Abstract
The analgesic effect of buprenorphine is mediated via the mu opioid receptor (MOP). In the present study, using mice lacking the MOP and their wild-type littermates, we determined the role of the MOP in buprenorphine-induced locomotor stimulation and conditioned place preference (CPP). Buprenorphine (3 mg/kg) increased motor activity in wild-type but not in MOP knockout mice, showing the motor stimulatory action of buprenorphine is mediated via the MOP. When the mice were given the same treatment once daily for 5 consecutive days and challenged with buprenorphine on day 11, the motor stimulatory action of buprenorphine was enhanced in wild-type but not in MOP knockout mice, showing sensitization developed to the motor stimulatory action of buprenorphine and this phenomenon was mediated via the MOP. Likewise, buprenorphine induced CPP in wild-type mice after four alternate-day saline/buprenorphine (3 mg/kg) injections paired with olfactory and visual cues. However, buprenorphine failed to induce CPP in MOP knockout mice. In contrast, amphetamine (1 mg/kg) induced a comparable CPP in wild-type and MOP knockout mice. Together, the present results suggest that the ability of buprenorphine to increase motor activity and induce locomotor sensitization and CPP is mediated via the MOP.
Keywords: Buprenorphine, Mu opioid receptor (MOP), Knockout mouse, Locomotor sensitization, Conditioned place preference (CPP), Amphetamine
1. Introduction
Opioids and other drugs of abuse exert their rewarding and addictive effects via modulation of the mesolimbic dopaminergic reward circuitry (Di Chiara and Imperato, 1988a; for reviews see Koob and Le Moal, 1997; Koob and Nestler, 1997; Nestler and Malenka, 2004). For example, morphine, a mu opioid receptor (MOP) agonist, increases extracellular dopamine in the nucleus accumbens, a response that is thought to play an important role in its rewarding actions (Di Chiara et al., 2004; Matthes et al., 1996; but see Hnasko et al., 2005). The increase in accumbal dopamine is also important for the motor stimulatory action of morphine (Hnasko et al., 2005). The motor stimulatory action of morphine and other drugs of abuse is enhanced following their repeated intermittent administration, a phenomenon referred to as locomotor sensitization which is thought to play an important role in the development and maintenance of drug dependency, particularly craving (for review see Robinson and Berridge, 1993, 2000).
Buprenorphine, a mixed agonist/antagonist at the opioid receptors, is used clinically as an analgesic and for the management of opiate dependency. Although buprenorphine is described as a partial agonist at the MOP (Martin et al., 1976; for reviews see Cowan, 2003; Lutfy and Cowan, 2004; Ohlsen and Pilowsky, 2005; Robinson, 2002; Tzschentke, 2002), its mechanism of action is not fully understood For example, there is evidence showing its interaction with the kappa and delta opioid receptors as well as with the opioid receptor-like (ORL-1) receptor (Bloms-Funke et al., 2000; Hawkinson et al., 2000; Huang et al., 2001; Lutfy et al., 2003; Negus et al., 1989, 2002; Sadee et al., 1982; Wnendt et al., 1999; for review see Lutfy and Cowan, 2004). Thus, buprenorphine represents an opioid with unique and complex pharmacology because it can simultaneously act as an agonist and/or antagonist at different classes of opioid receptors. However, the contribution of each receptor in the actions of buprenorphine remains poorly understood.
There is ample evidence indicating the analgesic effect of buprenorphine to its activity at the MOP. Kamei and colleagues, for example, have demonstrated that the antinociceptive effect of buprenorphine was blocked by naloxonazine (Kamei et al., 1995), a MOP1 antagonist, as well as in MOP1 deficient CXBK mice (Kamei et al., 1997). Previously, we have also reported that the antinociceptive effect of buprenorphine was abolished in mice lacking the MOP (Lutfy et al., 2003), raising the possibility that other actions of buprenorphine could also be altered in these mice. Therefore, using mice lacking the MOP and their wild-type littermates, the present study was designed to determine the role of the MOP in locomotor stimulation and conditioned place preference (CPP) induced by buprenorphine.
2. Methods
2.1. Subjects
Male MOP knockout (Matthes et al., 1996) and wild-type offspring (3–6 months) of heterozygous mice were used for all experiments. Mice were housed 2–4 per cage with free access to food and water and maintained under a 12-h light/12-h dark cycle. All experiments were conducted according to the NIH guideline for the proper use of animals in research and approved by the Institutional Animal Care and Use Committee.
