Abstract
The regions of the supraspinal network that controls urinary bladder behavior are well known, but little is known about their interconnections. We tested the feasibility of using physiophysiological interaction to explore the effective connections of the network and to seek disease-related differences in connectivity. This was a secondary analysis of fMRI data obtained from women aged 26 - 85 years, 11 with urge urinary incontinence and 10 with normal bladder function. In each subject, fMRI BOLD images were obtained during a period with full bladder and strong bladder sensation (without detrusor overactivity) while repeatedly infusing and withdrawing a small amount of liquid in and out of the bladder. Regions of interest included right insula (RI) and anterior cingulate gyrus (ACG), both consistently involved in bladder control. Other regions effectively connected to them were identified by significant correlation between their fMRI signal and the interaction RIxACG. Among normal subjects, many regions involved in bladder control were effectively connected with RI/ACG, including frontotemporal and sensorimotor cortex, forebrain, midbrain and pontine regions. The sign of the correlation with RIxACG was near-uniformly positive, perhaps suggesting mainly inhibitory connections. Among urge-incontinent subjects the effective connectivity was shifted to a parieto-temporal complex, while the sign of the correlation was predominantly negative, perhaps consistent with excitation (recruitment) of accessory pathways in an attempt to maintain bladder control. Thus, physiophysiological interaction yields potentially important information about the connectivity of the bladder control network and its changes in disease.
Keywords: functional magnetic resonance imaging, urinary incontinence, connectivity, physiophysiological interaction, insula, anterior cingulate
Introduction
Urge incontinence is a common clinical problem that is typically associated with urgency and with involuntary bladder contraction (detrusor overactivity, DO), suggesting a failure of the brain to control the bladder. Bladder control depends on an extensive supraspinal neuronal network. The anatomic locations of its main regions, and their responses during urine storage and voiding, have been identified in normal subjects by functional imaging using PET or fMRI (Athwal et al., 2001; Blok et al., 1997; Di Gangi Herms et al., 2006; Griffiths et al., 2005; Kuhtz-Buschbeck et al., 2005; Nour et al., 2000; Zhang et al., 2005) (see (Kavia et al., 2005) for a review). However, the function of the network as an integrated system is less well understood than its anatomy. In particular, knowledge of the effective connections among the regions is lacking, although it is a prerequisite for understanding how the system operates and how it may fail in urge incontinence.
Two important regions of the network are the insula, particularly on the right (RI), which is believed to map normal sensation (Craig, 2003), and the anterior cingulate gyrus (ACG), which is involved in various functions including monitoring of autonomic, emotional and motor arousal (Bush et al., 2000; Critchley et al., 2003; Devinsky et al., 1995; Margulies et al., 2007). We have previously reported fMRI observations showing RI response to bladder filling in both normal and urge-incontinent subjects experiencing strong sensation (Griffiths et al., 2005; Griffiths et al., 2007). In addition, in urge-incontinent subjects ACG response was abnormally pronounced when sensation was strong, suggesting that it corresponded to urgency. (Urgency is an abnormal and difficult- to-define sensation that includes fear of leakage (Abrams et al., 1988; Abrams et al., 2002).) At the same time, there was widespread strong activation of other brain regions, probably indicating recruitment of accessory pathway in an attempt to prevent threatened urine loss (Griffiths et al., 2007). This change in the pattern of responses in incontinent subjects suggests changes in the network of effective connections among brain regions involved in bladder control. Therefore, following this primary analysis (Griffiths et al., 2007), we have performed a secondary, exploratory analysis of connectivity, using the physiophysiological interaction method (PPI), an approach described in 1997 (Friston et al., 1997) but new to this field. PPI assesses the effective connectivity in a network of brain regions by testing to what extent the statistical interaction between the time-varying fMRI BOLD (blood oxygen level dependent) signals in 2 regions, chosen a priori, can explain the variation in activity in other regions (Friston et al., 1997).
Physiophysiological interaction is similar to the psychophysiological interaction analysis used in cognitive research (Friston et al., 2000; Friston et al., 1997) but is a more natural fit to our bladder filling protocol, which does not include a specific cognitive task but involves physiological stimulation. PPI is superior to simple correlation for assessing the connections between different brain regions (Stephan, 2004; Stephan et al., 2004). Its usefulness has been demonstrated in several reports (Das et al., 2005; Friston et al., 1997; Williams et al., 2006).
Methods
Study subjects
Responses to bladder filling were examined in 24 female volunteers with no overt neurological disease, aged from 26 - 85 years, half with urge urinary incontinence and half age-matched controls. Exclusion criteria included dementia, spinal cord disease with gross neurological deficit, pelvic irradiation or bladder cancer, mobility or mentation problems precluding scanning, claustrophobia, implanted ferromagnetic or electronic device, positive pregnancy test or current urinary tract infection. Subjects were recruited by advertising or from another study and signed written informed consent. The University of Pittsburgh Institutional Review Board approved all research procedures.
Controls with normal bladder function had no symptoms suggestive of lower urinary tract dysfunction (i.e. increased voiding frequency, urgency or urge incontinence; (Abrams et al., 2002)) on history or bladder diary and no detrusor overactivity on urodynamic study prior to scanning. Urge-incontinent subjects had symptomatic urge incontinence on history and diary and detrusor overactivity on urodynamics prior to scanning.
