Abstract
Myocardial infarction and ischemic heart disease are among the most common causes of morbidity and mortality in the industrial world. Surgical and percutaneous intravascular approaches are commonly used to treat these diseases. Regrettably, a significant number of patients are either ineligible or demonstrate suboptimal responses to these therapies. In an attempt to provide such patients improved therapeutic options, much effort has been spent developing noninvasive approaches to restore coronary vascular perfusion. One such strategy, termed therapeutic revascularization or angiogenesis, involves administration of proangiogenic factors, which improve coronary perfusion by promoting growth of the coronary vasculature. Thus far, two potential proangiogenic factors have been intensively examined, fibroblast growth factor and vascular endothelial growth factor. Unfortunately, despite their apparent efficacy in animal models, neither factor has performed adequately in the clinic to date. Within the past year a new factor, hedgehog, has been shown to effectively promote the growth of the coronary vasculature and thus has been proposed as a novel candidate for therapeutic revascularization. In this review, we discuss the discovery of the hedgehog pathway as an essential regulator of the development of the coronary vasculature, as an inducer of adult coronary vascular growth, and as a therapeutic in the treatment of ischemic heart disease.
Ischemic Heart Disease
Myocardial infarction and ischemic heart disease are the leading cause of death in the industrial world. Therapies used for treating these diseases are aimed at restoring and/or improving blood flow to ischemic cardiac tissue. Coronary artery bypass grafting and percutaneous coronary interventions are the mainstays of treatment for these patients and significantly reduce morbidity and mortality (Caines et al. 2004). Unfortunately, roughly 10% of all patients are ineligible for these procedures owing to the presence of diffuse or intractable lesions. Furthermore, significant numbers of diabetic patients that undergo these procedures show insufficient improvement. Without treatment, these patients do poorly, with mortality rates of 8% to 10% per year (Syed et al. 2004).
Recently, a noninvasive approach aimed at promoting growth of new coronary blood vessels has been proposed to treat patients who are not eligible for coronary artery bypass grafting and percutaneous coronary interventions. This strategy, termed pharmacological revascularization or angiogenesis, involves either systemic or local administration of proangiogenic agents that promote growth of the established vasculature and/or formation of new coronary blood vessels. The search for molecules that possess this activity is actively underway.
Over the past 10 years, it has been realized that expression of a number of known proangiogenic factors can promote coronary vessel growth in animal models. Overexpression of fibroblast growth factor-2 (FGF2), vascular endothelial growth factor-A (VEGF-A), and angiopoietin-2 (ANG2) in the myocardium of adult mice leads to significant increases in coronary artery number (House et al. 2003, Landau et al. 1995, Rajanayagam et al. 2000, Syed et al. 2004, Tammela et al. 2005, Uchida et al. 1995, Visconti et al. 2002). Since these initial observations, the effects of Fgf2 and Vegf-A in the adult heart have been intensively investigated and proposed as candidates for the treatment of ischemic heart disease (Scheinowitz et al. 1997, Syed et al. 2004). However, despite their ability to promote new blood vessel growth in both normal and ischemic hearts, clinical trails using either protein or gene therapy have been disappointing (Henry et al. 2000, Losordo et al. 2002, Simons et al. 2002, Syed et al. 2004).
In light of these disappointments, it has been postulated that monoagent therapies may be unable to efficiently stimulate the signaling pathways necessary to adequately trigger a therapeutic level of blood vessel growth in the adult human heart (Syed et al. 2004). To this end, it has been proposed that combinations of proangiogenic agents may be required to accomplish these goals. Consistently, transgenic overexpression of both VEGF-A and ANG2 leads to significantly greater levels of vascular growth than does either factor alone (Visconti et al. 2002). To this end, combination therapy is currently being tested, and new proangiogenic agents capable of achieving higher levels of vascular growth are actively being pursued.
A Developmental Approach
An alternative approach to identify new molecules that can effectively promote coronary vascular growth is to investigate how the coronary vascular system develops. As developmental programs are often recapitulated in adult physiology and tissue repair, this approach offers an opportunity to develop novel or improved therapeutic agents. Several FGFs (FGF1, FGF2, FGF9, FGF16, and FGF20), VEGFs (VEGF-A and VEGF-B), and ANGs (ANG1 and ANG2) are expressed in the embryonic heart during coronary vessel formation (Detillieux et al. 2003, Lavine et al. 2005, Tomanek et al. 2002, Ward and Dumont, 2002). However, until recently, little was known about whether or how these molecules orchestrate the development of the coronary vascular system.