2.2. Experimental procedure
2.2.1. Buprenorphine-induced motor stimulation and locomotor sensitization
Mice were habituated to activity testing chambers (3.8 L Plexiglas cylinders) for 1 h, injected with buprenorphine (3 mg/kg, s.c.) and distance traveled (cm), used as a measure of motor activity, was recorded for 1 h (4 × 15-min sessions). The Videomex-V system (Columbus Instruments Inc., Columbus, OH, USA) was used to measure motor activity. Mice were given the same treatment for 4 additional days and then left untreated until day 11 (test day). On the test day, mice were habituated for 1 h, then injected with buprenorphine (3 mg/kg, s.c.) and motor activity recorded for 1 h (4 × 15-min sessions).
2.2.2. Buprenorphine-induced conditioned place preference (CPP)
The CPP apparatus and paradigm were as described previously (Marquez et al., 2006). Mice lacking the MOP and their wild-type littermates were tested for baseline preference toward the CPP chambers on day 1. On this day, each mouse was individually placed in the neutral (central gray) chamber of a three-chambered CPP apparatus and allowed to freely explore all three chambers of the CPP apparatus for 15 min. The amount of time that the mice spent in each chamber was recorded. On days 2–9, mice received alternate-day saline/buprenorphine (3 mg/kg, s.c.) conditioning sessions. During the conditioning sessions, mice were daily injected with saline or buprenorphine and confined to the vehicle-paired or drug-paired conditioning chambers for 1 h. The conditioning chambers were distinguishable by the presence of olfactory (almond or orange scent) or visual (decorated with 1-inch horizontal or vertical black and white stripes). Every attempt was made to balance the exposure of the mice to the treatments and conditioning chambers including the olfactory and visual cues. On day 10, mice were tested for post-conditioning preference in which each mouse was placed in the neutral chamber and allowed to freely explore all the CPP chambers for 15 min. The amount of time that the mice spent in each chamber was recorded and used for data analysis. For comparison, we also assessed amphetamine-induced CPP in mice lacking the MOP and their wild-type littermates. A separate group of naive mice were used for this experiment. Mice were tested for baseline preference on day 1, received alternate-day saline/amphetamine (1 mg/kg, i.p.) conditioning sessions on days 2–9 and then tested for post-conditioning preference on day 10, as described above for buprenorphine-induced CPP.
2.2.3. Data analysis
