Abstract
Sulforaphane (SFN) is an isothiocyanate found in cruciferous vegetables such as broccoli. This anticarcinogen was first identified as a potent inducer of Phase 2 enzymes, but evidence is mounting that SFN acts through other cancer chemopreventive mechanisms. We recently reported on a novel mechanism of chemoprotection by SFN in human colon cancer cells and prostate epithelial cells, namely the inhibition of histone deacetylase (HDAC). In the present investigation, we sought to test whether SFN also might inhibit HDAC activity in vivo. When consumed in the diet at an average daily dose of 7.5 μmol per animal for 21 days, SFN suppressed the growth of human PC-3 prostate cancer cells by 40% in male nude mice. There was a significant decrease in HDAC activity in the xenografts, as well as in the prostates and mononuclear blood cells (MBC), of mice treated with SFN, compared to controls. There also was a trend towards increased global histone acetylation in the xenografts, prostates, and MBC. In human subjects, a single dose of 68 g BroccoSprouts inhibited HDAC activity significantly in peripheral blood mononuclear cells (PBMC) 3 and 6 hrs following consumption. These findings provide evidence that one mechanism through which SFN acts as a cancer chemopreventive agent in vivo is through the inhibition of HDAC activity. Moreover, the data suggest that HDAC activity in PBMC may be used as a biomarker for assessing exposure to novel dietary HDAC inhibitors in human subjects.
Keywords: prostate cancer, histone deacetylase inhibitor, sulforaphane mechanisms of prevention, diet and cancer
Introduction
Targeting the epigenome, including the use of histone deacetylase (HDAC) inhibitors, is a novel strategy for cancer chemoprevention. Recently, drugs classified as HDAC inhibitors have shown promise as potential chemoprevention agents (1–5). HDAC inhibitors are interesting candidates for prevention because they enable re-expression of epigenetically-silenced genes involved in differentiation, cell cycle regulation, apoptosis, angiogenesis, invasion, and metastasis.
HDAC changes have been linked to the progression of several cancers, including prostate cancer (1, 3, 4, 6). For example, HDAC1 mRNA levels are higher in several prostate cancer cell lines compared with benign prostate hyperplasia, with a concomitant increase in HDAC activity (7). Moreover, HDAC1 expression was increased in primary human prostate cancer tissue compared with prostate hyperplasia (7). Overexpression of HDAC1 in PC-3 cells resulted in an increase in cell proliferation and an overall decrease in cell differentiation (8). HDAC1 expression was highest in hormone refractory prostate cancer (8), suggesting that alterations in HDAC may be of particular importance in the progression to androgen independence. Importantly, inhibitors of HDAC, including suberoylanilide hydroxamic acid (SAHA), valproic acid, depsipeptide, and sodium butyrate have been demonstrated to be effective against prostate cancer in experimental models (9–11).
Epidemiologic evidence suggests that consumption of cruciferous vegetables decreases overall risk for prostate cancer, particularly during the early stages (12–15). Sulforaphane (SFN) is a constituent of cruciferous vegetables, found at high levels in broccoli and broccoli sprouts (16, 17). SFN has been identified as an effective cancer chemoprotective agent in animal models (17–19), as well as in xenograft models of prostate cancer (20). Although the majority of early studies focused on SFN as a potent Phase 2 enzyme inducer, recent work has implicated other mechanisms of SFN action (reviewed in Ref. 2). For example, studies using various cancer cell lines have shown either cell cycle arrest or apoptosis upon treatment with SFN (20–29).
We recently identified SFN as a novel HDAC inhibitor in colon and prostate cancer cells (30, 31). HDAC inhibition was associated with global increases in histone acetylation, enhanced interactions of acetylated histones with the promoter regions of the P21 and BAX genes, and elevated expression of p21Cip1/Waf1 and BAX proteins. In addition to a G2/M cell cycle arrest, there was evidence for loss of BCL-2 expression and increased multicaspase activity, resulting in apoptosis.
Based on these findings (30, 31), we sought to determine whether SFN might act as an HDAC inhibitor in vivo, using a prostate xenograft model, as well as in peripheral blood mononuclear cells (PBMC) from human volunteers consuming broccoli sprouts. Here, we report that dietary administration of SFN, in both mice and humans, inhibited HDAC activity significantly and suppressed the growth of human PC-3 xenografts in nude mice. These findings provide evidence that SFN may exert its chemopreventive effects, at least in part, through inhibition of HDAC activity in vivo.
Materials and Methods
Xenografts
Male athymic nude BALB/c (nu/nu) mice (5 weeks old) were purchased from Charles River (Wilmington, MA) and maintained in accordance with Institutional Animal Care and Use Committee guidelines. PC-3 cells were mixed in a 1:1 ratio of complete media (Roswell Park Memorial Institute-1640 + 10% FBS) and High Concentration Growth Factors Matrigel Matrix (Becton Dickinson, Bedford, MA). A suspension of 106 cells (50 μl) was injected subcutaneously into the right flank of each mouse. Tumor volume was calculated using the following formula for the volume of an ellipsoid: length × width2 × 0.5236 (π/6).
Treatments
Mice were randomized into groups of 10 animals. In the test group, SFN (LKT, St. Paul, MN) was mixed into pelleted AIN93G diet without t-butylhydroquinone at a concentration of 443 mg/kg (2.5 μmol/g) diet (Research Diets, New Brunswick, NJ). AIN93G diet does not contain any isothiocyanates. Control mice received the same diet without added SFN. Diets were γ-irradiated at 2.5 Mrads and fed to mice ad libitum. Food intake and body weights were monitored throughout the study, and the animals were killed by CO2 inhalation 21 days after xenograft implantation.
Mononuclear Blood Cell (MBC) Isolation
Spleens were homogenized with a Dounce homogenizer, and a single cell suspension was made by passing the homogenate through a 21-gauge needle followed by a 23-gauge needle. The cell suspension was placed on top of Fico/Lite LE (Atlanta Biologicals, Lawrenceville, GA) and centrifuged for 25 mins at 2000 g. The MBC layer was collected and washed in phosphate-buffered saline (PBS) by spinning for 10 mins at 900 g. The MBC pellet was placed in 90% FBS/10% dimethylsulfoxide and slow-frozen in isopropyl alcohol at −80°C overnight.