We and others have postulated that an understanding of how these pathways control coronary blood vessel formation in the embryonic heart may provide important information guiding the development of novel therapeutics (Kusano et al. 2005, Lavine et al. 2006). To this end we have sought to (1) understand the mechanism by which FGFs, VEGFs, and ANGs control coronary development; (2) identify factors that coordinately control the expression of proangiogenic factors during coronary development; and (3) determine whether reactivation of signaling pathways that promote coronary development can similarly trigger coronary vessel growth in the adult heart.
Development of the Coronary Vasculature
Vascular development is governed by two sequentially acting processes, vasculogenesis and angiogenesis. Vasculogenesis refers to the formation of blood vessels via de novo differentiation of either angioblast or hemangioblast precursors, whereas angiogenesis is defined as the growth or remodeling of established blood vessels. In general, vascular systems undergo a stereotyped pattern of development beginning with the formation of a primary capillary plexus that is later remodeled, giving rise to the mature vasculature. It is thought that the primary capillary plexus forms by vasculogenesis and is remodeled via angiogenesis (Flamme et al. 1997, Risau 1997).
Similar to other vascular systems, coronary development begins with the formation of a vascular network that is later remodeled, giving rise to the mature coronary tree (Kattan et al. 2004, Morabito et al. 2002) (Figure 1) Interestingly, the initial coronary vascular plexus consists of two sets of blood vessels located within different positions, the subepicardial mesenchyme and the myocardial wall (Figure 2).
Figure 1.
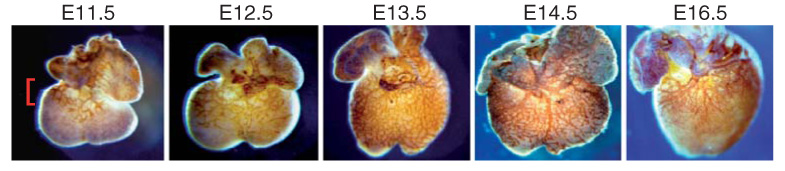
Development of the coronary vasculature. Platelet/endothelial cell adhesion molecule-1/CD31 immunostaining of murine embryonic day (E)11.5 to E14.5 and E16.5 hearts demonstrating the progression of coronary growth during development. Between E11.5 and E13.5, the developing coronary vasculature plexus emerges from the atrial ventricular groove (red bracket) and grows in a wavelike pattern to cover both ventricles by E13.5. Between E14.5 and E16.5, the coronary vascular plexus remodels, giving rise to the mature coronary vasculature.
Figure 2.

The coronary vascular plexus consists of two subsets of blood vessels. Left and middle, H&E-stained histological sections of murine embryonic hearts showing the formation of the subepicardial mesenchyme (SEM) between E11.5 and E13.5. Right, Histological section of a PECAM-stained E13.5 heart revealing two sets of developing coronary vessels. The intramyocardial blood vessels (intramyo. BV) course through the myocardial wall, whereas the subepicardial blood vessels (subepi. BV) grow within the subepicardial mesenchyme.
Morphological events that are involved in formation of the coronary vascular system have been well described, especially in avian systems (chick and quail) (Morabito et al. 2002, Reese et al. 2002, Wada et al. 2003). Before stage 14 in the chick (embryonic day 9.5 in the mouse), the heart consists of two layers, an outer myocardial layer and an inner endocardial layer. At stage 18 in the chick (embryonic day 10.5 in the mouse), the third cardiac layer, the epicardium, migrates to the embryonic heart and envelopes its outer surface to form a continuous layer of epithelium. Epicardial progenitors are derived from the proepicardial organ, an epithelium originally described in the chick that is associated with the septum transversum.
After investment of the heart by the epicardium, epicardial cells undergo an epithelial–mesenchymal transformation beginning at stage 26 in the chick (embryonic day 12.5 in the mouse). Epicardial epithelial–mesenchymal transformation leads to the formation of a mesenchyme situated between the epicardium and myocardium (subepicardial space). Within the subepicardial mesenchyme, endothelial cells will coalesce to form vascular channels that are ensheathed by mesenchymal progenitors (Figure 2). Vascular channel formation leads to the development of a vascular plexus that covers the heart. At later stages in development, this vascular plexus undergoes remodeling, giving rise to the mature coronary vascular system (Morabito et al. 2002, Reese et al. 2002, Wada et al. 2003).
Initial studies in avian systems have demonstrated that the proepicardial organ and the epicardium are required for coronary development because removal of either structure severely perturbs coronary vessel formation (Gittenberger-de Groot et al. 2000). More recently, genetic analysis in the mouse has confirmed this observation. Removal of genes required for formation (Gata4), migration, or attachment (Vcam, a4b1 integrin) of epicardial precursors to the myocardium leads to severe defects in coronary development (Kwee et al. 1995, Watt et al. 2004, Yang et al. 1995). In addition, deletion of genes necessary for survival and/or integrity (p300, Wt1) of the epicardium leads to similar phenotypes (Davies et al. 1999, Moore et al. 1999, Shao et al. 2005, Shikama et al. 2003).
