Abstract
Background
The incidence of ventricular fibrillation (VF) as the presenting rhythm in out-of-hospital cardiac arrest (OHCA) is declining, whereas pulseless electrical activity (PEA) is increasing. This changing epidemiology has occurred concomitant with an increase in beta blocker use.
Aims
The aim of this study was to measure the association of beta blocker use among prehospital cardiac arrest patients with PEA versus VF as presenting rhythm.
Materials and Methods
In this retrospective cohort study, records of all OHCA patients presenting to a single municipal hospital between January 1, 2001 through December 31, 2006 were reviewed. Age, sex, race, first documented rhythm, estimated down time, presence of bystander CPR, return of spontaneous circulation, beta blocker use, and comorbid illnesses were noted. A Mantel-Haenzel chi-square was computed to describe the association between beta blocker use and PEA, compared to beta blocker use and VF. A sensitivity analysis was also performed to account for missing data, misclassification of beta blocker use, misclassification of initial rhythm, confounding by unknown factors, and random error.
Results
After exclusion of patients with asystole and patients in whom beta-blocker use was unclear/unknown, a cohort of 179 arrests was evaluated. The odds ratio for beta-blocker use among PEA vs. VF patients was 3.7 (95%CI 1.9 -7.2), and probabilistic adjustment for exposure and outcome misclassification, confounding, and random error increased the odds ratio to 5.0 (95%CI 1.1 -31.0).
Conclusions
There appears to be an association between beta blockers and the changing epidemiology of arrest rhythms, which may account for the increasing incidence of PEA and concomitant decrease in VF.
Keywords: cardiac arrest, beta-blockers, ventricular fibrillation, pulseless electrical activity
INTRODUCTION
The proportion of ventricular fibrillation (VF) and pulseless ventricular tachycardia (VT) as the first documented rhythm in out-of-hospital cardiac arrests (OHCA) has been declining, while the proportion of pulseless electrical activity (PEA) has been increasing. (1–4) VF accounted for approximately 40–60% of out-of-hospital cardiac arrests in the United States in the 1980’s and early 1990’s. At present, VF is estimated to account for approximately 25% of all out-of-hospital cardiac arrests.(1, 2) It is thought that the decreasing number of VF arrests may be a contributing factor to the increasing proportion of out-of-hospital cardiac arrests due to asystole and PEA.(3) The reason for the declining proportion of VF as the first documented cardiac arrest rhythm is unclear.
Chronic beta-blockers usage has expanded over the last 1–2 decades and indications now include hypertension, cardio-protection after myocardial infarction, angina, congestive heart failure, and rate control for arrhythmias. Beta-blockers are the fourth most commonly prescribed medication for hypertension.(5) The prescription rate for beta-blockers after acute myocardial infarction is viewed as a hospital quality indicator, and an average of approximately 60% of post-MI patients at all hospitals are discharged on beta-blockers.(6) Beta-blockade is also independently associated with improved survival in patients with VF or symptomatic VT (7), and its use also appears to prevent shocks in patients with implanted defibrillators.(8)
Due to the apparent temporal relationship between the increasing use of chronic beta-blocker therapy in the population most at risk for sudden out-of-hospital cardiac arrest and the rising proportion of PEA as the initial rhythm among out-of-hospital arrests, we hypothesized an association between the two. If there is such an association, a measurable difference in beta blocker use between patients presenting with PEA vs. VF should exist.
MATERIALS AND METHODS
Study design
This is an observational retrospective cohort study of all consecutive adult patients (>18 years of age) with out-of-hospital cardiac arrests who presented to a single public hospital in Los Angeles County between January 1, 2001 through December 31, 2006. The institutional review board (IRB) at the participating hospital provided ethics approval and a waiver of consent, since all data were de-identified and the results would be reported in aggregate.
