Abstract
Although intranasal methamphetamine abuse has increased, there are no published data investigating the residual effects of the drug under controlled conditions. Thus, the current study examined the residual effects of single-dose intranasal methamphetamine administration on a broad range of behavioral and physiological measures. Non-treatment seeking methamphetamine abusers (n = 11) completed this two-week, in-patient, within-participant, double-blind study. The study consisted of 4 two-day blocks of sessions; each block was separated by at least 24 hrs. At approximately 1000 hrs, on the first day of each block, participants received one of four intranasal methamphetamine doses (0, 12, 25, 50 mg/70 kg). Lights were turned out at 2300 hrs that evening and sleep measures were assessed. On the morning of the second day of each block, methamphetamine plasma levels, cardiovascular measures, mood, subjective reports of the previous evening's sleep, and psychomotor performance were assessed to determine residual drug effects. The larger methamphetamine doses (25 and 50 mg) markedly disrupted subjective measures of that night's sleep and some indices of next-day mood, but only the largest dose (50 mg) dose decreased objective measures of that night's sleep and increased next-day physiological measures. Methamphetamine did not produce any negative residual effects on early next-day performance. Future studies should assess methamphetamine-related residual effects following repeated doses administered over consecutive days.
Keywords: methamphetamine abuse, sleep, cognitive performance, mood, humans, hangover
1. Introduction
Following several decades of dormancy, the abuse of amphetamines has again become an important public health problem in several countries, including Australia, Thailand, and the United States. In the U.S., for example, treatment admissions for methamphetamine use disorders have steadily increased since 2000. Although acute administration of relatively low oral methamphetamine doses reportedly improves mood and cognitive performance (e.g., Johnson et al. 1999, 2000; Hart et al. 2002), long-term abuse of larger doses, administered via routes other than oral, is associated with mood disturbances (London et al. 2004) and cognitive impairments (London et al. 2005). These deleterious effects appear to be exacerbated following abrupt discontinuation of methamphetamine use (Peck et al. 2005). In addition, hypersomnia, increased depression-related symptoms, anxiety and methamphetamine craving are reported after cessation of methamphetamine use (McGregor et al. 2005).
It should be noted, however, that there were a few methodological concerns that constrained the conclusions from studies indicating methamphetamine-related disruptive effects after discontinuation of drug use. For example, sleep behavior based on subjective reports may not correspond with objective sleep measures (e.g., Baker et al. 1999; Tworoger et al. 2005). Furthermore, previous studies relied on retrospective self-reported information regarding recency and amounts of methamphetamine use, making it difficult to precisely quantify patterns of methamphetamine use that are most likely to precipitate disruptive effects. Given these considerations, a systematic laboratory investigation, during which carefully controlled methamphetamine doses are administered and objective sleep measures are assessed, is needed. A better understanding of methamphetamine-related residual effects is an important initial step in developing effective methamphetamine abuse treatments because interventions can target specific symptoms. Therefore, we undertook a double-blind, in-patient, within-participant study to evaluate the residual effects of intranasal methamphetamine administration (0, 12, 25, and 50 mg/70 kg) on several dependent variables, including mood, cognitive/psychomotor performance, objective and subjective sleep behaviors. Residual effects were operationally defined as those occurring at least 12 hours after drug administration, at a time when plasma levels of methamphetamine are declining and acute subjective effects are negligible. These participants were previously described in an investigation of the acute effects of intranasal methamphetamine on the behavioral and physiological measures in abusers (Hart et al. 2007).
2. Methods
2.1. Participants
Eleven research participants (2F, 9M) completed this two-week inpatient study: their age ranged from 22 − 45 years (mean age = 30.7). Prior to study enrollment, participants signed a consent form that was approved by the Institutional Review Board of The New York State Psychiatric Institute (NYSPI); each passed comprehensive medical and psychiatric evaluations and were within normal weight ranges according to the 1983 Metropolitan Life Insurance Company height/weight table [body mass index: 24.1 ± 4.4 (mean ± SD)]. All participants met DSM-IV criteria for current methamphetamine abuse or dependence and stated they were not seeking treatment at the time of study participation. No participant met criteria for any other axis I disorder. All reported abusing methamphetamine primarily via the intranasal route, although nine reported having used via the smoked route and one via the intravenous route. Participants reported using methamphetamine 3.6 ± 1.7 (mean ± SD) days per week. Six participants reported current cocaine use (1−4 times/week), seven reported current alcohol use (1.5−15 drinks/week), seven reported current marijuana use (1−6 times/week), and eight smoked 2−20 tobacco cigarettes per day.
