Abstract
Until recently, quantitative studies of walking have typically focused on properties of a typical or average stride, ignoring the stride-to-stride fluctuations and considering these fluctuations to be noise. Work over the past two decades has demonstrated, however, that the alleged noise actually conveys important information. The magnitude of the stride-to-stride fluctuations and their changes over time during a walk – gait dynamics – may be useful in understanding the physiology of gait, in quantifying age-related and pathologic alterations in the locomotor control system, and in augmenting objective measurement of mobility and functional status Indeed, alterations in gait dynamics may help to determine disease severity, medication utility, and fall risk, and to objectively document improvements in response to therapeutic interventions, above and beyond what can be gleaned from measures based on the average, typical stride. This review discusses support for the idea that gait dynamics has meaning and may be useful in providing insight into the neural control of locomtion and for enhancing functional assessment of aging, chronic disease, and their impact on mobility.
Keywords: gait, gait variability, fractals, aging, falls, review, Parkinson's disease, executive function
1. Introduction
Human gait is remarkable. The healthy locomotor system integrates input from the motor cortex, cerebellum, and the basal ganglia, as well as feedback from visual, vestibular and proprioceptive sensors to produce carefully controlled motor commands that result in coordinated muscle firings and limb movements. When everything is working properly, this multi-level neural control system produces a stable gait and a highly consistent walking pattern. In fact, the kinetics, kinematics, and muscular activity of gait appear to remain relatively constant from one stride to the next, even during unconstrained walking. Given that the upright body may be viewed as a highly unstable, inverted pendulum, achievement of this dynamic balance and forward progression with a regular and steady gait is a rather impressive feat.
Close examination, however, reveals complex fluctuations in the gait pattern, even under constant environmental conditions (Frenkel-Toledo et al., 2005b; Hausdorff, Peng, Ladin, Wei, & Goldberger, 1995; Jordan, Challis, & Newell, 2007b; Pailhous & Bonnard, 1992). In the past, these fluctuations were generally considered to be ‘noise’ and something to be ignored and filtered out of any analysis. Thus, quantitative studies of walking typically focused on properties of each participant’s average stride, ignoring the within-subject stride-to-stride fluctuations. Work over the past two decades has demonstrated that this alleged noise actually conveys important information. The magnitude of the stride-to-stride fluctuations and their changes over time during a walk – gait dynamics – may be useful in understanding the motor control of gait, in quantifying pathologic and age-related alterations in the locomotor control system, and in augmenting objective measurement of mobility and functional status Indeed, alterations in gait dynamics may help to determine disease severity, and medication utility, and to objectively document improvements in response to therapeutic interventions, above and beyond what can be gleaned from measures based on the average, typical stride. To help frame this paradigm shift in how the stride-to-stride fluctuations are viewed and to provide a perspective for future investigations in this area, we here review some of the original studies that illustrate how gait dynamics has meaning as well as more recent investigations that demonstrate how this approach may be useful for providing insight into the neural control of locomotion and for enhancing functional assessment of aging, chronic disease and their impact on mobility. Before beginning, it is important to note that because of space constraints, this review considers only a subset of the reports on this topic. For complementary perspectives, the reader is referred to other pertinent reviews (Chau, 2001a, 2001b; Chau, Young, & Redekop, 2005; Hausdorff, 2005) as well as parallel studies by others (Buzzi, Stergiou, Kurz, Hageman, & Heidel, 2003; Costa, Peng, Goldberger, & Hausdorff, 2003; Dingwell & Cavanagh, 2001; Dingwell, Cusumano, Cavanagh, & Sternad, 2001; Dingwell, Gu Kang, & Marin, 2006; England & Granata, 2007; Georgoulis, Moraiti, Ristanis, & Stergiou, 2006; Granata & England, 2006; Kurz et al., 2003; Kurz & Stergiou, 2005; Stergiou, Harbourne, & Cavanaugh, 2006).
1.1 Early beginnings: A quantitative marker of gait instability
Our investigations of gait dynamics and instability began somewhat serendipitously. In 1991, we developed a new technique for simultaneous monitoring of the electrocardiogram (ECG) and walking stride rate in order to study the relationship between ECG changes (e.g., arrhythmias, ischemia, mean heart rate, and heart rate variability) and physical activity (e.g., supine, standing, walking, and walking rate) on a beat-by-beat and stride-by-stride basis (Hausdorff, Forman, Pilgrim, Rigney, & Wei 1992). Many investigations had shown that measures of heart rate dynamics and heart rate variability, an indicator of cardiovascular health, are sensitive to alterations in the autonomic nervous system's regulation of heart rate (Goldberger et al., 2002; Ho et al., 1997; Lipsitz, Mietus, Moody, & Goldberger, 1990; Peng et al., 1993). Indeed, one could suggest that the study of heart rate dynamics has turned into a discipline of its own, with literally thousands of research article s on this topic spanning those that have examined the basis for healthy heart rate dynamics to those that have examined clinical utility of measures based on heart rate dynamics. However, little was known about how gait affected these heart rate dynamics. Among other things, initial use of this technique revealed an inverse relationship between heart rate variability and stride rate (cadence) variability (r = −.73): healthier cardiovascular dynamics were associated with healthier gait dynamics. These findings suggested and later studies confirmed (Hausdorff, Herman, Baltadjieva, Gurevich, & Giladi, 2003; Hausdorff et al., 1994) that, at least in certain populations, gait instability is related to, and perhaps a function of, cardiovascular health and also demonstrate that stride variability reflects more than just musculo-skeletal mechanics. More generally, this initial work suggested that just like there is much to be gained by investigating heart rate dynamics, above and beyond the study of the average heart rate, similar investigations of gait dynamics may provide insight into locomotor control and may also have clinical applications.
1.2 Notes on nomenclature and data collection methods
In this review, the term ‘gait dynamics’ is used in a broad sense. It is meant to contrast to more traditional measures of walking that have focused on average values and as a general term, much the way ‘heart rate dynamics’ encompasses measures of heart rate variability as well as time series analysis indices that capture how the heart rate changes over time, independent of the variability. Thus, in the present context, ‘gait dynamics’ includes both measures of the stride-to-stride variability as well as other features of the stride-to-stride changes in walking.
Just like there are many approaches to the study of gait, so too there are many ways of measuring the within-subject stride-to-stride changes in gait. These include accelerometers, gyroscopes, goniometers, and video-based marker systems. Each of these approaches has advantages and disadvantages. When studying within-subject stride-to-stride changes, one important consideration is that the methods used must be able to record a relatively large number of strides. Whereas a few strides are sufficient to evaluate one's average or typical stride characteristics, investigation of stride-to-stride changes requires collection of many more strides (just how many is subject to some debate). The studies described below generally used force sensitive insoles placed inside the participant's shoes. One advantage of this approach is that the recording system is completely ambulatory; study participants can be assessed in clinical settings and ‘real-life’ environments without having to make their way to ‘gait labs’ or other sites with special equipment.
In general, in the studies described below, pressure-sensitive footswitches were used. Many used one footswitch under the heel and the other under the forefoot and the toes, combining the data from both sensors before the recording (but some investigations used 4, 8 and 100 sensors, with similar results). These inserts produce a measure of the force applied to the ground during ambulation. A small, lightweight (5.5 ✕ 2 ✕ 9 cm; 0.1 kg) recorder was worn on the ankle cuff of each foot and held in place using an ankle wallet. An on board A/D converter (12 bit) sampled the output of the ‘footswitches’ (actually an analog signal related to the force) at 300 Hz and stored the digitized force record. Subsequently, the digitized data were transferred to a computer workstation for analysis using software that extracts the initial contact time of each stride (Hausdorff, Ladin, & Wei, 1995). From the force signal, the stride time or duration of the gait cycle (time from initial contact of one foot to subsequent contact of the same foot) and stance and swing times were determined for each stride applying an algorithm that accurately locates initial and end contact times (and hence the stride time) by automatically finding large increases and changes in the slope of the force profile on a stride by stride basis (Hausdorff, Ladin et al., 1995).
To focus on the assessment of the dynamics of continuous, ‘normal’ walking and each participant’s ‘intrinsic’ dynamics and to insure that the analysis was not influenced by atypical strides, a median filter was applied to each participant’s time series to remove data points that were three standard deviations greater than or less than the median value (Hausdorff, Edelberg, Mitchell, Goldberger, & Wei, 1997; Hausdorff, Rios, & Edelberg, 2001). There is typically general agreement between the values obtained before and after application of the median filter, however, occasionally important differences occur that should be considered. Subsequently, the generated time series of stride time (and/or swing time or other parameters) can then become the input to algorithms that quantify the dynamics. For example, stride time variability, the magnitude of the stride-to-stride fluctuations in the gait cycle duration, is calculated by determining the standard deviation (SD) or the coefficient of variation (CV) of each subject's stride time time series (Maki, 1997; Hausdorff, Edelberg et al., 1997; Hausdorff, Rios et al., 2001). Similar methods can be used to study the variability of other measures of gait timing (e.g., swing time).
2. Fractal-like nature of gait: From ECG to DFA
Having identified a possible role of pathology in walking variability, we followed the lead of studies of the beat-to-beat fluctuations in heart rate to examine the fractal-like nature of the stride-to-stride fluctuations in gait timing. Studies highlighting key findings are described here.
2.1 First evidence of fractal-like fluctuations in gait
We first studied the stride-to-stride fluctuations present in healthy adults in order to gain insight into normal locomotor function and its control mechanisms and thereby better appreciate pathological deviations (Hausdorff, Ashkenazy et al., 2001; Hausdorff et al., 1996). Variability, the magnitude of the stride-to-stride fluctuations, and stride dynamics, how the stride fluctuates with time, independent of the magnitude, may rely upon different control mechanisms. Analysis of stride dynamics may therefore provide information that is independent of (and orthogonal to) the average stride. Many gait parameters fluctuate from stride-to-stride; we initially considered stride interval or gait cycle fluctuations (e.g., see Fig. 1) for several reasons including: 1) The stride interval can be viewed as one ‘final’ output of the multi-dimensional neuromuscular control system, an integrated effect of afferent and efferent components, similar to the way that the heart rate is the ‘output’ of the autonomic nervous system. 2) The time between heelstrikes, i.e., the stride interval or stride time, may function as a gait ‘clock’ and reflect the internal rhythmicity of the locomtor system. Thus, examination of the fluctuations in the stride interval could theoretically provide insight into the organization, regulation, interactions and stability of the entire locomotor system.
Fig. 1.
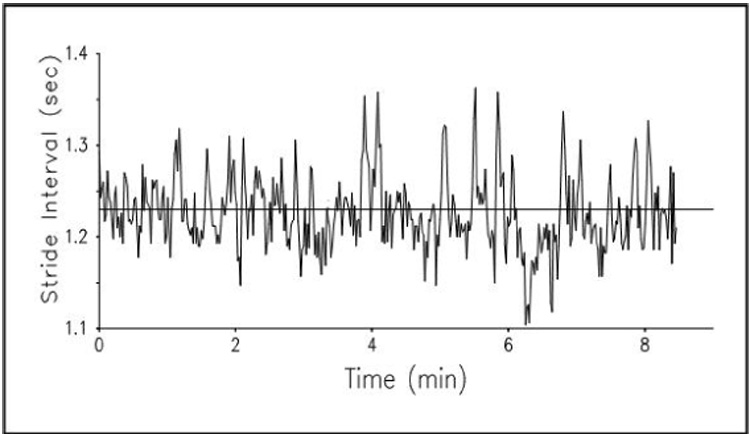
Example of the stride-to-stride fluctuations in the stride interval (i.e., the stride time or gait cycle duration). The stride time fluctuates around its mean (the horizontal line) in an apparently noisy, random fashion.
A priori, one likely explanation for stride-to-stride variations normally observed in the stride interval of healthy adults is that these fluctuations simply represent uncorrelated (white) noise superimposed on a basically regular process (i.e., the fluctuations are ‘random noise’). This is the implicit view of many investigations that examine only the average or typical stride. Alternatively, one might suggest that there are short-range correlations in the stride interval such that the current value is influenced by only the most recent stride intervals, but over the long term, the fluctuations are random. A third, less intuitive possibility is that the fluctuations in the stride interval exhibit long-range, fractal-like correlations, as seen in a class of scale -free phenomena including heart rate beat-to-beat fluctuations (Goldberger et al., 2002; Peng et al., 1993; Peng et al., 1998; Peng et al., 1994; Peng et al., 2002). In this case, the stride interval at any instant would ‘depend’ (in a statistical mechanics sense) on the interval at relatively remote times, and this dependence would decay in a scale-free (fractal-like), power-law fashion. We used spectral analysis and a modified random walk analysis, termed detrended fluctuation analysis (DFA) (Goldberger et al., 2002; Hausdorff, Peng et al., 1995; Hausdorff et al., 1996) to quantitatively characterize the stride interval fluctuations of healthy young men (aged: 20–30 years) as they walked continuously on level ground around a circular path.
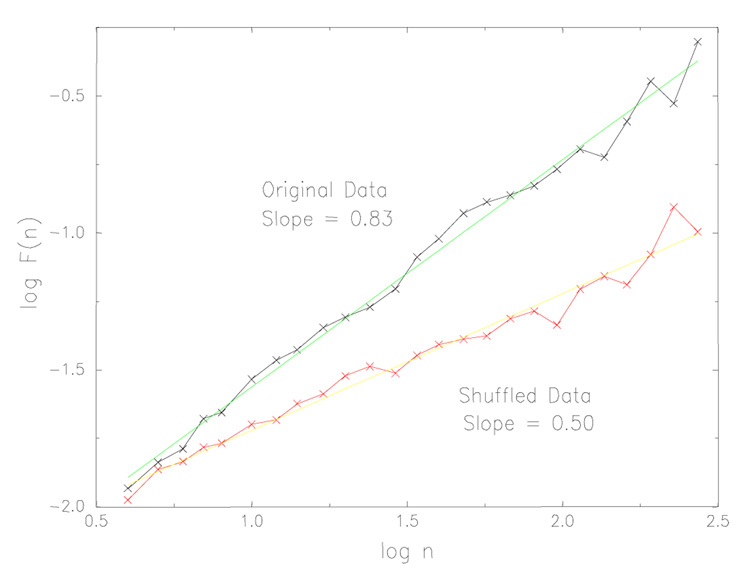
DFA is a modified random-walk analysis that makes use of the fact that a long-range (power-law) correlated time series can be mapped to a self-similar (fractal) process by simple integration. Briefly, each integrated time series is said to be self-similar if the fluctuations at different observation windows, F(n), scale as a power-law with the window size n (i.e , the number of strides in a window of observation or the time scale). Typically, F(n) will increase with window size n. A linear relationship on a double log graph indicates that F(n) ~ na, where the scaling index a (also called the self-similarity parameter) is determined by calculating the slope of the line relating log F(n) to log n. For a process where the value at one step is completely uncorrelated with any previous values, i.e., white noise, a = .5. In contrast, long-range, persistent and fractal-like correlations are present if .5 < a ≤ 1.0. An a < .5 signifies anti-persistent correlations (a large stride interval is more likely to be followed by a small one and vice versa over different time scales). Estimation of a may be applied over different window sizes, depending on the length of a given time series, to determine relatively short- or long-term scaling properties. In principle, the estimation of a will depend on the fitting range: n1 ≤ n ≤ n2. It is preferable to have n2 > > n1. However, due to the finite size of data, a large n2 may lead to bigger error in a. These considerations need to be accounted for when comparing values across different studies (that might use different fitting ranges) and explain some of the small differences among different reports.
