Abstract
A cDNA from adult female Onchocerca volvulus encoding the C-terminal portion of a tropomyosin isoform (termed MOv-14) has been shown previously to confer protective immunity in rodent models of onchocerciasis. The full-length sequence (designated Ov-tmy-1) obtained by PCR amplification, codes for a protein of 33 kDa and shares 91% identity with tropomyosins from other nematodes, falling to 57% identity with human α-tropomyosin. Ov-TMY-1 migrates with an apparent molecular mass of 42 kDa on SDS/PAGE and is present in all life-cycle stages, as determined by immunoblotting. Immunogold electron microscopy identified antigenic sites within muscle blocks and the cuticle of microfilariae and infective larvae. Anti-MOv14 antibodies were abundant in mice exhibiting serum-transferable protection against microfilariae conferred by vaccination with a PBS-soluble parasite extract. In contrast, little or no MOv14-specific antibody was present in mice inoculated with live microfilariae, in which resistance is mediated by antibody-independent mechanisms. In human infections, there was an inverse correlation between anti-tropomyosin IgG levels and densities of microfilariae in the skin. Seropositivity varied with the relative endemicity of infection. An immunodominant B cell epitope within Ov-TMY-1 (AQLLAEEADRKYD) was mapped to the N terminus of the MOv14 protein by using sera from protectively vaccinated mice. Intriguingly, the sequence coincides with an IgE-binding epitope within shrimp tropomyosin, believed to be responsible for hypersensitivity in individuals exhibiting allergy to shellfish. IgG and IgE antibodies reacting with the O. volvulus epitope were detected in human infections. It is concluded that antibody responses to tropomyosin may be important in limiting microfilarial densities in a proportion of individuals with onchocerciasis and have the potential to mediate hypersensitivity reactions to dead microfilariae, raising the possibility of a link with the immunopathology of infection.
The human filarial parasite Onchocerca volvulus is endemic in sub-Saharan Africa, Latin America, and Arabia, infecting an estimated 18 million people (1). Symptoms vary from mild skin irritation and edema, to gross dermal atrophy and blindness. Many of these manifestations of disease appear to be caused by immunopathological changes in response to the death of microfilariae in the tissues (2), so that the observed clinical spectrum may be in part a reflection of the host immunological spectrum (3).
The epidemiological evidence for acquired immunity to O. volvulus microfilariae in human populations, based on age-related infection intensity studies, is inconclusive (4–6). Nevertheless, parasite-specific IgG1 and IgG3 are inversely correlated with microfilarial load among infected individuals (7, 8), and IgE levels are particularly elevated in “localized onchocerciasis,” a form of the disease characterized by severe, focal skin pathology and few, or no, microfilariae (9, 10). Antibody-dependent cell cytotoxicity reactions mediated by human sera also have been shown to result in the death of microfilariae in vitro (11, 12). A role therefore is indicated for parasite-specific antibodies in host resistance to microfilariae. At the same time, high titers of certain antibody isotypes and/or specificities may correlate with the severity of clinical symptoms, so that a link may exist between antibody-mediated protection and pathology (8–10, 12).
Recent studies in this laboratory, using a mouse model designed to measure the establishment and survival of microfilariae in the skin, have revealed that vaccination with recombinant O. volvulus tropomyosin induces a 48–62% reduction in parasite recoveries compared with controls (13). Significant levels of protection also were conferred when the recombinant protein was used to vaccinate against challenge infections with infective larvae of the related filarial nematode Acanthocheilonema viteae in Mongolian jirds (13). These data accord with previous reports that tropomyosin induces acquired immunity in several helminthic infections of laboratory and domesticated animals (14–16). Here we describe the cloned cDNA from O. volvulus, examine the humoral response in vaccinated rodents, and present data from two independent foci of infection that suggest tropomyosin plays a role in host-protective responses in onchocerciasis.
METHODS
Parasite Material.
Adult worms and microfilariae of O. volvulus were recovered from nodules excised from patients attending a clinic at the Medical Research Laboratories in Bo, Sierra Leone (as part of a clinical management program). Microfilariae were purified on discontinuous Percoll gradients as described (17). O. volvulus infective larvae were obtained from infected blackflies (Simulium damnosum s.l.) in the Ivory Coast. These were supplied by Milan Trpis (Johns Hopkins University, Baltimore), operating an Onchocerciasis Resources Project on behalf of the Edna McConnell Clark Foundation (New York).
Polyspecific Antisera.
A high-titer antiserum to O. lienalis infective, third-stage (L3) larvae was raised by s.c. injection of a rabbit on multiple occasions with living or freeze-killed parasite preparations. The cattle parasite O. lienalis was used for this purpose, because it is known to generate strongly cross-reactive responses to O. volvulus (18) and has been used extensively as a model for the human parasite in laboratory studies (19).
Human sera were derived from two regions endemic for onchocerciasis. Sera from 64 individuals in Ecuador were obtained from Tom Nutman (National Institutes of Health, Bethesda, MD). These were classified with respect to age, sex, ethnic origin, and microfilarial skin densities. Sera from 23 individuals in Mali were provided by Jan Bradley (University of Salford, U.K.), for which data were available on age and skin densities of microfilariae.