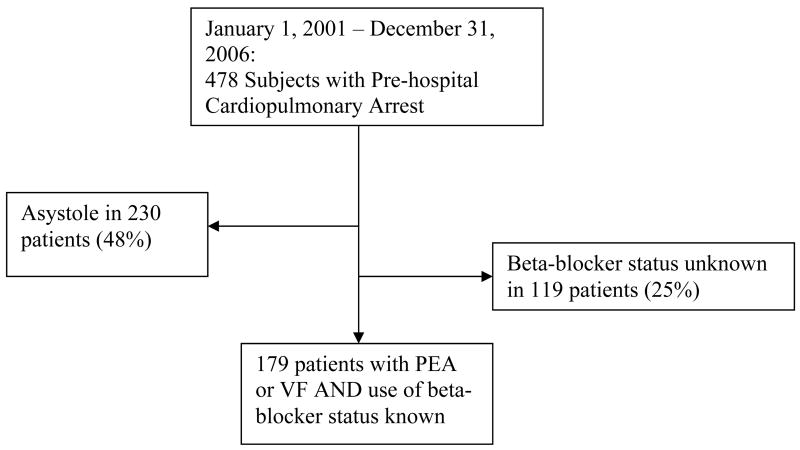
Two abstractors (SY and AHK) were trained to review and cull data from paramedic run sheets, nursing charts, and physician medical records. Neither abstractor was blinded to the hypothesis of the study. Exclusion criteria included patients with traumatic arrest, and those with an arrest related to a drug overdose (See Figure 1). The abstractors used a standardized form to record data, which included: beta-blocker use, age, sex, race, rhythm upon paramedic arrival, down time, whether there was bystander CPR or ROSC, and whether there was a history of chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), stroke or cerebrovascular accident (CVA), myocardial infarction (MI), chronic renal failure (CRF), cancer (CA), diabetes mellitus (DM), or hypertension (HTN). To assess the inter-rater reliability of the abstractors, a random sample of 20 charts were selected and a kappa coefficient was calculated.
Figure 1.
Enrollment Flow Chart
Statistical Analysis
Data were entered into a Microsoft Excel (Seattle, WA) spreadsheet, and DBMS Copy was used to convert the file into a SAS v.9.2 (Cary, NC) database. Descriptive statistics and odds ratios were calculated to evaluate associations. A Mantel-Haenzel chi-square was computed to describe the association between beta blocker use and PEA, compared to beta blocker use and VF. Additionally, a secondary exploratory analysis, using multivariable logistic regression modeling, adjusting for age, race, gender, rhythm upon paramedic arrival, down time, whether there was bystander CPR or ROSC, and presence of COPD, CHF, CVA, MI, CRF, cancer, DM, and HTN, was performed. An automated, probabilistic sensitivity analysis using Monte Carlo techniques was utilized to adjust for non-differential misclassification of both beta blocker use and initial rhythm and to account for random error, using the Excel macro SensTool.xls. (9–10) Trapezoidal probability distributions for sensitivity and specificity of beta blocker use (exposure) and initial rhythm (outcome) misclassification were defined as shown in tables 1 and 2. Once these distributions have been defined, the algorithm randomly samples from the distributions, combining the results to produce an estimate of the odds ratio in the cohort had it not been misclassified. Ten thousand iterations were performed to produce a distribution of adjusted odds ratios from which median and 95% confidence limits were calculated. In addition to simple misclassification analysis, we further included simultaneous adjustments for a hypothetical, unmeasured confounder with differential prevalence in the exposed of 30–50% and prevalence in the unexposed of 10–30%. We allowed the odds ratio for the confounder to range from 5–20, a rather strong confounding association. Uniform probability distributions were used for these parameters.
Table 1.