Applying DFA to gait time series, we discovered the presence of long-range, power-law correlations (see, for example, Fig. 2) (Hausdorff, Peng et al., 1995). The fractal scaling index, a values were around .75. That is, somewhat surprisingly, stride interval fluctuations at one time scale were statistically similar to those at multiple other time scales. From a neurophysiological control viewpoint, this behavior is of interest because it signifies the presence of long-term, non-trivial ‘dependence’ or ‘memory’ in the locomotor control system. Fluctuations in the stride interval are, on average, apparently related to variations in the stride interval hundreds of strides earlier in a scale-invariant, fractal manner.
Fig. 2.
Detrended fluctuation analysis (DFA) applied to time series of stride time from a healthy young adult. The slope of the line relating the size of the fluctuations to the window size, n, is defined as the fractal scaling exponent, a. For this participant, the slope is .83, indicating long-range, fractal correlations in the original data. When the data is randomly re-ordered (shuffled), the slope becomes .5, reflecting white noise and an absence of fractal scaling. Adapted from Hausdorff, Peng et al. (1995).
2.2 Robust nature of the fractal fluctuations
To study the stability and extent of these unexpected long-range correlations, we asked another group of healthy young (ages 18–29 years) men to walk for one hour at their usual, slow and fast paces around an outdoor track (Hausdorff et al., 1996). This data (as well as other data sets described here) and further details can be found at www.physionet.org. To our surprise, the stride interval exhibited long-range correlations with power-law decay for up to thousands of strides at all three walking speeds in all participants (see, for example, Fig. 3). Subsequent studies also demonstrated that the fractal-like fluctuations are present even when healthy participants run or walk on a treadmill (Frenkel-Toledo et al., 2005b; Jordan, Challis, & Newell, 2006, 2007a). Thus, the fractal dynamics of the stride interval are normally quite robust, they are apparently intrinsic to the locomotor system, and they exist at a wide range of gait speeds.
Fig. 3.
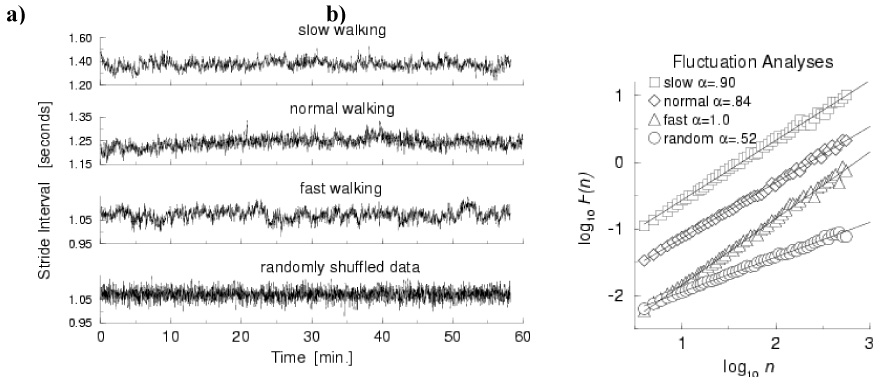
Effects of gait speed on long-range correlations and fractal dynamics. A) Example of time series of stride time during 1 hour of walking in a healthy young adult at slow, normal and fast walking rates, and below, after the fast data set is randomly shuffled. B) While there are subtle effects of gait speed, DFA shows that there is fractal scaling at all 3 gait speed. Adapted from (Hausdorff et al., 1996)
2.3 Changes in fractal gait dynamics with aging
Fluctuations in the duration of the gait cycle (the stride interval) display fractal dynamics and long-range correlations in healthy, young adults. We hypothesized that gait dynamics may be altered in younger children as the neuromuscular system matures (Hausdorff, Zemany, Peng, & Goldberger, 1999). Indeed, in very young children, immature control of posture and gait results in an obviously unsteady locomotion. By about three years of age, gait appears to be relatively mature; however, it is unknown whether the dynamics of walking change beyond this age. To test this hypothesis, we measured the gait cycle duration on a stride-by-stride basis in fifty healthy children (25 girls) that were 3 to 14 years old. Measures of stride-to-stride variability were significantly larger both in the 3 and 4 year olds compared to the 6 and 7 year olds and in the 6 and 7 year olds compared to the 11 to 14 year olds. Measures of the temporal organization of gait also revealed significant age-dependent changes. The fractal scaling index, a, was similar in the two youngest age groups and tended to decrease in the oldest children (a = .93 ± .04, .93 ± .03, .88 ± .04, in the 3 and 4 years olds, 6 and 7 year olds, and 11 to 14 year olds, respectively.) When this analysis was performed on the first difference of the time series (i.e., after removing any large trends), the effect of age became more pronounced and statistically significant (p < .01 and p < .05 comparing the 11 to 14 year olds to the 6 and 7 year olds and the 3 and 4 years olds, respectively). The effects of age persisted even after adjusting for height. In terms of neurophysiology, these findings indicate that mature stride dynamics may not be completely developed even in healthy 7 year old children whose gait appears perfectly adult-like and that different aspects of stride dynamics mature at different ages (Hausdorff et al., 1999). In addition, these results highlight the sensitivity of measures of gait dynamics to small changes in underlying function.
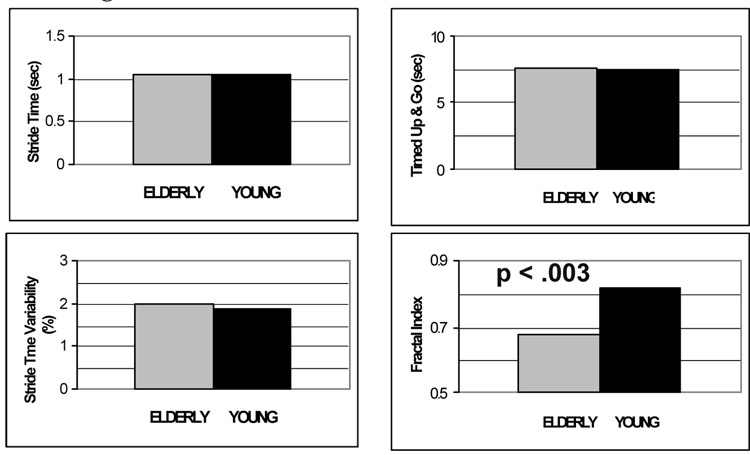
To gain further insight into the fractal properties of gait dynamics, we investigated the other side of the age spectrum (Hausdorff, Mitchell et al., 1997). We hypothesized that stride dynamics would be affected by subtle age-associated changes in physiology in older adults. To test this hypothesis, we compared the stride dynamics of healthy elderly participants, free of underlying disease, and young adult controls. The fractal scaling index a was significantly lower in the older adults compared to young adults, becoming much closer to .5, a value that indicates white noise, randomness and a lack of internal, long-range structure. Like the gait of children, these findings demonstrate that stride dynamics are altered in healthy older adults. However, unlike in children, in older adults, the stride-to-stride fluctuations become more random (i.e., less correlated) in healthy older adults compared to healthy young adults. Interestingly, in these healthy older adults, other measures of lower extremity function (e.g., Timed Up and Go times and the average stride time) were almost identical to those observed in young adults (see Fig. 4). Apparently, these measures of gait dynamics are sensitive to subtle changes in physiology that do not affect other aspects of gait.
Fig. 4.
Effects of healthy aging on gait: sensitivity of fractal dynamics. The average stride time, stride time variability, Timed Up and Go performance are similar in this group of healthy older adults and healthy young adults, while the fractal scaling index is reduced with aging. Adapted from Hausdorff, Mitchell et al. (1997).
2.4 Breakdown of the fractal-like structure
To further investigate the origin of long-range, fractal-like correlations, we examined the young adults who initially walked for one hour at three speeds under three additional conditions. Study participants were asked to walk for 0.5 h in time to a metronome that was set to each participant’s mean stride interval computed from each of the three previous 1-h walks (0.5 h was chosen instead of 1 h to minimize each participant's testing time while providing enough data for reliable analysis of the correlation properties). The purpose of these tests was to characterize the biological ‘clock’ that controls locomotion. A breakdown of long-range correlations during metronomic walking might suggest that some supraspinal locomotor clock is essential in generating this scale -free behavior or, at least, that centrally mediated entrainment of the clock can ‘overcome’ long-range correlations generated peripherally. Alternatively, persistence of the long-range correlations during metronomic walking might imply that the scaling property results either from neural circuits at or below the level of the spinal cord or from peripheral feedback influences.
Comparisons of the mean stride interval during metronomic walking and during unconstrained walking showed that participants were able to walk in time to the metronome. For example, the difference between the mean stride interval during free walking at the normal rate and metronomic walking was only 0.0 ± 1.0%. Paired t-tests showed no significant differences at all three walking rates (slow, normal, fast) for both mean stride interval and average gait speed. There was a statistically significant but small (~4 ms) decrease in the stride interval SD only during normal walking. Because fluctuation magnitude does not affect correlation properties, the only consequential difference between free walking and metronomic walking was the ‘clock’ regulating the timing of footfall.
Visual inspection of the stride interval time series already reveals significant differences in the fractal-nature of the stride-to-stride fluctuations during metronomic walking. Whereas there was a noticeable contrast between randomly shuffled data during a participant's free-walking time series (see Fig. 3), there was little visually appreciable difference between the randomly shuffled time series and the original time series during metronomic walking, suggesting break down of the long-range structure during metronomic walking. Quantitative analysis with DFA and spectral techniques confirmed that the long-range correlations break down and vanish during metronomic walking, i.e., a ˜ .5. Metronomic and unconstrained walking use the same actuators and effectors and feedback systems. Thus, the absence of a fractal scaling during metronomic walking supports the idea that surpraspinal networks contribute to the long-term, fractal-like fluctuations. Consistent with this idea, a recent study in patients with peripheral neuropathy reported the presence of the fractal fluctuations in gait timing, despite the changes in peripheral feedback (e.g., proprioception) and an increased stride-to-stride variability (Gates & Dingwell, 2006; Richardson, Thies, DeMott, & Ashton-Miller, 2004).
While peripheral neuropathy does not alter the fractal-scaling of gait, Huntington's disease does. Huntington’s disease is an autosomal-dominant neurodegenerative disease of the central nervous system. Most of the pathological changes are seen in the basal ganglia, with a loss of neural projection in the striatum (caudate nucleus and putamen). Huntington’s disease generally affects people in their 30s and 40s who are typically free from concomitant disease and age-related physiological changes. With impairment limited primarily to the central nervous system, Huntington’s disease offers a contrast to aging for the study of the conditions necessary for stride-interval correlations.
We hypothesized that the locomotor system’s ability to produce stride-interval correlations would be diminished in patients with Huntington’s disease. To test this hypothesis, we compared the stride-interval dynamics of patients with Huntington’s disease to that of healthy controls. The scaling component a was .60 ± .24 for the patients with Huntington’s disease and .88 ± .17 in the control participants (p < .005). Further, among the patients with Huntington’s disease, a was inversely correlated with disease severity (r = .78, p < .0005). The scaling exponent was significantly lower, indicative of more random stride-interval fluctuations, with more advanced disease.
Given the reduced stride-interval correlations in patients with Huntington’s disease, especially in the most impaired participants, one can infer that that the areas of the cerebrum that are affected by Huntington’s disease likely play an important role in generating stride-interval correlations. While some pathological changes have been found in the cortex, the primary pathology is in the basal ganglia. Perhaps, the striatal pathology that leads to a decrease in fine motor control in Huntington’s disease also impairs the ‘long-term dependence’ and fine control necessary for stride-interval correlations.
2.5 Fractal-scaling and additional measures of gait dynamics
Until now, we have primarily described two ways of quantifying the stride-to-stride fluctuations in gait: 1) calculating the magnitude of the variability (e.g., using the SD or CV) and 2) the fractal index, a measure of that captures the ordering of these fluctuations. These fluctuations can, however, be analyzed in many other ways. Application of other analysis methods allows one to capture other features of gait time series and generate an array or matrix of outcome measures, each evaluating different aspects of the dynamics. Studies in neurodegenerative disease support the idea that such a matrix of dynamical measures may be helpful in more fully characterizing different pathologic states (Hausdorff, Cudkowicz, Firtion, Wei, & Goldberger, 1998; Hausdorff et al., 2000 Hausdorff, Mitchell et al., 1997).
For example, we find that stride variability, the fractal index, a non-stationarity index and a measure based on the autocorrelation function provide information about the regulation of locomotor function that is distinct from each other as well as from conventional measures of walking, namely average gait speed and average stride time, and that these measures may be altered even before changes in average gait speed are evident. Patients with mild Lou Gehrig’s disease (amyotrophic lateral sclerosis, ALS) had an apparently normal gait pattern, but significant changes in a non-stationary index of the stride-to-stride fluctuations (p < .04), compared to healthy controls. Perhaps equally important, among participants with ALS, Parkinson's disease, and Huntington's disease, certain changes in gait dynamics appear to be common to neurological impairment while others are apparently disease specific (Hausdorff et al., 2000). For example, while an increased stride time variability appears to be common in all three groups, to varying degrees, changes in the fractal properties and other dynamic properties appear to be more sensitive to specific diseases. In support of this idea, a recent study demonstrated that a pair of measures of gait dynamics that characterize the stride-to-stride fluctuations can be used to distinguish among different neurodegenerative diseases (Carletti, Fanelli, & Guarino, 2006). In other words, by generating a matrix of parameters that reflect different features of gait dynamics, we may be able to improve our ability to evaluate gait dynamics and instability and its response to different therapeutic interventions (Hausdorff et al., 2000).
2.6 Modeling the stride-to-stride fractal fluctuations
A complementary approach to understanding the origin of the long-range correlations is to study models of the neural generation of rhythmic movement. Central pattern generators, groups of neurons located in the nervous system of animals and possibly in humans as well, are believed to be responsible for many rhythmic movements including locomotion. Although central pattern generator (CPG) models composed of simple oscillators do not produce long-range correlations, some CPG models or their derivatives may. For example, coupled nonlinear oscillator networks, under certain conditions, may produce a fractal-like output in phase space. (Interestingly, this can occur with a deterministic network without the addition of any external stochastic elements.) Even though these phase-space attributes do not necessarily guarantee that long-range correlations are present (these time series may have a white power spectrum), they may occur under some circumstances. An alternative possibility that would also account for the unexplained subtle changes during slow and fast walking is that the long-range correlations are the output of a correlated CPG model (Ashkenazy, Hausdorff, Ivanov, & Stanley, 2002; Hausdorff, Peng et al., 1995). In this stochastic model, transitions between different pattern generator frequencies can occur with different probabilities. If the arrangement of neighboring modes, i.e., stride frequencies that are most likely to switch from one to the other, are randomly predetermined, then the theoretical value of a is .75. However, if some constraints are imposed on the arrangement of the CPG frequencies, then a can be > .75 and give rise to the experimentally observed power-law scaling.
