Abstract
The purine nucleoside adenosine has been described as a ‘retaliatory metabolite' by virtue of its ability to function in an autocrine manner to modify the activity of a range of cell types following its extracellular accumulation during cell stress or injury. These effects are largely protective and are triggered by the binding of adenosine to any of four G-protein-coupled adenosine receptors. Most of the anti-inflammatory effects of adenosine have been assigned to the adenosine A2A receptor subtype, which is expressed in many immune and inflammatory cells. In this brief article, we will outline the growing evidence to support the hypothesis that the development of agonists selective for the A2A receptor is an effective strategy for suppressing the exaggerated inflammatory responses associated with many diseases by virtue of the receptor's ability to inhibit multiple pro-inflammatory signalling cascades.
Keywords: A2A adenosine, receptor, cyclic AMP, inflammation, immunosuppression
Introduction
Adenosine is an endogenous purine nucleoside that mediates a wide variety of physiological functions by interaction with four cell-surface receptors (A1, A2A, A2B and A3; Fredholm et al., 2000, 2001). Numerous studies have highlighted the anti-inflammatory role of the A2A receptor from studies of receptor distribution on inflammatory cells (Gessi et al., 2000) as well as from observations of inhibitory effects of A2A receptor-selective agonists in vitro and in vivo and enhanced inflammatory responses in vivo in A2A receptor-deficient mice (Sitkovsky, 2003; Hasko and Cronstein, 2004).
This article is written as an introduction to the symposium on New Insights into the Anti-inflammatory Effects of A2AAR Agonists presented at the Life Sciences Meeting in Glasgow on July 11, 2007. Its purpose is to introduce the reader to some key concepts and serve as a basis for other chapters in this journal from the symposium discussing the following: (1) A2A adenosine receptors in tissue protection from reperfusion injury, by Professor Joel Linden (University of Virginia, USA); (2) Adenosine receptors and asthma, by Professor Clive Page (King's College, London); (3) Moving out and turning tail: restricted collision of the A2A-adenosine receptor revisited, by Professor Michael Freissmuth (University of Vienna, Austria); and (4) Hypoxia-adenosinergic regulation of immune response and tissue damage by Professor Misha Sitkovsky (Northeastern University, Massachusetts, USA).
Generation of extracellular adenosine
Before considering what is currently known about A2A receptor activation and its intracellular effects on inflammatory and immune signalling, it is important to consider how the endogenous agonist is generated in vivo. Adenosine is released from all cells upon the degradation of ATP. Consequently, conditions of stress, hypoxia and increased tissue energy expenditure can lead to a rapid >200-fold elevation in tissue adenosine over basal levels (typically <50 nM) (Rivkees et al., 2001). Specifically, ATP is converted to adenosine at sites of inflammation and injury by the action of CD39, an ecto-apyrase, and the subsequent conversion of AMP to adenosine by the ecto-5′-nucleotidase CD73. These enzymes are abundantly expressed on endothelial cells as well as on leucocytes and regulatory T cells (Lennon et al., 1998; Eltzschig et al., 2004; Deaglio et al., 2007). The protective role of this pathway has been elegantly demonstrated by gene-targeting studies in mice, which have shown that the presence of functional CD39 and CD73 is necessary to maintain endothelial barrier function and thus prevent excessive vascular leakage following hypoxia (Eltzschig et al., 2003; Thompson et al., 2004). However, the accumulation of extracellular adenosine is transient due to its conversion to inosine by ecto-adenosine deaminase (ADA) or uptake into endothelial cells via specific transporters and conversion either to inosine by intracellular ADA or to AMP by adenosine kinase. Because adenosine exerts profound auto-/paracrine effects on critical aspects of the immune and inflammatory responses, it is perhaps not surprising that the expression of enzymes involved in its accumulation is subject to tight regulation. For example, both CD39 and CD73 are each strongly induced in vascular endothelial cells upon the onset of hypoxia, and CD73 expression can be potentiated also by interferon (IFN)-α and adenosine itself via receptor-mediated elevation of cyclic AMP (cAMP) (Narravula et al., 2000; Niemela et al., 2004). Thus, control of adenosine accumulation and initiation of protective signalling clearly represents an important adaptive mechanism by which hypoxia-mediated damage to the endothelium can be minimized.
Activation of signalling pathways by the A2A receptor: in vitro studies
The A2A receptor was originally identified by virtue of its ability to elevate intracellular levels of cAMP via receptor interaction with the heterotrimeric G-protein Gs and subsequent activation of adenylyl cyclase (Linden, 2001). Changes in cAMP levels are translated into pleiotropic intracellular effects by a panel of cAMP-binding effector proteins, which include cyclic nucleotide-gated ion channels, cAMP-dependent protein kinase (PKA) and exchange proteins directly activated by cAMP (Epacs) (Beavo and Brunton, 2002). Signal termination is achieved by hydrolysis of cAMP to 5′-AMP catalysed by the large superfamily of cyclic nucleotide phosphodiesterases (PDEs). A key aspect of cAMP's effects is the generation of intracellular cAMP gradients arising from the opposing effects of adenylyl cyclases and PDEs (Lynch et al., 2006). The ability of distinct regions within the cell to sample these gradients is dictated in part by specific A-kinase anchoring protein scaffolds that localize PDEs, RI and RII regulatory cAMP-binding subunits of PKA and Epacs to defined intracellular compartments. In the case of PKA, binding of cAMP to R subunits releases catalytic C subunits from the PKA holoenzyme and allows phosphorylation of nearby substrates (Baillie et al., 2005).