Data are expressed as mean ± S.E.M. The motor activity data were analyzed using a two-way randomized block analysis of variance (ANOVA). The CPP data were analyzed using two-factor ANOVA. The two factors were CPP chambers [vehicle-paired chamber (VPCh) versus drug-paired chamber (DPCh) and genotype (wild-type mice versus MOP knockout mice)]. Wherever it was appropriate, the post-hoc Student-Newman–Keuls test or the Least Squares of Means analysis was used to reveal significant differences between various groups. A p < 0.05 was considered statistically significant.
3. Results
3.1. Buprenorphine induced motor stimulation and locomotor sensitization in wild-type but not in MOP knockout mice
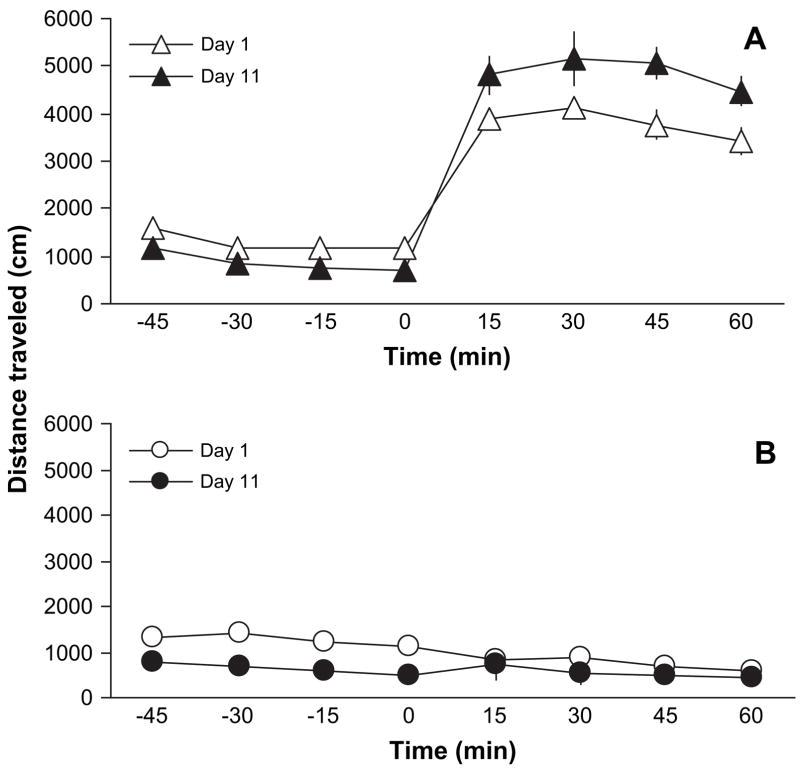
Fig. 1 illustrates the motor stimulatory action of buprenorphine in mice lacking the MOP and their wild-type littermates. A two-way randomized block ANOVA revealed a significant effect of time with regards to buprenorphine administration (F7,70 = 48.46; p < 0.001), a significant effect of genotype (F1,10 = 108.48; p < 0.0001) and a significant interaction between time and genotype (F7,70 = 105.21; p < 0.0001), showing that buprenorphine stimulated motor activity in wild-type but not in MOP knockout mice. Fig. 2 illustrates the development of locomotor sensitization in wild-type but not in MOP knockout mice. The motor stimulatory action of buprenorphine was enhanced upon its repeated intermittent administration in wild-type mice (Fig. 2A; compare day 1 versus day 11). A two-way randomized block ANOVA revealed a significant effect of test session in response to buprenorphine given on day 11 (F3,30 = 7.64; p < 0.006) and a significant effect of repeated buprenorphine administration (F1,10 = 5.69; p < 0.03), showing that buprenorphine increased motor activity and sensitization developed to this action in wild-type mice. However, such a sensitized response was not observed after repeated administration of buprenorphine in mice lacking the MOP (Fig. 2B; compare day 1 versus day 11). A two-way randomized block ANOVA revealed a significant effect of test session on day 11 (F3,30 = 3.92; p < 0.02) but no significant effect of repeated buprenorphine treatment (F1,10 = 0.56; p > 0.05), indicating that locomotor sensitization did not develop after repeated buprenorphine treatment in MOP knockout mice.
Fig. 1.

Buprenorphine failed to increase motor activity in mice lacking the MOP. Mice lacking the MOP [MOP (−/−)] and their wild-type littermates [MOP (+/+)] were habituated to motor activity chambers for 1 h, then injected with buprenorphine (3 mg/kg, s.c.) and motor activity recorded for an additional 1 h (4 × 15-min sessions). Data are presented as mean (±S.E.M.) of 6 mice per genotype.
Fig. 2.
Buprenorphine induced locomotor sensitization in wild-type but not in MOP knockout mice. Mice lacking the MOP [MOP (−/−)] and their wild-type littermates [MOP (+/+)] were tested on day 1 as described under legend to Fig. 1. The same treatment was given for 4 additional consecutive days. Mice were then left undisturbed until day 11 (test day). On the test day, mice were habituated to the motor activity cambers for 1 h, then injected with buprenorphine (3 mg/kg, s.c.) and motor activity recorded for an additional 1 h (4 × 15-min sessions). Data are presented as mean (±S.E.M.) of 6 wild-type (A) and 6 MOP knockout (B) mice.
