Abstract
Few studies have tested the benefits of using peripheral blood stem cell (PBSC) grafts versus bone marrow (BM) grafts for unrelated donor transplantation. Yet there has been a substantial change in clinical practice, with increasing numbers of adults receiving unrelated donor PBSC grafts. We compared outcomes after 331 PBSC and 586 BM transplants in adults with leukemia and myelodysplastic syndrome who were followed for a median of 3 years after transplantation. PBSC recipients were less likely to have chronic myeloid leukemia and more likely to have myelodysplastic syndrome, to have poor performance scores and to be transplanted more recently. Outcomes were analyzed using Cox regression models. Rates of grades 2–4 acute graft-versus-host disease (GVHD) (58% vs. 45%, p<0.001) and chronic GVHD (56% vs. 42%, p<0.001) were significantly higher with PBSC than with BM transplants. Rates of grade 3–4 acute GVHD were similar with PBSC and BM transplants. The 3-year probabilities of treatment-related mortality, leukemia recurrence, leukemia-free and overall survival were similar in the two groups with 3-year leukemia-free survival rates of 30% and 32% after transplantation of PBSC and BM, respectively. Unlike results after HLA-matched sibling donor PBSC transplants, we did not identify a survival advantage with PBSC grafts in patients receiving unrelated donor transplants for advanced leukemia. The higher rate of chronic GVHD after PBSC transplants and, consequently, more frequent late adverse events warrant extended follow up of PBSC recipients.
Keywords: peripheral blood graft, graft-versus-host disease, unrelated donor transplant
INTRODUCTION
Peripheral blood stem cells (PBSC) are increasingly used for related and unrelated donor hematopoietic stem cell transplantation. Collection of PBSC rather than bone marrow (BM) offers several advantages to the donor, namely, avoidance of anesthesia, hospitalization and potential exposure to blood products, though controlled comparisons of PBSC and BM donation do not indicate substantial differences in serious adverse effects. There are numerous reports, including randomized trials, comparing recipient outcomes after PBSC and BM transplants from HLA-matched sibling donors [1–4]. While these studies support a survival advantage with PBSC in recipients transplanted for advanced leukemia, a convincing survival advantage for those with early leukemia is not documented. Most data indicate a higher risk of chronic GVHD as well as severity with PBSC than with BM grafts [5,6]. There are few data available regarding outcomes of PBSC and BM transplants from unrelated donors and none have shown lower survival rates after PBSC transplants [7–10]. Data from the National Marrow Donor Program (NMDP) indicates that approximately 80% of unrelated donor transplants in adults in the U.S. now use PBSC grafts. Therefore, to address the role of PBSC grafts, we analyzed data on 331 recipients of unrelated donor PBSC and 586 recipients of unrelated donor BM transplants facilitated by the NMDP in the U.S. in 2000–2003 and reported to the Center for International Blood and Marrow Transplant Research (CIBMTR).
METHODS
Data collection
A formal affiliation of the research division of the NMDP (established in 1986) and the International Bone Marrow Transplant Registry (established in 1972) led to the establishment of the CIBMTR in 2004. The CIBMTR is a working group of more than 500 transplant centers worldwide that voluntarily contribute data on allogeneic transplant recipients to a Statistical Center at the Medical College of Wisconsin. Participating centers register and provide basic information on all consecutive transplantations. Detailed demographic, disease and transplant characteristics and outcome data are collected on all unrelated donor transplantations facilitated by the NMDP in the U.S. Patients are followed longitudinally. Computerized error checks, physician review of submitted data and on-site audits of participating centers ensure data quality.
Inclusion criteria
The study included patients 18–60 years of age who received PBSC or BM grafts from a volunteer unrelated donor for acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), myelodysplastic syndrome (MDS) or chronic myeloid leukemia (CML) in the U.S. All transplants were facilitated by the NMDP between January 1st 2000 and December 31st 2003 and donor-recipient pairs had to have allele-level typing at HLA-A, -B, -C, -DRB1. Excluded were recipients of T-cell depleted BM or CD34 selected PBSC grafts and reduced intensity preparative regimens. We defined reduced intensity regimens as follows: busulfan dose <9mg/kg, melphalan dose <150mg/m2, and total body irradiation dose <500 cGy (single or fractionated) or 500–800 cGy (fractionated).