Sensitivity Analysis Accounting for Exposure Misclassification
| Exposure Misclassification | ||||
|---|---|---|---|---|
| Beta blocker misclassification among PEA group | ||||
| Minimum | Mode 1 | Mode 2 | Maximum | |
| Sensitivity | 0.75 | 0.80 | 0.85 | 0.90 |
| Specificity | 0.90 | 0.94 | 0.97 | 1.00 |
| Beta blocker misclassification among VF group | ||||
| Minimum | Mode 1 | Mode 2 | Maximum | |
| Sensitivity | 0.75 | 0.80 | 0.85 | 0.90 |
| Specificity | 0.90 | 0.94 | 0.97 | 1.00 |
Table 2.
Sensitivity Analysis Accounting for Outcome Misclassification
| Outcome Misclassification | ||||
|---|---|---|---|---|
| PEA misclassification among the beta blocker group | ||||
| Minimum | Mode 1 | Mode 2 | Maximum | |
| Sensitivity | 0.80 | 0.85 | 0.90 | 0.95 |
| Specificity | 0.85 | 0.90 | 0.95 | 1.00 |
| PEA misclassification among the non-beta blocker group | ||||
| Minimum | Mode 1 | Mode 2 | Maximum | |
| Sensitivity | 0.80 | 0.85 | 0.90 | 0.95 |
| Specificity | 0.85 | 0.90 | 0.95 | 1.00 |
The potential effect of missing data on the effect estimate was explored by first assigning expected exposure counts to both excluded PEA and VF cohorts at a ratio equal to that of the complete cases. We then added these newly classified subjects to the non-missing cohort, and calculated an odds ratio. The proportion of exposed individuals in the missing cohort with PEA was then sequentially decreased by moving one count from the exposed to the unexposed category while simultaneously increasing by one count the number of exposed versus unexposed in the missing VF cohort. After each iteration, the newly classified cohort was aggregated with complete cases and a new odds ratio and 95% limits were calculated.
RESULTS
The median age of the 478 subjects was 70 with an interquartile range from 57–79, and approximately 59% were male. Comparisons of demographic variables for the groups are shown in table 3. Of the total 478 cases, 143 (30%) presented with PEA, and 104 (22%) presented with VF; 97 (20%) were taking beta blockers, whereas 262 (55%) were not. Two hundred thirty (48%) presented with asystole, and it was unclear/unknown whether beta-blockers were used by 119 (25%) patients. The inter-rater reliability was 100%. Since asystole is viewed as the final terminal rhythm for all failed resuscitations, patients whose initial rhythm was asystole were also excluded. Thus, the final cohort consisted of 179 arrests, of whom 100 (56%) had PEA, 79 had VF (44%), 65 (36%) were taking beta blockers and 114 (64%) were not (table 4).
Table 3.
Baseline Demographics of Subjects (n = 478)
| Variable name | Range of values |
|---|---|
| Age | Median = 70; IQR = (57,79) |
| Gender | M = 280 (59%)
F = 198 (41%) |
| Race | Asian = 74 (16%)
Black = 137 (29%) Caucasian = 130 (27%) Hispanic = 79 (17%) Other = 29 (6%) Unknown = 26 (5%) |
| Rhythm | Asystole = 230 (48%)
PEA = 143 (30%) VF = 105 (22%) |
| Beta-blocker Use | Yes = 97 (20%)
No = 262 (55%) Unknown = 119 (25%) |
| Calcium Channel blocker Use | Yes = 45 (9%)
No = 314 (66%) Unknown = 119 (25%) |
| Bystander CPR | Yes = 172 (36%)
No = 265 (55%) Unknown = 41 (9%) |
| Return of Spontaneous Circulation (ROSC) | Yes = 83 (17%)
No = 395 (83%) |
| History of Chronic Obstructive Pulmonary Disease (COPD) | Yes = 80 (17%)
No = 357 (75%) Unknown = 41 (9%) |
| History of Cerebro-vascular Accident | Yes = 52 (11%)
No = 383 (80%) Unknown = 43 (9%) |
| History of Congestive Heart Failure | Yes = 77 (16%)
No = 357 (75%) Unknown = 44 (9%) |
| History of Myocardial Infarction | Yes = 80 (17%)
No = 356 (75%) Unknown = 42 (9%) |
| History of Chronic Renal Failure | Yes = 65 (14%)
No = 370 (77%) Unknown = 43 (9%) |
| History of Cancer | Yes = 57 (12%)
No = 377 (79%) Unknown = 44 (9%) |
| History of Diabetes | Yes = 157 (33%)
No = 277 (58%) Unknown = 44 (9%) |
| History of Hypertension | Yes = 209 (44%)
No = 227 (47%) Unknown = 42 (9%) |
| Downtime | < 10 minutes = 151 (32%)
10 – 15 minutes = 133 (28%) 15 – 30 minutes = 24 (5%) > 30 minutes = 170 (36%) |
Table 4.
