Abstract
Intermedin (IMD) is a novel peptide related to calcitonin gene-related peptide (CGRP) and adrenomedullin (AM). Proteolytic processing of a larger precursor yields a series of biologically active C-terminal fragments, IMD1–53, IMD1–47 and IMD8–47. IMD shares a family of receptors with AM and CGRP composed of a calcitonin-receptor like receptor (CALCRL) associated with one of three receptor activity modifying proteins (RAMP). Compared to CGRP, IMD is less potent at CGRP1 receptors but more potent at AM1 receptors and AM2 receptors; compared to AM, IMD is more potent at CGRP1 receptors but less potent at AM1 and AM2 receptors. The cellular and tissue distribution of IMD overlaps in some aspects with that of CGRP and AM but is distinct from both. IMD is present in neonatal but absent or expressed sparsely, in adult heart and vasculature and present at low levels in plasma. The prominent localization of IMD in hypothalamus and pituitary and in kidney is consistent with a physiological role in the central and peripheral regulation of the circulation and water-electrolyte homeostasis. IMD is a potent systemic and pulmonary vasodilator, influences regional blood flow and augments cardiac contractility. IMD protects myocardium from the deleterious effects of oxidative stress associated with ischaemia-reperfusion injury and exerts an anti-growth effect directly on cardiomyocytes to oppose the influence of hypertrophic stimuli. The robust increase in expression of the peptide in hypertrophied and ischaemic myocardium indicates an important protective role for IMD as an endogenous counter-regulatory peptide in the heart.
Keywords: ischaemia, hypertrophy, cardiomyocyte, myocardium, kidney, intermedin, adrenomedullin, CGRP, RAMP
Emergence of a peptide superfamily comprising calcitonin, calcitonin gene-related peptide, amylin and adrenomedullin and the discovery of intermedin
The regulatory influence of the peptide, calcitonin (CT), on calcium metabolism has long been recognized. In 1983, alternative tissue-specific processing of primary mRNA from the rat calcitonin gene (CT) was shown to generate two distinct single-chain polypeptides, namely calcitonin (rCT) comprising 32 amino acids and calcitonin gene-related peptide (rαCGRP) comprising 37 amino acids; CGRP is expressed predominantly in the nervous system and CT in parafollicular cells of the thyroid (Rosenfeld et al., 1983). Existence of a structurally similar peptide in humans was confirmed by isolation of hαCGRP from a medullary thyroid carcinoma (Morris et al., 1984). A second CGRP/CT gene (β-gene), thought to have arisen by exon duplication, generates alternative β-forms of CGRP which differ from the α-forms by 1 and 3 amino acids in rats and humans, respectively. There is no evidence for expression of an alternative form of CT from this gene (Steenbergh et al., 1985; Alevizaki et al., 1986). Disparity in regional distribution of CGRP and its binding sites in the central nervous system (Kruger et al., 1988) provided strong indication of the likely existence of additional member(s) of this peptide superfamily. The superfamily was enlarged with the subsequent discovery of amylin (AMY), a peptide identical in length to CGRP, which was extracted from amyloid deposits in pancreatic tissue of diabetics (Rink et al., 1993), and adrenomedullin (AM), comprising 52 amino acids, extracted from human pheochromocytoma (Kitamura et al., 1993). The superfamily expanded to varying degrees in different vertebrate lineages probably due to variation between these in regard to prevailing living conditions (Ogoshi et al., 2003). Existence of multiple family members, capable of activating overlapping signalling mechanisms imparts enormous versatility and plasticity in responding to the environment. Members of this peptide superfamily play important roles in a variety of physiological processes including ionic balance, neurotransmission, glucose metabolism and cardiovascular and endocrine homeostasis.
Based on searches of genome sequences in humans and other mammals and non-vertebrates, Roh et al. (2004) identified a novel gene encoding a prepro peptide of 146–150 amino acids. Although its overall sequence possessed little similarity to other known proteins, the C-terminal region of this prepro-peptide encompassed a putative 47 amino-acid peptide sharing ∼28% sequence identity with AM and <20% with CGRP and predicted to possess the common structural features characteristic of members of the superfamily. This novel peptide was named ‘intermedin' (IMD) due to its abundant expression in the intermediate lobe of the anterior pituitary. In the same year, Takei et al. (2004b) examined mammalian complementary DNA libraries for potential mammalian homologues of five orthologues of AM (AM1–AM5) which they had identified in pufferfish. Using this approach, they also identified a 146–150 amino-acid mammalian prepro-peptide which yielded a mature peptide containing 47 amino acids; this displayed ∼30% sequence homology to AM and >70% homology to the pufferfish AM2 orthologue; this novel mammalian peptide was named adrenomedullin-2 (AM2). Confirmation that the primary sequences of human prepro-AM2 and human prepro-IMD, each 148 amino acids, were identical and that the mature peptides elicited similar biological effects led to general acceptance that IMD and AM-2, discovered independently by the two research groups, were the same peptide.
The purpose of this article is to summarize progress made in regard to understanding the biological significance of IMD since the review published shortly after the discovery of this peptide (Chang et al., 2004). The general distribution of IMD, the nature of its interaction with a common receptor signalling system shared with other members of this peptide superfamily, and emerging evidence of the unique physiological profile of IMD are addressed. Particular emphasis has been placed on appraising the effects of IMD in the heart and vasculature and in the kidney, and potential involvement in various cardiovascular pathologies. Detailed examination of the actions of AM and CGRP is beyond the scope of the current article; these have been reviewed recently elsewhere (for example, Brain and Grant, 2004; Ishimitsu et al., 2006). However, where appropriate their actions have been compared and contrasted with those of IMD, to inform discussion of the physiological and pathophysiological relevance and unique contribution of the newest member of this peptide superfamily.
Synthesis and structure of intermedin
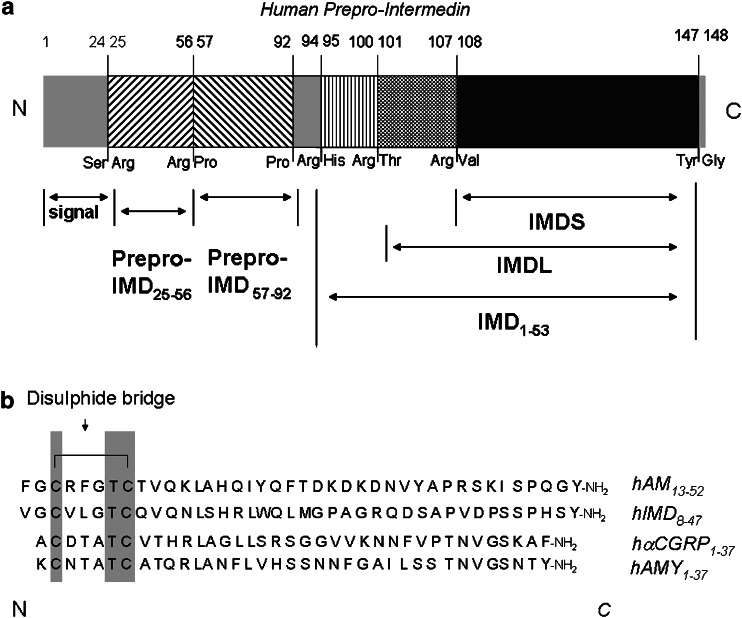
The 148 prepro-peptide encoded by a gene located on the distal arm of human chromosome 22q3 encompasses a putative signal sequence of 20 amino acids at the N terminal. Pairs of basic amino acids are found at Arg100–Thr101 and Arg107–Val108 (Figure 1); proteolytic cleavage at these sites followed by cleavage of Tyr147–Gly148 and amidation of the C-terminal amino acid yields a long 47 amino-acid form of the peptide, IMD1–47 (prepro-IMD101–147, IMDL) and a short 40 amino-acid peptide IMD8–47 (prepro-IMD108–147, IMDS) (Roh et al., 2004). Analysis of orthologous intermedins indicates that the position of the N-terminal dibasic cleavage site varies by several amino acids between species whereas the arginine residue seven amino acids downstream from this cleavage site in human IMD is conserved in all species, supporting an important physiological role for the shorter form (Chang et al., 2004; Roh et al., 2004). Identification of a further proteolytic cleavage site in rat prepro-IMD between Arg92–His93 indicates the existence of an additional larger peptide, prepro-IMD93–145 or rIMD1–53, which also possesses biological activity (Yang et al., 2005a; Ren et al., 2006). It has been noted that similar pairs of basic amino acids at Lys93–Arg94 and Arg146–Arg147 demark mature AM1–52 within prepro-AM (Kitamura et al., 1993) and that only the terminal 40 amino acids, AM13–52, are absolutely required for biological activity (Chu et al., 2000). Additional structurally distinct peptides are derived from the prepro-AM precursor and are also biologically active: an N-terminal peptide, prepro-AM22–41, named pro-AM N-terminal peptide (Kitamura et al., 1994), and a C-terminal peptide, prepro-AM153–185, named adrenotensin (Gumusel et al., 1996). A further peptide is demarked by prepro-AM45–92; its physiological function, if any, remains unknown (Morgenthaler et al., 2005). In contrast, the IMD gene is unlikely to encode additional active peptides since the putative prepro region is not conserved between species. IMD itself is highly conserved; mouse and rat IMD1–47 differ by one amino acid, while human IMD1–47 is 87% identical to rat and >60% to pufferfish (Roh et al., 2004; Takei et al., 2004b). Closer structural similarity of IMD to CGRP and AM than to AMY and CT indicates that CGRP, AM and IMD represent an evolutionary sub-branch of the superfamily (Chang et al., 2004). IMD shares the structural features characteristic of the superfamily, namely an N-terminal intramolecular loop of six amino acids flanked by a disulphide bond, followed by a well-defined α-helical region leading into a predominantly disordered structure terminating in a C-terminal amide (Roh et al., 2004). The length of the N-terminal sequence proximal to the intramolecular loop varies depending on the form of IMD, being greatest for IMD1–53. It is highly probable that several biologically active forms of IMD are generated within the same species dependent on post-translational processing events occurring within the cell or after secretion.
Figure 1.
Schematic diagram (a) of human prepro-IMD showing the fragments derived from processing of the 148 amino-acid prepro-peptide precursor at the putative cleavage sites indicated; (b) comparison of the primary sequences of adrenomedullin13–52, intermedin8–47 (IMDS), αCGRP1–37 and AMY1–37 in human; note the cysteine residues common to all of these peptides which contribute to the disulphide bridge giving rise to the characteristic intramolecular loop structure (adapted from Chang et al., 2004; Roh et al., 2004). AMY, amylin.
Distribution of intermedin in mammalian tissues and plasma
Evidence obtained from transcriptional expression and/or immunohistochemical investigation in rodents and human, indicates that IMD is found at particularly high levels in kidney, within the gastrointestinal tract, especially the muscularis mucosa of stomach and in jejunum, in brain, especially hypothalamus, where IMD is colocalized with arginine vasopressin, and intermediate and anterior lobes of pituitary (Roh et al., 2004; Takei et al., 2004b; Taylor et al., 2005; Takahashi et al., 2006), in skin (Kindt et al., 2007) and in submaxillary gland, pancreas, lung, spleen, thymus and ovary, but not in testis or adrenal gland (Takei et al., 2004b). Prepro-IMD is present, but much less abundantly so than prepro-AM in the heart of adult mouse (Takei et al., 2004b) but is not detected in myocardium of adult rat (Pan et al., 2005). In mouse, IMD is localized in heart to endothelial cells of coronary arteries and veins, and in kidney to endothelial cells of glomerular capillaries and the vasa recta running parallel to renal tubules (Takei et al., 2004a). At variance with this observation, prepro-IMD is expressed in rat in neonatal cardiomyocytes (Pan et al., 2005) and is detected, but present at substantially lower levels than prepro-AM, in adult cardiomyocytes (Zhao et al., 2006) consistent with greater prominence of IMD during embryonic development of the myocardium. In human tissue obtained at autopsy from subjects without known cardiac or renal disease, Takahashi et al. (2006) reported that IMD is localized to myocardial cells but not endocardium, pericardial adipose tissue or vasculature of heart, and to renal tubular cells in both cortex and medulla, but not glomeruli or vasculature of kidney; the transcript is detected in kidney and cardiac left ventricle although expression levels vary considerably between individuals. It is unclear if such observations reflect genuine interspecies variation in the distribution of IMD, or arise as a consequence of disparity in the nature and duration of experimental protocols employed to detect the peptide or its transcript. Indeed, further investigations by Morimoto et al. (2007) utilizing antiserum with higher sensitivity have indicated that IMD is localized, albeit less abundantly, in human pericardial adipocytes, vascular endothelial cells of pericardial veins and vascular smooth muscle of coronary arteries and renal arterioles in addition to cardiomyocytes and renal tubular cells. A general consensus is that the peptide is scarce in healthy adult myocardium. It is possible that the stress encountered leading up to death and the underlying pathology contributing to its cause influenced levels of IMD present in patients at autopsy.