However, similar to the other three receptors, the A2A receptor can also activate the extracellular signal-regulated kinase (ERK) pathway; this has been demonstrated in Chinese hamster ovary cells as well as in human embryonic kidney (HEK)-293, PC12 and vascular endothelial cells (reviewed by Schulte and Fredholm, 2003). Intriguingly, multiple mechanisms are responsible that appear to differ between cell types. For example, whereas A2A receptor activation of ERK in CHO cells occurs via an Src-mediated process that can be blocked by H-89 and mimicked by treatment with the cell-permeable cAMP analogue 8-bromo-cAMP, stimulation of ERK by either endogenous A2A receptors in human umbilical vein endothelial cells or recombinant A2A receptors expressed in HEK293 cells appears to occur via a Ras- and Src kinase-dependent mechanism independent of cAMP elevation (Seidel et al., 1999). Identification of the Arf6 guanine nucleotide exchange factor as an A2A receptor-binding protein revealed that its interaction with the receptor's unique cytoplasmic C-terminal domain was critical for the sustained cAMP-independent ERK activation observed upon receptor expression in HEK293 cells, although not affecting the initial spike of ERK phosphorylation detectable at 5 min (Gsandtner et al., 2005).
Suppression of immune and inflammatory events by the A2A receptor: in vitro studies
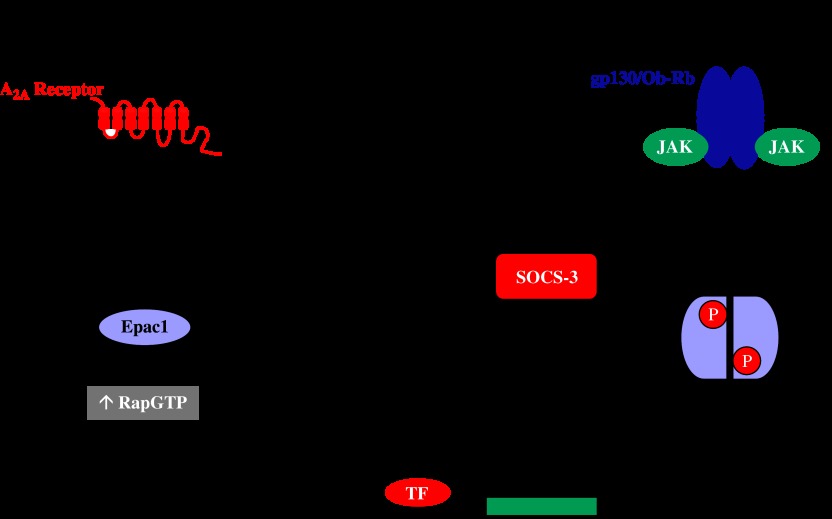
Functional and immunological approaches have shown that A2A receptors are expressed in specific haematopoietic cell populations. These include CD4+ and CD8+ T cells and natural killer cells as well as monocytes, macrophages and neutrophils. In contrast, the receptor is largely absent from B cells (Koshiba et al., 1999; Sullivan et al., 2001; Pinhal-Enfield et al., 2003; Zhang et al., 2005; Raskovalova et al., 2006). The majority of the A2A receptor's inhibitory effects on immune and inflammatory processes in these cell types have been proposed to occur via cAMP-/PKA-dependent pathways, which are known to have wide-ranging effects on immune cell function (Tasken and Stokka, 2006). For example, selective activation of A2A receptors expressed on lymphokine-activated killer cells using CGS21680 has been shown to suppress perforin- and FasL-mediated cytotoxicity via an RI PKA-dependent pathway (Raskovalova et al., 2006). Also, inhibition of both the oxidative burst response (Sullivan et al., 2001) and VLA-4 induction (Sullivan et al., 2004) in human neutrophils by A2A receptor-selective agonists can be reversed by the PKA-selective inhibitor H-89 and potentiated by inclusion of rolipram, an inhibitor of PDE-4. Similarly, the inhibitory effect of A2A receptor agonists on anti-CD3ɛ-mediated tyrosine phosphorylation of ZAP70, a critical step in T-cell activation, could also be reversed by H-89, indicative of a cAMP-/PKA-dependent mechanism (Sevigny et al., 2007). However, an alternative cAMP-activated pathway has recently emerged by which the A2A receptor can suppress JAK (Janus kinase)–STAT (signal transducer and activator of transcription) pathway activation by cytokines that utilize the transmembrane protein gp130 to trigger downstream signalling. In vascular endothelial cells and embryonic fibroblasts, cAMP-mediated activation of Epac1 results in the induction of the gene encoding ‘suppressor of cytokine signalling-3′ (SOCS-3) (Sands et al., 2006). SOCS-3 is one of the eight SOCS family members (cytokine-inducible SH2 domain protein, SOCS-1 to -7) defined by the presence of a distinct N-terminal region linked to a central SH2 and C-terminal SOCS box domains (Yoshimura et al., 2007). Upon induction, the SH2 domain binds to specific phosphor-Tyr residues present on target cytokine receptors, such as gp130 and the leptin receptor Ob-R (Heinrich et al., 2003) (Figure 1). Once bound, a so-called ‘kinase inhibitory region' within the N-terminal domain of SOCS-3 binds and inhibits the kinase activity of receptor-associated JAKs, thereby inhibiting STAT phosphorylation and activation (Yoshimura et al., 2007) (Figure 1). The exact mechanism by which Epac1 activation triggers SOCS-3 gene transcription remains to be elucidated, but it may provide a common mechanism by which Epac-mediated, PKA-independent effects of cAMP on the induction of genes such as those for AQP-2 and pro-glucagon can be rationalized (Lotfi et al., 2006; Umenishi et al., 2006).
Figure 1.