A number of approaches have been taken to model the stride-to-stride fluctuations in walking and the alterations in the fractal-nature of these fluctuations as well as other components of the other aspects of gait dynamics that change with aging and disease. These include deterministic models, stochastic models, as well as combinations of the two (Ashkenazy et al., 2002; Hausdorff, Ashkenazy et al., 2001; Hausdorff, Peng et al., 1995; Kurz & Stergiou, 2005; West & Latka, 2005; West & Scafetta, 2003). Some of these models are able to reproduce and explain the fractal-like fluctuations and many of the observed alterations with aging and disease have been captured as well. We developed a stochastic model of gait rhythm dynamics, based on transitions between different ‘neural centers’, that reproduces distinctive statistical properties of normal human walking (Ashkenazy et al., 2002). By tuning one model parameter, the transition (hopping) range, the model can describe alterations in gait dynamics from childhood to adulthood, including a decrease in the correlation and volatility exponents with maturation as well as an increase in the magnitude of the stride-to-stride changes and a decrease in the fractal exponent in older adults. The model also generates time series with multifractal spectra whose broadness depends only on this parameter. Moreover, the volatility exponent increases monotonically as a function of the width of the multifractal spectrum, suggesting the possibility of a change in multifractality with maturation. Furthermore, removal of ‘neural centers’ such like those that might be seen with aging or disease also mimics many features of the observed gait pattern among certain elderly fallers.
These and other models are useful for gaining insight into the mechanisms that may contribute to walking dynamics and its alterations. The models support the idea that neural mechanisms are critical to the long-range correlations in gait and that much of the diversity of the observed alterations in gait dynamics may be explained by changes in neural mechanisms, irrespective of any changes in mechanics or peripheral function. Still, to generate a deeper understanding of underlying neural control mechanisms, there is a need for models that more closely mimic the underlying physiology and the changes with aging and disease.
We have seen that the fractal nature of stride-to-stride fluctuations in gait timing are present in healthy adults, that they exist in a range of gait speeds, that they change in subtle ways with aging (at both ends of the spectrum) and, in addition, that they break down during metronomic walking and in patients with impaired basal ganglia function, especially as the disease progresses. This property of gait is apparently a robust marker of mature, healthy central nervous system (CNS) function, yet sensitive to alterations with aging and certain CNS diseases.
3. Fall risk and gait dynamics
Falls are a major health problem with significant social and economic ramifications (AGS Guidelines, 2001; Camicioli, Howieson, Lehman, & Kaye, 1997; Campbell et al., 1990; Condron & Hill, 2002; Kannus et al., 1999; Tinetti, 1994; Tinetti, Doucette, Claus, & Marottoli, 1995; 2006). Falls can lead to serious injuries like hip fractures and hospitalization (Kannus et al., 1999, 2006; Tinetti et al., 1988). Even when the immediate result is less dramatic, falls can have an important impact, bringing about self-imposed mobility restrictions, fear of falling, and dependency (Aizen, 2001; Fuller, 2000; Tinetti, Speechley, & Ginter, 1988). Falls are also an independent cause of institutionalization and mortality in older adults (Aizen, 2001; Fuller, 2000; Tinetti et al., 1988). During the past quarter century, much effort has been devoted to determining the risk factors for falls in ‘idiopathic’ elderly fallers and to identifying sensitive markers of fall risk. Here we describe several studies which support the idea measures of gait dynamics may be useful for assessing fall risk.
3.1 Quantifying fall risk and gait instability
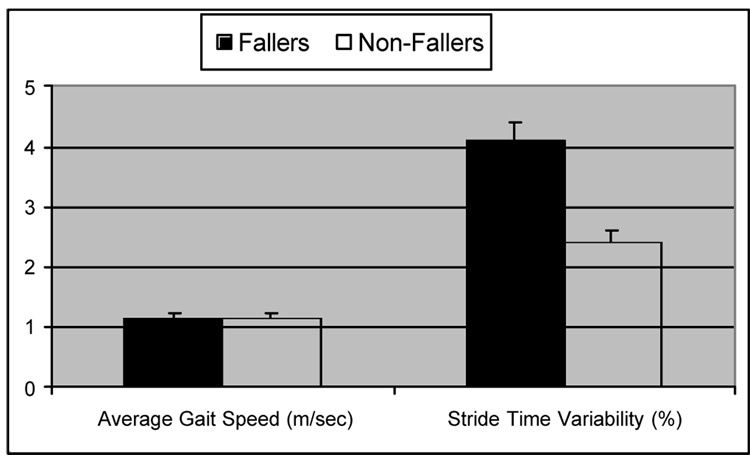
Although many gait parameters might appear to be useful for distinguishing between fallers and non-fallers, studies suggest that this is not actually the case; rather, features often associated with fallers more closely reflect fear of falling and a cautious gait (Feltner, MacRae, & McNitt-Gray, 1994; Hausdorff, Edelberg et al., 1997; Hausdorff, Rios et al., 2001; Maki, 1997). Building on the suggestions of Gabell and Nayak (1984), we hypothesized that quantitative measures of gait unsteadiness, as reflected in the temporal parameters of gait, might be increased in elderly fallers. To test this hypothesis, we performed a cross-sectional study to evaluate the variability of the temporal parameters of gait in a group of community living elderly fallers to groups of community living elderly non-fallers and young adult control participants (Hausdorff, Edelberg et al., 1997). All participants walked independently and participants had no overt neurological impairments and were relatively free from co-morbidities. As summarized in Fig. 5, stride time variability was significantly larger in the fallers compared to both the young adult and elderly participants (p < .0001). Similar results were observed for the variability of swing time and if the first or second half of the walk was examined. Thus, these findings indicate a marked increase in the stride-to-stride changes in the gait of elderly fallers, not only in the period of one stride cycle, but also in the sub-phases within each cycle. In contrast, gait speed was similar in the fallers and non-fallers. Although both elderly groups had similar walking speed and muscle strength, the elderly fallers had significant increases in stride variance and reduced long-range, fractal dependence. These findings show the potential clinical utility of measures based on gait dynamics and highlight the ability of these measures to provide quantitative assessment of gait instability and fall risk that may be more reflective of fall risk than traditional measures.
Fig. 5.
Among community-living older adults, gait speed was similar in fallers and non-fallers, while measures of stride variability were significantly increased in fallers. Adapted from Hausdorff, Edelberg et al. (1997).
To better appreciate this relationship between fall risk and gait variability, picture for a moment the drunken sailor. Each step is very different to the previous one, as he walks with an unsteady, stumbling gait, challenging the dynamic control of balance so that a fall to the ground becomes likely. Similarly, perhaps, relatively increased stride-to-stride variability may reflect an unsteady gait that predisposes to falls.
3.2 Gait instability prospectively identifies elderly fallers
To be useful as a clinical tool, at least in the assessment of falls, it is helpful not only to distinguish between those who have and have not fallen, but it also important to be able to use measures of gait dynamics for early identification of persons at risk of falls and to predict who is most likely to fall. We tested the hypothesis that in community-living older adults, specifically, patients attending an outpatient geriatric assessment clinic, altered gait dynamics predict future fallers (Hausdorff, Rios et al., 2001). Older adults were assessed at baseline and then followed for 12 months, monitoring fall status weekly. During the 12 month follow up period, 39% of the participants reported at least one fall. At baseline, fallers and non-fallers were similar in many ways. The two groups were not different with respect to age, gender, body-mass-index, Charlson Comorbidity Index, the use of medications, depressive symptoms, level of education, ability to perform activities of daily living (ADL's and IADL's), average gait speed, balance, and lower extremity function (e.g., Timed Up and Go, Performance Oriented Mobility).
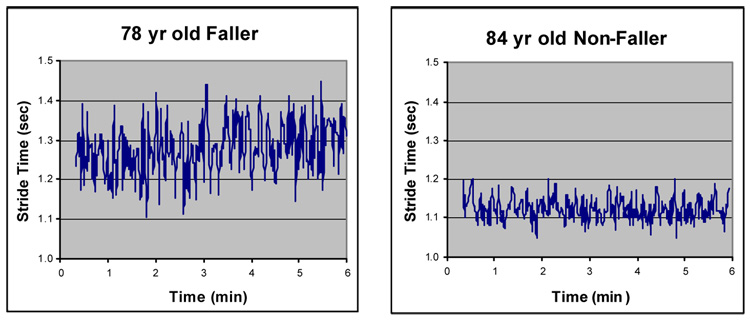
In contrast, at baseline, gait instability measures were significantly increased in those who subsequently fell compared to those who did not (e.g., see Fig. 6). Moreover, baseline measures of gait instability were also predictive of future falls (Table 1). Survival analysis also showed that participants were significantly more likely to fall sooner if gait instability was increased at baseline. These results indicate that measures of gait dynamics may be more sensitive than other measures of gait that are based on the average or typical stride pattern and that may provide a useful index of gait instability and fall risk in older adults.
Fig. 6.
Stride-to-stride fluctuations in the stride, as measured at baseline, were much larger in this participant who experienced a fall during the 12 month follow-up period, as compared to the non-fallers. Group results were similar. Adapted from Hausdorff, Rios et al. (2001).
Table 1.
Gait dynamics predict falls in older adults
| Odds Ratio | p-value | |
|---|---|---|
| Stride Time Variability | 5.3 | .04 |
| Swing Time Variability | 2.2 | .02 |
| Inconsistency of the Variance | 2.1 | .02 |
Adapted from Hausdorff, Rios et al. (2001c).
3.3 Falls in older adults with higher level gait disturbance (HLGD)
We have seen that gait variability is increased in elderly fallers compared to elderly non-fallers. Here we illustrate that the fractal property of the stride fluctuations also has utility for identifying fall risk, even among specific subgroups. Among many older adults with complaints of impaired gait, no obvious cause is identifiable, even after thorough examination. This disorder has been termed a ‘higher level gait disturbance’ (Nutt, Marsden, & Thompson, 1993) or cautious gait. In a study of 24 older adults (72 to 88 years of age; mean: 78) with a HLGD and age and sex matched controls (n = 28), we found that stride variability was significantly larger (p < .0001) in the participants with HLGD (55.6 ± 29.4 ms) compared to controls (24.1 ± 7.3 ms) (Giladi, Herman, Reider-Groswasser, Gurevich, & Hausdorff, 2005; Herman, Giladi, Gurevich, & Hausdorff, 2005). Participants with HLGD also more frequently showed extrapyramidal and frontal release signs compared to controls, but there was no difference in cerebellar or pyramidal function.
Among the patients with HLGD, about 50% reported falling while the rest of the group did not. Fear of falling, gait speed, the average stride time, gait variability and many other measures were similar in the two subgroups: those participants who reported falls (or multiple falls) and those who did not. However, the fractal scaling index of gait was successful in discriminating between fallers from non-fallers. In contrast to what was seen in the general elderly population, among patients with a HLGD, variability does not distinguish fallers from non-fallers, - it is increased all patients with a HLGD - while the fractal scaling index reflects changes in neuro-control that are related to fall risk. These findings further illustrate how evaluation of multiple measures of stride-to-stride fluctuations may improve discriminatory power and provide additional insight. The results also suggest that gait variability is markedly increased among older adults with HLGD (perhaps that explains the fear). Physical factors apparently do not to contribute to the level of instability, but neuro-psychological features, especially fear of falling, appear to be closely related to the degree of stride-to-stride variability in this population.
4. Parkinson’s disease
Gait dynamics have been examined in a variety of populations and settings. To more fully appreciate its potential, in this section, we describe by way of illustration how the study of gait dynamics has been applied to Parkinson's disease (PD) and, in addition, how such studies have provide a better understanding of locomotor control more generally.
PD is a progressive neurodegenerative disease due in part to loss of dopaminergic neurons in the basal ganglia. Impaired locomotion has been considered to be one of the cardinal symptoms of PD, along with rest tremor, bradykinesia, rigidity and postural instability. About 1% of the population over the age of 60 suffers from PD and about 20% of those over the age of 80 have parkinsonism associated gait disturbances. Because mobility impinges on quality of life and functional independence, it is not surprising that gait disturbances in PD often lead to wheel chair use and nursing home admission. Much is known about the gait changes in PD. The gait of patients with PD is typically marked by slowness, reduced stride length, reduced gait speed, small shuffling steps, flexed (stooped) posture, and reduced arm swing (Ebersbach et al., 1999; Morris, Huxham, McGinley, & Iansek, 2001; Morris, Iansek, Matyas, & Summers, 1994, 1996). Some have suggested that bradykinetic manifestations of the disease cause a reduced stride length and gait speed that is key to the gait changes commonly observed in PD (Morris et al., 1994, 1996). A reduced average stride length plays an important role in the gait disturbance of PD, however, another critical feature characteristic of PD is a loss of consistency and a decline in the ability to produce a steady gait rhythm. Patients with PD walk with increased stride-to-stride variability and have a decreased fractal scaling index (Blin, Ferrandez, & Serratrice, 1990; Frenkel-Toledo et al., 2005b; Hausdorff et al., 1998; Hausdorff, Peng et al., 1995; Hausdorff et al., 2000). Increased stride-to-stride variability, both in the stride length and stride time, has been observed in advanced PD (Blin et al., 1990; Hausdorff et al., 1998; Stolze et al., 2001) as well as in patients early in the disease process who have not even begun to take anti-parkinsonian medications (Baltadjieva, Giladi, Gruendlinger, Peretz, & Hausdorff, 2006; Hausdorff, Peng et al., 1995). In fact, in these patients with de novo PD, it has been shown that the increased stride-to-stride variability apparently is independent of a reduced stride length and apparently does not reflect inconsistent motor unit recruitment or muscle force output. Instead, this finding reflects alterations in rhythm generation and motor programming that are manifest even relatively early in the disease process. As we further discuss below, the reduced stride length in PD does not provide a full explanation of the observed gait changes; instead, measures based on gait dynamics provide a more complete picture while also conferring clinical utility.
4.1 Gait variability and fall risk in Parkinson’s Disease (PD)
Gait disturbances and falls are one of the most significant motor complaints in PD and a leading cause of loss of independence and institutionalization (Ashburn, Stack, Pickering, & Ward, 2001; Bloem, Hausdorff, Visser, & Giladi, 2004; Hausdorff et al., 2000). As discussed above, gait variability apparently predicts falls in older adults with ‘idiopathic falls’ as well as in patients with Alzheimer's disease (Azizah, Lajoie, & Teasdale, 2003; Hausdorff, Edelberg et al., 1997; Hausdorff, Rios et al., 2001; Maki, 1997; Nakamura, Meguro, & Sasaki, 1996; Visser, 1983;). In PD, there is a need for better predictors of falls (Bloem, Grimbergen, Cramer, Willemsen, & Zwinderman, 2001; Bloem, Van Vugt, & Beckley, 2001). Gait variability may help to enhance the early identification of potential fallers in PD, as it does in other populations. A few studies have observed increased variability in patients with PD compared to controls, noting that this variability tends to increase with disease severity (Blin et al., 1990; Blin, Ferrandez, Pailhous, & Serratrice, 1991; Hausdorff et al., 1998). In general, however, the relationships between gait variability, fall risk, and other parkinsonian features were not well-studied and the effect of levodopa on falls and gait variability were also largely unknown. To better understand the effects of PD on walking, especially gait instability, and the factors that contribute to gait variability and fall risk in PD, we studied patients with idiopathic PD in order to evaluate: (1) the relationship between gait variability, fall history and other parkinsonian features, and (2) the effect of levodopa on gait variability and fall frequency (Schaafsma et al., 2003). Forty-one percent of these patients reported a history of falls. In ‘off’ (off anti-parkinsonian medication), stride time variability was significantly (p < .009) larger among fallers compared to non-fallers (see Table 2). Stride time variability, total Unified Parkinson's Disease Rating Scale (UPDRS) scores, and total score on the timed motor tests of the Core Assessment Program for Intracerebral Transplantations (CAPIT) score significantly improved in response to levodopa, both in the fallers and non-fallers. In the ‘on’ state (on anti-parkinsonian medications), the stride time variability remained significantly increased in fallers compared to non-fallers (Table 2). The average stride time was similar in fallers and non-fallers (‘on’ or ‘off’) in both the ‘off’ and ‘on’ state (p > .27). Many of the measures of gait, disease severity, fall risk and stride variability were related to one another. In step-wise multiple regression analysis that included all measures of motor function and disease severity as potential co-variates, stride time variability and disease severity (UPDRS total score) were found to be the two significant predictors of fall frequency, explaining 68% of the variance of fall frequency in the ‘off’ state. From another perspective, among the sub-set of participants with a Hoehn and Yahr of 3.0 in the ‘off’ state (i.e., among participants with similar degree of disease severity), stride time variability was significantly increased in fallers compared to non-fallers (p < .041). UPDRS motor (Part III), disease duration and CAPIT scores were not different in this sub-set of fallers and non-fallers, further suggesting that the increased stride time variability among fallers is more than a simple reflection of disease severity.