IMD has been quantified in plasma of rat and human using primary antibodies which recognize a region common to all three bioactive fragments, IMD1–53, IMD1–47 and IMD8–47, but which do not crossreact with other members of this peptide superfamily. Plasma levels of IMD, like those of AM, are broadly similar or slightly greater in rat than human (Table 1), correlate positively with body mass index, particularly in females, but unlike those of AM are not increased with increasing age in adult human volunteers (Bell and Harbinson, unpublished observation). Plasma levels of IMD detected by radioimmunoassay are likely to be dependent on the sensitivity of the antibody used (Morimoto et al., 2007). Reliable quantification of AM in plasma is also influenced by its biological activity, short half-life, existence of a binding protein in plasma which facilitates the presence of high concentrations of AM at receptor sites and influences degradation (Morgenthaler et al., 2005). The extent to which such factors influence levels of IMD in plasma is not currently known. Neutral endopeptidase, a membrane-bound metallopeptidase, has been implicated in degradation of AM (Rademaker et al., 2002) and CGRP (Katayama et al., 1991). It is likely that IMD is also a substrate for neutral endopeptidase although CGRP, AM and IMD might not represent the preferred substrates for this enzyme: more specific enzymatic pathways probably remain to be identified. At least part of the post-translational processing of the prepro peptides is likely to occur extracellularly since fragments of AM and IMD precursor peptides have been identified in plasma. Mid-region pro-AM45–92 has attracted interest; the biological function, if any, of this fragment is unclear, it is less readily metabolized and may provide a more reliable biomarker in human plasma than the biologically active AM (Morgenthaler et al., 2005). Radioimmunoassay specifically recognizing fragments of the human IMD precursor, prepro-IMD25–56 and prepro-IMD57–92, have also become available recently and may offer similar utility.
Table 1. Peptide levels in mammalian plasma.
| Peptide | Species | Plasma level pg ml−1 mean+s.e. (n) | Reference |
|---|---|---|---|
| IMD | Rat, adult SD | 205.2+59.3 (5) | Taylor et al. (2006) |
| Rat, adult SD | 78.2+14.1 (10) | Bell et al. (2007) | |
| Rat, adult WKY | 106.6+8.7 (8) | McDermott et al. (2007) | |
| Human, adult | 7.2+0.6 (111) | Bell and Harbinsona (unpublished) | |
| Human, adult | 126.0+9.1 (3) | Morimoto et al. (2007) | |
| AM | Rat, adult SD | 121.1+6.3 (10) | Bell et al. (2007) |
| Rat, adult WKY | 92.5+10.4 (8) | McDermott et al. (2007) | |
| Human, adult | 27.1+1.5 (111) | Bell and Harbinsona (unpublished) | |
| Human, adult | 37.0+1.5 (46) | Kato et al. (1999) | |
| Human, adult | 39.7+8.3 (184) | Kato et al. (2002) | |
| Human, adult | 13.7+6.1 (21) | Caliumi et al. (2004) | |
| Human, adult | 8.7+2.1 (12) | Letizia et al. (2002) |
AM, adrenomedullin; IMD, intermedin; WKY, Wistar–Kyoto rat.
Measured in the same population of healthy individuals.
The cellular and tissue distribution of IMD overlaps in some aspects with that of CGRP and AM (reviewed in Bell and McDermott, 1996; Brain and Grant, 2004; Ishimitsu et al., 2006) but there are significant differences (for example, Kindt et al., 2007). The prominent localization of IMD in hypothalamus and pituitary is consistent with emerging evidence for involvement of IMD, like AM, in central regulation of thirst, fluid and electrolyte balance (Taylor et al., 2005; Taylor and Samson, 2005), like CGRP and AM, in the endocrine stress response (Chang et al., 2005; Taylor and Samson, 2005; Taylor et al., 2006), and secretion of oxytocin which influences reproductive function and complex, poorly defined behavioural responses in both males and females (Hashimoto et al., 2005; Taylor and Samson, 2005). The extensive distribution of the peptide throughout the digestive tract, notably in the mucosa of the stomach, supports involvement of IMD as a neurotransmitter or paracrine mediator in gastrointestinal regulation (Roh et al., 2004). Among other putative roles assigned to IMD are regulation of keratinocyte growth and maturation in skin (Kindt et al., 2007), regulation of fetal–placental growth (Chauhan et al., 2006), suppression of food intake (Roh et al., 2004) and release of cytokines from adipose tissue which modify insulin resistance and cardiovascular morbidity associated with obesity (Morimoto et al., 2007). Detailed discussion of these functions is not possible within the current review. Instead, emphasis has been placed on appraisal of the biological significance of IMD within the cardiovascular and renal systems.
Plasma levels of CGRP predominantly reflect overspill of this peptide from sensory nerves (Zaidi et al., 1985) and plasma levels of AM reflect secretion from atria and vasculature (Jougasaki and Burnett, 2000). The origin of IMD in plasma is unclear; the pituitary is likely to make a significant contribution. The relative paucity of IMD in the vicinity of the vasculature indicates that IMD is primarily an endocrine peptide. The prominent distribution of IMD in the renal tubule indicates a likely physiological role in local regulation of water-electrolyte balance and circulatory blood volume, while the robust expression of the peptide in diseased myocardium in contrast to the sparse levels found in healthy myocardium supports an important influence on cardiac pathology.
Receptor pharmacology
The biological actions of this peptide superfamily are mediated by two closely related G-protein-coupled receptors, the calcitonin receptor (CT) and calcitonin receptor-like receptor (CL) (Poyner et al., 2002). Gs-mediated activation of adenylate cyclase represents the major signalling pathway coupled with these receptors (Kuwasako et al., 2004), although Gq/11-mediated coupling to phospholipase C-β, Gi and/or Go-mediated regulation of potassium channels, activation of phosphatidylinositol 3-kinase and guanylate cyclase have been reported (reviewed in Poyner et al., 2002). A membrane-associated receptor component protein is required for optimum activation of signal transduction (Prado et al., 2001). In addition, receptor activity-modifying proteins (RAMPs) act as molecular chaperones for trafficking of CL and CT from endoplasmic reticulum and Golgi apparatus to the cell surface and exert a pivotal influence over the receptors' pharmacological characteristics (McLatchie et al., 1998).
With the discovery of AM it became apparent that CGRP and AM shared common receptors with varying degrees of affinity (Husmann et al., 2003; Hay et al., 2004). Coexpression of CL and RAMP1 constitutes a functional receptor with CGRP selectivity and the classical pharmacological characteristics of the CGRP1 receptor defined by Dennis et al. (1990); coexpression of CL with RAMP2 or RAMP3 gives rise to two subtypes of AM-selective receptors, AM1 and AM2 (reviewed by Poyner et al., 2002). While CGRP8–37 displays some selectivity as an antagonist at CGRP1 receptors, its ability to discriminate satisfactorily between CGRP1 and AM receptors is limited (Hay et al., 2003). Similarly, while AM22–52 displays some preference for AM receptors, it is a relatively low-affinity antagonist and cannot distinguish adequately between AM1 and AM2 receptors; high concentrations of this fragment also antagonize the CGRP1 receptor (Hay et al., 2003). In contrast, 1-piperidinecarboxamide N-[2-[[5-amino-1-[[4-(pyridinyl)-1-piperazinyl] carbonyl]pentyl]amino]-1-[(3,5-dibromo-4-hydroxyphenyl)-methyl]-2-oxoethyl]-4-(1,4-dihydro-2-oxo-3(2H)-quinazolinyl)-[R-(R*,S*)], the first non-peptide antagonist targeting this receptor family, is highly selective for the CGRP1 receptor and has greatly assisted elucidation of effects mediated by this receptor (Doods et al., 2000). Non-peptide antagonists displaying selectivity for AM1 and AM2 receptors, respectively, are eagerly awaited. RAMPs 1, 2 and 3 also interact with CT to form a series of receptors, AMY1, AMY2 and AMY3, which bind AMY with varying degrees of affinity and selectivity (Hay et al., 2005). It has been proposed (Hay et al., 2005) that the AMY1 receptor might account for the pharmacological profile of the classical CGRP2 receptor defined by Dennis et al. (1990), since there are no remaining orphan G-protein-coupled receptor in the human genome closely related to CT or CL.
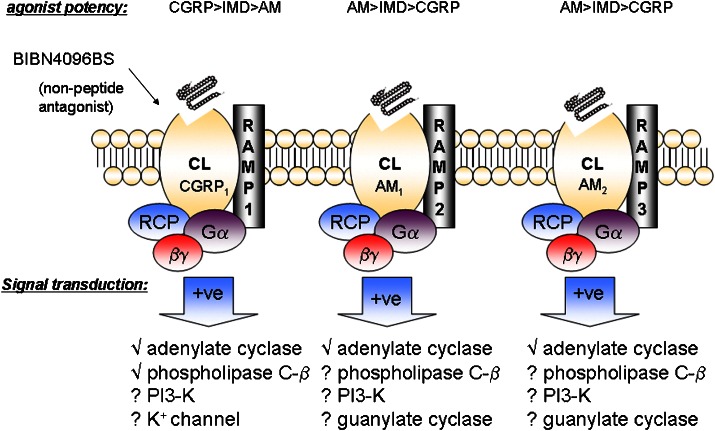
In the absence of convincing evidence that established or orphan G-protein-coupled receptor proteins form functional IMD-specific receptors by interacting with novel binding partners, it is hypothesized on account of the close structural similarity between IMD, AM and CGRP that IMD's biological effects are mediated via interaction with the various RAMP/CL complexes. Indeed, IMD interacts with RAMP/CL complexes in transfected 293T cells (Roh et al., 2004). Also, IMD1–47 and IMD8–47 stimulate accumulation of cyclic AMP in rat L6 myoblasts and human neuroblastoma SK-N-MC cells, both of which express RAMPs and CL, while the fragment IMD17–47 is devoid of activity but antagonizes the action of IMD (Chang et al., 2004; Roh et al., 2004). IMD interacts non-selectively with all three RAMP/CL complexes and possesses a pharmacological profile distinct from either AM or CGRP (Figure 2). Compared to CGRP, IMD has greater potency to stimulate cyclic AMP production in 293T cells transfected with RAMP3/CL (AM2) but lower potency in cells transfected with RAMP1/CL (CGRP1); compared to AM, IMD has lower potency in 293T cells transfected with either RAMP2/CL (AM1) or RAMP3/CL (AM2). The weak interaction of IMD at AMY1 receptors mirrors that of CGRP (Hay et al., 2005).
Figure 2.
