Abstract
The initial use of a tracer of phenylalanine was by Moss and Schoenheimer in rats in 1940 to determine that phenylalanine was hydroxylated to tyrosine, defining for the first time the primacy of this pathway. Phenylalanine and tyrosine kinetics were not measured in humans until the 1970–80s. The first application was for determination of the degree of blockage of phenylalanine hydroxylation in patients with hyperphenylalanemia and phenylketonuria, but this approach was expanded to determination of phenylalanine hydroxylation in normal subjects. Far more uses have been demonstrated for measuring rates of phenylalanine disposal and tyrosine production in relatively normal subjects than in patients with in-born errors of metabolism. Key to use of tracers to determine phenylalanine and tyrosine metabolic rates has been development of appropriate tracer models. Most applications have used relatively simple models ignoring the intracellular hydroxylation rate component. Because the liver is the primary site of hydroxylation in the body, the intracellular enrichment at the site of hydroxylation can be assessed from the tracer enrichments at isotopic steady state in rapid-turnover plasma proteins, such as Apo-B, made and secreted by the liver. Although there are potential problems with use of deuterated tracers of phenylalanine, suitable tracers are available and have been demonstrated for general measurement of phenylalanine and tyrosine kinetics in humans.
INDEXING WORDS: phenylalanine metabolism, phenylalanine kinetics, tyrosine metabolism, tyrosine kinetics, phenylketonuria, stable isotope tracers, protein breakdown
The most obvious reason to measure rates of phenylalanine and tyrosine kinetics in humans is to understand the derangements of the metabolism of these amino acids that occurs in patients with the in-born error phenylketonuria (PKU) and in diseases that affect their metabolism, such as in liver or renal disease. However, phenylalanine and tyrosine also have specific characteristics as amino acids that make them useful as markers of protein metabolism. Firstly, both phenylalanine and tyrosine are indispensable amino acids that are essential to our diet. In the postabsorptive state there is no entry of amino acids from dietary sources, and the flux of phenylalanine in the body is derived from entry of phenylalanine released from protein breakdown. That input is matched by phenylalanine removal via protein synthesis and via metabolic disposal by conversion to tyrosine. Therefore, the measurement of the whole body rate of appearance of phenylalanine in the postabsorptive state is a measure of the whole body rate of proteolysis (1). Additional reasons for determining phenylalanine and tyrosine kinetics are the determination of dietary requirements of phenylalanine and tyrosine and measurement of the production of tyrosine from dietary phenylalanine.
History of phenylalanine and tyrosine metabolic measurements
Prior to 1940, there was only circumstantial evidence that tyrosine was produced from phenylalanine. As reported by Moss and Schoenheimer (2), Embden and Blades had shown in 1913 that L-tyrosine was formed in livers perfused with DL-phenylalanine and assumed that phenylalanine metabolism proceeded by conversion to tyrosine. However, Shambaugh, Lewis and Tourtellotte in 1931 suggested that phenylalanine was not converted to tyrosine. Others in the 1920–30’s (2) had shown that hydroxylated metabolites of phenylalanine could be found in the urine of animals given a load of phenylalanine. Probably the most convincing evidence at that time came from the study of Womack and Rose (3) showing that phenylalanine was essential to the diet of rats, but that tyrosine was not, arguing that tyrosine is formed from phenylalanine, but not the reverse.
The 1940 study of Moss and Schoenheimer provided the key evidence and ended the speculation about the metabolism of phenylalanine. They added deuterated DL-phenylalanine to a casein-containing diet given to both growing and adult rats. Samples of tyrosine were isolated from proteins of the animals, and the 2H content determined. The tyrosine from protein in internal organs contained enough 2H to show that 20–30% of the tyrosine had come from the 2H-phenylalanine tracer, demonstrating that tyrosine was produced from phenylalanine and that phenylalanine was metabolized to tyrosine. Today we take these isotopic study results for granted, but in 1940 the use of a deuterated tracer by Moss and Schoenheimer was key in proving the metabolic link between phenylalanine and tyrosine and the ability of phenylalanine to substitute for tyrosine in the diet (3).
The 1940 Moss and Schoenheimer study was not really a kinetic study. Rittenberg, a colleague of Schoenheimer, later developed in the 1950’s a kinetic model using 15N-labeled amino acids to determine rates of whole body protein synthesis in humans. Grümer et al. in 1962 thinking that the Rittenberg approach to measuring the rate of human protein synthesis had too many assumptions and limitations gave a 14C-phenylalanine tracer to four PKU patients. Grümer et al. reasoned that if phenylalanine conversion to tyrosine was effectively blocked in these PKU patients, then the only route of 14C-phenylanine tracer disposal was through incorporation into newly synthesized protein and therefore used these data to develop an alternative model to measure whole body protein synthesis (4). Surprisingly, these authors did not use the 14C-phenylalanine tracer to try to assess residual phenylalanine hydroxylase enzyme activity in these PKU patients.
What quickly became clear from studies in the 1960’s was that measurement of whole body protein synthesis using tracers is actually very difficult. In contrast, whole body protein breakdown is easy to measure using an indispensable amino acid tracer. The dilution of the indispensable amino acid tracer in blood occurs due to release of unlabeled indispensable amino acid from protein breakdown and entry from the diet (1). Knowing the rate of indispensable amino acid intake in fed studies or neglecting this route in postabsorptive subjects, the rate of protein breakdown is readily calculated from the dilution of an intravenously infused indispensable amino acid tracer. The first demonstration in humans of measurement of whole body protein breakdown using an indispensable amino acid tracer was by Waterlow in 1967 using a 14C-lysine tracer. In 1974 O’Keefe et al. used a [1-14C]leucine tracer to measure whole body protein breakdown in humans (5), and other indispensable amino acid tracers have been used since then. However, it was not until 1988 that Darmaun et al. used a [phenyl-2H5]phenylalanine tracer to measure phenylalanine kinetics and whole body protein breakdown (6). Waterlow and colleagues had tried a 14C-tyrosine tracer in 1976 (7), but use of a tyrosine tracer to measure whole body protein breakdown via tracer dilution is inherently problematic because of the unknown amount of phenylalanine converted to tyrosine that also provides an input.