Beta blocker vs. Rhythm
| Beta-blocker Use | PEA | VF | Totals |
|---|---|---|---|
| Yes | 49 | 16 | 65 (36%) |
| No | 51 | 63 | 114 (64%) |
| 100 (56%) | 79 (44%) | 179 |
The unadjusted odds ratio for beta-blocker use and PEA compared to beta blocker use and VF was 3.7 (95%CI 1.9 –7.2). The secondary exploratory analysis, using multivariable logistic regression modeling, demonstrated an effect estimate of 6.1 (95% CI 2.4 –15.4), which is qualitatively similar to the Mantel-Haenzel chi-square odds ratio. Automated probabilistic adjustment for misclassification of exposure and outcome had the effect of increasing the odds ratio to 7.04 (95% CI of 2.8 – 24.1). This upward revision of the odds ratio is generally expected, as misclassification of binary variables will often bias the effect measure towards the null.(11) When simultaneous adjustments for misclassification, confounding, and random error were included, the resulting odds ratio was 5.01 (95% CI of 1.1 – 31.0).
For missing data, systematically varying the exposure prevalence among missing subjects revealed that substantial departures from the expected exposure prevalence are required to bring the overall odds ratio down to unity. The lower 95% confidence limit breaches 1 at an exposure prevalence of 60% in missing VF cases (compared with 20% in the measured cohort, p=0.002), and 23% in missing PEA cases (compared with 49% in the measured cohort, p=0.004), resulting in an overall odds ratio in the total cohort of 1.65 (95% CI of 0.97–2.83). For these new effect estimates to hold, we would have to assume an odds ratio of 0.2 (95% CI of 0.07–0.59) for the exposure-disease association among the missing cases, suggesting a preventive effect of beta blockers for PEA. This would necessitate a substantial, and unlikely reversal in direction of association.from what we found among the complete cases.
DISCUSSION
The results of this study demonstrate that only a minority of patients who present with VF are taking beta-blockers, whereas approximately half of all patients presenting with PEA are taking beta-blockers. In the study population, out-of-hospital cardiac arrest victims taking beta-blockers were approximately 5 times more likely to have PEA as the first documented rhythm than those not taking beta-blockers. Neither random nor systematic differential exposure prevalence between subjects with missing exposure data and complete cases can credibly account for the observed association between beta blockers and PEA.
Given that only patients with OHCA were compared in our study, our inferences apply only to the type of OHCA rhythm (PEA vs. VF) found on EMS arrival. We do not attempt to make any conclusions concerning the causes of OHCA or its incidence, only concluding that, when it occurs, the association between beta blockers and this type of presenting rhythm (PEA) appears to be strong.