Antibodies to Onchocerca microfilariae were raised in mice by using protocols that do, or do not, generate serum capable of conferring passive protection (20, 21). “Vaccination” serum was produced by s.c. immunization of mice with a PBS extract of O. lienalis microfilariae, which was given on successive occasions with Freund’s complete adjuvant, Freund’s incomplete adjuvant, and as an aqueous preparation. Animals were challenged with 5,000 microfilariae, and the serum was collected 15 days later. “Infection” serum was produced by s.c. inoculation of mice with 5,000 live O. lienalis microfilariae. One hundred days later, when the initial infection had cleared, the animals were challenged with a further 5,000 microfilariae and the serum was collected after 15 days. “Vaccination” serum conferred 50% protection against challenge in naive recipients, whereas “infection” serum conferred none. Both serum pools contained a wide range of anti-microfilarial specificities (21).
Immunoscreening of O. volvulus cDNA.
A cDNA library of O. volvulus constructed in λgt11 with mRNA derived from adult female worms was kindly provided by John Donelson from the University of Iowa (22). Bacteriophage plaques (5 × 105) were screened with rabbit anti-O. lienalis L3 antibodies by plaque immunoassay by using standard procedures (23). DNA sequencing identified one of the clones as tropomyosin. Because the cDNA originated from a Mali O. volvulus isolate, it was designated MOv14.
Monospecific Antibody Assays.
Antibodies specific to the protein encoded by MOv14 were isolated from the poly-specific anti-L3 larva rabbit serum by affinity purification against the recombinant protein, as described (24). A high-titer anti-MOv14 antiserum also was prepared by active immunization of a rabbit with MOv14 protein as a fusion with Escherichia coli maltose-binding protein (MBP) after subcloning of the insert from λgt11 into pMALcR1 (New England Biolabs) (25). For immunoblotting, parasite antigens were extracted by boiling in electrophoresis sample and were fractionated on 8–20% SDS/PAGE gels, as described (24). Nitrocellulose filters were probed with antiserum at 1:500 dilution (26). For immunogold electron microscopy, adult females and microfilariae of O. volvulus were fixed within nodules and infective larvae were fixed within blackflies (26). Sections (90 nm) mounted on formvar-coated nickel grids were reacted with rabbit anti-MBP-MOv14 serum (at 1:200 dilution) or affinity-purified antibodies. Antibody–antigen complexes were revealed with protein A-gold conjugate (Sigma; 10 nm gold colloid), and the sections were counterstained with uranyl acetate.
Sequence Determination.
The MOv14 cDNA was amplified by PCR using forward (5′-GGTGGCGACGACTCCTGGAGCCCG-3′) and reverse (5′-TTGACACCAGACCAACTGGTAATG-3′) λgt11 primers (New England Biolabs). The 611-bp product was subcloned into the sequencing vector pCR1000 (Invitrogen) (pCR-MOv14), and the nucleotide sequence was determined by the dideoxy nucleotide chain termination method (27). The deduced amino acid sequence was determined by the genejockey program (Biosoft, Cambridge, U.K. ). blast n and blast p programs were used to search the computer databases for sequence similarities.
blast searches revealed that MOv14 encodes part of a tropomyosin molecule, from which the amino terminus was missing. Primers were designed to derive the 5′ end of the transcript. These comprised an antisense primer (5′-CTCGTGTTTCTGCCTCCTTC-3′) based on sequence at the 5′ end of MOv14, and a sense primer (5′-CATGCGGCCGCGGTTTAATTACCCAAGTTTGAG-3′) based on SL1, the spliced leader sequence that is trans-spliced to the 5′ end of many species of O. volvulus mRNAs (28). Template DNA was prepared from bacteriophage of the O. volvulus cDNA library. The resulting PCR product (792 bp) was subcloned into pCR1000 (Invitrogen) (pCR-SLMOv14) and sequenced.
ELISA Analyses.
MOv14 was cleaved from the MBP-MOv14 fusion protein by digestion with Factor Xa according to the manufacturer’s instructions (New England Biolabs), and the digestion products were separated by ultrafiltration by using a 30-kDa exclusion Centricon tube (Amicon). MOv14 peptide (15 kDa) was recovered from the flow-through.
Human and mouse sera were examined by ELISA as described (26). Plates were coated at a concentration of 2 μg/ml cleaved MOv14 antigen in 0.05 M carbonate buffer (pH 9.6). For the IgG and IgG subclass ELISAs, individual sera were diluted 1:200 and applied to the plates for 2 hr at room temperature. For the human IgE assays, sera were diluted 1:10 and applied to the plates for 16–24 hr at 4°C. Horseradish peroxidase-conjugated goat anti-human IgG[H+L] (Nordic), goat anti-mouse IgG1, IgG2a, or IgM (Nordic), or mouse anti-human IgE (Southern Biotechnology) were used at a dilution of 1:1,000. Plates were developed by using the chromogenic substrate 2,2′-azino-bis (2-ethylbenzthiazoline-6-sulfonic acid; ABTS) (Sigma) and read at an absorbance of 405 nm.
