Abstract
Since in vitro study of Buse and Reid in 1975 showing a stimulatory effect of leucine upon rat muscle protein synthesis and reduction in proteolysis, a similar effect has been sought in humans. Sherwin demonstrated in humans in 1978 an improvement in N balance with infusion of leucine in obese subjects fasting to lose weight. A variety of subsequent studies have been performed in humans where leucine alone or the branched-chain amino acids (BCAAs) have been administered in varying amounts and durations and the effect upon protein metabolism has been measured. Measurement of changes in muscle amino acid metabolism were made by arterio-venous difference measurements and by biopsies. An anabolic effect of leucine and the BCAAs on reduction of muscle protein breakdown was found in these studies with no measured effect upon muscle protein synthesis. Later studies using stable isotope tracers to define both whole body protein turnover and leg or arm protein metabolism have similarly concluded that leucine administration specifically induces a reduction in protein breakdown without increasing protein synthesis. This anabolic effect produced through a reduction of protein breakdown in vivo in humans by leucine is contrary to in vitro studies of rat muscle where stimulation of protein synthesis has been demonstrated by leucine. Likewise an increase in protein synthesis has also been demonstrated by insulin in rat muscle that is not seen in humans. Of the various studies administering BCAAs or leucine to humans for varying periods of time and amount, the results have been consistent. In addition no untoward effects have been reported in any of these studies from infusion of the BCAAs at upwards three-times basal flux or six-times normal dietary intake during the fed portion of the day.
INDEXING WORDS: protein synthesis, protein breakdown, leucine
The branched-chain amino acids (BCAAs)2 share common enzymes for the first two degradative steps, transamination and subsequent decarboxylation of the branched-chain keto acids (BCKAs), and the BCAAs are the only amino acids to share common metabolic steps. The BCAAs are also the only indispensable amino acids to have degradative metabolic pathways active in muscle (1–3). After a meal containing protein, half of the BCAAs in the meal will pass through the splanchnic bed during absorption and appear directly in the systemic circulation (4,5). These observations have been confirmed by us and others (3) measuring the direct splanchnic extraction of tracer leucine in humans (6,7). Because muscle and other non-splanchnic tissues are the major sites of postprandial uptake of these BCAAs, it is easy to hypothesize that the BCAAs should be good signals to indicate to peripheral tissues postprandial amino acid availability. As such we could speculate that one or more of the BCAAs could have cell signaling capabilities to tell tissues to increase protein synthesis or reduce protein breakdown to store amino acids following a meal.
One of the earliest clues that one or more of the BCAAs may have signaling properties for promotion of anabolism came from an in vitro study of rat muscle protein metabolism in response to addition of the individual BCAAs (8). Rat hemi-diaphragm muscles were incubated with the individual amino acids and the uptake of 14C-lysine into protein measured, as an index of protein synthesis. Leucine increased 14C-lysine incorporation into muscle protein, but neither isoleucine, valine nor any other amino acid tested produced this effect. Studies blocking transcription with addition of actinomycin-D or translation with cycloheximide suggested that leucine both decreased protein breakdown and stimulated protein synthesis, but not through stimulation of transcription or translation, and that the effects observed were limited among amino acids to leucine (8).
A number of subsequent in vitro studies were completed in rat muscle and liver following the report of Buse and Reid. These studies were reviewed in 1989 (9). All of the papers listed for studies of muscle showed a positive effect of leucine upon stimulation of protein synthesis and reduction of protein breakdown. Similarly studies in liver showed stimulation of protein synthesis by leucine and a reduction in protein breakdown. The initial report of Buse and Reid and follow up studies by others set the stage for testing the effect of leucine in vivo in humans.