Western Blotting
Frozen portions of tumor tissue were thawed in lysis buffer and ground using a Potter-Elvehjem homogenizer (Teflon drill). Whole prostates were homogenized and centrifuged at 15,000 rpm for 5 mins, and the supernatant was collected. Protein concentrations were determined by the Lowry assay. Proteins (10–20 μg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis on a 4%–12% bis-Tris gel (Novex, San Diego, CA) and were transferred to nitrocellulose membrane (Invitrogen, Carlsbad, CA). Equal protein loading was confirmed with Amido black staining and β-actin levels. The membrane was blocked for 1 hr with 2% bovine serum albumin, followed by either overnight incubation with primary antibody at 4°C or 1 hr incubation with primary antibody at room temperature, and was finally incubated for 1 hr with secondary antibody conjugated with horseradish peroxidase (Bio-Rad, Hercules, CA). Antibody dilutions were as follows: acetylated histone H3, 1:100 (Upstate, Charlottesville, VA); acetylated histone H4, 1:100 (Upstate); Bax, 1:100 (Santa Cruz Biotechnologies, Santa Cruz, CA); and β-actin, 1:5000 (Sigma, St. Louis, MO). Antibodies were selected on the basis of their ability to recognize both human and mouse proteins. Detection was by Western Lightning Chemiluminescence Reagent Plus (PE Life Sciences, Boston, MA) with image analysis on an AlphaInnotech photodocumentation system (Hayward, CA). Image quantification was determined by National Institutes of Health ImageJ software (Bethesda, MD). Expression levels were normalized to β-actin.
HDAC Activity Assay
HDAC activity was determined using the Fluor-de-Lys HDAC activity assay kit (Biomol, Plymouth Meeting, PA), as reported previously (31). Homogenates (25 μg of total protein) from tumor tissue, prostates, or MBC were incubated with Fluor-de-Lys substrate in triplicate for 12 min at 37°C to initiate the HDAC reaction. Fluor-de-Lys Developer was then added, and the mixture was incubated for another 10 min at room temperature. Fluorescence was measured using a Spectra Max Gemini XS fluorescent plate reader (Molecular Devices, Sunnyvale, CA), with excitation at 360 nm and emission at 460 nm.
Human Study
The Institutional Review Board at Oregon State University approved the protocol for this investigation, and all participants provided written consent before enrollment. In brief, healthy volunteers (n = 3) were selected for this study on the basis of age (18–55 yrs), nonnutritional supplement use (>6 months), and exercise status (<5 hrs/week of aerobic activity). Volunteers were asked to refrain from cruciferous vegetable intake for 48 hrs. Each subject consumed 68 g BroccoSprouts broccoli sprouts (approximiately 105 mg SFN; equivalent to approximately 570 g of mature broccoli) with a bagel and cream cheese. Blood was collected at 0, 3, 6, 24, and 48 hrs after consumption of broccoli sprouts. Approximately 8 ml of blood was drawn into EDTA vacutainer blood collection tubes. The blood was layered on top of Histopaque (Sigma) and centrifuged at 400 g for 30 min at room temperature. The PBMC layer was removed, placed in a clean 15-ml tube, washed with PBS three times, and then frozen at −80°C until HDAC activity analysis.
Statistics
One-way analysis of variance or Student’s t test was used to assess the differences between groups. Differences among treatments were tested by Dunnett’s test.
Results
We recently identified SFN as an HDAC inhibitor in human prostate epithelial cells, with HDAC inhibition resulting in cell cycle arrest and apoptosis (30). In the present study, we sought to verify that SFN was an HDAC inhibitor in vivo, leading to retardation of PC-3 xenograft growth, and to determine whether human consumption of SFN-rich broccoli sprouts would result in inhibition of HDAC activity in PBMC. SFN administered to mice in the diet inhibited xenograft growth significantly compared with controls as early as 3 days after implantation (Fig. 1A). Although the protective effects diminished somewhat at later times, SFN retarded xenograft growth significantly throughout the 21-day duration of this study.
Figure 1.
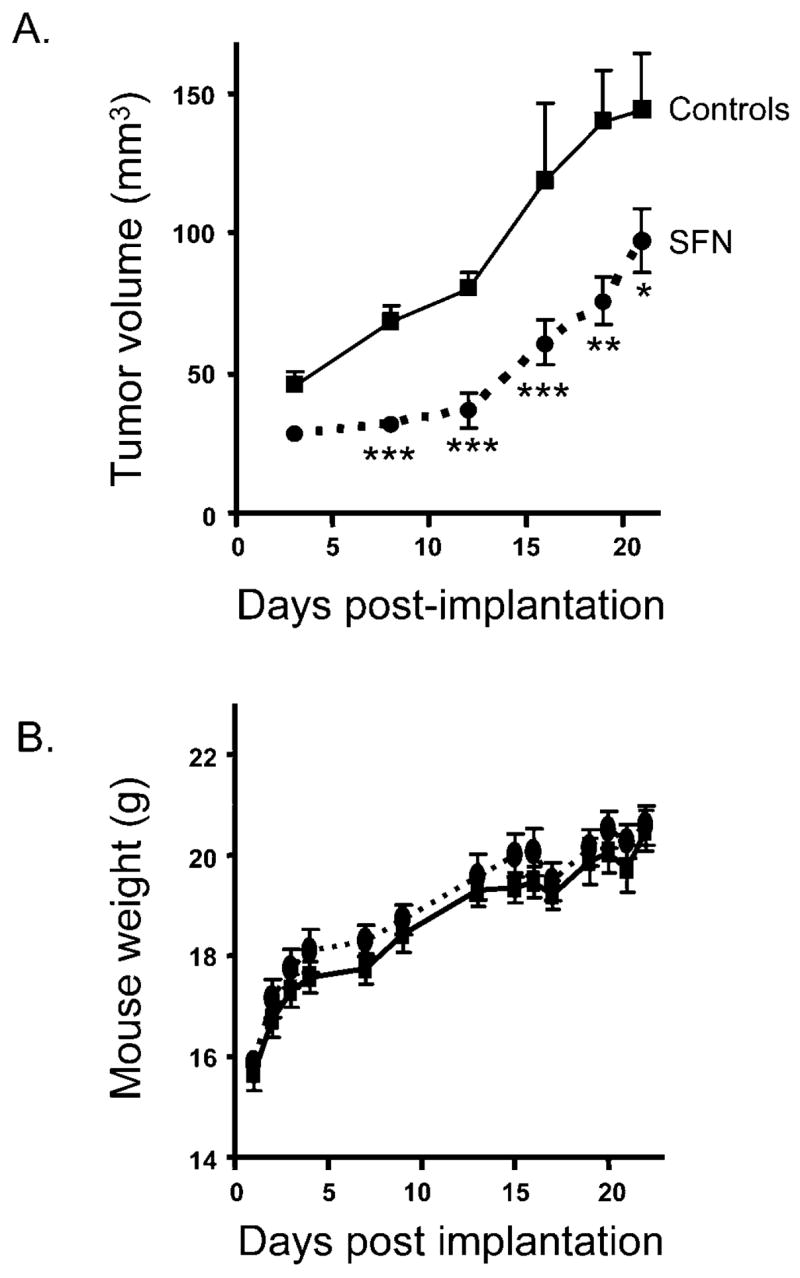
SFN retards the growth of PC-3 xenografts. (A) PC-3 cells were implanted into male nude mice (10 mice per group). SFN was administered in the diet (443 mg/kg; 2.5 μmol/g) beginning on the day of implantation. Tumor volume was determined as described in Materials and Methods. (B) No adverse effect of SFN on mouse body weight. Data are expressed as mean ± SE. *P < 0.05, **P < 0.01, ***P < 0.001.