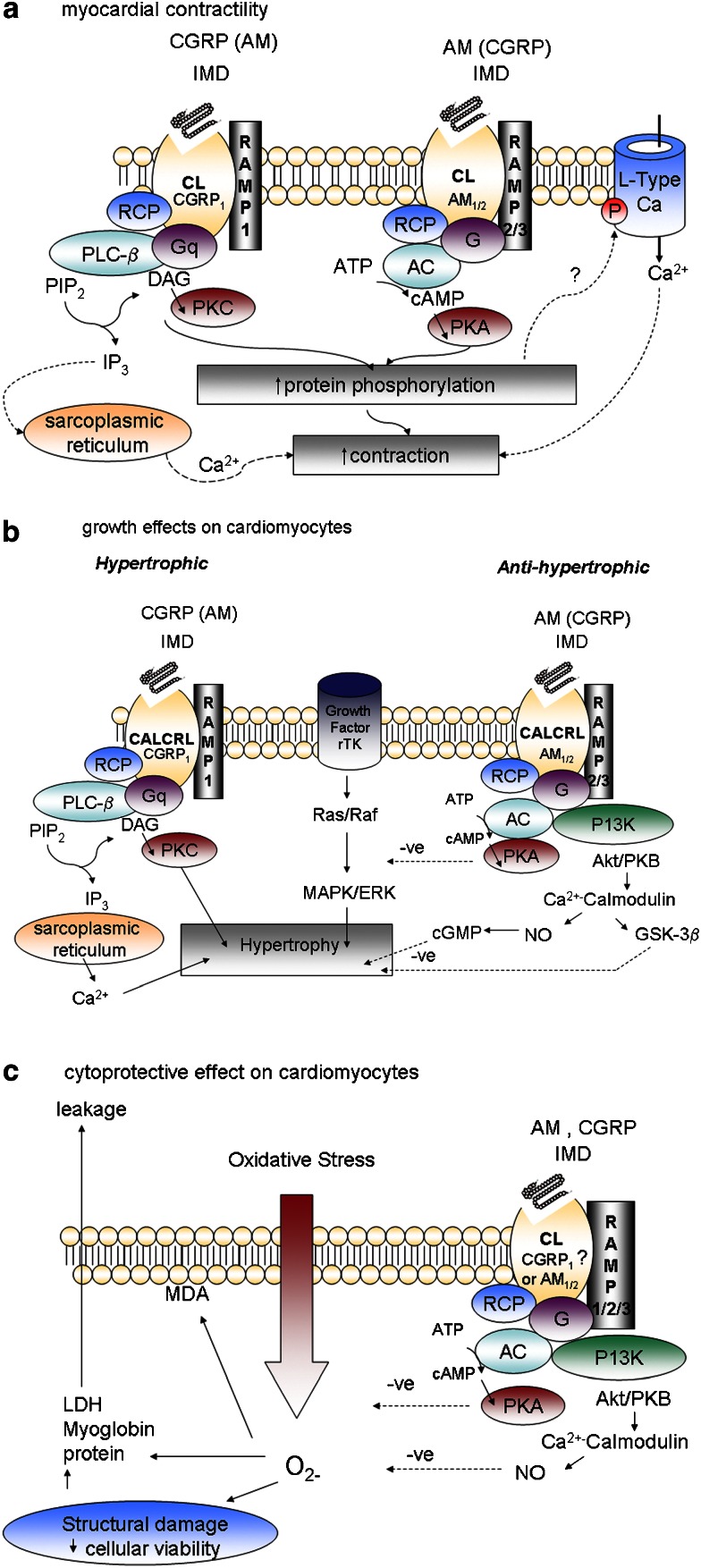
CGRP, AM and IMD share a common family of G-protein (Gαβγ)-coupled receptors formed by the association of the calcitonin receptor-like receptor (CL) with one of three receptor –activity-modifying proteins (RAMPs). A receptor component protein (RCP) is also required for optimum activation of signal transduction. Pharmacological characteristics and putative signalling mechanisms associated with each of the three recognized receptor subtypes (CGRP1, AM1 and AM2) are indicated (based on Poyner et al., 2002; Roh et al., 2004). CGRP, calcitonin gene-related peptide; AM, adrenomedullin; IMD, intermedin.
Distribution of receptors
CL is ubiquitously expressed, especially in areas of the central nervous system related to neuroendocrine and autonomic control, feeding and thirst, and in lung, adrenal gland, spleen, kidney and skin (Hagner et al., 2002; Cottrell et al., 2005). CL is also present within blood vessels, being extensively distributed throughout the systemic and pulmonary vasculature in endothelial cells and vascular smooth muscle cells, and in heart (Totsune et al., 2000; Cueille et al., 2002) including cardiomyocytes (Autelitano and Ridings, 2001; Zhao et al., 2006). RAMP1 is widely expressed in brain and spinal cord, gastrointestinal tract, adrenal gland, fat tissue, thymus and spleen (Nagae et al., 2000; Cottrell et al., 2005). Within the vasculature, RAMP1 is prominent in perivascular nerves and smooth muscle of smaller arteries and arterioles (Oliver et al., 2002), and is also expressed in cardiomyocytes and non-myocytes (Autelitano and Ridings, 2001), inferring the presence of CGRP1 receptors. RAMP2 is highly expressed in lung, spleen, fat tissue and kidney (Nagae et al., 2000). Within the cardiovascular system, RAMP2 is found in macrovascular and microvascular endothelial cells, vascular smooth muscle (Kamitani et al., 1999), cardiomyocytes, and less abundantly in cardiac non-myocytes (Autelitano and Ridings, 2001), inferring the presence of AM1 receptors. RAMP3 is less extensively distributed: high levels are found in kidney and to a lesser extent in lung, spleen and thymus (Nagae et al., 2000); RAMP3 is detected at low levels in adult myocardium and cardiomyocytes, being the least abundant RAMP (Zhao et al., 2006) which infers that AM2 receptors are sparse. However, RAMPs exhibit varying degrees of affinity for CL in different cell types in which they are coexpressed: the interaction of RAMP3 with CL predominates in rabbit aortic endothelial cells over that of RAMP1 or RAMP2 (Muff et al., 1998). In addition, the distribution of RAMPs extends further than CL, indicating a much broader role for RAMPs in cellular function; additional functions of RAMPs, unrelated to their interaction with CL have emerged (Christopoulos et al., 2003; Tfelt-Hansen et al., 2006).
The sharing of a receptor family by CGRP, AM and IMD has confounded interpretation of radioligand binding and functional data pertaining to these naturally occurring ligands. However, a general finding is that the distribution of binding sites within the cardiovascular system mirrors that of the individual peptides themselves and of their receptor components. CGRP binding is particularly evident in intima and media of smaller blood vessels, cardiac valves and conduction tissue and more abundant in atria than ventricular myocardium (reviewed by Bell and McDermott, 1996). AM binding is prominent in vascular endothelium, especially microvasculature, but is also found in myocardium (reviewed by Ishimitsu et al., 2006). There are few reports of binding studies using IMD as a radioligand. Within rat heart, IMD1–53 recognizes a single population of binding sites on myocardial sarcolemmal membranes (Jia et al., 2006). In view of the expression of all three RAMPs in myocardium, this finding could reflect inability of IMD to discriminate between CGRP1 and AM receptors.
Haemodynamic effects
The initial observations of Takei et al. (2004b) indicated that intravenous injection of IMD1–47 decreased arterial pressure more potently than AM in mice. Roh et al. (2004) reported that intraperitoneal injection of IMD1–47 produced a hypotensive effect in rat; IMD1–47 elicited a greater reduction in systolic blood pressure than an equivalent dose of IMD8–47 but was less effective than AM. The response to IMD1–47 was attenuated markedly by concurrent administration of CGRP8–37 in normotensive and spontaneously hypertensive rat (SHR). A weak antagonistic effect of AM22–52 on IMD response was demonstrated in SHR; the effect of this fragment was not examined in normotensive animals (Roh et al., 2004). Intravenous administration of IMD1–47 evokes a rapid and marked hypotensive effect in rat (Table 2): Taylor et al. (2005) found the response to 1 nmol kg−1 IMD1–47 to be of similar magnitude to that of CGRP but considerably less than that evoked by AM; Ren et al. (2006) reported that the hypotensive effect of IMD1–47 was more prominent than that of an equivalent dose (3 nmol kg−1) of either AM or IMD1–53, while Fujisawa et al. (2006) noted that the response to IMD was similar in magnitude to an equivalent dose of AM but less sustained. Pan et al. (2005), employing exceptionally large doses of the peptides (∼600 nmol kg−1), found IMD1–47 and IMD8–47 to elicit responses of similar magnitude. Overall, these findings indicate that the longer form of IMD possesses greater biological activity than the truncated form in vivo and that the hypotensive effect of IMD is qualitatively and quantitatively different to that of AM. In contrast, the influence of IMD1–47 on regional haemodynamics in rat is more similar to that of AM than CGRP but distinct from both (Fujisawa et al., 2007). IMD increases the percent distribution of cardiac output particularly to kidney, consistent with the marked expression of the peptide in this organ, but also like AM to heart, lungs and spleen and like CGRP to liver, stomach and small intestine. In rat, the hypotensive effect of IMD, like that of AM, is enhanced in pregnancy (Chauhan et al., 2007). In addition to an effect of IMD on resistance vessels, reduction of mean circulatory filling pressure in the absence of an effect on blood volume is evoked by IMD in rats subjected to ganglionic blockade, consistent with a direct action on capacitance vessels (Abdelrahman and Pang, 2006). Intravenous infusion of IMD into a large conscious mammal, namely the sheep, also results in marked reduction in mean arterial blood pressure; like that of AM, the effect of IMD is more pronounced on diastolic than systolic blood pressure (Charles et al., 2006).
Table 2. Infusion studies in rodents.
| Peptide | Dose | mABP maximum change (mm Hg) | |||
|---|---|---|---|---|---|
| IMD1–47 | 1.0 nmol kg−1 | iv | −7.9 | Rat | Taylor et al. (2005) |
| AM | 1.0 nmol kg−1 | iv | −15.0 | Rat | Taylor et al. (2005) |
| CGRP | 1.0 nmol kg−1 | iv | −5.0 | Rat | Taylor et al. (2005) |
| AM | 3.0 nmol kg−1 | iv | −18.3 | Rat | Ren et al. (2006) |
| IMD1–53 | 3.0 nmol kg−1 | iv | −15.8 | Rat | Ren et al. (2006) |
| IMD1–47 | 3.0 nmol kg−1 | iv | −19.2 | Rat | Ren et al. (2006) |
| IMD1–47 | 5.0 nmol kg−1 | iv | −12.0 | Rat | Fujisawa et al. (2006) |
| AM | 5.0 nmol kg−1 | iv | −10.0 | Rat | Fujisawa et al. (2006) |
| AM | 10 nmol kg−1 | iv | −17.0 | Mouse | Takei et al. (2004a) |
| IMD1–47 | 10 nmol kg−1 | iv | −27.0 | Mouse | Takei et al. (2004a) |
| IMD1–47 | ∼600 nmol kg−1 | iv | −39.0 | Rat | Pan et al. (2005) |
| IMD8–47 | ∼600 nmol kg−1 | iv | −45.0 | Rat | Pan et al. (2005) |
| IMD1–47 | 195 pmol | icv | +11.0 | Rat | Taylor et al. (2005) |
| CGRP | 195 pmol | icv | +9.0 | Rat | Taylor et al. (2005) |
| AM | 195 pmol | icv | No change | Rat | Taylor et al. (2005) |
AM, adrenomedullin; CGRP, calcitonin gene-related peptide; icv, intracerebroventricular; IMD, intermedin; iv, intravenous; mABP, mean arterial blood pressure.
It is probable that the actions of exogenous IMD in vasculature are associated with activation of both CGRP and AM receptors dependent on route of administration and ease of access to these receptors. The relative abundance of these receptor populations, their localization on endothelium or smooth muscle and associated coupling mechanisms vary with regard to species and vessel and influence the characteristics of responses evoked in specific vascular beds. The action of CGRP is independent of endothelium in the majority of tissues investigated and associated with CGRP1 receptor-stimulated accumulation of cyclic AMP and activation of potassium channels resulting in membrane hyperpolarization in vascular smooth muscle; less commonly CGRP, acting at CGRP1 receptors or AM receptors located on endothelial cells stimulates nitric oxide release (reviewed in Bell and McDermott, 1996; Brain and Grant, 2004) (Figure 3). AM is also heterologous in respect to species and vessel, both CGRP8–37- and AM22–52-sensitive and endothelium-dependent and -independent effects have been reported (reviewed in Brain and Grant, 2004). IMD1–47 and IMD8–47 increase cyclic AMP levels and relax norepinephrine preconstricted rat aorta in vitro (Pan et al., 2005). Vasodilatation of this vessel in response to IMD1–53 has been attributed to endothelium-dependent and -independent mechanisms (Yang et al., 2006). Indeed IMD1–53, like AM, stimulates constitutive, but not inducible nitric oxide synthase leading to enhanced nitric oxide production in aortic endothelial cells and also stimulates vascular L-arginine transport and expression of cationic amino-acid transporters, CAT-1 and CAT-2B (Yang et al., 2006). Rat aorta is one of the few vessels in which endothelium-dependent nitric oxide release has been demonstrated in response to CGRP (Brain and Grant, 2004). However, the effect of IMD in aorta might also be accounted for by activation of AM-preferring receptors on vascular smooth muscle since AM-stimulated accumulation of cyclic AMP in aortic smooth muscle cells is attenuated by AM22–52 (Eguchi et al., 1994), although AM22–52 lacks adequate specificity to enable CGRP1 receptor involvement to be discounted. In rat mesenteric artery, the action of IMD is dependent on production of nitric oxide, accumulation of cyclic AMP and cyclic GMP and activation of potassium channels, again consistent with both endothelium-dependent and -independent signalling events (Chauhan et al., 2007). Kandilci et al. (2006) reported that IMD1–47 decreases pulmonary arterial pressure and decreased pulmonary vascular resistance in rat under conditions of elevated pulmonary vasoconstrictor tone. The response to IMD is attenuated by but less sensitive to CGRP8–37 than that of CGRP. Although IMD induces tachyphylaxis to CGRP, IMD does not induce self-tachyphylaxis, and CGRP does not induce tachyphylaxis to IMD. Pulmonary vasodilatation in response to IMD is dependent largely on production of nitric oxide and not cyclooxygenase products or activation of KATP channels (Kandilci et al., 2006). This finding is consistent with the endothelial dependence of CGRP reported previously in rat pulmonary artery (Wisskirchen et al., 1998). However, there is conflicting evidence regarding involvement of nitric oxide in the vasorelaxant responses to CGRP in this vessel (Heaton et al., 1995; Wisskirchen et al., 1998). The action of IMD cannot be entirely accounted for by stimulation of CGRP1 receptors on endothelium coupled to production of nitric oxide. The action of AM in this vessel is not attenuated by CGRP8–37 (Wisskirchen et al., 1998) or NG-nitro-L-arginine-methyl-ester (L-NAME) (Heaton et al., 1995) consistent with the presence of additional receptors, coupled to alternative mechanisms, at which IMD might also act.