The NMDP retrospectively obtained consent for data submission and study participation from surviving patients or their parent/legal guardian for transplantations it facilitated in the U.S during the study period; the NMDP Institutional Review Board waived consent for patients who had died prior to soliciting consent. To address bias introduced by inclusion of only a proportion of surviving patients (those who consented) but all deceased recipients, a sample of deceased patients was selected using a weighted randomized scheme that adjusts for over-representation of deceased patients in the consented cohort. This weighted randomized scheme was developed based on all survivors in the NMDP database. A logistic regression model was fit to identify factors that predicted whether patients had consented or not consented to use of data collected by the NMDP. This analysis found the following factors were associated with the likelihood of a patient consenting: age, disease type, race, sex, cytomegalovirus serologic status and country of transplantation (U.S. vs. non-U.S.). Using estimated consenting probabilities from this model based on the characteristics of dead patients a biased coin method of randomization was performed to determine which dead patients are included in the final sample. Thus, this procedure ensures that the pre-consented dead patients are included in the sample with the same probability as the survivors who actually consented to participate in the study. Approximately 13% of the surviving patients failed to consent, and 12% of the dead patients were deleted by the weighted randomized method. The above-described method was tested several times and on every occasion the proportion of deleted dead patients was similar [11]. Each sample gave essentially the same set of regression parameters and survival estimates.
End points
Neutrophil recovery was defined as achieving an absolute neutrophil count of at least 500 cells per cubic millimeter for three consecutive days; platelet recovery was defined as achieving at least 20,000 platelets per cubic millimeter, unsupported by transfusions for seven days. Incidences of grades 2, 3 and 4 acute GVHD and chronic GVHD were determined in all patients [12–14]. Treatment-related mortality was defined as death during a continuous remission. Relapse was defined as recurrence of leukemia (hematological); patients in whom a remission failed to occur after transplantation were considered to have had a recurrence at day 1. Leukemia-free survival was defined as survival in a state of continuous complete remission.
Statistical analysis
Variables related to patient, disease, and transplant characteristics were compared using the chi-square test for categorical variables and the Kruskal-Wallis test for continuous variables. Probabilities of overall and leukemia-free survival were calculated using the Kaplan-Meier estimator [15]. For analyses of survival, death from any cause was considered an event and data on surviving patients were censored at last follow-up. For analyses of leukemia-free survival, relapse or death (i.e., treatment failure) was considered an event and data for patients alive in continuous remission were censored at last follow-up. Probabilities of neutrophil and platelet recovery, acute and chronic GVHD, treatment-related mortality, and relapse were calculated using the cumulative-incidence-function [15]. For neutrophil and platelet recovery and GVHD, death without an event (hematopoietic recovery or GVHD) was the competing event. For treatment-related mortality (death in continuous complete remission), relapse was the competing event. For relapse, treatment-related death was the competing event. Data on patients without either competing event were censored at last follow-up. Confidence intervals were calculated with the use of a log-transformation [15]. Adjusted probabilities of overall and leukemia-free survival were estimated by Cox’s proportional hazards regression model [16].
Multivariate models were built using a stepwise forward selection technique, using a p-value of 0.05 or less as the criterion for inclusion in the final model. The primary objective was to compare outcomes according to graft type, PBSC versus BM; this variable was included in all models. Other variables considered in the analyses were donor and recipient age, performance score pretransplant, serologic status of the donor and recipient with respect to cytomegalovirus (CMV) before transplantation, sex of the donor and recipient, type of leukemia (ALL vs. AML vs. MDS vs. CML), disease status at transplantation (first complete clinical remission, first chronic phase, refractory anemia vs. other), conditioning regimen (irradiation vs. none), GVHD prophylaxis (cyclosporine-based vs. tacrolimus-based), and HLA disparity (matched at HLA-A, -B, -C and DRB1 vs. 1-allele mismatch vs. 2-allele mismatch). All possible risk factors were checked for proportional hazards using a time-dependent covariate approach and there were no violations to the proportionality assumption. There were no first order interactions between graft type and these other variables. There were no statistically significant center effects [17]. P values are two-sided. Completeness of follow-up (the ratio of the sum of the observed follow up time to the sum of the potential follow up time for all patients in the study) for the study population was 97% [18]. Analyses were completed with the use of SAS software, version 9.1 (SAS Institute, Cary, NC).