3.2. Buprenorphine induced CPP in wild-type but not in MOP knockout mice
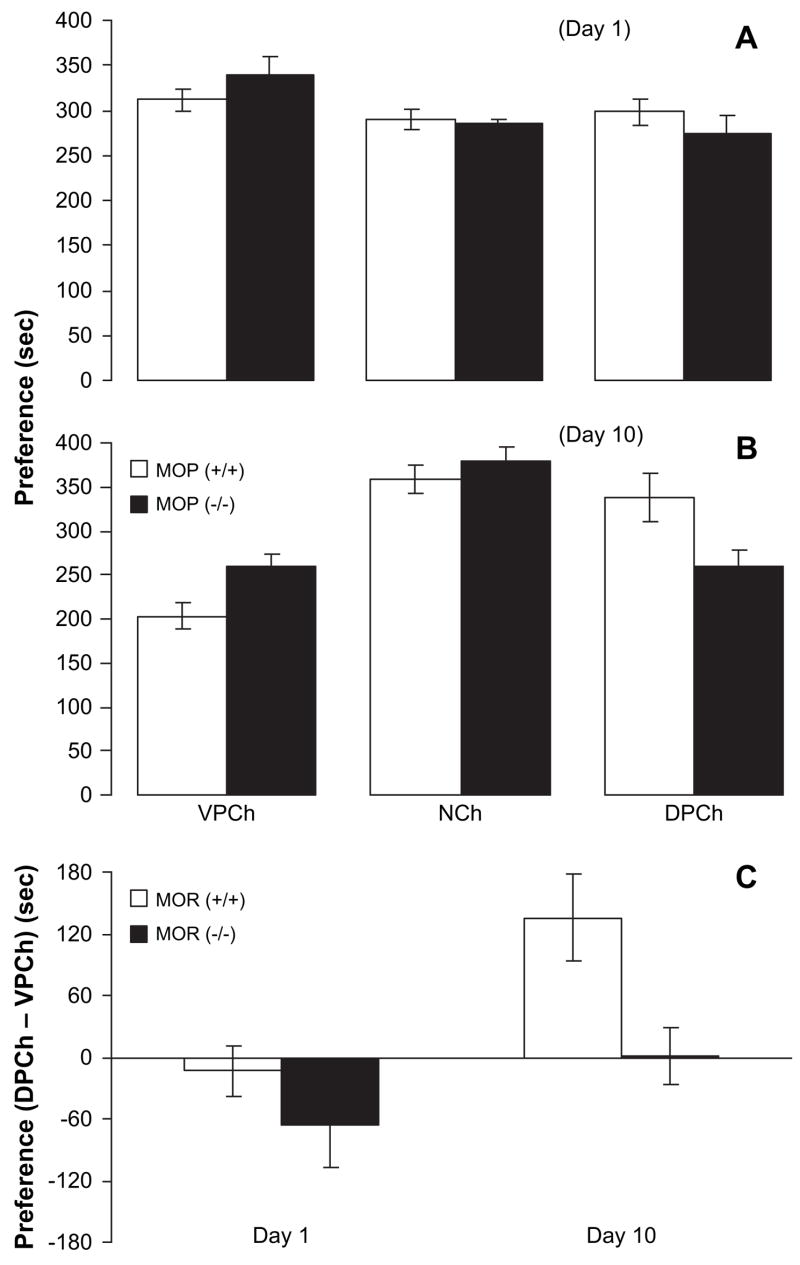
No significant difference was evident between the wild-type and MOP knockout mice in the amount of time spent in the conditioning chambers on day 1 (Fig. 3A). A two-factor ANOVA revealed a significant effect of CPP chamber (F2,39 = 4.02; p < 0.03) but no significant effect of genotype (F1,39 = 0.06; p > 0.05) or significant interaction between CPP chamber and genotype (F2,39 = 1.40; p > 0.05). Further analysis of the data showed that both wild-type and MOP knockout mice displayed similar basal preference toward the CPP chambers ( p > 0.05). However, repeated alternate-day saline/buprenorphine injection paired with olfactory and visual cues shifted the preference toward the drug-paired chamber in the wild-type but not in the MOP knockout mice (Fig. 3B). A two-factor ANOVA revealed a significant effect of CPP chamber (F2,39 = 15.96; p < 0.0001) and a significant interaction between CPP chamber and genotype (F2,39 = 4.49; p < 0.02). Further analysis of the data showed that wild-type mice displayed CPP which was evidenced as a significant increase in the amount of time that the mice spent in the buprenorphine-paired chamber over the saline-paired chamber (p < 0.05) but the same conditioning paradigm failed to induce CPP in MOP knockout mice (p > 0.05). For simplicity, we then calculated the difference in the amount of time that the mice spent in the conditioning chambers (DPCh – VPCh) for both preconditioning and post-conditioning days (Fig. 3C). This panel shows that there was no significant preconditioning preference in wild-type or MOP knockout mice on day 1. However, on day 10, a significant preference was observed in wild-type but not MOP knockout mice.
Fig. 3.
Buprenorphine induced CPP in wild-type but not in MOP knockout mice. Mice lacking the MOP [MOP (−/−)] and their wild-type littermates [MOP (+/+)] were tested for baseline preference on day 1 (A). Mice then received alternate-day saline/buprenorphine conditioning sessions on days 2–9. Mice were then tested for post-conditioning preference on day 10 (B). (C) Illustrates the difference in the amount of time (seconds) that the mice spent in the conditioning chambers (DPCh – VPCh) on days 1 and 10. Broken horizontal line indicates no preference. Data are expressed as mean (±S.E.M.) of 5–10 mice per genotype. VPCh, NCh and DPCh stand for vehicle-paired, neutral and drug-paired chambers, respectively.
