Abstract
Multidrug resistance due to reduced drug accumulation is a phenomenon predominantly caused by the overexpression of members of the ATP-binding cassette transporters, including ABCB1 (P-glycoprotein), ABCG2 and several ABCC family members (MRPs). We previously reported that a thiosemicarbazone derivative, NSC73306, is cytotoxic to carcinoma cells that overexpress functional P-glycoprotein and it re-sensitizes these cells to chemotherapeutics. In this study, we investigated the effect of NSC73306 on cells overexpressing other ABC drug transporters, including ABCG2, MRP1, MRP4 and MRP5. Our findings demonstrated that NSC73306 is not more toxic to cells that overexpress these transporters compared to their respective parental cells, and these transporters do not confer resistance to NSC73306 either. In spite of this, we observed that NSC73306 is a transport substrate for ABCG2 that can effectively inhibit ABCG2-mediated drug transport and reverse resistance to both mitoxantrone and topotecan in ABCG2-expressing cells. Interactions between NSC73306 and the ABCG2 drug-binding site(s) were confirmed by its stimulatory effect on ATPase activity (140–150 nM concentration required for 50% stimulation) and by inhibition of [125I]-Iodoarylazidoprazosin photolabeling (50% inhibition at 250–400 nM) of the substrate-binding site(s). Overall, NSC73306 appears to be a potent modulator of ABCG2 that does not interact with MRP1, MRP4 or MRP5. Collectively, these data suggest that NSC73306 can potentially be used, due to its dual mode of action, as an effective agent to overcome drug resistance by eliminating P-glycoprotein-overexpressing cells, and by acting as a potent modulator that re-sensitizes ABCG2-expressing cancer cells to chemotherapeutics.
Keywords: ABC transporter, ABCG2, ATP hydrolysis, chemosensitization, drug transport, multidrug resistance, photoaffinity labeling, thiosemicarbazones
Introduction
Multidrug resistance (MDR) due to reduced drug accumulation is a phenomenon associated with the overexpression of ATP-binding cassette (ABC) transporters such as P-glycoprotein (Pgp, ABCB1), multidrug resistance associated proteins (MRPs) and ABCG2 or mitoxantrone resistance-associated protein (MXR). These transporters have great clinical significance, given that they can actively efflux a structurally diverse range of chemotherapeutic agents with overlapping specificity (1, 2). ABCG2, also known as the placenta specific ABC transporter (ABCP), and as the breast cancer resistance protein (BCRP) (3, 4) is a ‘half transporter’ that most likely functions as a homo-dimer or higher form oligomer (5, 6) and can efflux a variety of anticancer drugs, including mitoxantrone, doxorubicin, topotecan and SN-38 (7–9). ABCG2 overexpression has been reported in drug-selected carcinoma cell lines (10, 11), and it is also ubiquitously expressed in a variety of stem cells (12, 13). While no clinical trials have yet demonstrated a drug resistance role for ABCG2 in cancer patients (14), inhibitors of ABCG2 have been shown to enhance the efficacy of drugs in mouse preclinical and human clinical trials (15, 16).
Strategies employed to circumvent or resolve the reduced drug accumulation conferred by these poly-specific efflux transporters has relied heavily on the development of clinical inhibitors of Pgp for concurrent administration with chemotherapeutics (17). Although a number of these agents have shown promise in vitro, they have not been successful in clinical trials, probably due to interference with the function of endogenous Pgp and sub-optimal trial design (18). As a result of this, alternative strategies are required to resolve this clinical issue.
Recently, the small molecule thiosemicarbazone NSC73306 was shown to possess a unique property that exploits Pgp expression and function to induce toxicity (19, 20), while not itself being a substrate for Pgp (19, 20). As such, NSC73306 represents a novel strategy to combat MDR in cancer therapy by selectively killing Pgp-expressing drug resistant carcinoma cells, and it is currently undergoing preclinical evaluation in mouse tumor xenografts alone, and in combination with conventional chemotherapeutics such as doxorubicin.
Due to the fact that the molecular target of NSC73306 is normally associated with drug resistance, it is imperative that its interactions with other drug efflux transporters be explored as part of its pre-clinical evaluation. Given the overlapping substrate specificity between Pgp, ABCG2 and to some extent with ABCC1 (16), we have investigated the effect of NSC73306 on transport mediated by a select group of ABCC subfamily members and on ABCG2-mediated transport. Spontaneous mutations in drug-selected cells at amino acid position 482 (from arginine in the wild-type to glycine or threonine in the mutant ABCG2) were shown to have a vital role in both substrate and inhibitor specificity for ABCG2 (21–25). Therefore, both wild-type and mutant ABCG2-overexpressing cells are used to study ABCG2-mediated transport in this study. We observed that NSC73306 does not interact with ABCC1, ABCC4 or ABCC5. However, we demonstrate that NSC73306 is transported by ABCG2 in short term assays, such as [3H]NSC73306 accumulation assays, where the level of [3H]NSC73306 inside the cell is significantly reduced by ABCG2 over a period of 30 min. This result is supported by the finding that NSC73306 stimulates ABCG2 ATPase activity in the nanomolar range and by the inhibitory effect of NSC73306 photolabeling of this transporter with [125I]-Iodoarylazidoprazosin (IAAP). Further, NSC73306 and/or modified forms of NSC73306 compete or interfere with ABCG2-mediated transport, and re-sensitize ABCG2-expressing cancer cells to chemotherapeutics. Thus, the dual mode of action of this compound including its ability to kill Pgp expressing cells and potent modulation of ABCG2 may be exploited to increase the efficacy of chemotherapeutics in the clinic.
Materials and Methods
Drugs and chemicals
NSC73306 and NSC251820 were obtained from the Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program (DTP), Division of Cancer Treatment and Diagnosis, National Cancer Institute, NIH. ZD1694 was a generous gift from AstraZeneca Pharmaceuticals (Macclesfield, Cheshire, UK). Calcein-AM was purchased from Molecular Probes (Eugene, OR). Radiolabeled [125I]IAAP (2200 Ci/mmol) was obtained from PerkinElmer Life Science (Wellesley, MA). [α-32P]-8-Azido-ATP (15–20 Ci/mmol) was obtained from Affinity Labeling Technologies (Lexington, KY). Radiolabeled [3H]NSC73306 (25 Ci/mmol) was purchased from American Radiolabeled Chemicals, Inc. (St. Louis, MO). G418 was procured from RPI Corp. (Mt. Prospect, IL). Cell Counting Kit-8 was purchased from Dojindo Molecular Technologies, Inc. (Gaithersburg, MD). The BXP-21 monoclonal antibody was obtained from Kamiya Biomedical Co. (Seattle, WA). Mouse anti-GAPDH monoclonal antibody was obtained from Zymed Laboratories (Invitrogen). Mitoxantrone and all other chemicals were purchased from Sigma (St. Louis, MO).