A2A receptor-mediated inhibition of cytokine receptor activation of the JAK-STAT pathway. Binding of adenosine or synthetic agonists to the A2A receptor triggers the activation of the stimulatory heterotrimeric G-protein Gs, which in turn activates adenylyl cyclase. The resulting elevation of cAMP levels is sensed by multiple intracellular cAMP-binding effectors, including Epac1. Epac1 functions as a cAMP-activated guanine nucleotide exchange factor for the Rap family of Ras-related small G-proteins, promoting the formation of GTP-bound Rap1a. This initiates transcription of the SOCS-3 gene via a transcription factor (TF) that has yet to be identified. In vascular endothelial cells, SOCS-3 inhibits JAK-mediated tyrosine phosphorylation of STATs after binding to tyrosine-phosphorylated Ob-Rb and gp130 following their activation by leptin and soluble IL-6 receptor α/IL-6 (sIL-6Rα/IL-6) trans-signalling complexes, respectively.
A key molecular mechanism that has emerged as being critical for the inhibitory effects of the A2A receptor on inflammatory and immune responses is suppression of the nuclear factor-κB (NF-κB) pathway activated by cytokines such as tumour necrosis factor (TNF)-α, IL-1β as well as pathogen-derived Toll-like receptor (TLR) agonists such as lipopolysaccharride (LPS). Interestingly, the molecular basis for this inhibition appears to be cell type-specific. Thus, A2A receptor expression in rat glioma cells prevents the accumulation of transcriptionally active NF-κB dimers in the nucleus in response to either LPS or TNFα by specifically blocking the phosphorylation and degradation of inhibitor of NF-κB (IκB) proteins, resulting in an abolition of inducible nitric oxide synthase (iNOS) induction in response to LPS or TNFα in combination with IFNγ (Sands et al., 2004). This would suggest that the receptor inhibits a common step in TNFα receptor/TLR signalling at or above the level of IκB kinases (IKKs). Similarly, deletion of the A2A receptor in macrophages increases the rate and extent to which TNFα triggers the degradation of IκBα, resulting in an enhanced accumulation of multiple κB-regulated transcripts (Lukashev et al., 2004). In contrast, overexpression of the A2A receptor in vascular endothelial cells blocks the TNFα-stimulated nuclear accumulation of p50/RelA heterodimers, thereby reducing κB-regulated adhesion molecule expression, without altering degradation of IκB proteins (Sands et al., 2004). Thus, presumably A2A receptor expression is either blocking the nuclear import of NF-κB dimers or accelerating chromosomal region maintenance 1 (CRM1)-dependent export back to the cytoplasm, although experiments utilizing CRM1 inhibitor leptomycin B suggest the former mechanism (EW Strong and TM Palmer, unpublished observations). Interestingly, recent work describing how the adenosine generated during hypoxic preconditioning suppresses cytokine activation of NF-κB has suggested that activation of adenosine receptors inactivates the Skp-cullin-F box-1 E3 ligase complex required for poly-ubiquitylation and degradation of IκBα by promoting the removal of the ubiquitin-like protein Nedd8 from the Cul1 component of the complex, although the identity of the adenosine receptor subtype involved and the relative contributions of cAMP or other signalling pathways have not been determined (Khoury et al., 2007). Nevertheless, such a mechanism could potentially account for the enhanced IκBα degradation observed in macrophages from A2A receptor-deficient mice, as one would predict that the absence of the A2A receptor should increase the proportion of active Cul1, thereby potentiating IκBα degradation (Lukashev et al., 2004). Finally, it is important to note that the expression of the A2A receptor is positively regulated by the pro-inflammatory stimuli whose responses it inhibits. For example, IL-1β, LPS and TNFα each increase A2A receptor mRNA and protein levels in microvascular endothelial cells, macrophages and THP-1 monocytic cells (Nguyen et al., 2003; Khoa et al., 2004; Murphree et al., 2005). The effect is inhibited, although not abolished, by pre-incubation with an IKK inhibitor (Murphree et al., 2005), indicative of a role for NF-κB in up-regulating A2A receptor gene transcription. This potentiates the ability of receptor activation to inhibit LPS-stimulated TNFα production in macrophages and also reduces TNFα-mediated up-regulation of vascular endothelial growth factor mRNA in endothelial cells (Nguyen et al., 2003; Murphree et al., 2005). Potentiation of A2A receptor expression therefore constitutes an additional negative feedback mechanism by which inflammatory responses may be limited in vivo.
Anti-inflammatory potential of the A2A receptor: in vivo evidence
The evidence that activation of the A2A receptor in vitro leads to suppression of inflammatory responses is overwhelming as reviewed above. Three lines of experimental evidence targeting the A2A receptor directly in animal models of inflammation–tissue damage should support the in vitro evidence if: (1) administration of an A2A receptor-selective antagonist enhances inflammation following induction of an inflammatory response, (2) inflammation is exaggerated in animals with targeted deletion of the A2A receptor and (3) application of selective agonists reduces inflammation and tissue damage.
Exacerbation of tissue damage and inflammation with A2A receptor antagonists has been shown in a variety of inflammatory models. For instance, treatment of mice with the selective A2A receptor antagonist ZM241385 enhanced liver injury and inflammation in response to concanavalin A, Pseudomonas aeruginosa and carbon tetrachloride (Ohta and Sitkovsky, 2001; Chan et al., 2006). Similarly, ZM241385 prevented both the anti-inflammatory effects and the increased survival rates induced by low-dose ketamine administration, which promotes adenosine accumulation, in mice where sepsis was induced by LPS or Escherichia coli (Mazar et al., 2005). In the mouse lung, treatment with ZM241385 enhanced lung neutrophilia in response to intratracheal administration of LPS (Thiel et al., 2005). However, in other models, ZM241385 did not enhance inflammation/tissue damage in response to an inflammatory stimulus (see, for example, Peirce et al., 2001; Fozard et al., 2002). This may reflect either varying amounts of endogenous adenosine in the different models or, alternatively, administration of a near-maximal dose of inflammatory stimulus, which would make it difficult to observe any further enhancement with an A2A receptor-selective antagonist.