Phenylalanine conversion to tyrosine
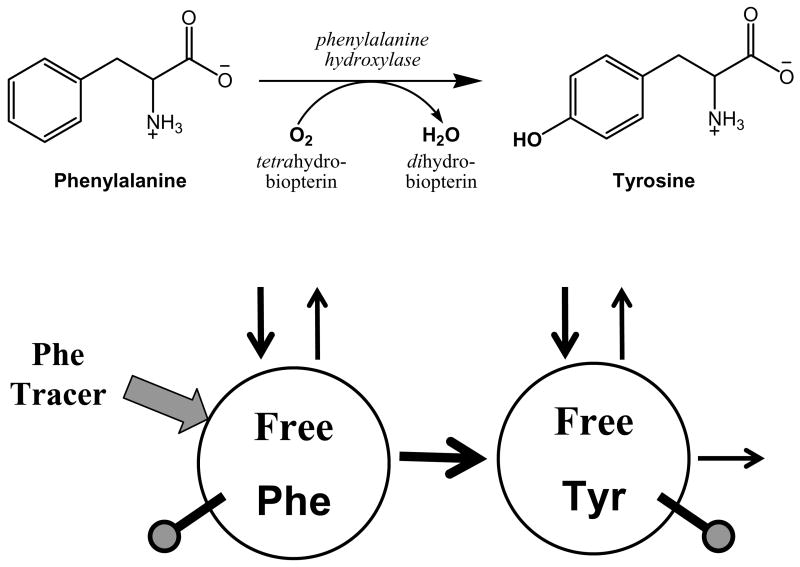
The early principal use of a deuterated phenylalanine tracer was not to measure whole body protein breakdown, but to assess the conversion of phenylalanine to tyrosine. While significant amounts of phenylalanine tracer are normally converted to tyrosine in humans, phenylalanine conversion to tyrosine is limited in PKU patients. Curtius et al. used a 2H-phenylalanine tracer administered to one patient as a proof of concept of this method in 1972 (8) and followed up this report with measurement of phenylalanine conversion to tyrosine in hyperphenylalanemic and PKU patients in 1977 (9). The approach used by Curtius et al. is shown in the model in Fig. 1. The key measurement gleaned is the enrichment of plasma 2H-tyrosine derived from conversation of the [phenyl-2H5]phenylalanine tracer normalized against the plasma 2H-phenylalanine enrichment. This tyrosine/phenylalanine enrichment ratio defines the fraction of free tyrosine that is produced from phenylalanine. In normal postabsorptive humans, ~15% of indispensable amino acids are oxidized in the postabsorptive state (1), and thus phenylalanine disposal through conversion to tyrosine should produce a normal tyrosine/phenylalanine enrichment ratio of ~15%. A PKU patient who has no phenylalanine hydroxylase activity would be expected to have undetectable amounts of 2H-tyrosine in plasma following administration of a 2H-phenylalanine tracer. Values in between 0–15% have been used to classify the degree of impairment of phenylalanine metabolism in patients with derangements of phenylalanine metabolism (10). Because of the very limited conversion capacity or most PKU patients, this method is difficult to apply with good accuracy in PKU due to the low enrichment of 2H-tyrosine.
FIGURE 1.
Model to measure conversion of phenylalanine to tyrosine using a phenylalanine tracer. The top half shows the reaction converting phenylalanine to tyrosine via phenylalanine hydroxylase. The bottom half shows the model equivalent. Circles denote the free phenylalanine (Phe) and tyrosine (Tyr) pools. Arrows indicate rates of appearance and disappearance from the free pools due to whole body protein synthesis and breakdown. The wide arrow indicates administration of a phenylalanine tracer (usually by continuous infusion) into the free phenylalanine pool. Tracer abundance (enrichment) is measured in free phenylalanine and in tyrosine (indicated by the ball and stick figure to denote sampling from the free pools). The ratio of the tyrosine tracer enrichment divided by the phenylalanine tracer enrichment defines the fraction of free tyrosine derived from phenylalanine.
Rather than using phenylalanine tracer conversion to tyrosine to assess derangements of phenylalanine metabolism, Clarke and Bier used the conversion to define normal metabolism (11). Although tyrosine is a dispensable amino acid, tyrosine synthesis in the body depends upon phenylalanine availability. When phenylalanine intake is limited, phenylalanine availability may be limited for production of tyrosine, and tyrosine can become a conditionally indispensable amino acid (1). The model using a phenylalanine tracer and measurement of the tracer in free phenylalanine and tyrosine determines the fraction of tyrosine produced from phenylalanine, but a tyrosine tracer is also needed to determine tyrosine flux and define the absolute amount of tyrosine produced from phenylalanine. Clarke and Bier infused a [1-13C]tyrosine tracer in conjunction with the [phenyl-2H5]phenylalanine tracer to measure simultaneously the turnover rates of both phenylalanine and tyrosine as well as the rate of phenylalanine conversation to tyrosine. The model for this approach is shown in Fig. 2.
FIGURE 2.