Epitope Mapping.
The Novatope system (Novagen R and D Systems, Abingdon, U.K.) for characterizing B cell epitopes within antigenic polypeptides was used to map sites of antibody binding within recombinant O. volvulus tropomyosin. PCR product spanning the complete ORF of Ov-tmy-1 was digested with DNase I [1:1,330–1:4,500 (U/v)], and the resulting oligonucleotides (average, 50–100 bp in length) were blunt-ended, tailed with a single dATP, and ligated into the plasmid pTOPE T (AMS Biotechnology). Plasmid DNA was introduced into E. coli NovaBlue (DE3) cells (AMS Biotechnology), and transformants were selected with ampicillin.
The library of random, overlapping inserts expressed by transformed cells was screened by colony immunoassay (29) by using a pool of “vaccination” serum from mice protectively immunized against microfilariae (described above). Bacterial colonies expressing immunoreactive peptides were selected and plasmid inserts were sequenced by using primers complementary to flanking regions of the cloning site in pTOPE T. The deduced amino acid sequences were aligned with the ORF of Ov-tmy-1.
Epitope ELISAs.
A multiple antigenic peptide (MAP) was synthesized by Merrifield solid-phase chemistry (30). This comprised a lysine core linking 8 repeats of a 13-aa sequence, identified by Novatope screening as the dominant B cell epitope recognized by “vaccination” serum in O. volvulus tropomyosin (see Fig. 1). IgG and IgE ELISAs were performed as described above, with MAP coated on the plates at a concentration of 5 μg/ml.
Figure 1.
Comparison of the deduced amino acid sequences of O. volvulus muscle tropomyosin (O.v TM), T. colubriformis muscle tropomyosin (T.c TM), C. elegans muscle isoform I (C.e TMI), D. melanogaster muscle isoform II (D.m TMII), and human skeletal α-tropomyosin (H.s α-TM). Amino acids identical to those in the O. volvulus tropomyosin sequence are represented by a dot. The first amino acid encoded by MOv14 is indicated with an arrow, and the mapped B cell epitope is indicated with a bar.
RESULTS AND DISCUSSION
Sequence Analysis.
Sequencing of Ov-tmy-1 cDNA (GenBank accession no. L41633) revealed that the transcript is trans-spliced with the SL1 spliced leader sequence (28), 54 nt upstream of the translation initiation codon. There is a stop codon (TAA) at nucleotide 928 (numbering from the first nucleotide of SL1) and another in-frame stop (TGA) at nucleotide 949. The original cDNA, MOv14, spans nucleotides 514-1122.
The deduced amino acid sequence of Ov-tmy-1 (Fig. 1) possesses the tropomyosin “signature” (LKEAExRAE) (31) at position 221–229, and the sequence contains two overlapping heptad series of hydrophobic residues, which enable tropomyosins to adopt their characteristic coiled-coil conformation (31). The ORF codes for 284 aa residues and has a predicted size of 33.2 kDa, consistent with a muscle tropomyosin (29, 30). The amino-terminal 9 residues (MDAIKKKMQ) are also characteristic of muscle tropomyosins and are thought to mediate muscle contraction via interaction with actin and troponin (31). Nonmuscle isoforms present in platelets, fibroblasts, pancreas, and brain tend to be smaller and are more variable at the N terminus (31, 32).
Ov-TMY-1 shares 91% identity at the amino acid level with the tropomyosins of the sheep intestinal nematode, Trichostrongylus colubriformis (33), and free-living nematode, Caenorhabditis elegans (34). The similarity to tropomyosins from nonnematode species is lower, at 66% identity with Drosophila melanogaster muscle tropomyosin II (35) and 57% identity with human skeletal α-tropomyosin (36) (Fig. 1).
Stage Specificity.
Immunoblotting of parasite extracts with rabbit antibodies raised against MBP-MOv14 revealed a single immunodominant polypeptide with an apparent molecular mass of 42 kDa in each life-cycle stage of O. volvulus examined (microfilariae, infective larvae, adult male, and female worms, data not shown). Similar results were obtained when blots were probed with rabbit anti-L3 serum affinity-purified against β-galactosidase (β-gal)-MOv14 fusion protein (data not shown). The predicted size of Ov-TMY-1 from sequence data is 33.2 kDa, suggesting that it may be posttranslationally modified. However, several tropomyosins have been recorded as migrating anomalously on SDS/PAGE gels, including those of C. elegans (37), Ascaris lumbricoides (38), and T. colubriformis (14). In the case of T. colubriformis, glycanase treatment of native tropomyosin indicated that it was not modified with sugars (14).
Immunoelectron Microscopy.