Studies in animals with targeted deletion of the A2A receptor largely support the conclusions from experiments using ZM241385. For instance, mice deficient in the A2A receptor displayed an exaggerated inflammatory response and cytokine release in response to a wide variety of inflammatory insults, such as concanavalin A- and carbon tetrachloride-induced liver damage, and LPS-induced inflammation in an air pouch model (Ohta and Sitkovsky, 2001; Chan et al., 2006). Mice lacking the A2A receptor were recently reported to have exaggerated lung inflammation in response to sensitization and inhalation with ragweed antigen (Nadeem et al., 2007). In addition, adoptive transfer studies employing inflammatory cells from mice lacking the A2A receptor have also supported the concept that this receptor functions as a physiological anti-inflammatory mechanism (Yang et al., 2006).
The above-mentioned two groups of studies, in which signalling via the A2A receptor has been inhibited, support the concept that in an inflammatory environment the A2A receptor functions as a ‘physiological brake' on inflammatory processes. A number of reviews have been written, highlighting the concept that the A2A receptor is involved in inflammation (e.g., Sitkovsky, 2003; Sitkovsky et al., 2004; Hasko and Cronstein, 2004). From a therapeutic perspective, these data also suggest that administration of selective A2A receptor agonists should inhibit the excessive inflammation and tissue damage associated with disease. There are many publications supporting the view that agonists of the A2A receptor can inhibit inflammation in a wide variety of in vivo models. In addition, CGS21680, the first reported selective agonist at the A2A receptor (Hutchinson et al., 1989), and the more recently developed ATL-146e (Sullivan et al., 2001) have also shown a broad spectrum of anti-inflammatory and tissue-protective effects in many different animal models of inflammation (Akkari et al., 2006; and see below).
Investigation of the anti-inflammatory mechanisms in vivo have supported many of the observations made in vitro and have provided evidence for the anti-inflammatory effects of A2A receptor agonists in a variety of target tissues, as discussed below.
Liver
Concanavalin A induces polyclonal T-cell activation and subsequently liver cell death (Tiegs et al., 1992). The suggested mechanism of injury involves CD4+ T cells with secretion of cytokines such as TNFα, IFNγ and IL-6 (Gantner et al., 1995; Ohta and Sitkovsky, 2001). TNFα and IL-6 are both known to be important for induction of liver cell damage, apoptosis and necrosis. CGS21680 and ATL-146e inhibit both liver damage and the associated elevation in serum cytokine levels induced by concanavalin A administration, suggesting that inhibition of cytokine production by T cells contributed to the protective effect observed (Odashima et al., 2006). This conclusion was supported by the observations of Ohta and Sitkovsky (2001), who demonstrated that concanavalin A-induced liver damage was enhanced in mice lacking the A2A receptor.
Kidney
A tissue protection mechanism via an inhibitory action on CD4+ T cells has also been supported by in vivo studies examining the protective effects of ATL-146e on ischaemia–reperfusion (IR) damage in the mouse kidney (Day et al., 2003, 2006). In these studies, the protective effect of ATL-146e was inhibited in mice whose bone marrow had been ablated and reconstituted with bone marrow from A2A receptor-deficient animals. Using adoptive transfer techniques, the mechanism of protection of ATL-146e was shown to be due to an action of A2A receptor agonists on CD4+ T cells and involved an inhibition of IFNγ release.
Lungs
In rats and mice, intratracheal or intranasal administration of CGS21680 has been shown to suppress lung inflammation in response to pro-inflammatory stimuli such as antigen (ovalbumin) and involved inhibition of neutrophil, eosinophil, macrophage and lymphocyte infiltration (Fozard et al., 2002; Bonneau et al., 2006). In the study by Fozard et al. (2002), the effects of CGS21680 were inhibited by the selective A2A receptor antagonist ZM241385, although this agent did not appear to exaggerate inflammation induced by antigen itself. In contrast, CGS21680 was much less effective in inhibiting lung inflammation in response to LPS–N-formyl-methionine-leucine-phenylalanine administration in mice, but it did appear to inhibit neutrophil activation, as assessed by elastase release (Bonneau et al., 2006). Thiel et al. (2005) recently reported that the deleterious effect of oxygen therapy on lung inflammation and survival in mice was prevented by treatment with CGS21680, and several publications have also documented the inhibitory effects of A2A receptor agonists on lung injury and inflammation in response to IR (Hasko et al., 2006).
Heart
Agonists at the A2A receptor reduce infarct size and inflammation in studies of IR in canine and mouse models of myocardial infarction. Initial studies in canine models demonstrated that inhibition of P-selectin induction, neutrophil infiltration and other indices of inflammation accompanied the reduction in infarct size (Glover et al., 2005, 2007). Subsequent studies in mouse models of cardiac IR have highlighted the potential central role of CD4+ T cells in orchestrating several aspects of the inflammatory process, such as neutrophil infiltration, in response to IR. For instance, infarct size in response to IR is smaller in Rag1 knockout mice, which lack mature lymphocytes, and is increased by adoptive transfer of CD4+ T cells from control animals and those lacking the A2A receptor but not in those T cells lacking the ability to secrete IFNγ. Importantly, activation of the A2A receptor reduced both infarct size and inflammation in Rag1 knockout mice reconstituted with mature T cells but not in those reconstituted with CD4+ T cells lacking the A2A receptor (Yang et al., 2006).
Overwhelmingly, therefore, the anti-inflammatory/tissue-protective effects of the A2A receptor have been repeatedly demonstrated in several organs in a wide variety of in vivo model systems. Indeed, some anti-inflammatory medications have been proposed to derive some of their clinical benefit by promoting adenosine release (Cronstein et al., 1991; Gadangi et al., 1996; Hwang et al., 2001), and data from animal models suggest that this triggers an essential activation of the A2A receptor (Cronstein et al., 1993; Montesinos et al., 2007).