Cell lines
The human large cell lung tumor line COR-L23P and its doxorubicin-selected MRP1-overexpressing variant COR-L23R were cultured in RPMI 1640 medium (Gibco, Invitrogen), supplemented with 10% fetal calf serum (FCS) and 100 units of penicillin/streptomycin/mL (Invitrogen, Carlsbad, CA) at 37°C in 5%CO2 humidified air. 0.2 μg/mL doxorubicin was added to the COR-L23R cell culture medium (26). Parental HEK293 cells, MRP4-expressing HEK293/4.63 cells and MRP5-expressing HEK293/5I (27, 28) were maintained in DMEM (Gibco, Invitrogen), supplemented with 10% FCS and 100 units of penicillin/streptomycin/mL at 37°C in 5% CO2 humidified air. G418 (80 μg/mL) was added to the MRP1-HEK293 cell culture medium (29). HEK293 cells stably transfected with either empty pcDNA3.1 vector (pcDNA-HEK293) or pcDNA3.1 containing ABCG2 coding arginine 482 (R482-HEK293) were cultured in Eagle’s MEM (Gibco, Invitrogen), supplemented with 10% FCS, 100 units of penicillin/streptomycin/mL and 2 mg/mL G418 at 37°C in 5% CO2 humidified air (25). MCF-7 cells and sublines were maintained in DMEM supplemented with 10% FCS and penicillin/streptomycin. MCF-7 AdVP3000 (T482-ABCG2) cells were maintained in the presence of 3 μg/mL doxorubicin and 5 μg/mL verapamil, and MCF-7 FLV1000 (R482-ABCG2) cells were cultured in the presence of 1 μg/mL flavopiridol (22, 30). The human colon carcinoma cell line S1 and its drug-resistant subline S1-M1-80 were maintained in RPMI supplemented with 10% FCS and100 units of penicillin/streptomycin/mL. S1-M1-80 was cultured in 80 μM of mitoxantrone as described previously (8).
Cytotoxicity assay
Cell Counting Kit-8 (CCK) assays were used to determine the sensitivities of cells to tested chemicals as described previously (31). Briefly, cells were plated at a density of 2000–3000 cells/well in 100 μL of culture medium into 96-well plates at 37°C for 24 h before adding drugs to make a final volume of 200 μL. Cells were incubated for an additional 72 h with various concentrations of drugs. CCK reagent was then added into each well and incubated for 2–4 h before reading at a wavelength of 450 nm. IC50 values were calculated from fitted dose-response curves obtained from at least three independent experiments.
Fluorescent drug accumulation assay
Efflux assays were carried out using a FACSort flow cytometer equipped with Cell Quest software (Becton-Dickinson, Franklin Lakes, NJ) as described previously (32, 33). Fluorescent substrates mitoxantrone and calcein were used to study ABCG2-mediated and MRP1-mediated efflux, respectively. Briefly, cells were harvested after trypsinization by centrifugation at 500× g and then resuspended in IMDM (Gibco, Invitrogen) supplemented with 5% FCS. 5 μM of mitoxantrone or 0.25 μM of calcein-AM was added to 3 × 105 cells in 4 mL of IMDM in the presence or absence of NSC73306 or Fumitremorgin C (FTC), the ABCG2-specific inhibitor, or MK-571, an inhibitor of MRP1. In the MRP1 efflux study, the cells were incubated in a 37°C water bath in the dark for 10 min, whereas for the ABCG2 efflux, cells were preloaded for 5 min and followed by 45 min incubation at 37°C in the dark prior to being pelleted by centrifugation at 500× g. The cell pellet was then suspended in 300 μL PBS containing 0.1% FCS and analyzed immediately by flow cytometry.
[3H]NSC73306 accumulation assays
MCF-7 or MCF-7 FLV1000 cells were grown in monolayers (0.25 × 106 per well in 24-well plates) at 37°C. The assay was initiated by incubating cells with 100 nmol/L [3H]NSC73306 (25 Ci/mmol). FTC or NSC73306 at 10 μmol/L was added to [125I]IAAP or [3H]NSC73306-treated wells and incubated at 32°C for 5 min under subdued light. After incubation, cells were washed with PBS and lysed by 0.3 mL/well trypsin/EDTA at 37°C for 30 min. The cell lysates were transferred to scintillation vials containing 15 mL Bio-Safe II scintillation fluid, and the radioactivity was measured in a scintillation counter. Cells washed with PBS immediately after addition of the assay mix were used as the 0-min time point. The value for accumulated [3H]NSC73306 at 0 min was subtracted from a given time point as nonspecific binding of these compounds to the cells. The accumulation of labeled compounds was expressed as pmol/106 cells. These assays were performed at 32°C to slow the rate of efflux from the cells so that the accumulation/efflux could be studied in a time-dependent manner.
Photoaffinity labeling of ABCG2 with [125I]IAAP
Crude membranes (50 μg/mL) made from the MCF-7 FLV1000 cells were incubated with varying concentrations of NSC73306 for 10 min at room temperature in 50 mM Tris-HCl, pH 7.5, and then 3–6 nM [125I]IAAP (2200 Ci/mmole) was added. The samples were incubated for an additional 5 min under subdued light. The samples were exposed to a UV lamp (365 nm) for 10 min at room temperature. The labeled ABCG2 was immunoprecipitated using the BXP-21 antibody and processed as described previously (34).
Photoaffinity labeling of ABCG2 with [α-32P]8-azidoATP
Crude membranes from MCF-7 FLV1000 cells were incubated with 25 μM or 50 μM of NSC73306 or 10 mM ATP for 10 min at 4°C in ATPase assay buffer. 10 μM [α-32P] 8-azidoATP (10 μCi/nmole) was added under subdued light and incubated for an additional 5 min at 4°C. The samples were then illuminated with a UV lamp (365 nm) for 10 min and were separated on a SDS-7% Tris-acetate polyacrylamide gel at constant voltage. The gel was dried under vacuum and was exposed to an X-ray film for 1–3 days at −70°C and exposed to Bio-Max MR film at −80°C for 12–24 h.
ATPase assays
ATPase activities of ABCG2 in High Five insect cell crude membranes were measured by endpoint Pi assay as described previously (35, 36). ABCG2-specific ATPase activity was recorded as beryllium fluoride (BeFx)-sensitive ATPase activity. Briefly, the assay measured the amount of Pi released over 20 min at 37°C in the ATPase assay buffer (50 mM MES-Tris, pH 6.8, 50 mM KCl, 5 mM NaN3, 1 mM EGTA, 1 mM ouabain, 2 mM dithiothreitol and 10 mM MgCl2) in the absence and presence of 2.5 mM NaF and 0.2 mM beryllium sulfate. The reaction was initiated by the addition of 5 mM ATP and terminated with SDS (2.5% final concentration). The amount of Pi released was quantified using a colorimetric method (35).
Western blot analysis
Crude membrane protein was prepared and subjected to electrophoresis on a 7.5% SDS-polyacrylamide gel and transferred onto a nitrocellulose membrane as described previously (30). Each blot was then incubated in blocking buffer [5% (w/v) milk powder in 0.1% TBS-Tween (25 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% Tween-20)] for an hour prior to the addition of the ABCG2-specific primary antibody (BXP-21, 1: 500 dilution) or anti-GAPDH primary antibody (1:2000 dilution). The secondary antibody used was the Horseradish peroxidase-conjugated goat anti-mouse IgG (1:10,000 dilution). Signals were detected using an enhanced chemiluminescence kit (GE Healthcare, Piscataway, NJ).