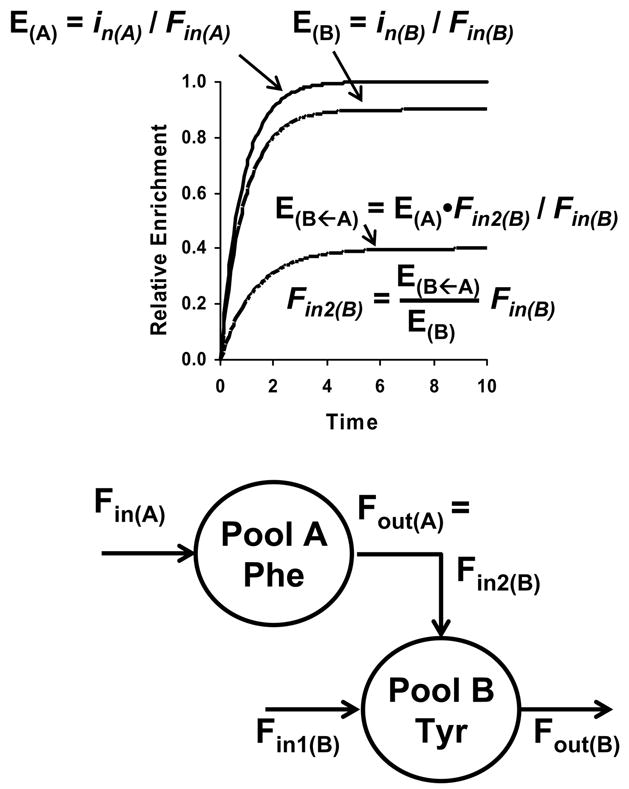
Two pool model of phenylalanine-tyrosine metabolism and tracer enrichments shown during continuous infusion of phenylalanine and tyrosine tracers. Theoretical time courses of the tracer enrichments for phenylalanine (E(A)), tyrosine (E(B)) and the phenylalanine tracer in tyrosine (E(B←A)) are shown in the upper half of the figure. The model is shown in the bottom half where the pools are for free phenylalanine (pool A) and tyrosine (pool B). Inputs into pool A (Fin(A)) and pool B (Fin(B)) are from phenylalanine and tyrosine release from protein breakdown. Phenylalanine disposal (Fout(A)) is by conversion to tyrosine, and this rate equals the rate of tyrosine production from phenylalanine (Fin2(B)). Tyrosine is removed via tyrosine degradation (Fout(B)). Phenylalanine has one input; tyrosine has two with the total rate of input being Fin = Fin1(B) + Fin2(B). Because only the entry of unlabeled phenylalanine and tyrosine dilutes the tracers, rates of phenylalanine and tyrosine uptake for protein synthesis do not affect tracer enrichments and are not shown in the model. The phenylalanine tracer enrichment in blood (E(A)) at isotopic steady state during a continuous infusion of a phenylalanine tracer is the ratio of the rate of phenylalanine tracer infusion (in(A)) and rate of phenylalanine release from protein breakdown (Fin(A)). The rise of the phenylalanine enrichment to reach isotopic steady-state (“plateau”) is shown in the upper half of the figure and is the curve with the plateau enrichment of 1.0. Similarly a tyrosine tracer may be infused, and its enrichment (E(B)) will also be reflective of the rate of tyrosine infusion (in(B)) and rate of tyrosine appearance (Fin(B)). The enrichment of the phenylalanine tracer as tyrosine (E(B←A)) will also rise and reach a plateau during infusion of the phenylalanine tracer (shown as the enrichment curve with a plateau enrichment of 0.4). This enrichment can be used to calculate the rate of tyrosine production from phenylalanine (Fin2(B)).
Tyrosine oxidation
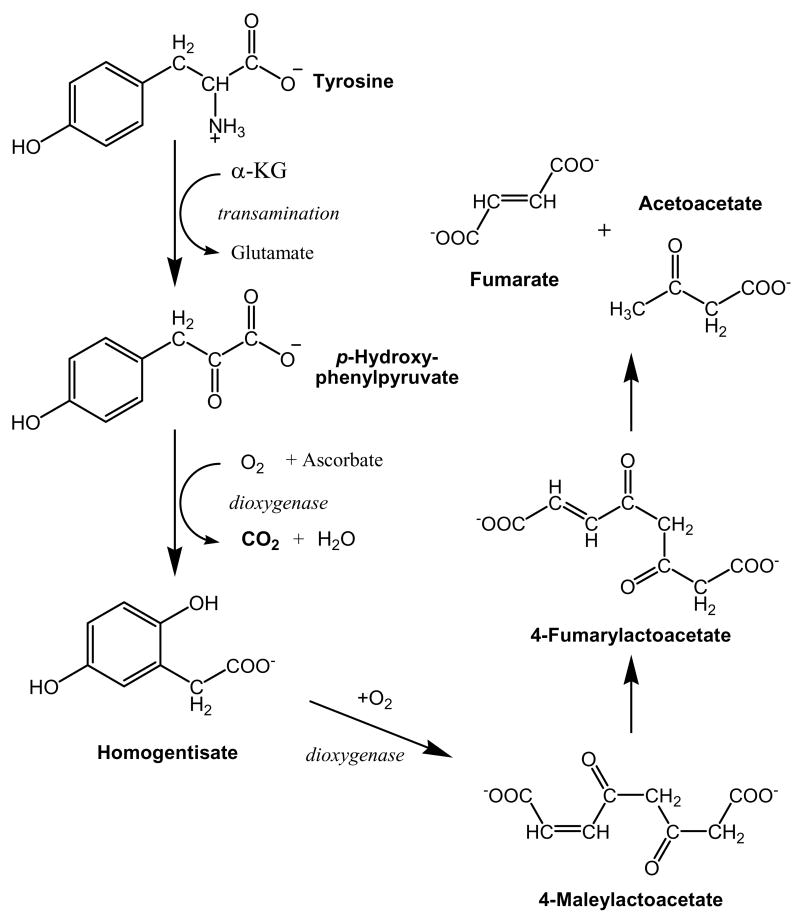
The only rate left unsolved in Fig. 2 as applied by Clarke and Bier is the rate of tyrosine oxidation (Fout(B)). The normal approach to measure whole body oxidation of a tracer would be to use a 13C or 14C tracer that can be recovered in exhaled CO2. The best tracers to measure oxidation are those that are quickly liberated as CO2 in an early metabolic step of the compound being measured, rather than in a distal step in its metabolism when it is more likely for the tracer to enter other compounds rather than being recovered in CO2. Fig. 3 shows the metabolic pathway of tyrosine degradation. The carboxyl carbon of the tyrosine is almost immediately released as CO2 during tyrosine degradation. The remainder of the molecule either ends up as fumarate or acetoacetate. Although both molecules may enter the TCA cycle and the label recovered as CO2, there are also alternative non-oxidative fates. Therefore, a carbon label placed in the phenyl ring of tyrosine will have a lower recovery in CO2 than a carbon isotope placed in the carboxyl position.
FIGURE 3.
Pathway of tyrosine degradation. After the first step of transamination of tyrosine with α-ketoglutarate (α-KG) to form glutamate and the ketoacid of tyrosine, the ketoacid is decarboxylated releasing the carboxyl-C as CO2. From there a series of steps opens up the aromatic ring, eventually forming fumarate and acetoacetate as end products.