The biochemical mechanism(s) of protection in vivo have also supported the in vitro observations. For instance, mice lacking the A2A receptor display an enhanced release of TNFα and other cytokines, which is associated with increased gene transcription in response to injection of LPS. NF-κB is the major transcription factor responsible for production of pro-inflammatory cytokines in immune cells and, as described previously, studies in A2A receptor-deficient mice have demonstrated an enhanced NF-κB activation associated with elevated IKK-mediated phosphorylation and proteasomal degradation of IκBα (Lukashev et al., 2004). In wild-type mice, administration of CGS21680 and ZM241385 were able to inhibit and enhance, respectively, CpG-mediated increases in inflammatory gene transcripts, presumably by modulating activation of the NF-κB pathway (Lukashev et al., 2004). Influences on the mitogen-activated protein kinase activation pathways and, in particular, activation of ERK1/2 have also been investigated, as these have been suggested to play a role in cell protection. For example, studies in lung have demonstrated that administration of A2A receptor agonists induce a robust activation of the ERK pathway that is associated with reduced levels of pro-apoptotic markers (Rivo et al., 2007).
Clinical potential of A2A receptor agonists
Despite all the overwhelming pre-clinical evidence that A2A receptor-selective agonists can reduce inflammation and tissue damage, the most advanced agents (Regadenson from CV Therapeutics and Apadenoson from Adenosine Therapeutics) are actually in late-phase development as imaging agents/cardiac stress agents for coronary artery disease. This is based on the principle that activation of the A2A receptor in the coronary circulation produces a profound vasodilation. Thus, infusion with a compound with a longer half-life (in the order of a few minutes) and better selectivity for the A2A receptor than adenosine, which is currently utilized for such applications, would be expected to have a better and safer clinical profile in these conditions (Cerqueira, 2004).
Why then have the anti-inflammatory and tissue-protective properties of A2A receptor agonists not yet been adequately tested in clinical trials? The A2A receptor has a widespread tissue/cell-type distribution, and therefore activating this receptor might lead to unwanted side effects. For instance, the A2A receptor mediates inhibition of platelet aggregation, hypotension and a variety of effects within the CNS, some of which involve interactions with dopamine D2 receptors (see Ledent et al., 1997; Schwarzschild et al., 2006). Thus, chronic treatment of inflammatory conditions with an A2A receptor-selective agonist might have an unacceptable side-effect liability. In support of this concept, Fozard et al. (2002) demonstrated that, following intratracheal administration, inhibition of lung inflammation by CGS21680 was accompanied by falls in blood pressure, and these authors speculated that strategies would have to be developed to target the action of adenosine A2A receptor agonists within the lung to avoid the observed systemic side effects. Not withstanding the above consideration, at least two companies (Adenosine Therapeutics and Pfizer) are reported to be testing the anti-inflammatory potential of A2A receptor-selective agonists in a variety of clinical situations.
Concluding remarks
Many studies have now established the ability of selective A2A receptor activation to repress the exaggerated immune and inflammatory responses associated with many diseases. Although several high-affinity agonists with strong selectivity for the A2A receptor have now been developed and tested in several animal models, a major aim in future will be to assess whether these will have a sufficiently low side-effect liability to be suitable for systemic administration in patients. The identification of adenosine as a critical factor that accumulates in tumours in response to hypoxia, and which prevents their destruction by inhibiting anti-tumour T-cell function via A2A receptor activation (Lukashev et al., 2007), would also suggest that selective antagonists would be beneficial in many types of cancers. Finally, although the vast majority of the A2A receptor's effects appear to be mediated via elevation of cAMP, unravelling the detailed molecular mechanisms by which it suppresses specific pro-inflammatory signalling pathways will be essential if we are to (1) understand why A2A receptor activation is such a powerful strategy for turning off immune and inflammatory responses and (2) devise ways to minimize unwanted side effects of receptor activation arising from initiation of cAMP-independent signalling pathways.
Acknowledgments
TMP thanks the British Heart Foundation and UK Biotechnology and Biological Sciences Research Council for financial support.
Glossary
- ADA
adenosine deaminase
- cAMP
cyclic AMP
- CRM1
chromosomal region maintenance 1
- Epac
exchange protein directly activated by cAMP
- ERK
extracellular signal-regulated kinase
- HEK
human embryonic kidney
- IκB
inhibitor of NF-κB
- IFN
interferon
- IKK
IκB kinase
- IL
interleukin
- IR
ischaemia–reperfusion
- JAK
Janus kinase
- LPS
lipopolysaccharride
- NF-κB
nuclear factor-κB
- NK
natural killer
- PDE
phosphodiesterase
- PKA
cAMP-dependent protein kinase
- SOCS
suppressor of cytokine signalling
- STAT
signal transducer and activator of transcription
- TLR
Toll-like receptor
- TNFα
tumour necrosis factor-α
- TNFR
TNFα receptor
Footnotes
Conflict of interest
The authors state no conflict of interest.