3.3. Amphetamine induced a comparable CPP in wild-type and MOP knockout mice
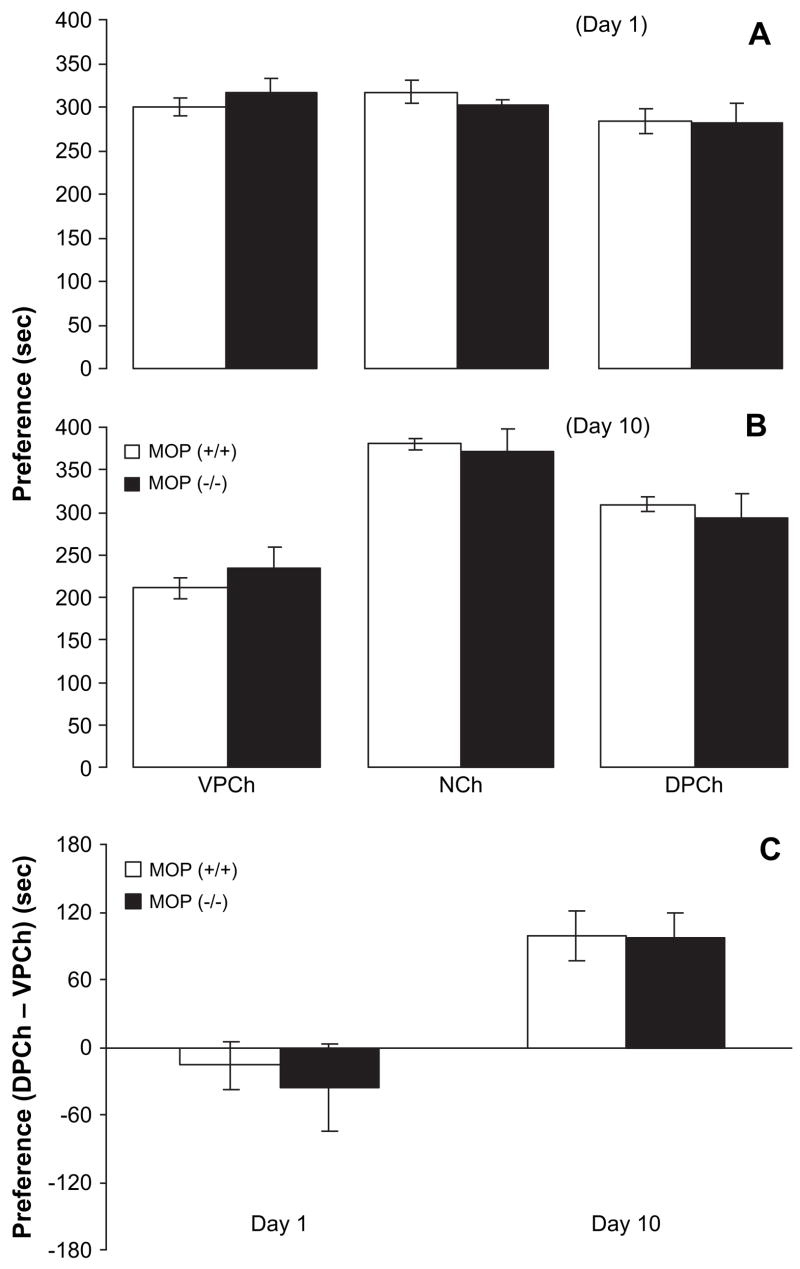
There was no significant difference between the wild-type and MOP knockout mice in the amount of time spent in the conditioning chambers on day 1 (Fig. 4A). A two-factor ANOVA revealed no significant effect of CPP chamber (F2,27 = 2.47; p > 0.05), or effect of genotype (F1,27 = 0.01; p > 0.05) or an interaction between CPP chamber and genotype (F2,27 = 0.68; p > 0.05). Further analysis of the data demonstrated no difference in basal preference toward the CPP chambers between MOP knockout mice and their wild-type littermates ( p > 0.05). However, four alternate-day saline/amphetamine injections paired with odor and visual cues shifted the preference toward the drug-paired chamber in both the wild-type and MOP knockout mice (Fig. 4B). A two-factor ANOVA revealed a significant effect of CPP chamber (F2,27 = 41.00; p < 0.0001), but no significant effect of genotype (F1,27 = 0.05; p > 0.05) or interaction between CPP chamber and genotype (F2,27 = 0.76; p > 0.05). Post-hoc analysis of the data showed that both wild-type and knockout mice displayed a comparable ( p > 0.05) amphetamine-induced CPP. Fig. 4C shows the difference in the amount of time that the mice spent in the two conditioning chambers (DPCh – VPCh) for days 1 and 10. This panel depicts that there was no preference in wild-type or knockout mice on day 1; however, a comparable preference was observed in both genotypes on day 10.
Fig. 4.
Amphetamine induced comparable CPP in wild-type and MOP knock-out mice. MOP knockouts [MOP (−/−)] and their wild-type littermates [MOP (+/+)] were tested for baseline preference on day 1 (A). Mice then received alternate-day saline/amphetamine (1 mg/kg, i.p.) conditioning sessions on days 2–9. Mice were then tested for post-conditioning preference on day 10 (B). (C) Illustrates the difference in the amount of time (seconds) that the mice spent in the conditioning chambers (DPCh – VPCh) on days 1 and 10. Broken horizontal line indicates no preference. Data are presented as mean (±S.E.M.) of 5–6 mice per genotype. VPCh, NCh and DPCh stand for vehicle-paired, neutral and drug-paired chambers, respectively.
