at position 8, and an amidated carboxy-terminus. The blocked termini make it only accessible to endopeptidases. Present in all life stages, these peptides are synthesised and secreted from a distinct region of the corpora cardiaca (CC), a neurohaemal gland connected to the brain that also contains intrinsic neurosecretory cells. Other cell types in the brain and ganglia may also synthesize such peptides, as shown by immunocytochemistry in females of the yellow fever mosquitoes Aedes aegypti (Brown and Lea, 1988), and of the African malaria mosquito Anopheles gambiae (Kaufmann and Brown, 2006), as well as in other insect species (Schooneveld et al., 1985; Kodrik et al., 2003).
The primary endocrine function of AKH is to mobilize metabolites from storage to the haemolymph, and this has been demonstrated in several different insects. In the migratory locust, Locusta migratoria, injection of species-specific AKH mobilized lipids, an adipokinetic or hyperlipaemic effect (van der Horst, 2003). In the American cockroach, Periplaneta americana, it increased the trehalose in the haemolymph, a hypertrehalosaemic effect (Scarborough et al., 1984). Similarly, it mobilized proline in dung beetles, Scarabaeus spp. and the fruit beetle, Pachnoda sinuata, revealing a hyperprolinaemic effect (Gäde and Auerswald, 2002). In larvae of D. melanogaster, manipulation of AKH gene expression altered not only circulating levels of trehalose and lipid, but also affected general locomotor activity and survival of imagos during starvation (Lee and Park, 2004).
The expression of two AKH genes (Anoga-AKHs) and a putative G protein-coupled receptor for AKH (Anoga-AKHR) in female A. gambiae (Kaufmann and Brown, 2006) suggest that at least one of the AKHs may be involved in the mobilization of metabolites for flight, as known for other insects (Gäde and Auerswald, 2002; van der Horst, 2003). Both carbohydrates and lipids are used as flight substrates in females of this species (Kaufmann and Briegel, 2004). Other dipteran AKHs have been identified (Table 1), and their bioactivity reported for the blow fly, Phormia terraenovae (Gäde et al., 1990), the horse fly (Tabanus spp., Woodring and Leprince, 1992), the tsetse fly, Glossina morsitans (Pimley, 1984; Mwangi and Awiti, 1989), and the fruit fly, D. melanogaster (Lee and Park, 2004). In this study, we first tested the synthetic Anoga-AKHs for effects on metabolite mobilization in female A. gambiae, and then examined whether these peptides also affected female flight performance. In addition, this in vivo approach was combined with targeted RNA interference of Anoga-AKHR gene expression to link the endocrine effect of an Anoga-AKH to the putative AKHR.
Table 1.
AKH family members known for Diptera
| Name | Sequence | Species |
|---|---|---|
| Tabat–AKH | pQLTFTPGWa | T. atratus1 and other Tabanus spp.2 |
| –HoTH | pQLTFTPGWGYa | T. atratus1 and other Tabanus spp.2 |
| Phote–HrTH | pQLTFSPDWa | P. terraenovae3, D. melanogaster4, and N. bullata5 |
|
Anoga–HrTH
(Anoga–AKH–I) |
pQLTFTPAWa | A. gambiae6 |
| –AKH–II | pQVTFSRDWNAa | A. gambiae6, A. aegypti7, and C. pipiens8 |
| Aedae–AKH–I | pQLTFTPSWa | A. aegypti7 and C. pipiens8 |
Bold letters illustrate conserved amino acids.
AKHs in T. calens T. lineola T. proximus T. suluciforns same as in T. atratus (Woodring and Leprince, 1992).
Predicted in the A. aegypti genome data base, Aedae-AKH-I: GeneID, AAEL011996; and AKH-II same in A. gambiae and A. aegypti: GeneID, AAEL010950.
Predicted in the C. pipiens genome data base, Aedae-AKH-I or Culpi-AKH-I: GeneID, 145648990; and AKH-II same in A. gambiae, A. aegypti, and C. pipens: GeneID, 145648990.
Material and Methods
Mosquitoes
The colony of Anopheles (Cellia) gambiae s.s. (Giles), strain 16CSS from Lagos, Nigeria, was maintained at 26 ± 1°C under long-day conditions (16 h light, 8 h dark). Larvae were raised in trays (24 × 16 × 6 cm) with 350 ml distilled water and fed pulverized Tetramin® daily (Timmermann and Briegel, 1993). Imagos (200 – 300 in cages of 24 × 19 × 18.5 cm) had access to 10% fructose solution or distilled water, depending on experimental conditions. For experiments, large females with wing lengths between 3.2 to 3.5 mm (measured from the alula to the tip, including the fringes) were used. For egg production and experiments, females were given blood meals from a human arm.
Injection of Anoga-AKHs
Synthetic Anoga-AKH-I and -II (Table 1) were dissolved first in dimethyl sulfoxide (DMSO) and then diluted to 2.5% DMSO in Aedes saline (7.5 g NaCl, 0.35 g KCl, and 0.21 g CaCl2 in 1 l nanopure H2O; adjusted to pH 6.5). For control injections, 2.5% DMSO in Aedes saline alone was used. Known amounts of peptide in 0.25 μl of DMSO/saline were injected with fine glass needles into the first abdominal segment of females to ensure the least damage. In blood-fed female mosquitoes, haemolymph volume doubles as water is drawn from the midgut and excreted in the first hour (1 to 2.4 μl in Aedes aegypti, Clements, 1992), so an injection of 0.25 μl is within physiological tolerance and resulted in <10% mortality in control and experimental females. The needles were made from borosilicate glass capillaries (TW100-6, World Precision Instruments) on a Flaming/Brown Micropipette puller (Model P-97, Sutter Instruments Co.). After injection, females were caged, held at 27°C in a humidified chamber, and not given access to fructose solution or water.
Biochemical analyses
To quantify nutrient reserves, two abdomens from experimental sugar-fed females were pooled, and for experimental blood-fed females, a whole individual or haemolymph from two females was used. Haemolymph was collected by incubating two individuals together in 100 μl Aedes saline on ice. Each female was carefully opened at the segmental line between the last two abdominal segments to allow haemolymph to bleed or diffuse into the saline. After 10 min, 90 μl of the haemolymph solution was collected for assay. Sugar, glycogen and total lipids were measured in the samples using a modified version of Van Handel and Day (1988). For the separation of sugar and lipids 1.6 ml CHCl3-MeOH (v:v, 1:1) and 0.6 ml of distilled H2O was used. Glycogen in the precipitate and sugar in the aqueous fraction were measured with the hot anthrone reaction (2 ml anthrone/tube), with glucose standards (Merck 8337) 0.1% in EtOH (25%). Lipid was quantified by a vanillin-phosphoric acid reaction (1 ml vanillin (Merck 818718)/tube) with 0.1% soybean oil (Sigma S-7381) in chloroform as a standard. Absorbance values for 100 μl/well from processed experimental females and standard samples were measured in 96 well plates by a microplate reader (SpectraMax, at λ = 630/525 nm for carbohydrate/lipid), and converted to μg per female, based on a formula calculated from a regression line derived from the standard sample values. To compare energy content, flight metabolites in Figure 5 were converted to Joules. Bioassays were replicated for two or three cohorts (N = 8 to 10 individuals per cohort). Statistical analyses were performed with ANOVA (JMP Version 5.1.2).