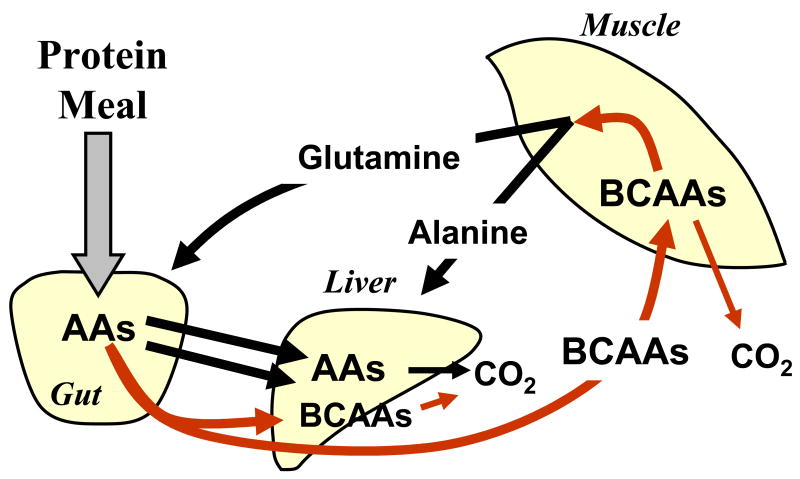
In 1976 Wahren and colleagues, picking up on the earlier observations that muscle extensively oxidizes BCAAs and releases significant amounts of glutamine and alanine, performed a study to measure splanchnic bed balance through use of an hepatic vein catheter and leg balance through arteriovenous (AV) measurements across the leg following feeding of a protein meal in normal postabsorptive humans (4). They determined that the protein meal produced a large absorption and release of amino acids from the splanchnic bed into systemic circulation, largely from the BCAAs (Fig. 1). The BCAAs made up half of the amino acids released following the protein meal. At the same time there was a large uptake by muscle of the BCAAs. Alanine and glutamine were continuously released by muscle and taken up by the splanchnic bed (presumably glutamine by the gut and alanine by the liver), but there was no significant stimulation of release of these amino acids by leg following the meal. The results of this paper established that dietary BCAAs are largely extracted by muscle following a meal and that other amino acids are not extracted postprandially by muscle to the same extent as the BCAAs (4).
Figure 1.
Diagram of the ingestion of protein on splanchnic and leg metabolism in humans. The figure is based upon data contained within the paper by Wahren et al. (4). Muscle released glutamine and alanine prior to the meal and during the meal, while the splanchnic bed extracted glutamine and alanine continuously. Many of the amino acids (AAs) and especially the indispensable amino acids were retained in the splanchnic bed during meal absorption. The BCAAs accounted for about half of the amino acids released from the splanchnic bed post meal, and muscle extracted most of the BCAAs that entered systemic circulation during meal absorption. Excess dietary amino acids that are not incorporated into protein were oxidized to CO2, and muscle is a primary site for BCAA oxidation.
Sherwin infused normal subjects intravenously (iv) for 3 h with leucine at 75 μmol/m2/min (10). This leucine infusion rate corresponds to ≈120 μmol/kg/h in these subjects and is approximately equal to the endogenous leucine flux (3,7). Plasma leucine concentration approximately doubled during the leucine infusion, blood glucose concentration decreased slightly, and plasma insulin and glucagon remained unchanged. These results are typical to what others have found in subsequent studies: leucine infusion does not significantly alter hormone levels, including insulin. Sherwin also noted that insulin infusion caused a significant fall in the plasma concentrations of the other two BCAAs, isoleucine and valine, and decreases in several other amino acids, of which declines in phenylalanine, tyrosine, methionine, threonine, and serine were particularly noted.
Sherwin also infused obese subjects who were fasting for weight loss with leucine for 4 h at 75 μmol/m2/min on the fourth day of fasting and on the 29th and 30th days of fasting (10). He noted that urinary N loss was reduced by ≈25% on the day that leucine was infused (4th day of fasting) and again when leucine was infused on two days after a month of fasting. Because urinary 3-methylhistidine excretion was not altered on the days of leucine infusion, Sherwin concluded that the reduction of urinary N loss was not due to reduced muscle protein breakdown and could be due to a stimulation of protein synthesis, supporting the in vitro observations of Buse. However, there were no direct measures of protein breakdown in this study, and we cannot rule out the possibility that a reduction in non-muscle protein breakdown (e.g. liver protein) could be partially responsible.