Table 2.
Gait dynamics in patients with Parkinson's disease, fallers and non-fallers
| Gait Measure | Fallers | Non-Fallers | p-value |
|---|---|---|---|
| "Off" Stride time variability (CV %) | 8.8 ± 7.9 | 4.2 ± 1.3 | .009 |
| “On” Stride time variability (CV %) | 5.0 ± 1.9 | 3.3 ± 1.6 | .013 |
| “Off“ Average stride time (msec) | 970 ± 191 | 996 ± 131 | .520 |
| “On“ Average stride time (msec) | 992 ± 195 | 1035 ± 82 | .293 |
Adapted from Schaafsma et al. (2003). CV: coefficient of variation.
These results are consistent with earlier findings that reported increased stride-to-stride variability among patients with PD (Blin et al., 1990; Blin et al., 1991; Hausdorff et al., 1998), but extend these results in a number of ways. These findings demonstrate that the locomotor control system’s ability to regulate the stride-to-stride variations in gait timing is especially impaired among PD participants with a history of falls. We found that fall frequency and stride-to-stride variability are not related to tremor, rigidity, or bradykinesia in the ‘off’ state. Perhaps this explains, in part, why traditional measures of motor function have not been successful at evaluating fall risk in PD. In addition, we clearly demonstrate that levodopa has a positive effect on stride-to-stride variability. This underscores the role of levodopa and the basal ganglia, more generally, in the regulation of gait rhythmicity. This study of gait dynamics in PD indicates that impairment in the ability to regulate stride-to-stride variability is apparently further increased among PD participants who fall. This finding suggests the possibility of exaggerated impairment of internal clock function in PD fallers and the potential of using gait variability as a clinical measure of fall risk for PD.
4.2 Freezing of gait
Patients with PD often experience freezing of gait (FOG), a debilitating phenomenon that inexplicably affects about 50% of patients with PD. For no apparent reason, patients suddenly become unable to start walking or to continue to move forward. A feeling of being glued to the floor is typically expressed. FOG can be quite disabling, impairing function, health-related quality of life, mobility, and independence (Gray & Hildebrand, 2000; Giladi et al., 2001). Because of its episodic nature, patients with FOG have an increased risk for falls, nursing home admission, and mortality (Gray & Hildebrand, 2000).
Little is known about the gait of those patients with PD who experience FOG or the patho-physiology of freezing or why some patients experience FOG and some do not. One possibility is that FOG is a truly paroxysmal phenomenon and that the usual walking pattern of patients who experience FOG is not different than that of other patients with PD who do not experience these transient episodes of freezing of gait. On the other hand, some have observed gait changes just prior to freezing and suggested that freezing is caused by a combination of an increasing inability to generate stride length superimposed on a dyscontrol of the cadence of walking (Nieuwboer et al., 2001). To address this question, we compared the gait of PD patients with freezing of gait to PD patients without freezing of gait. Given the potential importance of the dyscontrol of the cadence of walking in freezing, we focused on two aspects of gait: the average stride time (the inverse of cadence, a measure of the walking pace or rate) and the variability of the stride time (a measure of ‘dyscontrol’ and arrhythmicity). We found that although the average stride time was similar in patients with and without FOG, stride-to-stride variability was markedly increased among PD patients with FOG compared to those without FOG, both while ‘on’ (p < .020) and ‘off’ (p < .002) anti-parkinsonian medications. Further, we found that increased gait variability was not related to other measures of motor control (while off medications) and levodopa apparently reduced gait variability, both in patients with and without freezing.
These results suggest that a paradigm shift should take place in how FOG is viewed. PD patients with FOG have a continuous gait disturbance, above and beyond what is typically seen in PD: the ability to regulate the stride-to-stride variations in gait timing and maintain a stable walking rhythm is markedly impaired in patients with FOG, even in between freezing episodes. In addition, these findings suggest that the inability to control cadence, i.e., stride time variability, might play an important role in this debilitating phenomenon and highlight the key role of dopamine-mediated pathways in the stride-to-stride regulation of walking.
4.3 Cognitive function and gait variability
Traditional biomechanical studies of gait have focused on musculo-skeletral mechanics and have largely ignored ‘higher-level’ cognitive function. A growing body of literature has shown, however, that walking is not simply an automated task, but rather regulation of gait is influenced by cognitive function, in particular executive function (EF). The dual task paradigm, in which participants are asked to perform a secondary task and divide their attention, is often used to study this relationship. In a healthy participant, one task may be performed automatically (unconsciously) requiring minimal attention reservoirs, while the person is attentive to the second task (Bond & Morris, 2000). However, with aging or disease, performance and automaticity may deteriorate, giving rise to the ‘dual-task decrement.’ Studies in PD demonstrate this connection between executive function and gait variability.
To better understand the role of attention on gait in PD, we tested the hypothesis that gait variability increases when participants with PD walk while performing a cognitively challenging task (Hausdorff, Balash, & Giladi, 2003). Participants with idiopathic PD walked on level ground under normal conditions and during dual tasking, i.e., serial 7 subtractions, a procedure widely used to provide distraction and a cognitive challenge When walking while cognitively challenged, gait variability increased significantly, more than twofold (p < .002). These results highlight the profound effects of attention and ‘dual tasking’ on walking, exacerbating gait variability and impairing the ability of patients with PD to maintain a stable walk.
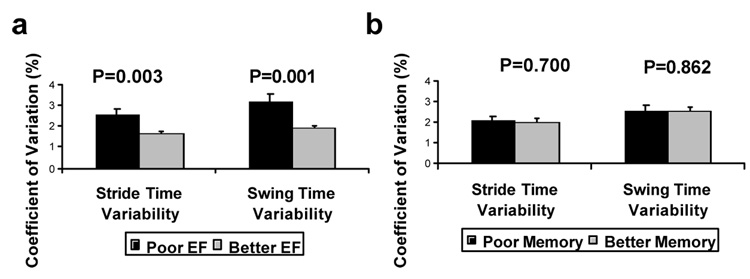
In a follow-up study (Yogev et al., 2005), we examined patients with idiopathic PD (mean age: 70.9 yrs; mean Mini-Mental Status Exam, MMSE, Scores: 28.0) with moderate disease severity (Hoehn and Yahr 2–3) and age and gender-matched healthy controls. Gait variability was measured under four walking conditions: usual walking and walking with three different levels of cognitive loading. Compared to the control participants, gait variability was significantly increased in the PD group in all four conditions (p < .01). Both the controls and the patients walked more slowly during dual tasking. As the degree of cognitive loading increased, gait variability increased compared to usual walking, but only in the PD patients (e.g., see Fig. 7). In PD, swing time variability was 2.8 ± 1.4 % during usual walking and increased to 4.3 ± 2.6 % during the most difficult mental loading (p < .0005). EF was significantly worse in the PD group (p < .0002), while memory, information processing, and MMSE scores were not different between the two groups. EF and gait variability were moderately related during usual walking, and this association became stronger during mental loading (see Table 3). Gait variability was not related to MMSE scores or to memory or information processing abilities during any walking condition.
Fig. 7.
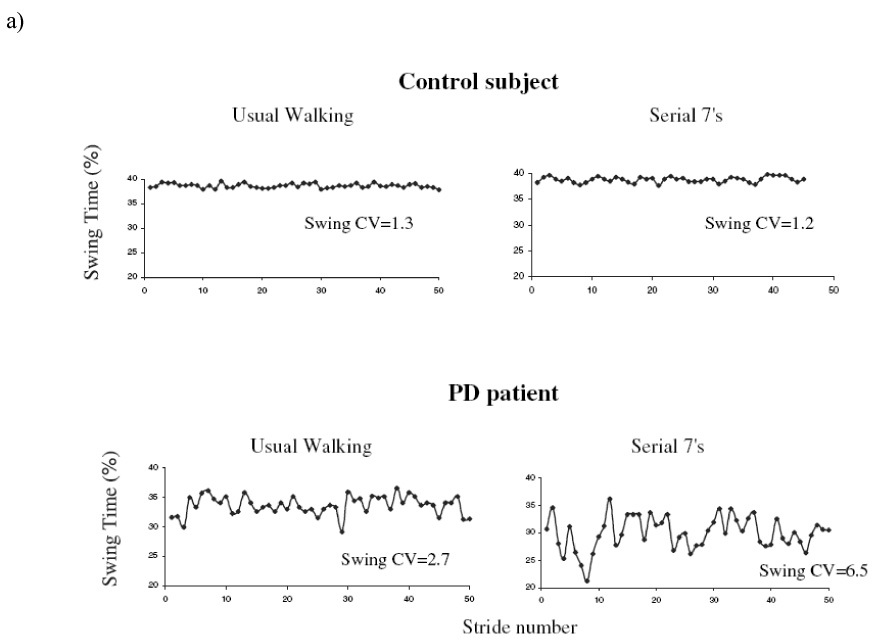
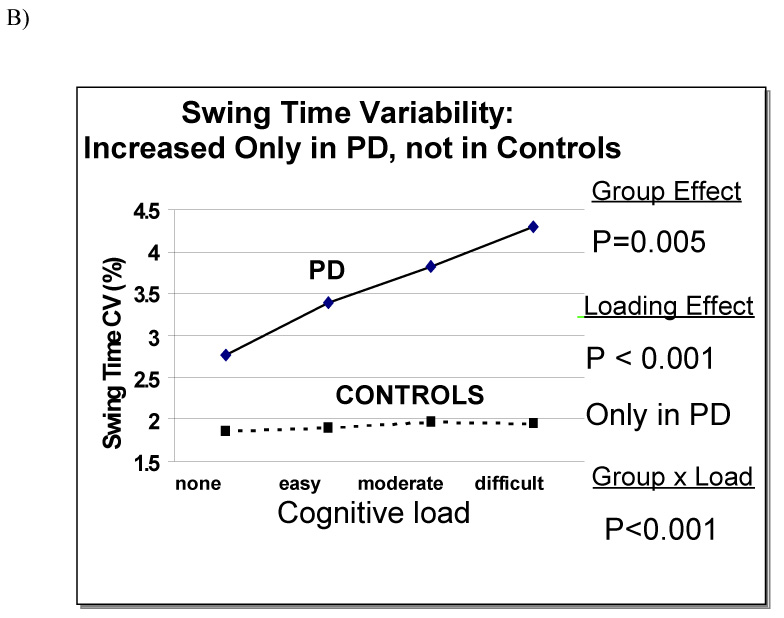
a Example of swing time series from a patient with PD and a control, under usual walking conditions and when performing serial 7 subtractions. Under usual walking conditions, variability is larger in the patient with PD (CV = 2.7%), compared to the control (CV = 1.3%). Variability increases during dual tasking in the participant with PD (CV = 6.5%), but not in the control (CV = 1.2%). b) For all levels of dual tasking difficulty, gait variability values among the PD participants were significantly increased compared to the controls. In PD, but not in controls, variability increased with the level of difficulty of the dual task. In contrast, gait speed (not shown) responded similarly in both groups. Adapted from Yogev et al. (2005).
Table 3.
Effect of dual tasking on the association between stride variability and executive function in patients with Parkinson's disease
| Cognitive Load Level | |||
|---|---|---|---|
| None | Moderate | Difficult | |
| Correlation | r = −.39 | r = −.42 | r = −.49 |
| Probability | p = .007 | p = .002 | p = .002 |
Adapted from Yogev et al. (2005).
These findings demonstrate that not only does dual task performance exacerbate the bradykinetic manifestations of PD, it also apparently markedly heightens gait variability and instability. In fact, the magnitude of the effect on variability is apparently much larger than the effect on bradykinetic manifestations (i.e., 10% change in average stride time vs. 150% change in stride time variability). In addition, the reduced gait speed (and stride length) in the control group, without any parallel change in variability illustrates how these stride length and rhythmicity are independent of one another. These results also indicate that in PD, the ability to maintain a steady walk worsens as participants perform secondary mental tasks. Gait variability is not ‘automatically’ regulated, but apparently basal ganglia input is needed along with cortical function and attention. In fact, there is also an association between EF and gait variability that is suggestive of a cause and effect. The lack of an association between memory, information processing, or other aspects of cognitive function and gait variability demonstrates that EF may drive the relationship between cognitive function and gait variability. In contrast to other cognitive domains, the results specifically suggest that EF may be critical to dual-tasking gait variability in PD.
Interestingly, similar results have been observed in ‘idiopathic’ elderly fallers, suggesting that EF and cognitive resources are important contributors to fall risk (see also Table 4) and that gait rhythmicity requires attention in older adults with largely intact basal ganglia function (Springer et al., 2006). To more fully assess this relationship between cognitive function and gait variability, we also studied relatively healthy older adults (who did not have PD) and stratified participants first with respect to EF and memory scores. As shown in Fig. 8, gait variability was strongly dependent on EF, but not on memory, underscoring the idea that among older adults, the regulation of gait timing may rely on higher-level cognitive input. We also compared the ability of participants to regulate gait (stride-to-stride variability) with their performance on a relatively simple, ‘thoughtless’, motor task (i.e., rhythmic tapping) and on a more complex task (i.e., catching of a falling object on the computer screen). Tapping, both the average and the variability of the tapping interval, was not associated with gait variability. However catch-game performance was strongly associated with these measures, suggesting that walking may be more like catching, a non-rhythmic motor task that requires planning and EF, than it is like tapping (Hausdorff, Yogev, Springer, Simon, & Giladi, 2005).
Table 4.
Effect of dual tasking on the association between stride variability and executive function in elderly fallers. As loading increases, the association becomes tighter. Compare with Table 3.
| Cognitive Load Level | |||
|---|---|---|---|
| None | Moderate | Difficult | |
| Correlation | r = −.34 | r = −.40 | r = −.57 |
| p-Value | p = .054 | p = .01 | p = .003 |
Adapted from Springer et al. (2006).
Fig. 8.
Among older adults, a) measures of gait variability are associated with executive function (EF), but not with memory b). Adapted from Hausdorff et al. (2005).
In general, the results of these dual tasking studies demonstrate the significant effects of EF, attention and ‘dual tasking’ on walking stability. Further, they explain, in part, why walking while cognitively challenged markedly exacerbates gait variability and impairs the ability of patients with high risk of falls, such as patients with Parkinson's disease, to maintain a stable walk. In addition, because EF is mediated by the frontal lobe (Stuss & Levine, 2002), these findings also demonstrate the importance of frontal lobe function in the control of gait rhythmicity
4.4 Plasticity and sensitivity to therapeutic interventions
Can gait dynamics be altered via intervention? We have already described how a metronome alters the fractal gait pattern in healthy young adults, and how treatment with levodopa reduces gait variability in patients with Parkinson's disease. In this section, we summarize representative studies which support the idea that appropriate interventions can be used to influence gait dynamics, again using patients with PD as an example.