Statistical analysis
Data are mean values +/− standard deviation from at least three independent experiments. Differences between any mean values were analyzed by two-sided Student’s t-test and results were considered statistically significant at P < 0.05.
Results
MRP1-, MRP4-, MRP5- or ABCG2-overexpressing cells are equally sensitive to thiosemicarbazone NSC73306
To investigate whether NSC73306 is specifically toxic to cells that overexpress ABC transporters linked to MDR other than Pgp, the intrinsic cytotoxicity of NSC73306 in cells overexpressing the ABC transporters such as Pgp, MRP1, MRP4, MRP5 and ABCG2 were evaluated (Table 1). To eliminate the possible variability among cell lines, multiple MRP1- and ABCG2-overexpressing cell lines were used in this study. ABCG2-expressing human embryonic kidney (R482-HEK293) cells, human breast carcinoma (MCF7-AdVP3000 and MCF7-FLV1000) cells and human colon carcinoma (S1-M1-80) cells were used to study ABCG2 function. MRP1-expressing HEK293 cells (MRP1-HEK293) and human large-cell lung tumor (COR-L23/R) cells were used to evaluate MRP1 function. MRP4- and MRP5-expressing HEK293 cells (HEK293/4.63 and HEK293/5I) were used to study MRP4- and MRP5-mediated drug sensitivity, respectively. Pgp-expressing MCF-7 (MCF7-ADR) cells were used to confirm the effect of NSC73306 on Pgp-overexpressing cells as described previously (19). The expression levels of each ABC transporter in respective cell lines was confirmed by Western blot analysis (data not shown) as detailed in the Materials and Methods section.
Table 1.
Sensitivity of cells overexpressing ABC drug transporters and their respective parental cells to the thiosemicarbazone NSC73306
| Cell line | Transporter over-expressed | IC50 [μM] a NSC73306 | RF b |
|---|---|---|---|
| pcDNA3.1-HEK293 | - | 8.51 ± 1.71 | - |
| R482-HEK293 | ABCG2 | 8.97 ± 1.60 | 1.05 |
|
| |||
| MCF7 | - | 59.3 ± 12.8 | - |
| MCF7-ADR | ABCB1 (Pgp) | 9.91 ± 1.30* | 0.17 |
| MCF7-AdVp3000 | ABCG2 | 77.0 ± 13.4 | 1.30 |
| MCF7-FLV1000 | ABCG2 | 72.3 ± 13.2 | 1.22 |
|
| |||
| S1 | - | 12.6 ± 3.8 | - |
| S1-M1-80 | ABCG2 | 12.2 ± 2.8 | 0.97 |
|
| |||
| pcDNA-HEK293 | - | 5.19 ± 1.15 | - |
| MRP1-HEK293 | ABCC1 (MRP1) | 4.26 ± 0.85 | 0.82 |
|
| |||
| COR-L23/P | - | 36.1 ± 3.8 | - |
| COR-L23/R | ABCC1 (MRP1) | 44.9 ± 7.0 | 1.24 |
|
| |||
| HEK293 | - | 7.28 ± 2.00 | - |
| HEK293/4.63 | ABCC4 (MRP4) | 9.17 ± 2.79 | 1.26 |
| HEK293/5I | ABCC5 (MRP5) | 7.38 ± 1.17 | 1.01 |
IC50 values are mean ± SD calculated from dose response curves obtained from three duplicate determinations using cytotoxicity assay as detailed in Materials and Methods section
(P < 0.05).
Resistance factors (RF) were calculated by dividing the IC50 values of the ABC transporter over-expressing cells by the IC50 values of the respective parental cells.
The resistance factor (RF) was used to compare the relative toxicity of NSC73306 to cells overexpressing selected ABC transporters (Table 1). The RF indicates the degree of sensitivity due to the expressed ABC transporter, and is calculated by dividing the IC50 against cells which overexpress the ABC transporter by the IC50 of the respective parental cells. An RF value > 1.0 indicates the ability of the particular ABC transporter to confer resistance to a compound such as NSC73306; an RF = 1.0 means that NSC73306 is equally toxic to both cell lines; and an RF value < 1.0 signifies that NSC73306 is more toxic to cells which overexpress a particular ABC transporter than to the parental cells. For instance, the resistance factor of MCF7-ADR cells to NSC73306 is calculated as 0.17, which indicates that NSC73306 is more toxic to the Pgp-overexpressing cells, MCF7-ADR, than to the parental MCF-7 cells, confirming the results which were previously reported (19). In contrast, cells overexpressing ABCG2, MRP1, MRP4 or MRP5 did not show any selective sensitivity to NSC73306 compared to their respective parental cell lines (RF values are all close to 1.0). Therefore, although it appears that NSC73306 is selectively toxic to Pgp-overexpressing cancer cells, this is not the case concerning cancer cells that overexpress ABCG2, MRP1, MRP4 or MRP5.
Effect of NSC73306 on the ABCG2- and MRP-mediated drug resistance
To determine if non-toxic concentrations (0.5 μM) of NSC73306 can re-sensitize drug resistant cells to chemotherapeutics, drug resistance mediated by ABCG2, MRP1, MRP4 and MRP5 was evaluated. At this concentration, NSC73306 was able to restore sensitivity of ABCG2-overexpressing cells to mitoxantrone and topotecan, which are both well-established ABCG2 substrates with relative resistance factors of 24 and 14, respectively (30, 37). The relative resistance factor was calculated by dividing the IC50 value of drug resistant cells by the IC50 value of the respective parental cells in the presence of a particular drug. NSC73306 significantly restored the sensitivity of ABCG2 expressing cells to mitoxantrone and topotecan by approximately 6- and 3-fold, respectively (Table 2). As a positive control, a high dose (5 μM) of fumitremorgin C (FTC) was used to demonstrate complete reversal of ABCG2-mediated drug resistance.
Table 2.
Chemosensitizing effect of NSC73306 on ABC transporter-mediated drug resistance in HEK293 cells
| IC50 [nM] a | ||||
|---|---|---|---|---|
| Treatment | Conc. [μM] | pcDNA-HEK293 | R482-HEK | RR b |
| Mitoxantrone alone | - | 6.5 ± 0.6 | 155.6 ± 10.1 | 24 |
| + NSC73306 | 0.5 | 8.7 ± 1.6 | 27.7 ± 1.9* | 4 |
| + FTC | 5 | 6.9 ± 0.7 | 10.8 ± 0.6* | 2 |
| Topotecan alone | - | 12.5 ± 2.1 | 174.3 ± 26.3 | 14 |
| + NSC73306 | 0.5 | 10.2 ± 2.9 | 46.7 ± 5.1** | 5 |
| + FTC | 5 | 10.5 ± 3 | 8.9 ± 1.8* | 1 |
|
| ||||
|
IC50 [μM]a
| ||||
| Conc. [μM] | pcDNA-HEK293 | MRP1-HEK293 (MRP1) | RRb | |
|
| ||||
| Etoposide alone | - | 0.28 ± 0.04 | 39.29 ± 8.53 | 140 |
| + NSC73306 | 0.5 | 0.23 ± 0.03 | 43.80 ± 11.28 | 190 |
| + MK-571 | 25 | 0.21 ± 0.03 | 15.56 ± 1.56** | 74 |
|
| ||||
| Conc. [μM] | HEK293 | HEK293/4.63 (MRP4) | RRb | |
|
| ||||
| NSC251820 alone | - | 5.54 ± 0.86 | 39.21 ± 4.61 | 7 |
| + NSC73306 | 0.5 | 5.49 ± 0.34 | 38.94 ± 4.09 | 7 |
| + MK-571 | 25 | 2.18 ± 0.54** | 4.84 ± 0.72* | 2 |
|
| ||||
| Conc. [μM] | HEK293 | HEK293/5I (MRP5) | RRb | |
|
| ||||
| ZD1694 alone | - | 9.64 ± 2.01 | 65.25 ± 14.30 | 7 |
| + NSC73306 | 0.5 | 8.90 ± 1.15 | 70.11 ± 16.19 | 8 |
| + MK-571 | 25 | 9.55 ± 1.73 | 44.16 ± 19.52 | 5 |
| + Quercetin | 10 | 7.64 ± 1.63 | 27.97 ± 12.29*** | 4 |
IC50 values are mean ± SD in the presence and absence of NSC73306 or other tested compounds. The IC50 values were calculated from dose-response curves obtained from at least three independent experiments
P < 0.001,
P < 0.01,
P < 0.05).