There were no adverse effects of SFN treatment on animal health, food intake, or body weight. The average daily food intake did not differ between controls and treatment groups (Table 1). Moreover, body weight was not significantly different throughout the duration of the study, suggesting that SFN was essentially nontoxic at the dietary concentration used in the present investigation (Fig. 1B). Mice consumed approximately 7.5 μmol SFN (1.3 mg) per day.
Table 1.
No Effect of SFN on Food Intake and Body Weight Gaina
| Average daily food intake (g) | Average body weight at end of study (g) | |
|---|---|---|
| Control | 3.11 ± 0.99 | 20.48 ± 1.22 |
| SFN | 3.26 ± 0.98 | 20.58 ± 1.23 |
Nude mice (10 animals per group) were given γ-irradiated AIN93G diet (controls), or the same diet containing 443 mg SFN per kg, from the day that PC-3 cells were implanted as xenografts until the study was terminated at 3 weeks. Food and water were provided ad libitum. For body weights at earlier time points, see Figure 1B.
At the end of the study, xenografts were examined for possible systemic effects of SFN treatment on HDAC activity. Compared with xenografts recovered from animals on the control diet, HDAC activity was inhibited significantly in xenografts of mice fed SFN (P < 0.001; Fig. 2A). Interestingly, immunoblotting of xenografts showed a trend towards increased acetylation of histones H3 and H4 among the treatment groups, compared with controls (Fig. 2B and C). These changes, however, were not statistically significant. In the PC-3 xenografts, induction of BAX expression was observed, and this was significant in animals fed SFN (Fig. 2B and C). Because PC-3 cells lack P53, the increase in BAX may be attributed to increased histone acetylation at the BAX promoter, as observed recently in BPH-1 cells treated with SFN in vitro (30).
Figure 2.
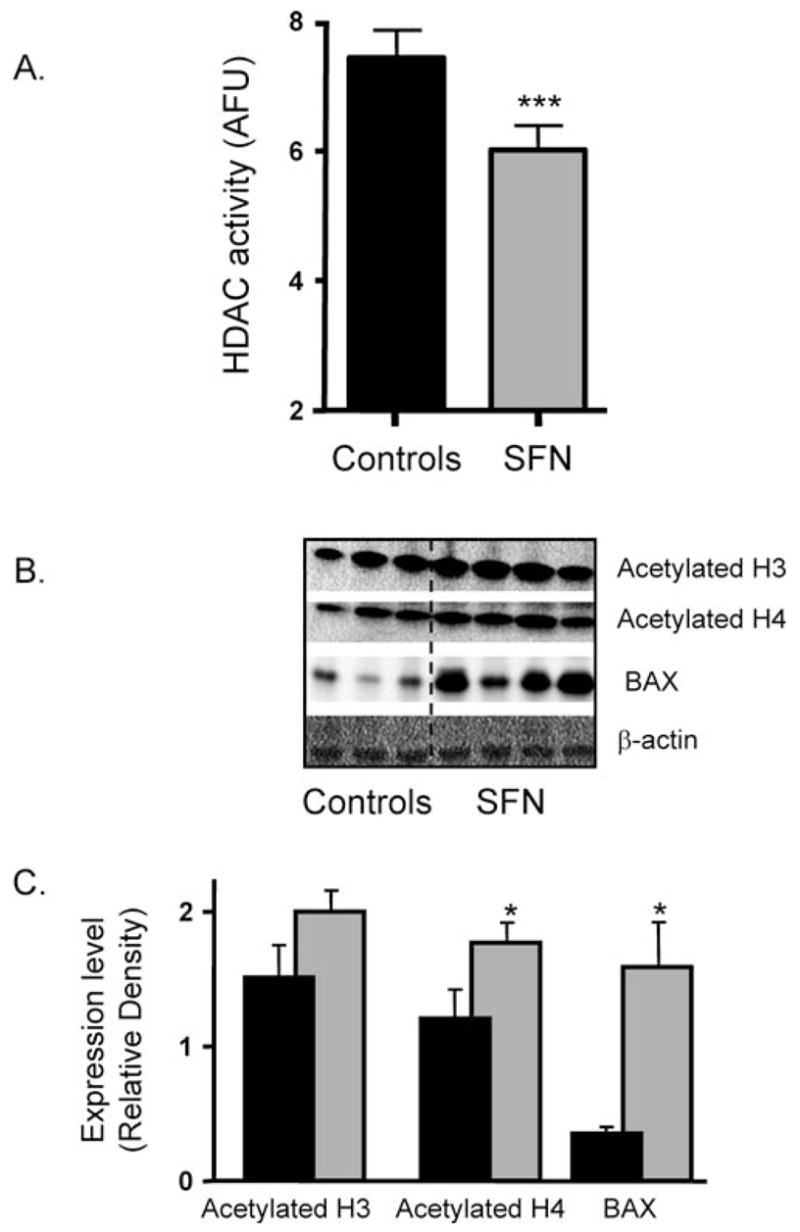
SFN inhibits HDAC activity in PC-3 xenografts. (A) HDAC activity (arbitrary fluorescent units [AFU]) was determined in xenografts as described in Materials and Methods. Data are expressed as mean ± SE. n = 10. ***P < 0.001. (B) Xenografts were immunoblotted for acetylated H3, acetylated H4, and BAX; β-actin was used as the loading control. Each lane represents a xenograft from an individual animal, and the blot is a representative sample from each group of 10 animals. (C) Quantification of immunoblots using National Institutes of Health Image J, normalized for β–actin. Data are expressed as mean ± SE. *P < 0.05.