RESULTS
Patients
Table 1 shows patient, disease and transplant characteristics. As compared with BM recipients, PBSC recipients were less likely to have CML, more likely to have MDS, more likely to have a poor performance score pre-transplantation, and were transplanted more recently. We observed no differences in patient selection with respect to disease status at transplantation. We could not examine whether cytogenetic risk classification affected the choice of graft type, as this data is not available for two-thirds of patients with acute leukemia. The median total nucleated cell dose of PBSC grafts was higher than that of BM grafts (6.7 x 108/kg, range, 2.0–25.2 vs. 2.9 x 108/kg, range, <1.0 – 21.8, p<0.001). The median period of follow-up for survivors after PBSC and BM transplantation was 34 and 38 months, respectively.
Table 1.
Characteristics of patients who received peripheral blood stem cell and bone marrow transplantations
| Peripheral blood stem cells | Bone marrow | P-value | |
|---|---|---|---|
| Characteristics of patients | Number (%) | Number (%) | |
| Number of patients | 331 | 586 | |
| Age at transplant, years | 0.141 | ||
| 18–30 | 85 (26) | 168 (29) | |
| 31–40 | 79 (24) | 147 (25) | |
| 41–50 | 104 (31) | 193 (33) | |
| 51–60 | 63 (19) | 78 (13) | |
| Male recipient | 184 (56) | 317 (54) | 0.663 |
| Performance score | 0.005 | ||
| 90–100 | 111 (34) | 384 (66) | |
| 10–80 | 181 (55) | 150 (26) | |
| Unknown | 39 (12) | 52 ( 9) | |
| Disease | 0.007 | ||
| ALL | 77 (23) | 138 (24) | |
| AML | 143 (43) | 225 (38) | |
| CML | 66 (20) | 170 (29) | |
| MDS | 45 (14) | 53 ( 9) | |
| Disease status | |||
| AML | 0.006 | ||
| CR1 | 47 (33) | 62 (28) | |
| CR2 | 49 (34) | 114 (51) | |
| Relapse | 47 (33) | 49 (22) | |
| ALL | 0.299 | ||
| CR1 | 28 (36) | 45 (33) | |
| CR2 | 37 (48) | 59 (43) | |
| Relapse | 12 (16) | 34 (25) | |
| MDS | 0.038 | ||
| RA | 20 (44) | 13 (25) | |
| RAEB/RAEBT | 25 (56) | 40 (75) | |
| CML | 0.019 | ||
| CP1 | 28 (42) | 103 (60) | |
| CP2, AP | 33 (50) | 52 (31) | |
| Blast phase | 5 ( 8) | 15 ( 9) | |
| Year of infusion | <0.001 | ||
| 2000 | 45 (14) | 220 (38) | |
| 2001 | 77 (23) | 158 (27) | |
| 2002 | 77 (23) | 96 (16) | |
| 2003 | 132 (40) | 112 (19) | |
| Conditioning regimen | 0.061 | ||
| TBI- regimen | 232 (70) | 444 (76) | |
| Busulfan +cyclophosphamide | 99 (30) | 142 (24) | |
| GVHD Prophylaxis | 0.500 | ||
| Tacrolimus ± other | 163 (49) | 275 (47) | |
| Cyclosporine ± other | 168 (51) | 311 (53) | |
| Donor age at transplant, years | 0.178 | ||
| 18–30 | 102 (31) | 204 (35) | |
| 31–40 | 126 (38) | 221 (38) | |
| 41–50 | 74 (22) | 130 (22) | |
| 51–60 | 29 ( 9) | 31 ( 5) | |
| Donor-recipient sex match | 0.330 | ||
| Male → Male | 113 (34) | 215 (37) | |
| Male → Female | 79 (24) | 159 (27) | |
| Female → Male | 71 (21) | 102 (17) | |
| Female → Female | 68 (21) | 110 (19) | |
| Donor-recipient CMV status | 0.104 | ||
| Donor (−)/Recipient (−) | 90 (27) | 192 (33) | |
| Donor (−)/Recipient (+) | 99 (30) | 155 (26) | |
| Donor (+)/Recipient (−) | 50 (15) | 74 (13) | |
| Donor (+)/Recipient (+) | 59 (18) | 134 (23) | |
| Unknown | 33 (10) | 31 (5) | |
| HLA-A, B, C, DRB1 (allele-level typing) | 0.967 | ||
| Matched | 192 (58) | 341 (58) | |
| 1-allele mismatch | 62 (11) | 44 (13) | |
| ≥2-allele mismatch | 183 (32) | 95 (29) | |
Abbreviations: ALL=acute lymphoblastic leukemia; AML=acute myeloid leukemia; CML=chronic myeloid leukemia; MDS=myelodysplastic syndrome; TBI=total body irradiation; CR=complete clinical remission; CP=chronic phase; AP=accelerated phase; GVHD=graft-versus-host disease; CMV=cytomegalovirus; HLA=human leukocyte antigen.