These analyses indicate that the epicardium is absolutely required for coronary vascular development. It has been proposed that the epicardium is important because it physically contributes vascular cell types to the heart (Mikawa and Fischman 1992, Mikawa and Gourdie 1996, Perez-Pomares et al. 2002). However, lineage analysis of the epicardium reveals contributions to only perivascular fibroblast and smooth muscle cell lineages and not the coronary endothelial cell lineage (Dettman et al. 1998, Vrancken Peeters et al. 1999).
An alternative explanation for why the epicardium is essential for coronary development is that the epicardium acts as a signaling center. Support for this hypothesis stems from the observation that the epicardium promotes cardiomyoblast proliferation by secreting mitogens (Chen et al. 2002, Stuckmann et al. 2003). Interestingly, not only is retinoic acid (RA) signaling in the epicardium necessary for the secretion of such mitogens but it also is required for coronary development (Sucov et al. 1994).
To date only one such set of epicardially derived mitogens, FGFs, has been identified. Previously, we have demonstrated that FGF9 (and likely FGF16 and FGF20) is a RA regulated epicardially derived mitogen that is essential for cardiomyoblast proliferation (Lavine et al. 2005). From this and extensive studies showing that FGF signaling can promote vascular formation and growth (Auguste et al. 2003, Kanda et al. 2004, Seghezzi et al. 1998), we have hypothesized that RA signaling within the epicardium may regulate coronary development by controlling the expression of proangiogenic factors such as FGFs. Interestingly, recent studies have shown that FGFs only indirectly control vascular development, suggesting that yet unidentified factor(s) may be involved in coordinating FGF-induced vascular growth (Lavine et al. 2006).
An FGF-hedgehog-VEGF/ANG Signaling Pathway Controls Coronary Development
Through both conditional gene targeting and organ culture assays, we have demonstrated that an FGF-hedgehog (HH)-VEGF/ANG signaling pathway controls the formation of the coronary vascular system (Figure 3). Epicardial and endocardial sources of FGF9, 16, and 20 signal to the myocardium through redundant function of FGFR1c and FGFR2c. Fibroblast growth factor signaling to the myocardium directly regulate cardiomyoblast proliferation and indirectly trigger the development of the coronary vasculature. Myocardial FGF signaling controls formation of the coronary vascular system by activating a wavelike pattern of HH signaling. This wavelike expansion of HH activation originates from the atrial ventricular groove (at E12.5) and extends toward the ventricular apex (by E13.5), almost exactly tracking the progression of the developing coronary vascular plexus. Hedgehog ligands signal to the cardiomyoblast and perivascular mesenchymal cell and induce the expression of Vegf-A, Vegf-B, Vegf-C, and Ang2, all of which signal to and promote growth of the developing coronary vascular endothelium.
Figure 3.
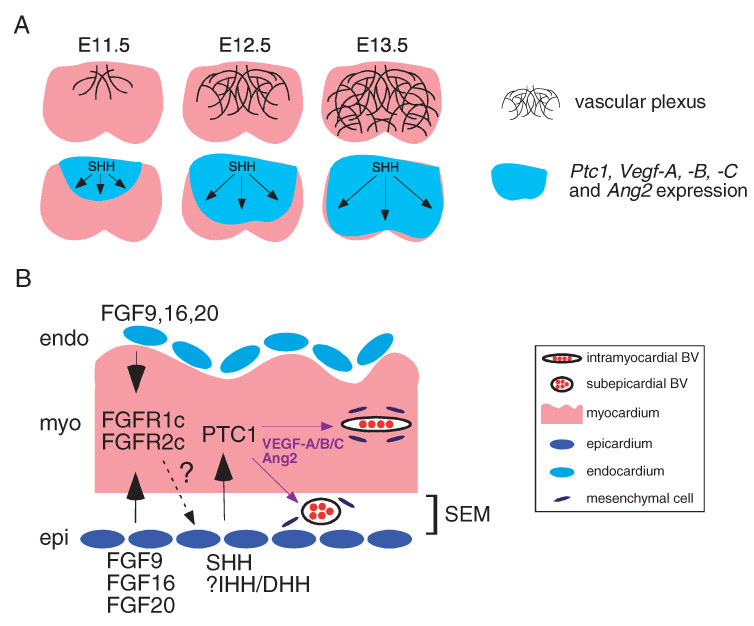
Hedgehog signaling controls the development of the coronary vasculature. (A) Schematic highlighting the simultaneous wavelike expansion of the coronary vascular plexus and HH activation between E11.5 and E13.5. The blue-colored area denotes the expression domain of Ptc1, Vegf-A, Vegf-B, Vegf-C, and Ang2. (B) An FGF-HH-VEGF/ANG signaling cascade controls embryonic coronary development. Model depicting the spatial relationship between FGF, HH, VEGF, and ANG growth factors during coronary development. Dashed arrow and question mark represent the unidentified myocardial to epicardial signal controlling SHH expression.