Because HDAC inhibition was detected in the xenografts, we next examined systemic effects of SFN in mouse prostate tissues. HDAC activity was significantly inhibited in the prostates from mice treated with SFN, compared with animals fed the control diet (Fig. 3A). Paralleling the inhibition of HDAC activity, prostates from the SFN-treated mice showed increased acetylated histones H3 and H4, and a concomitant increase in BAX expression (Fig. 3B and C).
Figure 3.
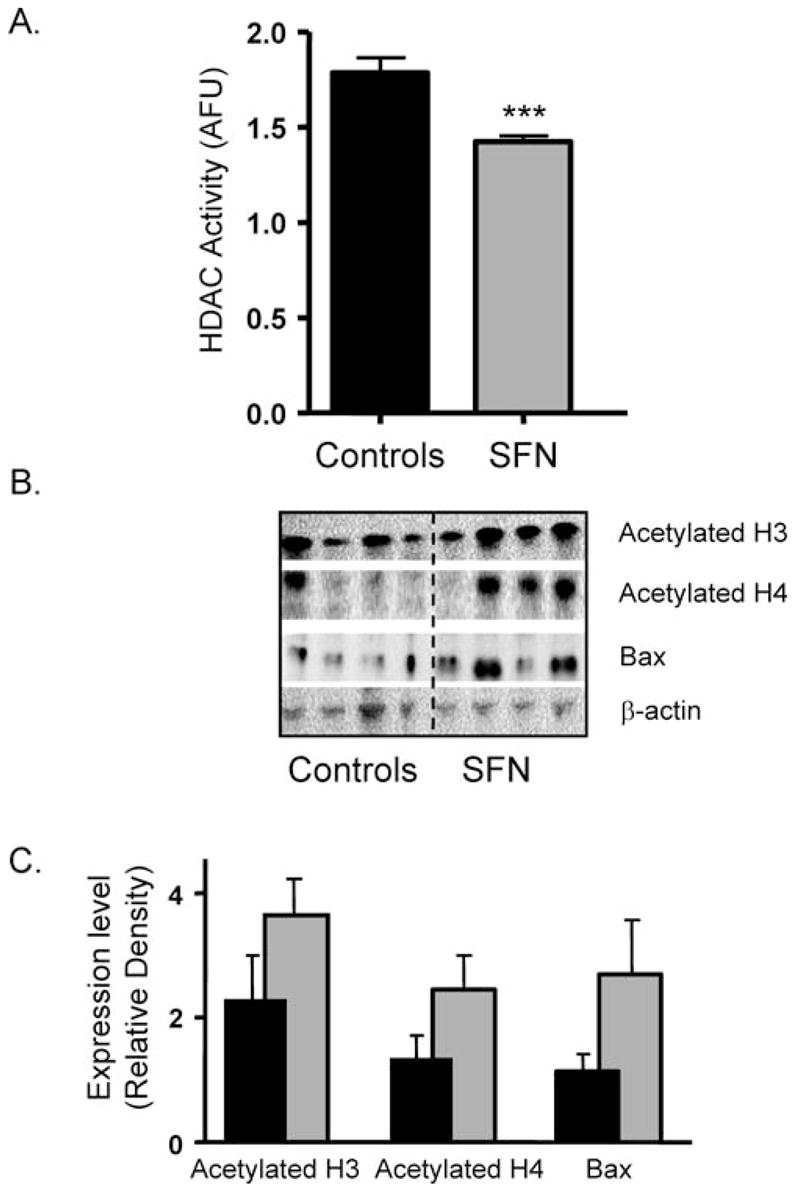
SFN increases acetylated histones and induces BAX expression in mouse prostates. (A) HDAC activity was determined in mouse prostate tissues as described in Materials and Methods. Data are expressed as mean ± SE. n = 10. ***P < 0.001. (B) Prostates were immunoblotted for acetylated H3, acetylated H4, and BAX; β-actin was included as a loading control. Each lane represents a prostate from an individual animal, and the blot is a representative sample from each group of 10 animals. (C) Quantification of immunoblots using National Institutes of Health Image J, normalized for β–actin. Data are expressed as mean ± SE.
Several HDAC inhibitors are currently undergoing clinical trials; an important biomarker of HDAC inhibition used in these trials is acetylated histone status from PBMC (32, 33). In the present investigation, HDAC activity was inhibited significantly in MBC obtained from SFN-fed mice, compared with controls (**P < 0.01, Fig. 4A). Moreover, in human subjects, HDAC activity was significantly inhibited in the PBMC of all three subjects as early as 3 hrs after consumption of 68 g of broccoli sprouts (Fig. 4B). In two of the subjects, HDAC inhibition also was seen at the 6-hr time point, and by 24–48 hrs HDAC activity had returned to the level detected at 0 hrs (Fig. 4C). In a third subject, the starting HDAC activity at 0 hrs was lower than expected, and for this reason the highest HDAC activity was seen at 48 hrs. (Fig. 4C). A longer period of restriction from cruciferous vegetable intake might be required in some subjects to attain peak HDAC activity levels in BMC.
Figure 4.