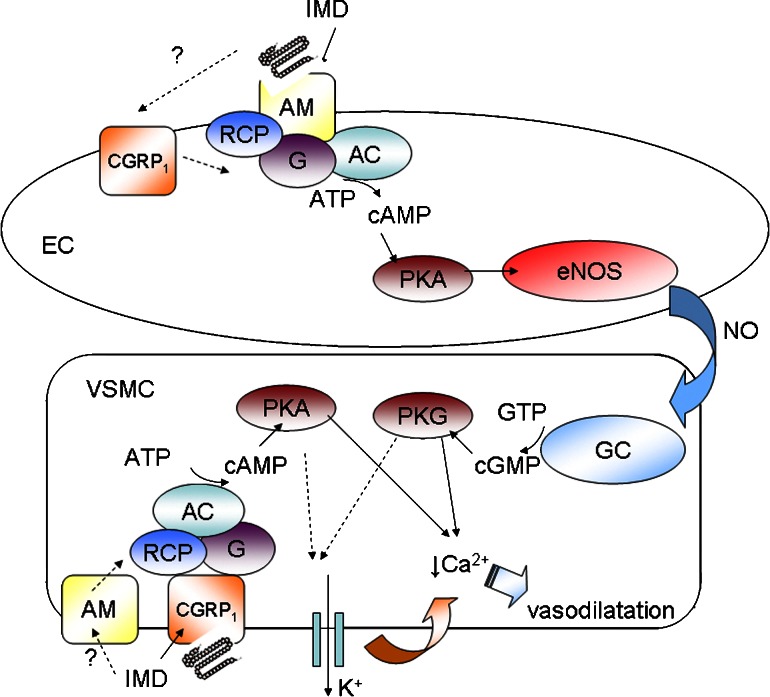
Figure 3.
Schematic diagram outlining the proposed endothelium-dependent and -independent signalling pathways by which IMD might evoke vasodilatation. The relative contribution of such processes is likely to vary significantly with respect to vessel and species. Endothelial cell (EC); vascular smooth muscle cell (VSMC); adenylate cyclase (AC); nitric oxide synthase (NOS); protein kinase A (PKA); protein kinase G (PKG); guanylate cyclase (GC). The endothelium-dependent effect of IMD is attributed to cyclic AMP-dependent activation of constitutive endothelial NOS and synthesis of nitric oxide which then diffuses to VSMC to stimulate GC leading to accumulation of cyclic GMP and activation of PKG. Accumulation of cyclic AMP in EC is stimulated mainly by activation of endothelial AM receptors although a possible contribution of CGRP1 receptors, at least in some vessels, cannot be discounted. Stimulation of CGRP1 receptors, or alternatively in some vessels of AM receptors, on VSMC directly is associated with accumulation of cyclic AMP and activation of PKA. PKA and PKG trigger processes in VSMC which lead to reduced intracellular calcium ion concentration resulting in vasodilatation; such processes may involve activation of outward potassium currents leading to membrane hyperpolarization and reduced calcium entry. CGRP, calcitonin gene-related peptide; IMD, intermedin; NOS, nitric oxide synthase.
Hypotension evoked by IMD1–47 in rat in vivo is not attenuated by L-NG-monomethyl arginine (L-NMMA), prompting Taylor et al. (2005) to conclude that the systemic vasodilator response to IMD, like that to CGRP, is much less dependent on nitric oxide production than that of AM. Taken together with the similar magnitude of the responses to IMD and CGRP, this observation is more consistent with an action of IMD at CGRP1 receptors, located predominantly on smooth muscle, than at AM receptors, found mainly on endothelium. However, the lack of effect of L-NMMA could be interpreted as relative inhibition since vasoconstriction evoked by L-NMMA directly could mask an inhibitory effect by raising tone thereby favouring the action of a vasodilator mediator (Gardiner et al., 1995). In all in vitro studies to date IMD has been applied to vessels in which endothelium-dependence of CGRP's action has been demonstrated, at least in part. Further studies are warranted to characterize the vasorelaxant effect of IMD in smaller resistance arteries and arterioles in which the vasorelaxant effect of CGRP is endothelium-independent and CGRP1 receptors are found abundantly on smooth muscle (Oliver et al., 2002).
The physiological as opposed to pharmacological influence of members of this family on haemodynamics is unclear. CGRP8–37 does not affect basal blood pressure (Gardiner et al., 1991) and results obtained from measurement of blood pressure in various strains of CT/αCGRP gene knockout mice relative to wild-type mice have been conflicting (Lu et al., 1999; Gangula et al., 2000; Ohhashi et al., 2001). CGRP8–37 does influence regional blood flow (Gardiner et al., 1990) which indicates that CGRP might regulate tissue blood flow under physiological conditions and provide a braking system for microvascular resistance to protect tissues from injury. Homozygous knockout of the AM gene in mice results in a lethal phenotype due to insufficient development of blood vessels (Caron and Smithies, 2001; Hay and Smith, 2001). Evidence obtained from viable heterozygous AM gene knockout mice indicates a role for endogenous AM in regulation of blood pressure (Shindo et al., 2001). The physiological influence of IMD is unclear in the absence of suitable transgenic mice in which to investigate the unique contribution of this peptide.
Effects on the kidney
Takei et al. (2004b) initially reported that bolus injection of a hypotensive dose of IMD, but not AM, inhibited urine flow and reduced urine sodium concentration in mice. In conscious sheep infusion of a markedly hypotensive dose of IMD, like AM, also evoked a modest reduction in urine sodium concentration but did not influence urine volume (Charles et al., 2006). In contrast, diuretic and natriuretic effects of AM have been reported previously in other species, including human (Nagaya et al., 1999: Lainchbury et al., 2000). It is difficult to dissociate effects exerted locally on the kidney from the consequences of the systemic hemodynamic effects of a vasodilator peptide. It is possible that the systemic hypotensive effect of IMD resulted in reduced renal perfusion thereby masking a direct diuretic and natriuretic effect of IMD. Furthermore, the strain of mouse used by Takei et al. (2004b) originated in a dry region of Central Asia and these mice urinate scarcely to conserve sodium and water; some natriuresis and diuresis was evoked by IMD at higher rates of infusion in overhydrated mice. Direct infusion of IMD into rat renal artery at doses sufficient to profoundly lower systemic blood pressure also inhibits urine flow (Fujisawa et al., 2004), while intravenous administration of less hypotensive doses of IMD, like AM, increases renal blood flow and the proportion of cardiac output distributed to the kidney indicative of a direct role for IMD in renal vasculature (Fujisawa et al., 2006, 2007). Renal vasodilatation in response to AM has been attributed to a nitric oxide-dependent mechanism (Edwards et al., 1996; Majid et al., 1996). Activation of renal sympathetic nerve activity (RSNA) accompanies the hypotensive actions of IMD, AM and also of sodium nitroprusside; however, the effects of the peptides on RSNA are not completely suppressed while that of sodium nitroprusside is abolished by baroreceptor denervation (Fujisawa et al., 2006). Similarly in sheep, enhancement of RSNA precedes the maximum reduction in blood pressure (Charles et al., 2006). The baroreceptor reflex cannot therefore account for all of the augmentation of RSNA by these peptides and additional mechanisms such as direct enhancement of central sympathetic outflow are likely to contribute. The actions of IMD on renal blood flow persist when blood pressure, heart rate and RSNA return to normal (Fujisawa et al., 2006); furthermore, subdepressor doses of the peptide also increase renal blood flow and decrease renal vascular resistance strongly supporting a direct effect on the renal vasculature (Fujisawa et al., 2004). Lack of influence of IMD on glomerular filtration rate infers that IMD influences renal afferent and efferent arterioles equally; in contrast, intrarenal infusion of subdepressor doses of CGRP increases renal blood flow and glomerular filtration rate (Kurtz et al., 1989; Edwards and Trizna, 1990). Increased urinary flow and urinary sodium excretion in response to IMD in the absence of an increase in glomerular filtration rate is indicative of a direct inhibitory influence of IMD on tubular re-absorption of sodium (Fujisawa et al., 2004). The actions of AM are qualitatively similar but more persistent. At variance with these findings, AM stimulates sodium uptake in distal tubule luminal membranes in vitro, indicative of an antinatriuretic effect (Leclerc and Brunette, 2000). The direct action of IMD on tubular sodium handling, receptor involvement and associated signalling mechanism remains unresolved. Central administration of IMD, like AM, increases plasma levels of arginine vasopressin (Taylor and Samson, 2005) which exerts an action predominantly on distal tubule and collecting duct to conserve water, but not salt, by concentrating the urine and reducing urine volume. The influence of IMD on renal tubular water and electrolyte balance is likely to reflect a composite of the direct and indirect actions of the peptide.
Effects on the heart
IMD influences cardiac output as a consequence of direct and indirect action on the heart (Figure 4). Hypotension evoked by intraperitoneal or intravenous administration of IMD, like that of AM and CGRP, is accompanied by increased heart rate (Roh et al., 2004; Takei et al., 2004b; Charles et al., 2006). Tachycardia evoked by intravenous administration of IMD1–47 in rat is blunted by ganglionic blockade consistent with autonomic nervous system involvement (Abdelrahman and Pang, 2006). In rat, tachycardia evoked by IMD is qualitatively similar but less sustained than that of AM (Roh et al., 2004) but greater than that of sodium nitroprusside despite an equivalent decrease in mean arterial pressure. Tachycardia evoked by sodium nitroprusside is abolished by baroreceptor denervation while that of IMD is attenuated but not abolished (Fujisawa et al., 2006). In sheep, augmentation of heart rate occurs before the maximum extent of reduction in blood pressure by IMD is achieved (Charles et al., 2006). The action of IMD clearly cannot be accounted for solely by baroreceptor activation in response to hypotension and additional mechanism(s) are likely to contribute. Indeed, intracerebroventricular administration of IMD1–47 (Taylor et al., 2005) evokes a marked increase in blood pressure and heart rate in rat; in regard to potency and duration the actions of IMD1–47 are more similar to those of CGRP than AM, are antagonized by CGRP8–37 suggestive of CGRP1 receptor involvement, and attenuated by phentolamine indicating that IMD's effect is attributable to sympathetic outflow from the brain (Taylor et al., 2005). Intracerebroventricular administration of IMD1–53 also increases blood pressure and heart rate; these actions are markedly attenuated by an anti-prepro-IMD antibody, or by either CGRP8–37 or AM22–52 (Ren et al., 2006). However, the antibody did not influence blood pressure or heart rate in the absence of exogenous IMD1–53 indicating that the endogenous peptide is unlikely to participate in central cardiovascular regulation under physiological conditions. IMD8–47 also increases heart rate in isolated perfused hearts of rats consistent with a direct chronotropic action (Pan et al., 2005). It is possible that the direct component of the positive chronotropic effect of IMD8–47 is associated with activation of CGRP1 receptors since CGRP evokes a direct chronotropic response in spontaneously beating right atria and neonatal cardiomyocytes of rat (Fisher et al., 1988; Wang and Fiscus, 1989).