Relative resistance (RR) values were obtained by dividing the IC50 values of the ABC-transporter transfected HEK293 cells by the IC50 values of the empty vector pcDNA-HEK293 transfected cells.
In contrast, NSC73306 was not able to restore drug sensitivity in cells overexpressing MRP1, MRP4 or MRP5 (Table 2). For instance, the presence of NSC73306 was unable to restore MRP1-mediated etoposide resistance, MRP4-mediated NSC251820 resistance or MRP5-mediated ZD1694 resistance. Etoposide, NSC251820 and ZD1694 are established substrates of MRP1, MRP4 and MRP5, with relative resistance factors of 140, 7 and 7, respectively (27, 38, 39). MK-571 (25 μM) and/or quercetin (10 μM) were used as positive controls to reverse drug resistance conferred by MRPs (39).
NSC73306 inhibits the efflux of mitoxantrone from both wild-type and mutant ABCG2-expressing cells
Considering the significant effect of NSC73306 to re-sensitize ABCG2-overexpressing cells to both mitoxantrone and topotecan, we evaluated the capability of NSC73306 to block ABCG2-mediated mitoxantrone efflux. Using flow cytometry with wild type R482-HEK293 cells (Fig. 1, top panel), ABCG2-expressing MCF-7 FLV1000 cells and ABCG2 mutant R482T MCF-7 AdVp3000 cells (Fig. 1, bottom panel), the intensities of the accumulated fluorescent substrates were then analyzed as described in Materials and Methods. The presence of NSC73306 significantly increased the accumulation of mitoxantrone in both wild-type and mutant ABCG2-expressing cells while it had no effect on the control parental cells (Fig. 1). 5 μM and 10 μM of NSC73306 were shown to have the maximum inhibitory effect in ABCG2-overexpressing HEK293 (Fig. 1, top panel) and MCF7 cells (Fig. 1 bottom panel), respectively. As a positive control, a high concentration of an ABCG2 inhibitor FTC (20 μM) was used to achieve complete block on ABCG2-mediated efflux. Interestingly, it appears that NSC73306 was more effective in blocking the efflux of mitoxantrone from the wild-type 482R (MCF-7 FLV1000) than the mutant 482T (MCF-7 AdVp3000) ABCG2-overexpressing cells (Fig. 1, bottom panel). However, mutant 482T cells only exist in in vitro drug-selected cell culture conditions (22). In addition, the effect of NSC73306 on MRP1-mediated calcein efflux was also evaluated. As expected based on the cytotoxicity studies, NSC73306 had no significant effect on MRP1-mediated calcein efflux, while the MRP1-inhibitor MK-571 effectively blocked the MRP1-mediated calcein efflux (Fig. 1, suppl. data3).
Figure 1. Effect of NSC73306 on mitoxantrone accumulation in wild-type ABCG2-overexpressing R482-HEK293 cells(A); wild-type MCF-7 FLV1000 and mutant ABCG2-overexpressing MCF-7 AdVp3000 cells (B).

Cells were resuspended in IMDM supplemented with 5% fetal bovine serum. 5 μM mitoxantrone was preloaded in the dark at room temperature and then incubated at 37°C for 45 min, in the absence (solid line) or presence of 5 μM (A, dotted line) or 10 μM NSC73306 (B, dotted line), or 20 μM FTC (grey solid line). The cells were pelleted by centrifugation at 500× g and resuspended in 300 μL of PBS containing 0.1% bovine serum albumin. Samples were analyzed immediately by using flow cytometry. Representative histograms from three independent experiments are shown. For these experiments, concentrations ranging from 1–50 μM of NSC73306 were used. For clarity, histograms with only indicated concentration of this compound are shown.
NSC73306 inhibits the photoaffinity labeling of ABCG2 with [125I]Iodoarylazidoprazosin (IAAP) but not with [α-32P]8-azidoATP
Typically, ABC transport modulators can either bind and compete with substrate binding at the substrate-binding site(s) or interfere with ATP binding or hydrolysis by binding to the ATP-binding site. Both mechanisms result in the inhibition of drug efflux and drug resistance. [125I]-IAAP is a photoaffinity analogue of prazosin that has been used to characterize the substrate-binding sites of ABCG2 (34). We used the [125I]IAAP photolabeling assay to explore the binding of NSC73306 and its relative binding affinity to ABCG2 substrate-binding site(s). NSC73306 inhibits the photolabeling in a concentration-dependent manner, with a calculated IC50 value of 319 ± 96 nM (n=3) (Fig. 2A), which is almost 20-fold more effective than FTC (IC50 ≈ 5.7 μM) (Fig. 2B). Moreover, the photoaffinity ATP analogue [α-32P]8-azidoATP has been shown to bind specifically to the nucleotide-binding domains of numerous ABC transporters (33, 36), and thus it was used to test whether NSC73306 interacts with the nucleotide-binding site of ABCG2. Even at high concentrations of 25 μM and 50 μM (Fig. 2C, lane 2 and 3), NSC73306 had no effect on the photolabeling of ABCG2 with [α-32P]8-azidoATP (Fig. 2C, lane 1). Excess ATP (10 mM) was used as a positive control to show the displacement of [α-32P]8-azidoATP (Fig. 2C, lane 4). Collectively, these results suggest that NSC73306 binds specifically to the ABCG2 substrate-binding site(s) but not to the nucleotide-binding sites.
Figure 2. Effect of NSC73306 (A) or FTC (B) on photoaffinity labeling of ABCG2 with [125I]IAAP or with [α-32P]8-azidoATP (C).