2.2. Design and Procedures
This two-week, double-blind, within-participant study consisted of 4 two-day blocks of sessions with each block of sessions separated by at least 48 hrs. On the first day of each block, at approximately 1000 hrs, participants received one of four intranasal methamphetamine doses (0, 12, 25, 50 mg/70 kg). Methamphetamine dosing was counterbalanced across participants. Subsequently that evening, lights were turned off at 2300 hrs for an 8-hr sleep period and objective sleep was assessed. During this period, participants were instructed to remain in bed. On the second day of each block, at approximately 1000 hrs, heart rate (HR), systolic (SP) diastolic pressure (DP) and methamphetamine plasma levels were assessed. Then, a sleep questionnaire, subjective-effect ratings, and a cognitive/psychomotor battery were completed to determine residual drug effects.
At least two days before beginning experimental sessions, participants were admitted onto the General Clinical Research Service (GCRS) at the NYSPI, where they resided until study completion. This arrangement ensured a sufficient drug washout period before study commencement and decreased the likelihood that non-study drugs would be consumed during the study.
2.3. Sleep Monitoring
Objective measures of sleep were obtained by tracking gross motor activity using Actiwatch® Activity Monitoring System (Actiwatch: Respironics Company, Bend, OR), worn throughout the study by six of the 11 participants (Kushida et al. 2001). Actiwatch® data from the first five participants were not available due to equipment malfunctions with another portable sleep system. Each morning, all participants were asked to estimate the number of hours they slept the previous night and to complete a visual analog sleep questionnaire consisting of a 100 mm lines labeled “not at all” at one end and “extremely” at the other end. The six items were: “I slept well last night,” “I woke up early this morning,” “I fell asleep easily last night,” “I feel clear-headed this morning,” “I woke up often last night,” “I am satisfied with my sleep last night.”
2.4. Subjective-effects and Cognitive/Psychomotor Battery
The computerized visual analog questionnaire consisted of a series of 100 mm lines labeled “not at all” at one end and “extremely” at the other end (described in Hart et al. 2007). The lines were labeled with adjectives describing a mood (e.g., “Anxious,” “Depressed,” “Frustrated”), a drug effect (e.g., “Bad Drug Effect,” “Good Drug Effect,” “High”), or a physical symptom (e.g., “Headache”, “Muscle Pain,” “Stomach Upset”). Three items were also used to operationalize drug craving and were labeled “I want meth,” “I want alcohol,” and “I want a cigarette.”
Computerized psychomotor tasks (Haney et al. 1999) consisted of a 3-min Digit-Symbol Substitution Task (DSST; McLeod et al. 1982), a 3-min repeated-acquisition task (RA; Kelly et al. 1993), a 10-min divided attention task (DAT; Miller et al. 1988), a 10-min rapid information task (RIT; Wesnes and Warburton 1983), and a 3-min immediate and delayed digit-recall task (Hart et al. 2001).
2.5. Drugs
Methamphetamine HCl, was provided by the National Institute on Drug Abuse (NIDA). Lactose (60 mg/70kg) was used as a placebo and lactose was also added to each methamphetamine dose (12, 25, and 50 mg/70 kg) to achieve a final weight of 60 mg/70 kg. As a safety precaution, the maximum single methamphetamine dose administered did not exceed 60 mg. Three participants' weight exceeded 84 kg, and as a result, they were administered 41−48 mg/70 kg. Each dose was provided in a small medicine cup, along with a plastic straw (∼ 7 cm) and participants were instructed to insufflate the entire dose within a 30-sec period. All drug administrations occurred in a double-blind manner.
2.6. Data Analysis
Repeated-measures analyses of variance (ANOVA) with planned comparisons were conducted to determine the residual effects of intranasal methamphetamine (0, 12, 25, 50 mg/70 kg) on sleep measures, subjective-effect ratings, cognitive/psychomotor performance, and physiological measures. For all analyses, ANOVAs provided the error terms needed to calculate planned comparisons that were designed to determine the effects of methamphetamine dose. Data were considered statistically significant at p < .05, using Huynh-Feldt corrections.
3. Results
Data collected from the three participants who weighed greater than 84 kg were consistent with the results of the other participants.