The HH signaling system is composed of three ligands, namely sonic hedgehog (SHH), indian hedgehog, and desert hedgehog. All three HH ligands are expressed in the embryonic and adult heart, with SHH being the most abundant (Kusano et al. 2005, Lavine et al. 2006). Hedgehog ligands are essentially interchangeable, all of which signal through the same receptors, patched 1 and 2 (PTC1 and PTC2). Binding of an HH ligand to the PTC receptor stimulates the downstream effector, smoothened (SMO), which in turn signals through an ill-defined pathway involving several different molecules (fused, suppressor of fused, protein kinase A) eventually leading to the activation of one or several of the three GLI transcription factors, GLI1-3 (Dellovade et al. 2006).
Intriguingly, this FGF-HH-VEGF/ANG signaling cascade coordinately controls the growth of both subepicardial and intramyocardial blood vessels (Lavine et al. 2006). Preliminary studies in our laboratory using transgenic mice that mark specific vascular lineages indicate that these two sets of blood vessels represent distinct vascular subtypes: veins and arteries, respectively. It is currently unclear when arterial and venous lineages are established in the developing heart. Moreover, whether the development of coronary arteries and veins is coordinated and the mechanism by which this might occur is unknown. Intriguingly, if a single molecule or signaling pathway (such as HH) does coordinate arterial and venous growth during development, would expression of this molecule or activation of this signaling pathway in the adult heart lead to a similarly coordinated expansion of these vascular subtypes?
Despite the identification of several key components of this signaling axis, a number of important questions remained unanswered. For instance, do additional receptor tyrosine kinase ligands function in parallel with FGFs? This is clearly a possibility because loss of FGF signaling leads to only a delay but not an arrest in both Shh expression and coronary vascular development. In addition, how FGF signaling controls the expression of Shh is unclear, and whether VEGF ligands and ANG2 are direct targets of HH signaling remains unknown. How this signaling axis might interact with that of classic angiogenic pathways, such as HIF1α, is unclear. Moreover, whether this signaling axis functions during remodeling of the coronary vascular plexus is unexplored.
Several studies have provided evidence that HH signaling functions more broadly in vascular development. Mouse embryos lacking Smoothened (Smo, transducer of HH signaling) and zebra-fish embryos lacking Shh display defects in vasculogenesis, SHH promotes vascular plexus formation in cell culture, and activation of HH signaling in the adult mouse are sufficient to promote neovascularization in several different tissues (Kanda et al. 2003, Lawson et al. 2002, Pola et al. 2001, Vokes et al. 2004). Based on these studies demonstrating that HH signaling promotes vascular growth by inducing expression of various VEGF and ANG molecules, it is likely that HH governs blood vessel formation and growth throughout the embryo and potentially the adult organism by activating a conserved growth factor signaling cascade.
HH-Mediated Vessel Growth in Adult Heart
Activation of HH signaling is both necessary for coronary development and sufficient to promote formation of new coronary vessels in the embryonic and adult heart. We and others have discovered that activation of HH signaling in the adult heart promotes coronary neovascularization and provides protection from ischemia (Kusano et al. 2005, Lavine et al. 2006). Moreover, Shh gene therapy can promote coronary neovascularization and protection from ischemic injury in rodent and large animal models (Kusano et al. 2005). These studies directly implicate the HH signaling pathway as a potential therapeutic target for pharmacological revascularization and make a compelling case for the potential therapeutic use of HH agonists in patients with ischemic heart disease.
Hedgehog signaling orchestrates both coronary development and adult coronary neovascularization by controlling the expression of multiple proangiogenic genes including Vegf-A, Vegf-B, Vegf-C, and Ang2 (Figure 4). Based on findings indicating that HH signaling regulates coronary vascular formation, promotes neovascularization in the adult heart, and induces expression of numerous signaling molecules, it has become evident that the HH pathway represents a powerful regulator capable of orchestrating coronary vascular growth in numerous settings.
Figure 4.