4. Discussion
The main finding of the present study is that buprenorphine increased motor activity, and induced locomotor sensitization and CPP in wild-type but not in MOP knockout mice. The rewarding action of amphetamine, on the other hand, was not altered in mice lacking the MOP. Together, the present results suggest that buprenorphine produces its motor stimulatory and rewarding action primarily via the MOP.
Previous studies have shown that MOP agonists increase extracellular dopamine in the nucleus accumbens (Di Chiara and Imperato, 1988b), a response that is thought to mediate their motor stimulatory and rewarding actions. Along this line, morphine was found to induce no motor activation in mice lacking dopamine (Hnasko et al., 2005). Furthermore, the presence of the MOP was necessary for this response because morphine did not induce motor stimulation in mice lacking the MOP (Matthes et al., 1996). Likewise, the existence of the MOP was required for the antinociceptive and rewarding effects of morphine (Matthes et al., 1996). We have previously shown that the antinociceptive effect of buprenorphine was abolished in mice lacking the MOP (Lutfy et al., 2003), raising the possibility that the motor stimulatory and rewarding actions of buprenorphine could also be altered in these mice. Consistent with this notion, we found that buprenorphine stimulated motor activity in wild-type but not in MOP knockout mice, showing that the motor stimulatory action of buprenorphine is mediated via the MOP.
Repeated intermittent opioid administration leads to the development of locomotor sensitization, a response that is thought to play an important role in the development and maintenance of drug dependency (for reviews see Robinson and Berridge, 1993, 2000). Wild-type mice treated with buprenorphine once daily for 5 days and challenged with buprenorphine on day 11 exhibited locomotor sensitization. In contrast, this phenomenon did not develop in MOP knockout mice, suggesting that the MOP is also necessary for the development of locomotor sensitization to buprenorphine.
The conditioned place preference (CPP) paradigm is widely used as an animal model of drug reward (Bardo and Bevins, 2000). Thus, repeated administration and pairing of MOP agonists with a particular environment increases their incentive value, thereby leading to preference toward that environment. The observation that the motor stimulatory action of buprenorphine was abolished in mice lacking the MOP, prompted us to propose that the rewarding action of buprenorphine would also be abolished in these mice because motor activity is considered as an indirect measure of motivational behaviors (Wise, 1987). As expected, wild-type mice displayed a significant buprenorphine-induced CPP; however, MOP knockout mice failed to exhibit any CPP, suggesting that the rewarding action of buprenorphine is also mediated via the MOP. Surprisingly, a recent study showed that buprenorphine was able to induce CPP in mice lacking the MOP (Ide et al., 2004). Although the reason for this discrepant result is not known at the present time, we investigated the possibility that our MOP knockout mice might lack motivated behaviors and would not respond to other reinforcing agents as well. Thus, for comparison, we tested whether MOP knockout mice would show CPP following amphetamine. Interestingly, amphetamine induced a comparable CPP in MOP knockout and wild-type mice. Thus, these results clearly demonstrate that MOP knockout mice respond to another reinforcing agent that produces its rewarding action independently of the MOP activation, leading to the conclusion that the rewarding action of buprenorphine is selectively mediated via the MOP.
The ability of morphine and other drugs of abuse to induce psychomotor stimulation and reward could be indicative of their abuse potentials. Previous studies have shown that buprenorphine can induce psychomotor stimulation and reward (Rowlett et al., 1994; Smith et al., 2003; Sorge and Stewart, 2006; Sorge et al., 2005; Tzschentke, 2004). The results of the present study demonstrate that these actions of buprenorphine are mediated via the MOP. Interestingly, morphine also induces its motor stimulatory and rewarding actions via the same receptor since these actions of morphine were abolished in MOP knockout mice (Matthes et al., 1996). However, the central question remains to be asked is that what makes buprenorphine different from morphine then? One probable explanation is that buprenorphine acts as a high affinity partial agonist at the MOP (Cowan et al., 1977a,b; Lutfy et al., 2003; Martin et al., 1976; for review see Cowan, 2003; Lutfy and Cowan, 2004; Robinson, 2002; Tzschentke, 2002). The other possibility could be the slow dissociation rate of buprenorphine from the MOP (for reviews see Cowan, 2003; Lutfy and Cowan, 2004; Robinson, 2002; Tzschentke, 2002). A third explanation is that buprenorphine interacts with receptors other than the MOP (Bloms-Funke et al., 2000; Hawkinson et al., 2000; Ide et al., 2004; Kajiwara et al., 1986; Lutfy et al., 2003; Negus et al., 1989, 2002; Sadee et al., 1982; Wnendt et al., 1999; for review see Cowan, 2003; Lutfy and Cowan, 2004). The ability of buprenorphine to interact with the ORL-1 receptor (also known as NOP) is of a particular interest in this regard since orphanin FQ/nociceptin, the endogenous ligand of the ORL-1 receptor (Meunier et al., 1995; Reinscheid et al., 1995), have been shown to block the analgesic (for review see Mogil and Pasternak, 2001) and rewarding (Ciccocioppo et al., 2000; Murphy et al., 1999) action of morphine. Interestingly, we have previously shown that the antinociceptive effect of buprenorphine was enhanced in mice lacking the ORL-1 receptors (Lutfy et al., 2003). Thus, further studies are required to characterize the role of the ORL-1 receptor in buprenorphine-induced reward and in the ability of the drug to curb opiate dependency.