Crude membranes (50 μg/mL) from the MCF-7 FLV1000 were incubated with various concentrations of NSC73306 (A) or FTC (B) or 10 mM ATP for 10 min at room temperature, and 3–6 nM [125I]IAAP (2200 Ci/mmole) (A and B) or 10 μM [α-32P] 8-azidoATP (10 μCi/nmole) (C), was then added. The samples were incubated for an additional 5 min under subdued light. The samples were illuminated with a UV lamp (365 nm) for 10 min at room temperature. The labeled ABCG2 was processed and visualized as described in the Materials and Methods. Representative gels from three independent experiments are shown. In all three panels, lane 1 is control without NSC73306 or FTC. In panel A, lanes 2 to 9 were incubated in the presence of 0.25, 0.5, 1, 2.5, 5, 10 and 20 μM of NSC73306, respectively. In panel B, lanes 2 to 9 were incubated in the presence of 1, 5, 10, 20, 50, 75, 100 and 200 μM of FTC, respectively. In panel C, lanes 2, 3 and 4 were incubated with 25 μM, 50 μM of NSC73306 and 10 mM ATP, respectively.
The reduced level of [3H]-NSC73306 accumulation in ABCG2-overexpressing cells is caused by ABCG2-mediated efflux
Since NSC73306 binds specifically to the substrate-binding site(s) of ABCG2, a [3H]-NSC73306 accumulation assay was performed to determine whether NSC73306 is a transport substrate of ABCG2. The initial linear rate (0–5 min) of 25 nM [3H]-NSC73306 accumulation in the parental MCF-7 cells (Fig. 3, open squares) was significantly higher (approximately 4-fold higher) than in the ABCG2-overexpressing MCF-7 FLV1000 cells (open circles), while the level of accumulation remained constant after approximately 5 min. Moreover, the rate and the total accumulation of [3H]-NSC73306 in MCF-7 and MCF-7 FLV1000 cells are identical in the presence of the known ABCG2 inhibitor FTC, 10 μM (filled symbols) or if the assays were carried out at 4°C (data not shown).
Figure 3. The accumulation of [3H]NSC73306 in parental MCF-7 and ABCG2-overexpressing MCF-7 FLV1000 cells.
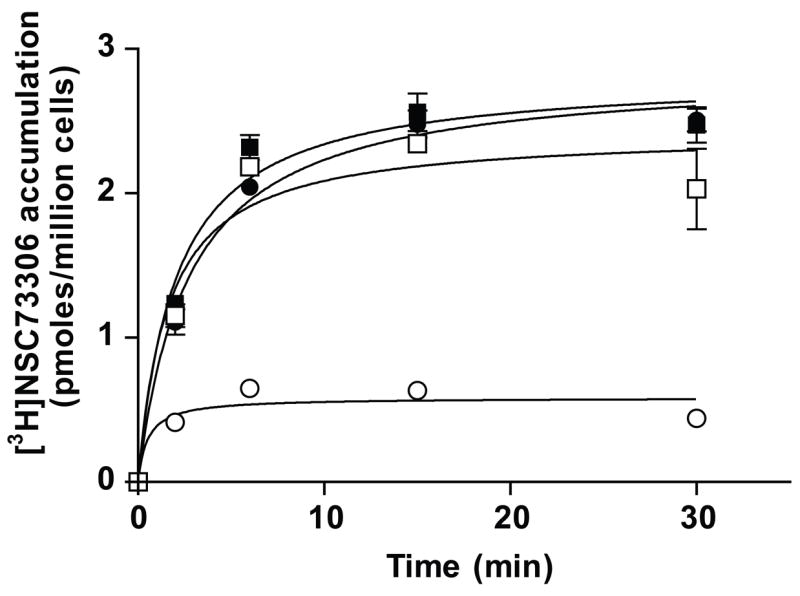
MCF-7 (squares) and MCF-7 FLV1000 (circles) cells were incubated with 25 nmol/L of [3H]NSC73306 in the absence (open symbols) or presence (filled symbols) of 10 μmol/L FTC at 32°C and monitored over 30 min in RPMI medium supplemented with 5% FBS. Immediately after incubation, cells were washed with 1 mL of ice-cold PBS and lysed by trypsinization in 0.3 mL of trypsin-EDTA solution. The amount of radioactive material that accumulated inside the cells was measured by liquid scintillation counting. The graphs represent the amount of radiolabeled materials that accumulated (picomoles per 1 million cells) as a function of time in the absence or presence of NSC73306 or FTC. The values represent the mean, and the error bars are standard deviation (n = 3).
NSC73306 stimulates ABCG2 ATPase activity
The effect of NSC73306 on ABCG2-mediated ATP hydrolysis was examined using High Five insect cells overexpressing ABCG2. NSC73306 stimulates BeFx-sensitive basal ABCG2-ATPase activity in a concentration-dependent manner, with maximum stimulation of 3-fold with the concentration required for 50% stimulation in the range of 140–150 nM (n=3) (Fig. 4A), which is significantly lower than FTC, with the apparent Ki ≈ 1.0 μM (40). In comparison, the ABCG2 substrate mitoxantrone also stimulated ABCG2-ATPase activity in a concentration-dependent manner, but only to approximately 2-fold of the basal level with the concentration required for 50% stimulation at 12.3 μM (Fig. 4B). Moreover, increasing concentrations of mitoxantrone (up to 20 μM) had no significant effect on NSC73306 (0.2 μM)-stimulated ABCG2 ATPase activity (Fig. 4B). In contrast to mitoxantrone, the presence of another known ABCG2 substrate pheophorbide A (up to 20 μM) and ABCG2 inhibitor FTC (up to 5 μM) inhibited NSC73306-stimulated ATP hydrolysis significantly in a concentration-dependent manner, with respective IC50 values of approximately 1.31 μM and 0.55 μM (Fig. 4C and 4D). Pheophorbide A itself has a biphasic effect on ABCG2 ATPase activity, stimulating ATP hydrolysis at lower concentrations, but inhibiting ATP hydrolysis at higher concentrations (Fig. 4C). The effect of several ABCG2 modulators and substrates such as curcumin I, XR9576, GF120918, flavopiridol and nifedipine on NSC73306-stimulated ABCG2 ATP hydrolysis was also tested (Fig. 4D). FTC itself inhibits ABCG2 ATP hydrolysis (41) whereas curcumin I (41) and nifedipine (34) stimulate it. XR9576, GF120918 and flavopiridol stimulate ABCG2 ATP hydrolysis with 3-fold, 4-fold and 2-fold maximum stimulation (data not shown). Despite stimulating ABCG2 ATP hydrolysis, XR9576, GF120918, flavopiridol and nifedipine failed to have any significant effect on the NSC73306-stimulated ABCG2 ATPase activity. Interestingly, the presence of curcumin I actually further stimulates ABCG2 ATP hydrolysis slightly (Fig. 4D). Moreover, NSC73306 failed to stimulate either BeFx-sensitive basal MRP1 or MRP4 ATPase activity as expected (Fig. 2, suppl. data).
Figure 4. Effect of ABCG2 modulators on BeFx-sensitive ABCG2 ATPase activity.
Effect of 0–20 μM NSC73306 (A) and 0–2 μM (A, insert), on BeFx-sensitive ABCG2 ATPase activity. Effect of mitoxantrone (B, open circles) and pheophorbide A (C, open circles) on BeFx-sensitive ABCG2 ATPase activity, as well as the effect of mitoxantrone (B, filled circles), pheophorbide A (C, filled circles), FTC (D, open squares), curcumin I (D, filled squares), XR9576 (D, open triangles), GF120918 (D, filled triangles), flavopiridol (D, open circles) and nifedipine (D, filled circles) on NSC73306-stimulated BeFx-sensitive ABCG2 ATPase activity was measured. Crude membranes of ABCG2 baculovirus infected High Five insect cells (100 μg/mL protein) were incubated at 37°C for 5 min with the indicated compound or 0.2 μM NSC73306 plus a given concentration of indicated compound in the presence and absence of BeFx. The reaction was initiated by addition of 5 mM ATP and terminated with SDS (2.5% final concentration) after 20 min incubation at 37°C. The amount of Pi released was quantitated using a colorimetric method (36). Values represent mean ± S.E.M. from at least three independent experiments.