Arteriovenous exchange measurements
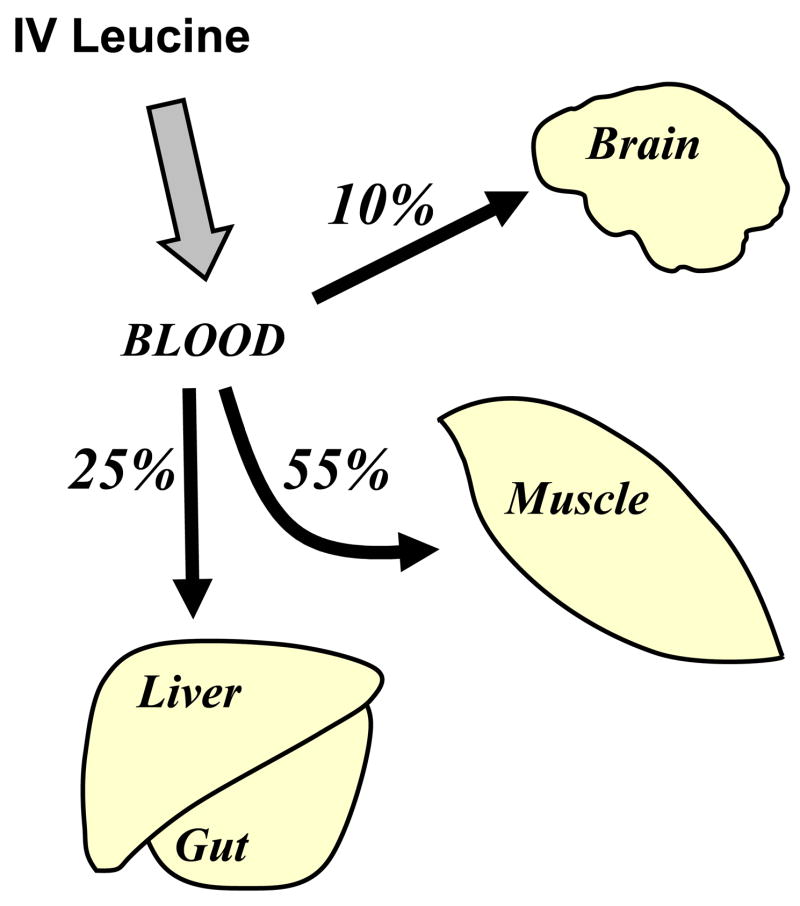
In 1980, Hagenfeldt et al. began a series of studies of leucine and/or BCAA infusions into normal subjects, measuring uptake by various organ beds using AV difference measurements across each bed (11). They first infused leucine iv for 2.5 h at 300 μmol/min, i.e. ≈ 260 μmol/kg/h, which is double the anticipated endogenous leucine turnover (causing leucine flux to triple). They noted large falls in the blood concentrations of valine and isoleucine as well as significant declines in phenylalanine, tyrosine, and methionine. From their AV exchange measurements they noted (Fig. 2) that half of the infused leucine was extracted by muscle (when leg uptake is extrapolated to whole body muscle mass), one quarter of the infused leucine was extracted by the splanchnic bed (gut and liver) and 10% was extracted by the brain. Presumably the remaining 10% was extracted by other tissues (e.g. kidney). They could account for much of the fall of blood concentration of valine and isoleucine through splanchnic bed uptake of these amino acids in response to leucine infusion, but the fall in blood phenylalanine and tyrosine, for example, could not be directly attributed organ uptake. They concluded that elevated leucine through leucine infusion may be altering L-system amino acid transport, essentially restricting free movement of these amino acids, such as phenylalanine and tyrosine, out of cells and causing a reduction of blood concentrations.
Figure 2.
Diagram of the removal of intravenously (iv) infused leucine by different organ beds in humans. The figure is based upon data contained within the paper by Hagenfeldt et al. (11). Leucine was infused for 2.5 h into postabsorptive humans at 300 μmol/min. From AV balance measurements, Hagenfeldt et al. determined that muscle extracted over half of the infused leucine, splanchnic bed extracted about one quarter with the remainder removed by brain and other tissues.