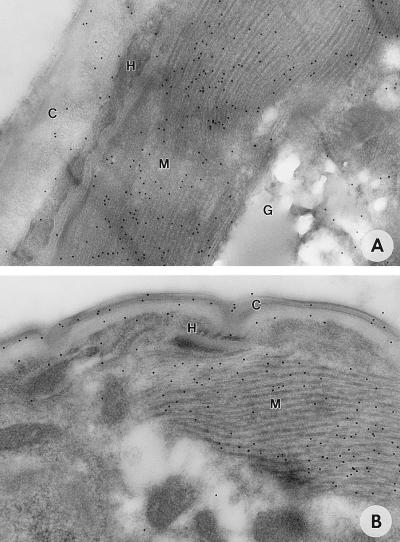
As expected, a high concentration of antigenic sites was detected in parasite muscle by monospecific rabbit anti-MBP-MOv14 antibodies (Fig. 2A). Similar labeling was seen when sections were probed with rabbit anti-L3 serum affinity-purified to β-gal-MOv14 fusion protein (data not shown). No reactivity above background was observed when sections were probed with preimmune rabbit serum or jird anti-MBP serum (data not shown). The sequence similarity between muscle and nonmuscle isoforms of tropomyosin is high, and it is likely that antibodies to muscle tropomyosin will cross-react with nonmuscle tropomyosin. One nonmuscle tissue labeled with rabbit anti-MBP-MOv14 was the cuticle, particularly that of the skin-derived microfilariae (Fig. 2B). Uniform cuticular labeling occurred in every microfilariae examined (n = 20). No labeling was seen in control sections probed with preimmune rabbit serum. Antigenic sites within the cuticle of the infective larva also were observed, but with less regularity (Fig. 2A).
Figure 2.
Immunogold electron micrographs of Onchocerca life-cycle stages probed with rabbit anti-MOv14 antibodies. (A) Infective larva in the insect vector. (×114,000.) (B) Microfilariae in an excised onchocercoma. (×156,000.) Indicated are: C, cuticle; G, glycogen; H, hypodermis; M, muscle block.
Similar to these observations are reports describing the presence of muscle-associated proteins in the tegument of Schistosoma mansoni (39, 40). Indeed, the tegumental localization of paramyosin was postulated to account for the successful immunization of mice against schistosome infection with the invertebrate muscle protein (39, 41, 42). Tropomyosin also has been localized to the tegument of S. mansoni by immunocytochemistry (40), but whether it plays a role in the nematode cuticle has not been established. Previous studies from this laboratory have revealed the presence of a low-abundance, cross-reactive 40-kDa antigen in larval secretions (13). This may account for the cuticular localization of antigen, if secretion occurs via the hypodermis and cuticle as described for other secreted products of filarial infective larvae (e.g., ref. 26). It may also underpin the partial host protection induced by recombinant MOv14 protein in rodent models of onchocerciasis (13) and possibly in other parasitic nematode infections, such as T. colubriformis and Haemonchus contortus in sheep (15).
Mouse Serology.
Recent work in a mouse model of onchocerciasis that mimics the phase of skin invasion by microfilariae has pointed to a possible link between antitropomyosin antibodies and the expression of acquired resistance. First, mice can be partially protected against microfilariae by active immunization with recombinant MOv14 protein (13). Second, animals vaccinated with crude parasite extracts that exhibit serum-transferable resistance have antibodies that recognize an antigen comigrating with Onchocerca tropomyosin on Western blots (21). Significantly, this antibody specificity appears to be absent from mice multiply exposed to live microfilariae, in which resistance is mediated by immune mechanisms that are not transferable with serum (20, 21).
To explore this relationship further, sera from mice protectively vaccinated with parasite extracts were examined by subclass ELISA by using recombinant protein (Table 1). Levels of anti-MOv14 antibodies in the vaccinated group greatly exceeded those in animals that had been infected multiply with live microfilariae. The dominant isotype induced by vaccination was IgG1, although significant levels of MOv14-specific IgG2a were also detected. Levels of antitropomyosin IgM were negligible in the sera of both groups of mice (Table 1).
Table 1.
Mean (±SD) serum antibody levels measured by ELISA (optical density, 405 nm) of the isotype-specific response against MOv14 protein in mice protected by vaccination or multiple infection with Onchocerca microfilariae
| Antibody isotype | Vaccinated | Infected | Control |
|---|---|---|---|
| IgG1 | 2.65 (0.19) | 0.24 (0.38) | 0.06 (0.01) |
| IgG2a | 0.76 (0.56) | 0.01 (0.02) | 0.01 (0.01) |
| IgM | 0.09 (0.05) | 0.07 (0.05) | 0.11 (0.03) |
These data confirm the positive association between serum-transferable protection and antitropomyosin antibodies in the mouse model. Because tropomyosin is a conserved molecule shared between parasite and host (see Fig. 1), we wished to define sequences within the protein mediating antibody binding. Consequently, sera from animals vaccinated with parasite extracts containing native tropomyosin were used to map putative host-protective epitope(s) in the O. volvulus antigen.
Epitope Mapping.