Hematopoietic recovery
The probability of neutrophil recovery at day 28 was higher after transplantation of PBSC than after BM, 94% (95% CI 91–96) and 87% (95% CI 84–90), respectively, p<0.001. Sixteen of 311 PBSC recipients and 55 of 531 BM recipients did not achieve neutrophil recovery. Similarly, platelet recovery at day-100 was higher after PBSC than after BM transplants, 80% (95% CI 75–84) and 69% (95% CI 65–72), respectively, p<0.001. A similar trend was observed at 1-year (82% [95% CI 77 – 86] vs. 72% [95% CI 68 – 76], p<0.001). Fifty-nine of 330 PBSC recipients and 162 of 586 BM recipients did not achieve platelet recovery.
Graft-versus-host disease
Grade 2–4 acute GVHD occurred in 194 of 330 PBSC and 268 of 585 BM recipients; risks of grade 2–4 acute GVHD were higher after transplantation of PBSC than BM (RR 1.50, 95% CI 1.27–1.81, p<0.001). Grade 3–4 acute GVHD risks were similar in the two groups (RR 1.17, 95% CI 0.90–1.51, p=0.249). The day-100 probabilities of grade 2–4 acute GVHD were 58% (95% CI 53 – 64) and 45% (95% CI 41 – 49) after transplantation of PBSC and BM, respectively, p<0.001 (Figure 1). probabilities of grade 3–4 acute GVHD were 28% (95% CI 24 – 33) and 25% (95% CI 21 – 28).
Figure 1.
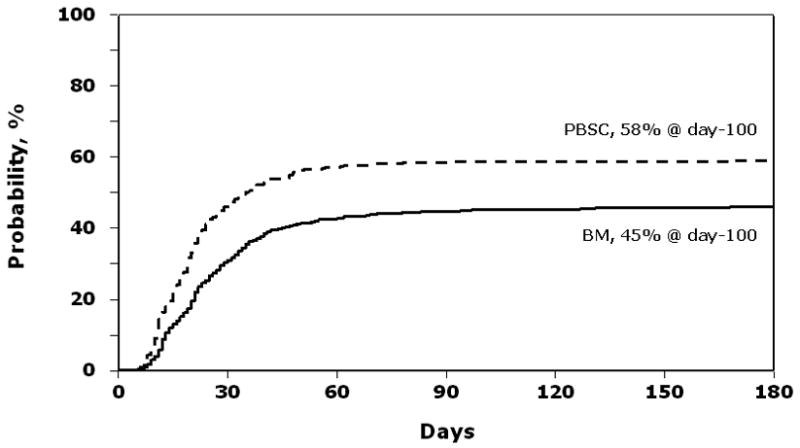
Probability of grade 2–4 acute GVHD after peripheral blood stem cell (PBSC) and bone marrow (BM) transplants
Chronic GVHD occurred in 186 of 331 PBSC and 249 of 586 BM recipients. Risks of chronic GVHD were significantly higher after transplantation of PBSC than after BM (RR 1.72, 95% CI 1.41–2.10, p<0.001). The severity of chronic GVHD did not differ by graft type. The 3-year probabilities of chronic GVHD were 56% (95% CI 51 – 61) and 42% (95% CI 38 – 46) after PBSC and BM transplants, respectively, p<0.001 (Figure 2).
Figure 2.