Although the choice for a tyrosine label for measurement of tyrosine oxidation should be one with a carboxyl-label, it has not been an easy tracer to obtain for carbon-14. Thus, the earliest measurement of human tyrosine oxidation used a [U-14C]tyrosine label (7), and this choice has continued for carbon-14. The situation is different for stable isotopes. A carboxyl-label is available as the non-radioactive [1-13C]tyrosine, making this label the preferred choice for measuring tyrosine oxidation. The first reported use of the [1-13C]tyrosine label for oxidation was the report of Cortiella et al. in 1992 who also used a [1-13C]phenylalanine label to measure phenylalanine oxidation as well (12). They performed two series of experiments: one where six volunteers were infused with [1-13C]phenylalanine and [2,2-2H2]tyrosine on one day and where they were infused with [2,2-2H2]phenylalanine and [1-13C]tyrosine on another. In both cases, exhaled 13CO2 was determined to define the rate of 13C-tracer oxidation.
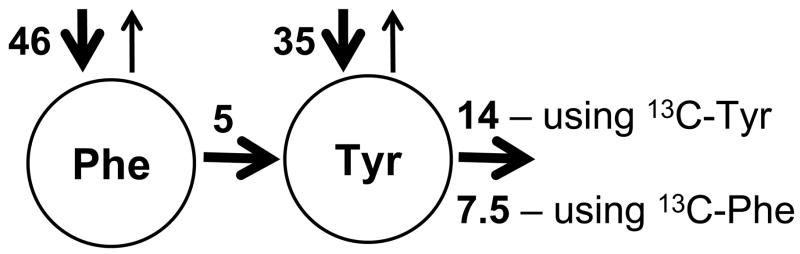
The key results of the Cortiella et al. study (12) are summarized in Fig. 4. While a variety of studies using dual tracers have been performed to study phenylalanine to tyrosine conversion and oxidation of these amino acids, this is the only study where the same subjects were infused with the different 13C labels on different days to obtain a complete picture of oxidation of a phenylalanine label as well as that of a tyrosine label. The results of this study highlight several general points. The first is that in the postabsorptive state, we anticipate that the appearance rate of indispensable amino acids into plasma will be proportional to the rate of protein breakdown and the abundance of each amino acid in protein. For example, leucine is more than twice as abundant as phenylalanine in protein on a molar basis, and the leucine whole body flux should be more than double the phenylalanine flux. This relationship has be been shown in general to be linear for dispensable amino acids (1). On this basis, we would expect the rate of tyrosine appearance from protein breakdown would be ~60% that of phenylalanine, and if phenylalanine flux is 46 μmol kg−1 h−1 in Fig. 4, then tyrosine appearance from protein breakdown should be ~29 μmol kg−1 h−1 – compared to the observed value by Cortiella et al. (12) of 35 μmol kg−1 h−1. The observed value is the measured tyrosine flux minus the rate determined to be phenylalanine hydroxylation.
FIGURE 4.
A model showing the kinetic results reported by Cortiella et al. for six volunteers infused on one day with [1-13C]phenylalanine and [2,2-2H2]tyrosine on another day with [2,2-2H2]phenylalanine and [1-13C]tyrosine (12). Phenylalanine flux was determined from the dilution of the phenylalanine tracer in plasma and is represented by the bold arrow entering the free phenylalanine pool. Tyrosine flux was similarly determined from the dilution of the tyrosine tracer in plasma, but its flux is the sum of the two bold arrows entering the free tyrosine pool (tyrosine from protein breakdown and tyrosine from phenylalanine hydroxylation). The rate of phenylalanine hydroxylation was determined for each tracer pair from the phenylalanine tracer appearing as tyrosine as described in Fig. 2. The oxidation rates of phenylalanine and tyrosine were determined from the recovery of tracer as 13CO2 in exhaled air. All values are approximate because results from the two groups of infusions have been combined; all values are reported as μmol kg−1 h−1.
We would expect based upon measurements of indispensable amino acids using 13C or 14C tracers in postabsorptive humans that oxidation should be in the range of 15–20% of the flux, or 7–9 μmol kg−1 h−1 for phenylalanine, compared to the observed value of ~5 μmol kg−1 h−1 (Fig 4). More interestingly, the rate of phenylalanine oxidation measured by recovery of the [1-13C]phenylalanine label in exhaled air (~7.5 μmol kg−1 h−1) was higher than the rate determined for phenylalanine hydroxylation (~5μmol kg−1 h−1), but appropriate for the expected phenylalanine oxidation rate. These results (12) would suggest that the rate of phenylalanine hydroxylation was underestimated – especially because the release rate of the 13C-phenylalanine trace has to be underestimated by trapping of 13C label as 13C-tyrosine in newly synthesized protein. Adjusting the rate of phenylalanine hydroxylation upwards would reduce the rate of tyrosine flux entering from protein breakdown toward the expected amount.
The final parameter we can glean from Fig. 4 is the rate of tyrosine oxidation determined from the 13C-tyrosine tracer. As discussed above, we would expect that tyrosine oxidation would be ~6 μmol kg−1 h−1 based upon the tyrosine appearance rate from protein breakdown and the anticipated obligatory oxidation of amino acids in the postabsorptive state. Added on top of this value would be that phenylalanine that must be oxidized via tyrosine, estimated to be ~8 μmol kg−1 h−1 for a total expected tyrosine oxidation rate of ~14 μmol kg−1 h−1. This expected value for tyrosine oxidation agrees with the measured value for tyrosine oxidation shown in Fig. 4.
The [1-13C]tyrosine tracer appears to be an appropriate marker for tyrosine oxidation, but is the [1-13C]phenylalanine tracer an appropriate marker for phenylalanine oxidation? Almost for sure the 13C-phenylalanine tracer underestimates oxidation because to oxidize the [1-13C]phenylalanine tracer, it must first be hydroxylated to form [1-13C]tyrosine. At that point, there are two possible fates for the tracer: oxidation, as shown in Fig. 2, or incorporation into newly synthesized protein. The 13C-phenylalanine tracer will underestimate phenylalanine oxidation by the amount that the tracer is trapped into newly synthesized protein. The appropriate measure of phenylalanine catabolism is using a phenylalanine-tyrosine tracer pair as described originally by Clarke and Bier (11) and shown in Fig. 2.