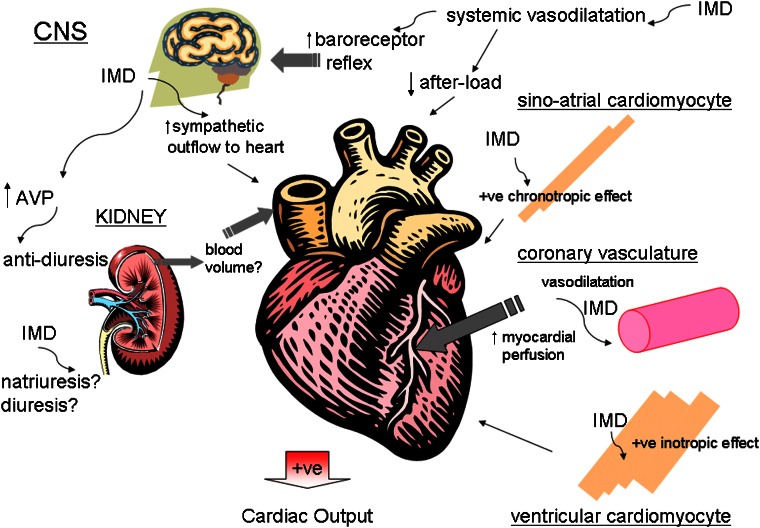
Figure 4.
Schematic diagram depicting the combination of direct and indirect effects exerted upon the heart by IMD, which influence cardiac output. IMD exerts chronotropic and inotropic effects directly on myocardium. Coronary vasodilatation enhances myocardial perfusion, which augments contractility indirectly. Systemic vasodilatation reduces afterload and activates the baroreceptor mechanism to increase central sympathetic outflow, which also enhances cardiac contractility. The actions of IMD centrally and locally within the kidney influence blood volume which in turn influences venous return and cardiac output. IMD, intermedin.
Intravenous administration of IMD8–47 or IMD1–47 in rat at concentrations sufficiently large to evoke marked hypotension reduce left ventricular end-systolic pressure and maximum rates of pressure development and decline, indicative of a negative inotropic action (Pan et al., 2005). IMD8–47 evoked similar effects in isolated perfused hearts while IMD1–47 was almost without effect; baseline parameters were greater in the IMD8–47-treated group which may have accounted for the difference observed. Myocardial cyclic AMP content is elevated in response to intravenous administration in vivo or perfusion of isolated hearts with the peptides (Pan et al., 2005); this observation is inconsistent with a negative inotropic action. In subsequent studies in the same laboratory, intraperitoneal injection of IMD1–53 over four consecutive days in rat had no influence on cardiac function (Jia et al., 2006) but paradoxically IMD1–53 evoked a positive inotropic effect acutely in isolated perfused rat heart at variance with earlier findings (Yang et al., 2005a). Fujisawa et al. (2007) also report that intravenous administration of IMD1–47 enhances cardiac output in rats. Reports of augmented cardiac output in response to CGRP and AM are influenced by variations in regard to species studied, dose chosen, and therefore the extent of reduction in afterload and sympathetic activation. The latter influences are absent in isolated perfused hearts. CGRP elicits a positive inotropic action in isolated perfused hearts of guinea-pigs but not of rats or rabbits (reviewed in Bell and McDermott, 1996). AM evokes a positive inotropic effect with gradual onset in isolated perfused hearts of rats; this effect is not attenuated by CGRP8–37, indicative of an AM receptor-dependent response. Furthermore, the effect is independent of cyclic AMP and associated with initial discharge of calcium stores and subsequent protein kinase C-dependent phosphorylation of L-type calcium channels (Szokodi et al., 1998). The positive inotropic response to IMD in rat might be attributed in part to enhanced myocardial perfusion since IMD8–47 and IMD1–53 dilate coronary arteries and enhance coronary flow in isolated perfused rat hearts (Pan et al., 2005; Yang et al., 2005a). The vasorelaxant effect of AM in human coronary artery is attenuated by AM22–52 (Terata et al., 2000). However, the effect of AM in rat is less potent than that of CGRP and sensitive to CGRP8–37, suggesting CGRP1 receptor involvement, at least in this species, in the response to IMD. Evidence for a direct action on myocardium requires use of isolated atria, papillary muscle or cardiomyocytes. IMD evokes a positive inotropic effect in adult rat (Bell, unpublished observation) and mouse ventricular cardiomyocytes (Dong et al., 2006). In the latter, both PKA- and protein kinase C-dependent mechanisms are implicated; the inotropic effect is associated with intracellular calcium release and is qualitatively similar to those of CGRP and AM, but receptor involvement has not been explored. It is not known if IMD evokes similar responses directly in cardiomyocytes of other species including man. CGRP evokes a direct positive inotropic action in atria of rat, guinea-pig and human, human myocardial trabeculae and in ventricular cardiomyocytes of rat, but not rabbit or human (reviewed in Bell and McDermott, 1996; Saetrum Opgaard et al., 2000). In adult rat ventricular cardiomyocytes, the action of CGRP is attenuated by CGRP8–37 indicative of CGRP1 receptor involvement, but independent of cyclic AMP (Bell and McDermott, 1994) and associated with calcium-induced calcium release (Huang et al., 1999). AM elicits a positive inotropic effect on papillary muscle and atrial cardiomyocytes of rat (Ihara et al., 2000), has no activity on human ventricular cardiomyocytes (Saetrum Opgaard et al., 2000) and evokes a negative response in rabbit cardiomyocytes attributed to nitric oxide synthesis and activation of a cyclic GMP-dependent mechanism (Ikenouchi et al., 1997). Ability to demonstrate a contractile response directly in myocardium is dependent on species, age, cardiac chamber and frequency and duration of electrical stimulation. Heterogeneity in regard to receptor involvement and signalling mechanism is likely to be influenced by selectivity of the antagonists and signal transduction inhibitors chosen and concentrations at which these are employed. Activation of CGRP1 receptors coupled to phosphotidylinositol signalling and mobilization of intracellular calcium is likely to contribute to the inotropic effect of IMD in rodent ventricular cardiomyocytes; the involvement of additional receptors and/or signalling mechanisms is possible (Figure 5a).
Figure 5.
Schematic diagram outlining the proposed signalling pathways by which IMD might influence (a) contraction; (b) hypertrophic remodelling; (c) protection against the deleterious consequences of oxidative stress directly in ventricular cardiomyocytes. Adenylate cyclase (AC); protein kinase A (PKA); protein kinase C (PKC); diacylglycerol (DAG); mitogen-activated protein kinase (MAPK); receptor tyrosine kinase (rTK); phospholipase C-β (PLC-β); extracellular signal-regulated kinase (ERK); phosphoinositide 3-kinase (PI3K); receptor component protein (RCP). IMD, intermedin.
Pathophysiological significance in the cardiovascular system
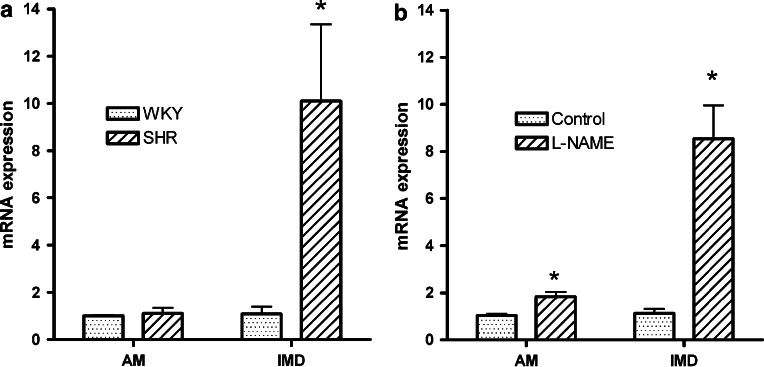
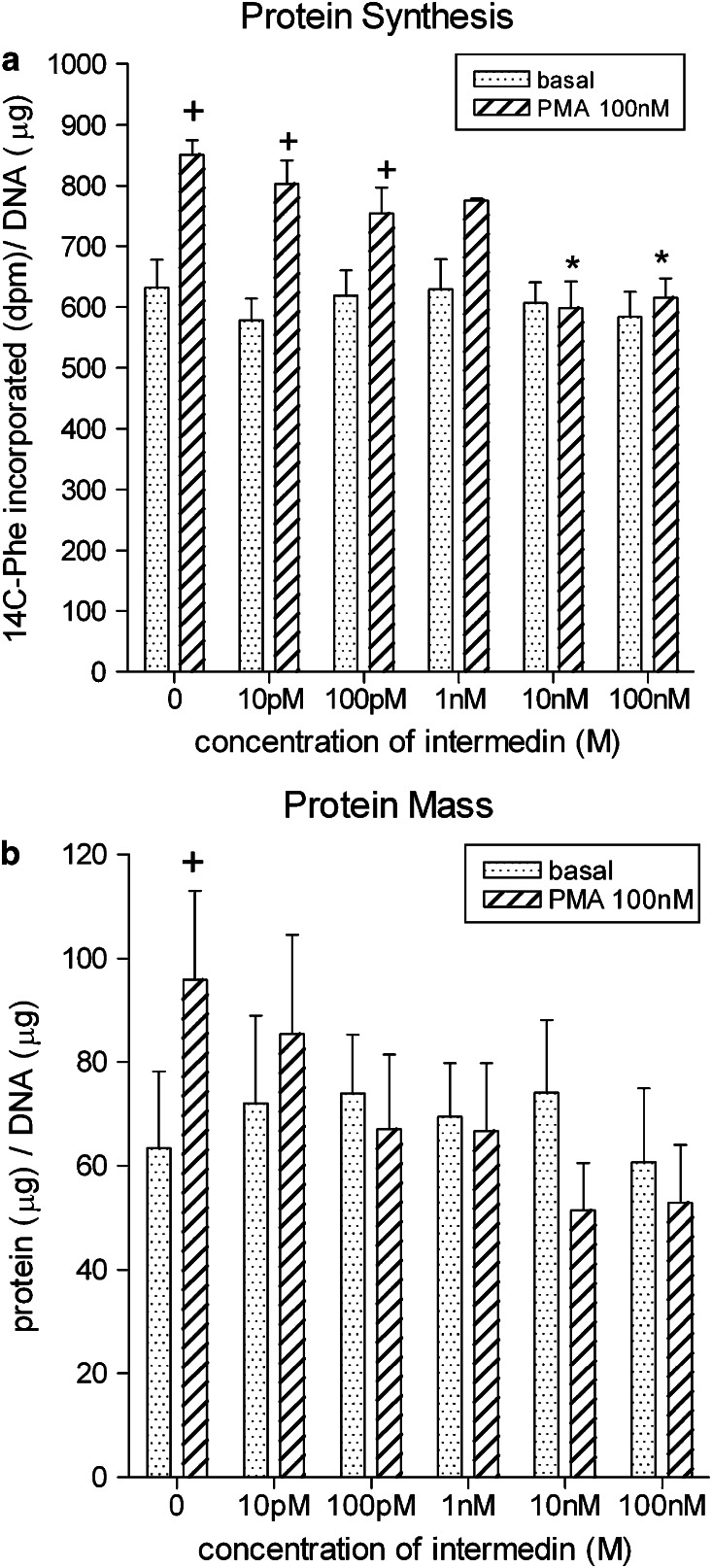
Expression of IMD is augmented in hypertrophied myocardium induced by chronic administration of isoprenaline in rat (Pan et al., 2005). The transcript is also upregulated while that of AM is unchanged (Figure 6) and neutral endopeptidase reduced (McDermott et al., 2007) in hypertrophied left ventricular cardiomyocytes of SHR relative to that of normotensive Wistar–Kyoto rat. IMD does not alter basal protein synthesis in neonatal (Pan et al., 2005) or adult (Figure 7) rat cardiomyocytes but abolishes protein synthesis in response to a hypertrophic stimulus. These findings support an important counter-regulatory influence of IMD locally on hypertrophic growth and remodelling. It is probable that this action of IMD is mediated by AM-preferring receptors since AM attenuates cardiomyocyte hypertrophy (Bell et al., 2006) while CGRP is a prohypertrophic stimulus in rat (Bell et al., 1995) (Figure 5b). Increased expression of RAMP1, and to a lesser extent RAMP3 mRNA, is evident but RAMP2 mRNA is unchanged in hypertrophied cardiomyocytes of SHRs. Paradoxically, at protein level, all three RAMPs are less abundant in sarcolemmal membranes of SHR cardiomyocytes than those of Wistar–Kyoto rat (McDermott et al., 2007); the consequences for IMD signalling and other functions regulated by RAMP proteins is unclear. Such changes could represent a primary defect in IMD receptor signalling or downregulation secondary to augmented levels of IMD.