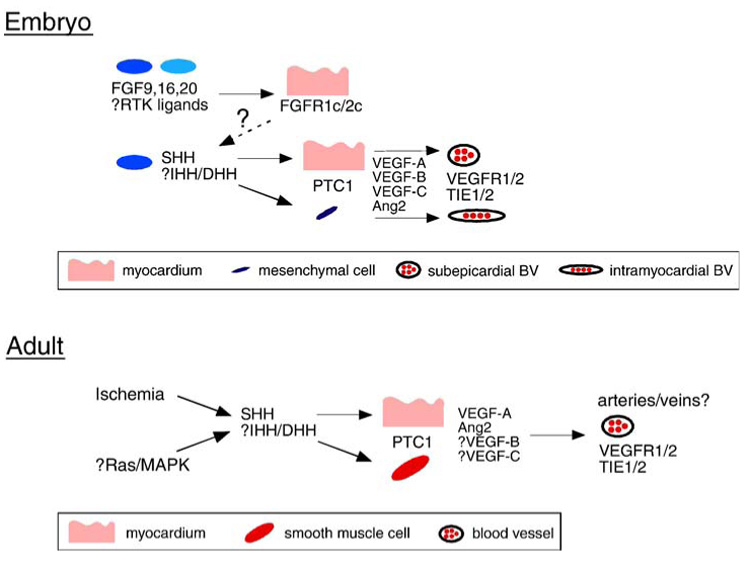
Comparison between HH signaling during embryonic and adult coronary vascular growth. Top, Model displaying the source and target tissues of FGF, HH, VEGF, and ANG signals. Dashed arrow and question mark represent the unidentified myocardial to epicardial signal controlling SHH expression. Bottom, Model describing the mechanism by which HH signaling controls coronary growth in the adult heart. Question marks represent components proposed to be involved in this signaling cascade.
Therapeutics aimed at such a molecule and/or pathway have the potential to succeed where other pharmacological modalities have failed. It has been postulated that monotherapy with FGF2 or VEGF-A165 has been unsuccessful because expression of multiple factors is required for efficient coronary neovascularization (Syed et al. 2004). In support of this concept, coexpression of Vegf-A and Ang2 in the myocardium causes significantly more robust increases in coronary vessel density than that of Vegf-A or Ang2 alone (Visconti et al. 2002). Intriguingly, HH signaling regulates expression of not only Vegf-A and Ang2 but also Vegf-B and Vegf-C, making HH signaling an attractive candidate for therapy aimed at promoting coronary vascular growth. Consistent with a role for HH in controlling multiple proangiogenic factors, coapplication of VEGF-A and ANG2 is required to rescue coronary development in hearts treated with an HH antagonist (Lavine et al. 2006). Furthermore, unlike VEGF agonist therapies, activation of HH signaling promotes the growth of multiple blood vessels types, including both larger smooth muscle encased vessels and capillaries, whereas VEGF only promotes the growth of capillary-sized blood vessels (Kusano et al. 2005, Visconti et al. 2002).
HH-Mediated Vessel Growth in Other Organ Systems
In addition to the heart, activation of the HH pathway has been to shown to promote therapeutic blood vessel growth in various other tissues, including the cornea, skin, skeletal muscle, and peripheral nerve. Injection of recombinant SHH protein into adult mice leads to increased blood vessel numbers in the heart, skeletal muscle, and cornea (Pola et al. 2001). Consistent with what has been observed in the heart, activation of HH signaling promoted growth of multiple blood vessel types compared with the uniform increase in capillary-sized blood vessels after treatment with VEGF agonists (Pola et al. 2001).
Consistent with the ability of HH signaling to promote the growth of functional vasculature, several studies have demonstrated that activation of HH signaling protects from ischemic damage and leads to functional improvements in a diversity of tissues. Injection of an HH small molecule agonist decreases infarct size by 40% to 50% and leads to improved behavior and body weight in a middle cerebral artery occlusion model (Dellovade et al. 2006). Moreover, treatment of diabetic mice with recombinant SHH protein not only promoted vascular growth in the skin and peripheral nervous tissue but also resulted in accelerated wound healing and improved peripheral nerve conduction velocity, two processes that are defective at baseline in diabetic mouse models (Asai et al. 2006, Kusano et al. 2004). These data implicate the HH signaling pathway as a useful target for therapeutics aimed to minimize ischemic damage and improve tissue function in conjunction with both macrovascular and microvascular disease.
Transitioning HH Agonists into the Clinic
Data published thus far clearly implicate the potential therapeutic use of HH agonists in several different clinical settings. However, before HH agonists are used in such circumstances, several issues should be addressed. First, it is currently unclear which type of HH agonist (recombinant SHH protein, gene therapy, or small molecule agonist) is best suited for clinical use. To date there are no studies comparing the efficacy of recombinant SHH protein, gene therapy, and small molecule agonists in coronary angiogenesis or myocardial ischemia models. Moreover, issues of dosing, length of treatment, and route of administration are for the most part unexplored. Key points include whether systemic or local administration of HH agonists is most efficacious and whether the effects of HH on the vasculature are lost upon discontinuation of the therapeutic agent.