Discussion
Thiosemicarbazone related compounds are well-known to have active antiviral, antibacterial, antimalarial, and antihypertensive properties (42), as well as antitumor activity that can overcome resistance to chemotherapeutics (19, 43). Recently, we reported that the small-molecule thiosemicarbazone NSC73306 is significantly more cytotoxic to cells that overexpress Pgp than to cells that do not, and this unique property is directly proportional to functional Pgp protein expression (19). Therefore, we proposed that NSC73306 could be used to re-sensitize Pgp-expressing multidrug resistant carcinoma cells to chemotherapy. However, in addition to Pgp, the presence of MRPs and ABCG2 is well-documented in numerous types of the cancer cells (4) and has been shown to play a major role in the development of MDR in cancer cells (16, 18, 44, 45). For example, ABCG2 and MRP1 are known to transport numerous anti-cancer chemotherapeutics, while MRP4 and MRP5 can transport cyclic nucleotides, as well as prostaglandins, glutathione-conjugated molecules and various antiviral drugs (16, 21, 44, 46). This prompted us to investigate the potential interactions of NSC73306 with ABCG2, MRP1, MRP4 and MRP5, and the effect of their expression in cell lines on the cytotoxicity of NSC73306.
While NSC73306 was more cytotoxic to Pgp-overexpressing cells (R.F = 0.17) (19), this selective cytotoxic property of NSC73306 was absent in selected cell lines that overexpress ABCG2, MRP1, MRP4 or MRP5 (Table 1). We did observe that some cell lines are naturally more resistant to NSC73306. It is not unusual for a particular drug to display varying levels of toxicity in cell lines originating from different tissues (41), but in this instance, intrinsic Pgp levels in the different cell lines may be responsible for differential cytotoxicity. Low levels of MDR1 mRNA are present in HEK293 cells, which are more NSC73306 sensitive (NSC7330 IC50 = 7.29 μM), but MDR1 mRNA is undetectable by RT-PCR in MCF-7 (NSC73306 IC50 = 59.3 μM) or COR-L23 (NSC73306 IC50 = 36.1μM) cells (data not shown), consistent with the Pgp-selective cytotoxicity of NSC73306. Despite the lack of selective intrinsic cytotoxicity towards ABCG2-expressing cells, NSC73306 is able to re-sensitize these cells to both mitoxantrone and topotecan (Table 2). This chemosensitizing effect is absent in cells overexpressing MRP1, MRP4 or MRP5. We next demonstrated that NSC73306 not only binds to the substrate-binding site(s) of ABCG2 selectively (Fig. 2), but is also transported by ABCG2 (Fig. 3). ATPase assays showed that NSC73306 elicited a 3-fold stimulation of ABCG2-mediated ATP hydrolysis (Fig. 4), confirming the interaction between NSC73306 and ABCG2; ATP hydrolysis and substrate transport are closely linked events in ABC transporters (47). It is important to note that NSC73306 has much higher affinity for ABCG2 when compared with the known inhibitor FTC (Fig. 2 and 4). In addition, NSC73306 has no effect on the BeFx-sensitive MRP1 or MRP4 ATPase activity (Fig. 2, suppl. data), confirming the lack of interaction between NSC73306 and the MRPs tested.
Our results suggest that different ABCG2 modulators have different effects on the NSC73306-stimulated ABCG2 ATPase activity (Fig. 4). The ABCG2 substrate mitoxantrone has no effect on NSC73306-stimulated ABCG2 ATPase activity (Fig. 4B). In contrast, NSC73306-stimulated ABCG2 ATPase activity is abolished by increasing concentrations of the ABCG2 substrate, pheophorbide A (Fig. 4C), and the ACBG2 inhibitor FTC (Fig. 4D). This suggests that both pheophorbide A and FTC compete with and displace NSC73306 at the ABCG2 substrate-binding site(s), hence eliminating the stimulated ABCG2 ATPase activity. While mitoxantrone, curcumin I, XR9576, GF120918, flavopiridol and nifedipine all stimulate ABCG2-ATPase activity by themselves, they have no effect on NSC73306-stimulated ABCG2-ATPase activity (Fig. 4D), either because they bind to an ABCG2 drug-binding site(s) distinct from that of NSC73306, or because they have a lower binding affinity for the same site as NSC73306. Our results are consistent with the a recent report by McDevitt et al. (48) that suggested the presence of at least two drug-binding sites on ABCG2, with possible allosteric communication between them.
The transport data reported here support the notion that that reduced accumulation in MCF-7 FLV1000 cells was caused by ABCG2-mediated efflux of [3H]NSC73306 (Fig. 3). A known transport substrate of ABCG2, [125I]IAAP (34) was used in our assay to confirm that functional ABCG2 is present in MCF-7 FLV1000 cells. ABCG2-mediated [125I]-IAAP transport was reduced by both FTC and NSC73306, suggesting that IAAP, FTC and NSC73306 all bind in the same substrate binding pocket of ABCG2 (data not shown). Despite NSC73306 is a transported substrate of ABCG2, this transporter does not confer resistance to it in either HEK293 or MCF-7 cells (Table 1). There are several possible explanations that can produce this result. One possibility is that in long term assays, such as the cytotoxicity assay (72 h drug incubation), it may be a metabolite of NSC73306 (including metal complexes such as iron and copper (49)) that is not an ABCG2 substrate that confers cytotoxicity. Alternatively, NSC73306 insensitivity to lowered cellular accumulation may be due to NSC73306 interacting with an extracellular target. We have shown previously that growing Pgp expressing KB-V1 cells in the presence of NSC73306 results in the loss of Pgp expression and consequently the loss of the MDR phenotype (19). In contrast, we found no change in ABCG2 mRNA levels, ABCG2 protein surface expression, or its function after growing ABCG2-overexpressing MCF7-FLV1000 cells in 20 μM NSC73306 for as long as 21 days (data not shown).
In summary, the cytotoxic action of NSC73306 requires the functional expression of Pgp. Although it is not a substrate or modulator for some of the ABCC (ABCC1, C4 and C5) transporters implicated in conferring multidrug resistance, this compound interacts with ABCG2 with high affinity and it is transported by this transporter. It has been shown previously that long-term incubation of NSC73306 with Pgp-expressing cells results in the reduced expression of Pgp (14), resensitizing them to conventional chemotherapeutics. Further, NSC73306 can reduce ABCG2-mediated drug efflux of other agents, even at very low concentrations, thus sensitizing ABCG2-expressing cancer cells to chemotherapeutics and also selectively killing Pgp-expressing multidrug resistant cancer cells. Further work with a mouse model system will provide insight into the suitability of NSC73306 as an agent that can circumvent MDR in cancer cells by inhibiting ABCG2 function and by killing ABCB1-expressing cells.