3.1. Sleep
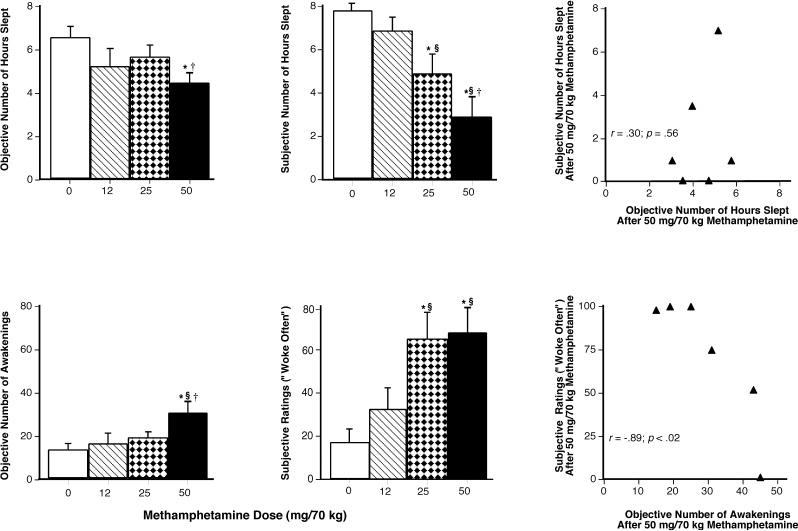
Figure 1 (upper left panel) shows that, relative to placebo, objective total sleep duration was significantly decreased by the 50-mg dose (p < .01); the average reduction was 2 hrs (50 mg: 4.4 ± 1.03 vs placebo: 6.4 ± 1.16 hrs). Similarly, the number of awakenings was increased by the largest methamphetamine dose compared with all other doses (p < .01: Figure 1, lower left panel).
Figure 1.
Left Panels: Mean values for total number of hours slept and number of awakenings as measured by the Actiwatch® Activity Monitoring System as a function of methamphetamine dose (n = 6). Middle Panels: Mean values for sleep questionnaire estimates of hour slept and ratings of “woke often” as a function of methamphetamine dose (n = 11). Right Panels: Values for subjective and objective number of hours slept for each participant and values for subjective and objective number of awakenings following 50 mg/70 kg methamphetamine (n = 6). Error bars represent one SEM. An * indicates significantly different from placebo (p < .05). An § indicates significantly different from 12 mg/70 kg (p < .05). An † indicates significantly different from 25 mg/70 kg (p < .05).
Figure 1 (upper middle panel) shows that participants estimated that they had slept approximately 2.9 and 4.9 fewer hours the previous night when they received 25 and 50 mg, respectively, compared to when they had received placebo (p < .001). Additionally, when participants received the larger methamphetamine doses (25 and 50 mg), they reported waking more frequently during the sleep period (p < .05: Figure 1, lower middle panel). Table 1 shows additional significant effects produced by methamphetamine on subjective sleep measures.
Table 1.
Residual effects of methamphetamine on the sleep questionnaire, subjective-effect ratings, and physiological measures
|
Methamphetamine Conditions | |||||||
|---|---|---|---|---|---|---|---|
| |
Placebo |
12 mg |
|
25 mg |
|
50 mg |
|
|
Measure |
Mean (SEM) |
Mean (SEM) |
F Value |
Mean (SEM) |
F Value |
Mean (SEM) |
F Value |
| Sleep questionnaire | |||||||
| Clear-headed | 78.20 (7.38) | 71.17 (8.98) | 0.39 | 63.80 (8.08) | 1.64 | 49.44 (9.10) | 6.55* |
| Estimated # of hrs Slept | 7.70 (0.31) | 6.77 (0.56) | 1.42 | 4.83 (0.85) | 13.78*§ | 2.84 (0.89) | 39.41*§† |
| Fell Asleep Easily | 57.50 (9.74) | 56.06 (12.51) | 0.01 | 27.40 (11.34) | 4.38* | 10.22 (4.56) | 10.80*§ |
| Satisfied with Sleep | 80.30 (7.75) | 64.00 (10.77) | 1.37 | 42.40 (12.43) | 7.38* | 25.33 (6.46) | 15.53*§ |
| Slept Well | 85.20 (3.82) | 75.72 (8.57) | 0.72 | 49.60 (11.68) | 10.08*§ | 21.44 (7.41) | 32.34*§† |
| Woke Often | 16.50 (6.66) | 32.28 (10.04) | 1.15 | 64.80 (12.48) | 10.78*§ | 67.56 (11.52) | 12.04*§ |
| Subjective-effect ratings | |||||||
| Alert | 55.55 (9.76) | 51.09 (9.90) | 0.32 | 53.36 (8.85) | 0.08 | 38.09 (9.15) | 4.40* |
| Content | 53.91 (6.46) | 46.09 (6.63) | 1.56 | 42.09 (4.63) | 3.56 | 37.36 (5.00) | 6.98* |