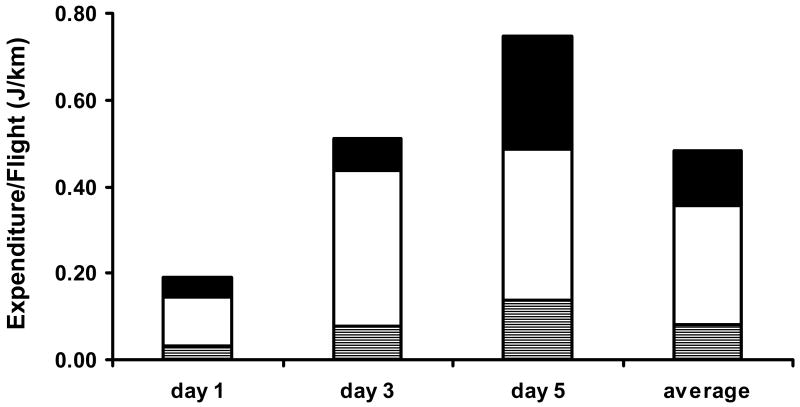
Figure 5.
Energy expenditure (cal/km) by sugar-fed A. gambiae females at day 1, 3, and 5 after eclosion (N = 11-13 per day) after a 4 h flight: glucose (gray), glycogen (white), and lipid (black).
Flight-mill system
To determine whether Anoga-AKH affects female flight performance, a modified flight-mill system, originally described by Rowley at al. (1968), was used to measure the flight distance over a certain time period (Kaufmann and Briegel, 2004). A female mosquito was mounted on one arm of the flight-mill, and a female unable to fly (wings glued together) acted as a counterbalance on the other arm. The number of revolutions (32.7 cm circumference) powered by the female was registered by a computer at 30 s intervals to give total flight distances over a known time period and simultaneously the temporal pattern of flight activities. Resting periods were also recorded; for additional details, see Briegel et al. (2001).
Flight-trials were set up with 5 to 8 cohorts of up to 10, 4 day old females, given continuous access to a 10% fructose solution after eclosion. It was found that decapitated females flew as well as intact ones, and because decapitation eliminated hormonal factors released from the brain and CC that may affect flight performance, decapitated females also were tested in the flight experiments. After decapitation, females were held for 1 h to allow the wound to close, and then half of these or the intact females were injected with saline and the others with 50 pmol of the Anoga-AKHs. Flights started at around 3:00 pm and lasted for 20 h at room temperature (24 ± 2°C) in a natural light regimen. Only flights of more than 500 m per 20 h trial were included in the data set that was analyzed for just the first 4 h of flight. Statistical analysis for all time points (1, 1.5, 2, 4 h) were performed with 2-way ANOVA (N = 10-15, JMP Version 5.1.2). In addition, flight metabolites in females given 10% fructose for up to 5 days after eclosion were quantified after 4 hours on the flight mill, as described in Kaufmann and Briegel (2004).
Reverse Transcriptase Polymerase Chain Reaction and RNA interference
To obtain a cDNA template for the reverse transcriptase polymerase chain reaction (RT-PCR), abdominal body walls were dissected in Aedes saline, immediately transferred into 50 μl RNAlater™ (Sigma) for storage at 4°C overnight and then at -80°C until processed. Total RNA was extracted with the RNeasy® Mini kit, treated with DNase digestion (Qiagen), and used to make cDNA with the Clontech Advantage™ RT-for-PCR Kit and the oligo (dT)18 reverse primer.
For the RNA interference (RNAi) experiment, cDNA fragments (∼200 bp) of the amino (N)- and carboxy (C)-termini of the Anoga-AKHR (GeneID: 1269400, Locus tag: ENSANGG00000016873, GenBank: DQ396551) were amplified by RT-PCR from the abdomen cDNA and specific primer pairs tailed by the T7-promotor sequence (Table 2). Conditions were set according to Titanium Tag PCR kit: initial denaturation, 4 min at 95°C; denaturation for 20 s at 95°C; 20 s at 60°C for annealing, and 45 s extension for the amplification at 72°C for 32 cycles, followed by a 5 min 72°C incubation. dsRNA was generated from the Anoga-AKHR cDNA fragments with the MEGAscript® RNAi Kit (Ambion). Concentrations of dsRNA were estimated after running a 1.5% agarose gel with a low mass leader as marker (Invitrogen, Cat. No. 10068-013). Approximately 200 ng Anoga-AKHR dsRNA/0.25 μl were injected into freshly eclosed females from three different cohorts (N = 2 to 5 individuals/cohort), and the same amount of enhanced green fluorescent protein (EGFP) dsRNA was injected into females, as a control because this gene does not exist in the mosquito genome. The females had access to 10% fructose, and two days after dsRNA injection, Anoga-AKHR expression in females was visualized by RT-PCR of the ORF regions of the Anoga-AKHR gene. PCR conditions were the same as above, except an amplification time of 1 min and 30 s was used, and set up included lanes for single primer and “no template” PCR controls, as well as amplification of an Anoga-actin gene (GeneID: 1275175, Locus tag: ENSANGG00000012550, primers in Table 2) to check cDNA integrity. PCR products were separated in 1.5% agarose gels, and pictures of the gels were taken with GeneSnap 6.03 (SynGene) and transferred to Photoshop 7.0 (Adobe).
Table 2.
Primers for RT-PCR
| Primer name: | Sequence 5′-3′ | ORF (bp) |
|---|---|---|
| AKHR-for | CAG CCA GCC AGC CAG AAC | |
| AKHR-rev | GGA GCG TTA GTA ACA TGG AAT GAA GTG | 1110 |
| Actin-for | CCC GCT GAA CCC GAA GGC TAA CC | |
| Actin-rev | GTA CCA CCG GAC AGG ACA G | 585 |
| N-term-T7-for | T7– CAG CCA GAA CAA TGC CCA ACA CAA | |
| N-term-T7-rev | T7– GCC AGT ATG CTG AGC ACC GTA A | 267 |
| C-term-T7-for | T7– TAT GGT ACT GGC TCG ACA AGG AGT | |
| C-term-T7-rev | T7– ATG TTA CTA ACG CTC CTA CGC CCT | 253 |
| T7-sequence | TAA TAC GAC TCA CTA TAG G | – |
ORF: open reading frame spanning of the forward (for) and reverse (rev) primer pair; AKHR: adipokinetic hormone receptor of Anopheles gambiae (Kaufmann and Brown, 2006); N-term and C-term-T7 primer set represent the forward and reverse primer set of the N- and C-terminus of the AKHR and T7 sequence was added for dsRNA synthesis.