Supplementary Material
Supplementary material for this article is available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).
Acknowledgments
We thank Drs. Susan E. Bates (Cancer Therapeutics Branch, Center for Cancer Research, NCI, NIH) for the parental and ABCG2 expressing cell lines, Piet Borst (The Netherlands Cancer Institute) for the HEK294/4.63 and HEK293/5I cells, Margery A. Barrand (University of Cambridge, UK) for the human COR-L23P and its MRP1-overexpressing variant COR-L23R cells, Kapil Mehta (MD Anderson, Houston, TX) for the MCF-7 and MCF-7/ADR cell lines and Krishnamachary Nandigama (LCB, NCI, NIH) for providing the crude membranes of HighFive cells expressing ABCG2. We also thank Developmental Therapeutics Program, DCT&D, NCI for providing NSC251820 and NSC73306, as well as AstraZeneca Pharmaceuticals (Macclesfield, Cheshire, UK) for providing ZD1694. The editorial assistance provided by George Leiman is gratefully acknowledged.
Grant Support: This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. AMC was supported by the NIGMS Pharmacology Research Associate (PRAT) Program.
Abbreviations
- ABC
ATP-binding cassette
- BeFx
beryllium fluoride
- FTC
fumitremorgin C
- IAAP
Iodoarylazidoprazosin
- MDR
multidrug resistance
- Pgp
P-glycoprotein
References
- 1.Gottesman MM, Pastan I, Ambudkar SV. P-glycoprotein and multidrug resistance. Curr Opin Genet Dev. 1996;6:610–7. doi: 10.1016/s0959-437x(96)80091-8. [DOI] [PubMed] [Google Scholar]
- 2.Gottesman M, Ambudkar SV. Overview: ABC transporters and human disease. J Bioenerg Biomembr. 2001;33:453–8. doi: 10.1023/a:1012866803188. [DOI] [PubMed] [Google Scholar]
- 3.Allen JD, Brinkhuis RF, van Deemter L, Wijnholds J, Schinkel AH. Extensive contribution of the multidrug transporters P-glycoprotein and Mrp1 to basal drug resistance. Cancer Res. 2000;60:5761–6. [PubMed] [Google Scholar]
- 4.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 5.Henriksen U, Gether U, Litman T. Effect of Walker A mutation (K86M) on oligomerization and surface targeting of the multidrug resistance transporter ABCG2. J Cell Sci. 2005;118:1417–26. doi: 10.1242/jcs.01729. [DOI] [PubMed] [Google Scholar]
- 6.Xu J, Peng H, Chen Q, Liu Y, Dong Z, Zhang JT. Oligomerization domain of the multidrug resistance-associated transporter ABCG2 and its dominant inhibitory activity. Cancer Res. 2007;67:4373–81. doi: 10.1158/0008-5472.CAN-06-3169. [DOI] [PubMed] [Google Scholar]
- 7.Bates SE, Medina-Perez WY, Kohlhagen G, et al. ABCG2 mediates differential resistance to SN-38 (7-ethyl-10-hydroxycamptothecin) and homocamptothecins. J Pharmacol Exp Ther. 2004;310:836–42. doi: 10.1124/jpet.103.063149. [DOI] [PubMed] [Google Scholar]
- 8.Miyake K, Mickley L, Litman T, et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 1999;59:8–13. [PubMed] [Google Scholar]
- 9.Yoshikawa M, Ikegami Y, Sano K, et al. Transport of SN-38 by the wild type of human ABC transporter ABCG2 and its inhibition by quercetin, a natural flavonoid. J Exp Ther Oncol. 2004;4:25–35. [PubMed] [Google Scholar]
- 10.Maliepaard M, van Gastelen MA, de Jong LA, et al. Overexpression of the BCRP/MXR/ABCP gene in a topotecan-selected ovarian tumor cell line. Cancer Res. 1999;59:4559–63. [PubMed] [Google Scholar]
- 11.Scheffer GL, Maliepaard M, Pijnenborg AC, et al. Breast cancer resistance protein is localized at the plasma membrane in mitoxantrone- and topotecan-resistant cell lines. Cancer Res. 2000;60:2589–93. [PubMed] [Google Scholar]
- 12.Kim M, Turnquist H, Jackson J, et al. The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin Cancer Res. 2002;8:22–8. [PubMed] [Google Scholar]
- 13.van Herwaarden AE, Schinkel AH. The function of breast cancer resistance protein in epithelial barriers, stem cells and milk secretion of drugs and xenotoxins. Trends Pharmacol Sci. 2006;27:10–6. doi: 10.1016/j.tips.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Robey RW, Polgar O, Deeken J, To KW, Bates SE. ABCG2: determining its relevance in clinical drug resistance. Cancer Metastasis Rev. 2007;26:39–57. doi: 10.1007/s10555-007-9042-6. [DOI] [PubMed] [Google Scholar]
- 15.Jonker JW, Smit JW, Brinkhuis RF, et al. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J Natl Cancer Inst. 2000;92:1651–6. doi: 10.1093/jnci/92.20.1651. [DOI] [PubMed] [Google Scholar]
- 16.Sarkadi B, Homolya L, Szakacs G, Varadi A. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev. 2006;86:1179–236. doi: 10.1152/physrev.00037.2005. [DOI] [PubMed] [Google Scholar]
- 17.Tsuruo T, Iida H, Tsukagoshi S, Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981;41:1967–72. [PubMed] [Google Scholar]
- 18.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–34. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 19.Ludwig JA, Szakacs G, Martin SE, et al. Selective toxicity of NSC73306 in MDR1-positive cells as a new strategy to circumvent multidrug resistance in cancer. Cancer Res. 2006;66:4808–15. doi: 10.1158/0008-5472.CAN-05-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szakacs G, Annereau JP, Lababidi S, et al. Predicting drug sensitivity and resistance: profiling ABC transporter genes in cancer cells. Cancer Cell. 2004;6:129–37. doi: 10.1016/j.ccr.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 21.Chen ZS, Robey RW, Belinsky MG, et al. Transport of methotrexate, methotrexate polyglutamates, and 17beta-estradiol 17-(beta-D-glucuronide) by ABCG2: effects of acquired mutations at R482 on methotrexate transport. Cancer Res. 2003;63:4048–54. [PubMed] [Google Scholar]
- 22.Honjo Y, Hrycyna CA, Yan QW, et al. Acquired mutations in the MXR/BCRP/ABCP gene alter substrate specificity in MXR/BCRP/ABCP-overexpressing cells. Cancer Res. 2001;61:6635–9. [PubMed] [Google Scholar]
- 23.Miwa M, Tsukahara S, Ishikawa E, Asada S, Imai Y, Sugimoto Y. Single amino acid substitutions in the transmembrane domains of breast cancer resistance protein (BCRP) alter cross resistance patterns in transfectants. Int J Cancer. 2003;107:757–63. doi: 10.1002/ijc.11484. [DOI] [PubMed] [Google Scholar]