A year later this group (12) infused the individual BCAAs into postabsorptive subjects and measured the effect upon blood amino acid levels. The BCAAs were infused at rates relative to their plasma concentrations to each other (300 μmol/min leucine, 600 μmol/min valine, and 150 μmol/min isoleucine) for 2.5 h. They measured the change in concentration of other amino acids as a function of the change in concentration of the BCAAs and determined that leucine alone produced the most significant changes (decreases) on the concentrations of other amino acids such as phenylalanine, tyrosine, and methionine. Valine and isoleucine had little effect. They concluded that the depression of amino acid concentrations was a leucine-specific, not a BCAA-specific effect. They also concluded, as per the prior study, that the significant fall in the levels of specific amino acid could be from a leucine-specific alteration in L-system amino acid transport. Because there was no general fall in all amino acid concentrations, the authors could not assign any stimulation of protein synthesis effect to the leucine infusion (11).
Because protein breakdown must exceed protein synthesis in the postabsorptive state to accommodate obligatory amino acid oxidation (3), there will be a net release of amino acids from protein into the intracellular compartments. If plasma concentrations of some amino acid fall as a result of leucine infusion restricting amino acid transport, then the intracellular concentrations of the affected amino acids must subsequently rise due to the net release of amino acids from protein. This hypothesis can be directly tested by measuring amino acid intracellular concentrations during a leucine infusion.
The Swedish group then infused leucine iv for 2.5 h at 300 μmol/min (≈ 260 μmol/kg/h and tripling the endogenous leucine flux). Besides measuring AV exchange of amino acids across the leg, they also obtained muscle biopsies prior to and at the end of the leucine infusion in half of the subjects to determine intracellular free amino acid concentrations (13). Based upon their leg AV exchange measurements, they concluded that ≈40% of the infused leucine was extracted by muscle and about half accumulated as intracellular free leucine. Presumably the remainder of the muscle-extracted leucine was oxidized or incorporated into protein. Plasma amino acid concentrations in general fell during leucine infusion with the greatest declines being in those previously shown to be affected. There was a reduced release of alanine and threonine, but no significant changes in the release of other amino acids from the leg were found with leucine infusion. The intracellular muscle concentration of leucine increased threefold with leucine infusion, but the concentration of most other intracellular muscle amino acids either had no change or decreased. These observations indicate that the depression in the plasma concentration of amino acids (e.g. phenylalanine, tyrosine, methionine and threonine) cannot be caused by an altered intracellular/extracellular gradient from altered amino acid transport. The measurement of amino acid exchange across the leg suggested an increase in uptake of amino acids due to a net increase in protein balance (either increased protein synthesis or decreased protein breakdown), but the uncertainty in the measurements precluded making any conclusions.
Arteriovenous exchange measurement using tracers
Louard et al. tested in humans the observation of Buse and Reid in rats that elevating leucine increases muscle protein synthesis and decreases protein breakdown by performing an AV-balance study across the forearm, but adding an infusion of a [phenyl-2,6-3H]phenylalanine and [1-14C]leucine tracers (14). The tracers were used to determine both whole body protein turnover (reflective of protein breakdown in the postabsorptive state) and to measure forearm muscle protein synthesis and breakdown using the phenylalanine tracer exchange measurements across the forearm model (15). They infused iv either saline (control subjects) or BCAAs (100 μmol/kg/h of each BCAA) into postabsorptive subjects for 3 h. This rate of BCAA infusion approximately matches endogenous leucine or valine flux and doubles isoleucine flux (3). Of course BCAA infusion greatly increased the individual plasma concentrations of the BCAAs. Significant declines were observed in plasma concentrations of several amino acids, such as phenylalanine. The AV exchange across the forearm of the indispensable amino acids tended to move toward less release and more uptake. Whole body protein breakdown measured with leucine declined during BCAA infusion, but a nearly similar decline was also noted in the saline control subjects, making this determination not significant. Subtraction of the large leucine infusion rate from the BCAA-infused subject’s leucine turnover made a clean measurement difficult for leucine kinetics. However, phenylalanine flux clearly declined ≈20% with BCAA infusion, defining a reduction in whole body proteolysis. The forearm phenylalanine tracer exchange data supported this result: muscle phenylalanine appearance from protein breakdown declined about one-third with BCAA infusion. There was a nonsignificant decline in the rate of phenylalanine uptake for muscle protein synthesis with BCAA infusion. No stimulation of protein synthesis was measured with BCAA infusion (14). Note also that BCAA infusion did not alter insulin concentrations, thus assigning the reduction of protein breakdown to the leucine component of the BCAA infusion and independent of insulin, which is known to decrease protein breakdown (16,17).