Results
Metabolic effects of Anoga-AKHs
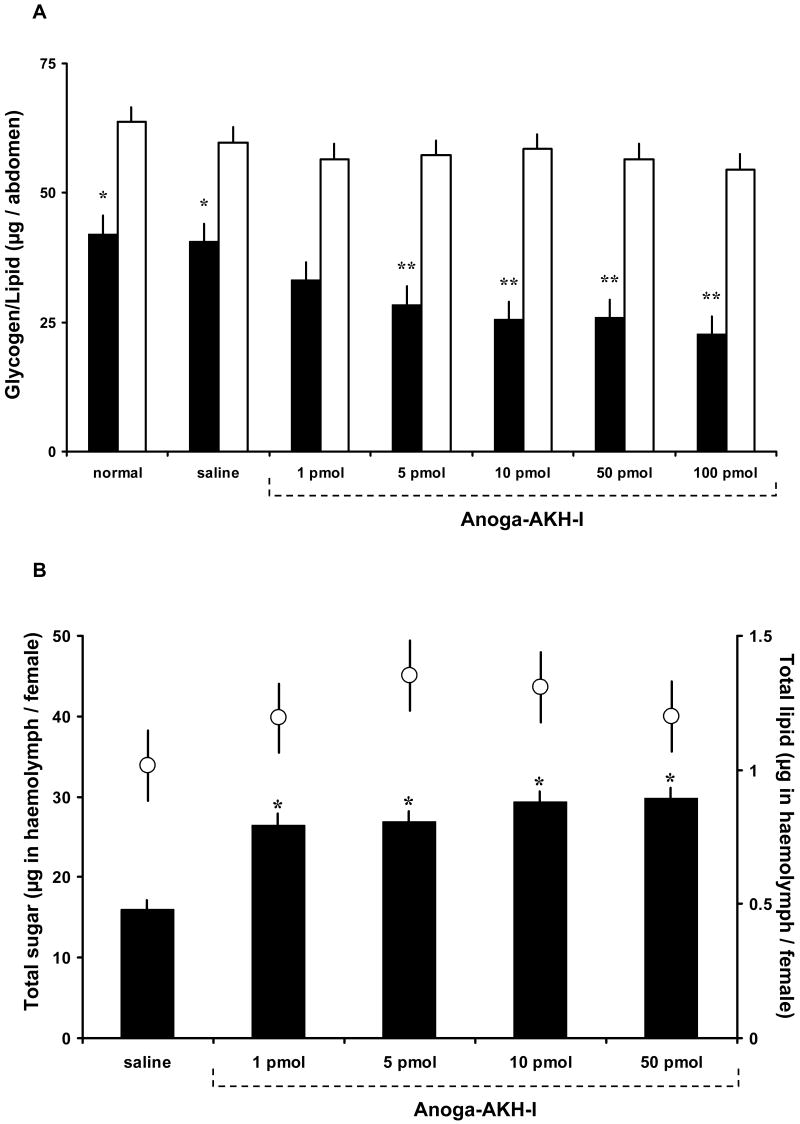
Synthetic Anoga-AKH-I and -II were injected over a dose range of 1 to 100 pmol separately into groups of four day old A. gambiae females given access to 10% fructose solution for the first three days. After 1.5 h, total glycogen and lipid content of abdomens and sugar content in haemolymph were assayed. Anoga-AKH-I doses of 5 pmol and higher significantly reduced glycogen levels but not lipid levels, relative to that of control females (Fig. 1 A). As well, haemolymph trehalose content was significantly increased in females given 1 pmol and higher doses of Anoga-AKH-I, but there was no effect on lipid levels (Fig. 1B). Anoga-AKH-II had no effect on glycogen, lipid, or trehalose levels in females over the dose range shown in Figures 1 A and B (data not shown).
Figure 1.
Glycogen and lipid levels in sugar-fed female A. gambiae treated with different doses of Anoga-AKH-I. (A) Amount of glycogen (black bars) and total lipid (white bars) in abdomens of females injected with Anoga-AKH-I after 1.5 h incubation. Columns with one asterisk differ significantly from columns with two asterisks (Tukey-Kramer HSD test, p ≤ 0.05; M±S.E.; 3 cohorts, n = 10/cohort). (B) Amount of soluble carbohydrates (black bars) and lipid (white circles) in haemolymph of females treated as in A. Carbohydrates increased significantly in females treated with all doses of Anoga-AKH-I, relative to that of control females. Lipid levels (average of 1.1 ± 0.2 μg / female) of Anoga-AKH-I and control females showed no statistical difference. Asterisks indicate significant differences to saline controls (Tukey-Kramer HSD test, p ≤ 0.05; M±S.E.; 3 cohorts, N = 8-10/cohort).
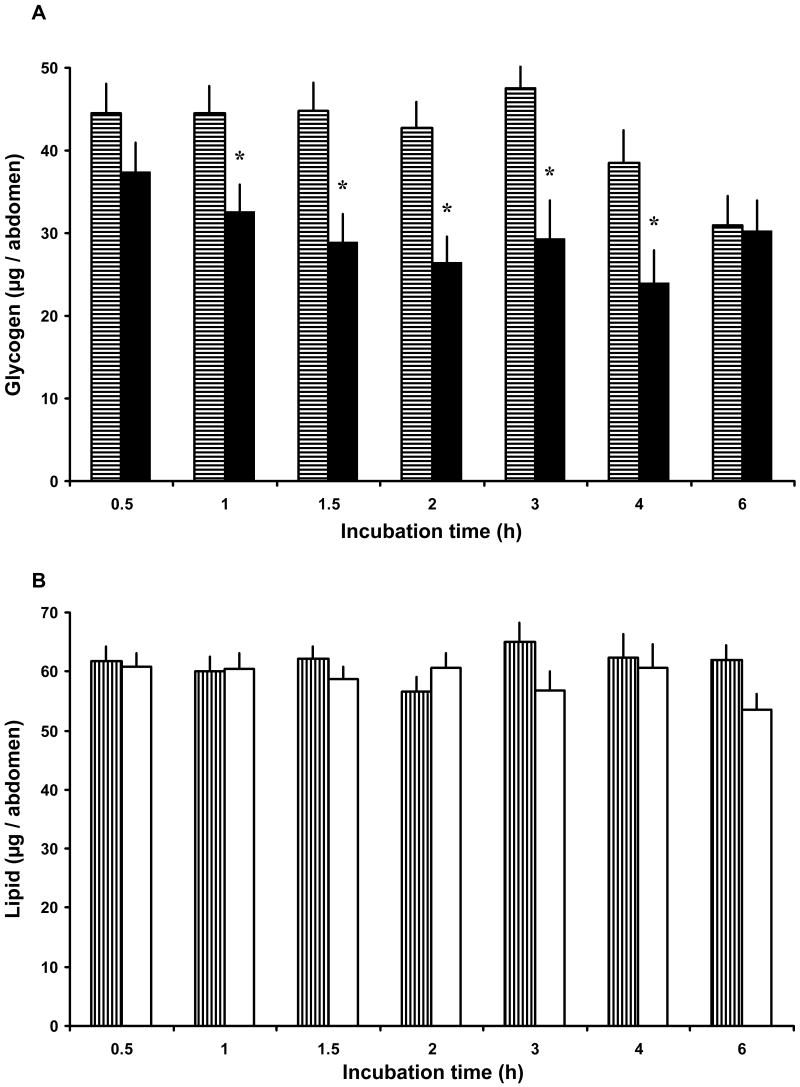
To determine the temporal efficacy of Anoga-AKH-I treatment, 4 day old females, previously given access to the fructose solution, were injected with 50 pmol and assayed at different times thereafter. This high dose was administered with the hope that its action would persist for a few hours, because the half-life of AKHs is limited to tens of minutes by the action of endopeptidases in locusts (Oudejans et al., 1996). By 1 h post injection, glycogen levels had significantly decreased relative to that of control females, and this effect persisted up to 4 h post injection. By 6 h post injection, there was no difference in the glycogen levels of saline and peptide-treated females (Fig. 2 A). Because this dose had a significant effect for up to 4 h, it was used in subsequent flight performance experiments. As before, this dose had no detectable effect on lipid levels over the 6 h period (Fig. 2 B). Anoga-AKH-II at this same dose had no effect on carbohydrate or lipid levels over a 6 h period (data not shown).
Figure 2.