References
- Akkari R, Burbiel JC, Hockemeyer J, Muller E. Recent progress in the development of adenosine receptor ligands as anti-inflammatory agents. Curr Top Med Chem. 2006;6:1375–1399. doi: 10.2174/15680266106061375. [DOI] [PubMed] [Google Scholar]
- Baillie GS, Scott JD, Houslay MD. Compartmentalisation of phosphodiesterases and protein kinase A: opposites attract. FEBS Lett. 2005;579:3264–3270. doi: 10.1016/j.febslet.2005.03.089. [DOI] [PubMed] [Google Scholar]
- Beavo JA, Brunton LL. Cyclic nucleotide research—still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- Bonneau O, Wyss D, Ferretti S, Blaydon C, Stevenson CS, Trifilieff A. Effect of A2AAR activation in murine models of respiratory disorders. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1036–L1043. doi: 10.1152/ajplung.00422.2005. [DOI] [PubMed] [Google Scholar]
- Cerqueira MD. The future of pharmacologic stress: selective A2A adenosine receptor agonists. Am J Cardiol. 2004;94:33D–40D. doi: 10.1016/j.amjcard.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Chan ES, Montesinos MC, Fernandez P, Desai A, Delano DL, Yee H, et al. Adenosine A(2A) receptors play a role in the pathogenesis of hepatic cirrhosis. Br J Pharmacol. 2006;148:1144–1155. doi: 10.1038/sj.bjp.0706812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein BN, Eberle MA, Gruber HE, Levin RI. Methotrexate inhibits neutrophil function by stimulating adenosine release from connective tissue cells. Proc Natl Acad Sci USA. 1991;88:2441–2445. doi: 10.1073/pnas.88.6.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein BN, Naime D, Ostad E. The anti-inflammatory mechanism of methotrexate. Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. Clin Invest. 1993;92:2675–2682. doi: 10.1172/JCI116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day YJ, Huang L, McDuffie MJ, Rosin DL, Ye H, Chen JF, et al. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest. 2003;112:883–891. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day YJ, Huang L, Ye H, Li L, Linden J, Okusa MD. Renal ischemia-reperfusion injury and adenosine A2A receptor-mediated tissue protection: the role of CD4+ T cells and IFN-gamma. J Immunol. 2006;176:3108–3114. doi: 10.4049/jimmunol.176.5.3108. [DOI] [PubMed] [Google Scholar]
- Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, et al. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Thompson LF, Karhausen J, Cotta RJ, Ibla JC, Robson SC, et al. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood. 2004;104:3986–3992. doi: 10.1182/blood-2004-06-2066. [DOI] [PubMed] [Google Scholar]
- Fozard JR, Ellis KM, Villela Dantas MF, Tigani B, Mazzoni L. Effects of CGS21680, a selective A2AAR agonist, on allergic airways inflammation in the rat. Br J Pharmacol. 2002;438:183–188. doi: 10.1016/s0014-2999(02)01305-5. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Arslan G, Halldner L, Kull B, Schulte G, Wasserman W. Structure and function of adenosine receptors and their genes. Naunyn Schmiedeberg's Arc Pharmacol. 2000;362:364–374. doi: 10.1007/s002100000313. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Gadangi P, Longaker M, Naime D, Levin RI, Recht PA, Montesinos MC, et al. The anti-inflammatory mechanism of sulfasalazine is related to adenosine release at inflamed sites. J Immunol. 1996;156:1937–1941. [PubMed] [Google Scholar]
- Gantner F, Leist M, Lohse AW, Germann PG, Tiegs G. Concanavalin A induced T cell mediated hepatic injury in mice: the role of tumour necrosis factor. Hepatology. 1995;21:190–198. doi: 10.1016/0270-9139(95)90428-x. [DOI] [PubMed] [Google Scholar]
- Gessi S, Varani K, Merighi S, Ongini E, Borea PA. A(2A) adenosine receptors in human peripheral blood cells. Br J Pharmacol. 2000;129:2–11. doi: 10.1038/sj.bjp.0703045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover DK, Riou LM, Ruiz M, Sullivan GW, Linden J, Rieger JM, et al. Reduction of infarct size and postischemic inflammation from ATL-146e, a highly selective A2AAR agonist, in reperfused canine myocardium. Am J Physiol Heart Circ Physiol. 2005;288:H1851–H1858. doi: 10.1152/ajpheart.00362.2004. [DOI] [PubMed] [Google Scholar]
- Glover DK, Ruiz M, Takehana K, Petruzella FD, Rieger JM, Macdonald TL, et al. Cardioprotection by adenosine A2A agonists in a canine model of myocardial stunning produced by multiple episodes of transient ischemia. Am J Physiol Heart Circ Physiol. 2007;292:H3164–H3171. doi: 10.1152/ajpheart.00743.2005. [DOI] [PubMed] [Google Scholar]
- Gsandtner I, Charalambous C, Stefan E, Ogris E, Freissmuth M, Zezula J. Heterotrimeric G protein-independent signaling of a G protein-coupled receptor. Direct binding of ARNO/cytohesin-2 to the carboxyl terminus of the A2A adenosine receptor is necessary for sustained activation of the ERK/MAP kinase pathway. J Biol Chem. 2005;280:31898–31905. doi: 10.1074/jbc.M506515200. [DOI] [PubMed] [Google Scholar]
- Hasko G, Cronstein BM. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hasko G, Xu DZ, Lu Q, Németh ZH, Jabush J, Berezina TL, et al. A2AAR activation reduces lung injury in trauma/hemorrhagic shock. Crit Care Med. 2006;34:1119–1125. doi: 10.1097/01.CCM.0000206467.19509.C6. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson AJ, Webb RL, Oei HH, Ghai GR, Zimmerman MB, Williams M. CGS21680, an A2-selective adenosine receptor agonist with preferential hypotensive activity. J Pharmacol Exp Ther. 1989;251:47–55. [PubMed] [Google Scholar]