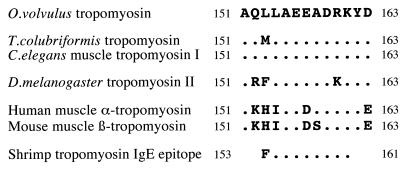
Using the Novatope method of screening for antigenic peptides, immunoreactive plasmids were selected and their inserts sequenced for alignment with the ORF of tropomyosin. Inserts mapped to the same region of the O. volvulus tropomyosin sequence, encompassing amino acid residues 148–163. The shortest peptide mediating antibody binding was 13 aa long, beginning with Ala-151 (Fig. 1). The protein coded by MOv14 cDNA possesses these residues close to the amino terminus of the truncated gene product.
A comparison of the B cell epitope sequence with equivalent regions in homologous genes from other organisms was undertaken to explore the potential for immunological cross-reactivities (Fig. 3). The O. volvulus epitope was between 54 and 100% identical to the various tropomyosins examined, displaying highest identity with both free-living (C. elegans) and parasitic (T. colubriformis) nematode species, and lowest similarity with sequences from the mammalian hosts. Interestingly, the allergen responsible for the adverse affects experienced by some individuals after eating shellfish has been characterized as tropomyosin (43). The IgE-binding epitope recognized by human allergic sera maps to residues 153–161, which resides within the sequence mapped for the IgG-binding epitope of O. volvulus tropomyosin (Fig. 3). All but one of the residues is conserved. This raises the possibility that responses to O. volvulus tropomyosin may also be allergenic and hence could have the potential to drive both protection and pathology in human onchocerciasis. Immediate hypersensitivity has long been recognized to mediate many of the adverse responses to dying or dead microfilariae in the tissues and to be a particular problem after chemotherapy with the microfilaricidal drug diethylcarbamazine (2, 44). In shrimp tropomyosin, the IgG- and IgE-binding epitopes coincide, in which Phe-153 and Leu-154 are thought to be critical in mediating IgE binding (43). In O. volvulus there is a Leu-for-Phe substitution at position 153. To test whether this influenced the binding of human IgE, MBP-MOv14 fusion protein was used in a competitive immunoassay with intact shrimp tropomyosin and shrimp-specific antibodies from individuals presenting with shellfish allergy. MOv14 recombinant protein strongly inhibited IgE binding (Subba Rao, personal communication), indicating the existence of cross-reactivity between the epitopes in spite of the dissimilarity in the amino acid residue at position 153. These data encouraged us to examine the humoral immune response to recombinant O. volvulus tropomyosin in human infections.
Figure 3.
Comparison of the B cell epitope of O. volvulus tropomyosin with the equivalent region of tropomyosins from other organisms. The deduced amino acid sequence shown is that of the shortest peptide recognized by IgG in serum from mice vaccinated with a PBS extract of microfilariae. Aligned are representative sequences from nematodes, an insect, and mammals. The IgE-binding epitope of shrimp tropomyosin also is shown. Amino acids identical to those of the O. volvulus epitope are represented by a dot.
Human Anti-Tropomyosin Antibody Responses.
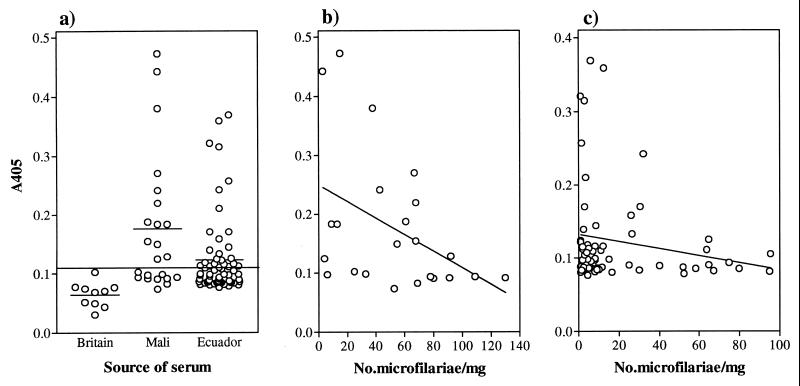
The anti-MOv14 IgG responses of individuals infected with O. volvulus are shown in Fig. 4a. The range of antibody levels among the Ecuadorian sera was broader than that among the British controls, and the means were significantly different (P < 0.001, Mann–Whitney U test) (Fig. 4a). Forty-two percent of the Ecuadorian cases generated an IgG response above the threshold level for positivity, calculated as the mean of British control values plus 2 SD. Among the Mali sera there was a broader range of antibody levels, with a mean value significantly greater than that of either the British (P = 0.001, Mann–Whitney U test) or Ecuadorian (P = 0.008, Mann–Whitney U test) sera (Fig. 4a). Fifty-seven percent of the cases from Mali contained levels of antitropomyosin IgG above the threshold value. The apparently higher reactivity of Mali sera to tropomyosin may reflect differences in the force of transmission between the two regions. In Mali, onchocerciasis is hyperendemic and rates of infection can exceed 80% (6). In contrast, the Ecuadorian sera were derived from people living in the region of the Cayapas River, where the prevalence of infection is closer to 50% (45).
Figure 4.