The median age of 70 in our cohort reflects a population for whom the OHCA event occurred slightly later in life when compared to historical literature (age 59–65). Other series have noted a temporal trend of increasing age at presentation for OHCA in general (12) and have further found that the proportion of patients presenting in VF decreases with increasing age,(13) especially among those with a cardiac aetiology.(14) In our cohort, the median age of VF victims was not significantly different from the PEA victims: 67 years of age with an interquartile range (IQR) of 54–83 vs. 70 years of age with an IQR of 58–80, respectively. Based on published literature, it appears that beta blockers extend life when given for certain indications. It is plausible that beta blockers may have prevented earlier arrest due to VF and that the presence of beta blockade resulted in later PEA, an arrest rhythm for which current resuscitative therapies are generally inadequate. While we found no differences in age distributions between the VF and PEA cohorts, the current study is ultimately not designed to answer such questions.
Most victims of sudden cardiac death have extensive multivessel coronary atherosclerosis and more than 50% of cases have evidence of acute coronary pathology, i.e., plaque rupture, thrombosis, or both, at autopsy.(15) This substrate is a predisposition for ventricular arrhythmias and VF. Numerous clinical trials evaluating the effect of beta-blockers during acute ischemia and after acute myocardial infarction or in the management of congestive heart failure have demonstrated a lower incidence of sudden death, presumably the result of the antiarrhythmic properties of this class of drugs.(16–18) The lower rate of VF in our study population receiving chronic beta-blocker therapy is therefore not unexpected. Why this population should have a higher incidence of pulseless electrical activity as the first documented cardiac arrest mechanism is uncertain. Acute occlusion of a large epicardial coronary artery is known to result in an almost immediate decline in cardiac contractile function and cardiac output. Contractile dysfunction is likely to be more profound in the setting of preocclusion beta-blockade.(19–20) If the beta-blocking agent is pharmacologically “nonselective” with associated alpha-blockade, it is likely that the compensatory vasoconstriction mediated by catecholamine release will be attenuated, resulting in hypotension and a low pulse pressure characteristic of PEA. In our study, we were often unable to determine the type of beta-blocker. Therefore, it was not possible to determine if PEA was more common in patients taking a nonselective agent, eg, carvedilol, or a beta-1 selective agent, eg, metoprolol.(21) Nonetheless, the association of beta-blocker use with PEA is fairly robust. Neither misclassification nor the presence of a large unmeasured confounder, as demonstrated with sensitivity analysis, can credibly account for the association between beta blockers and an initial arrest rhythm of PEA.
Due to the limited number of survivors, we were unable to determine if prearrest beta-blocker therapy impacted outcome in the PEA subgroup. Several laboratory investigations suggest that beta-blockers given at the start of resuscitation efforts or after resuscitation from VF improve short term survival.(22–25) However, these studies utilized cardiac electrical stimulation to induce VF in animals with normal coronary arteries and, with the exception of one study, beta-blockers were administered after restoration of circulation rather than before onset of arrest. It is uncertain if beta-blocker use prior to cardiac arrest will impact the effectiveness of currently recommended drugs, particularly if the beta-blocker is nonselective with an alpha-blockade effect. The benefit of epinephrine in cardiac arrest is largely ascribed to its peripheral alpha agonist effect.(26)
Study Limitations
There are several limitations in our study design. The subjects in our cohort all presented to one facility, thus limiting the generalizability of our results. Furthermore, neither of the abstractors was blinded to the study hypothesis, potentially biasing our findings. In addition, the first documented ECG finding in the study population was often asystole and these patients were excluded from analysis, due to the fact that asystole may follow either VF or PEA. Our adjustments for misclassification bias involved assumptions of non-differentiality and independence. We found no association between calcium channel blocker usage and PEA. However, the role of other medications, such as aspirin and ACE-inhibitors was not investigated in the current analysis. These and other factors may merit consideration in future studies. Finally, data were missing in a large proportion of cases. In these instances, it was unclear from the chart review what medications the patient was taking. However, it is unlikely that these excluded cases were systematically different from the included subjects.
Conclusions
The findings of this study suggest that there is an association between beta blockers and the changing epidemiology of arrest rhythms, accounting for a decreasing incidence of VF and a concomitant increase in PEA. It is unknown if prearrest treatment with beta-blockers affords survival benefit or impacts the effectiveness of drugs administered during resuscitative efforts.