Bladder filling protocol
Details of our methods have been described previously (Griffiths et al., 2005). Briefly, two 8 F urethral catheters were introduced, for bladder filling/emptying and for bladder pressure measurement to monitor detrusor overactivity. A structural MRI was recorded, followed by functional scanning during a series of bladder filling and withdrawal maneuvers as follows (Griffiths et al., 2007), A small volume of saline solution (50-100 ml) was introduced into the empty bladder. Saline solution was then repeatedly infused into and withdrawn from the bladder while functional brain imaging (fMRI) was performed (reverse spiral imaging; 3 T magnet; one whole brain scan per 1.5 s; 3×3×3 mm resolution). Each infusion/withdrawal cycle comprised: pause (10.5 s); infusion (22 ml in 10.5 s); pause (10.5 s); withdrawal (15 ml in 10.5 s); four such cycles followed by a 10.5 s runout period formed one measurement block. After performing 2 such measurement blocks, the bladder was filled further, without scanning, until the subject signaled strong bladder sensation. If the subject permitted, further measurement blocks were then performed, with scanning, until sensation became uncomfortable or DO developed. In most subjects this resulted in a further 2 to 4 blocks of measurement. The fMRI BOLD signal acquired in the final measurement block (or the last measurement block prior to the onset of DO) was used for PPI analysis. In general this measurement block yielded the strongest signals, facilitating analysis of connectivity. This final block of measurements was comparable in different subjects because it was always performed in the same situation: with a full bladder and just tolerable sensation, but without DO.
fMRI analysis
After image acquisition, pre-processing and all further analyses were done using Statistical Parametric Mapping (SPM2) (Wellcome Department of Imaging Neuroscience, 2003) with the software toolbox INRIAlign (INRIAlign, 2005). As in our previous work (Griffiths et al., 2005), regional brain responses to bladder filling were determined by comparing the fMRI BOLD signal during fluid infusion with the baseline signal attained during fluid withdrawal from the bladder (contrast = infusion minus withdrawal).
Connectivity analysis - basic principles
The mathematical basis of PPI is illustrated in Figure 1A. Initially, two regions of interest (ROIs) are selected, in this case right insula (RI) and anterior cingulate gyrus (ACG). The statistical interaction (RI×ACG) for the 2 chosen ROIs is an element-by-element product of the mean vectors containing the activities in these two regions. It is large when RI and ACG signals change in the same direction (in parallel) and small when they change in opposite directions. A map is constructed showing brain regions where the BOLD signal is significantly correlated with RI×ACG. In Figure 1A one such region (‘new’) is shown. The correlation coefficient may be positive or negative.
Figure 1.
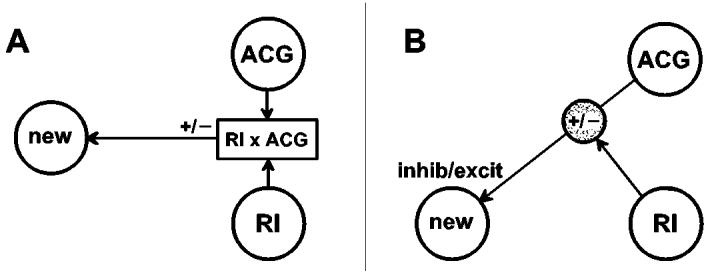
Basis of physiophysiological (PPI) connectivity analysis. A. Mathematical scheme: interaction between regions RI and ACG (chosen a priori) is correlated positively or negatively to activity in ‘new’ region revealed by analysis. B. Physiological interpretation: effective connection between ACG and ‘new’ region is modulated positively or negatively by RI. In addition, the connection may tend either to excite or to inhibit activity in the new region.
Figure 1B shows a physiological interpretation. ACG activity affects activity in the new region via the effective connection shown at the top of the diagram. Another effective connection enables RI to modulate the effect of ACG on the new region. If the correlation is positive, parallel changes in RI and ACG activity increase the signal in the new region. If it is negative, parallel RI and ACG changes tend to reduce the signal level in the new region.
Some variations are possible. (1) The modulatory connection from RI, shown in Figure 1B, could act at any synapse from the ACG up to and including the new region. (2) In Figure 1A RI and ACG play interchangeable roles. In Figure 1B therefore the roles of RI and ACG could equally well be interchanged, with RI as the origin of the effective pathway to the new region, and ACG as the source of the modulatory connection.
Connectivity analysis - practical realization
For connectivity analyses, the choice of initial ROIs (RI and ACG) was based partly on the observation that these regions showed activation in nearly every subject when sensation was strong, thus confirming their importance and also facilitating group analyses. Based on a review of previous imaging studies that examined bladder filling or withholding of urine with full bladder (Athwal et al., 2001; Griffiths et al., 2005; Kuhtz-Buschbeck et al., 2005; Seseke et al., 2006), we selected for right insula a spherical ROI with radius 12 mm, centered at Montreal Neurological Institute (MNI) coordinates 38, 16, 6 mm; for anterior cingulate gyrus the ROI was centered at 2, 12, 42 with radius 18 mm.
After performing standard main-effects analyses of the data in the final measurement block, to confirm the main regions involved in bladder control, we proceeded with PPI analysis, creating two 3-dimensional brain images for each subject (one for positive and one for negative modulation). For group analyses, individual data were combined as for standard random-effects analyses (Holmes et al., 1998). Results were thresholded at an uncorrected P-value of 0.05 and statistical significance was assessed after correction for multiple comparisons at cluster level (P < 0.05). A few responses that met only the uncorrected level of significance have been reported as trends. Locations of brain regions are given in MNI coordinates. Their anatomical locations were identified using the Talairach Daemon (Research Imaging Center UTHSCSA, 2005) after conversion to Talairach coordinates with the program mni2tal (Brett, 2006).