In summary, the present data demonstrate that buprenorphine induced motor stimulation and reward via the MOP because these actions of buprenorphine were abolished in mice lacking the MOP. In contrast, the rewarding effect of amphetamine was comparable in wild-type and MOP knockout mice. Together, the present results indicate that the motor stimulatory and rewarding actions of buprenorphine are mediated via the MOP.
Acknowledgments
The authors wish to thank Mr. Nagaraju Gajawada for technical support. The present study was supported in part by a NIDA Grant DA 086682 to K.L.
References
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bloms-Funke P, Gillen C, Schuettler AJ, Wnendt S. Agonistic effects of the opioid buprenorphine on the nociceptin/OFQ receptor. Peptides. 2000;21:1141–1146. doi: 10.1016/s0196-9781(00)00252-7. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Sanna PP, Weiss F, Massi M. Effect of nociceptin/orphanin FQ on the rewarding properties of morphine. Eur J Pharmacol. 2000;404:153–159. doi: 10.1016/s0014-2999(00)00590-2. [DOI] [PubMed] [Google Scholar]
- Cowan A. Buprenorphine: new pharmacological aspects. Int J Clin Pract. 2003;(Suppl):3–8. [PubMed] [Google Scholar]
- Cowan A, Doxey JC, Harry EJ. The animal pharmacology of buprenorphine, an oripavine analgesic agent. Br J Pharmacol. 1977a;60:547–554. doi: 10.1111/j.1476-5381.1977.tb07533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan A, Lewis JW, Macfarlane IR. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol. 1977b;60:537–545. doi: 10.1111/j.1476-5381.1977.tb07532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988a;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988b;244:1067–1080. [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Hawkinson JE, Costa-Burruel M, Espitia SA. Opioid activity profiles indicate similarities between the nociceptin/orphanin FQ and opioid receptors. Eur J Pharmacol. 2000;389:107–114. doi: 10.1016/s0014-2999(99)00904-8. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Sotak BN, Palmiter RD. Morphine reward in dopamine-deficient mice. Nature. 2005;438:854–857. doi: 10.1038/nature04172. [DOI] [PubMed] [Google Scholar]
- Huang P, Kehner GB, Cowan A, Liu-Chen LY. Comparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonist. J Pharmacol Exp Ther. 2001;297:688–695. [PubMed] [Google Scholar]
- Ide S, Minami M, Satoh M, Uhl GR, Sora I, Ikeda K. Buprenorphine antinociception is abolished, but naloxone-sensitive reward is retained, in muopioid receptor knockout mice. Neuropsychopharmacology. 2004;29:1656–1663. doi: 10.1038/sj.npp.1300463. [DOI] [PubMed] [Google Scholar]
- Kajiwara M, Aoki K, Ishii K, Numata H, Matsumiya T, Oka T. Agonist and antagonist actions of buprenorphine on three types of opioid receptor in isolated preparations. Jpn J Pharmacol. 1986;40:95–101. doi: 10.1254/jjp.40.95. [DOI] [PubMed] [Google Scholar]
- Kamei J, Saitoh A, Suzuki T, Misawa M, Nagase H, Kasuya Y. Buprenorphine exerts its antinociceptive activity via mu 1-opioid receptors. Life Sci. 1995;56:L285–L290. doi: 10.1016/0024-3205(95)00078-x. [DOI] [PubMed] [Google Scholar]
- Kamei J, Sodeyama M, Tsuda M, Suzuki T, Nagase H. Antinociceptive effect of buprenorphine in mu1-opioid receptor deficient CXBK mice. Life Sci. 1997;60:L-7. doi: 10.1016/s0024-3205(97)00170-7. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Nestler EJ. The neurobiology of drug addiction. J Neuropsychiatry Clin Neurosci. 1997;9:482–497. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- Lutfy K, Cowan A. Buprenorphine: a unique drug with complex pharmacology. Neuropsychopharmacology. 2004;2:395–402. doi: 10.2174/1570159043359477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Eitan S, Bryant CD, Yang YC, Saliminejad N, Walwyn W, Kieffer BL, Takeshima H, Carroll FI, Maidment NT, Evans CJ. Buprenorphine-induced antinociception is mediated by muopioid receptors and compromised by concomitant activation of opioid receptor-like receptors. J Neurosci. 2003;23:10331–10337. doi: 10.1523/JNEUROSCI.23-32-10331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez P, Baliram R, Gajawada N, Friedman TC, Lutfy K. Differential involvement of enkephalins in analgesic tolerance, locomotor sensitization, and conditioned place preference induced by morphine. Behav Neurosci. 2006;120:10–15. doi: 10.1037/0735-7044.120.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the muopioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Pasternak GW. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol Rev. 2001;53:381–415. [PubMed] [Google Scholar]