Even if phenylalanine oxidation using a 13C-phenylalanine tracer underestimates phenylalanine oxidation, the oxidation of the 13C-phenylalanine tracer still has found important uses. The most extensive use of the [1-13C]phenylalanine tracer has been of the indicator amino acid oxidation (IAAO) method of determining the requirements of individual indispensable amino acids. The IAAO method was developed by Ball et al. in growing pigs (13,14) using a 14C-lysine or 14C-phenylalanine tracer. The concept of the IAAO method is simple: when a single indispensable amino acid is deficient in the diet, the body is limited in how much protein can be synthesized. Under these conditions, there is an excess of the other indispensable amino acids, and the body has no choice other than to oxidize them. The more deficient the diet is in a single indispensable amino acid, the greater the oxidation of the other indispensable amino acids. However, at requirement or just above, the oxidation of indispensable amino acids will be at a minimum. The result is a two-line curve when the oxidation of the indicator amino acid is plotted versus the intake of the indispensable amino acid being manipulated in the test animals or human subjects. An example of this type of plot is shown in the paper of Wilson et al. where the recovery of [1-13C]phenylalanine as 13CO2 (the indicator amino acid) is plotted versus dietary threonine intake in young adult men consuming different levels of dietary threonine (15). The intake of all other amino acids was held constant (including that of the indicator, phenylalanine). At threonine intakes above requirement, phenylalanine oxidation is constant, but phenylalanine oxidation progressively increases as threonine intake is decreased below the threonine requirement. A breakpoint between the two curves is realized indicating a threonine requirement of 19 mg/kg/d. It is not important in the IAAO method that the measured indicator amino acid accurately determines oxidation, rather the measured oxidation value only needs to be responsive and produce a breakpoint as a function of amino acid intake. For this purpose the groups of Ball and Pencharz have focused upon the use of [1-13C]phenylalanine (13–17).
Pitfalls of measuring phenylalanine and tyrosine kinetics
Given the complexities of the methods described above for determining phenylalanine and tyrosine metabolism, what are the pitfalls and limitations of the existing methods? The paper of Cortiella et al. (12) was used as one example to highlight both the power of the measurements and problems where some of the measured phenylalanine and tyrosine kinetic parameters using the model (Fig. 4) were not congruent. There are several possible problems that can affect the kinetic results and produce disparate results. The problem is that most studies do not use a complete range of tracers with multiple infusions as per the Cortiella et al. study (12), and tracer problems will go unnoticed.
Problems with D-amino acid tracer impurities
The first possible problem that can affect almost every amino acid tracer kinetic measurement is the presence of some tracer that is not of the “L” stereoisomer, i.e. is of the opposite “D” configuration. Most synthetic routes to produce either stable or radioisotope tracers of amino acids produce initially racemic mixtures of the “D” and “L” stereoisomers that must subsequently be resolved either by enzymatic degradation or purification via a physical chemical property such as crystallization or chromatography. The original studies of phenylalanine and tyrosine metabolism used racemic “DL” mixtures of tracers (e.g. (2)), but studies since the 1960’s have generally used tracers administered only as the “L” stereoisomer. However, it is not simple to determine whether small amounts of optical “D” stereoisomers are present in the tracer, and even small amounts of “D” stereoisomer can produce untoward effects. Darling et al. reported a study of the effect of some D-stereoisomer in the L-[1-13C]phenylalanine administered (18). However, in general, the effect of D-stereoisomer contamination can be reduced to minimal levels with adequate assay of each lot of tracer before use to determine that it is correctly manufactured as the “L”-only form.
Plasma sampling versus intracellular metabolism
A much more important consideration is that we infuse our tracers into plasma and sample their enrichments from plasma, but the metabolism and interconversion of the tracers is intracellular. We have discussed this problem previously with respect to leucine (1,19,20). The branched-chain amino acids have their first step of metabolism being the reversible transamination step to produce α-ketoacids. The only source of these ketoacids in the body is from these amino acids. As we have illustrated for leucine, the measurement of leucine’s ketoacid, α-ketoisocaproate (KIC), in plasma can be used as an intracellular index of the tracer enrichment of intracellular leucine. In general the plasma KIC enrichment is ~75% that of the plasma leucine tracer, indicating that the rate of leucine transport into and out of cells is about four times faster than the rate of leucine entry into cells from protein breakdown. Therefore, there is a significant difference in the rate of leucine kinetics calculated when the KIC enrichment is used for the calculations compared to the leucine enrichment.
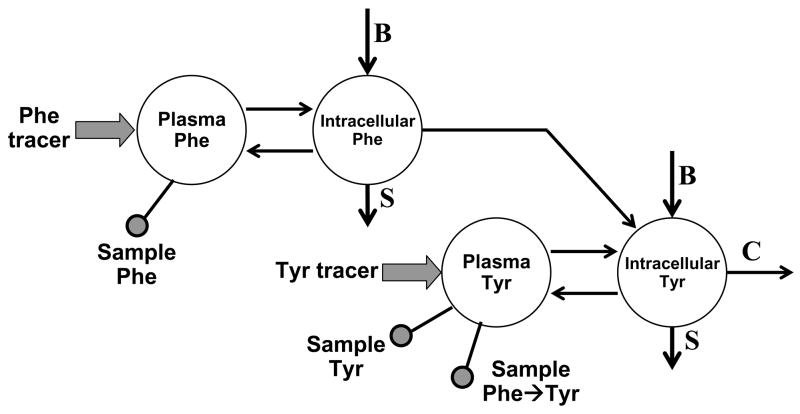
The intracellular compartments have been added in Fig. 5 to the phenylalanine-tyrosine tracer model. Given that amino acid transport for phenylalanine is expected to be similar to that for leucine, we would expect that the intracellular enrichments of both phenylalanine and tyrosine to be about 75% that found in plasma. Arbitrary corrections could be applied to the model in Fig. 5, but it would be better if we had a way to assess the intracellular enrichment directly. In some respects, we are in luck because, in contrast to leucine, the majority of phenylalanine metabolism occurs through hydroxylation in the liver. Therefore, the organ that we need to focus upon is the liver for phenylalanine and tyrosine kinetics.
FIGURE 5.