Results
Among 24 subjects who entered the scanner, two could not tolerate scanning and one was eliminated because of large head movements (> 3 mm). Thus, data were obtained and main effects were analyzed for 10 subjects with normal bladder control and 11 with urge incontinence. For connectivity analysis, data from one incontinent subject were excluded because there were too few activated voxels in one of the designated ROIs, thus leaving 10 incontinent and 10 age-matched normal subjects for analysis. The maximum cystometric capacity on pre-fMRI urodynamics (median and range) was 599 ml [475-980 ml] among normals and 369 ml [246-961 ml] among urge-incontinent subjects. Corresponding values for the volume in the bladder following completion of measurements were 675 ml [270-1100 ml] and 600 ml [300-842 ml] respectively. See Table 1 in (Griffiths et al., 2007) for further details. For the measurement block used in this analysis the median block number was 5 (range 4-6) in normal controls and 4 (range 2-6) among urge-incontinent cases. Thus - as expected - there was a tendency to lower volumes and also to a lower block number in urge-incontinent subjects, in whom it was limited by DO in 2 cases and in one case by extremely strong sensation in Block #2.
Main effects
The main effects have been described in detail previously (Griffiths et al., 2005; Griffiths et al., 2007). Tables 1A and 1B and Figures 2A and 2B summarize the main effects of bladder filling in the final measurement block. In normal subjects there were the expected responses to bladder filling in RI and adjacent fronto-temporal regions (Figure 2A) and also in parietal regions, hypothalamus, ACG and pons (Table 1A). There was little evidence of deactivation in response to bladder filling.
Table 1A.
Regions with main-effects responses to bladder filling, for subjects with normal bladder function (n=10, threshold P = 0.05 uncorrected, FDR* = 0.49)
| coordinates | Z-value | |||
|---|---|---|---|---|
| x | y | z | ||
| Cluster: P < 0.000 (corrected) | ||||
| R/L. Inferior frontal gyrus (BA44, 45) | 58, -30 | 6, 28 | 14, -6 | 3.18, 3.73 |
| R. Superior temporal gyrus (BA22) | 50 | 2 | 2 | 3.32 |
| ACG (BA32) | -4, -10 | 6, 12 | 42, 40 | 2.45, 2.81 |
| R. Insula (BA13) | 44 | 6 | 4 | 3.15 |
| Hypothalamus | -14, -4 | -8, -14 | 6, -8 | 4.84, 4.12 |
| Brain stem | 10 | 12 | -8 | 4.18 |
FDR = false discovery rate
Table 1B.
Regions with main-effects responses to bladder filling, for subjects with urge incontinence (n=10, threshold P = 0.01* uncorrected, FDR = 0.17)
| coordinates | Z-value | |||
|---|---|---|---|---|
| x | y | z | ||
| Cluster 1: fronto-temporal lobes, RI, ACG+claustrum, P < 0.000 (corrected) | ||||
| R. Inferior frontal gyrus (BA9, 44) | 52, 50 | 6, 8 | 32, 14 | 3.37, 3.53 |
| R. frontal lobe (BA6, precentral gyrus) | 40, 40 | -6, -2 | 54, 34 | 3.79, 3.37 |
| R/L. Medial frontal gyrus (BA6) | 12, -18 | 0, -4 | 56, 58 | 3.32, 3.32 |
| R. Frontal lobe (BA8) | 6 | 18 | 50 | 3.72 |
| R. sup.temporal gyrus (BA22) | 54 | 8 | -2 | 2.70 |
| ACG (BA32) | -4 | 16 | 38 | 4.33 |
| R.insula (BA13) | 38, 40 | -4, 6 | 16, 2 | 3.81, 3.80 |
| Claustrum | 30 | 14 | 8 | 4.35 |
| Cluster 2: parietal lobe+ paracentral/postcentral lobule, P < 0.000 (corrected) | ||||
| R. Inferior parietal lobule (BA40) | 36 | -44 | 44 | 3.92 |
| R. parietal lobe (BA5) | 20 | -44 | 50 | 3.72 |
| R.frontal lobe (paracentral lobule, BA5) | 16 | -40 | 56 | 2.80 |
| R. parietal lobe/postcentral gyrus (BA40) | 62, 62 | -34, -28 | 24, 18 | 2.84, 2.71 |
| Cluster 3: cerebellum, P < 0.02 | ||||
| Cerebellum (anterior lobe, culmen) | 24, 34 | -42, -54 | -30, -26 | 3.37, 3.22 |
| Cerebellum (posterior lobe, declive) | 20, 4 | -64, -66 | -20, -12 | 3.00, 2.93 |
| Trends: posterior cortex, P < 0.002 uncorrected, individually | ||||
| R. Occipital lobe (cuneus, BA17) | 16 | -92 | 8 | 3.09 |
| R. Occipital lobe (lingual gyrus, BA17) | 10 | -86 | 0 | 3.39 |
significant clusters better separated at this threshold level than at P = 0.05
Figure 2.