- 24.Ozvegy-Laczka C, Koblos G, Sarkadi B, Varadi A. Single amino acid (482) variants of the ABCG2 multidrug transporter: major differences in transport capacity and substrate recognition. Biochim Biophys Acta. 2005;1668:53–63. doi: 10.1016/j.bbamem.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Robey RW, Honjo Y, Morisaki K, et al. Mutations at amino-acid 482 in the ABCG2 gene affect substrate and antagonist specificity. Br J Cancer. 2003;89:1971–8. doi: 10.1038/sj.bjc.6601370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrand MA, Heppell-Parton AC, Wright KA, Rabbitts PH, Twentyman PR. A 190-kilodalton protein overexpressed in non-P-glycoprotein-containing multidrug-resistant cells and its relationship to the MRP gene. J Natl Cancer Inst. 1994;86:110–7. doi: 10.1093/jnci/86.2.110. [DOI] [PubMed] [Google Scholar]
- 27.Wielinga PR, Reid G, Challa EE, et al. Thiopurine metabolism and identification of the thiopurine metabolites transported by MRP4 and MRP5 overexpressed in human embryonic kidney cells. Mol Pharmacol. 2002;62:1321–31. doi: 10.1124/mol.62.6.1321. [DOI] [PubMed] [Google Scholar]
- 28.Wijnholds J, Mol CA, van Deemter L, et al. Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc Natl Acad Sci U S A. 2000;97:7476–81. doi: 10.1073/pnas.120159197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller M, Yong M, Peng XH, Petre B, Arora S, Ambudkar SV. Evidence for the role of glycosylation in accessibility of the extracellular domains of human MRP1 (ABCC1) Biochemistry. 2002;41:10123–32. doi: 10.1021/bi026075s. [DOI] [PubMed] [Google Scholar]
- 30.Robey RW, Medina-Perez WY, Nishiyama K, et al. Overexpression of the ATP-binding cassette half-transporter, ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast cancer cells. Clin Cancer Res. 2001;7:145–52. [PubMed] [Google Scholar]
- 31.Ishiyama M, Tominaga H, Shiga M, Sasamoto K, Ohkura Y, Ueno K. A combined assay of cell viability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet. Biol Pharm Bull. 1996;19:1518–20. doi: 10.1248/bpb.19.1518. [DOI] [PubMed] [Google Scholar]
- 32.Robey RW, Steadman K, Polgar O, et al. Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Res. 2004;64:1242–6. doi: 10.1158/0008-5472.can-03-3298. [DOI] [PubMed] [Google Scholar]
- 33.Sauna ZE, Muller M, Peng XH, Ambudkar SV. Importance of the conserved Walker B glutamate residues, 556 and 1201, for the completion of the catalytic cycle of ATP hydrolysis by human P-glycoprotein (ABCB1) Biochemistry. 2002;41:13989–4000. doi: 10.1021/bi026626e. [DOI] [PubMed] [Google Scholar]
- 34.Shukla S, Robey RW, Bates SE, Ambudkar SV. The calcium channel blockers, 1,4-dihydropyridines, are substrates of the multidrug resistance-linked ABC drug transporter, ABCG2. Biochemistry. 2006;45:8940–51. doi: 10.1021/bi060552f. [DOI] [PubMed] [Google Scholar]
- 35.Ambudkar SV. Drug-stimulatable ATPase activity in crude membranes of human MDR1-transfected mammalian cells. Methods Enzymol. 1998;292:504–14. doi: 10.1016/s0076-6879(98)92039-0. [DOI] [PubMed] [Google Scholar]
- 36.Sauna ZE, Nandigama K, Ambudkar SV. Multidrug resistance protein 4 (ABCC4)-mediated ATP hydrolysis: effect of transport substrates and characterization of the post-hydrolysis transition state. J Biol Chem. 2004;279:48855–64. doi: 10.1074/jbc.M408849200. [DOI] [PubMed] [Google Scholar]
- 37.Chen YN, Mickley LA, Schwartz AM, Acton EM, Hwang JL, Fojo AT. Characterization of adriamycin-resistant human breast cancer cells which display overexpression of a novel resistance-related membrane protein. J Biol Chem. 1990;265:10073–80. [PubMed] [Google Scholar]
- 38.Grant CE, Valdimarsson G, Hipfner DR, Almquist KC, Cole SP, Deeley RG. Overexpression of multidrug resistance-associated protein (MRP) increases resistance to natural product drugs. Cancer Res. 1994;54:357–61. [PubMed] [Google Scholar]
- 39.Wu CP, Calcagno AM, Hladky SB, Ambudkar SV, Barrand MA. Modulatory effects of plant phenols on human multidrug-resistance proteins 1, 4 and 5 (ABCC1, 4 and 5) Febs J. 2005;272:4725–40. doi: 10.1111/j.1742-4658.2005.04888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robey RW, Honjo Y, van de Laar A, et al. A functional assay for detection of the mitoxantrone resistance protein, MXR (ABCG2) Biochim Biophys Acta. 2001;1512:171–82. doi: 10.1016/s0005-2736(01)00308-x. [DOI] [PubMed] [Google Scholar]
- 41.Chearwae W, Shukla S, Limtrakul P, Ambudkar SV. Modulation of the function of the multidrug resistance-linked ATP-binding cassette transporter ABCG2 by the cancer chemopreventive agent curcumin. Mol Cancer Ther. 2006;5:1995–2006. doi: 10.1158/1535-7163.MCT-06-0087. [DOI] [PubMed] [Google Scholar]
- 42.Pandeya SN, Dimmock JR. Recent evaluations of thiosemicarbazones and semicarbazones and related compounds for antineoplastic and anticonvulsant activities. Pharmazie. 1993;48:659–66. [PubMed] [Google Scholar]
- 43.Whitnall M, Howard J, Ponka P, Richardson DR. A class of iron chelators with a wide spectrum of potent antitumor activity that overcomes resistance to chemotherapeutics. Proc Natl Acad Sci U S A. 2006;103:14901–6. doi: 10.1073/pnas.0604979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annu Rev Biochem. 2002;71:537–92. doi: 10.1146/annurev.biochem.71.102301.093055. [DOI] [PubMed] [Google Scholar]
- 45.Kuppens IE, Beijnen J, Schellens JH. Topoisomerase I inhibitors in the treatment of gastrointestinal cancer: from intravenous to oral administration. Clin Colorectal Cancer. 2004;4:163–80. doi: 10.3816/ccc.2004.n.017. [DOI] [PubMed] [Google Scholar]
- 46.Deeley RG, Westlake C, Cole SP. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev. 2006;86:849–99. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- 47.Ambudkar SV, Cardarelli CO, Pashinsky I, Stein WD. Relation between the turnover number for vinblastine transport and for vinblastine-stimulated ATP hydrolysis by human P-glycoprotein. J Biol Chem. 1997;272:21160–6. doi: 10.1074/jbc.272.34.21160. [DOI] [PubMed] [Google Scholar]
- 48.Clark R, Kerr ID, Callaghan R. Multiple drugbinding sites on the R482G isoform of the ABCG2 transporter. Br J Pharmacol. 2006;149:506–15. doi: 10.1038/sj.bjp.0706904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beraldo H, Gambino D. The wide pharmacological versatility of semicarbazones, thiosemicarba-zones and their metal complexes. Mini Rev Med Chem. 2004;4:31–9. doi: 10.2174/1389557043487484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).