Supplementary Material
Acknowledgments
Funding source
Funded, in part, by a grant from the National Institutes of Health, NHLBI R01 HL076671, Bethesda, MD, and the Los Angeles County Emergency Medical Services Agency, Los Angeles, CA.
Footnotes
Conflict of interest statement
The authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kette F. Increased survival despite a reduction in out-of-hospital ventricular fibrillation in north-east Italy. Resuscitation. 2007;72:52–8. doi: 10.1016/j.resuscitation.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 2.Polentini MS, Pirrallo RG, McGill W. The changing incidence of ventricular fibrillation in Milwaukee, Wisconsin (1992–2002) Prehospital Emerg Care. 2006;10:52–60. doi: 10.1080/10903120500366961. [DOI] [PubMed] [Google Scholar]
- 3.Bunch TJ, White RD, Friedman PA, Kotke TE, Wu LA, Packer DL. Trends in treated ventricular fibrillation out-of-hospital cardiac arrest: a 17-year population-based study. Heart Rhythm. 2004;1:255–9. doi: 10.1016/j.hrthm.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Hess EP, Campbell RL, White RD. Epidemiology, trends, and outcome of out-of-hospital cardiac arrest of non-cardiac origin. Resuscitation. 2007;72:200–6. doi: 10.1016/j.resuscitation.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 5.Stafford RS, Monti V, Furberg CD, Ma J. Long-term and short-term changes in antihypertensive prescribing by office-based physicians in the United States. Hypertension. 2006;48:196–7. doi: 10.1161/01.HYP.0000229653.73128.b6. [DOI] [PubMed] [Google Scholar]
- 6.Bradley EH, Herrin J, Mattera JA, et al. Quality Improvement efforts and hospital performance: rates of beta-blocker prescription after acute myocardial infarction. Med Care. 2005;43:282–92. doi: 10.1097/00005650-200503000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Exner DV, Reiffel JA, Epstein AE, et al. Beta-blocker use and survival in patients with ventricular fibrillation or symptomatic ventricular tachycardia: the antiarrhythmics versus implantable defibrillators (AVID) trial. J Am Coll Cardiol. 1999;34:325–33. doi: 10.1016/s0735-1097(99)00234-x. [DOI] [PubMed] [Google Scholar]
- 8.Connolly SJ, Dorian P, Roberts RS, Gent M, et al. Comparison of beta-blockers, amiodarone plus beta-blockers, or sotalol for prevention of shocks from implantable cardioverter defibrillators: the OPTIC study: a randomized trial. JAMA. 2006;295:165–71. doi: 10.1001/jama.295.2.165. [DOI] [PubMed] [Google Scholar]
- 9.http://sph.bu.edu/images/stories/scfiles/epidemiology/senstoolv1.01.xls, accessed on 3/15/07.