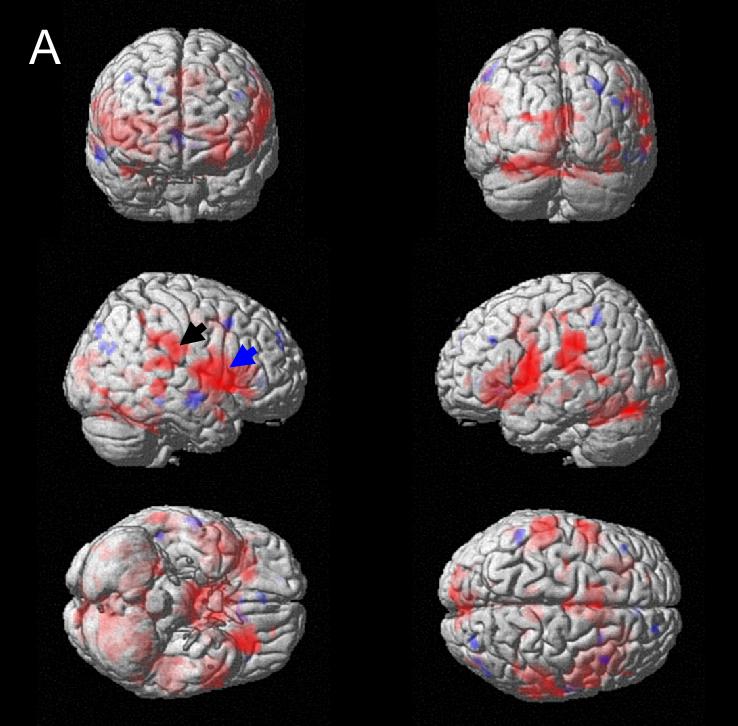
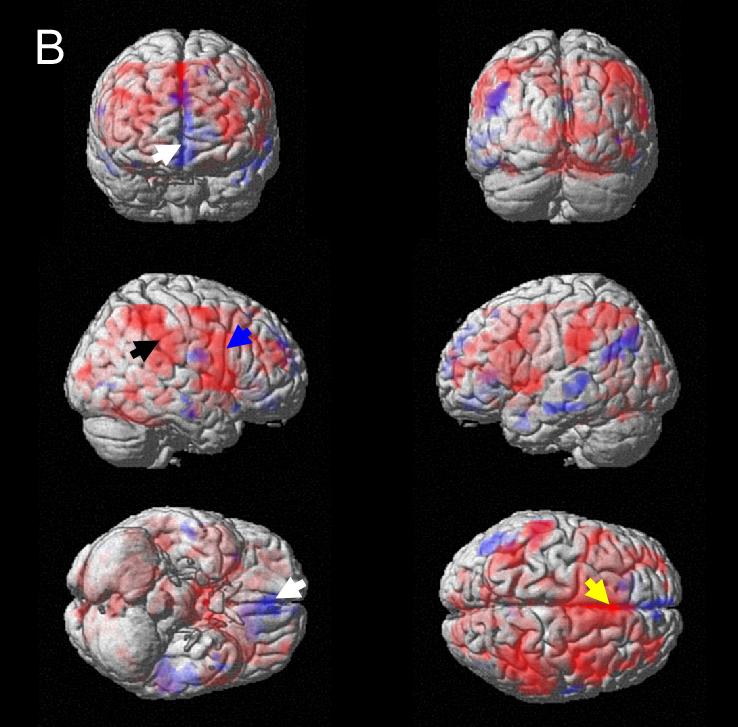
Responses to bladder filling (main effects, thresholded at P = 0.05) rendered on standard brain surface (red = activation, blue = deactivation). A. Normal subjects; blue arrow: right insula and adjacent regions; black arrow: inferior parietal region. B. Urge-incontinent subjects; blue arrow: insula and frontotemporal; black arrow: parietal; white arrow: medial orbitofrontal; yellow arrow: anterior cingulate (ACG).
In subjects with urge incontinence (Figure 2B), in addition to fronto-temporal and RI activity (blue arrow), there was abnormally strong activation of ACG (with adjacent frontal regions), and also of parietal regions, suggesting recruitment of accessory pathways. There was deactivation in response to bladder filling in the medial orbitofrontal cortex and a chain extending posteriorly and rostrally from the left temporal lobe (Supplementary Figure 4).
Connectivity analysis
Results of connectivity analyses are shown in Tables 2A and 2B and Figures 3A and 3B. Both the pattern of regions with effective connections from RI and ACG, and the sign of their main associations with the RI×ACG interaction, differed between subjects with normal bladder control and subjects with urge incontinence.
Table 2A.