Time course response to a single high dose of Anoga-AKH-I (50 pmol) injected into sugar-fed female A. gambiae (4 day old with continuous access to 10% fructose). (A) Glycogen levels in Anoga-AKH-I treated females (black bars) and ones injected with saline (bars with horizontal shading). Total glycogen content was significantly lower in Anoga-AKH-I female between 1 to 4 hours post injection (asterisks indicate significant difference; Tukey-Kramer HSD test, p ≤ 0.05; M±S.E.; 3 cohorts, N = 8/cohort). (B) Total lipid content in Anoga-AKH-I treated females (white bars) did not differ from that of control females (bars with vertical shading) over the 6 h period.
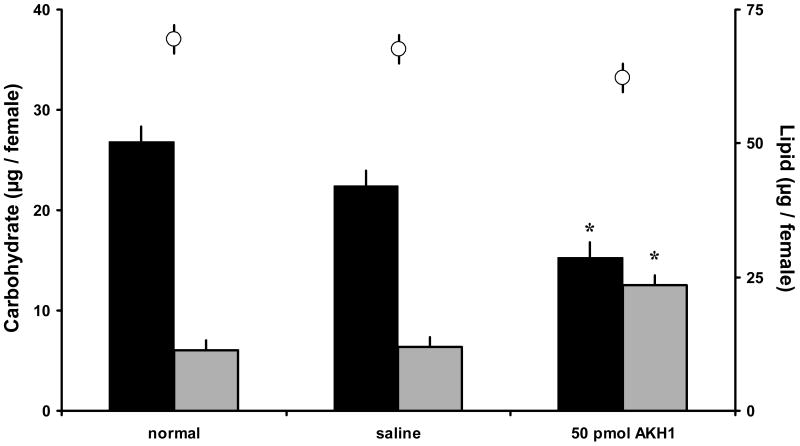
The bioactivity of the two peptides also was tested in females given only water after blood-feeding and oviposition, so that the nutrient reserves were obtained from the blood meal and not a sugar meal (Briegel, 1990). As before, a 50 pmol dose of Anoga-AKH-I significantly decreased glycogen levels and increased haemolymph carbohydrate levels after 1.5 h incubation (Fig. 3), but had no effect on lipid levels in haemolymph.
Figure 3.
Carbohydrate and total lipid levels in blood-fed A. gambiae females (5 days old - post blood-feeding and oviposition; no prior sugar feeding) treated with 50 pmol of Anoga-AKH-I. Glycogen (black bars), haemolymph soluble carbohydrates (gray bars), and lipid (white circles) assayed in individual females after 1.5 h incubation. Anoga-AKH-I significantly increased haemolymph carbohydrate levels and reduced glycogen reserves. Asterisks indicate significant differences to the other treatments (Tukey-Kramer HSD test, p ≤ 0.05; M±S.E.; 3 cohorts, N = 10/cohort).
The mosquito AKHs were assayed for bioactivity in L. migratoria and P. americana to examine their structure-activity relationships for mobilizing metabolites (Gäde, 1992, 1993). These tests were performed in laboratory of Prof. G. Gäde following the procedure of Gäde (1980). Anoga-AKH-I stimulated a hyperlipaemic response in Locusta (Table 3). Anoga-AKH-II was tested because it shares 80% similarity with the fourth Locusta AKH (Locmi-HrTH), which showed hypertrehalosaemic activity in P. americana (Siegert, 1999), and in the assay with this species, the mosquito AKH decapeptide was inactive (Table 4).
Table 3.
Lipid levels in the haemolymph (mg/ml) of Locusta migratoria males (14 to 20 days old) before and 1.5 h after treatment of the same individuals. Anoga-AKH-I significantly increased lipid levels, but not Anoga-AKH-II (M ± S.D., n = 8, paired t-test; bioassay performed in the Gäde laboratory (Gäde, 1980).
| Incubation time | ||||
|---|---|---|---|---|
| Treatment | 0 h | 1.5 h | difference | paired t-test |
| Control | 16.3 ± 4.4 | 15.1 ± 4.1 | -1.2 ± 3.8 | p ≤ 0.55 |
| 0.1 pCC L. migratoria | 14.4 ± 2.6 | 57.4 ± 13.5 | 43.0 ± 11.5 | p ≤ 0.000015 |
| 35 pmol Anoga-AKH-I | 17.7 ± 7.0 | 26.4 ± 5.2 | 8.7 ± 4.4 | p ≤ 0.00080 |
| 70 pmol Anoga-AKH-I | 17.6 ± 6.4 | 41.8 ± 7.2 | 24.2 ± 11.7 | p ≤ 0.00065 |
|
| ||||
| Control | 11.5 ± 2.6 | 14.9 ± 5.1 | 3.4 ± 3.7 | n.s. |
| 0.1 pCC L. migratoria | 11.5 ± 2.2 | 41.2 ± 10.7 | 29.7 ± 10.3 | p ≤ 0.00008 |
| 52 pmol Anoga-AKH-II | 11.1 ± 4.1 | 13.8 ± 5.7 | 2.7 ± 2.1 | n.s. |
Table 4.
Carbohydrate levels in the haemolymph (mg/ml) of Periplaneta americana males (unspecified age) before and 2 h after treatment of the same individuals. Anoga-AKH-II had no effect. (M ± S.D., n = 5, paired t-test; bioassay performed in the Gäde laboratory (Gäde, 1980).
| Incubation time | ||||
|---|---|---|---|---|
| Treatment | 0 h | 2 h | difference | paired t-test |
| control | 10.7 ± 2.9 | 11.7 ± 4.3 | 1.0 ± 2.0 | n.s. |
| 0.1 pCC P. americana | 13.7 ± 2.8 | 28.0 ± 9.1 | 14.3 ± 8.5 | p ≤ 0.04 |
| 52 pmol Anoga-AKH-II | 14.9 ± 2.7 | 16.0 ± 3.5 | 1.1 ± 2.0 | n.s. |
AKHs and flight performance
Given that Anoga-AKH-I mobilized glycogen stores in relatively immobile females, the next step was to determine whether this peptide would enhance female flight performance. Because the effect of a 50 pmol dose of Anoga-AKH-I persisted for at least 4 h in females (Fig. 2 A), both intact and decapitated females were injected with this dose, prior to being mounted on the flight mills. Another reason for using this higher dose was that the half-life of AKHs in flying locusts is much shorter that in resting ones (Oudejans et al., 1996). To optimize the effect of injected peptides, females were decapitated to remove the CC, the putative source of Anoga-AKH-I, and the brain, the source of Anoga-AKH II (Kaufmann and Brown, 2006). By pulling the head back and stretching the neck, the CC is pulled away from the thorax, so that when the head is cut off, both AKH sources are removed, along with other neurohaemal factors that may be released in response to flight.