ELISA analysis of the IgG response to MOv14 of human onchocerciasis infection sera. (a) Comparison of the response of individuals from two onchocerciasis endemic foci with uninfected British controls. Group mean values are represented by a short, horizontal line. The cut-off value is represented by a horizontal line across the graph and is derived from the mean of the control values plus 2 SD. Relationship between microfilarial skin density and anti-MOv14 IgG levels in a Mali focus (b) and an Ecuador focus (c).
Densities of microfilariae in the skin generally are lower in people from New World foci compared with those from most parts of Africa (1). In the study populations examined here, the mean microfilarial density was 19 microfilariae/mg skin in the Ecuadorians and 57 microfilariae/mg in the Malians. The two groups of sera were analyzed for significant correlations between the anti-MOv14 IgG antibody response and age, sex, ethnic origin, and number of nodules in individuals. No significant correlations were found for any of these parameters (data not shown). However, there was an inverse correlation between the anti-MOv14 response and the density of microfilariae in the skin (Figs. 4 b and c). A similar trend was present in each cohort of sera, although only in the population from Mali which had the greater proportion of responders did this reach statistical significance (P = 0.019, Spearman Rank Correlation Coefficient). Taken together with the evidence for protective immunity in the animal models, these data further support the case that antitropomyosin antibodies play a role in the clearance of microfilariae.
IgE antibody responses to MOv14 protein were measured in the same populations. In the majority of individuals there was no detectable antigen-specific IgE, but 22% of the cohort from Mali and 14% of the cohort from Ecuador had antibody levels above the threshold value for positivity, calculated as for IgG above (data not shown). There were insufficient responders to analyze the data for statistically significant associations. Nevertheless, the trend contrasted with that seen for IgG, conforming more closely to a positive correlation between IgE and microfilarial densities. An inverse relationship between IgG and IgE levels was evident in responder individuals. Such a reciprocal relationship between the isotypes might be expected if T cell help mediating class switching during antibody production is polarized as a consequence of infection and can vary among infected individuals as a function of interhost variation and/or of previous infection experience. Differences in the dominant CD4+ T cell population among individuals exposed to infection certainly exist, as illustrated by heterogeneity in cytokine production after antigenic restimulation of peripheral blood leukocytes in vitro (46).
Human Anti-MAP Responses.
The antiepitope IgG response of individuals from the two study populations was investigated in parallel. Fifty-seven percent of the cohort from Mali and 26% from Ecuador had IgG responses that exceeded the threshold value for positivity, with optical densities ranging from 0.19 to 0.68 (data not shown). Mean antibody levels in each population were significantly greater than those among British controls (P < 0.002 and P = 0.001, respectively, Mann–Whitney U test). Although there was an equivalent number of responders to the MAP and to MOv14 protein in the Mali population, fewer reacted with the MAP compared with the protein among the Ecuadorian group. This indicates that there may be additional B cell epitopes within the MOv14 sequence (including conformation-dependent epitopes) that are recognized by some infected individuals. Possibly because of this, a significant correlation was not observed between anti-MAP antibody levels and microfilarial burden. Alternatively, a distinct epitope repertoire may be involved in the expression of immunity to microfilariae in humans and mice.
The anti-MAP IgE response also was examined. Ten percent of individuals from Mali and 8% from Ecuador exhibited responses above the threshold value for positivity, with optical densities ranging from 0.12 to 0.46 (data not shown). Because the clinical presentation of these individuals was not recorded at the time of serum collection, an association with specific pathological manifestations cannot be made. However, the existence of IgE responders to this epitope may warrant further attention that, in view of their frequency in the population, may need to be approached as a case-controlled study.
In conclusion, data from both human and animal studies implicate O. volvulus tropomyosin in host resistance to microfilariae in onchocerciasis. It seems unlikely that responses to tropomyosin act alone to regulate microfilarial densities, and we assume that other mechanisms exist, some of which may act in parallel and may even exacerbate infection intensity by promoting parasite survival, as suggested in other helminth infections (47). It is hard to envisage that the target of functional antibodies in a parasitic nematode, such as O. volvulus, is a muscle isoform of tropomyosin. It is unlikely to be exposed in living microfilariae. Rather, the muscle isoform from dying or dead microfilariae may induce a humoral response that gives rise to cross-reactivity with a nonmuscle form, leading to active parasite destruction most likely through an antibody-dependent cell cytotoxicity reaction. This possibility is supported by the binding of antitropomyosin antibodies to the cuticle of microfilariae, as observed by immunoelectron microscopy. Although antibody appears to have a role in protective responses induced by tropomyosin, antibody-independent mechanisms of host resistance also have been identified in the mouse model (20, 48). Moreover, identification of an immunodominant B cell epitope in tropomyosin that binds both human IgG and IgE raises the intriguing possibility that protection and pathology may be closely linked phenomena, distinguished more by the nature of the host effector mechanism than by the antigenic target of the response. This implies that the host immune response to microfilariae is a complex and dynamic process, consistent with the clinicoparasitological presentation of infection in human populations. Dissection of the fine detail of responses to defined molecules and epitopes is likely to be needed to fully characterize immunological pathways leading to protection and pathology.