| Energetic | 35.20 (9.71) | 45.00 (10.48) | 1.04 | 29.20 (4.91) | 0.39 | 22.80 (5.54) | 5.33§ |
| Friendly | 65.00 (7.79) | 60.36 (6.41) | 0.48 | 59.09 (7.43) | 0.77 | 43.00 (9.77) | 10.69*§† |
| Social | 67.09 (7.59) | 50.36 (6.17) | 4.46* | 55.82 (6.49) | 2.02 | 40.82 (6.60) | 10.99* |
| Talkative | 58.46 (4.63) | 47.00 (8.62) | 2.11 | 55.64 (6.10) | 0.12 | 37.36 (6.43) | 7.16*† |
| Physiological measures | |||||||
| MA Plasma Levels (ng/ml) | 0.00 (0.00) | 6.72 (0.42) | 4.33 | 13.67 (2.90) | 17.91* | 38.08 (4.11) | 139.00*§† |
| Heart Rate (bpm) | 78.36 (2.28) | 84.18 (2.90) | 3.07 | 85.27 (3.09) | 4.33 | 90.46 (4.44) | 13.26* |
| Systolic Pressure (mmHg) | 118.00 (2.58) | 119.64 (2.15) | 0.42 | 123.00 (3.04) | 3.96 | 124.00 (2.65) | 5.70* |
| Diastolic Pressure (mmHg) | 69.00 (2.69) | 71.36 (1.82) | 0.84 | 73.82 (2.83) | 3.51 | 78.55 (2.85) | 13.76*§ |
MA = Methamphetamine
df= 1, 30
P < .05, significantly different from placebo
P < .05, significantly different from 12 mg
P < .05, significantly different from 25 mg
When the relationship between objective and subjective sleep measures was examined for the 25-and 50-mg dose, only the number of awakenings as measured by the Actiwatch° and subjective ratings of “Woke Often” following the 50-mg dose was significantly correlated (r = −.89 and p < .02: Figure 1; 25 mg data not shown). There were no significant correlations following the 25-mg dose.
3.2. Subjective effects
Table 1 displays the residual effects of methamphetamine on subjective-effect ratings. The 50-mg dose administered 24 hrs earlier, significantly decreased rating of “Alert,” “Content,” “Energetic,” “Friendly,” “Social,” and “Talkative” (p < .05).
3.3. Cognitive/Psychomotor effects
Methamphetamine did not produce any significant residual effects on cognitive/psychomotor performance.
3.4. Physiological measures
Table 1 summarizes next-day physiological effects of methamphetamine. Notably, all physiological measures were significantly increased by the 50-mg dose compared with placebo (p < .05).
4. Discussion
The present data show that objective and subjective sleep behaviors were disrupted by a single intranasal methamphetamine dose administered to experienced methamphetamine users 12 − 14 hours before bedtime. Objective measures of sleep (i.e., total number of hours slept and number of awakenings) reached statistical significance only following the largest methamphetamine dose (50 mg), whereas subjective measures (e.g., ratings of “Slept Well”) were markedly decreased by both the 25- and 50-mg doses. Consistent with objective sleep results, some measures of next-day mood (e.g., “Content” and “Friendly”) were decreased and next-day HR, SP, DP, and methamphetamine plasma levels were increased by the largest methamphetamine dose. Despite sleep and limited mood alterations caused by methamphetamine, next-day cognitive performance was not negatively impacted.