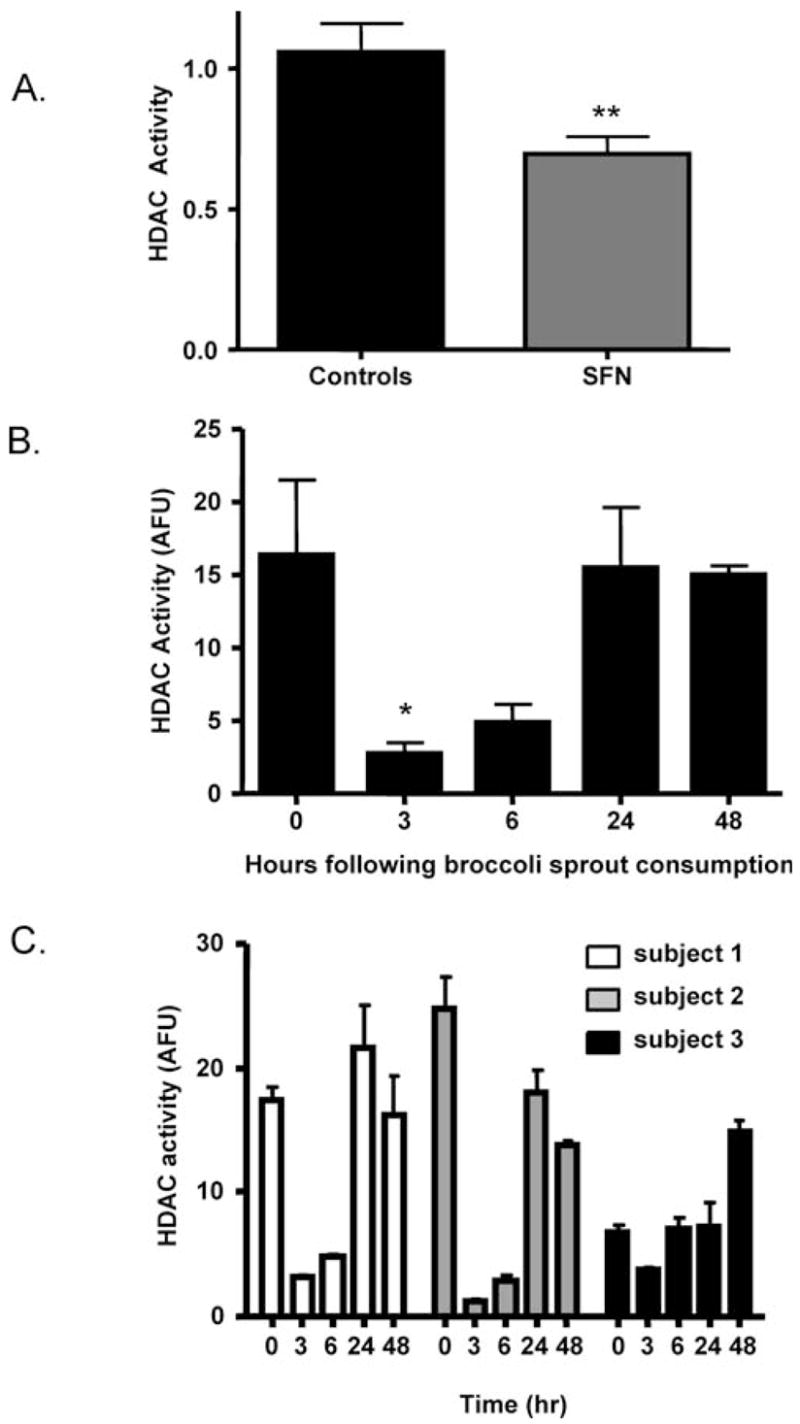
SFN inhibits HDAC activity in mouse MBC and human PBMC. (A) HDAC activity in mouse MBC was determined as described in Materials and Methods; mean ± SE. **P < 0.01 for SFN versus controls. (B) Human subjects consumed 68 g of BroccoSprouts and blood was obtained at the times indicated in the figure. PBMC were isolated and HDAC activity assays were performed as described in Materials and Methods. Data are expressed as mean ± SE. n = 3. *P < 0.05. (C) Data for HDAC activity in PBMC from each subject.
Discussion
We recently reported that SFN inhibited HDAC activity in BPH-1, LnCaP, and PC-3 prostate epithelial cells, with accumulation of acetylated histones associated with the BAX promoter, increased levels of BAX mRNA and protein, loss of procaspase-3, and induction of multicaspase activity leading to apoptosis (30). Other groups have reported that SFN given to mice at a dose of 5.6 μmol three times per week by gavage slowed the growth of PC-3 xenografts and caused induction of apoptosis (20). In the present study, we confirmed that dietary SFN also retarded the growth of PC-3 xenografts in vivo, with evidence for HDAC inhibition and induction of BAX in xenografts and prostate tissues of mice. Importantly, HDAC activity also was inhibited in MBC from mice given SFN, and in PBMC from human subjects consuming broccoli sprouts, which are a rich source of SFN (17–20).
SFN is an effective chemopreventive agent and induces Phase 2 enzymes involved in carcinogen detoxification and excretion (17–20). However, SFN also has been shown to suppress colonic aberrant crypts when administered solely after the carcinogen exposure period (18), suggesting a role for mechanisms postinitiation. SFN induced apoptosis in, and slowed the growth of, PC-3 xenografts in vivo (20), and cell culture models have demonstrated cell cycle arrest and apoptosis using SFN as a test agent (20–22, 24, 25, 27–30, 36–39). Several mechanisms by which SFN modulates cell cycle arrest and apoptosis in cancer cells have been proposed (reviewed in Refs. 2, 40). Based on the present findings and our previous work in colon and prostate cancer cells (30, 31), we propose that SFN acts as an HDAC inhibitor, augmenting histone acetylation and derepressing genes such as P21 and Bax, leading to cancer prevention via cell cycle arrest and apoptosis.
Interestingly, the HDAC activity levels varied between tissue types, based on the fluorescent values that measure relative HDAC activity. For example, the arbitrary fluorescent units for average HDAC activity in the xenografts (human PC-3 prostate cancer) from control mice were approximately 8-fold higher than the arbitrary fluorescent units for average HDAC activity in the prostates in control mice. PC-3 cells are derived from an androgen-independent, late-stage human prostate carcinoma; the higher HDAC activity levels in these cells compared with normal murine prostate tissue confirms the importance of HDAC activity in the progression to tumorigenesis. Interestingly, there seemed to be a difference in the extent of accumulation of acetylated histones in the prostate compared with the PC-3 xenografts (Figs. 2B and 3B). This may be because of differences in relative HDAC activity levels before the SFN intervention, metabolism of SFN in the two sites, or the accumulation and retention of SFN in xenografts versus murine prostates. It is also possible that cancerous cells have a greater ability to resist the downstream effects of HDAC inhibition, that is, accumulation of acetylated histones, than normal cells. This would suggest that targeting epigenetic alterations early in the carcinogenesis process through prevention may be a better strategy than using HDAC inhibitiors as chemotherapeutic agents in a more advanced stage of cancer. Further studies should address the efficacy of prevention versus treatment.