Acknowledgments
This research was funded by the Wellcome Trust and Edna McConnell Clark Foundation. M.J.T. is supported by a Wellcome Trust Career Development Fellowship in Basic Biomedical Science. We acknowledge the contributions of Drs. Subba Rao and Eric Ottesen in evaluating MOv14 protein in the IgE-binding assay with shrimp tropomyosin.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: MBP, maltose-binding protein; MAP, multiple antigenic peptide.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database [accession no. L41633 (Ov-tmy-1)].
References
- 1.World Health Organization. WHO Tech Rep Ser. 1995;852:1–104. [PubMed] [Google Scholar]
- 2.Kershaw W E, Duke B O L, Budden F H. Brit Med J. 1954;2:724–729. doi: 10.1136/bmj.2.4890.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ottesen E A. Trans R Soc Trop Med Hyg. 1984;78:9–15. doi: 10.1016/0035-9203(84)90309-2. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. WHO Tech Rep Ser. 1976;597:1–94. [PubMed] [Google Scholar]
- 5.Kirkwood B, Smith P, Marshall T, Prost A. Trans R Soc Trop Med Hyg. 1983;77:857–861. doi: 10.1016/0035-9203(83)90307-3. [DOI] [PubMed] [Google Scholar]
- 6.Karam M, Weiss N. Am J Trop Med Hyg. 1985;34:907–917. doi: 10.4269/ajtmh.1985.34.907. [DOI] [PubMed] [Google Scholar]
- 7.Boyer A E, Tsang V C W, Eberhard M L, Zea-Flores G, Hightower A, Pilcher J, Zea-Flores R, Zhou W, Reimer C B. J Immunol. 1991;146:4001–4010. [PubMed] [Google Scholar]
- 8.Dafa’alla T H, Ghalib H W, Abdelmageed A, Williams J F. Clin Exp Immunol. 1992;88:258–263. doi: 10.1111/j.1365-2249.1992.tb03070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kouemeni L-E, Haque A, Capron A. Clin Exp Immunol. 1982;50:541–548. [PMC free article] [PubMed] [Google Scholar]
- 10.Büttner D W, von Laer G, Mannweiler E, Büttner M. Tropenmed Parasitol. 1982;33:201–212. [PubMed] [Google Scholar]
- 11.Greene B M, Taylor H R, Aikawa M. J Immunol. 1981;127:1611–1618. [PubMed] [Google Scholar]
- 12.MacKenzie C D, Williams J F, Sisley B M, Steward M W, O’Day J. Rev Infect Dis. 1985;7:802–808. doi: 10.1093/clinids/7.6.802. [DOI] [PubMed] [Google Scholar]
- 13.Taylor M J, Jenkins R E, Bianco A E. Parasite Immunol. 1996;18:219–225. doi: 10.1046/j.1365-3024.1996.d01-93.x. [DOI] [PubMed] [Google Scholar]
- 14.O’Donnell I J, Dineen J K, Wagland B M, Letho S, Werkmeister J A, Ward C W. Int J Parasitol. 1989;19:327–335. doi: 10.1016/0020-7519(89)90144-6. [DOI] [PubMed] [Google Scholar]
- 15.Emery D L, Wagland B M. Parasitol Today. 1991;7:347–349. doi: 10.1016/0169-4758(91)90217-c. [DOI] [PubMed] [Google Scholar]
- 16.Hartmann S, Adam R, Marti T, Kirsten C, Seidinger S, Lucius R. Parasitol Res. 1997;83:390–393. doi: 10.1007/s004360050269. [DOI] [PubMed] [Google Scholar]
- 17.Medina-De la Garza C E, Brattig N W, Tischendorf F W. Tropenmed Parasitol. 1987;38:53–54. [PubMed] [Google Scholar]
- 18.Kuo Y M, Bianco A E. Am J Trop Med Hyg. 1995;53:624–632. doi: 10.4269/ajtmh.1995.53.624. [DOI] [PubMed] [Google Scholar]
- 19.Bianco A E. In: Parasitic Helminths, Zoonoses and Human Health in Africa. Macpherson C M L, Craig P S, editors. London: Unwin Hayman Press; 1991. pp. 138–203. [Google Scholar]
- 20.Folkard S G, Bianco A E. Parasite Immunol. 1995;17:541–553. doi: 10.1111/j.1365-3024.1995.tb00885.x. [DOI] [PubMed] [Google Scholar]
- 21.Folkard S G, Jenkins R E, Bianco A E. Trop Med Int Health. 1996;1:359–362. doi: 10.1046/j.1365-3156.1996.d01-48.x. [DOI] [PubMed] [Google Scholar]
- 22.Donelson J E, Duke B O L, Moser D, Zeng W, Erondu N E, Lucius R, Renz A, Karam M, Flores G Z. Mol Biochem Parasitol. 1988;31:241–250. doi: 10.1016/0166-6851(88)90154-5. [DOI] [PubMed] [Google Scholar]