- Hwang KK, Hall CS, Spielman WS, Sparks HV. FK506 promotes adenosine release from endothelial cells via inhibition of adenosine kinase. Eur J Pharmacol. 2001;425:85–93. doi: 10.1016/s0014-2999(01)01179-7. [DOI] [PubMed] [Google Scholar]
- Khoa ND, Montesinos MC, Reiss AB, Delano D, Awadallah N, Cronstein BN. Inflammatory cytokines regulate function and expression of adenosine A2A receptors in human monocytic THP-1 cells. J Leukoc Biol. 2004;76:727–734. doi: 10.4049/jimmunol.167.7.4026. [DOI] [PubMed] [Google Scholar]
- Khoury J, Ibla JC, Neish AS, Colgan SP. Antiinflammatory adaptation to hypoxia through adenosine-mediated cullin-1 deneddylation. J Clin Invest. 2007;117:703–711. doi: 10.1172/JCI30049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba M, Rosin DL, Hayashi N, Linden J, Sitkovsky MV. Patterns of A2A extracellular adenosine receptor expression in different functional subsets of human peripheral T cells. Flow cytometry studies with anti-A2A receptor monoclonal antibodies. Mol Pharmacol. 1999;55:614–624. [PubMed] [Google Scholar]
- Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, et al. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2A receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- Lennon PF, Taylor CT, Stahl GL, Colgan SP. Neutrophil-derived 5′-adenosine monophosphate promotes endothelial barrier function via CD73-mediated conversion to adenosine and endothelial A2B receptor activation. J Exp Med. 1998;188:1433–1443. doi: 10.1084/jem.188.8.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- Lotfi S, Li Z, Sun J, Zuo Y, Lam PP, Kang Y, et al. Role of the exchange protein directly activated by cyclic adenosine 5′-monophosphate (Epac) pathway in regulating proglucagon gene expression in intestinal endocrine L cells. Endocrinology. 2006;147:3727–3736. doi: 10.1210/en.2006-0056. [DOI] [PubMed] [Google Scholar]
- Lukashev D, Ohta A, Apasov S, Chen JF, Sitkovsky M. Cutting edge: physiologic attenuation of proinflammatory transcription by the Gs protein-coupled A2A adenosine receptor in vivo. J Immunol. 2004;173:21–24. doi: 10.4049/jimmunol.173.1.21. [DOI] [PubMed] [Google Scholar]
- Lukashev D, Ohta A, Sitkovsky M. Hypoxia-dependent anti-inflammatory pathways in protection of cancerous tissues. Cancer Metastasis Rev. 2007;26:273–279. doi: 10.1007/s10555-007-9054-2. [DOI] [PubMed] [Google Scholar]
- Lynch MJ, Hill EV, Houslay MD. Intracellular targeting of phosphodiesterase-4 underpins compartmentalized cAMP signaling. Curr Top Dev Biol. 2006;75:225–259. doi: 10.1016/S0070-2153(06)75007-4. [DOI] [PubMed] [Google Scholar]
- Mazar J, Rogachev B, Shaked G, Ziv NY, Czeiger D, Chaimovitz C, et al. Involvement of adenosine in the anti-inflammatory action of ketamine. Anesthesiology. 2005;102:1174–1181. doi: 10.1097/00000542-200506000-00017. [DOI] [PubMed] [Google Scholar]
- Montesinos MC, Takedachi M, Thompson LF, Wilder TF, Fernández P, Cronstein BN. The antiinflammatory mechanism of methotrexate depends on extracellular conversion of adenine nucleotides to adenosine by ecto-5′-nucleotidase: findings in a study of ecto-5′-nucleotidase gene-deficient mice. Arthritis Rheum. 2007;56:1440–1445. doi: 10.1002/art.22643. [DOI] [PubMed] [Google Scholar]
- Murphree LJ, Sullivan GW, Marshall MA, Linden J. Lipopolysaccharide rapidly modifies adenosine receptor transcripts in murine and human macrophages: role of NF-κB in A2A adenosine receptor induction. Biochem J. 2005;391:575–580. doi: 10.1042/BJ20050888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeem A, Fan M, Ansari HR, Ledent C, Mustafa SJ. Enhanced airway reactivity and inflammation in A2A adenosine receptor-deficient allergic mice. Am J Physiol Lung Cell Mol Physiol. 2007;2:L1335–L1344. doi: 10.1152/ajplung.00416.2006. [DOI] [PubMed] [Google Scholar]
- Narravula S, Lennon PF, Mueller BU, Colgan SP. Regulation of endothelial CD73 by adenosine: paracrine pathway for enhanced endothelial barrier function. J Immunol. 2000;165:5262–5268. doi: 10.4049/jimmunol.165.9.5262. [DOI] [PubMed] [Google Scholar]
- Nguyen DK, Montesinos MC, Williams AJ, Kelly M, Cronstein BN. Th1 cytokines regulate adenosine receptors and their downstream signaling elements in human microvascular endothelial cells. J Immunol. 2003;171:3991–3998. doi: 10.4049/jimmunol.171.8.3991. [DOI] [PubMed] [Google Scholar]
- Niemela J, Henttinen T, Yegutkin GG, Airas L, Kujari AM, Rajala P, et al. IFN-α induced adenosine production on the endothelium: a mechanism mediated by CD73 (ecto-5′-nucleotidase) up-regulation. J Immunol. 2004;172:1646–1653. doi: 10.4049/jimmunol.172.3.1646. [DOI] [PubMed] [Google Scholar]
- Odashima M, Otaka M, Jin M, Horikawa Y, Matsuhashi T, Ohba R, et al. A selective A2AAR agonist, ATL-146e, prevents concanavalin A-induced acute liver injury in mice. Biochem Biophys Res Commun. 2006;347:949–954. doi: 10.1016/j.bbrc.2006.06.185. [DOI] [PubMed] [Google Scholar]