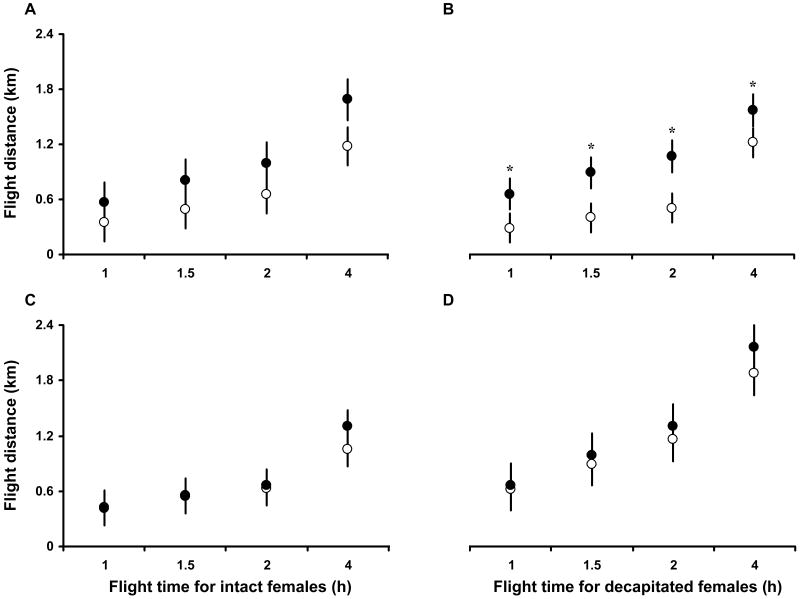
The total distance flown after 4 h was approximately the same for the peptide- and saline-injected intact and decapitated females. The mean distance flown was 1.12 ± 0.20 km and 1.50 ± 0.21 km for intact and decapitated females respectively, thus showing that the flight capacity of decapitated females was not diminished by head removal. The mean difference in distance flown over the time points 1, 1.5, 2, and 4 h was for saline vs. Anoga-AKH-I injected intact or decapitated females 0.35 ± 0.12 km and 0.44 ± 0.10 km, respectively. The distance flown by decapitated females was significantly increased by the injection of Anoga-AKH-I, relative that of control females, over the 4 h period (Fig. 4 B; p ≤ 0.0002, 2-way ANOVA). This effect was less pronounced in intact females injected with this peptide (Fig. 4 A, p ≤ 0.03, 2-way ANOVA). The flight performance of intact and decapitated females was not significantly affected by the injection of Anoga-AKH-II (Figure 4 C and D). For saline vs. Anoga-AKH-II treated intact or decapitated females the mean difference in distance flown was 0.06 ± 0.12 km and 0.14 ± 0.11 km, respectively.
Figure 4.
Distances flown by A. gambiae females (4 day old with access to 10% fructose; attached to flight mills) for up to 4 h post injection with 50 pmol of Anoga-AKH-I (solid circles: A, intact; B, decapitated), Anoga-AKH-II (solid circle: C, intact; D, decapitated), or saline (open circles). Decapitated, Anoga-AKH-I treated females flew a significantly greater distance than controls (B. p ≤ 0.0002; 2-way ANOVA; M±S.E.; N = 10-15), and similarly treated intact females showed the same but non-significant trend (A. p ≤ 0.03).
An additional experiment showed that carbohydrates were the main flight substrate used during a 4 hour flight by intact females up to 5 days old (Fig. 5). Total energy expenditure (Joules used per km flown) increased as females aged from day 1 to day 5, as did the use of lipids. When averaged for the three age groups of females, 74% of the energy used for flight per km was carbohydrate (glucose and glycogen combined) and 26% lipid. This result suggests that the enhanced flight performance of decapitated females injected with Anoga-AKH-I is due to the hypertrehalosaemic effect of this peptide.
RNA interference
To establish the functional pairing of Anoga-AKHR and Anoga-AKH-I, we used RNA interference to silence the Anoga-AKHR gene to test whether the hypertrehalosaemic effect of injected peptide was diminished. Double-stranded RNA for the N- and C-termini of the Anoga-AKHR was injected into newly emerged A. gambiae females, and 48 h later, transcription of the gene in abdomens was greatly reduced, as judged by the faint Anoga-AKHR PCR products (Fig. 6 A). In comparison, the gene products were clearly evident, when amplified from the cDNA of females injected with the control EGFP dsRNA (EGFP; Fig. 6 A), as was the expression of an actin gene in females injected with the receptor dsRNA (Fig. 6 A). When Anoga-AKH-I (50 pmol/female) was injected into dsRNA-treated females from the same cohort as those with lowered Anoga-AKHR expression, it failed to lower glycogen levels in females receiving the receptor dsRNA (N-, and C-terminus; Fig. 6 B). In those females treated with the EGFP dsRNA, it significantly lowered glycogen levels in comparison to the levels in females injected with saline after the earlier treatment with EGFP and Anoga-AKHR dsRNA (Fig. 6 B). These results show that the action of Anoga-AKH-I requires a high expression level of AKHR in female abdomens, and as such indicates that this peptide is a likely ligand for the receptor in vivo.
Figure 6.
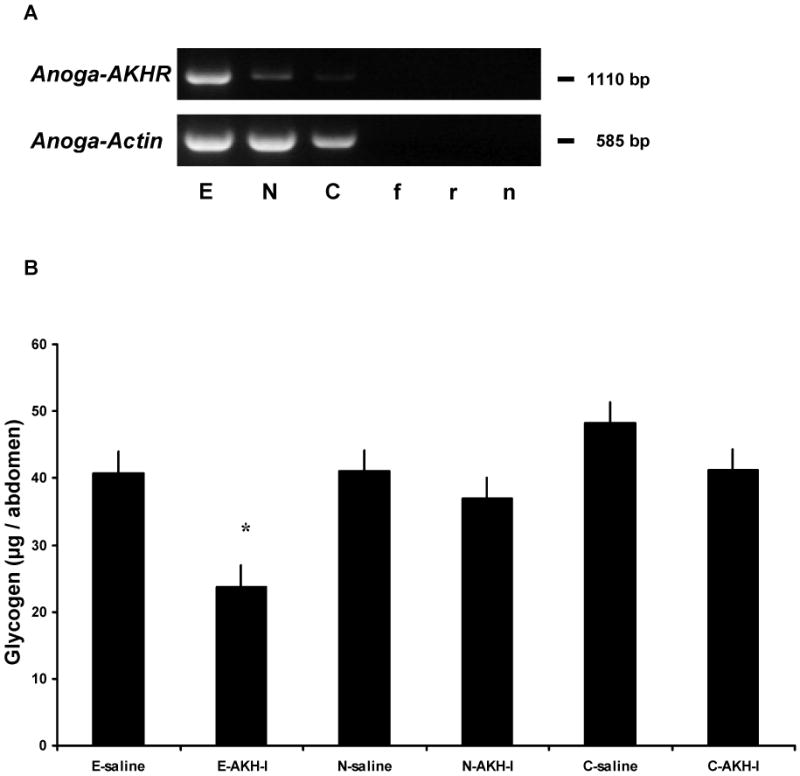
Effects of RNAi in sugar-fed A. gambiae females injected first with Anoga-AKHR-dsRNA and EGFP dsRNA (control) and, 2 days later, 50 pmol of Anoga-AKH-I. (A) Representative gels of Anoga-AKHR and actin PCR products amplified from abdomen cDNA of females treated with dsRNA: E – EGFP; N – amino terminus Anoga-AKHR; and C – carboxy terminus Anoga-AKHR. Bottom of gel, controls for PCR: f – forward Anoga-AKHR primer alone, r – reverse Anoga-AKHR primer, n – no template. Staining intensity of the Anoga-AKHR PCR product was greatly reduced in females injected with Anoga-AKHR dsRNA- lane N and C, versus the control females, lane E. (B) Glycogen content of female A. gambiae injected with EGFP dsRNA followed by Anoga-AKH-I (E-AKH-I) was significantly reduced, but not in females treated with both types of Anoga-AKHR dsRNA (N-AKH-I and C-AKH-I) and then the peptide. Asterisk indicates a significant difference from saline controls (Tukey-Kramer HSD test, p ≤ 0.05; M±S.E.; 2 cohorts, n = 8-10/cohort). This shows that receptor specific dsRNA reduced receptor tissue expression to such a level that injection of Anoga-AKH-I failed to elicit a hypertrehalosaemic response. There was no significant difference in the glycogen content of females treated with the different dsRNA and saline (E-saline, N-saline, and C-saline), thus indicating that the dsRNA treatment had no effect.