The finding that methamphetamine-related effects on objective and subjective sleep measures were not entirely consistent is interesting and underscores an important limitation of past studies. Previous research investigating the residual effects of methamphetamine have relied exclusively on self-reports of sleep behavior (McGregor et al. 2005; Peck et al. 2005), despite the fact that a large database comparing subjective estimates of sleep with objective sleep measures suggests that the two modalities may tap different aspects of the sleep experience (e.g., Coates et al. 1982; Vitiello et al. 2004). Indeed, the current data demonstrate that self-reports may dramatically overestimate the extent of sleep disruptions produced by methamphetamine. For example, participants reported they had slept nearly 5 fewer hours the morning after they had received the 50-mg dose. Data from the Actiwatch, however, showed that they had slept only 2 fewer hours. Similarly, participants reported a general decrease in sleep quality after the two larger methamphetamine doses, but objective sleep measures were significantly altered only following the largest dose. A caveat related to these observations is that objective sleep data was available for only six of the 11 participants, and this might limit generality of the current results. Despite this, the current results suggest that there may be a dissociation between objective and subjective measures of sleep disruption, a finding that should be assessed with a larger cohort of participants.
Although some next-day subjective ratings were decreased by the 50-mg methamphetamine dose, the overall pattern of effects produced by methamphetamine were limited and do not suggest that next-day mood disturbances are a major feature associated with a single intranasal dose administration. Importantly, ratings of “depressed,” “anxious,” and “I want meth” were not altered by the methamphetamine dose administered the previous day. Because no earlier study has assessed the residual effects of intranasal methamphetamine under laboratory conditions, it is difficult to relate the current findings to previous data. Nevertheless, the next-day performance data are congruent with the majority of next-day subjective ratings. That is, methamphetamine, administered 24 hours earlier, did not produce disruptions in any of the cognitive domains assessed, including memory, reaction time, and sustained attention.
Anecdotally, illicit methamphetamine is used in multiple dose cycles, which may continue over the course of several consecutive days (Angrist 1994; Cho et al. 2001). In the present investigation, the residual effects of methamphetamine were evaluated following only a single dose administration, possibly decreasing the likelihood of observing methamphetamine-associated disruptions. A related point is that 24 hours following methamphetamine administration (primarily the 50-mg dose), physiological measures remained significantly elevated and this might have potential implications for toxicity. For example, de Wit and colleagues showed that tolerance develops more rapidly to oral amphetamine-related subjective effects relative to physiological effects (Brauer et al. 1996). Thus, in the natural environment, some users may take repeated methamphetamine doses in order to achieve a certain level of intoxication, which might increase the likelihood of physiological harm (e.g., cardiotoxicity). Future studies should determine the impact of the residual effects of methamphetamine following repeated dosing over consecutive days. Another caveat of this study was that next-day mood and performance were assessed at only one time point in the morning. It is possible that performance disruptions, for example, are subtle and require multiple assessments over the course of an entire day in order to be detected.
In conclusion, the present data show that a single intranasal methamphetamine dose produced marked reductions on measures of subjective sleep quality and, to a lesser extent, objective sleep. These findings highlight the importance of assessing both subjective and objective measures of sleep when determining the impact of methamphetamine-associated effects. Despite methamphetamine-related alterations of sleep and physiological measures, the drug produced few residual effects on mood and cognitive performance. Further study of the residual effects of methamphetamine following repeated dosing is needed to better understand the purported abstinence syndrome associated with methamphetamine abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- Angrist B. Amphetamine psychosis: clinical variations of the syndrome. In: Amphetamine and its analogues. In: Cho AK, Segal DS, editors. Academic Press.; San Diego: 1994. pp. 387–414. [Google Scholar]
- Baker FC, Maloney S, Driver HS. A comparison of subjective estimates of sleep with objective polysomnographic data in healthy men and women. J Psychosom Res. 1999;47:335–341. doi: 10.1016/s0022-3999(99)00017-3. [DOI] [PubMed] [Google Scholar]
- Brauer HL, Ambre J, deWitt H. Acute tolerance to subjective but not cardiovascular effects of d-amphetamine in normal, healthy man. Journal of Clinical Psychopharmacology. 1996;16:72–76. doi: 10.1097/00004714-199602000-00012. [DOI] [PubMed] [Google Scholar]