Regions with connectivity with RI and/or ACG (PPI analysis, positive modulation), for subjects with normal bladder function (n=10, threshold P = 0.05 uncorrected, FDR = 0.41)
| coordinates | Z-value | |||
|---|---|---|---|---|
| x | y | z | ||
| Cluster 1: predominantly {[fronto-temporal + adjacent postcentral gyrus (parietal)] + thalamus, insula, putamen, claustrum}, P < 0.000 | ||||
| R.Inferior frontal gyrus (BA46) | 48 | 40 | 12 | 3.10 |
| L. Inferior frontal gyrus (BA47, 9) | -36, -44 | 24, 0 | 2, 24 | 2.83, 3.21 |
| R/L. Middle frontal gyrus (BA11, 6) | 32, -46 | 34, 0 | -12, 40 | 3.00, 2.89 |
| L. Precentral gyrus (frontal lobe, BA44) | -46 | 0 | 10 | 3.20 |
| L. Precentral gyrus (frontal lobe, BA6) | -44, -60 | -4, 0 | 42, 14 | 2.77, 3.32 |
| R/L. Precentral gyrus (frontal lobe, BA4) | -58, 58 | -12, -4 | 26, 18 | 3.39, 3.21 |
| L. Postcentral gyrus (parietal lobe, BA2, 40) | -62, -62 | -20, -28 | 32, 20 | 3.45, 2.80 |
| R/L. Superior temporal gyrus (BA22) | 56, 54, | -50-6, -2, 2 | 0, 4, -2 | 3.62,3.39, 2.96 |
| L. Temporal lobe (BA42) | -58 | -10 | 10 | 3.35 |
| L. Insula (BA13) | -34 | 16 | -2 | 3.49 |
| Claustrum | 26 | -8 | 20 | 2.78 |
| Thalamus | -6, 8, 6 | -4, -4, -18 | 8, 8, 2 | 2.80, 2.70, 2.83 |
| Putamen /lent.nucl. | 26 | 4 | 8 | 2.70 |
| Cluster 2: posterior cortex + cerebellum + PMC/PAG, P < 0.000 | ||||
| R. Lingual gyrus (occipital lobe, BA18) | 20 | -78 | -4 | 3.71 |
| R/L. Cuneus (occipital lobe, BA18, 17) | 10, -6 | -82, -82 | 16, 12 | 2.73, 3.14 |
| R. Precuneus (parietal lobe, BA31) | 12 | -70 | 22 | 2.81 |
| L. Fusiform gyrus (temporal lobe) | -36, -46 | -38, -56 | -18, -18 | 3.20, 3.33 |
| L. Middle temporal gyrus (BA39) | -50 | -66 | 10 | 2.80 |
| Cerebellum (anterior lobe, culmen) | -40, 4 | -44, -60 | -20, -20 | 3.08, 2.58 |
| PMC | 4 | -36 | -34 | 2.75 |
| PAG | 2, 6 | -22, -22 | -18, -18 | 3.06, 2.70 |
Table 2B.
Regions with connectivity with RI and/or ACG (PPI analysis, negative modulation), for subjects with urge incontinence (n=10, threshold p = 0.05 uncorrected, FDR = 0.60)
| coordinates | Z-value | |||
|---|---|---|---|---|
| x | y | z | ||
| Cluster: parieto-temporal, P < 0.000 | ||||
| L. Superior parietal lobule (BA7) | -34, -40 | -56, -64 | 50, 48 | 3.23, 3.07 |
| L. Inferior Parietal lobe (BA40) | -40, -54 | -52, -50 | 52, 48 | 3.21, 2.85 |
| L. Postcentral gyrus (parietal lobe, BA2, 3) | -54, -56 | -22, -20 | 28, 36 | 2.85, 2.32 |
| L. Superior/middle temporal gyrus (BA41, 22) | -38 -58,-52 | -40,-42,-44 | 8,18,-2 | 3.07,3.11,3.0 |
| L. insula (BA13) | -30 | -30 | 14 | 2.62 |
| Trends: cerebellum and (para)hippocampal, P = 0.007, 0.007, 0.02 uncorrected, respectively | ||||
| Cerebellum (anterior lobe) | -2, -8 | -48, -54 | -24, -14 | 3.46, 2.43 |
| Cerebellum | -12 | -60 | -16 | 2.44 |
| (Para)hippocampal | 32, 20 | -22, -12 | -14, -14 | 3.14, 2.62 |
Figure 3.
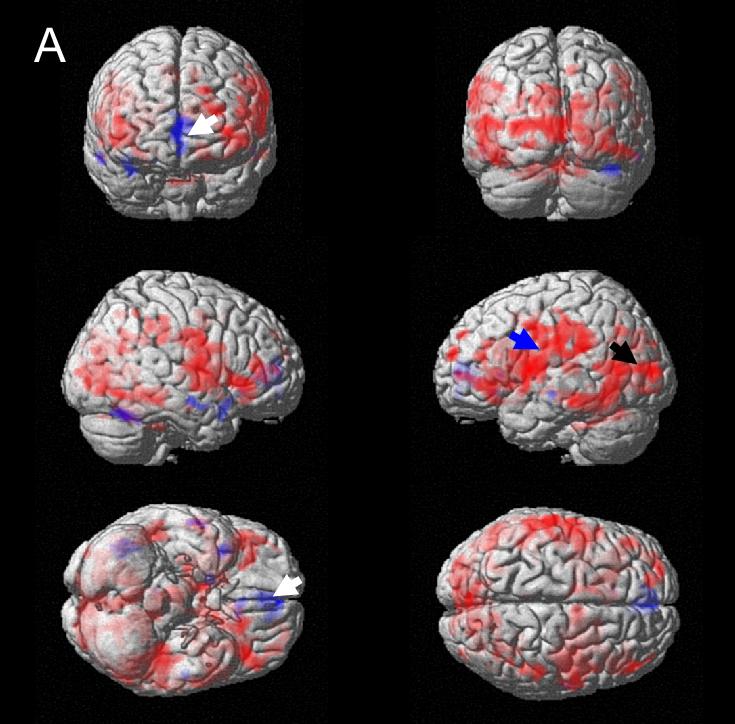

Regions with effective connectivity to right insula and/or ACG (thresholded at P = 0.05) rendered on standard brain surface (red = positive modulation, blue = negative modulation). A. Normal subjects; blue arrow: left insula and adjacent regions, including parietal; black arrow: posterior cortex; white arrow: medial orbitofrontal cortex. B. Urge-incontinent subjects; black arrow: parietal regions.