- Murphy NP, Lee Y, Maidment NT. Orphanin FQ/nociceptin blocks acquisition of morphine place preference. Brain Res. 1999;832:168–170. doi: 10.1016/s0006-8993(99)01425-0. [DOI] [PubMed] [Google Scholar]
- Negus SS, Picker MJ, Dykstra LA. Kappa antagonist effects of buprenorphine in the rat drug-discrimination procedure. NIDA Res Monogr. 1989;95:518–519. [PubMed] [Google Scholar]
- Negus SS, Bidlack JM, Mello NK, Furness MS, Rice KC, Brandt MR. Delta opioid antagonist effects of buprenorphine in rhesus monkeys. Behav Pharmacol. 2002;13:557–570. doi: 10.1097/00008877-200211000-00005. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Malenka RC. The addicted brain. Sci Am. 2004;290:78–85. doi: 10.1038/scientificamerican0304-78. [DOI] [PubMed] [Google Scholar]
- Ohlsen RI, Pilowsky LS. The place of partial agonism in psychiatry: recent developments. J Psychopharmacol. 2005;19:408–413. doi: 10.1177/0269881105053308. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr, Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Robinson SE. Buprenorphine: an analgesic with an expanding role in the treatment of opioid addiction. CNS Drug Rev. 2002;8:377–390. doi: 10.1111/j.1527-3458.2002.tb00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Gibson TR, Bardo MT. Dissociation of buprenorphine-induced locomotor sensitization and conditioned place preference in rats. Pharmacol Biochem Behav. 1994;49:241–245. doi: 10.1016/0091-3057(94)90484-7. [DOI] [PubMed] [Google Scholar]
- Sadee W, Rosenbaum JS, Herz A. Buprenorphine: differential interaction with opiate receptor subtypes in vivo. J Pharmacol Exp Ther. 1982;223:157–162. [PubMed] [Google Scholar]
- Smith MA, Gordon KA, Craig CK, Bryant PA, Ferguson ME, French AM, Gray JD, McClean JM, Tetirick JC. Interactions between opioids and cocaine on locomotor activity in rats: influence of an opioid’s relative efficacy at the mu receptor. Psychopharmacology (Berl) 2003;167:265–273. doi: 10.1007/s00213-003-1388-z. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Stewart J. The effects of long-term chronic buprenorphine treatment on the locomotor and nucleus accumbens dopamine response to acute heroin and cocaine in rats. Pharmacol Biochem Behav. 2006;84:300–305. doi: 10.1016/j.pbb.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Rajabi H, Stewart J. Rats maintained chronically on buprenorphine show reduced heroin and cocaine seeking in tests of extinction and drug-induced reinstatement. Neuropsychopharmacology. 2005;30:1681–1692. doi: 10.1038/sj.npp.1300712. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Behavioral pharmacology of buprenorphine, with a focus on preclinical models of reward and addiction. Psychopharmacology (Berl) 2002;161:1–16. doi: 10.1007/s00213-002-1003-8. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Reassessment of buprenorphine in conditioned place preference: temporal and pharmacological considerations. Psychopharmacology (Berl) 2004;172:58–67. doi: 10.1007/s00213-003-1626-4. [DOI] [PubMed] [Google Scholar]
- Wise RA. The role of reward pathways in the development of drug dependence. Pharmacol Ther. 1987;35:227–263. doi: 10.1016/0163-7258(87)90108-2. [DOI] [PubMed] [Google Scholar]
- Wnendt S, Kruger T, Janocha E, Hildebrandt D, Englberger W. Agonistic effect of buprenorphine in a nociceptin/OFQ receptor-triggered reporter gene assay. Mol Pharmacol. 1999;56:334–338. doi: 10.1124/mol.56.2.334. [DOI] [PubMed] [Google Scholar]