- 23.Young R A, Davis R W. Proc Natl Acad Sci USA. 1983;80:1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozaki L S, Mattei D, Jendoubu M, Druihle P, Blisnick T, Guillotte M, Puijalon O, DaSilva L P. J Immunol Methods. 1986;89:213–219. doi: 10.1016/0022-1759(86)90360-1. [DOI] [PubMed] [Google Scholar]
- 25.Maina C V, Riggs P D, Grandea A G, III, Slatko B E, Moran L S, Tagliamonte J A, McReynolds L A, Guan C. Gene. 1988;74:365–373. doi: 10.1016/0378-1119(88)90170-9. [DOI] [PubMed] [Google Scholar]
- 26.Jenkins R E, Taylor M J, Gilvary N, Bianco A E. Parasite Immunol. 1996;18:29–42. doi: 10.1046/j.1365-3024.1996.d01-10.x. [DOI] [PubMed] [Google Scholar]
- 27.Sanger F, Nicklen S, Coulson A R. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donelson J E, Zeng W. Parasitol Today. 1990;6:327–334. doi: 10.1016/0169-4758(90)90177-6. [DOI] [PubMed] [Google Scholar]
- 29.Hanahan D, Meselson M. Gene. 1980;10:63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- 30.Tam J P, Zavala F J. J Immunol Methods. 1989;124:53–61. doi: 10.1016/0022-1759(89)90185-3. [DOI] [PubMed] [Google Scholar]
- 31.Smillie L B. Trends Biochem Sci. 1979;4:151–155. [Google Scholar]
- 32.Cote G P. Mol Cell Biochem. 1983;57:127–146. doi: 10.1007/BF00849190. [DOI] [PubMed] [Google Scholar]
- 33.Frenkel M J, Savin K W, Bakker R E, Ward C W. Mol Biochem Parasitol. 1989;37:191–200. doi: 10.1016/0166-6851(89)90151-5. [DOI] [PubMed] [Google Scholar]
- 34.Kagawa H, Sugimoto K, Matsumoto H, Inoue T, Imadzu H, Takuwa K, Sakube Y. J Mol Biol. 1995;251:603–613. doi: 10.1006/jmbi.1995.0459. [DOI] [PubMed] [Google Scholar]
- 35.Hanke P D, Storti R V. Gene. 1986;45:211–214. doi: 10.1016/0378-1119(86)90256-8. [DOI] [PubMed] [Google Scholar]
- 36.Reinach F C, MacLeod A R. Nature (London) 1986;322:648–650. doi: 10.1038/322648a0. [DOI] [PubMed] [Google Scholar]
- 37.Harris H E, Tso M-Y W, Epstein H F. Biochemistry. 1977;16:859–864. doi: 10.1021/bi00624a008. [DOI] [PubMed] [Google Scholar]
- 38.Kimura K, Tanaka T, Nakae H, Obinata T. Comp Biochem Physiol. 1987;88B:399–407. [Google Scholar]
- 39.Matsumoto Y, Perry G, Levine R J C, Blanton R, Mahmoud A A F, Aikawa M. Nature (London) 1988;333:76–78. doi: 10.1038/333076a0. [DOI] [PubMed] [Google Scholar]
- 40.MacGregor A N, Shore S J. Int J Parasitol. 1990;20:279–284. doi: 10.1016/0020-7519(90)90141-9. [DOI] [PubMed] [Google Scholar]
- 41.Pearce E J, James S L, Dalton J, Barrall A, Ramos C, Strand M, Sher A. J Immunol. 1986;137:3959–3600. [PubMed] [Google Scholar]
- 42.Pearce E J, James S L, Hieny S, Lanar D E, Sher A. Proc Natl Acad Sci USA. 1988;85:5678–5682. doi: 10.1073/pnas.85.15.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shanti K N, Martin B M, Nagpal S, Metcalfe D D, Rao P V. J Immunol. 1993;151:5354–5363. [PubMed] [Google Scholar]
- 44.Ackerman S J, Kephart G M, Francis H, Awadzi K, Gleich G J, Ottesen E A. J Immunol. 1990;144:3961–3969. [PubMed] [Google Scholar]
- 45.Guderian R H, Molea J, Swanson D, Proano R, Carrillo R, Swanson W L. Tropenmed Parasitol. 1983;34:143–148. [PubMed] [Google Scholar]
- 46.Elson L H, Calvopina H M, Parades Y W, Araujo N E, Bradley J E, Guderian R H, Nutman T B. J Inf Dis. 1995;171:652–658. doi: 10.1093/infdis/171.3.652. [DOI] [PubMed] [Google Scholar]
- 47.Pritchard D I. Parasite Immunol. 1993;15:5–9. doi: 10.1111/j.1365-3024.1993.tb00566.x. [DOI] [PubMed] [Google Scholar]
- 48.Folkard S G, Taylor M J, Butcher G A, Bianco A E. Inf Immunol. 1997;65:2846–2851. doi: 10.1128/iai.65.7.2846-2851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]