Discussion
The primary endocrine function of AKH-related peptides across the great diversity of insects is to mobilize metabolites stored in tissues for energy-requiring activities (Gäde et al., 1997). This includes hyperglycaemia and hyperlipaemia that resulted from the injection of a species-specific CC extract, first reported for the cockroach (Steele, 1961) and locust (Mayer and Candy, 1969), and later from a synthetic AKH, after its characterization from Locusta (Stone et al., 1976). Carbohydrates are mobilized mainly from glycogen reserves in the fat body, resulting in an increased level of soluble carbohydrates in haemolymph. If an AKH induces this mobilization, it is defined as a hypertrehalosaemic hormone (Gäde, 1991), because the main ‘blood-sugar’ in insects is trehalose and not glucose (Wyatt, 1967). As reported herein, Anoga-AKH-I induced this effect in female A. gambiae. A single injection of 1 pmol resulted in a significant increase in soluble carbohydrates in haemolymph, but to achieve an equivalent decrease in glycogen, 5 pmol was required (Fig. 1). A 1 pmol dose is approximately equivalent to the Anoga-AKH content of 4 to 6 female thoraces containing the CC, as determined with a radioimmunoassay (RIA) based on an antiserum to the D. melanogaster AKH (Kaufmann and Brown, 2006), and the total AKH content is likely to be greater, given this amount was determined with a heterologous AKH RIA. As in other insects, trehalose is the main carbohydrate in the haemolymph of adult mosquitoes (Van Handel, 1969), and injection of up to 50 pmol of Anoga-AKH-I increased haemolymph carbohydrates to as high as 30 μg/female (Fig. 1B). These results lead us to designate Anoga-AKH-I as a hypertrehalosaemic hormone in female A. gambiae (abbreviated as Anoga-HrTH). This hormone-induced mobilization of the carbohydrates is similar to the action of glucagon in mammals, and both the propeptide and processed forms of insect AKHs share sequence homology with the glucagon peptide family of vertebrates (Clynen et al., 2004).
On the other hand, pharmalogical doses of Anoga-AKH-I had no effect on lipid levels stored in the body or circulating in the haemolymph of the experimental females (Fig. 1 and 2).
Dipteran AKHs and functions
Mobilization of carbohydrate and lipid stores
Relatively few AKHs are known for Diptera, including two now identified in the genome database of another mosquito, A. aegypti and C. pipiens (Table 1). In mosquitoes (Suborder Nematocera) and horse flies, Tabanus spp. (Suborder Brachycera), there are two processed forms of AKH, an octapeptide and a decapeptide (Jaffe et al., 1988; Kaufmann and Brown, 2006), Table 1). In tests with an intact adult tabanid species, T. atratus AKH decapeptide at 100 pmol/fly induced a significant hyperlipaemic and hyperglycaemic response; and the T. atratus AKH octapeptide (same dose), only a weak hyperglycaemic response (Woodring and Leprince, 1992). To date, only a processed AKH octapeptide with the same sequence has been identified in three higher dipteran species (Suborder Brachycera): blow fly, P. terraenovae (Gäde et al., 1990), fruit fly, D. melanogaster (Schaffer et al., 1990), and flesh fly, Neobellieria bullata (Verleyen et al., 2004) (Table 1). This AKH octapeptide had a hypertrehalosaemic effect in male blow flies (0.75 to 3 pmol/fly) and did not affect lipid levels (Gäde et al., 1990). Only for D. melanogaster larvae are both a hypertrehalosaemic and a hyperlipaemic effect ascribed to this AKH (Lee and Park, 2004). These effects were not achieved by AKH injection, but indirectly by gene ablation of AKH producing cells in the CC and by over expression of the AKH gene in the fat bodies. In the tsetse fly, G. morsitans, only a hypertrehalosaemic response was obtained with species-specific CC extract injected into females (Mwangi and Awiti, 1989), but in vitro studies with female fat bodies and crude CC extracts produced a hyperlipaemic effect (Pimley, 1984). The processed structure of AKH octapeptides is highly conserved across the insects, so much so that Anoga-HrTH produced a significant hyperlipaemic response in Locusta (Table 3), as does another dipteran AKH octapeptide (Gäde, 1993).
The mosquito AKH decapeptide had no activity in our bioassays. Although the Anoga-AKH-II gene structure clearly shows it to be a member of the AKH family (Kaufmann and Brown, 2006), its processed sequence is dissimilar to that of Anoga-HrTH and other dipteran AKHs (Table 3). The lack of bioactivity was not surprising given that the Anoga-AKH-II sequence is highly similar to that of Locusta AKH IV (pQVTFSRDWSPa), which has no known function in Locusta. Both decapeptides have an Arg (R) at position 6, which probably changes its structure that it will not bind to the AKHR. This and the lack of a Gly (G) at position 9 that is typical for larger AKHs (Gäde et al., 1997), might be the reason why no detectable metabolic effect was seen. The locust peptide induced a weak hypertrehalosaemic effect in the cockroach and was named Locmi-HrTH (P. americana; Siegert, 1999), but Anoga-AKH-II had no such activity in the same cockroach (Table 4). Since it has been speculated that both of these decapeptides are synthesised in the brain and not the corpora cardiaca, they are most probably not typical AKHs (Siegert, 1999; Kaufmann and Brown, 2006), and their function remains unknown.
Flight metabolism
For several insect species, AKH octapeptides play an important role during flight (Gäde and Auerswald, 2002; van der Horst, 2003) and other energy consuming activities, such as walking, ball rolling, or swimming (Gäde and Auerswald, 2002; Gäde et al., 2007). Locusts have two phases of flight metabolism (van der Horst, 2003). For flights of a short duration, they mainly depend on carbohydrates, and then for a longer flight, there is a transition to mobilization of lipids for energy, which is regulated by these AKHs.
Typically, adult dipterans do not engage in long flights, but even for short flights, different metabolites provide energy. For the tsetse fly, the main flight substrate is proline (Bursell et al., 1974). Mosquitoes normally utilize carbohydrates during flight (Clements, 1992; Briegel et al., 2001), but when attached to a flight mill, A. gambiae females can fly up to 22 h and use both lipids and carbohydrates over this period (0.38 and 0.29 J/km respectively; (Kaufmann and Briegel, 2004). In our experiments, females of this species used only a third of the lipid (0.13 J/km, Fig. 5) and an equal amount of carbohydrate (0.33 J/km; Fig. 5) for short flights (4 h) compared to longer flight. These results suggest that when A. gambiae females are forced to fly on a mill, carbohydrates are primarily used during the first few hours, which agrees with flight mill experiments performed with a variety of other mosquito species (Clements, 1992). In A. gambiae, however, it was shown that when carbohydrates are exhausted, lipids are mobilized and used (Kaufmann and Briegel, 2004). The mechanism for mobilizing lipids is not known at this time, and it is unlikely that Anoga-HrTH plays a role, because it clearly had no hyperlipaemic effect even in carbohydrate starved, blood-fed, females, which have high lipid and low carbohydrate reserves in comparison to sugar-fed females (Kaufmann and Briegel, 2004). It is also possible that A. gambiae females may use proline as a flight metabolite, as reported for A. aegypti (Scaraffia and Wells, 2003). If this is the case also in A. gambiae, then Anoga-HrTH may be involved in the regulation in proline re-synthesis as well, this is reminiscent to the situation in two beetle species (Gäde and Auerswald, 2002).