We also tested a prototypical HDAC inhibitor, trichostatin A (TSA), in the mouse xenograft model, starting on the day of implantation and administered by subcutaneous injection (0.5 mg/kg body weight) every day for 21 days; TSA was not as effective as SFN at inhibiting xenograft growth, and there was no evidence for additive or synergistic effects of SFN plus TSA coexposure (data not shown). TSA is a potent HDAC inhibitor in vitro, but it can be converted rapidly to inactive metabolites in vivo (41). In previous studies, an inhibitor of the mercapturic acid pathway attenuated the HDAC inhibitory effects of SFN in vitro, but increased the effectiveness of TSA (31), indicating that TSA parent compound is required for HDAC inhibition. Further work is needed on the possible synergistic effects of TSA plus SFN in vivo.
Phase 1 clinical trials have been completed for HDAC inhibitors such as SAHA and depsipeptide in which the test agents were administered by continuous intravenous (iv) infusion for 2 hrs (32, 33), and acetylated histone H3 levels in PBMC were used as a biomarker of effectiveness (32, 33). In the present investigation, SFN was given in the diet for 3 weeks and HDAC inhibition was observed in MBC and systemic tissues at the end of the study, hinting at a possible sustained effect of SFN compared with pharmacologic HDAC inhibitors given via the iv route of administration. However, we noted that the inhibition of xenograft development by SFN was most obvious early after implantation (Fig. 1A). Compensatory HDAC or histone acetyltransferase (HAT) expression might reduce the efficacy of SFN over time, but it will be necessary to confirm this possibility in follow-up studies. Such a finding could have important implications for human clinical trials in individuals who might develop resistance to HDAC inhibitors administered for prolonged periods. Cell culture studies are currently in progress to further understand the kinetics of HDAC inhibition by SFN, in order to determine whether there are changes in HDAC protein levels over time in response to SFN and to characterize potential HDAC subtypes that may be more sensitive to inhibition by SFN.
The xenograft model is useful for testing potential chemopreventive and chemotherapeutic agents in vivo, but we considered it important to examine biological effects in the intended target tissue. The accumulation of acetylated histones in the prostate of SFN-treated mice and the activation of BAX suggested that SFN indeed entered the prostate in a bioactive form and attained concentrations necessary for HDAC inhibition. HDAC activity assays confirmed the inhibition by SFN in prostate and MBC of mice. We therefore conducted a pilot study with human volunteers to determine whether consumption of broccoli sprouts would inhibit HDAC activity in human PBMC. Based on the approximate SFN content in BroccoSprouts, we calculated that 68 g of broccoli sprouts (approximately 1 cup) would provide a dose of SFN in humans comparable to that which inhibited HDAC activity in colonic mucosa, prostate, and PBMC of mice (42). During the period 3–6 hrs after ingestion, broccoli sprouts strongly inhibited HDAC activity in human PBMC (Fig. 4B and C). To our knowledge, this is the first study to show that a naturally-consumed food product, namely broccoli sprouts, exerted such a marked effect on HDAC activity in humans. A recent study reported the pharmacokinetics of SFN in humans and suggested that after consumption of broccoli containing high levels of SFN, some SFN or its metabolites may be retained in various tissues rather than rapidly excreted (43). Further work will be necessary to characterize the concentration of SFN metabolites in human plasma and urine and the concordance with HDAC activity, acetylated histones, and other acetylated proteins after single or repeated consumption of broccoli sprouts.
Clinical trials with pharmacologic HDAC inhibitors utilize the degree of histone acetylation as a biomarker for inhibition of HDAC activity (32, 33). In this small preliminary pilot study, we chose to use a more direct measure of HDAC activity through the use of an HDAC activity assay. In the results reported here from the xenograft study and in our previous studies (30, 31, 42), we have found that a decrease in HDAC activity correlates with an increase in accumulation of acetylated histones. Although clinical trials have focused on alterations in acetylated histone status as a biomarker for HDAC inhibition, the use of an HDAC activity assay requires less sample processing and the potential for high throughput analysis. Further studies are underway to validate this assay and confirm similar alterations in acetylated histone status in human volunteers consuming broccoli sprouts. In addition to acetylation of histones, HDACs also target various nonhistone proteins for deacetylation, such as p53, α-tubulin, and estrogen receptor α (44), ultimately affecting the activity of the proteins. Because the alterations in the activities of these proteins play a role in cell cycle progression and apoptosis, it will be important to examine the acetylation status of nonhistone proteins in future studies.
Importantly, this study found that administration of both purified SFN and SFN from broccoli sprouts resulted in HDAC inhibition. Because of the cost of purified SFN, the use of SFN-rich foods, such as broccoli sprouts, will greatly increase the accessibility of this important chemopreventive agent. The purified SFN used in this study was a racemic mixture of two stereoisomers, whereas SFN found in nature is only the L enantiomer. In cell culture, there was no difference in HDAC inhibition by the purified D or L enantiomers or by a 50:50 mixture (data not shown), suggesting that the inhibition of HDAC activity by SFN is not dependent on the specific isomer. Crystallization studies with SFN and human HDAC proteins would confirm this observation.
In summary, the present investigation showed that dietary administration of SFN retarded the growth of human PC-3 prostate cancer cells in nude mice. SFN inhibited HDAC activity in the xenografts, prostates, and MBC, and there was a trend towards increased histone acetylation. Consumption of a SFN-rich food (broccoli sprouts) strongly inhibited HDAC activity in MBC of human subjects. We conclude that further studies are warranted on dietary HDAC inhibitors and prostate cancer prevention, and on the use of HDAC activity as a biomarker in clinical trials. When combined with pharmacologic HDAC inhibitors or other chemopreventive agents, dietary HDAC inhibitors might improve the efficacy of intervention strategies and reduce the effective dose used in more conventional therapeutic treatments, thereby ameliorating untoward side effects.