Louard, Barrett and Gelfand repeated their study, but this time addressed the question of whether the acute effects seen with a BCAA infusion would produce a longer term effect or whether the acute effect would wane. Changes in intracellular pool sizes in response to BCAA infusion that could have affected some of the results seen during a short infusion period should stabilize during a longer infusion period. Thus, Louard et al. infused BCAAs at a rate of 100 μmol/kg/h per each BCAA, but this time as a 16-h overnight infusion (18). The tracers [phenyl-2,6-3H]phenylalanine and [1-14C]leucine tracers were infused at the end of the BCAA infusion period to determine both whole body protein turnover and to measure forearm muscle protein synthesis and breakdown using the phenylalanine tracer exchange measurements across the forearm. Subjects who had been infused with saline for a shorter period were used as control subjects. The BCAA infusion increased the individual plasma concentrations of the BCAAs by 5 – 8 times. Significant declines were observed in plasma concentrations of most of the indispensable amino acids, such as phenylalanine. The AV exchange across the forearm of the indispensable amino acids tended to move toward less release and more uptake, but reached significance only for phenylalanine and methionine. Whole body protein breakdown measured with leucine was not different between the subjects infused with BCAAs or saline, but a 37% decline was noted in phenylalanine appearance (a measure of protein breakdown) in the BCAA infused subjects compared to the saline control subjects. The forearm phenylalanine tracer exchange data also showed a reduced rate of phenylalanine appearance from protein breakdown in the BCAA infusion group compared to the saline controls, and there was a nonsignificant decline in the rate of phenylalanine uptake for muscle protein synthesis with BCAA infusion. These results complement the earlier results and demonstrate that a BCAA infusion will produce a reduction in protein breakdown without a change in protein synthesis in humans infused with BCAAs for an extended 16-h period (18).
A similar study was performed by Nair et al. using only a leucine infusion (150 μmol/kg/h) or infusion of saline for 7 h into postabsorptive subjects (19). They measured AV balance across the arm during an infusion of [phenyl-2H5]phenylalanine and [1-13C]valine tracers. Plasma leucine increased four times with the leucine infusion, and there was no change in any measured hormone. Significant declines were observed in plasma concentrations of the other BCAAs, phenylalanine, tyrosine, and methionine. The AV balance across the leg shifted toward less release and more uptake for indispensable amino acids, suggesting a change in muscle protein balance. The whole body release of valine and phenylalanine from protein breakdown measured with tracers was significantly lower in the subjects infused with leucine compared to those receiving saline. The leg phenylalanine tracer exchange data showed a similar result: muscle phenylalanine appearance from protein breakdown was significantly lower in the leucine-infused subjects. There was also a nonsignificant decline in the rate of phenylalanine uptake for muscle protein synthesis with leucine infusion. These results (19) are concordant with the both studies of Louard et al. and also demonstrate that leucine administration produces an anabolic effect by reducing protein breakdown without causing an increase in protein synthesis.