- Cho AK, Melega WP, Kuczenski R, Segal DS. Relevance of pharmacokinetic parameters in animal models of methamphetamine abuse. Synapse. 2001;39:161–166. doi: 10.1002/1098-2396(200102)39:2<161::AID-SYN7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Coates TJ, Killen JD, George J, Marchini E, Silverman S, Thoresen C. Estimating sleep parameters: a multitrait--multimethod analysis. J Consult Clin Psychol. 1982;50:345–52. [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology. 1999;22:504–512. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- Hart CL, Gunderson EW, Perez A, Kirkpatrick MG, Thurmond A, Comer SD, Foltin RW. Acute physiological and behavioral effects of intranasal methamphetamine in humans. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301578. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Haney M, Foltin RW, Fischman MW. Effects of the NMDA antagonist memantine on human methamphetamine discrimination. Psychopharmacology. 2002;164:376–384. doi: 10.1007/s00213-002-1225-9. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Comer SD, Gunderson E, Foltin RW. Modafinil attenuates disruptions in cognitive performance during simulated night-shift work. Neuropsychopharmacology. 2006;31:1526–1536. doi: 10.1038/sj.npp.1300991. [DOI] [PubMed] [Google Scholar]
- Hart CL, van Gorp W, Haney M, Foltin FW, Fischman MW. Effects of acute smoked marijuana on complex cognitive performance. Neuropsychopharmacology. 2001;25:757–765. doi: 10.1016/S0893-133X(01)00273-1. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Foltin RW, Emurian CS, Fischman MW. Performance-based testing for drugs of abuse: dose and time profiles of marijuana, amphetamine, alcohol, and diazepam. Journal of Analytical Toxicology. 1993;17:264–272. doi: 10.1093/jat/17.5.264. [DOI] [PubMed] [Google Scholar]
- Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Medicine. 2001;2:389–396. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- London ED, Berman SM, Voytek B, Simon SL, Mandelkern MA, Monterosso J, Thompson PM, Brody AL, Geaga JA, Hong MS, Hayashi KM, Rawson RA, Ling W. Celebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biological Psychiatry. 2005;58:770–778. doi: 10.1016/j.biopsych.2005.04.039. [DOI] [PubMed] [Google Scholar]
- London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, Shinn A,K, Miotto K, Learn J, Dong Y, Matochik JA, Kurian V, Newton T, Woods R, Rawson R, Walter L. Mood disturbances and regional celebral metabolic abnormalities in recently abstinent methamphetamine abusers. Archives of General Psychiatry. 2004;61:73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Wells LT. Effects of isradipine, a dihydropyridine-class calcium channel antagonist, on d-methamphetamine-induced cognitive and physiological changes in humans. Neuropsychopharmacology. 2000;22:504–512. doi: 10.1016/S0893-133X(99)00116-5. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Roache JD, Bordnick PS, Ait-Daoud N. Isradipine, a dihydropyridine-class calcium channel antagonist, attenuates some of d-methamphetamine's positive subjective effects: a preliminary study. Psychopharmacology. 1999;144:295–300. doi: 10.1007/s002130051007. [DOI] [PubMed] [Google Scholar]
- McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM. The nature, time course and severity of methamphetamine withdrawal. Addiction. 2005;100:1320–1329. doi: 10.1111/j.1360-0443.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST). Behavioral Research Methods Instrument. 1982;14:463–466. [Google Scholar]
- Miller TP, Taylor JL, Tinklenberg JR. A comparison of assessment techniques measuring the effects of methylphenidate, secobarbital, diazepam and diphenhydramine in abstinent alcoholics. Neuropsychobiology. 1988;19:90–96. doi: 10.1159/000118441. [DOI] [PubMed] [Google Scholar]
- Peck JA, Shoptaw S, Rotheram-Fuller E, Reback CJ, Bierman B. HIV-associated medical, behavioral, and psychiatric characteristics of treatment-seeking, methamphetamine-dependent men who have sex with men. 24. J Addict Dis. 2005:115–132. doi: 10.1300/J069v24n03_10. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Office of Applied Studies. Treatment Episode Data Set (TEDS): 1995−2005. National Admissions to Substance Abuse Treatment Services, DASIS Series: S-37, DHHS Publication No. (SMA) 07−4234. Rockville, MD: 2007. [Google Scholar]
- Tworoger SS, Davis S, Vitiello MV, Lentz MJ, McTiernan A. Factors associated with objective (actigraphic) and subjective sleep quality in young adult women. J Psychosom Res. 2005;59:11–19. doi: 10.1016/j.jpsychores.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Vitiello MV, Larsen LH, Moe KE. Age-related sleep change: Gender and estrogen effects on the subjective-objective sleep quality relationships of healthy, noncomplaining older men and women. J Psychosom Res. 2004;56:503–510. doi: 10.1016/S0022-3999(04)00023-6. [DOI] [PubMed] [Google Scholar]
- Wesnes K, Warburton DM. Effects of smoking on rapid information processing performance. Neuropsychologybiology. 1983;9:223–229. doi: 10.1159/000117969. [DOI] [PubMed] [Google Scholar]