Acknowledgments
We thank Roland Corden for processing of tissues; Lucas Tilley for help with animal treatments; Karin Hardin, Gayle Orner, David Yu, Qingjie Li, Violet Depoe, Mandy Louderback, Christine Larsen, and Rong Wang for assistance with the necropsy; the staff of Oregon State University Laboratory Animal Care for the care of the mice; and the human volunteers.
This work was supported in part by the National Institutes of Health grants CA66525 (R.H.D.), CA80176 (R.H.D.), CA90890 (R.H.D.), and CA107693 (E.H.), and by the National Institute of Environmental Health Sciences Center grant P30 ES00210. Additional support was provided by the Oregon Agricultural Experiment Station and the Medical Research Foundation of Oregon.
References
- 1.Myzak MC, Dashwood RH. Histone deacetylases as targets for dietary cancer preventive agents: lessons learned with butyrate, diallyl disulfide, and sulforaphane. Curr Drug Targets. 2006;7:443–452. doi: 10.2174/138945006776359467. [DOI] [PubMed] [Google Scholar]
- 2.Myzak MC, Dashwood RH. Chemoprotection by sulforaphane: keep one eye beyond Keap1. Cancer Lett. 2006;233:208. doi: 10.1016/j.canlet.2005.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov. 2002;1:287–299. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- 4.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 5.Marks PA, Richon VM, Rifkind RA. Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst. 2000;92:1210–1216. doi: 10.1093/jnci/92.15.1210. [DOI] [PubMed] [Google Scholar]
- 6.De Ruijter AJM, Van Gennip AH, Caron HN, Kemp S, Van Kuilenburg ABP. Histone deacetylases (HDACS): characterization of the classical HDAC family. Biochem J. 2003;370:737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patra SK, Patra A, Dahiya R. Histone deacetylase and DNA methyltransferase in human prostate cancer. Biochem and Biophys Res Comm. 2001;287:705–713. doi: 10.1006/bbrc.2001.5639. [DOI] [PubMed] [Google Scholar]
- 8.Halkidou K, Gaughan L, Cook S, Leung HY, Neal DE, Robson CN. Upregulation and nuclear recruitment of HDAC1 in hormone refractory prostate cancer. Prostate. 2004;59:177–189. doi: 10.1002/pros.20022. [DOI] [PubMed] [Google Scholar]
- 9.Fronsdal K, Saatcioglu F. Histone deacetylase inhibitors differentially mediate apoptosis in prostate cancer cells. Prostate. 2004;62:299–306. doi: 10.1002/pros.20140. [DOI] [PubMed] [Google Scholar]
- 10.Thelen P, Schweyer S, Hemmerlein B, Wuttke W, Seseke F, Ringert RH. Expressional changes after histone deacetylase inhibition by valproic acid in LNCaP human prostate cancer cells. Int J Oncol. 2004;24:25–31. [PubMed] [Google Scholar]
- 11.Butler LM, Agus DB, Scher HI, Higgins B, Rose A, Cordon-Cardo C, Thaler HT, Rifkind RA, Marks PA, Richon VM. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res. 2000;60:5165–5170. [PubMed] [Google Scholar]
- 12.Kolonel LN, Hankin JH, Whittemore AS, Wu AH, Gallagher RP, Wilkens LR, John EM, Howe GR, Dreon DM, West DW, Paffenbarger RS., Jr Vegetables, fruits, legumes and prostate cancer: a multiethnic case-control study. Cancer Epidemiol Biomarkers Prev. 2000;9:795–804. [PubMed] [Google Scholar]
- 13.Cohen JH, Kristal AR, Stanford JL. Fruit and vegetable intakes and prostate cancer risk. J Natl Cancer Inst. 2000;92:61–68. doi: 10.1093/jnci/92.1.61. [DOI] [PubMed] [Google Scholar]
- 14.Kristal AR, Lampe JW. Brassica vegetables and prostate cancer risk: a review of the epidemiological evidence. Nutr Cancer. 2002;42:1–9. doi: 10.1207/S15327914NC421_1. [DOI] [PubMed] [Google Scholar]
- 15.Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC. A prospective study of cruciferous vegetables and prostate cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:1403–1409. [PubMed] [Google Scholar]
- 16.Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci U S A. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung FL, Conaway CC, Rao CV, Reddy BS. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis. 2000;21:2287–2291. doi: 10.1093/carcin/21.12.2287. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci U S A. 1994;91:3147–3150. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh AV, Xiao D, Lew KL, Dhir R, Singh SV. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis. 2004;25:83–90. doi: 10.1093/carcin/bgg178. [DOI] [PubMed] [Google Scholar]
- 21.Chiao JW, Chung FL, Kancherla R, Ahmed T, Mittelman A, Conaway CC. Sulforaphane and its metabolite mediate growth arrest and apoptosis in human prostate cancer cells. Int J Oncol. 2002;20:631–636. doi: 10.3892/ijo.20.3.631. [DOI] [PubMed] [Google Scholar]
- 22.Gingras D, Gendron M, Boivin D, Moghrabi A, Theoret Y, Beliveau R. Induction of medulloblastoma cell apoptosis by sulforaphane, a dietary anticarcinogen from Brassica vegetables. Cancer Lett. 2004;203:35–43. doi: 10.1016/j.canlet.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 23.Bonnesen C, Eggleston IM, Hayes JD. Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res. 2001;61:6120–6130. [PubMed] [Google Scholar]
- 24.Gamet-Payrastre L, Li P, Lumeau S, Cassar G, Dupont MA, Chevolleau S, Gasc N, Tulliez J, Terce F. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000;60:1426–1433. [PubMed] [Google Scholar]