Administration of BCAAs with other amino acids
One of the points that have been demonstrated from several studies is that insulin does not stimulate protein synthesis directly in humans. The fall in amino acid pools with insulin administration is attributed primarily to a reduction in protein breakdown (20). One thought is that protein synthesis cannot be stimulated without provision of amino acid substrate and that a protein stimulatory effect of insulin will not be seen when amino acids levels fall. To see a stimulatory effect, amino acids must also be infused with administration of insulin (21). Nonetheless, it appears that administration of amino acids promote a stimulatory effect on protein synthesis, not insulin (22).
In this context, McNurlan et al. tested the effect of iv administration of BCAAs in addition to the provision of iv amino acids (23). The subjects in this study were patients who were to have colorectal tumors removed. As such, it gave these researchers a chance to also measure tumor rates of synthesis in response to BCAA administration. The patients received for 20 h prior to surgery either (i) saline, (ii) conventional amino acid formula infusion (0.2 g N/kg) or (iii) amino acids with addition of extra BCAAs (30% of the 0.2 g N/kg came from BCAAs). Amino acid infusion stimulated fractional protein synthetic rates equally in muscle and tumor. However, increasing the fraction of administered amino acids from BCAAs did not produce any additional stimulation. If anything, the synthetic rates were slightly reduced compared to the conventional amino acid infusion group. The results from this study showed that amino acid administration will stimulate muscle protein synthesis, but increasing the proportion of administered amino acids as BCAAs has no effect.
In vitro results of leucine stimulation of protein synthesis
Jefferson’s group have performed collectively a series of elegant studies investigating the signaling of protein synthesis stimulation in muscle (24–28). They have identified a leucine-mediated signaling pathway that stimulates protein synthesis in rat muscle through a stimulation of initiation of mRNA translation via activation of the mammalian target of rapamycin (mTOR) protein kinase. This pathway has been shown to be stimulated also through insulin’s cell signaling pathway via the insulin receptor. The activation of mTOR results in the translational repression of eukaryotic initiation factor (eIF) 4E binding protein 1 (4E-BP1) that increases active 4F (eIF4F) complex for initiation of translation and simultaneously enhances phosphorylation of ribosomal protein S6 kinase. The 2001 paper by Anthony et al. provides a good figure diagramming these signaling processes (27). The strong conclusion from this body of work is that leucine very clearly stimulates the processes increasing protein synthesis in at least two ways.
However, these observations from in vitro studies in rat muscle are not supported by results in humans. There are several reasons for these interspecies differences. One possibility is that rat physiology is regulated by different pathways than in humans, and the pathway through mTOR does not show the same level of activity in human muscle. Another possibility is that in vivo regulation is more complex than that defined in in vitro preparations, and other factors may limit the ability of leucine to stimulate protein synthesis through mTOR. Regardless of the explanation, the original in vitro work of Buse and Reid in 1975 through the current work in this decade from Jefferson’s group show a stimulatory effect of leucine on rat muscle protein synthesis that is not shown in studies in humans. The prominent effect in humans is a reduction of protein breakdown with administration of the BCAAs or leucine alone. This situation is analogous to the results found for insulin in humans (causing a reduction in proteolysis) versus animal experiments (observation of stimulation of protein synthesis). It will take additional work to reconcile these differences.
CONCLUSIONS
BCAAs or leucine alone have been administered to humans in a variety of studies. Most studies have provided the BCAAs intravenously rather than enterally. Administration periods ranged from an hour or two to almost a day. The amounts of the BCAAs administered were typically double to triple the normal turnover of the BCAAs and several-fold greater than that with respect to normal daily intake. None of these studies reported any untoward effects of either BCAA administration or administration of leucine alone. Neither BCAA nor leucine administration significantly altered concentrations of circulating hormones. However, both BCAA and/or leucine administration significantly reduced the plasma concentration of several indispensable amino acids. This reduction is not caused by a direct alteration of amino acid transport across cells, but by a reduction of protein breakdown and reduced release of amino acids from cells. This effect has been identified predominantly in muscle. No alteration or stimulation of protein synthesis with either BCAA or leucine administration has been defined in humans. The observed effects for BCAA administration can largely be reproduced by infusion of leucine alone.