Figure 6.
Expression of prepro-IMD and prepro-AM mRNAs in left ventricular cardiomyocytes of (a) SHR and WKY rat at 20 weeks of age (mean+s.e., n=8), data taken from McDermott et al., 2007; (b) 8-week-old SD rat given L-NAME (35 mg kg−1 day−1) in drinking water for 8 weeks and age-matched untreated rat for comparison, (mean+s.e., n=10), data taken from Bell et al. (2007). Data were expressed relative to glyceraldehyde 3-phosphate dehydrogenase mRNA levels. *Denotes significant difference between treatment groups, *P<0.05. AM, adrenomedullin; IMD, intermedin; SHR, spontaneously hypertensive rat; WKY, Wistar–Kyoto; L-NAME, NG-nitro-L-arginine-methyl-ester.
Figure 7.
Effect of IMD (10 pM–100 nM) on (a) protein synthesis; (b) protein mass in ventricular cardiomyocytes (combined population obtained from left and right ventricles of 12-week-old normotensive SD control rat) and maintained in short-term (24 h) culture under basal conditions and in the presence of the hypertrophic stimulus, phorbol 12-myristate 13-acetate (PMA) (100 nM). Data are the mean values+s.e. of 4–6 heart cell preparations. *Denotes significant difference between PMA response in the absence and presence of IMD. +Denotes significant difference between basal value and PMA-stimulated response (P<0.05). Unpublished observation; methodology employed as described for AM in Bell et al. (2006). IMD, intermedin.
Global ischaemia followed by reperfusion in rat heart in vitro is associated with bradycardia, inhibition of contractile function and significant myocardial injury. Calcium overload and excessive production of reactive oxygen species are central to ischaemia–reperfusion injury. IMD, administered during the reperfusion period ameliorates the reduction in cardiac contractility, demonstrated by reversal of bradycardia and of the decline in maximum change in left ventricular pressure and rates of pressure development and decay (Yang et al., 2005b). IMD attenuates ischaemia–reperfusion injury, as evidenced by reduced leakage of lactate dehydrogenase, total protein and myoglobin from myocardium and less formation of malondialdehyde, an end product of lipid peroxidation by reactive oxygen species. The cardio-protective effects of IMD are similar to those of CGRP and AM (Kato et al., 2003), which are mediated in part by cyclic AMP (Beltowski and Jamroz, 2004). IMD also stimulates accumulation of cyclic AMP in myocardium (Yang et al., 2005a). The number of binding sites for IMD in myocardium is increased during ischaemia–reperfusion, indicating transport of more receptors to the cell surface; receptor affinity is also increased. It is not clear which receptor subtype(s) are involved in the protective effects of IMD (Yang et al., 2005a). Levels of the prepro-IMD precursor are reduced, indicative of enhanced processing to the active form. All three forms of the peptide, IMD1–53, IMD1–47 and IMD8–47 are equally protective (Yang et al., 2005a, 2005b). At least part of the beneficial action of IMD could be attributed to improved coronary perfusion (Yang et al., 2005a), but it is likely that a positive inotropic effect and cytoprotective effect is also exerted directly on myocardium (Figure 5c).
Subcutaneous injection of a high dose of isoprenaline over two consecutive days provides an alternative means of inducing myocardial ischaemic injury. This approach increases plasma lactate dehydrogenase activity and plasma and myocardial malondialdehyde content, lowers maximum rate of increase and decrease of left ventricular pressure development and raises left ventricular end diastolic pressure, indicative of severe heart failure and ischaemic injury. Myocardial structural disorder, subendocardial necrosis, fibroblast hyperplasia, capillary dilatation and leukocyte infiltration are evident. Concurrent administration of IMD1–53 improves myocardial contractile function, as evidenced by reversal of isoprenaline-evoked decline in functional parameters, attenuated myocardial lactate dehydrogenase leakage and malondialdehyde formation and isoprenaline-induced decline in myocardial cyclic AMP content (Jia et al., 2006). IMD1–53 binding capacity is increased in myocardial sacrolemmal membranes and this is associated with augmented mRNA expression of CL and each of the RAMPs, especially RAMP3. This finding is indicative of enhanced synthesis of receptor components, in addition to an influence on transport of receptors to the cell surface, demonstrated in the acute setting in vitro. The absence of an effect of IMD1–53 on contractile function in the absence of isoprenaline-induced injury, together with marked upregulation of the IMD receptor system indicates that IMD plays a pivotal cardio-protective role in response to ischaemic insult.
Chronic administration of the inhibitor of nitric oxide synthase, Nω-nitro-L-arginine methyl ester (L-NAME), to rats results in hypertension which depending on the dose given is accompanied by activation of the renin–angiotensin system, myocardial remodelling (cardiac hypertrophy and fibrosis), vascular remodelling (medial thickening and perivascular fibrosis), cardiac ischaemia and necrosis, and mechanical dysfunction (reviewed in Zatz and Baylis, 1998; Bell et al., 2006). In our own laboratory in which L-NAME (35 mg kg−1 day−1) was administered to rats for 8 weeks, myocardial hypertrophy is evidenced by increased cardiomyocyte width and acquisition of a hypertrophic phenotype (Zhao et al., 2006) while oxidative stress is shown by increased cardiomyocyte membrane protein oxidation and augmented expression of pro-oxidant enzymes (Bell et al., 2007). Robust increase in prepro-IMD mRNA relative to prepro-AM in cardiomyocytes of rats treated chronically with L-NAME (Figure 6) indicates an important role for IMD in the cardiac pathology of nitric oxide deficiency. Furthermore, IMD, AM and their receptor components are differentially regulated: upregulation of AM, RAMP2 and RAMP3 expression in cardiomyocytes of rats subjected to chronic nitric oxide deficiency is prevented when blood pressure is normalized by the smooth muscle relaxant, hydralazine given concurrently with the diuretic, hydrochlorothiazide (Zhao et al., 2006) while that of IMD and RAMP1 is not attenuated. Conversely, upregulation of cardiomyocyte IMD and RAMP1 is abolished when oxidative stress is normalized by vitamin C and Tempol; this antioxidant combination does not lower blood pressure and does not attenuate the augmented expression of AM, RAMP2 and RAMP3 in L-NAME-treated rats (Bell et al., 2007). These studies indicate two important influences on AM and IMD signalling: cardiomyocyte expression of AM, RAMP2 and RAMP3 is influenced by pressure loading on the myocardium while that of IMD/RAMP1 is regulated by the extent of local oxidative stress in the absence of pressure loading. Neuronal release of CGRP from perivascular nerves is enhanced in response to myocardial ischaemia (Franco-Cereceda et al., 1987). CGRP enhances blood flow to ischaemic myocardium, improves contractile indices, attenuates ischaemia–reperfusion arrhythmias and protects cardiomyocytes directly against the detrimental effects of oxidative stress (Ren et al., 1993; Zhang et al., 1994). Enhanced levels of non-neuronally derived IMD acting on CGRP1 receptors, may, like neuronal CGRP, serve primarily an anti-ischaemic cardio-protective function in cardiomyocytes subjected to chronic nitric oxide deficiency. An additional attenuating influence for IMD on cardiomyocyte hypertrophy in this model, mediated by stimulation of AM2 receptors cannot be discounted, particularly since RAMP3 expression is also enhanced.
Oxidative stress within the cerebral circulation is a common cardiovascular complication of diabetes and is also observed in response to restriction in cerebral blood flow due to stroke or transient ischaemic attack. Cerebral endothelial cells, a major component of blood–brain barrier, normally form tight junctions and have specialized transport systems to maintain strict ionic and metabolic homeostasis of brain parenchyma. Cerebral endothelial cells have a large energy requirement by virtue of the abundance and activity of active transport systems operational across the blood–brain barrier. Oxidative stress is associated with endothelial cell contraction, reduced blood–brain barrier integrity and leakage of serum components into the central nervous system resulting in oedema and neuronal damage. IMD, like AM, preserves cerebral endothelial cell viability in vitro as evidenced by attenuation of hydrogen peroxide induced reduction in trans-endothelial electrical resistance and increased intercellular gap formation leading to increased permeability (Chen et al., 2006). IMD (and AM) increases cyclic AMP and nitric oxide production, enhances peripheral localization of F-actin bands improving stability of tight junctions and preserves mitochondrial membrane potential and integrity. Prepro-AM is abundantly expressed in cerebral endothelium, indicative of an important regulatory role (Kis et al., 2002). These effects of exogenously applied IMD are likely to be mediated by stimulation of endothelial AM receptors. Less is known currently about the presence and physiological influence of IMD in the cerebral vasculature in vivo, particularly whether expression of IMD is augmented locally in response to oxidative stress.
Future perspectives
Emerging evidence indicates that IMD is a potent vasodilator and enhances cardiac contractility and renal function directly and indirectly. Many of the effects demonstrated can be accounted for by the propensity of IMD to crossreact at receptors for CGRP and AM. It is unclear whether IMD reaches such receptors at appreciable levels to activate similar responses under physiological conditions. IMD is present constitutively at very low levels in adult myocardium and vasculature: plasma IMD could primarily reflect secretion from the pituitary, where the peptide is expressed abundantly. Species variation and lack of consistency in regard to use of IMD fragments of varying length has added to the current confusion in the literature. Elucidation of receptor involvement in the actions of IMD (and AM, CGRP) has also been hampered by lack of antagonists of sufficient potency and selectivity to distinguish between each receptor subtype. Non-peptide antagonists with selectivity for CGRP1 receptors (Doods et al., 2000) represent a major advance and identification of suitable antagonists displaying selectivity for AM1 and AM2 receptors is urgently required. The use of such pharmacological tools cannot however by itself provide information regarding which member of this peptide family might serve as the endogenous ligand at a particular receptor population in a given cell or tissue, or indeed the extent to which family members interact at the same receptor. Functional characterization of viable strains of mice in which the IMD gene has been deleted should provide clarity regarding the physiological role of IMD; the availability of such mice is eagerly awaited.
Potentially more interesting is the robust upregulation of the prepro-IMD transcript specifically in hypertrophied and ischaemic myocardium. Oxidative stress regulates expression of transcription factors such as nuclear factor-κB, c-jun/c-fos heterodimer (AP-1) hypoxia-inducible factor HIF-1α and endothelial PAS domain protein 1 (Valko et al., 2007). The AM and CL genes possess binding motifs for one or more of these factors within their promoter regions (Nikitenko et al., 2003; Ishimitsu et al., 2006) and confirmation of the presence of similar motifs within the promoter region of the IMD gene could explain induction of this gene within cardiomyocytes in response to oxidative stress. Reactive oxygen species also modify activity of various signalling kinases implicated in hypertrophic remodelling including protein kinase C, src tyrosine kinase and mitogen-activated protein kinase all of which influence gene expression. Taken together with the beneficial effects on contractile function and attenuation of cardiomyocyte hypertrophy and ischaemia–reperfusion injury, it is likely that IMD provides the myocardium with an endogenous mechanism to counter the detrimental consequences of oxidative stress and ischaemic insult. The importance of IMD in this regard should now be examined through investigation of the consequences of cardiac-restricted deletion of the IMD gene or localized application of small interfering RNA or IMD-specific antibodies in suitable experimental models of myocardial ischaemic insult. Confirmation of a crucial beneficial role of IMD should pave the way for translational research to the clinical arena. Firstly, the potential of measuring IMD as a cardiac biomarker could be addressed. Secondly, strategies could be devised to deliver IMD in the acute or chronic setting, such as infusion of the recombinant peptide or adenoviral vector-mediated delivery of the IMD gene to prevent or lessen the extent of myocardial injury in patients with ischaemic heart disease.
Acknowledgments
Experiments described in this article, which were undertaken in the authors' laboratory, were funded by a project grant awarded by Heart Research UK.