(1) In normal subjects there were significant positive effective connections with many of the regions involved in supraspinal bladder control, including left insula and fronto-temporal and parietal regions (Figure 3A), thalamus, putamen and claustrum, posterior cortex, cerebellum, PMC and PAG (Table 2A). There were few negative connections except for a trend in medial orbitofrontal cortex (data not shown).
(2) In urge-incontinent subjects connections were preponderantly negative (Figure 3B). There were significant connections to left parieto-temporal lobes, and those to hippocampus, parahippocampal gyrus and cerebellum showed a trend to significance (Table 2B). Activation of sensorimotor cortex was also visible, although it did not reach significance at cluster level. Furthermore, right frontal cortex showed a trend to negative connection. There were few positive connections (data not shown).
c) A 2-sample t-test confirmed that the connectivity differences between normal and urge-incontinent subgroups, suggested by Figures 3A and 3B, were significant in a cluster that included the posterior cortex (cuneus; data not shown).
Discussion
The analyses based on the physiophysiological interaction method confirm that RI and ACG are important regions of the supraspinal bladder control network. They modulate, via effective connections, activity in other regions of the network in response to bladder afferent signals. The effective connections differ between subjects with normal bladder function and subjects with urge incontinence, both in the regions involved and in the sign of modulation. They differ in a way that suggests that, in urge-incontinent subjects, accessory pathways are recruited (Griffiths et al., 2007).
In subjects with normal bladder function, the pattern of connections from RI and/or ACG (Figure 3A) is similar to the pattern of responses (Figure 2A), implying that RI and/or ACG have effective connections with many of the brain regions involved in bladder control: the frontotemporal and sensorimotor regions (Athwal et al., 2001; Blok et al., 1998; Blok et al., 1997; Matsuura et al., 2002; Nour et al., 2000; Seseke et al., 2006; Zhang et al., 2005); the thalamus and putamen (Athwal et al., 2001; Seseke et al., 2006; Zhang et al., 2005); the cerebellum (Athwal et al., 2001; Griffiths et al., 2005; Matsuura et al., 2002; Nour et al., 2000; Seseke et al., 2006; Zhang et al., 2005); and the pons (PMC) and midbrain (PAG) (Athwal et al., 2001; Blok et al., 1997; Nour et al., 2000). The analysis also reveals effective connections from RI and/or ACG to the posterior cortex, a region only recently discussed in the context of bladder function (Blok et al., 2006).
For subjects with urge incontinence, connections are shifted to an alternative complex of brain regions: parieto-temporal lobes, especially on the left, together with parahippocampal gyrus and parts of cerebellum. Activity in regions of this alternative complex is related in healthy volunteers to voluntary control of voiding by pelvic floor contraction (Zhang et al., 2005); to suppression of the voluntary urge to void (Kuhtz-Buschbeck et al., 2005); to withholding of urine (Yin et al., 2006); and to use of pelvic floor muscles (Di Gangi Herms et al., 2006; Kuhtz-Buschbeck et al., 2005; Seseke et al., 2006). This regional and functional pattern, along with the changed sign of connectivity, suggests that activity in the alternative complex represents excitation (recruitment) of accessory pathways in an attempt to control urgency and the voiding reflex when loss of bladder control threatens.
The change in the pattern of connectivity in urge-incontinent subjects is consistent also with the emotional charge carried by the abnormal sensation of urgency (fear of impending leakage). Activation of the parietal region may be related to unpleasant and emotionally charged stimuli (Liotti et al., 2001), and (para)hippocampal activity is associated with emotional and recognition memory (Jack et al., 1999; Manns et al., 2003). The deactivation observed in other parts of the limbic system, in response to bladder filling (Figure 2B), may be another sign of emotional involvement in these subjects.
The striking difference between subject groups in the sign of the modulation associated with connectivity deserves comment. As for all brain activity measurements, observed activation may represent either excitation or inhibition. If the modulation is positive the effective connection from ACG and/or RI is perhaps more likely to be inhibitory, since reinforcement of an excitatory pathway could lead to runaway excitation, an unstable situation. Thus many connections in normal subjects may be inhibitory (Figure 3A). Conversely, in urge-incontinent subjects most of the connections are negative and thus possibly excitatory (Figure 3B), facilitating recruitment of regular and accessory pathways to suppress urgency. On the other hand, the change of sign of the modulation might merely reflect the limitations of a quasi-linear approach to an inherently non-linear problem (Friston et al., 2000), especially since one of the regions involved (ACG) is very much more strongly activated in abnormal than in normal subjects, thus exaggerating non-linear effects.
The effective connection of RI and/or ACG to the pontine micturition center (PMC; see Table 1A) is noteworthy, since the PMC is the final efferent nucleus in the central micturition pathway. This connection therefore confirms that ACG and RI are closely involved in control of the voiding reflex. (PMC activation during storage was noted in the main-effects analysis also, see Supplementary Figure 5.) Because the connection is observed in normal subjects with perfect bladder control, it is likely to be inhibitory in nature, so preventing premature triggering of the voiding reflex. Correspondingly, the associated modulation has a positive sign.