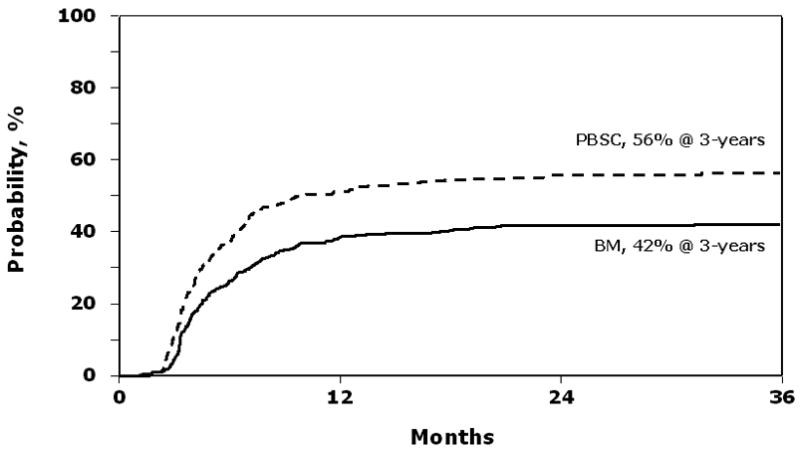
Probability of chronic GVHD after peripheral blood stem cell (PBSC) and bone marrow (BM) transplants
Transplant-related mortality
Transplant-related mortality rates were similar after PBSC and BM transplants (Table 2, 3). Early (within 3 months) transplant-related mortality rates were similar after PBSC and BM transplants, 22% (95% CI 18 – 27) and 26% (95% CI 24 – 28), respectively. Corresponding 3-year probabilities were 44% (95% CI 38 – 50) and 44% (95% CI 40 – 48) after PBSC and BM transplants, respectively.
Table 2.
Results of multivariate analysis of transplant-outcomes after unrelated donor peripheral blood stem cell and bone marrow transplantations
| Outcome | N | Relative Risk (95% confidence interval) | P-value |
|---|---|---|---|
| Transplant-related mortality | |||
| Bone marrow | 577 | 1.00 | |
| Peripheral blood stem cells | 327 | 0.89 (0.73 – 1.10) | 0.293 |
| Relapse | |||
| Bone marrow | 577 | 1.00 | |
| Peripheral blood stem cells | 327 | 0.99 (0.74 – 1.31) | 0.938 |
| Treatment failure | |||
| Bone marrow | 577 | 1.00 | |
| Peripheral blood stem cells | 327 | 0.92 (0.78 – 1.09) | 0.352 |
| Overall mortality | |||
| Bone marrow | 586 | 1.00 | |
| Peripheral blood stem cells | 331 | 0.92 (0.78 – 1.09) | 0.359 |
N=number of evaluable recipients
The relative risks shown above are adjusted for the following:
Transplant related mortality: performance score at transplant, donor-recipient cytomegalovirus status, recipient age and donor-recipient HLA-match.
Relapse: leukemia type, disease status at transplant and donor-recipient HLA-match
Treatment failure and overall mortality: performance score at transplant, recipient age, leukemia type, disease status at transplant, donor-recipient cytomegalovirus status and HLA-match
Table 3.
Results of multivariate analysis of transplant-outcomes after unrelated donor peripheral blood stem cell and bone marrow transplantations by leukemia type and disease status at transplantation
| Outcome | N1/N2 | Relative Risk* (95% confidence interval) | P-value |
|---|---|---|---|
| Transplant-related mortality | |||
| AL/MDS, CR1, RA | 95/119 | 0.78 (0.52 – 1.19) | 0.256 |
| AL/MDS, CR2, Relapse, RAEB, RAEBT | 170/296 | 0.86 (0.65 – 1.16) | 0.328 |
| CL, CP1 | 28/103 | 1.71 (0.97 – 3.01) | 0.062 |
| CL, CP2, AP, BP | 38/67 | 0.97 (0.49 – 1.90) | 0.928 |
| Relapse | |||
| AL/MDS, CR1, RA | 95/119 | 0.83 (0.44 – 1.55) | 0.554 |
| AL/MDS, CR2, Relapse, RAEB, RAEBT | 170/296 | 0.85 (0.60 – 1.19) | 0.345 |
| CL, CP1 | 28/103 | 0.76 (0.09 – 6.31) | 0.800 |
| CL, CP2, AP, BP | 38/67 | 1.30 (0.64 – 2.64) | 0.462 |
| Treatment failure | |||
| AL/MDS, CR1, RA | 95/119 | 0.78 (0.55 – 1.11) | 0.172 |
| AL/MDS, CR2, Relapse, RAEB, RAEBT | 170/296 | 0.85 (0.68 – 1.06) | 0.151 |
| CL, CP1 | 28/103 | 1.64 (0.95 – 2.82) | 0.074 |
| CL, CP2, AP, BP | 38/67 | 1.12 (0.69 – 1.82) | 0.650 |
| Overall mortality | |||
| AL/MDS, CR1, RA | 95/119 | 0.78 (0.55 – 1.11) | 0.174 |
| AL/MDS, CR2, Relapse, RAEB, RAEBT | 170/296 | 0.86 (0.68 – 1.07) | 0.174 |
| CL, CP1 | 28/103 | 1.89 (1.09 – 3.27) | 0.023 |
| CL, CP2, AP, BP | 38/67 | 0.96 (0.58 – 1.59) | 0.889 |
N1=number of evaluable PBSC recipients; N2=number of evaluable BM recipients
Baseline: bone marrow; relative risk of less than 1.00 and p≤0.05 indicate an advantage for peripheral blood stem cell.