Glossary
- AM
adrenomedullin
- AMY
amylin
- CGRP
calcitonin gene-related peptide
- CL
calcitonin receptor-like receptor
- CT
calcitonin
- L-NAME
NG-nitro-L-arginine-methyl-ester
- RAMP
receptor activity-modifying protein
- SHR
spontaneously hypertensive rat
Footnotes
Conflict of interest
The authors state no conflict of interest.
References
- Abdelrahman AM, Pang CCY. Effect of intermedin/adrenomedullin-2 on venous tone in conscious rats. Naunyn Schmied Arch Pharmacol. 2006;373:376–380. doi: 10.1007/s00210-006-0076-z. [DOI] [PubMed] [Google Scholar]
- Alevizaki M, Shiraishi A, Rassool FV, Ferrier GJM, Macintyre I, Legon S. The calcitonin-like sequence if the β-CGRP gene. FEBS Lett. 1986;206:47–52. doi: 10.1016/0014-5793(86)81338-2. [DOI] [PubMed] [Google Scholar]
- Autelitano DJ, Ridings R. Adrenomedullin signalling in cardiomyocytes is dependent upon CRLR and RAMP2 expression. Peptides. 2001;22:1851–1857. doi: 10.1016/s0196-9781(01)00536-8. [DOI] [PubMed] [Google Scholar]
- Bell D, McDermott BJ. CGRP stimulates a positive contractile response in rat ventricular cardiomyocytes. J Cardiovasc Pharmacol. 1994;23:1011–1021. doi: 10.1097/00005344-199406000-00021. [DOI] [PubMed] [Google Scholar]
- Bell D, McDermott BJ. Calcitonin gene-related peptide in the cardiovascular system: characterisation of receptor populations and their pathophysiological significance. Pharmacol Rev. 1996;48:253–288. [PubMed] [Google Scholar]
- Bell D, Schluter KD, Zhou XJ, McDermott BJ, Piper HM. Hypertrophic effects of CGRP and amylin in adult mammalian ventricular cardiomyocytes. J Mol Cell Cardiol. 1995;27:2433–2443. doi: 10.1006/jmcc.1995.0231. [DOI] [PubMed] [Google Scholar]
- Bell D, Zhao YY, Kelso EJ, McHenry EM, Rush LM, Nicholls DP, et al. Up-regulation of adrenomedullin and its receptor components during cardiomyocyte hypertrophy induced by chronic inhibition of nitric oxide synthesis. Am J Physiol. 2006;290:H904–H914. doi: 10.1152/ajpheart.00152.2005. [DOI] [PubMed] [Google Scholar]
- Bell D, Zhao YY, McCoy FGP, Devine AB, McDermott BJ. Differential effects of an anti-oxidant intervention on cardiomyocyte expression of adrenomedullin and intermedin and their receptor components in chronic nitric oxide deficiency. Cell Physiol Biochem. 2007;20:269–282. doi: 10.1159/000107513. [DOI] [PubMed] [Google Scholar]
- Beltowski J, Jamroz A. Adrenomedullin what we know ten years since its discovery. Pol J Pharmacol. 2004;56:5–27. [PubMed] [Google Scholar]
- Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004;84:903–934. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- Caliumi C, Cianci R, Celi M, Cerci S, Cotesta D, Petramala L, et al. Plasma adrenomedullin concentrations in patients with renovascular or malignant hypertension. Min Cardioangiol. 2004;52:313–322. [PubMed] [Google Scholar]
- Caron KM, Smithies O. Extreme hydrops fetalis and cardiovascular abnormalities in mice lacking a functional adrenomedullin gene. Proc Natl Acad Sci USA. 2001;98:615–619. doi: 10.1073/pnas.021548898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CL, Roh J, Hsu SYT. Intermedin, a novel calcitonin family peptide that exists in teleosts as well as in mammals: a comparison with other calcitonin/intermedin family peptides in vertebrates. Peptides. 2004;25:1633–1642. doi: 10.1016/j.peptides.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Chang LC, Roh J, Park JI, Klein C, Cushman N, Haberberger RV, et al. Intermedin functions as a pituitary paracrine factor regulating prolactin release. Mol Endocrinol. 2005;19:2824–2838. doi: 10.1210/me.2004-0191. [DOI] [PubMed] [Google Scholar]
- Charles CJ, Rademaker MT, Richards AM. Hemodynamic, hormonal and renal actions of adrenomedullin-2 in normal conscious sheep. Endocrinology. 2006;147:1871–1877. doi: 10.1210/en.2005-1403. [DOI] [PubMed] [Google Scholar]
- Chauhan M, Ross GR, Yallampalli U, Yallampalli C. Adrenomedullin-2, a novel calcitonin/CGRP family peptide, relaxes rat mesenteric artery: influence of pregnancy. Endocrinology. 2007;148:1727–1735. doi: 10.1210/en.2006-1105. [DOI] [PubMed] [Google Scholar]
- Chauhan M, Yallampalli U, Reed L, Yallampalli C. Adrenomedullin-2 antagonist infusion to rats during midgestation causes fetoplacental growth restriction through apoptosis. Biol Reprod. 2006;75:940–947. doi: 10.1095/biolreprod.106.053322. [DOI] [PubMed] [Google Scholar]
- Chen L, Kis B, Hashimoto H, Busija DW, Takei Y, Yamashita H, et al. Adrenomedullin-2 protects rat cerebral endothelial cells from oxidative damage in vitro. Brain Res. 2006;1086:42–49. doi: 10.1016/j.brainres.2006.02.128. [DOI] [PubMed] [Google Scholar]
- Christopoulos A, Christopoulos G, Morfis M, Udawela M, Laburthe M, Couvineau A, et al. Novel receptor partners and function of receptor activity-modifying proteins. J Biol Chem. 2003;278:3293–3297. doi: 10.1074/jbc.C200629200. [DOI] [PubMed] [Google Scholar]
- Chu DQ, Choy M, Foster P, Cao T, Brain SD. A comparative study of the ability of CGRP and adrenomedullin (13-52) to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589–1596. doi: 10.1038/sj.bjp.0703502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell GS, Roosterman D, Marvizon JC, Song B, Wick E, Pikios S, et al. Localisation of calcitonin receptor-like receptor and receptor activity modifying protein 1 in enteric neurons, dorsal root ganglia and the spinal cord of rat. J Comp Neurol. 2005;490:239–255. doi: 10.1002/cne.20669. [DOI] [PubMed] [Google Scholar]
- Cueille C, Pidoux E, de Vernejoul MC, Ventura-Clapier R, Garel JM. Increased myocardial expression of RAMP1 and RAMP3 in rats with chronic heart failure. Biochem Biophys Res Commun. 2002;294:340–346. doi: 10.1016/S0006-291X(02)00487-4. [DOI] [PubMed] [Google Scholar]
- Dennis T, Fournier A, Cadieaux A, Pomerlau F, Jolicoeur FB, St Pierre S, et al. hCGRP8–37: a CGRP antagonist revealing CGRP receptor heterogeneity in the brain and periphery. J Pharmacol Exp Ther. 1990;254:123–128. [PubMed] [Google Scholar]
- Dong F, Taylor MM, Samson WK, Ren J. Intermedin (adrenomedullin-2) enhances cardiac contractile function via a protein kinase C and protein kinase A-dependent pathway in murine ventricular myocytes. J Appl Physiol. 2006;101:778–784. doi: 10.1152/japplphysiol.01631.2005. [DOI] [PubMed] [Google Scholar]
- Doods H, Hallermayer G, Wu D, Entzeroth M, Rudolf K, Engel W, et al. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br J Pharmacol. 2000;129:420–423. doi: 10.1038/sj.bjp.0703110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RM, Trizna W. CGRP: effects on renal arteriolar tone and tubular cyclic AMP levels. Am J Physiol. 1990;258:F121–F125. doi: 10.1152/ajprenal.1990.258.1.F121. [DOI] [PubMed] [Google Scholar]
- Edwards RM, Trizna W, Stack E, Aiyar N. Effect of adrenomedullin on cAMP levels along the rat nephron: comparison with CGRP. Am J Physiol. 1996;271:F895–F899. doi: 10.1152/ajprenal.1996.271.4.F895. [DOI] [PubMed] [Google Scholar]
- Eguchi S, Hirata Y, Iwasaki H, Sato K, Watanabe TX, Inui T, et al. Structure–activity relationship of adrenomedullin, a novel vasodilatory peptide in cultured rat vascular smooth muscle cells. Endocrinology. 1994;135:2454–2458. doi: 10.1210/endo.135.6.7988431. [DOI] [PubMed] [Google Scholar]
- Fisher RA, Robertson SM, Olson MS. Stimulation and homologous desensitisation of CGRP receptors in cultured beating rat heart cells. Endocrinology. 1988;123:106–112. doi: 10.1210/endo-123-1-106. [DOI] [PubMed] [Google Scholar]
- Franco-Cereceda A, Saria A, Lundberg JM. Ischemia and changes in contractility induce release of CGRP but nor NPY from the isolated perfused guinea-pig heart. Acta Physiol Scand. 1987;132:181–190. doi: 10.1111/j.1748-1716.1987.tb08244.x. [DOI] [PubMed] [Google Scholar]
- Fujisawa Y, Nagai Y, Miyatake A, Miura K, Nishiyama A, Kimura S, et al. Effects of adrenomedullin-2 on regional haemodynamics in conscious rats. Eur J Pharmacol. 2007;558:128–132. doi: 10.1016/j.ejphar.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Fujisawa Y, Nagai Y, Miyatake A, Miura K, Shokoji T, Nishiyama A, et al. Roles of adrenomedullin-2 in regulating the cardiovascular and sympathetic nervous systems in conscious rats. Am J Physiol. 2006;290:H1120–H1127. doi: 10.1152/ajpheart.00461.2005. [DOI] [PubMed] [Google Scholar]
- Fujisawa Y, Nagai Y, Miyatake A, Takei Y, Miura K, Shoukouji T, et al. Renal effects of a new member of adrenomedullin family, adrenomedullin-2 in rats. Eur J Pharmacol. 2004;497:75–80. doi: 10.1016/j.ejphar.2004.06.039. [DOI] [PubMed] [Google Scholar]
- Gangula PR, Zhao H, Supowit SC, Wimalawansa SJ, DiPette DJ, Westlund KN, et al. Increased blood pressure in alpha-CGRP/CT gene knockout mice. Hypertension. 2000;35:470–475. doi: 10.1161/01.hyp.35.1.470. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, Compton AM, Kemp PA, Bennett T, Bose C, Foulkes R, et al. Antagonistic effect of human alphaCGRP(8-37) on the in vivo regional haemodynamic actions of human alphaCGRP. Biochem Biophys Res Commun. 1990;171:938–943. doi: 10.1016/0006-291x(90)90774-h. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, Compton AM, Kemp PA, Bennett T, Bose C, Foulkes R, et al. Antagonistic effect of human alpha-CGRP (8-37) on regional haemodynamic actions of rat islet amyloid polypeptide in conscious Long-Evans rats. Diabetes. 1991;40:948–951. doi: 10.2337/diab.40.8.948. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, Kemp PA, March JE, Bennett T. Regional haemodynamic effects of human and rat adrenomedullin in conscious rats. Br J Pharmacol. 1995;114:584–591. doi: 10.1111/j.1476-5381.1995.tb17179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumusel B, Chang JK, Hyman A, Lippton H. Adrenotensin: an adrenomedullin gene product with the opposite effect to adrenomedullin. Life Sci. 1996;57:87–90. doi: 10.1016/0024-3205(95)02012-8. [DOI] [PubMed] [Google Scholar]
- Hagner S, Knauer J, Haberberger R, Goke B, Voigt K, McGregor GP. Calcitonin receptor-like receptor: identification and distribution in human peripheral tissues. Cell Tissue Res. 2002;310:41–50. doi: 10.1007/s00441-002-0616-x. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Hyodo S, Kawasaki M, Mera T, Chen L, Soya A, et al. Centrally administered adrenomedullin-2 activates hypothalamic ocytocin-secreting neurons causing elevated plasma oxytocin level in rats. Am J Physiol. 2005;289:E753–E761. doi: 10.1152/ajpendo.00042.2005. [DOI] [PubMed] [Google Scholar]