- 10.Fox MP, Lash TL, Greenland S. A method to automate probabilistic sensitivity analysis of misclassified binary variables. Int J Epidemiol. 2005;34:1370–6. doi: 10.1093/ije/dyi184. [DOI] [PubMed] [Google Scholar]
- 11.Greenland S, Gustafson P. Accounting for independent nondifferential misclassification does not increase certainty that an observed association is in the correct direction. Am J Epidemiol. 2006;164:63–8. doi: 10.1093/aje/kwj155. [DOI] [PubMed] [Google Scholar]
- 12.Herlitz J, Engdahl J, Svensson L, Young M, et al. Changes in demographic factors and mortality after out-of-hospital cardiac arrest in Sweden. Coron Artery Dis. 2005;16:51–7. doi: 10.1097/00019501-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Polentini MS, Pirrallo RG, McGill W. The changing incidence of ventricular fibrillation in Milwaukee, Wisconsin (1992–2002) Prehosp Emerg Care. 2006;10:52–60. doi: 10.1080/10903120500366961. [DOI] [PubMed] [Google Scholar]
- 14.Herlitz J, Eek M, Engdahl J, Holmberg M, et al. Factors at resuscitation and outcome among patients suffering from out of hospital cardiac arrest in relation to age. Resuscitation. 2003;58:309–17. doi: 10.1016/s0300-9572(03)00155-2. [DOI] [PubMed] [Google Scholar]
- 15.Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. J Am Coll Cardiol. 2006;48:e247–e346. doi: 10.1016/j.jacc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Freemantle N, Cleland J, Young P, Mason J, Harrison J. β-blockade after myocardial infarction: systematic review and meta regression analysis. BMJ. 1999;318:1730–7. doi: 10.1136/bmj.318.7200.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonet S, Agusti A, Arnau JM, et al. Beta-adrenergic blocking agents in heart failure: benefits of vasodilating and non-vasodilating agents according to patients’ characteristics: a meta-analysis of clinical trials. Arch Intern Med. 2000;160:621–7. doi: 10.1001/archinte.160.5.621. [DOI] [PubMed] [Google Scholar]
- 18.Foody JM, Farrell MH, Krumholz HM. β-blocker therapy in heart failure. Scientific review JAMA. 2002;287:883–9. doi: 10.1001/jama.287.7.883. [DOI] [PubMed] [Google Scholar]
- 19.Aupetit JF, Frassati D, Biu-Xuan B, Freysz M, Faucon G, Timour Q. Efficacy of a β-adrenergic receptor antagonist, propranolol, in preventing ischaemic ventricular fibrillation : dependence on heart rate and ischaemia duration. Cardiovasc Res. 1998;37:656–55. doi: 10.1016/s0008-6363(97)00304-0. [DOI] [PubMed] [Google Scholar]
- 20.Coetzee A, Coetzee G. β-adrenergic blockade during severe ischemia. J Cardiothorac Vasc Anesthe. 2002;16:670–8. doi: 10.1053/jcan.2002.128420. [DOI] [PubMed] [Google Scholar]
- 21.Reiter MJ. Cardiovascular drug class specificity: β-blockers. Prog Cardiovasc Dis. 2004;47:11–33. doi: 10.1016/j.pcad.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Menegazzi JJ, Davis EA, Yealy DM, et al. An experimental algorithm versus standard advanced cardiac life support in a swine model of out-of-hospital cardiac arrest. Ann Emerg Med. 1993;22:235–9. doi: 10.1016/s0196-0644(05)80211-2. [DOI] [PubMed] [Google Scholar]
- 23.Killingsworth CR, Wei CC, Dell’Italia LJ, et al. Short-acting beta-adrenergic antagonist esmolol given at reperfusion improves survival after prolonged ventricular fibrillation. Circulation. 2004;109:2469–74. doi: 10.1161/01.CIR.0000128040.43933.D3. [DOI] [PubMed] [Google Scholar]
- 24.Huang L, Weil MH, Cammarata G, Sun S, Tang W. Nonselective β-blocking agent improves the outcome of cardiopulmonary resuscitation in a rat model. Crit Care Med. 2004;32(Suppl):S378–80. doi: 10.1097/01.ccm.0000134266.65164.7c. [DOI] [PubMed] [Google Scholar]
- 25.Cammarata G, Weil MH, Sun S, Tang W, Wang J, Huang L. β1-adrenergic blockade during cardiopulmonary resuscitation improves survival. Crit Care Med. 2004;32(Suppl):S440–3. doi: 10.1097/01.ccm.0000134263.32657.34. [DOI] [PubMed] [Google Scholar]
- 26.Cao L, Weil MH, Sun S, Tang W. Vasopressor agents for cardiopulmonary resuscitation. J Cardiovasc Pharmacol Therpeut. 2003;8:115–21. doi: 10.1177/107424840300800204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.