- Ohta A, Sitkovsky M. Role of G protein coupled adenosine receptors in down regulation of inflammation and tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- Peirce SM, Skalak TC, Rieger JM, Macdonald TL, Linden J. Selective A(2A) adenosine receptor activation reduces skin pressure ulcer formation and inflammation. Am J Physiol Heart Circ Physiol. 2001;281:H67–H74. doi: 10.1152/ajpheart.2001.281.1.H67. [DOI] [PubMed] [Google Scholar]
- Pinhal-Enfield G, Ramanathan M, Hasko G, Vogel SN, Salzman AL, Boons GJ, et al. An angiogenic switch in macrophages involving synergy between Toll-like receptors 2, 4, 7, and 9 and adenosine A2A receptors. Am J Pathol. 2003;163:711–721. doi: 10.1016/S0002-9440(10)63698-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskovalova T, Lokshin A, Huang X, Jackson EK, Gorelik E. Adenosine-mediated inhibition of cytotoxic activity and cytokine production by IL-2/NKp46-activated NK cells: involvement of protein kinase A isozyme I (PKA I) Immunol Res. 2006;36:91–99. doi: 10.1385/IR:36:1:91. [DOI] [PubMed] [Google Scholar]
- Rivkees SA, Zhao Z, Porter G, Turner C. Influences of adenosine on the fetus and newborn. Mol Gen Metab. 2001;74:160–171. doi: 10.1006/mgme.2001.3217. [DOI] [PubMed] [Google Scholar]
- Rivo J, Zeira E, Galun E, Einav S, Linden J, Matot I. Attenuation of reperfusion lung injury and apoptosis by A2A adenosine receptor activation. Shock. 2007;27:266–273. doi: 10.1097/01.shk.0000235137.13152.44. [DOI] [PubMed] [Google Scholar]
- Sands WA, Martin AF, Strong EW, Palmer TM. Specific inhibition of nuclear factor-kappaB-dependent inflammatory responses by cell type-specific mechanisms upon A2A adenosine receptor gene transfer. Mol Pharmacol. 2004;66:1147–1159. doi: 10.1124/mol.104.001107. [DOI] [PubMed] [Google Scholar]
- Sands WA, Woolson HD, Milne GR, Rutherford C, Palmer TM. Exchange protein activated by cyclic AMP (Epac)-mediated induction of suppressor of cytokine signaling 3 (SOCS-3) in vascular endothelial cells. Mol Cell Biol. 2006;26:6333–6346. doi: 10.1128/MCB.00207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte G, Fredholm BB. Signalling from adenosine receptors to mitogen-activated protein kinases. Cell Signal. 2003;15:813–827. doi: 10.1016/s0898-6568(03)00058-5. [DOI] [PubMed] [Google Scholar]
- Schwarzschild MA, Agnati L, Fuxe K, Chen JF, Morelli M. Targeting adenosine A2A receptors in Parkinson's disease. Trends Neurosci. 2006;29:647–654. doi: 10.1016/j.tins.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Seidel MG, Klinger M, Freissmuth M, Holler C. Activation of mitogen-activated protein kinase by the A2A-adenosine receptor via a Rap1-dependent and via a p21ras-dependent pathway. J Biol Chem. 1999;274:25833–25841. doi: 10.1074/jbc.274.36.25833. [DOI] [PubMed] [Google Scholar]
- Sevigny CP, Li L, Awad AS, Huang L, McDuffie M, Linden J, et al. Activation of adenosine 2A receptors attenuates allograft rejection and alloantigen recognition. J Immunol. 2007;178:4240–4249. doi: 10.4049/jimmunol.178.7.4240. [DOI] [PubMed] [Google Scholar]
- Sitkovsky MV. Use of the A2A adenosine receptor as a physiological immunosuppressor and to engineer inflammation in vivo. Biochem Pharmacol. 2003;65:493–501. doi: 10.1016/s0006-2952(02)01548-4. [DOI] [PubMed] [Google Scholar]
- Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, et al. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- Sullivan GW, Lee DD, Ross WG, DiVietro JA, Lappas CM, Lawrence MB, et al. Activation of A2A adenosine receptors inhibits expression of α4/β1 integrin (very late antigen-4) on stimulated human neutrophils. J Leukoc Biol. 2004;75:127–134. doi: 10.1189/jlb.0603300. [DOI] [PubMed] [Google Scholar]
- Sullivan GW, Rieger JM, Scheld WM, Macdonald TL, Linden J. Cyclic AMP-dependent inhibition of human neutrophil oxidative activity by substituted 2-propynylcyclohexyladenosine A(2A) receptor agonists. Br J Pharmacol. 2001;132:1017–1026. doi: 10.1038/sj.bjp.0703893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasken K, Stokka AJ. The molecular machinery for cAMP-dependent immunomodulation in T-cells. Biochem Soc Trans. 2006;34:476–479. doi: 10.1042/BST0340476. [DOI] [PubMed] [Google Scholar]
- Thiel M, Chouker A, Ohta A, Jackson E, Caldwell C, Smith P, et al. Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PLoS Biol. 2005;6:e174. doi: 10.1371/journal.pbio.0030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, et al. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiegs G, Hentschel A, Wendel A. T cell dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest. 1992;90:196–203. doi: 10.1172/JCI115836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umenishi F, Narikiyo T, Vandewalle A, Schrier RW. cAMP regulates vasopressin-induced AQP2 expression via protein kinase A-independent pathway. Biochim Biophys Acta. 2006;1758:1100–1105. doi: 10.1016/j.bbamem.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Yang Z, Day YJ, Toufektsian MC, Xu Y, Ramos SI, Marshall MA, et al. Myocardial infarct-sparing effect of A2AAR activation is due to its action on CD4+ T lymphocytes. Circulation. 2006;114:2056–2064. doi: 10.1161/CIRCULATIONAHA.106.649244. [DOI] [PubMed] [Google Scholar]
- Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- Zhang JG, Hepburn L, Cruz G, Borman RA, Clark KL. The role of adenosine A2A and A2B receptors in the regulation of TNF-α production by human monocytes. Biochem Pharmacol. 2005;69:883–889. doi: 10.1016/j.bcp.2004.12.008. [DOI] [PubMed] [Google Scholar]