The findings of the work to date suggest that leucine and the other BCAAs can be safely consumed in large amounts relative to the other amino acids in protein with no effect upon hormone or protein metabolism. Although large doses of leucine do not appear to stimulate protein synthesis in humans, the observations reported in animals and in vitro discussed above, cannot be dismissed. Thus, any study designed to define the upper limits of tolerance in humans should include measurement of circulating hormones as well as measurement of protein metabolism using isotopically labeled tracers to confirm that high doses of BCAAs do not cause untoward effects with respect to hormonal regulation and protein metabolism in humans.
Footnotes
Supported by grants from the National Institute of Health R01-DK38429 and M01-RR00109.
Abbreviations used: AV, arteriovenous; BCAAs, branched-chain amino acids; BCKAs, branched-chain keto acids; eIF, eukaryotic initiation factor; iv, intravenous; mTOR, mammalian target of rapamycin; 4E-BP, 4E binding protein
References
- 1.Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr. 1984;4:409–454. doi: 10.1146/annurev.nu.04.070184.002205. [DOI] [PubMed] [Google Scholar]
- 2.Wagenmakers AJM, Soeters PB. Metabolism of branched-chain amino acids. In: Cynober LA, editor. Amino Acid Metabolism and Therapy in Health and Nutritional Disease. CRC Press; Boca Raton, FL: 1995. pp. 67–87. [Google Scholar]
- 3.Matthews DE. Proteins and amino acids. In: Shils ME, Olson JA, Shike M, Ross AC, editors. Modern Nutrition in Health and Disease. Williams and Wilkins; Baltimore: 1999. pp. 11–48. [Google Scholar]
- 4.Wahren J, Felig P, Hagenfeldt L. Effect of protein ingestion on splanchnic and leg metabolism in normal man and in patients with diabetes mellitus. J Clin Invest. 1976;57:987–999. doi: 10.1172/JCI108375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elia M, Livesey G. Effects of ingested steak and infused leucine on forelimb metabolism in man and the fate of the carbon skeletons and amino groups of branched-chain amino acids. Clin Sci. 1983;64:517–526. doi: 10.1042/cs0640517. [DOI] [PubMed] [Google Scholar]
- 6.Hoerr RA, Matthews DE, Bier DM, Young VR. Effects of protein restriction and acute refeeding on leucine and lysine kinetics in young men. Am J Physiol Endocrinol Metab. 1993;264:E567–E575. doi: 10.1152/ajpendo.1993.264.4.E567. [DOI] [PubMed] [Google Scholar]
- 7.Matthews DE, Marano MA, Campbell RG. Splanchnic bed utilization of leucine and phenylalanine in humans. Am J Physiol Endocrinol Metab. 1993;264:E109–E118. doi: 10.1152/ajpendo.1993.264.1.E109. [DOI] [PubMed] [Google Scholar]
- 8.Buse MG, Reid SS. Leucine: A possible regulator of protein turnover in muscle. J Clin Invest. 1975;56:1250–1261. doi: 10.1172/JCI108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.May ME, Buse MG. Effects of branched-chain amino acids on protein turnover. Diabetes Metab Rev. 1989;5:227–245. doi: 10.1002/dmr.5610050303. [DOI] [PubMed] [Google Scholar]
- 10.Sherwin RS. Effect of starvation on the turnover and metabolic response to leucine. J Clin Invest. 1978;61:1471–1481. doi: 10.1172/JCI109067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagenfeldt L, Eriksson LS, Wahren J. Influence of leucine on arterial concentrations and regional exchange of amino acids in healthy subjects. Clin Sci. 1980;59:173–181. doi: 10.1042/cs0590173. [DOI] [PubMed] [Google Scholar]
- 12.Eriksson LS, Hagenfeldt L, Wahren J. A comparison of the effects of intravenous infusion of individual branched-chain amino acids on blood amino acid levels in man. Clin Sci. 1981;60:95–100. doi: 10.1042/cs0600095. [DOI] [PubMed] [Google Scholar]