Importantly, the possibility that activation of HH signaling either locally or systemically may have deleterious consequences and whether small molecule HH agonists have undesirable off-target effects have not been adequately explored. This is important, given the prominent role of HH signaling in several neoplastic processes (Dellovade et al. 2006). Moreover, whereas acute activation of HH signaling seems to be therapeutic and potentially protective for the heart, it is not known whether chronic activation of HH signaling is equally advantageous or potentially harmful to either the heart or any other organ. Studies addressing these issues and others will need to be addressed before HH agonists are brought to the clinic.
In addition to HH agonists, the use of HH antagonists therapeutics against medulloblastoma has been explored and discussed as potential treatment for pancreatic carcinoma, small cell lung carcinoma, and basal cell carcinoma (Dellovade et al. 2006). Given that SHH expression is induced during myocardial and skeletal muscle ischemia (Kusano et al. 2005, Pola et al. 2003), it is possible that HH signaling may be an important endogenous regulator of vascular growth and potentially maintenance. This possibility is further supported by the finding that inhibition of HH activity, with the use of neutralizing anti-SHH antibodies, adversely affected recovery in a hind limb ischemia model (Pola et al. 2003). It would therefore be prudent to examine whether therapeutic doses of HH antagonists impair coronary vascular growth and maintenance in both normal and diseased hearts before the use of these HH antagonists in the clinic.
Acknowledgments
The authors of this study thank all of the authors who have contributed to their understanding of coronary vascular development and adult angiogenesis. They are grateful for the generous contributions provided by grants from the NIH (HL076664, HD39952, DK52574 [microinjection]), from the American Heart Association (0415469Z), and a generous contribution from the Virginia Friedhofer Charitable Trust.
References
- Asai J, Takenaka H, Kusano KF, et al. Topical sonic hedgehog gene therapy accelerates wound healing in diabetes by enhancing endothelial progenitor cell-mediated microvascular remodeling. Circulation. 2006;113:2413–2424. doi: 10.1161/CIRCULATIONAHA.105.603167. [DOI] [PubMed] [Google Scholar]
- Auguste P, Javerzat S, Bikfalvi A. Regulation of vascular development by fibroblast growth factors. Cell Tissue Res. 2003;314:157–166. doi: 10.1007/s00441-003-0750-0. [DOI] [PubMed] [Google Scholar]
- Caines AE, Massad MG, Kpodonu J, et al. Outcomes of coronary artery bypass grafting versus percutaneous coronary intervention and medical therapy for multi-vessel disease with and without left ventricular dysfunction. Cardiology. 2004;101:21–28. doi: 10.1159/000075982. [DOI] [PubMed] [Google Scholar]
- Chen TH, Chang TC, Kang JO, et al. Epicardial induction of fetal cardiomyocyte proliferation via a retinoic acid-inducible trophic factor. Dev Biol. 2002;250:198–207. doi: 10.1006/dbio.2002.0796. [DOI] [PubMed] [Google Scholar]
- Davies R, Moore A, Schedl A, et al. Multiple roles for the Wilms’ tumor suppressor WT1. Cancer Res. 1999;59:1747s–1750s. [discussion 1751s] [PubMed] [Google Scholar]
- Dellovade T, Romer JT, Curran T, Rubin LL. The hedgehog pathway and neurological disorders. Annu Rev Neurosci. 2006;29:539–563. doi: 10.1146/annurev.neuro.29.051605.112858. [DOI] [PubMed] [Google Scholar]
- Detillieux KA, Sheikh F, Kardami E, Cattini PA. Biological activities of fibroblast growth factor-2 in the adult myocardium. Cardiovasc Res. 2003;57:8–19. doi: 10.1016/s0008-6363(02)00708-3. [DOI] [PubMed] [Google Scholar]
- Dettman RW, Denetclaw W, Jr, Ordahl CP, Bristow J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Dev Biol. 1998;193:169–181. doi: 10.1006/dbio.1997.8801. [DOI] [PubMed] [Google Scholar]
- Flamme I, Frolich T, Risau W. Molecular mechanisms of vasculogenesis and embryonic angiogenesis. J Cell Physiol. 1997;173:206–210. doi: 10.1002/(SICI)1097-4652(199711)173:2<206::AID-JCP22>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Gittenberger-de Groot AC, Vrancken Peeters MP, Bergwerff M, et al. Epicardial outgrowth inhibition leads to compensatory mesothelial outflow tract collar and abnormal cardiac septation and coronary formation. Circ Res. 2000;87:969–971. doi: 10.1161/01.res.87.11.969. [DOI] [PubMed] [Google Scholar]