Abbreviations: AL=acute leukemia; MDS=myelodysplastic syndrome; CL=chronic myeloid leukemia; CR= clinical remission; RA=refractory anemia; RAEB/RAEBT=refractory anemia with blasts or in transformation; CP=chronic phase; AP=accelerated phase; BP=blast phase
Relapse
Relapse rates were similar after transplantation of PBSC and BM regardless of type of leukemia or whether the transplantation was done for early, intermediate, or advanced disease (Table 2, 3). 3-year probabilities of relapse were 26% (95% CI 21 – 31) and 24% (95% CI 21 – 28) after PBSC and BM transplants, respectively.
Leukemia-free survival
Risks of treatment failure were similar after transplantation of PBSC and BM regardless of type of leukemia or whether the transplantation was done for early, intermediate, or advanced disease (Table 2, 3). 3-year probabilities of leukemia-free survival were 30% (95% CI 25 –36) and 32% (95% CI 28 – 36) after PBSC and BM transplants, respectively (Figure 3).
Figure 3.
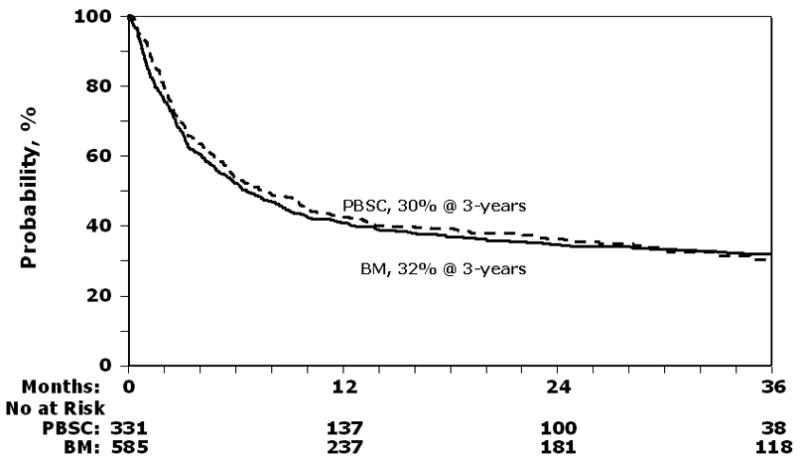
Probability of leukemia-free survival after peripheral blood stem cell (PBSC) and bone marrow (BM) transplants
Overall mortality
Overall, deaths occurred in 216 of 331 PBSC and 384 of 586 BM recipients. of overall mortality were similar after transplantation of PBSC and BM (Table 2). However, for patients with good risk CML (first chronic phase), overall mortality rates appear to be higher after PBSC transplants (Table 3). The 3-year probabilities of overall survival were 32% (95% CI 26 – 37) and 33% (95% CI 29 – 37) after PBSC and BM transplants, respectively. There were no differences in causes of death by graft type. Causes of death included recurrent leukemia (36% vs. 33%), GVHD (14% vs. 13%), interstitial pneumonitis (8% vs. 8%), infections (18% vs. 20%), organ failure (13% vs. 14%) and other causes (11% vs. 12%) after PBSC and BM transplants, respectively.
DISCUSSION
Our primary objective was to compare the effectiveness of transplantation of PBSC and BM grafts from unrelated donors in adults with leukemia and MDS. Despite faster hematopoietic recovery after PBSC transplantation, the net differences in the 3-year probabilities of transplant-related mortality, relapse, leukemia-free and overall survival between the groups were negligible. Transplantation of PBSC was associated with higher grade 2–4 acute GVHD and chronic GVHD than transplantation of BM. Mortality rates were higher in recipients with chronic GVHD regardless of the type of graft used for transplantation.