The medial prefrontal (orbitofrontal) cortex is responsible for voluntary decisions about voiding based on time, place and social context as well as bladder volume (Blok et al., 1997; Kavia et al., 2005). In our normal subjects it showed essentially no activation by bladder filling, but it was negatively modulated by connections from RI and ACG (Figures 2A and 3A). In urge-incontinent subjects, although deactivation appeared pronounced, the connectivity was less clear (Figures 2B and 3B). It is thus possible that effective connections to the prefrontal cortex are weaker in incontinent subjects, perhaps contributing to their ineffective bladder control.
This exploratory analysis has several limitations. First, although the stimulus - rapid and repeated bladder infusion and withdrawal - produces a substantial change in the BOLD signal and mimics physiological urine storage to a certain extent, it is not truly physiological. Second, because the number of study subjects is small, we had to choose liberal threshold probability levels in order to see responses and connections clearly; consequently the false discovery rates are relatively high (Tables 1A and 1B). Third, the physiophysiological interaction approach is based on an analytical model that may be oversimplified (Friston et al., 2000). On the other hand, it requires choice of only 2 initial brain regions, and then explores the rest of the brain to find new regions effectively connected to them. Given the current limited knowledge about the bladder control network, this is an essential first step toward generating more complete models of supraspinal control that can be tested by sophisticated methods such as dynamic causal modeling (Friston et al., 2003) or driving/modulatory component analysis (Friston et al., 2000).
Conclusions
This exploratory study has shown that physiophysiological interaction methods yield biologically plausible information about the connectivity of the bladder control network. In women with normal bladder function, there are effective connections from right insula and/or anterior cingulate gyrus to many of the brain regions responding to bladder filling. The positive sign of the associated modulation suggests that they may be largely inhibitory. In urge-incontinent women, brain responses to bladder filling and the effective connections from right insula and/or anterior cingulate gyrus differ from normal, even in the absence of detrusor overactivity. Diversion of effective connections to a parieto-temporal complex, together with modulation of negative sign, suggests recruitment of accessory pathways in an attempt to maintain bladder control when the bladder is full.
Supplementary Material
Supplementary Figure 4. A: Regions where response to infusion increased at large bladder volumes, in normal subjects. B: Regions where response was weaker in subjects with poor bladder control than in normals, at large bladder volumes. z = -6 mm. R = right. PH: preoptic hypothalamus. Arrowheads: orbitofrontal cortex, showing significant clusters (P < 0.0001 to 0.01 corrected). Fixed-effects analyses thresholded at P < 0.01 uncorrected. Data from (Griffiths et al., 2005).
Supplementary Figure 5. Activation of pontine micturition center (M) in response to bladder infusion at smaller bladder volumes. Coordinates -6, -36, -32. Random-effects analysis in all subjects, P < 0.01 uncorrected. R = right side. Data from (Griffiths et al., 2005).
Acknowledgements
We are grateful to Mary Alyce Riley BSN, Kelly Goode and Mary Jo Sychak for their dedicated and enthusiastic help, to Werner Schaefer DI and Neil Resnick MD for their helpful comments, and to the staff of the UPMC Magnetic Resonance Research Center. This work was supported by US Public Health Service grants RO3AG25166, RO1AG020629 and P01AG04390, and by University of Pittsburgh Competitive Medical Research Fund. Dr Tadic was supported by NIH training grant 5T32AG021885.
Supported by US Public Health Service grants RO3AG25166, RO1AG020629 and P01AG04390, and by University of Pittsburgh Competitive Medical Research Fund. Dr Tadic is supported by NIH training grant 5T32AG021885-03
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams P, Blaivas JG, Stanton S, Andersen JT. The standardisation of terminology of lower urinary tract function. Neurourology and Urodynamics. 1988;7:403–426. [Google Scholar]
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, Victor A, Wein A. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourology & Urodynamics. 2002;21:167–178. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- Athwal BS, Berkley KJ, Hussain I, Brennan A, Craggs M, Sakakibara R, Frackowiak RS, Fowler CJ. Brain responses to changes in bladder volume and urge to void in healthy men. Brain. 2001;124:369–377. doi: 10.1093/brain/124.2.369. [DOI] [PubMed] [Google Scholar]
- Blok BF, Sturms LM, Holstege G. Brain activation during micturition in women. Brain. 1998;121:2033–2042. doi: 10.1093/brain/121.11.2033. [DOI] [PubMed] [Google Scholar]
- Blok BF, Willemsen AT, Holstege G. A PET study on brain control of micturition in humans. Brain. 1997;120:111–121. doi: 10.1093/brain/120.1.111. [DOI] [PubMed] [Google Scholar]
- Blok BFM, Groen J, Bosch JLHR, Veltman DJ, Lammersma AA. Different brain effects during chronic and acute sacral modulation in urge incontinent patients with implanted neurostimulators. BJU Int. 2006;08:1238–1243. doi: 10.1111/j.1464-410X.2006.06521.x. [DOI] [PubMed] [Google Scholar]
- Brett M. The MNI brain and the Talairach Atlas. 2006 http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach.