Model of whole body phenylalanine and tyrosine metabolism including intracellular pools. The model is similar to that shown in Fig. 2. The arrows are labeled with “B” for rate of phenylalanine or tyrosine entry into the intracellular phenylalanine and tyrosine pools from protein breakdown, “S” for rate of phenylalanine or tyrosine uptake from the intracellular pools for new protein synthesis, and “C” for rate of tyrosine oxidation. The model shows infusion inputs for a phenylalanine and a tyrosine tracer (wide arrows) and for sampling from plasma (ball and stick indicator) for phenylalanine tracer enrichment, tyrosine tracer enrichment, and phenylalanine tracer converted to tyrosine (Phe→Tyr).
Although the liver is very difficult to sample in humans, it is also fortuitous to study for amino acid metabolism because it makes a variety of secreted proteins that can be sampled from blood. One of the proteins that is made in significant abundance that has a relatively fast turnover rate in plasma is Apo-B packaged into VLDL. Several groups have measured labeled amino acid incorporation into Apo-B for the purpose of determining its synthetic rate. However, Reeds et al. recognized another purpose for measuring tracer enrichment into Apo-B: the plasma Apo-B tracer enrichment will serve as an intracellular marker for liver intracellular amino acid tracer enrichments (21). Although the enrichment in tyrosine was not measured in this study, Reeds et al. did infuse and measure the enrichment of a phenylalanine tracer in plasma phenylalanine and Apo-B. Based upon the work of Clarke and Bier (11) and Reeds et al. (21), the intracellular compartments outlined in Fig. 5 can be addressed by using a sufficiently long infusion of tracer (≥ 8 h) both to establish a steady state equilibrium of phenylalanine tracer in tyrosine and to define a plateau enrichment in VLDL Apo B phenylalanine and tyrosine.
Isotope effects
The final concern in measuring phenylalanine and tyrosine kinetics is the possibility of an isotope effect affecting these measurements. Although it was not until 1988 that a [phenyl-2H5]phenylalanine tracer was first used in humans to measure phenylalanine kinetics (6), it has been a popular tracer since that time because the multiple deuteriums increases the mass of the tracer well above natural abundance isotopomer levels when measured by gas chromatography-mass spectrometry (GCMS), and it is cost effective. Alternative tracers do not increase mass significantly (e.g. [1-13C]- or [15N]phenylalanine) and have to be measured against a significant natural isotopomer background, especially when a silylated derivative is used. Tracers that increase mass significantly, such as [phenyl-13C6]phenylalanine, are also significantly more expensive.
There is a potential, non-isotope effect, problem with the [15N]phenylalanine tracer in that the same transaminase that begins the degradation of tyrosine may also affect phenylalanine. Branched-chain amino acids also have transamination as the first step in their metabolism, and 15N tracers of these amino acids produce much higher fluxes than found for 13C or 2H labeled tracers, reflective of a rapid and reversible transamination process for branched-chain amino acids (22,23). Presumably a 15N label of a [15N]tyrosine tracer would be similarly affected by transamination in the first step of tyrosine degradation, as per the metabolic process shown in Fig. 3. Because phenylpyruvate, the transamination product of phenylalanine, is found in small concentrations in blood and increases dramatically in hyperphenylalanemia or PKU, we assume that some transamination of phenylalanine occurs normally. The extent of the transamination has not been quantified in humans, but would be expected to be dependent upon phenylalanine concentration, as it is for the branched-chain amino acids or alanine (22–24). Krempf et al. (25) infused 6 volunteers simultaneously with [1-13C]- and [15N]phenylalanine both intravenously and via an intragastric route. They found no significant difference in the measured phenylalanine flux between these tracers. The power of the measurement was such that they should have been able to define a difference of 5% between the fluxes measured with the two tracers if it existed. These results (25) suggest that phenylalanine transamination plays a minor metabolic role and that the 15N-phenylalanine tracer provides an accurate measure of phenylalanine kinetics in normal subjects.
The concern of an actual isotope effect is primarily with the use of the [phenyl-2H5]phenylalanine tracer. As indicated in Fig. 1, one of the deuteriums of this tracer must be removed to hydroxylate the phenylalanine. Depending upon the enzymatic mechanism of the hydroxylation reaction, an isotope effect slowing the rate of 2H-bond breaking could occur (a primary isotope effect) or a secondary isotope effect could arise via one of the adjacent phenyl-deuteriums in the molecule. A primary isotope effect could slow the reaction by half (26). Such an isotope effect for a [2H5]phenylalanine tracer would affect primarily the measurement of the rate of hydroxylation of phenylalanine to tyrosine because this step involves removal of a deuterium. We would expect weak force interactions (e.g. van der Waals, hydrogen-bonding or pi-bond interactions) to be limited upon the [2H5]phenylalanine in the free state in circulating plasma or inside cells and therefore, not to be sufficient to alter the dilution of the [2H5]phenylalanine label in the body (measurement of flux).
Two studies have been completed with the [2H5]phenylalanine tracer to define whether this tracer produces an isotope effect when used to measure phenylalanine kinetics in humans. Krempf et al. (25) also co-infused intravenously or via an intragastric route [2H5]phenylalanine along with [15N]phenylalanine and [1-13C]phenylalanine. There was no significant difference in the postabsorptive state in the flux of phenylalanine in six subjects when the tracers were infused intravenously (power of measurement being approximately 5% of the flux). However, the [2H5]phenylalanine tracer produced lower plasma enrichments (higher flux) in five subjects when given intragastrically compared to the other two tracers. Differences could also be seen within this limited group of subjects between the 15N and 13C tracers, albeit the difference was not as great as produced by the [2H5]phenylalanine tracer.
Marchini et al. infused 4 subjects simultaneously with [1-13C]- and [2H5]phenylalanine and [2,2-2H2]tyrosine in the postabsorptive and fed states (27). No significant difference in flux was observed in the postabsorptive state between the 13C and 2H phenylalanine tracers. However, the [2H5]phenylalanine tracer produced a slightly higher enrichment (lower flux) in the fed state than the 13C-phenylalanine tracer did, implying an isotope effect. Because the effect observed in this study with a [2H5]phenylalanine tracer is the opposite that observed by this same group in their earlier study (25), we cannot conclude from the two studies that the [2H5]phenylalanine tracer measures a different phenylalanine flux than the other tracers or whether the results from these studies are artifactual due to the small numbers of subjects studied.