The results of this analysis, indicating higher GVHD rates without a survival advantage, suggest a need for randomized clinical trials to better define the role of PBSC grafts in unrelated donor transplantation. Published data in this area are few [7–10]. Unlike two other reports of unrelated donor PBSC transplants [8,10], we observed higher chronic GVHD rates after PBSC transplants compared to BM transplants but with a similar proportion of patients with extensive chronic GVHD in both groups. The probability of chronic GVHD after PBSC and BM transplants reported by Remberger and colleagues [10] was higher than in the current report. The etiology for the higher rate of chronic GVHD in their population of patients is not readily explained. In this report, transplantations were more recent (2000–2003), 60% of patients in both treatment groups were matched (allele-level) at HLA A-, -B, -C and DRB1 and approximately 50% received tacrolimus-containing regimen for GVHD prophylaxis. patients reported by Remberger and colleagues were transplanted earlier and patients and donors were matched at HLA A, B (low resolution) and DRB1. We anticipate that with allele-level typing 30% of these patients will be mismatched at HLA-A and/or –B and 92% will carry at least one allele-mismatch at one of the eight loci [19].
The presumed protective effect of GVHD in preventing recurrent leukemia was not observed in this study and that of others [7–10] even though rates of acute and chronic GVHD were significantly higher after PBSC transplants. Garderet and colleagues [9] observed lower leukemia-free survival rates in patients with ALL, a trend not observed in the current analysis or other reports [7,8,10]. As expected, disease status at transplantation adversely affected leukemia relapse, overall and leukemia-free survival regardless of the type of graft. There was no overall advantage in survival for one graft type over another in patients with intermediate and advanced leukemia at transplantation. This is consistent with other reports of unrelated donor PBSC transplants [7–10] but differs from transplantation of PBSC grafts from HLA-matched sibling donors where patients with advanced leukemia appear to benefit from PBSC grafts [1–4]. There might be a GVHD threshold above, which there is no additional benefit from graft-versus-leukemia responses, and with unrelated donor transplantation such a threshold may be achieved with BM grafts.
It remains to be seen whether, with longer follow-up, the observed higher acute and chronic GVHD after unrelated donor PBSC transplants will adversely affect long-term survival in good risk patients with CML as observed after HLA-matched sibling donor PBSC transplants [20]. In the current report, mortality rates appear to be higher after PBSC transplants in patients with good risk CML. As there are only 28 PBSC recipients in this group our findings must be confirmed in a larger series and preferably a randomized clinical trial. Extended follow-up of the current cohort may also better define the role of PBSC grafts and is planned. We did not perform analysis of total nucleated cell dose or CD34 cell dose, as these are surrogates for graft type. PBSC recipients received higher cell doses compared to BM recipients. Nevertheless, most BM recipients received a cell dose adequate to achieve hematopoietic recovery.
All aspects of the transplantation regimen including choice of graft were determined by transplant centers. Any observational study of a therapeutic intervention is subject to bias owing to the complex selection process that underlies the choice of intervention and our study is no exception. Nevertheless, our ability to adjust for key risk factors made a controlled comparison of the groups possible. Additionally, randomized trials to compare these two graft types from unrelated donors are necessary. One such trial, sponsored by the Blood and Marrow Transplant Clinical Trials Network was initiated in 2004. This study is expected to enroll 550 patients over the next 3 years with extensive evaluation of both donor and recipient outcomes.
Acknowledgments
Supported by Public Health Service Grant U24-CA76518-08 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute, the National Marrow Donor Program of the U.S and the Department of Navy, Office of Naval Research grant #N00014-99-2-0006 to the National Marrow Donor Program. The findings, conclusions or recommendations expressed in this material are those of the authors and do not reflect the views of the Office of Naval Research or the National Marrow Donor Program.