- Henry GD, Byrne R, Hunyh TT, et al. Intracavernosal injections of vascular endothelial growth factor protects endothelial dependent corpora cavernosal smooth muscle relaxation in the hypercholesterolemic rabbit: a preliminary study. Int J Impot Res. 2000;12:334–339. doi: 10.1038/sj.ijir.3900621. [DOI] [PubMed] [Google Scholar]
- House SL, Bolte C, Zhou M, et al. Cardiac-specific overexpression of fibroblast growth factor-2 protects against myocardial dysfunction and infarction in a murine model of low-flow ischemia. Circulation. 2003;108:3140–3148. doi: 10.1161/01.CIR.0000105723.91637.1C. [DOI] [PubMed] [Google Scholar]
- Kanda S, Mochizuki Y, Suematsu T, et al. Sonic hedgehog induces capillary morphogenesis by endothelial cells through phosphoinositide 3-kinase. J Biol Chem. 2003;278:8244–8249. doi: 10.1074/jbc.M210635200. [DOI] [PubMed] [Google Scholar]
- Kanda S, Miyata Y, Kanetake H. Fibroblast growth factor-2-mediated capillary morphogenesis of endothelial cells requires signals via Flt-1/vascular endothelial growth factor receptor-1: possible involvement of c-Akt. J Biol Chem. 2004;279:4007–4016. doi: 10.1074/jbc.M307569200. [DOI] [PubMed] [Google Scholar]
- Kattan J, Dettman RW, Bristow J. Formation and remodeling of the coronary vascular bed in the embryonic avian heart. Dev Dyn. 2004;230:34–43. doi: 10.1002/dvdy.20022. [DOI] [PubMed] [Google Scholar]
- Kusano KF, Allendoerfer KL, Munger W, et al. Sonic hedgehog induces arteriogenesis in diabetic vasa nervorum and restores function in diabetic neuropathy. Arterioscler Thromb Vasc Biol. 2004;24:2102–2107. doi: 10.1161/01.ATV.0000144813.44650.75. [DOI] [PubMed] [Google Scholar]
- Kusano KF, Pola R, Murayama T, et al. Sonic hedgehog myocardial gene therapy: tissue repair through transient reconstitution of embryonic signaling. Nat Med. 2005;11:1197–1204. doi: 10.1038/nm1313. [DOI] [PubMed] [Google Scholar]
- Kwee L, Baldwin HS, Shen HM, et al. Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (VCAM-1) deficient mice. Development. 1995;121:489–503. doi: 10.1242/dev.121.2.489. [DOI] [PubMed] [Google Scholar]
- Landau C, Jacobs AK, Haudenschild CC. Intrapericardial basic fibroblast growth factor induces myocardial angiogenesis in a rabbit model of chronic ischemia. Am Heart J. 1995;129:924–931. doi: 10.1016/0002-8703(95)90113-2. [DOI] [PubMed] [Google Scholar]
- Lavine KJ, Yu K, White AC, et al. Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Dev Cell. 2005;8:85–95. doi: 10.1016/j.devcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Lavine KJ, White AC, Park C, et al. Fibroblast growth factor signals regulate a wave of hedgehog activation that is essential for coronary vascular development. Genes Dev. 2006;20:1651–1661. doi: 10.1101/gad.1411406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson ND, Vogel AM, Weinstein BM. Sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- Losordo DW, Vale PR, Hendel RC, et al. Phase 1/2 placebo-controlled, double-blind, dose-escalating trial of myocardial vascular endothelial growth factor 2 gene transfer by catheter delivery in patients with chronic myocardial ischemia. Circulation. 2002;105:2012–2018. doi: 10.1161/01.cir.0000015982.70785.b7. [DOI] [PubMed] [Google Scholar]
- Mikawa T, Fischman DA. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc Natl Acad Sci U S A. 1992;89:9504–9508. doi: 10.1073/pnas.89.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol. 1996;174:221–232. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- Moore AW, McInnes L, Kreidberg J, et al. YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development. 1999;126:1845–1857. doi: 10.1242/dev.126.9.1845. [DOI] [PubMed] [Google Scholar]
- Morabito CJ, Kattan J, Bristow J. Mechanisms of embryonic coronary artery development. Curr Opin Cardiol. 2002;17:235–241. [Google Scholar]
- Perez-Pomares JM, Carmona R, Gonzalez-Iriarte M, et al. Origin of coronary endothelial cells from epicardial mesothelium in avian embryos. Int J Dev Biol. 2002;46:1005–1013. [PubMed] [Google Scholar]
- Pola R, Ling LE, Silver M, et al. The morphogen sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7:706–711. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- Pola R, Ling LE, Aprahamian TR, et al. Postnatal recapitulation of embryonic hedgehog pathway in response to skeletal muscle ischemia. Circulation. 2003;108:479–485. doi: 10.1161/01.CIR.0000080338.60981.FA. [DOI] [PubMed] [Google Scholar]