The key parameter to focus upon remains the measurement of the phenylalanine hydroxylation rate. The Marchini et al. study (27) also measured phenylalanine hydroxylation (via tyrosine enrichment) and found a substantial difference between the [1-13C]- and [2H5]phenylalanine tracers. In both the fasted and fed state, the hydroxylation rate measured with the [2H5]phenylalanine tracer was substantially lower (indicative of a much lower [2H4]tyrosine enrichment compared to [1-13C]tyrosine enrichment). These results depend upon accurate measurement by GCMS of the 3 simultaneous tyrosine tracer species ([1-13C]-, [2,2-2H2]- and [phenyl-2H4]tyrosine) that will have overlapping isotopomer patterns and will be difficult to measure simultaneously in the same sample (28). If there were no measurement problems of the tyrosine sample enrichments, then these results provide the clearest evidence for an isotope effect on the [phenyl-2H5]phenylalanine conversion to [phenyl-2H4]tyrosine in humans (27).
Summary
The most obvious application of determination of phenylalanine and tyrosine kinetics is in patients with impaired ability to metabolize phenylalanine or tyrosine (e.g. in PKU patients). However, the degree of impairment of phenylalanine hydroxylation is usually so large in PKU patients that the whole body tracer approach has not been terribly effective in defining hydroxylation rate differences due to the difficulty of measuring small enrichments of the phenylalanine tracer in tyrosine. The tracer method has been far more effective when applied to normal subjects. Obvious applications are in subjects whose phenylalanine or tyrosine kinetics are altered, as in the case when tyrosine is limited in the diet or in patients who have mild but significant alterations in phenylalanine-tyrosine metabolism (e.g. in patients with liver disease).
A complete picture of phenylalanine-tyrosine metabolism can be accomplished with two tracers (a phenylalanine and a tyrosine tracer) and measurement of the phenylalanine tracer in tyrosine. Although a [1-13C]tyrosine tracer is ideal for determining tyrosine kinetics because we also get a direct measurement of tyrosine oxidation, it may not necessarily be used when combined with a phenylalanine tracer. Although [phenyl-2H5]phenylalanine is an obvious economical choice as a tracer, it may produce a significant isotope effect during conversion to [2H4]tyrosine, making this tracer less desirable for measuring phenylalanine hydroxylation. Therefore, a [1-13C]phenylalanine becomes the desired choice, forcing use of a [2,2-2H2]tyrosine tracer and forfeit of a tyrosine oxidation rate measurement. The [1-13C]phenylalanine tracer has also seen use in the IAAO method of determining indispensable amino acid requirements in humans.
A key to an accurate picture of whole body phenylalanine-tyrosine metabolism requires determination of intracellular enrichments at the site of phenylalanine hydroxylation. Determination of enrichments of faster turnover proteins secreted by liver is an obvious approach to get information about intracellular hepatic enrichments. Apo-B from VLDL has been demonstrated to be useful for phenylalanine in this regard and should be applicable to measurement of tyrosine as well.
Because phenylalanine is an indispensable amino acid, its flux provides a good representation of whole body protein breakdown. One of the more consistent uses of phenylalanine tracers has been measurement or rates of protein breakdown in the whole body and in tissues that do not hydroxylate phenylalanine, e.g. muscle. There are a limited number of indispensable amino acids whose metabolism and availability of tracers coincide for use of determining protein kinetics in humans, and phenylalanine and phenylalanine tracers are important in this regard.
Footnotes
Supported by grants from the National Institute of Health R01-DK38429 and M01-RR00109.
Abbreviations used: GCMS, gas chromatography-mass spectrometry; KIC, ketoisocaproate; Phe, phenylalanine; PKU, phenylketonuria; Tyr, tyrosine; VLDL, very low density lipoproteins
Literature Cited
- 1.Matthews DE. Proteins and amino acids. In: Shils ME, Shike M, Olson JA, Ross AC, Caballero B, Cousins RJ, editors. Modern Nutrition in Health and Disease. 10. Philadelphia: Lippincott, Williams & Wilkins; 2006. pp. 23–60. [Google Scholar]
- 2.Moss AR, Schoenheimer R. The conversion of phenylalanine to tyrosine in normal rats. J Biol Chem. 1940 Sep 1;135:415–29. [Google Scholar]
- 3.Womack M, Rose WC. Feeding experiments with mixtures of highly purified amino acids. VI. the relation of phenylalanine and tyrosine to growth. J Biol Chem. 1934 Nov 1;107:449–58. [Google Scholar]
- 4.Grümer HD, Koblet H, Woodard C. Phenylalanine metabolism in the phenylpyruvic condition. II. An attempt to calculate the daily incorporation of phenylalanine into proteins. J Clin Invest. 1962;41:61–6. doi: 10.1172/JCI104467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Keefe SJD, Sender PM, James WPT. “Catabolic” loss of body nitrogen in response to surgery. Lancet. 1974;304:1035–43. doi: 10.1016/s0140-6736(74)92149-7. [DOI] [PubMed] [Google Scholar]
- 6.Darmaun D, Matthews DE, Bier DM. Physiological hypercortisolemia increases proteolysis, glutamine and alanine production. Am J Physiol Endocrinol Metab. 1988;255:E366–E373. doi: 10.1152/ajpendo.1988.255.3.E366. [DOI] [PubMed] [Google Scholar]