Rhythmic auditory stimulation (RAS) improves gait rhythmicity
Previous studies have shown that rhythmic auditory stimulation (RAS) can improve the spatiotemporal features of gait in patients with PD (Lim et al., 2005; Rubenstein, Giladi, & Hausdorff, 2002). When using RAS, administered in the form of a metronome, gait speed and stride length improved in PD patients both at ‘on’ and ‘off’ states (McIntosh, Brown, Rice, & Thaut, 1997; Willems et al., 2006). However, while there is evidence indicating that RAS improves stride length and enhances other spatiotemporal features of gait in PD, the effects of RAS on stride-to-stride variability are largely unknown. A priori, one could argue that if key features of gait in PD such as stride length and gait speed improve, then rhythmicity would also improve with RAS. Further, one could suggest that because RAS sets the pace, it can act like an external rhythm generator and help to restore neurally controlled rhythmicity, thereby reducing stride-to-stride variability. Conversely, in PD, gait rhythmicity and stride variability often behave differently from stride length, gait speed, and other parkinsonian features (Hausdorff, 2005; Frenkel-Toledo et al., 2005a; Blin et al., 1991; Hausdorff et al., 1998; Schaafsma et al., 2003). Thus, one could suggest that while RAS restores the ability of PD patients to generate a ‘normal’ stride length, damage to the circuits that regulate rhythmicity may prevent these patients from walking with a consistent pattern, even in the presence of RAS.
We addressed these questions in a study of 21 non-demented patients with idiopathic PD (Hoehn & Yahr stage: 2–3; mean age: 67.2 yrs) with independent ambulation and intact hearing, and 26 age-matched healthy controls (Lowenthal et al., 2004). Participants were tested under the following conditions: 1) Baseline: (usual walking for 100 meters); 2) RAS set to each participant’s normal cadence; 3) Usual walking (to assess any carryover effect); 4) RAS set to 110% of usual cadence; 5) Usual walking (to assess immediate carryover effect); and 6) Usual walking 15 min later (to assess longer carryover). As in previous studies (Blin et al., 1990; Hausdorff et al., 1998; Hausdorff et al., 2000; Schaafsma et al., 2003), both stride time variability and swing time variability were significantly increased in the participants with PD compared to the controls under usual walking conditions (p < .01). When RAS was set to each participant’s usual cadence, the variability of the stride time and swing time tended to decrease among the participants with PD, but the effect was not significant. When the frequency of the RAS was increased further, by 10%, there was a significant reduction in the stride-to-stride variability of both the swing time variability (p < .04) and stride time variability (p < .004). While RAS enhanced gait stability in the participants with PD, it tended to increase stride-to-stride variability in the controls, suggesting that the positive effect of RAS is specific to participants who have impaired internal cueing and increased variability. Otherwise, RAS apparently interferes with normal gait sequencing. This different response of the control and PD participants was significant (mixed model Group x RAS interaction p < .01). The positive effects of RAS on the stride-to-stride variability apparently persisted even after the RAS was removed. Most intriguingly, in the patients with PD, stride variability remained low when gait was retested 15 minutes after being turned off. In contrast, among control participants, stride variability was unchanged (compared to baseline values) when it was re-evaluated 15 minutes later. Two conclusions can be drawn from these results: A) RAS can reduce stride-to-stride variability, i.e., RAS can enhance gait stability among patients with PD patients; B) There appears to be a carryover effect lasting at least 15 minutes after the cessation of RAS.
These effects of RAS on variability could be explained in at least three different ways. Perhaps the most straight forward explanation for the observed effects of RAS on stride-to-stride variability is that in patients with PD, RAS acts like a pacemaker and provides an external rhythm that is able to stabilize the defective internal rhythm of the basal ganglia (McIntosh et al., 1997). RAS may circumvent the pallidal-supplementary motor area (SMA) pathway, possibly via the premotor cortex, and provide external cues to guide movement (Elsinger et al., 2003; Hanakawa et al., 1999). This explanation, that RAS works simply by acting as an external time keeper, is rather intuitive, but the rate dependent nature of the effect of RAS on variability, the group specific response, and the observed carryover effects present difficulties to this theory. Other explanations of the observed effects of RAS revolve around its influence on the neural circuitry regulating gait. For example, the rate dependent and enduring effects could be achieved by affecting striatal activation (Brown, Martinez, & Parsons, 2006) or via an effect on the cerebellum (Del Olmo & Cudeiro, 2005; Eckert et al., 2005). Indeed, extensive training of speech in PD has demonstrated evidence for striatal plasticity, changes in the cerebellum, and long-term, carryover effects that persist well beyond the training period (Liotti et al., 2003; Liotti & Ramig, 2003).
Rhythmic stimulation via treadmill improves gait in PD
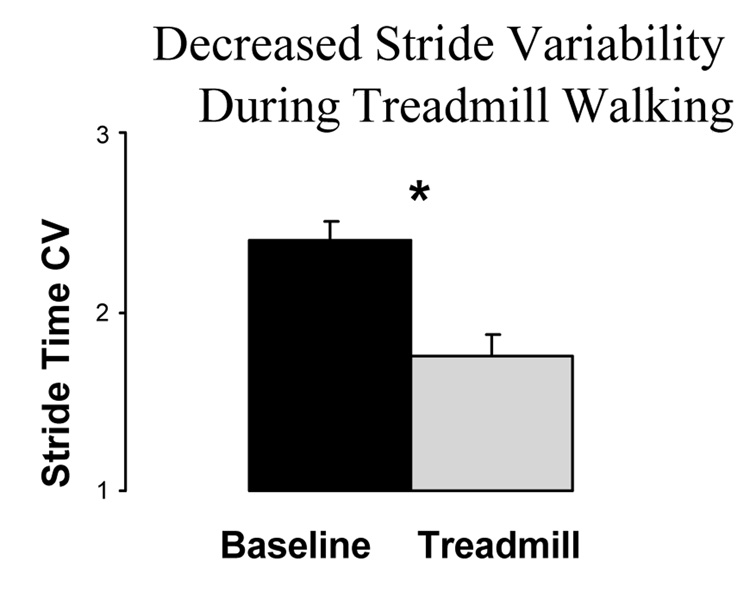
We hypothesized that, like a metronome, a treadmill can also be used as an external cue in PD. To test this hypothesis, we studied the effects of treadmill walking on the gait of 36 participants with PD and compared their performance to that of 30 age-matched healthy elderly controls (Frenkel-Toledo et al., 2005b; Frenkel-Toledo et al., 2005a). The primary question was: does treadmill walking lower stride-to-stride variability in patients with PD? There was a significant reduction in the stride time variability (see Fig. 9) and swing time variability during treadmill walking (p < .001). It is important to note that this reduction was not simply a result of a change in gait speed. The speed of the treadmill was set to each participant’s usual walking speed.
Fig. 9.
Effects of treadmill walking on stride time variability of patients with PD. During treadmill walking, stride-to-stride variability was significantly lower compared to usual walking (at the same gait speed). * p < .001. Adapted from Frenkel-Toledo et al. (2005b).
The changes in gait during treadmill walking also shed light on the origins of the increased stride-to-stride variability in PD. Some have suggested that this change is due to an inability to consistently generate sufficient muscle force (Blin et al., 1991). The present results, however, indicate that the changes are more likely the result of alterations in the pacing mechanisms. Treadmill walking likely places greater or similar demands on muscle forces, compared with overground walking at the same speed (Alton, Baldey, Caplan, & Morrissey, 1998; Perry, 1992). Thus, these findings support the idea that reduced stride-to-stride variability on the treadmill and the increased stride-to-stride variability typically seen in PD seems likely to be a result of changes in locomotor rhythmicity.
Effects of methylphenidate (MPH)
Given the decline in EF and attention abilities in PD (Cooper, Sagar, Jordan, Harvey, & Sullivan, 1991; Elias & Treland, 1999; Hausdorff et al., 2006; Yogev et al., 2005) and the putative reliance of a steady gait on this cognitive domain, we hypothesized that MPH may improve gait and reduce fall risk in patients with PD. MPH has a long history as the drug of choice for improving attention in children with ADHD, however, its potential to modify motor function is less known. In a pilot open label study, we evaluated the effect of a single dose (20 mg) of MPH on cognitive function, gait performance and markers of fall risk in 21 patients with PD (Auriel, Hausdorff, Herman, Simon, & Giladi, 2006) (mean age 70.2 ± 9.2 years).
In response to MPH treatment, there was a significant increase in a computerized cognitive battery attention index (p < .0 ) and the EF index (pp < .0 ). In contrast, scores of memory, visual-spatial orientation, and hand-eye coordination were unchanged. Measures of gait before and after intake of MPH are shown in Table 5. Significant improvements were observed for the Timed Up and Go times (p < .001), gait speed (p < .005) and stride time variability (p < .013). There was no significant improvement in tapping (rate or variability), a more simple, less cognitively demanding task. Interestingly, in response to MPH, the dual tasking effect on stride-to-stride variability, as measured by the swing time, was also significantly reduced (p < .05). The results indicate that a single dose (20 mg) of MPH administered to patients with PD receiving L-dopa is associated with a significant improvement in attention and EF, gait speed, stride variability and Timed Up and Go, markers of abnormal gait and fall risk, and a reduction in the dual tasking effect on gait variability.
Table 5.
Effects of MPH on gait and mobility in Parkinson's disease
| Pre | Post | p-Value | |
|---|---|---|---|
| Timed up and go (sec) | 11.9±3.8 | 10.6±2.3 | .0001 |
| Gait speed (m/sec) | 1.07±0.19 | 1.13±0.21 | .005 |
| Average stride time (msec) | 1114±85 | 1100±79 | .068 |
| Stride time variability (%) | 2.28±0.63 | 2.00±0.47 | .013 |
Adapted from Auriel et al. (2006).
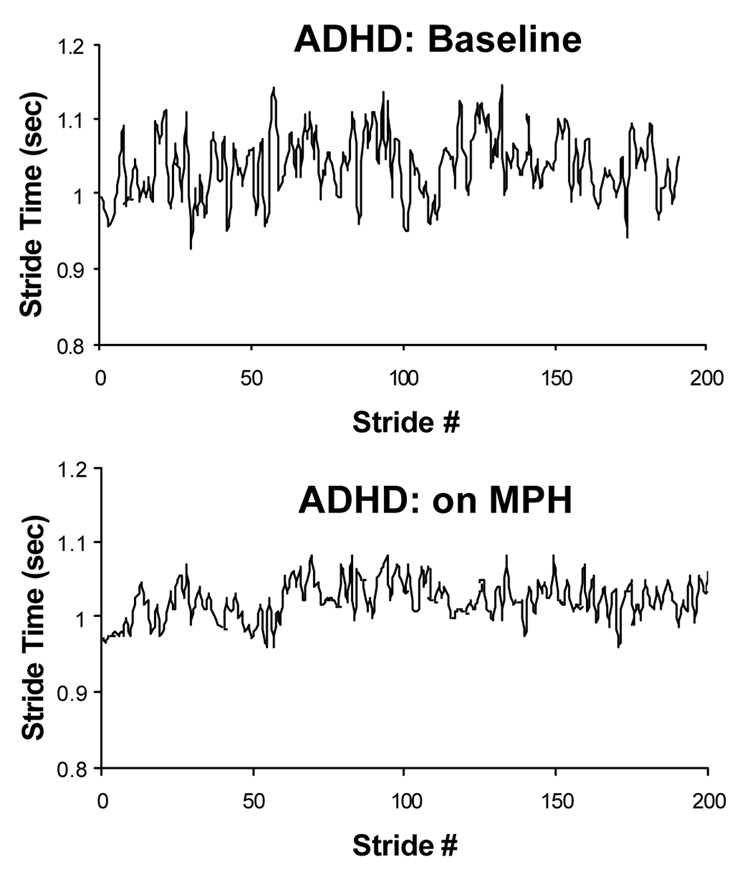
MPH may have a widespread effect on parkinsonism by promoting dopamine uptake in the presence of L-Dopa. In the present study, however, there was no significant improvement in finger tapping rate or variability, despite significant changes observed in gait. This suggests that the effects on gait were not simply due to enhanced basal ganglia and dopamine activity in general, but that they were achieved through a more selective effect. MPH may have worked by enhancing cognitive function. As a result of enhanced attention and EF abilities, relatively complex motor tasks that rely upon attention improved, while a more simple motor task requiring minimal cognitive input was not affected. The significant effects on attention and gait suggest a novel way of treating gait disturbances and perhaps reducing fall risk in PD, i.e., by enhancing cognitive function. In addition, these results underscore the dependence of gait on EF. Interestingly, in studies in children with ADHD (see, for example, Fig. 11) and in older adults (without Parkinson's disease), the effects of MPH on gait were very similar to those seen in patients with PD (Ben-Itzhak, Giladi, & Hausdorff, 2007; Leitner et al., 2007), further supporting the connection between EF and gait. Nonetheless, further evaluation is needed to assess the role of MPH as a possible line of treatment for fall risk and gait deficits.
Fig. 11.
Example of the effects of MPH (MPH) on stride time variability during usual walking in a child with ADHD. Above: baseline (72 hours off MPH). Below: after treatment with MPH. Note how the stride-to-stride fluctuations are reduced in response to MPH. Adapted from (Leitner et al. (2007).
5. Summary and concluding remarks
5.1 Contributing factors
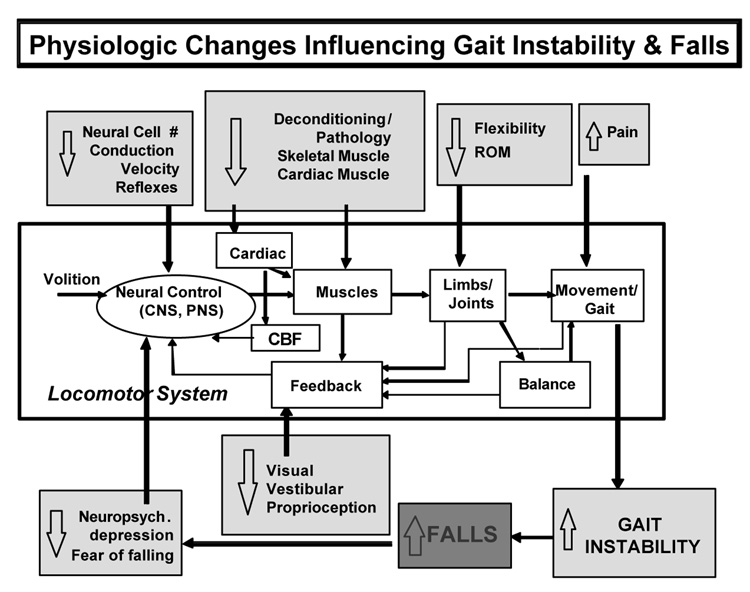
What contributes to and regulates the stride-to-stride to fluctuations in gait? A number of factors have already been mentioned. These include central nervous system function as seen with maturation and with aging. and as demonstrated by alterations in Parkinson's disease, Huntington's disease as well as in Alzheimer's disease (Nakamura et al., 1997; Nakamura et al., 1996; Sheridan, Solomont, Kowall, & Hausdorff, 2003). Gait variability has been related to cardiovascular function and associated with heart rate variability. We have also noted how a metronome reduces gait variability in patients with Parkinson's disease, while it alters (becoming more abnormal) the fractal scaling index. Randomized control trials in older adults and in patients with osteoarthritis have shown that the regulation of gait dynamics, variability as well as other measures such as non-stationary indices, are responsive to resistance training (Hausdorff, Nelson et al., 2001) and that improvement in physiologic capacity (e.g., muscle strength) is associated with reduced gait instability. These findings also demonstrate that while measures of variability are sensitive markers of fall risk, they are also sensitive to physiologic function and amenable to change with the appropriate intervention. A picture of the multi-factorial nature of the contributors to variability of gait thus begins to emerge (see Fig. 10). Interestingly, in many ways, the multi-factorial contributors to gait variability are parallel to the multi-factorial influences on fall risk. While it was once thought that a single generally cause lies behind fall risk in older adults, more often, it has been shown that several factors combine to increase fall risk (AGS Guidelines, 2001).