Footnotes
These data were presented at the 47th annual meeting of the American Society of Hematology in Atlanta, Georgia, December 2005.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Champlin RE, Schmitz N, Horowitz MM, et al. Blood stem cells compared with bone marrow as a source of hematopoietic cells for allogeneic transplantation. IBMTR Histocompatibility and Stem Cell Sources Working Committee and the European Group for Blood and Marrow Transplantation (EBMT) Blood. 2000;95:3702–3709. [PubMed] [Google Scholar]
- 2.Bensinger WI, Martin PJ, Storer B, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344:175–181. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz N, Beksac M, Hasenclever D, et al. Transplantation of mobilized peripheral blood cells to HLA-identical siblings with standard-risk leukemia. Blood. 2002;100:761–767. doi: 10.1182/blood-2001-12-0304. [DOI] [PubMed] [Google Scholar]
- 4.Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. J Clin Oncol. 2005;23:5074–5087. doi: 10.1200/JCO.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flowers ME, Parker PM, Johnston LJ, et al. Comparison of chronic graft-versus-host disease after transplantation of peripheral blood stem cells versus bone marrow in allogeneic recipients: long-term follow-up of a randomized trial. Blood. 2002;100:415–419. doi: 10.1182/blood-2002-01-0011. [DOI] [PubMed] [Google Scholar]
- 6.Mohty M, Kuentz M, Michallet M, et al. Chronic graft-versus-host disease after allogeneic blood stem cell transplantation: long-term results of a randomized study. Blood. 2002;100:3128–3134. doi: 10.1182/blood.V100.9.3128. [DOI] [PubMed] [Google Scholar]
- 7.Blau IW, Basara N, Lentini G, et al. Feasibility and safety of peripheral blood stem cell transplantation from unrelated donors: results of a single-center study. Bone Marrow Transplant. 2001;27:27–33. doi: 10.1038/sj.bmt.1702734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Remberger M, Ringden O, Blau IW, et al. No difference in graft-versus-host disease, relapse, and survival comparing peripheral stem cells to bone marrow using unrelated donors. Blood. 2001;98:1739–1745. doi: 10.1182/blood.v98.6.1739. [DOI] [PubMed] [Google Scholar]
- 9.Garderet L, Labopin M, Gorin NC, et al. Patients with acute lymphoblastic leukaemia allografted with a matched unrelated donor may have a lower survival with a peripheral blood stem cell graft compared to bone marrow. Bone Marrow Transplant. 2003;31:23–29. doi: 10.1038/sj.bmt.1703778. [DOI] [PubMed] [Google Scholar]
- 10.Remberger M, Beelen DW, Fauser A, et al. Increased risk of extensive chronic graft-versus-host disease after allogeneic peripheral blood stem cell transplantation using unrelated donors. Blood. 2005;105:548–551. doi: 10.1182/blood-2004-03-1000. [DOI] [PubMed] [Google Scholar]
- 11.Farag SS, Bacigalupo A, Eapen M, et al. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol Blood Marrow Transplant. 2006;12:876–884. doi: 10.1016/j.bbmt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–30. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 14.Atkinson K, Horowitz MM, Gale RP, et al. Risk factors for chronic graft-versus-host disease after HLA-identical sibling bone marrow transplantation. Blood. 1990;75:2459–2464. [PubMed] [Google Scholar]
- 15.Klein JP, Moeschberger ML. Survival Analysis: Techniques of censored and truncated data. 2. New York, NY: Springer-Verlag; 2003. [Google Scholar]
- 16.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;4:187–200. [Google Scholar]
- 17.Andersen PK, Klein JP, Zhang MJ. Testing for centre effects in multi-centre survival studies: a Monte Carlo comparison of fixed and random effects tests. Stat Med. 1999;18:1489–1500. doi: 10.1002/(sici)1097-0258(19990630)18:12<1489::aid-sim140>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet. 2002;359:1309–1310. doi: 10.1016/s0140-6736(02)08272-7. [DOI] [PubMed] [Google Scholar]
- 19.Hurley CK, Frenandez-Vina M, Hildebrand WH, et al. A high degree of HLA disparities arises from limited allelic diversity: Analysis of 1775 unrelated bone marrow transplant donor-recipient pairs. Human Immunology. 2007;68:30–40. doi: 10.1016/j.humimm.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Schmitz N, Eapen M, Horowitz MM, et al. Long-term outcome of patients given transplants of mobilized blood or bone marrow: A report from the International Bone Marrow Transplant Registry and the European Group for Blood and Marrow Transplantation. Blood. 2006;108:4288–4290. doi: 10.1182/blood-2006-05-024042. [DOI] [PMC free article] [PubMed] [Google Scholar]