- 7.James WPT, Garlick PJ, Sender PM, Waterlow JC. Studies of amino acid and protein metabolism in normal man with L-[U-14C]tyrosine. Clin Sci Mol Med. 1976;50:525–32. doi: 10.1042/cs0500525. [DOI] [PubMed] [Google Scholar]
- 8.Curtius HC, Völlmin JA, Baerlocher K. The use of deuterated phenylalanine for the elucidation of the phenylalanine-tyrosine metabolism. Clin Chim Acta. 1972;37:277–85. doi: 10.1016/0009-8981(72)90442-1. [DOI] [PubMed] [Google Scholar]
- 9.Curtius HC, Zagalak MJ, Baerlocher K, Schaub J, Leimbacher W, Redweik U. In vivo studies of the phenylalanine-4-hydroxylase system in hyperphenylalaninemics and phenylketonurics. Helv Paediatr Acta. 1977;32:461–9. [PubMed] [Google Scholar]
- 10.Matalon R, Matthews DE, Michals K, Bier DM. The use of deuterated phenylalanine for the in vivo assay of phenylalanine hydroxylase activity in children. J Inherited Metab Dis. 1982;5:17–9. doi: 10.1007/BF01799749. [DOI] [PubMed] [Google Scholar]
- 11.Clarke JTR, Bier DM. The conversion of phenylalanine to tyrosine in man. Direct measurement by continuous intravenous tracer infusions of L-[ring-2H5]phenylalanine and L-[1-13C]tyrosine in the postabsorptive state. Metabolism. 1982;31:999–1005. doi: 10.1016/0026-0495(82)90142-1. [DOI] [PubMed] [Google Scholar]
- 12.Cortiella J, Marchini JS, Branch S, Chapman TE, Young VR. Phenylalanine and tyrosine kinetics in relation to altered protein and phenylalanine and tyrosine intakes in healthy young men. Am J Clin Nutr. 1992;56:517–25. doi: 10.1093/ajcn/56.3.517. [DOI] [PubMed] [Google Scholar]
- 13.Ball RO, Bayley HS. Tryptophan requirement of the 2.5-kg piglet determined by the oxidation of an indicator amino acid. J Nutr. 1984;114:1741–6. doi: 10.1093/jn/114.10.1741. [DOI] [PubMed] [Google Scholar]
- 14.Ball RO, Bayley HS. Influence of dietary protein concentration on the oxidation of phenylalanine by the young pig. Br J Nutr. 1986;55:651–8. doi: 10.1079/bjn19860071. [DOI] [PubMed] [Google Scholar]
- 15.Wilson DC, Rafii M, Ball RO, Pencharz PB. Threonine requirement of young men determined by indicator amino acid oxidation with use of L-[1-13C]phenylalanine. Am J Clin Nutr. 2000;71:757–64. doi: 10.1093/ajcn/71.3.757. [DOI] [PubMed] [Google Scholar]
- 16.Zello GA, Pencharz PB, Ball RO. Dietary lysine requirement of young adult males determined by oxidation of L-[1-13C]phenylalanine. Am J Physiol Endocrinol Metab. 1993;264:E677–E685. doi: 10.1152/ajpendo.1993.264.4.E677. [DOI] [PubMed] [Google Scholar]
- 17.Zello GA, Wykes LJ, Ball RO, Pencharz PB. Recent advances in methods of assessing dietary amino acid requirements for adult humans. J Nutr. 1995;125:2907–15. doi: 10.1093/jn/125.12.2907. [DOI] [PubMed] [Google Scholar]
- 18.Darling PB, Bross R, Wykes LJ, Ball RO, Pencharz PB. Isotopic enrichment of amino acids in urine following oral infusions of L-[1-13C]phenylalanine and L-[1-13C]lysine in humans: Confounding effect of D-[13C]amino acids. Metabolism. 1999;48:732–7. doi: 10.1016/s0026-0495(99)90172-5. [DOI] [PubMed] [Google Scholar]
- 19.Matthews DE, Schwarz HP, Yang RD, Motil KJ, Young VR, Bier DM. Relationship of plasma leucine and α-ketoisocaproate during a L-[1-13C]leucine infusion in man: A method for measuring human intracellular leucine tracer enrichment. Metabolism. 1982;31:1105–12. doi: 10.1016/0026-0495(82)90160-3. [DOI] [PubMed] [Google Scholar]
- 20.Matthews DE. Stable isotope methodologies in studying human amino acid and protein metabolism. Ital J Gastroenterol. 1993;25:72–8. [PubMed] [Google Scholar]
- 21.Reeds PJ, Hachey DL, Patterson BW, Motil KJ, Klein PD. VLDL apolipoprotein B-100, a potential indicator of the isotopic labeling of the hepatic protein synthetic precursor pool in humans: Studies with multiple stable isotopically labeled amino acids. J Nutr. 1992;122:457–66. doi: 10.1093/jn/122.3.457. [DOI] [PubMed] [Google Scholar]
- 22.Matthews DE, Bier DM, Rennie MJ, Edwards RHT, Halliday D, Millward DJ, Clugston GA. Regulation of leucine metabolism in man: A stable isotope study. Science. 1981;214:1129–31. doi: 10.1126/science.7302583. [DOI] [PubMed] [Google Scholar]
- 23.Staten MA, Bier DM, Matthews DE. Regulation of valine metabolism in man: A stable isotope study. Am J Clin Nutr. 1984;40:1224–34. doi: 10.1093/ajcn/40.6.1224. [DOI] [PubMed] [Google Scholar]
- 24.Yang RD, Matthews DE, Bier DM, Wen Z-M, Young VR. Response of alanine metabolism in humans to manipulation of dietary protein and energy intakes. Am J Physiol Endocrinol Metab. 1986;250:E39–E46. doi: 10.1152/ajpendo.1986.250.1.E39. [DOI] [PubMed] [Google Scholar]
- 25.Krempf M, Hoerr RA, Marks L, Young VR. Phenylalanine flux in adult men: Estimates with different tracers and route of administration. Metabolism. 1990;39:560–2. doi: 10.1016/0026-0495(90)90018-8. [DOI] [PubMed] [Google Scholar]
- 26.Melander L, Saunders WH. Reaction Rates of Isotopic Molecules. New York: John Wiley; 1980. [Google Scholar]
- 27.Marchini JS, Castillo L, Chapman TE, Vogt JA, Ajami A, Young VR. Phenylalanine conversion to tyrosine: Comparative determination with L-[ring-2H5]phenylalanine and L-[1-13C]phenylalanine as tracers in man. Metabolism. 1993;42:1316–22. doi: 10.1016/0026-0495(93)90131-7. [DOI] [PubMed] [Google Scholar]
- 28.Jennings ME, II, Matthews DE. Determination of complex isotopomer patterns in isotopically labeled compounds by mass spectrometry. Anal Chem. 2005;77:6435–44. doi: 10.1021/ac0509354. [DOI] [PMC free article] [PubMed] [Google Scholar]