Fig. 10.
Simplified block diagram depicting some of the factors that contribute to gait stability and fall risk. Adapted from Hausdorff, Nelson et al. (2001).
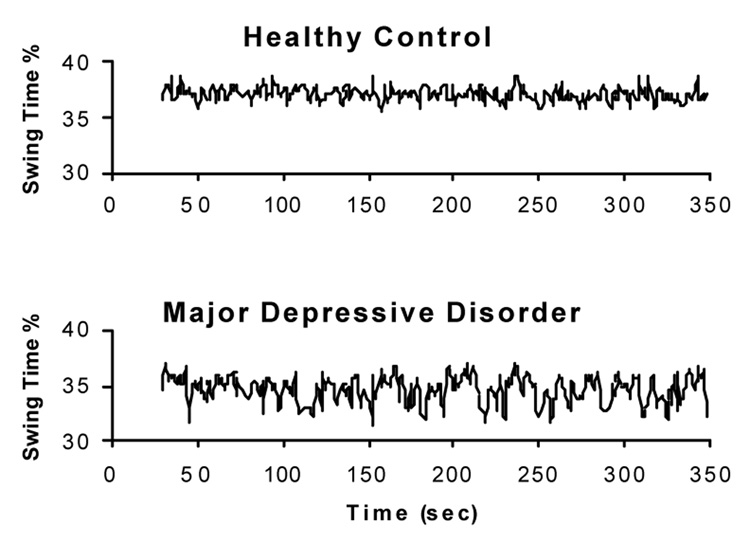
It is important to stress that while increased variability is associated with CNS function, cardiovascular function, and muscle function, we have also seen strong evidence supporting the idea that stride-to-stride variability is sensitive to cognitive function and neuropsychological status. We have noted the relationship between fear of falling and gait variability, the relationship between EF and gait variability, as well as the positive impact of an attention-enhancing drug. Gait variability has also been shown to be increased in patients with depression (see for example, Fig. 12), independent of gait speed (Hausdorff, Peng, Goldberger, & Stoll, 2004), and a prospective study has shown that anti-depressive therapy reduces gait variability (Paleacu et al., 2007). Like heart rate variability, changes in gait variability are apparently sensitive to multiple factors but not necessarily specific, for example, to a single rhythm generator.
Fig. 12.
Representative swing time series from a patient with major depressive disorder (38 yr old male; swing time CV = 3.2%), and a healthy control participant (25 yr old male; swing time CV = 1.6%). Note the relatively large stride-to-stride fluctuations in the participant with major depressive disorder. CV: coefficient of variation. Adapted from Hausdorff, Peng et al. (2004).
Extrapolating from the above, it is safe to say that multiple neural networks influence the regulation of gait dynamics. Some have suggested that in man, the caudal cholinergic mesencephalic nucleus, also referred to as the pedunculopontine nucleus (PPN), is considered as the human mesencephalic locomotion center and believed to control such spinal pattern generators, or to play a similar role as the spinal CPG in animals (Pahapill & Lozano, 2000). The magnitude of the variability (at least in terms of stride time and swing time) is regulated in part by the basal ganglia and dopamine-sensitive networks, as well as by the frontal lobe. The RAS study cited above also supports the possibility that the cerebellar function plays a role in the stride-to-stride regulation. If the PPN is the source of rhythmicity, it clearly is influenced by other networks as well. The fractal scaling index of gait timing is also apparently sensitive to the alterations in these networks, however, not all studies have reported how this measure changes as a function of different effects (in part because estimation of the fractal index requires many more strides than estimation of variability). However, while the magnitude of the variability is apparently highly sensitive to a fairly large number of influences, including central as well as peripheral influences, the fractal index appears to be more dependent on central than on peripheral influences.
5.2 Gait speed and gait dynamics
Many aspects of gait are, understandably, strongly related to each other. This is not surprising since all gait measures are designed to characterize features of the same motor system and because there is often a degree of redundancy and overlap among different gait parameters. For example, gait speed is, by definition, a simple linear function of stride length and stride time, so it is only natural that average gait speed and average stride time are associated. The question arises then: how are measures of dynamics related to other, more traditional measures, based on the average value of a given parameter? Several studies have observed a relationship between measures of variability and average values, at least in relatively homogenous groups (Danion, Varraine, Bonnard, & Pailhous, 2003; Frenkel-Toledo et al., 2005a; Jordan et al., 2007b; Yamasaki, Sasaki, Tsuzuki, & Torii, 1983). Findings from these studies would support the notion that variability measures are dependent on average values. From this perspective, it would make sense to adjust for and take into account gait speed (or stride length), when studying measures of variability. Such an approach has indeed been taken by some investigators (Moe-Nilssen & Helbostad, 2004).
It is, however, an oversimplification to suggest that variability measures are merely a mirror of average values (Hausdorff, 2004). In this regard, a few things should be kept in mind. First, it is important to remember that the average value of any fluctuating (1st moment) parameter and its standard deviation (2nd moment) are, theoretically, totally independent. Similarly, the standard deviation and higher moments and measures of the dynamics (e.g., fractal scaling) are also, theoretically, independent of one another. Thus, for example, hypothetically, one can have relatively large stride time variability, with a small or large fractal scaling index, and conversely, a large fractal index while the magnitude of the stride-to-stride fluctuations are large or small. Multiplication (scaling) of the stride-to-stride fluctuations has no effect on measures of the dynamics. Empirically, this independence has been shown in numerous reports. For example, as noted above, Gates and Dingwell (2006) recently observed that fractal scaling is not altered in patients with peripheral neuropathy, despite the fact that these patients tend to walk slowly and with increased stride-to-stride variability (Gates & Dingwell, 2006; Richardson, Thies, DeMott, & Ashton-Miller, 2004). This finding supports the idea that changes in fractal scaling are largely a reflection of central nervous system impairment, and not simply a reflection of a slower walking speed. Moreover, as mentioned, there are many studies that have demonstrated that measures of variability and dynamics are more sensitive to underlying, sometimes subtle changes, better discriminators of ‘healthy’ and ‘sick’ populations, and more successful at predicting events such as falls (Frenkel-Toledo et al., 2005a; Grabiner, Biswas, & Grabiner, 2001; Hausdorff, Edelberg et al., 1997; Hausdorff, Mitchell et al., 1997; Hausdorff, Rios et al., 2001; Maki, 1997; Nakamura et al., 1996; Sheridan et al., 2003). While there is a relationship between measures of variability and average values, the one does not always fully represent the other. Therefore, for a more complete description of gait, it is best to characterize average values and the typical gait cycle as well as dynamic features.
It is also important to keep in mind that while different measures of the stride-to-stride dynamics often behave similarly, there are often exceptions to this general rule (Frenkel-Toledo et al., 2005a; Gates & Dingwell, 2006; Hausdorff et al., 2000; Hausdorff, Mitchell et al., 1997). Thus, on the one hand, stride time variability and swing time variability were both increased in elderly fallers, compared to non-fallers ( Hausdorff, Edelberg et al., 1997; Hausdorff, Rios et al., 2001). On the other hand, in a study that looked at the effects of gait speed on stride time variability and swing time variability during treadmill walking (Frenkel-Toledo et al., 2005a), stride time variability was associated with gait speed, while swing time variability was not. As another example, we described above how a metronome altered the fractal scaling index in healthy adults, without affecting the average gait speed, or average stride time or stride time variability during slow and fast walking.
A recent study by Brach and colleagues (Brach, Studenski, Perera, VanSwearingen, & Newman, 2007) provides strong empirical support for the idea that it is helpful to study average values as well as measures of variability. In a prospective study of 379 older adults, stance time variability was a significant predictor of future mobility-related disability, even after adjusting for gait speed, age, health status and physical activity level. In other words, to fully estimate disability among the elderly, it is necessary to assess variability along with the characterization of the average features of gait.
5.3 Good vs. bad variability
A priori, variability of physiologic outputs may reflect adaptability and flexibility (i.e., more is better) or the inability of the physiologic control system to regulate a given parameter (i.e., more variability is worse) (Moe-Nilssen & Helbostad, 2005; Newell & Corcos, 1993; Stergiou et al., 2006). In studies of heart rate variability (HRV), more variability (larger magnitude) has generally been found to be better, i.e., increased variability reflects greater adaptability and a wider ability to respond (Goldberger et al., 2002; Ho et al., 1997; Lipsitz et al., 1990; Peng et al., 1993). In many of the gait studies cited above, larger stride-to-stride fluctuations were associated with poor outcomes or states, somewhat in contrast to what has been reported for HRV. Indeed, several groups have investigated the ‘regularity’ of gait fluctuations (Auvinet, Chaleil, & Barrey, 1999; Auvinet et al., 2002; Le et al., 2006; Moe-Nilssen & Helbostad, 2004), implicitly imparting value to the regularity: the more regular, the better. Alternatively put, for gait, reduced variability, at least to a certain degree, is generally better. On the other hand, both dynamical features of heart rate and gait share similar features. For example, both heart rate and gait timing exhibit fractal scaling that change, in a similar fashion, with aging and certain diseases (Goldberger et al., 2002). For such measures, ‘healthy’ values lie in between extremes of white noise and brown noise and may represent a balance between adaptability and regularity. Interestingly, in their studies of stride width variability in older adults, Brach and colleagues (Brach, Berlin, VanSwearingen, Newman, & Studenski, 2005) also found that too much and too little variability were predictive of falls, while healthy values were positioned in the middle (Brach et al., 2005). In other words, for some measures of gait dynamics, less variability is better (e.g., stride time variability), whereas others may reflect adaptability and an appropriate middle value is optimal (e.g., the fractal scaling index and perhaps stride width variability). In a similar light, Beauchet et al. have argued that Lyapunov exponent indices of joint kinematics reflect adaptability (higher is ‘better’) while stride time variability represents automaticity and rhytmicity (lower is better). In short, for stride time variability (and closely related measures like swing time variability and stance time variability), lower values generally reflect a healthier, more rhythmic and more consistent state. For the fractal scaling index of gait timing, a healthy value that likely reflects adaptability is found in between the extremes values (closer to .5 or 1.0).
5.4 Future directions and summary
Future investigations of gait dynamics are needed to address a number of as yet unanswered questions. While advances in our understanding of the factors that contribute to the stride-to-stride fluctuations have been made, much is still unknown about the physiologic and pathophysiologic mechanisms that shape these dynamics. We have some insight into differences among various parameters, but basic questions are not fully resolved. For example, even Gabell and Nayak's (1984) hypothesis that variations in the stride time represent the output of a central pattern generator and internal rhythmicity while fluctuations in the stance and swing (or double support) timing indicate ‘dynamic balance’ has not yet been fully tested. The degree to which the pedunculopontine nucleus mimics the CPG's found in animals and the role it plays in the regulation of gait rhythmicity remain to be determined. More questions arise when we try to understand the neuroscience behind higher moments and other measures of gait dynamics such as the fractal scaling index, the non-stationarity index or multi-fractal parameters. To this end, it may be helpful to study animal models or the effects of specific interventions to tease apart and identify the neural and other substrates that contribute to the regulation of the stride-to-stride fluctuations in walking. In addition, investigations that can relate the stride-to-stride fluctuations of different aspects of gait to underlying changes in biomechanics as well as to other contributing factors may help to provide a more comprehensive understanding of the factors that influence these stride-to-stride changes. Similarly, if measures of gait dynamics are going to find a home in clinical settings, work needs to be done to develop age-dependent norms and confidence intervals, to establish guidelines and procedures for standardization, and to identify practical ways of measuring gait fluctuations in clinical settings. As an example, to some degree, measures of variability depend on and become more robust as the length of the recording and the time series length increases (Owings & Grabiner, 2003). Yet, in studies reporting measures of gait variability, we find a wide range in the number of strides examined; some investigators base their measures on less than ten strides while others measure over hundreds of strides, even when quantifying the same aspects of the stride-to-stride fluctuations. This makes comparisons across studies difficult.
While there is much still to be learned, interim analysis of work to date suggests the following: 1) Gait instability is multi-factorial. Not surprisingly, neural control and muscle function influence gait instability and fall risk. Less intuitively, neuropsychological status and cognitive function also play an important role in gait instability, even in relatively healthy older adults. 2) To some degree, in certain populations, gait instability retains its plasticity and is modifiable. 3) Measures of gait dynamics, how walking varies from stride-to-stride, characterize a dimension of locomotor function that is often distinct from more traditional measures. For example, stride-to-stride variability prospectively identifies elderly fallers, even when standard measures (e.g., average gait speed) may not. Similarly, a fractal measure of gait identifies subtle changes in ‘successful’ aging and distinguishes between older adults with a ‘higher-level’ gait disturbance who fall and those who do not, while other indices of mobility fail to discriminate between these groups. All in all, it is fair to suggest that measures of gait dynamics may serve as a sensitive and clinically relevant parameter in the evaluation of mobility and fall risk and the response to therapeutic interventions.