- 25.Fimognari C, Nusse M, Cesari R, Iori R, Cantelli-Forti G, Hrelia P. Growth inhibition, cell-cycle arrest and apoptosis in human T-cell leukemia by the isothiocyanate sulforaphane. Carcinogenesis. 2002;23:581–586. doi: 10.1093/carcin/23.4.581. [DOI] [PubMed] [Google Scholar]
- 26.Fimognari C, Nusse M, Berti F, Iori R, Cantelli-Forti G, Hrelia P. Cyclin D3 and p53 mediate sulforaphane-induced cell cycle delay and apoptosis in non-transformed human T lymphocytes. Cell Mol Life Sci. 2002;59:2004–2012. doi: 10.1007/PL00012523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson SJ, Singletary KW. Sulforaphane: a naturally occurring mammary carcinoma mitotic inhibitor which disrupts tubulin polymerization. Carcinogenesis. 2003;25:219–227. doi: 10.1093/carcin/bgg192. [DOI] [PubMed] [Google Scholar]
- 28.Gamet-Payrastre L, Lumeau S, Gasc N, Cassar G, Rollin P, Tulliez J. Selective cytostatic and cytotoxic effects of glucosinolates hydrolysis products on human colon cancer cells in vitro. Anticancer Drugs. 1998;9:141–148. doi: 10.1097/00001813-199802000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Liu D, Ahmed T, Chung FL, Conaway C, Chiao JW. Targeting cell cycle machinery as a molecular mechanism of sulforaphane in prostate cancer prevention. Int J Oncol. 2004;24:187–192. [PubMed] [Google Scholar]
- 30.Myzak MC, Hardin K, Wang R, Dashwood RH, Ho E. Sulforaphane inhibits histone deacetylase activity in BPH-1, LnCaP and PC-3 prostate epithelial cells. Carcinogenesis. 2006;27:811–819. doi: 10.1093/carcin/bgi265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myzak MC, Karplus PA, Chung FL, Dashwood RH. A novel mechanism of chemoprotection by sulforaphane: inhibition of histone deacetylase. Cancer Res. 2004;64:5767–5774. doi: 10.1158/0008-5472.CAN-04-1326. [DOI] [PubMed] [Google Scholar]
- 32.Kelly WK, Richon VM, O’Connor O, Curley T, MacGregor-Curtelli B, Tong W, Klang M, Schwartz L, Richardson S, Rosa E, Drobnjak M, Cordon-Cordo C, Chiao JH, Rifkind R, Marks PA, Scher H. Phase I clinical trial of histone deacetylase inhibitor: suberoylanilide hydroxamic acid administered intravenously. Clin Cancer Res. 2003;9:3578–3588. [PubMed] [Google Scholar]
- 33.Sandor V, Bakke S, Robey RW, Kang MH, Blagosklonny MV, Bender J, Brooks R, Piekarz RL, Tucker E, Figg WD, Chan KK, Goldspiel B, Fojo AT, Balcerzak SP, Bates SE. Phase I trial of the histone deacetylase inhibitor, depsipeptide ( FR901228, NSC 630176), in patients with refractory neoplasms. Clin Cancer Res. 2002;8:718–728. [PubMed] [Google Scholar]
- 34.Jackson SJ, Singletary KW. Sulforaphane inhibits human MCF-7 mammary cancer cell mitotic progression and tubulin polymerization. J Nutr. 2004;134:2229–2236. doi: 10.1093/jn/134.9.2229. [DOI] [PubMed] [Google Scholar]
- 35.Misiewicz I, Skupinska K, Kasprzycka-Guttman T. Sulforaphane and 2-oxohexyl isothiocyanate induce cell growth arrest and apoptosis in L-1210 leukemia and ME-18 melanoma cells. Oncol Rep. 2003;10:2045–2050. [PubMed] [Google Scholar]
- 36.Parnaud G, Li P, Cassar G, Rouimi P, Tulliez J, Combaret L, Gamet-Payrastre L. Mechanism of sulforaphane-induced cell cycle arrest and apoptosis in human colon cancer cells. Nutr Cancer. 2004;48:198–206. doi: 10.1207/s15327914nc4802_10. [DOI] [PubMed] [Google Scholar]
- 37.Pham NA, Jacobberger JW, Schimmer AD, Cao P, Gronda M, Hedley DW. The dietary isothiocyanate sulforaphane targets pathways of apoptosis, cell cycle arrest, and oxidative stress in human pancreatic cancer cells and inhibits tumor growth in severe combined immuno-deficient mice. Mol Cancer Ther. 2004;3:1239–1248. [PubMed] [Google Scholar]
- 38.Singh SV, Herman-Antosiewicz A, Singh AV, Lew KL, Srivastava SK, Kamath R, Brown KD, Zhang L, Baskaran R. Sulforaphane-induced G2/M phase cell cycle arrest involves checkpoint kinase 2-mediated phosphorylation of cell division cycle 25C. J Biol Chem. 2004;279:25813–25822. doi: 10.1074/jbc.M313538200. [DOI] [PubMed] [Google Scholar]
- 39.Tseng E, Scott-Ramsay EA, Morris ME. Dietary organic isothiocyanates are cytotoxic in human breast cancer MCF-7 and mammary epithelial MCF-12A cell lines. Exp Biol Med (Maywood) 2004;229:835–842. doi: 10.1177/153537020422900817. [DOI] [PubMed] [Google Scholar]
- 40.Gamet-Payrastre L. Signaling pathways and intracellular targets of sulforaphane mediating cell cycle arrest and apoptosis. Curr Cancer Drug Targets. 2006;6:135–145. doi: 10.2174/156800906776056509. [DOI] [PubMed] [Google Scholar]
- 41.Sanderson L, Taylor GW, Aboagye EO, Alao JP, Latigo JR, Coombes RC, Vigushin DM. Plasma pharmacokinetics and metabolism of the histone deacetylase inhibitor trichostatin a after intraperitoneal administration to mice. Drug Metab Dispos. 2004;32:1132–1138. doi: 10.1124/dmd.104.000638. [DOI] [PubMed] [Google Scholar]
- 42.Myzak MC, Dashwood WM, Orner GA, Ho E, Dashwood RH. Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apc-minus mice. FASEB J. 2006;20:506–508. doi: 10.1096/fj.05-4785fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gasper AV, Al-janobi A, Smith JA, Bacon JR, Fortun P, Atherton C, Taylor MA, Hawkey CJ, Barrett DA, Mithen RF. Glutathione S-transferase M1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. Am J Clin Nutr. 2005;82:1283–1291. doi: 10.1093/ajcn/82.6.1283. [DOI] [PubMed] [Google Scholar]
- 44.Gloznak MASN, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. Gene. 2005;363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]