- 13.Alvestrand A, Hagenfeldt L, Merli M, Oureshi A, Eriksson LS. Influence of leucine infusion on intracellular amino acids in humans. Eur J Clin Invest. 1990;20:293–298. doi: 10.1111/j.1365-2362.1990.tb01858.x. [DOI] [PubMed] [Google Scholar]
- 14.Louard RJ, Barrett EJ, Gelfand RA. Effect of infused branched-chain amino acids on muscle and whole-body amino acid metabolism in man. Clin Sci. 1990;79:457–466. doi: 10.1042/cs0790457. [DOI] [PubMed] [Google Scholar]
- 15.Barrett EJ, Revkin JH, Young LH, Zaret BL, Jacob R, Gelfand RA. An isotopic method for measurement of muscle protein synthesis and degradation in vivo. Biochem J. 1987;245:223–228. doi: 10.1042/bj2450223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukagawa NK, Minaker KL, Rowe JW, Goodman MN, Matthews DE, Bier DM, Young VR. Insulin-mediated reduction of whole body protein breakdown: Dose-response effects of leucine metabolism in postabsorptive men. J Clin Invest. 1985;76:2306–2311. doi: 10.1172/JCI112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tessari P, Trevisan R, Inchiostro S, Biolo G, Nosadini R, De Kreutzenberg SV, Duner E, Tiengo A, Crepaldi G. Dose-response curves of effects of insulin on leucine kinetics in humans. Am J Physiol Endocrinol Metab. 1986;251:E334–E342. doi: 10.1152/ajpendo.1986.251.3.E334. [DOI] [PubMed] [Google Scholar]
- 18.Louard RJ, Barrett EJ, Gelfand RA. Overnight branched-chain amino acid infusion causes sustained suppression of muscle proteolysis. Metabolism. 1995;44:424–429. doi: 10.1016/0026-0495(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 19.Nair KS, Schwartz RG, Welle S. Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Am J Physiol Endocrinol Metab. 1992;263:E928–E934. doi: 10.1152/ajpendo.1992.263.5.E928. [DOI] [PubMed] [Google Scholar]
- 20.Matthews DE, Battezzati A. Substrate kinetics and catabolic hormones. In: Kinney JM, Tucker HN, editors. Organ Metabolism and Nutrition: Ideas for Future Critical Care. Raven Press; New York: 1994. pp. 1–22. [Google Scholar]
- 21.Wolfe RR. Effects of insulin on muscle tissue. Curr Opin Clin Nutr Metab Care. 2000;3:67–71. doi: 10.1097/00075197-200001000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Rennie MJ, Bohe J, Wolfe RR. Latency, duration and dose response relationships of amino acid effects on human muscle protein synthesis. J Nutr. 2002;132:3225S–3227S. doi: 10.1093/jn/131.10.3225S. [DOI] [PubMed] [Google Scholar]
- 23.McNurlan MA, Heys SD, Park KGM, Broom J, Brown DS, Eremin O, Garlick PJ. Tumour and host tissue responses to branched-chain amino acid supplementation of patients with cancer. Clin Sci. 1994;86:339–345. doi: 10.1042/cs0860339. [DOI] [PubMed] [Google Scholar]
- 24.Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr. 2000;130:139–145. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- 25.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413–2419. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- 26.Shah OJ, Anthony JC, Kimball SR, Jefferson LS. 4E-BP1 and S6K1: translational integration sites for nutritional and hormonal information in muscle. Am J Physiol Endocrinol Metab. 2000;279:E715–E729. doi: 10.1152/ajpendo.2000.279.4.E715. [DOI] [PubMed] [Google Scholar]
- 27.Anthony JC, Anthony TG, Kimball SR, Jefferson LS. Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. J Nutr. 2001;131:856S–860S. doi: 10.1093/jn/131.3.856S. [DOI] [PubMed] [Google Scholar]
- 28.Jefferson LS, Kimball SR. Amino acid regulation of gene expression. J Nutr. 2001;131:2460S–2466S. doi: 10.1093/jn/131.9.2460S. [DOI] [PubMed] [Google Scholar]