- Rajanayagam MA, Shou M, Thirumurti V, et al. Intracoronary basic fibroblast growth factor enhances myocardial collateral perfusion in dogs. J Am Coll Cardiol. 2000;35:519–526. doi: 10.1016/s0735-1097(99)00550-1. [DOI] [PubMed] [Google Scholar]
- Reese DE, Mikawa T, Bader DM. Development of the coronary vessel system. Circ Res. 2002;91:761–768. doi: 10.1161/01.res.0000038961.53759.3c. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Scheinowitz M, Abramov D, Eldar M. The role of insulin-like and basic fibroblast growth factors on ischemic and infarcted myocardium: a mini review. Int J Cardiol. 1997;59:1–5. doi: 10.1016/s0167-5273(96)02902-6. [DOI] [PubMed] [Google Scholar]
- Seghezzi G, Patel S, Ren CJ, et al. Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis. J Cell Biol. 1998;141:1659–1673. doi: 10.1083/jcb.141.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Lu J, Zhang G, et al. Histone acetyltransferase p300 promotes the activation of human WT1 promoter and intronic enhancer. Arch Biochem Biophys. 2005;436:62–68. doi: 10.1016/j.abb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Shikama N, Lutz W, Kretzschmar R, et al. Essential function of p300 acetyltransferase activity in heart, lung and small intestine formation. Embo J. 2003;22:5175–5185. doi: 10.1093/emboj/cdg502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Annex BH, Laham RJ, et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation. 2002;105:788–793. doi: 10.1161/hc0802.104407. [DOI] [PubMed] [Google Scholar]
- Stuckmann I, Evans S, Lassar AB. Erythropoietin and retinoic acid, secreted from the epicardium, are required for cardiac myocyte proliferation. Dev Biol. 2003;255:334–349. doi: 10.1016/s0012-1606(02)00078-7. [DOI] [PubMed] [Google Scholar]
- Sucov HM, Dyson E, Gumeringer CL, et al. RXR alpha mutant mice establish a genetic basis for vitamin A signaling in heart morphogenesis. Genes Dev. 1994;8:1007–1018. doi: 10.1101/gad.8.9.1007. [DOI] [PubMed] [Google Scholar]
- Syed IS, Sanborn TA, Rosengart TK. Therapeutic angiogenesis: a biologic bypass. Cardiology. 2004;101:131–143. doi: 10.1159/000075994. [DOI] [PubMed] [Google Scholar]
- Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005;65:550–563. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Tomanek RJ, Holifield JS, Reiter RS, et al. Role of VEGF family members and receptors in coronary vessel formation. Dev Dyn. 2002;225:233–240. doi: 10.1002/dvdy.10158. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Yanagisawa-Miwa A, Nakamura F. Angiogenic therapy of acute myocardial infarction by intrapericardial injection of basic fibroblast growth factor and heparin sulfate: an experimental study. Am Heart J. 1995;130:1182–1188. doi: 10.1016/0002-8703(95)90140-x. [DOI] [PubMed] [Google Scholar]
- Visconti RP, Richardson CD, Sato TN. Orchestration of angiogenesis and arteriovenous contribution by angiopoietins and vascular endothelial growth factor (VEGF) Proc Natl Acad Sci U S A. 2002;99:8219–8224. doi: 10.1073/pnas.122109599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vokes SA, Yatskievych TA, Heimark RL, et al. Hedgehog signaling is essential for endothelial tube formation during vasculogenesis. Development. 2004;131:4371–4380. doi: 10.1242/dev.01304. [DOI] [PubMed] [Google Scholar]
- Vrancken Peeters MP, Gittenberger-de Groot AC, Mentink MM, Poelmann RE. Smooth muscle cells and fibroblasts of the coronary arteries derive from epithelial-mesenchymal transformation of the epicardium. Anat Embryol (Berl) 1999;199:367–378. doi: 10.1007/s004290050235. [DOI] [PubMed] [Google Scholar]
- Wada AM, Willet SG, Bader D. Coronary vessel development: a unique form of vasculogenesis. Arterioscler Thromb Vasc Biol. 2003;23:2138–2145. doi: 10.1161/01.ATV.0000098645.38676.CC. [DOI] [PubMed] [Google Scholar]
- Ward NL, Dumont DJ. The angiopoietins and Tie2/Tek: adding to the complexity of cardiovascular development. Semin Cell Dev Biol. 2002;13:19–27. doi: 10.1006/scdb.2001.0288. [DOI] [PubMed] [Google Scholar]
- Watt AJ, Battle MA, Li J, Duncan SA. GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proc Natl Acad Sci U S A. 2004;101:12573–12578. doi: 10.1073/pnas.0400752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]