- Bush G, Luu P, Posner M. Cognitive and emotional inflences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Das P, Kemp AH, Liddell BJ, Brown KJ, Olivieri G, Peduto A, Gordon E, Williams LM. Pathways for fear perception: modulation of amygdala activity by thalamo-cortical systems. NeuroImage. 2005;26:141–148. doi: 10.1016/j.neuroimage.2005.01.049. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behavior. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Di Gangi Herms AMR, Veit R, Reisenauer C, Herms A, Grodd W, Enck P, Stenzl A, Birbaumer N. Functional imaging of stress urinary incontinence. NeuroImage. 2006;29:267–275. doi: 10.1016/j.neuroimage.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Büchel C. Attentional modulation of effective connectivity from V2 to V5/MT in humans. Proceedings National Academy Sciences. 2000;97:7591–7596. doi: 10.1073/pnas.97.13.7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Büchel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modeling. NeuroImage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Griffiths D, Derbyshire S, Stenger A, Resnick N. Brain control of normal and overactive bladder. J Urol. 2005;174:1862–1867. doi: 10.1097/01.ju.0000177450.34451.97. [DOI] [PubMed] [Google Scholar]
- Griffiths D, Tadic SD, Schaefer W, Resnick NM. Cerebral control of the bladder in normal and urge-incontinent women. NeuroImage. 2007;37:1–7. doi: 10.1016/j.neuroimage.2007.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AP, Friston KJ. Generalisability, random effects and population inference. Neuroimage. 1998;7:S754. [Google Scholar]
- INRIAlign Toolbox for fMRI realignment in SPM99. 2005 http://www.madic.org/people/roche/
- Jack CR, Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavia RB, Dasgupta R, Fowler CJ. Functional imaging and the central control of the bladder. J Comp Neurol. 2005;493:27–32. doi: 10.1002/cne.20753. [DOI] [PubMed] [Google Scholar]
- Kuhtz-Buschbeck JP, van der Horst C, Pott C, Wolff S, Nabavi A, Jansen O, Junemann KP. Cortical representation of the urge to void: a functional magnetic resonance imaging study. J Urol. 2005;174:1477–1481. doi: 10.1097/01.ju.0000173007.84102.7c. [DOI] [PubMed] [Google Scholar]
- Liotti M, Brannan S, Egan G, Shade R, Madden L, Abplanalp B, Robillard R, Lancaster J, Zamarripa FE, Fox PT, Denton D. Brain responses associated with consciousness of breathlessness (air hunger) Proc. Natl. Acad. Sci. USA. 2001;98:2035–2040. doi: 10.1073/pnas.98.4.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Hopkins RO, Reed JM, Kitchener EG, Squire LR. Recognition memory and the human hippocampus. Neuron. 2003;37:171–180. doi: 10.1016/s0896-6273(02)01147-9. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. NeuroImage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Matsuura S, Kakizaki H, Mitsui T, Shiga T, Tamaki N, Koyanagi T. Human brain region response to distention or cold stimulation of the bladder: a positron emission tomography study. J Urol. 2002;168:2035–2039. doi: 10.1016/S0022-5347(05)64290-5. [DOI] [PubMed] [Google Scholar]
- Nour S, Svarer C, Kristensen JK, Paulson OB, Law I. Cerebral activation during micturition in normal men. Brain. 2000;123:781–789. doi: 10.1093/brain/123.4.781. [DOI] [PubMed] [Google Scholar]
- Research Imaging Center UTHSCSA Talairach Daemon Client. 2005 ric.uthscsa.edu/projects/talairachdaemon.html.
- Seseke S, Baudewig J, Kallenberg K,R-H,R, Seseke F, Dechent P. Voluntary pelvic floor muscle control - an fMRI study. Neuroimage. 2006;31:1399–1407. doi: 10.1016/j.neuroimage.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Stephan KE. On the role of general system theory for functional neuroimaging. J Anat. 2004;205:443–470. doi: 10.1111/j.0021-8782.2004.00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Harrison LM, Penny WD, Friston KJ. Biophysical models of fMRI responses. Current Opinion in Neurobiology. 2004;14:629–635. doi: 10.1016/j.conb.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Wellcome Department of Imaging Neuroscience Statistical parametric mapping: SPM2. 2003 http://www.fil.ion.ucl.ac.uk/spm/spm2.html.
- Williams LM, Das P, Liddell BJ, Kemp AH, Rennie CJ, Gordon E. Mode of functional connectivity in amygdala pathways dissociates level of awareness for signals of fear. Journal Neuroscience. 2006;26:9264–9271. doi: 10.1523/JNEUROSCI.1016-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Shuke N, Okizaki A, Sato J, Aburano1 T, Li Y, Kaneko S, Mizunaga M, Yachiku S. Cerebral activation during withholding urine with full bladder in healthy men using 99mTc-HMPAO SPECT. J Nucl Med. 2006;47:1093–1098. [PubMed] [Google Scholar]
- Zhang H, Reitz A, Kollias S, Summers P, Curt A, Schurch B. An fMRI study of the role of suprapontine brain structures in the voluntary voiding control induced by pelvic floor contraction. Neuroimage. 2005;24:174–180. doi: 10.1016/j.neuroimage.2004.08.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 4. A: Regions where response to infusion increased at large bladder volumes, in normal subjects. B: Regions where response was weaker in subjects with poor bladder control than in normals, at large bladder volumes. z = -6 mm. R = right. PH: preoptic hypothalamus. Arrowheads: orbitofrontal cortex, showing significant clusters (P < 0.0001 to 0.01 corrected). Fixed-effects analyses thresholded at P < 0.01 uncorrected. Data from (Griffiths et al., 2005).
Supplementary Figure 5. Activation of pontine micturition center (M) in response to bladder infusion at smaller bladder volumes. Coordinates -6, -36, -32. Random-effects analysis in all subjects, P < 0.01 uncorrected. R = right side. Data from (Griffiths et al., 2005).