Since the half-life of AKHs has been reported to vary from a few minutes to one hour in locusts during flight (Oudejans et al., 1996) and around 18 min in the firebug, Pyrrhocoris apterus (Goldsworthy et al., 2002), a pharmacological dose of Anoga-HrTH (50 pmol/female) was used to achieve a long-lasting hypertrehalosaemic response in the flight experiments (Fig. 4). Decapitated females injected with this dose of Anoga-HrTH had a significantly stronger flight performance and in total flew an average of 0.4 ± 0.1 km more than the control females, and than the control females. This enhanced performance is due to the hypertrehalosaemic effect of the Anoga-HrTH. Interestingly, flight performance was enhanced but not statistically significant in intact females injected with Anoga-HrTH, relative to controls, which suggests that other factors released from the brain or increased protease action may counter the effect of the injected peptide.
The 50 pmol dose of Anoga-HrTH would produce an initial concentration of ∼100 μM in a female containing approximately 0.3 μl of haemolymph (approximate volume reported for newly emerged Anopheles stephensi female, Mack et al., 1979) and 0.25 μl of saline solvent. This is certainly not considered to be a physiological dose, but its effect persisted up to 4 h but not 6 h post injection in non-flying females (Fig. 2), indicating that it did not produce a desensitization response but was eventually degraded to an ineffective concentration.
RNA interference
RNA interference (RNAi) is a highly favoured method for determining the function of gene products in mosquitoes (Brown and Catteruccia, 2006), because injection of gene-specific dsRNA reduces expression of the gene and creates a ‘temporary null mutation’. This approach was used to silence expression of a putative A. gambiae AKHR, previously identified as a homolog of the D. melanogaster AKHR that is generously expressed throughout the body of all A. gambiae life stages (Kaufmann and Brown, 2006). Expression of the Anoga-AKHR likely is fat body-specific in females, and genes in this tissue are amendable to RNAi. Heterologous expression of this receptor did verify that Anoga-HrTH is a ligand (Belmont et al., 2006), but its in vivo action was not reported. As demonstrated in this study, Anoga-AKHR expression was reduced in females by dsRNA injection (Fig. 6A), and a subsequent injection of Anoga-HrTH had no significant effect on their carbohydrate metabolism. Together these results indicate that high expression of the identified AKHR is necessary for Anoga-HrTH to activate a signalling pathway that mobilizes carbohydrates. To our knowledge, this is first time that this approach has been used to investigate peptide hormone/receptor signalling in insects.
This study establishes a foundation for future studies of AKH signalling and function in A. gambiae and other mosquito species. The fitness of female mosquitoes depends on their ability to fly, so that they can find mates, nectar or blood sources, and sites for eggs. Flights to accomplish these activities may be short or long in duration, and as demonstrated herein, the AKH octapeptide mobilizes carbohydrates most likely for short duration flights. Longer flights require lipids as previously demonstrated for A. gambiae females (Kaufmann and Briegel, 2004), and proline may be used, as reported for A. aegypti (Scaraffia and Wells, 2003). These metabolites may be readily used by females in the field where nutrients are more limited. Investigation of proline and lipid metabolism during long flights or in wild females may reveal that AKH signalling is also involved, as reported in the fruit beetle, P. sinuata (Gäde and Auerswald, 2002). Defining the AKH signalling pathway and where it intercedes to mobilize carbohydrates in mosquitoes will allow us to understand the degree to which these mechanisms are conserved across insects.
Acknowledgments
This research was funded by an NIH grant (AI33108) to MRB. The authors wish to thank Gerd Gäde for his advice and comments on this manuscript, and for his tests of Anoga-AKHs in the migratory locust and American cockroach; Kevin Clark for synthesizing the Anoga-AKHs; Michael Strand and Gang Chen for assistance in RNAi experiment; Hans Briegel for reviewing the manuscript. Special thanks to Bruno Betschart for his support as advisor, and Sara Bailey for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belmont M, Cazzamali G, Williamson M, Hauser F, Grimmelikhuijzen CJ. Identification of four evolutionarily related G protein-coupled receptors from the malaria mosquito Anopheles gambiae. Biochemical and Biophysical Research Communications. 2006;344:160–165. doi: 10.1016/j.bbrc.2006.03.117. [DOI] [PubMed] [Google Scholar]
- Briegel H. Fecundity, metabolism, and body size in Anopheles (Diptera: Culicidae), vectors of malaria. Journal of Medical Entomology. 1990;27:839–850. doi: 10.1093/jmedent/27.5.839. [DOI] [PubMed] [Google Scholar]
- Briegel H, Knüsel I, Timmermann SE. Aedes aegypti: size, reserves, survival, and flight potential. Journal of Vector Ecology. 2001;26:21–31. [PubMed] [Google Scholar]
- Brown AE, Catteruccia F. Toward silencing the burden of malaria: progress and prospects for RNAi-based approaches. Biotechniques Supplement. 2006:38–44. doi: 10.2144/000112117. [DOI] [PubMed] [Google Scholar]
- Brown MR, Lea AO. FMRFamide- and adipokinetic hormone-like immunoreactivity in the nervous system of the mosquito, Aedes aegypti. Journal of Comparative Neurology. 1988;270:606–614. doi: 10.1002/cne.902700413. [DOI] [PubMed] [Google Scholar]
- Bursell E, Billing KC, Hargrove JW, McCabe CT, Slack E. Metabolism of the bloodmeal in tsetse flies (a review) Acta Tropica. 1974;31:297–230. [PubMed] [Google Scholar]
- Clements AN. The Biology of Mosquitoes. Chapman & Hall; London: 1992. [Google Scholar]
- Clynen E, de Loof A, Schoofs L. New insights into the evolution of the GRF superfamily based on sequence similarity between the locust APRPs and human GRF. General and Comparative Endocrinology. 2004;139:173–178. doi: 10.1016/j.ygcen.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Gäde G. Characteristics of adipokinetic and hyperglycemic factors of stick insects. Journal of Insect Physiology. 1980;26:351–360. [Google Scholar]
- Gäde G. Hyperglycaemia of hypertrehalosaemia? The effect of insect neuropeptides on haemolymph sugars. Journal of Insect Physiology. 1991;37:483–487. [Google Scholar]
- Gäde G. Structure-activity-relationships for the carbohydrate-mobilizing action of further bioanalogs of the adipokinetic hormone red pigment-concentrating hormone family of peptides. Journal of Insect Physiology. 1992;38:259–266. [Google Scholar]
- Gäde G. Structure-activity-relationships for the lipid-mobilizing action of further bioanalogs of the adipokinetic hormone red pigment-concentrating hormone family of peptides. Journal of Insect Physiology. 1993;39:375–383. [Google Scholar]
- Gäde G. Regulation of intermediary metabolism and water balance of insects by neuropeptides. Annual Review of Entomology. 2004;49:93–113. doi: 10.1146/annurev.ento.49.061802.123354. [DOI] [PubMed] [Google Scholar]