Acknowledgments
The author is indebted to Drs. Goldberger, Peng and Giladi for their invaluable collaborative efforts as well as to Tuli Herman and many others who have contributed to the work that is the basis of this review. This work was supported in part by NIH grants AG-14100, RR-13622, HD-39838 and AG-08812, by the European Union Sixth Framework Program, FET contract no 018474-2, Dynamic Analysis of Physiological Networks (DAPHNet), and by the National Parkinson Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Fatalities and injuries from falls among older adults – United States, 1993–2003 and 2001–2005. MMWR Morb.Mortal.Wkly.Rep. 2006;55:1221–1224. [PubMed] [Google Scholar]
- AGS Guidelines. Guideline for the prevention of falls in older persons. American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. Journal of the American Geriatrics Society. 2001;49:664–672. [PubMed] [Google Scholar]
- Aizen E. Risk factors for falls in the elderly. Harefuah. 2001;140:271–276. [PubMed] [Google Scholar]
- Alton F, Baldey L, Caplan S, Morrissey MC. A kinematic comparison of overground and treadmill walking. Clinical Biomechenics (Bristol, Avon) 1998;13:434–440. doi: 10.1016/s0268-0033(98)00012-6. [DOI] [PubMed] [Google Scholar]
- Ashburn A, Stack E, Pickering RM, Ward CD. A community-dwelling sample of people with Parkinson's disease: Characteristics of fallers and non-fallers. Age Ageing. 2001;30:47–52. doi: 10.1093/ageing/30.1.47. [DOI] [PubMed] [Google Scholar]
- Ashkenazy Y, Hausdorff JA, Ivanov PC, Stanley HE. A stochastic model of human gait dynamics. Physica A-Statistical Mechanics and Its Applications. 2002;316:662–670. [Google Scholar]
- Auriel E, Hausdorff JM, Herman T, Simon ES, Giladi N. Effects of methylphenidate on cognitive function and gait in patients with Parkinson's disease: A pilot study. Clinical Neuropharmacology. 2006;29:15–17. doi: 10.1097/00002826-200601000-00005. [DOI] [PubMed] [Google Scholar]
- Auvinet B, Berrut G, Touzard C, Moutel L, Collet N, Chaleil D, et al. Reference data for normal subjects obtained with an accelerometric device. Gait and Posture. 2002;16:124–134. doi: 10.1016/s0966-6362(01)00203-x. [DOI] [PubMed] [Google Scholar]
- Auvinet B, Chaleil D, Barrey E. Accelerometric gait analysis for use in hospital outpatients. Revue du Rhumatisme (English ed.) 1999;66:389–397. [PubMed] [Google Scholar]
- Azizah MG, Lajoie Y, Teasdale N. Step length variability at gait initiation in elderly fallers and non-fallers, and young adults. Gerontology. 2003;49:21–26. doi: 10.1159/000066506. [DOI] [PubMed] [Google Scholar]
- Baltadjieva R, Giladi N, Gruendlinger L, Peretz C, Hausdorff JM. Marked alterations in the gait timing and rhythmicity of patients with de novo Parkinson's disease. European Journal of Neuroscience. 2006;24:1815–1820. doi: 10.1111/j.1460-9568.2006.05033.x. [DOI] [PubMed] [Google Scholar]
- Ben-Itzhak R, Giladi N, Hausdorff JM. Methylphenidate enhances gait and reduces fall risk in community living older adults with non-amnestic mild cognitive impairment. Journal of the American Geriatrics Society. 2007 doi: 10.1111/j.1532-5415.2007.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin O, Ferrandez AM, Pailhous J, Serratrice G. Dopa-sensitive and dopa-resistant gait parameters in Parkinson's disease. Journal of the Neurological Sciences. 1991;103:51–54. doi: 10.1016/0022-510x(91)90283-d. [DOI] [PubMed] [Google Scholar]
- Blin O, Ferrandez AM, Serratrice G. Quantitative analysis of gait in Parkinson patients: increased variability of stride length. Journal of the Neurological Sciences. 1990;98:91–97. doi: 10.1016/0022-510x(90)90184-o. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Grimbergen YA, Cramer M, Willemsen M, Zwinderman AH. Prospective assessment of falls in Parkinson's disease. Journal of Neurology. 2001;248:950–958. doi: 10.1007/s004150170047. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson's disease: a review of two interconnected, episodic phenomena. Movement Disorders. 2004;19:871–884. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Van Vugt JP, Beckley DJ. Postural instability and falls in Parkinson's disease. Advances in Neurology. 2001;87:209–223. [PubMed] [Google Scholar]
- Bond JM, Morris M. Goal-directed secondary motor tasks: Their effects on gait in subjects with Parkinson disease. Archives of Physical Medicine and Rehabilitation. 2000;81:110–116. doi: 10.1016/s0003-9993(00)90230-2. [DOI] [PubMed] [Google Scholar]
- Brach JS, Berlin JE, VanSwearingen JM, Newman AB, Studenski SA. Too much or too little step width variability is associated with a fall history in older persons who walk at or near normal gait speed. Journal of Neuroengineering and Rehabilitation. 2005;2:21. doi: 10.1186/1743-0003-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brach JS, Studenski SA, Perera S, VanSwearingen JM, Newman AB. Gait variability and the risk of incident mobility disability in community-dwelling older adults. The Journals of Gerontol. Series A Biological Sciences and Medical Sciences. 2007 doi: 10.1093/gerona/62.9.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Martinez MJ, Parsons JM. The neural basis of human dance. Cerebral Cortex. 2006;16:1157–1167. doi: 10.1093/cercor/bhj057. [DOI] [PubMed] [Google Scholar]
- Buzzi UH, Stergiou N, Kurz MJ, Hageman PA, Heidel J. Nonlinear dynamics indicates aging affects variability during gait. Clinical Biomechanics (Bristol, Avon.) 2003;18:435–443. doi: 10.1016/s0268-0033(03)00029-9. [DOI] [PubMed] [Google Scholar]
- Camicioli R, Howieson D, Lehman S, Kaye J. Talking while walking: The effect of a dual task in aging and Alzheimer's disease. Neurology. 1997;48:955–958. doi: 10.1212/wnl.48.4.955. [DOI] [PubMed] [Google Scholar]
- Campbell AJ, Borrie MJ, Spears GF, Jackson SL, Brown JS, Fitzgerald JL. Circumstances and consequences of falls experienced by a community population 70 years and over during a prospective study. Age and Ageing. 1990;19:136–141. doi: 10.1093/ageing/19.2.136. [DOI] [PubMed] [Google Scholar]
- Carletti T, Fanelli D, Guarino A. A new route to non invasive diagnosis in neurodegenerative diseases? Neuroscience Letters. 2006;394:252–255. doi: 10.1016/j.neulet.2005.10.065. [DOI] [PubMed] [Google Scholar]
- Chau T. A review of analytical techniques for gait data. Part 1: Fuzzy, statistical and fractal methods. Gait and Posture. 2001a;13:49–66. doi: 10.1016/s0966-6362(00)00094-1. [DOI] [PubMed] [Google Scholar]
- Chau T. A review of analytical techniques for gait data. Part 2: neural network and wavelet methods. Gait and Posture. 2001b;13:102–120. doi: 10.1016/s0966-6362(00)00095-3. [DOI] [PubMed] [Google Scholar]
- Chau T, Young S, Redekop S. Managing variability in the summary and comparison of gait data. Journal of Neuroengineering and Rehabilitation. 2005;2:22. doi: 10.1186/1743-0003-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condron JE, Hill KD. Reliability and validity of a dual-task force platform assessment of balance performance: effect of age, balance impairment, and cognitive task. Journal of the American Geriatrics Society. 2002;50:157–162. doi: 10.1046/j.1532-5415.2002.50022.x. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Sagar HJ, Jordan N, Harvey NS, Sullivan EV. Cognitive impairment in early, untreated Parkinson's disease and its relationship to motor disability. Brain. 1991;114(Pt 5):2095–2122. doi: 10.1093/brain/114.5.2095. [DOI] [PubMed] [Google Scholar]
- Costa M, Peng CK, Goldberger AL, Hausdorff JM. Multiscale entropy analysis of human gait dynamics. Physica A - Statistical Mechanics and Its Applications. 2003;330:53–60. doi: 10.1016/j.physa.2003.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danion F, Varraine E, Bonnard M, Pailhous J. Stride variability in human gait: the effect of stride frequency and stride length. Gait and Posture. 2003;18:69–77. doi: 10.1016/s0966-6362(03)00030-4. [DOI] [PubMed] [Google Scholar]
- Del Olmo MF, Cudeiro J. Temporal variability of gait in Parkinson disease: effects of a rehabilitation programme based on rhythmic sound cues. Parkinsonism and Related. 2005;11:25–33. doi: 10.1016/j.parkreldis.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Dingwell JB, Cavanagh PR. Increased variability of continuous overground walking in neuropathic patients is only indirectly related to sensory loss. Gait and Posture. 2001;14:1–10. doi: 10.1016/s0966-6362(01)00101-1. [DOI] [PubMed] [Google Scholar]
- Dingwell JB, Cusumano JP, Cavanagh PR, Sternad D. Local dynamic stability versus kinematic variability of continuous overground and treadmill walking. Journal of Biomechanical Engineering. 2001;123:27–32. doi: 10.1115/1.1336798. [DOI] [PubMed] [Google Scholar]
- Dingwell JB, Gu Kang H, Marin LC. The effects of sensory loss and walking speed on the orbital dynamic stability of human walking. Journal of Biomechanics. 2006 doi: 10.1016/j.jbiomech.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Ebersbach G, Sojer M, Valldeoriola F, Wissel J, Muller J, Tolosa E, et al. Comparative analysis of gait in Parkinson's disease, cerebellar ataxia and subcortical arteriosclerotic encephalopathy. Brain. 1999;122(Pt 7):1349–1355. doi: 10.1093/brain/122.7.1349. [DOI] [PubMed] [Google Scholar]
- Eckert T, Barnes A, Dhawan V, Frucht S, Gordon MF, Feigin AS, et al. FDG PET in the differential diagnosis of parkinsonian disorders. NeuroImage. 2005;26:912–921. doi: 10.1016/j.neuroimage.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Elias JW, Treland JE. Executive function in Parkinson's disease and subcortical disorders. Seminars in Clinical Neuropsychiatry. 1999;4:34–40. doi: 10.1053/SCNP00400034. [DOI] [PubMed] [Google Scholar]
- Elsinger CL, Rao SM, Zimbelman JL, Reynolds NC, Blindauer KA, Hoffmann RG. Neural basis for impaired time reproduction in Parkinson's disease: An fMRI study. Journal of the International Neuropsychological Society. 2003;9:1088–1098. doi: 10.1017/S1355617703970123. [DOI] [PubMed] [Google Scholar]
- England SA, Granata KP. The influence of gait speed on local dynamic stability of walking. Gait and Posture. 2007;25:172–178. doi: 10.1016/j.gaitpost.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltner ME, MacRae PG, McNitt-Gray JL. Quantitative gait assessment as a predictor of prospective and retrospective falls in community-dwelling older women. Archives of Physical Medicine and Rehabilitation. 1994;75:447–453. doi: 10.1016/0003-9993(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Frenkel-Toledo S, Giladi N, Peretz C, Herman T, Gruendlinger L, Hausdorff JM. Effect of gait speed on gait rhythmicity in Parkinson's disease: variability of stride time and swing time respond differently. Journal of Neuroengineering and Rehabilitation. 2005a;2 doi: 10.1186/1743-0003-2-23. doi:10.1186/1743-0003-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel-Toledo S, Giladi N, Peretz C, Herman T, Gruendlinger L, Hausdorff JM. Treadmill walking as an external pacemaker to improve gait rhythm and stability in Parkinson's disease. Movement Disorders. 2005b;20:1109–1114. doi: 10.1002/mds.20507. [DOI] [PubMed] [Google Scholar]
- Fuller GF. Falls in the elderly. American Family Physician. 2000;61:2159–2164. [PubMed] [Google Scholar]
- Gabell A, Nayak US. The effect of age on variability in gait. Journal of Gerontology. 1984;39:662–666. doi: 10.1093/geronj/39.6.662. [DOI] [PubMed] [Google Scholar]
- Gates DH, Dingwell JB. Peripheral neuropathy does not alter the fractal dynamics of stride intervals of gait. Journal of Applied Physiology. 2006 doi: 10.1152/japplphysiol.00413.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgoulis AD, Moraiti C, Ristanis S, Stergiou N. A novel approach to measure variability in the anterior cruciate ligament deficient knee during walking: The use of the approximate entropy in orthopaedics. Journal of Clinical Monitoring and Computing. 2006;20:11–18. doi: 10.1007/s10877-006-1032-7. [DOI] [PubMed] [Google Scholar]
- Giladi N, McDermont M, Fahn S, Przedborski S, Jankovic J, Stern M, et al. Freezing of gait in Parkinson's disease. Neurology. 2001;56:1712–1721. doi: 10.1212/wnl.56.12.1712. [DOI] [PubMed] [Google Scholar]
- Giladi N, Herman T, Reider-Groswasser II, Gurevich T, Hausdorff JM. Clinical characteristics of elderly patients with a cautious gait of unknown origin. Journal of Neurology. 2005;252:300–306. doi: 10.1007/s00415-005-0641-2. [DOI] [PubMed] [Google Scholar]
- Goldberger AL, Amaral LA, Hausdorff JM, Ivanov PC, Peng CK, Stanley HE. Fractal dynamics in physiology: alterations with disease and aging. Proceedings of the National Academy of Sciences U.S.A. 2002;99 Suppl 1:2466–2472. doi: 10.1073/pnas.012579499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabiner PC, Biswas ST, Grabiner MD. Age-related changes in spatial and temporal gait variables. Archives of Physical Medicine and Rehabilitation. 2001;82:31–35. doi: 10.1053/apmr.2001.18219. [DOI] [PubMed] [Google Scholar]
- Granata KP, England SA. Stability of dynamic trunk movement. Spine. 2006;31:E271–E276. doi: 10.1097/01.brs.0000216445.28943.d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P, Hildebrand K. Fall risk factors in Parkinson's disease. The Journal of Neuroscience Nursing. 2000;32:222–228. doi: 10.1097/01376517-200008000-00006. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Katsumi Y, Fukuyama H, Honda M, Hayashi T, Kimura J, et al. Mechanisms underlying gait disturbance in Parkinson's disease: a single photon emission computed tomography study. Brain. 1999;122(Pt 7):1271–1282. doi: 10.1093/brain/122.7.1271. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM. Stride variability: Beyond length and frequency. Gait & Posture. 2004;20:304. doi: 10.1016/j.gaitpost.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM. Gait variability: Methods, modeling and meaning. Journal of Neuroengineering and Rehabilitation. 2005;2 doi: 10.1186/1743-0003-2-19. doi:10.1186/1743-0003-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff JM, Ashkenazy Y, Peng CK, Ivanov PC, Stanley HE, Goldberger AL. When human walking becomes random walking: Fractal analysis and modeling of gait rhythm fluctuations. Physica A. 2001;302:138–147. doi: 10.1016/s0378-4371(01)00460-5. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Balash J, Giladi N. Effects of cognitive challenge on gait variability in patients with Parkinson's disease. Journal of Geriatric Psychiatry and Neurology. 2003;16:53–58. doi: 10.1177/0891988702250580. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Cudkowicz ME, Firtion R, Wei JY, Goldberger AL. Gait variability and basal ganglia disorders: Stride-to-stride variations of gait cycle timing in Parkinson's disease and Huntington's disease. Movement Disorders. 1998;13:428–437. doi: 10.1002/mds.870130310. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Doniger GM, Springer S, Yogev G, Giladi N, Simon ES. A common cognitive profile in elderly fallers and in patients with Parkinson's disease: The prominence of impaired executive function and attention. Experimental Aging Research. 2006;32:411–429. doi: 10.1080/03610730600875817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff JM, Edelberg HK, Mitchell SL, Goldberger AL, Wei JY. Increased gait unsteadiness in community-dwelling elderly fallers. Archives of Physical Medicine and Rehabilitation. 1997;78:278–283. doi: 10.1016/s0003-9993(97)90034-4. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Forman DE, Ladin Z, Goldberger AL, Rigney DR, Wei JY. Increased walking variability in elderly persons with congestive heart failure. Journal of the American Geriatrics Society. 1994;42:1056–1061. doi: 10.1111/j.1532-5415.1994.tb06209.x. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Forman DE, Pilgrim DM, Rigney DR, Wei JY. A new technique for simultaneous monitoring of electrocardiogram and walking cadence. American Journal of Cardiology. 1992;70:1064–1071. doi: 10.1016/0002-9149(92)90362-3. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Herman T, Baltadjieva R, Gurevich T, Giladi N. Balance and gait in older adults with systemic hypertension. American Journal of Cardiology. 2003;91:643–645. doi: 10.1016/s0002-9149(02)03332-5. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Ladin Z, Wei JY. Footswitch system for measurement of the temporal parameters of gait. Journal of Biomechanics. 1995;28:347–351. doi: 10.1016/0021-9290(94)00074-e. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Lertratanakul A, Cudkowicz ME, Peterson AL, Kaliton D, Goldberger AL. Dynamic markers of altered gait rhythm in amyotrophic lateral sclerosis. Journal of Applied Physiology. 2000;88:2045–2053. doi: 10.1152/jappl.2000.88.6.2045. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Mitchell SL, Firtion R, Peng CK, Cudkowicz ME, Wei JY, et al. Altered fractal dynamics of gait: Reduced stride-interval correlations with aging and Huntington's disease. Journal of Applied Physiology. 1997;82:262–269. doi: 10.1152/jappl.1997.82.1.262. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Nelson ME, Kaliton D, Layne JE, Bernstein MJ, Nuernberger A, et al. Etiology and modification of gait instability in older adults: A randomized controlled trial of exercise. Journal of Applied Physiology. 2001;90:2117–2129. doi: 10.1152/jappl.2001.90.6.2117. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Peng CK, Goldberger AL, Stoll AL. Gait unsteadiness and fall risk in two affective disorders: A preliminary study. BMC Psychiatry. 2004;4:39. doi: 10.1186/1471-244X-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff JM, Peng CK, Ladin Z, Wei JY, Goldberger AL. Is walking a random walk? Evidence for long-range correlations in stride interval of human gait. Journal of Applied Physiology. 1995;78:349–358. doi: 10.1152/jappl.1995.78.1.349. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Purdon PL, Peng CK, Ladin Z, Wei JY, Goldberger AL. Fractal dynamics of human gait: stability of long-range correlations in stride interval fluctuations. Journal of Applied Physiology. 1996;80:1448–1457. doi: 10.1152/jappl.1996.80.5.1448. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Rios D, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Archives of Physical Medicine and Rehabilitation. 2001c;82:1050–1056. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Yogev G, Springer S, Simon ES, Giladi N. Walking is more like catching than tapping: Gait in the elderly as a complex cognitive task. Experimental Brain Research. 2005;164:541–548. doi: 10.1007/s00221-005-2280-3. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Zemany L, Peng C, Goldberger AL. Maturation of gait dynamics: stride-to-stride variability and its temporal organization in children. Journal of Applied Physiology. 1999;86:1040–1047. doi: 10.1152/jappl.1999.86.3.1040. [DOI] [PubMed] [Google Scholar]
- Herman T, Giladi N, Gurevich T, Hausdorff JM. Gait instability and fractal dynamics of older adults with a "cautious" gait: Why do certain older adults walk fearfully? Gait and Posture. 2005;21:178–185. doi: 10.1016/j.gaitpost.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Ho KK, Moody GB, Peng CK, Mietus JE, Larson MG, Levy D, et al. Predicting survival in heart failure case and control subjects by use of fully automated methods for deriving nonlinear and conventional indices of heart rate dynamics. Circulation. 1997;96:842–848. doi: 10.1161/01.cir.96.3.842. [DOI] [PubMed] [Google Scholar]
- Jordan K, Challis JH, Newell KM. Long range correlations in the stride interval of running. Gait & Posture. 2006;24:120–125. doi: 10.1016/j.gaitpost.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Jordan K, Challis JH, Newell KM. Walking speed influences on gait cycle variability. Gait & Posture. 2007a;26:87–102. doi: 10.1016/j.gaitpost.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Jordan K, Challis JH, Newell KM. Speed influences on the scaling behavior of gait cycle fluctuations during treadmill running. Human Movement Science. 2007b;26:87–102. doi: 10.1016/j.humov.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Kannus P, Parkkari J, Koskinen S, Niemi S, Palvanen M, Jarvinen M, et al. Fall induced injuries and deaths among older adults. JAMA. 1999;281:1895–1899. doi: 10.1001/jama.281.20.1895. [DOI] [PubMed] [Google Scholar]
- Kurz MJ, Stergiou N. An artificial neural network that utilizes hip joint actuations to control bifurcations and chaos in a passive dynamic bipedal walking model. Biological Cybernetics. 2005;93:213–221. doi: 10.1007/s00422-005-0579-6. [DOI] [PubMed] [Google Scholar]
- Kurz MJ, Stergiou N, Blanke D. Spanning set defines variability in locomotive patterns. Medical and Biological Engineering and Computing. 2003;41:211–214. doi: 10.1007/BF02344891. [DOI] [PubMed] [Google Scholar]
- Le BR, Billat V, Auvinet B, Chaleil D, Hamard L, Barrey E. Effect of fatigue on stride pattern continuously measured by an accelerometric gait recorder in middle distance runners. The Journal of Sports Medicine and Physical Fitness. 2006;46:227–231. [PubMed] [Google Scholar]
- Leitner Y, Barak R, Giladi N, Peretz C, Eshel R, Gruendlinger L, et al. Gait in attention deficit hyperactivity disorder: Effects of methylphenidate and dual tasking. Journal of Neurology. 2007 doi: 10.1007/s00415-006-0522-3. [DOI] [PubMed] [Google Scholar]
- Lim I, Van Wegen E, De Goede C, Deutekom M, Nieuwboer A, Willems A, et al. Effects of external rhythmical cueing on gait in patients with Parkinson's disease: A systematic review. Clinical Rehabilitation. 2005;19:695–713. doi: 10.1191/0269215505cr906oa. [DOI] [PubMed] [Google Scholar]
- Liotti M, Ramig L. Improvement of voicing in patients with Parkinson's disease by speech therapy. Neurology. 2003;61:1316–1317. doi: 10.1212/wnl.61.9.1316. [DOI] [PubMed] [Google Scholar]
- Liotti M, Ramig LO, Vogel D, New P, Cook CI, Ingham RJ, et al. Hypophonia in Parkinson's disease: Neural correlates of voice treatment revealed by PET. Neurology. 2003;60:432–440. doi: 10.1212/wnl.60.3.432. [DOI] [PubMed] [Google Scholar]
- Lipsitz LA, Mietus J, Moody GB, Goldberger AL. Spectral characteristics of heart rate variability before and during postural tilt. Relations to aging and risk of syncope. Circulation. 1990;81:1803–1810. doi: 10.1161/01.cir.81.6.1803. [DOI] [PubMed] [Google Scholar]
- Lowenthal J, Gruedlinger L, Baltadjieva R, Herman T, Hausdorff JM, Giladi N. Effects of rhythmic auditory stimulation on gait dynamics in Parkinson's disease. Movement Disorders. 2004;19:S139. [Google Scholar]
- Maki BE. Gait changes in older adults: Predictors of falls or indicators of fear. Journal of the American Geriatrics Society. 1997;45:313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- McIntosh GC, Brown SH, Rice RR, Thaut MH. Rhythmic auditory-motor facilitation of gait patterns in patients with Parkinson's disease. Journal of Neurology, Neurosurgery, and Psychiatry. 1997;62:22–26. doi: 10.1136/jnnp.62.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe-Nilssen R, Helbostad JL. Estimation of gait cycle characteristics by trunk accelerometry. Journal of Biomechanics. 2004;37:121–126. doi: 10.1016/s0021-9290(03)00233-1. [DOI] [PubMed] [Google Scholar]
- Moe-Nilssen R, Helbostad JL. Interstride trunk acceleration variability but not step width variability can differentiate between fit and frail older adults. Gait & Posture. 2005;21:164–170. doi: 10.1016/j.gaitpost.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Morris ME, Huxham FE, McGinley J, Iansek R. Gait disorders and gait rehabilitation in Parkinson's disease. Advances in Neurology. 2001;87:347–361. [PubMed] [Google Scholar]
- Morris ME, Iansek R, Matyas TA, Summers JJ. The pathogenesis of gait hypokinesia in Parkinson's disease. Brain. 1994;117(Pt 5):1169–1181. doi: 10.1093/brain/117.5.1169. [DOI] [PubMed] [Google Scholar]
- Morris ME, Iansek R, Matyas TA, Summers JJ. Stride length regulation in Parkinson's disease. Normalization strategies and underlying mechanisms. Brain. 1996;119(Pt 2):551–568. doi: 10.1093/brain/119.2.551. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Meguro K, Sasaki H. Relationship between falls and stride length variability in senile dementia of the Alzheimer type. Gerontology. 1996;42:108–113. doi: 10.1159/000213780. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Meguro K, Yamazaki H, Okuzumi H, Tanaka A, Horikawa A, et al. Postural and gait disturbance correlated with decreased frontal cerebral blood flow in Alzheimer disease. Alzheimer Disease and Associated Disorders. 1997;11:132–139. doi: 10.1097/00002093-199709000-00005. [DOI] [PubMed] [Google Scholar]
- Newell KM, Corcos DM. Variability and motor control. Human Kinetics Publishers; 1993. [Google Scholar]
- Nieuwboer A, Dom R, De WW, Desloovere K, Fieuws S, Broens-Kaucsik E. Abnormalities of the spatiotemporal characteristics of gait at the onset of freezing in Parkinson's disease. Movement Disorders. 2001;16:1066–1075. doi: 10.1002/mds.1206. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Marsden CD, Thompson PD. Human walking and higher-level gait disorders, particularly in the elderly. Neurology. 1993;43:268–279. doi: 10.1212/wnl.43.2.268. [DOI] [PubMed] [Google Scholar]
- Owings TM, Grabiner MD. Measuring step kinematic variability on an instrumented treadmill: How many steps are enough? Journal of Biomechanics. 2003;36:1215–1218. doi: 10.1016/s0021-9290(03)00108-8. [DOI] [PubMed] [Google Scholar]
- Pahapill PA, Lozano AM. The pedunculopontine nucleus and Parkinson's disease. Brain. 2000;123(Pt 9):1767–1783. doi: 10.1093/brain/123.9.1767. [DOI] [PubMed] [Google Scholar]
- Pailhous J, Bonnard M. Steady-state fluctuations of human walking. Behavioral Brain Research. 1992;47:181–189. doi: 10.1016/s0166-4328(05)80124-x. [DOI] [PubMed] [Google Scholar]
- Paleacu D, Shutzman A, Giladi N, Herman T, Simon ES, Hausdorff JM. Effects of pharmacologic therapy on gait and cognitive function in depressed patients. Clinical Neuropharmacology. 2007 doi: 10.1097/01.wnf.0000240949.41691.95. [DOI] [PubMed] [Google Scholar]
- Peng CK, Buldyrev SV, Hausdorff JM, Havlin S, Mietus JE, Simons M, et al. Non-equilibrium dynamics as an indispensable characteristic of a healthy biological system. Integrative Physiological and Behavioral Science. 1994;29:283–293. doi: 10.1007/BF02691332. [DOI] [PubMed] [Google Scholar]
- Peng CK, Hausdorff JM, Havlin S, Mietus JE, Stanley HE, Goldberger AL. Multiple-time scales analysis of physiological time series under neural control. Physica A. 1998;249:491–500. doi: 10.1016/s0378-4371(97)00508-6. [DOI] [PubMed] [Google Scholar]
- Peng CK, Mietus JE, Liu Y, Lee C, Hausdorff JM, Stanley HE, et al. Quantifying fractal dynamics of human respiration: age and gender effects. Annals of Biomedical Engineering. 2002;30:683–692. doi: 10.1114/1.1481053. [DOI] [PubMed] [Google Scholar]
- Peng C-K, Mietus J, Hausdorff JM, Havlin S, Stanley HE, Goldberger AL. Long-range anticorrelations and non-Gaussian behavior of the heartbeat. Physical Review Letters. 1993;70:1343–1346. doi: 10.1103/PhysRevLett.70.1343. [DOI] [PubMed] [Google Scholar]
- Perry J. Gait analysis: Normal and pathological function. Thorofare, NJ: SLACK Inc.; 1992. [Google Scholar]
- Richardson JK, Thies SB, DeMott TK, Ashton-Miller JA. A comparison of gait characteristics between older women with and without peripheral neuropathy in standard and challenging environments. Journal of the American Geriatrics Society. 2004:1532–1537. doi: 10.1111/j.1532-5415.2004.52418.x. [DOI] [PubMed] [Google Scholar]
- Rubenstein TC, Giladi N, Hausdorff JM. The power of cueing to circumvent dopamine deficits: A review of physical therapy treatment of gait disturbances in Parkinson's disease. Movement Disorders. 2002;17:1148–1160. doi: 10.1002/mds.10259. [DOI] [PubMed] [Google Scholar]
- Schaafsma JD, Giladi N, Balash Y, Bartels AL, Gurevich T, Hausdorff JM. Gait dynamics in Parkinson's disease: relationship to Parkinsonian features, falls and response to levodopa. Journal of the Neurological Sciences. 2003;212:47–53. doi: 10.1016/s0022-510x(03)00104-7. [DOI] [PubMed] [Google Scholar]
- Sheridan PL, Solomont L, Kowall N, Hausdorff JM. Influence of executive function on locomotor function: divided attention increases gait variability in Alzheimer's disease. Journal of the American Geriatrics Society. 2003;51:1633–1637. doi: 10.1046/j.1532-5415.2003.51516.x. [DOI] [PubMed] [Google Scholar]
- Springer S, Giladi N, Peretz C, Yogev G, Simon ES, Hausdorff JM. Dual-tasking effects on gait variability: the role of aging, falls, and executive function. Movement Disorders. 2006;21:950–957. doi: 10.1002/mds.20848. [DOI] [PubMed] [Google Scholar]
- Stergiou N, Harbourne R, Cavanaugh J. Optimal movement variability: a new theoretical perspective for neurologic physical therapy. Journal of Neurologic Physical Therapy. 2006;30:120–129. doi: 10.1097/01.npt.0000281949.48193.d9. [DOI] [PubMed] [Google Scholar]
- Stolze H, Kuhtz-Buschbeck JP, Drucke H, Johnk K, Illert M, Deuschl G. Comparative analysis of the gait disorder of normal pressure hydrocephalus and Parkinson's disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2001;70:289–297. doi: 10.1136/jnnp.70.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Levine B. Adult clinical neuropsychology: Lessons from studies of the frontal lobes. Annual Review of Psychology. 2002;53:401–433. doi: 10.1146/annurev.psych.53.100901.135220. [DOI] [PubMed] [Google Scholar]
- Tinetti ME. Prevention of falls and fall injuries in elderly persons: A research agenda. Preventive Medicine. 1994;23:756–762. doi: 10.1006/pmed.1994.1130. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Doucette J, Claus E, Marottoli R. Risk factors for serious injury during falls by older persons in the community. Journal of the American Geriatrics Society. 1995;43:1214–1221. doi: 10.1111/j.1532-5415.1995.tb07396.x. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. New England Journal of Medicine. 1998;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- Visser H. Gait and balance in senile dementia of Alzheimer's type. Age and Ageing. 1983;12:296–301. doi: 10.1093/ageing/12.4.296. [DOI] [PubMed] [Google Scholar]
- West BJ, Latka M. Fractional Langevin model of gait variability. Journal of Neuroengineering and Rehabilitation. 2005;2:24. doi: 10.1186/1743-0003-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West BJ, Scafetta N. Nonlinear dynamical model of human gait. Physical Review E. 2003;67:051917. doi: 10.1103/PhysRevE.67.051917. [DOI] [PubMed] [Google Scholar]
- Willems AM, Nieuwboer A, Chavret F, Desloovere K, Dom R, Rochester L, et al. The use of rhythmic auditory cues to influence gait in patients with Parkinson's disease, the differential effect for freezers and non-freezers, an explorative study. Disability and Rehabilitation. 2006;28:721–728. doi: 10.1080/09638280500386569. [DOI] [PubMed] [Google Scholar]
- Yamasaki M, Sasaki T, Tsuzuki S, Torii M. Effect of mental activity during walking on autoregressive power spectrum of step length. Journal of Human Ergology (Tokyo) 1983;12:95–98. [PubMed] [Google Scholar]
- Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM. Dual tasking, gait rhythmicity, and Parkinson's disease: Which aspects of gait are attention demanding? European Journal of Neuroscience. 2005;22:1248–1256. doi: 10.1111/j.1460-9568.2005.04298.x. [DOI] [PubMed] [Google Scholar]