- Gäde G, Auerswald L. Beetles' choice--proline for energy output: control by AKHs. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 2002;132:117–129. doi: 10.1016/s1096-4959(01)00541-3. [DOI] [PubMed] [Google Scholar]
- Gäde G, Wilps H, Kellner R. Isolation and structure of a novel charged member of the red-pigment-concentrating hormone-adipokinetic hormone family of peptides isolated from the corpora cardiaca of the blowfly Phormia terraenovae (Diptera) Biochemical Journal. 1990;269:309–313. doi: 10.1042/bj2690309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gäde G, Hoffmann KH, Spring JH. Hormonal regulation in insects: facts, gaps, and future directions. Physiological Reviews. 1997;77:963–1032. doi: 10.1152/physrev.1997.77.4.963. [DOI] [PubMed] [Google Scholar]
- Gäde G, Simek P, Marco HG. A novel adipokinetic peptide in a water boatman (Heteroptera, Corixidae) and its bioanalogue in a saucer bug (Heteroptera, Naucoridae) Peptides. 2007;28:594–601. doi: 10.1016/j.peptides.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Goldsworthy GJ, Kodrik D, Comley R, Lightfoot M. A quantitative study of adipokinetic hormone of the firebug, Pyrrhocoris apterus. Journal of Insect Physiology. 2002;48:1103–1109. doi: 10.1016/s0022-1910(02)00203-2. [DOI] [PubMed] [Google Scholar]
- Jaffe H, Raina AK, Fraser BA, Keim P, Rao KR, Zhang YS, Lancaster JL, Hayes DK. Isolation of two neuropeptides in the AKH/RPCH-family from horseflies (Diptera) Biochemical and Biophysical Research Communications. 1988;151:656–663. doi: 10.1016/s0006-291x(88)80331-0. [DOI] [PubMed] [Google Scholar]
- Kaufmann C, Briegel H. Flight performance of the malaria vectors Anopheles gambiae and Anopheles atroparvus. Journal of Vector Ecology. 2004;29:140–153. [PubMed] [Google Scholar]
- Kaufmann C, Brown MR. Adipokinetic hormones in the African malaria mosquito, Anopheles gambiae: identification and expression of genes for two peptides and a putative receptor. Insect Biochemistry and Molecular Biology. 2006;36:466–481. doi: 10.1016/j.ibmb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Kodrik D, Socha R, Syrova Z. Developmental and diel changes of adipokinetic hormone in CNS and haemolymph of the flightless wing-polymorphic bug, Pyrrhocoris apterus (L.) Journal of Insect Physiology. 2003;49:53–61. doi: 10.1016/s0022-1910(02)00245-7. [DOI] [PubMed] [Google Scholar]
- Lee G, Park JH. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 2004;167:311–323. doi: 10.1534/genetics.167.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack SR, Foley DA, Vanderberg JP. Hemolymph volume of noninfected and Plasmodium berghei-infected Anopheles stephensi. Journal of Invertebrate Pathology. 1979;34:105–109. doi: 10.1016/0022-2011(79)90088-0. [DOI] [PubMed] [Google Scholar]
- Mayer RJ, Candy DJ. Control of hemolymph lipid concentration during locust flight: an adipokinetic hormone from the corpora cardiaca. Journal of Insect Physiology. 1969;15:611–620. [Google Scholar]
- Mwangi RW, Awiti LRS. Hypertrehalosaemic activity in corpus cardiacum-corpus allatum-aorta complex and adipokinetic response of Glossina morsitans. Physiological Entomology. 1989;14:61–66. [Google Scholar]
- Oudejans RC, Vroemen SF, Jansen RF, van der Horst DJ. Locust adipokinetic hormones: carrier-independent transport and differential inactivation at physiological concentrations during rest and flight. Proceedings of the National Academy of Sciences. 1996;93:8654–8659. doi: 10.1073/pnas.93.16.8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimley RW. Chromatographic separation of some corpora cardiaca peptides that influence fat cell activity in female Glossina morsitans. Insect Biochemistry. 1984;14:521–525. [Google Scholar]
- Rowley WA, Graham CL, Williams RE. A flight mill system for the laboratory study of mosquito flight. Annual Entomological Society of America. 1968;61:1507–1514. [Google Scholar]
- Scaraffia PY, Wells MA. Proline can be utilized as an energy substrate during flight of Aedes aegypti females. Journal of Insect Physiology. 2003;49:591–601. doi: 10.1016/s0022-1910(03)00031-3. [DOI] [PubMed] [Google Scholar]
- Scarborough RM, Jamieson GC, Kalish F, Kramer SJ, McEnroe GA, Miller CA, Schooley DA. Isolation and primary structure of two peptides with cardioacceleratory and hyperglycemic activity from the corpora cardiaca of Periplaneta americana. Proceedings of the National Academy of Sciences. 1984;81:5575–5579. doi: 10.1073/pnas.81.17.5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer MH, Noyes BE, Slaughter CA, Thorne GC, Gaskell SJ. The fruitfly Drosophila melanogaster contains a novel charged adipokinetic-hormone-family peptide. Biochemical Journal. 1990;269:315–320. doi: 10.1042/bj2690315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooneveld H, Romberg-Privee HM, Veenstra JA. Adipokinetic hormone-immunoreactive peptide in the endocrine and central nervous system of several insect species: a comparative immunocytochemical approach. General and Comparative Endocrinology. 1985;57:184–194. doi: 10.1016/0016-6480(85)90262-x. [DOI] [PubMed] [Google Scholar]
- Siegert KJ. Locust corpora cardiaca contain an inactive adipokinetic hormone. Federation of European Biochemical Societies Letters. 1999;447:237–240. doi: 10.1016/s0014-5793(99)00299-9. [DOI] [PubMed] [Google Scholar]
- Steele JE. Occurrence of a hyperglycaemic factor in the corpus cardiacum of an insect. Nature. 1961;192:680–681. [Google Scholar]
- Stone JV, Mordue W, Batley KE, Morris HR. Structure of locust adipokinetic hormone, a neurohormone that regulates lipid utilisation during flight. Nature. 1976;263:207–211. doi: 10.1038/263207a0. [DOI] [PubMed] [Google Scholar]
- Timmermann SE, Briegel H. Water depth and larval density affect development and accumulation of reserves in laboratory populations of mosquitoes. Bulletin of the Society for Vector Ecology. 1993;18:174–187. [Google Scholar]
- van der Horst DJ. Insect adipokinetic hormones: release and integration of flight energy metabolism. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 2003;136:217–226. doi: 10.1016/s1096-4959(03)00151-9. [DOI] [PubMed] [Google Scholar]
- Van Handel E. Metabolism of hexoses in the intact mosquito: Exclusion of glucose and trehalose as intermediates. Comparative Biochemistry and Physiology. 1969;29:413–421. doi: 10.1016/0010-406x(69)91760-5. [DOI] [PubMed] [Google Scholar]
- Van Handel E, Day JF. Assay of lipids, glycogen and sugars in individual mosquitoes: correlations with wing length in field collected Aedes vexans. Journal of the American Mosquito Control Association. 1988;4:549–550. [PubMed] [Google Scholar]
- Verleyen P, Huybrechts J, Sas F, Clynen E, Baggerman G, de Loof A, Schoofs L. Neuropeptidomics of the grey flesh fly, Neobellieria bullata. Biochemical and Biophysical Research Communications. 2004;316:763–770. doi: 10.1016/j.bbrc.2004.02.115. [DOI] [PubMed] [Google Scholar]
- Woodring J, Leprince DJ. The function of corpus cardiacum peptides in horse flies. Journal of Insect Physiology. 1992;38:775–782. [Google Scholar]
- Wyatt GR. The biochemistry of sugars and polysacharides in insects. Advances in Insect Physiology. 1967;4:287–360. [Google Scholar]