- Hay DL, Christopoulos G, Christopoulos A, Poyner DR, Sexton PM. Pharmacological discrimination of calcitonin receptor: receptor activity modifying protein complexes. Mol Pharmacol. 2005;67:1655–1665. doi: 10.1124/mol.104.008615. [DOI] [PubMed] [Google Scholar]
- Hay DL, Conner AC, Howitt SG, Takhshid MA, Simms J, Mahmoud K, et al. The pharmacology of CGRP-responsive receptors in cultured and transfected cells. Peptides. 2004;25:2019–2026. doi: 10.1016/j.peptides.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Hay DL, Howitt SG, Conner AC, Schindler M, Smith DM, Poyner DR. CL/RAMP2 and CL/RAMP3 produce pharmacologically distinct adrenomedullin receptors; a comparison of effects of adrenomedullin22-52, CGRP8-37 and BIBN4096BS. Br J Pharmacol. 2003;140:477–486. doi: 10.1038/sj.bjp.0705472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DL, Smith DM. Knockouts and transgenics confirm the importance of adrenomedullin in the vasculature. Trends Pharmacol Sci. 2001;22:57–59. doi: 10.1016/s0165-6147(00)01617-5. [DOI] [PubMed] [Google Scholar]
- Heaton J, Lin B, Chang JK, Steinberg S, Hyman A, Lippton H. Pulmonary vasodilation to adrenomedullin: a novel peptide in humans. Am J Physiol. 1995;268:H2211–H2215. doi: 10.1152/ajpheart.1995.268.6.H2211. [DOI] [PubMed] [Google Scholar]
- Huang MH, Knight PR, Izzo JL. Calcium-induced calcium release involved in positive inotropic effect mediated by CGRP in ventricular cardiomyocytes. Am J Physiol. 1999;276:R259–R264. doi: 10.1152/ajpregu.1999.276.1.R259. [DOI] [PubMed] [Google Scholar]
- Husmann K, Born W, Fischer JA, Muff R. Three receptor-activity modifying proteins define calcitonin gene-related peptide or adrenomedullin selectivity of the mouse calcitonin-like receptor in COS-7 cells. Biochem Pharmacol. 2003;66:2107–2115. doi: 10.1016/j.bcp.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Ihara T, Ikeda U, Tate Y, Ishibashi S, Shimada K. Positive inotropic effects of adrenomedullin on rat papillary muscle. Eur J Pharmacol. 2000;390:167–172. doi: 10.1016/s0014-2999(00)00011-x. [DOI] [PubMed] [Google Scholar]
- Ikenouchi H, Kangawa K, Matsuo H, Hirata Y. Negative inotropic effect of adrenomedullin in isolated adult rabbit cardiac ventricular myocytes. Circulation. 1997;95:318–2324. doi: 10.1161/01.cir.95.9.2318. [DOI] [PubMed] [Google Scholar]
- Ishimitsu T, Ono H, Minami J, Matsuoka H. Pathophysiologic and therapeutic implications of adrenomedullin in cardiovascular disorders. Pharmacol Ther. 2006;111:909–927. doi: 10.1016/j.pharmthera.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Jia YX, Yang JH, Pan CS, Geng B, Zhang J, Xiao Y, et al. Intermedin 1–53 protects the heart against isoproterenol-induced ischemic injury in rats. Eur J Pharmacol. 2006;549:117–123. doi: 10.1016/j.ejphar.2006.07.054. [DOI] [PubMed] [Google Scholar]
- Jougasaki M, Burnett JC. Adrenomedullin: potential in physiology and pathophysiology. Life Sci. 2000;66:855–872. doi: 10.1016/s0024-3205(99)00358-6. [DOI] [PubMed] [Google Scholar]
- Kamitani S, Asakawa M, Shimekake Y, Kuwasako K, Nakahara K, Sakata T. The RAMP2/CRLR complex is a functional adrenomedullin receptor in human endothelial and vascular smooth muscle cells. FEBS Lett. 1999;448:111–114. doi: 10.1016/s0014-5793(99)00358-0. [DOI] [PubMed] [Google Scholar]
- Kandilci HB, Gumusel B, Wasserman A, Witriol N, Lippton H. Intermedin/adrenomedullin-2 dilates the rat pulmonary vascular bed: dependence on CGRP receptors and nitric oxide release. Peptides. 2006;27:1390–1396. doi: 10.1016/j.peptides.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Katayama M, Nadel JA, Bunnett NW, Maria GU, Haxhiu M, Borson DB. Catabolism of CGRP and substance P by neutral endopeptidase. Peptides. 1991;12:563–567. doi: 10.1016/0196-9781(91)90102-u. [DOI] [PubMed] [Google Scholar]
- Kato J, Kitamura K, Matsui M, Tanaka M, Ishizaka Y, Kita T, et al. Plasma adrenomedullin and natriuretic peptides in patients with essential or malignant hypertension. Hypertens Res. 1999;22:61–65. doi: 10.1291/hypres.22.61. [DOI] [PubMed] [Google Scholar]
- Kato J, Kitamura K, Uemura T, Kuwasako K, Kita T, Kangawa K, et al. Plasma levels of adrenomedullin and atrial and brain natriuretic peptides in the general population: their relations to age and pulse pressure. Hypertens Res. 2002;25:887–892. doi: 10.1291/hypres.25.887. [DOI] [PubMed] [Google Scholar]
- Kato K, Yin H, Agata J, Yoshida H, Chao L, Chao J. Adrenomedullin gene delivery attenuates myocardial infarction and apoptosis after ischemia and reperfusion. Am J Physiol. 2003;285:H1506–H1514. doi: 10.1152/ajpheart.00270.2003. [DOI] [PubMed] [Google Scholar]
- Kindt F, Wiegand S, Loser C, Nilles M, Niemeier V, Hsu SYT, et al. Intermedin: a skin peptide that is downregulated in atropic dermatitis. J Invest Dermatol. 2007;127:605–613. doi: 10.1038/sj.jid.5700576. [DOI] [PubMed] [Google Scholar]
- Kis B, Kaiya H, Nishi R, Deli MA, Abraham CS, Yanagita T, et al. Cerebral endothelial cells are a major source of adrenomedullin. J Neuroendocrinol. 2002;14:283–293. doi: 10.1046/j.1365-2826.2002.00778.x. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Kangawa K, Ishiyama Y, Washimine H, Ichiki Y, Kawamoto M, et al. Identification and hypotensive activity of pro-adrenomedullin N-terminal 20 peptide (PAMP) FEBS Lett. 1994;351:35–37. doi: 10.1016/0014-5793(94)00810-8. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, et al. Adrenomedullin: a novel hypotensive peptide from human phaeochromocytoma. Biochem Biophys Res Commun. 1993;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- Kruger L, Mantyh PW, Sternini C, Brecha NC, Mantyh CR. Calcitonin gene-related peptide (CGRP) in the rat central nervous system: patterns of immunoreactivity and receptor binding sites. Brain Res. 1988;463:223–244. doi: 10.1016/0006-8993(88)90395-2. [DOI] [PubMed] [Google Scholar]
- Kurtz A, Schurek HJ, Jelkmann W, Muff R, Lipp HP, Heckmann U, et al. Renal mesangium as a target for CGRP. Kidney Int. 1989;36:222–227. doi: 10.1038/ki.1989.183. [DOI] [PubMed] [Google Scholar]
- Kuwasako K, Cao YN, Nagoshi Y, Tsuruda T, Kitamura K, Eto T. Characterisation of the human calcitonin gene-related peptide receptor subtypes associated with receptor activity-modifying proteins. Mol Pharmacol. 2004;65:207–213. doi: 10.1124/mol.65.1.207. [DOI] [PubMed] [Google Scholar]
- Lainchbury JG, Troughton RW, Lewis LK, Yandle TG, Richards AM, Nicholls MG. Hemodynamic, hormonal, and renal effects of short-term adrenomedullin infusion in healthy volunteers. J Clin Endocrinol Metab. 2000;85:1016–1020. doi: 10.1210/jcem.85.3.6422. [DOI] [PubMed] [Google Scholar]
- Leclerc M, Brunette MG. The paradoxical effect of adrenomedullin on sodium transport by the renal distal tubule luminal membrane. Mol Cell Endocrinol. 2000;164:159–167. doi: 10.1016/s0303-7207(00)00225-2. [DOI] [PubMed] [Google Scholar]
- Letizia C, Subioli S, Cerci S, Caliumi C, Verrelli C, Delfini E, et al. High plasma adrenomedullin concentrations in patients with high-renin essential hypertension. J Ren Ang Aldo Sys. 2002;3:126–129. doi: 10.3317/jraas.2002.014. [DOI] [PubMed] [Google Scholar]
- Lu JT, Son YJ, Lee J, Jetton TL, Shiota M, Moscoso L, et al. Mice lacking alpha-CGRP exhibit normal cardiovascular regulation and neuromuscular development. Mol Cell Neurosci. 1999;14:99–120. doi: 10.1006/mcne.1999.0767. [DOI] [PubMed] [Google Scholar]
- Majid D, Kadowitz PJ, Coy DH, Navar LG. Renal responses to intra-arterial administration of adrenomedullin in dogs. Am J Physiol. 1996;270:F200–F205. doi: 10.1152/ajprenal.1996.270.1.F200. [DOI] [PubMed] [Google Scholar]
- McDermott BJ, Zhao YY, McCoy FGP, Bell D. Upregulation of intermedin and reduced neutral endopeptidase expression in hypertensive cardiac hypertrophy. J Mol Cell Cardiol. 2007;42:5134. [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, et al. RAMPs regulate the transport and ligand specificity of the calcitonin receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- Morgenthaler NG, Struck J, Alonso C, Bergmann A. Measurement of mid-regional proadrenomedullin in plasma with an illumunoluminometric assay. Clin Chem. 2005;51:1823–1829. doi: 10.1373/clinchem.2005.051110. [DOI] [PubMed] [Google Scholar]
- Morimoto R, Satoh F, Murakami O, Totsune K, Suzuki T, Sasano H, et al. Expression of adrenomedullin-2/intermedin in human brain, heart and kidney. Peptides. 2007;28:1095–1103. doi: 10.1016/j.peptides.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Morris HR, Panico M, Etienne T, Tippens J, Girgis SI, Macintyre I. Isolation and characterisation of human CGRP. Nature. 1984;208:746–748. doi: 10.1038/308746a0. [DOI] [PubMed] [Google Scholar]
- Muff R, Leuthauser K, Buhlmann N, Foord SM, Fischer JA, Born W. Receptor activity modifying proteins regulate the activity of a calcitonin gene related peptide receptor in rabbit aortic endothelial cells. FEBS Lett. 1998;441:366–368. doi: 10.1016/s0014-5793(98)01587-7. [DOI] [PubMed] [Google Scholar]
- Nagae T, Mukoyama M, Sugawara A, Mori K, Yahata K, Kasahara M, et al. Rat receptor-activity modifying proteins (RAMPs) for adrenomedullin/CGRP receptor: cloning and upregulation in obstructive nephropathy. Biochem Biophys Res Commun. 2000;270:89–93. doi: 10.1006/bbrc.2000.2390. [DOI] [PubMed] [Google Scholar]
- Nagaya N, Nishikimi T, Horio T, Yoshihara F, Kanazawa A, Matsuo H, et al. Cardiovascular and renal effects of adrenomedullin in rats with heart failure. Am J Physiol. 1999;276:R213–R218. doi: 10.1152/ajpregu.1999.276.1.R213. [DOI] [PubMed] [Google Scholar]
- Nikitenko LL, Smith DM, Bicknell R, Rees MC. Transcriptional regulation of the CRLR gene in human microvascular endothelial cells by hypoxia. FASEB J. 2003;17:1499–1501. doi: 10.1096/fj.02-0993fje. [DOI] [PubMed] [Google Scholar]
- Ogoshi M, Inoue K, Takei Y. Identification of a novel adrenomedullin gene family in teleost fish. Biochem Biophys Res Commun. 2003;311:10721–11077. doi: 10.1016/j.bbrc.2003.10.111. [DOI] [PubMed] [Google Scholar]
- Ohhashi Y, Shindo T, Kurihara Y, Imai T, Wang Y, Morita H, et al. Elevates sympathetic nervous activity in mice deficient in alphaCGRP. Circ Res. 2001;89:983–990. doi: 10.1161/hh2301.100812. [DOI] [PubMed] [Google Scholar]
- Oliver KR, Wainwright A, Edvinsson L, Pickard JD, Hill RG. Immunohistochemical localisation of calcitonin receptor-like receptor and receptor activity modifying proteins in the human cerebral vasculature. J Cereb Blood Flow Metab. 2002;22:620–629. doi: 10.1097/00004647-200205000-00014. [DOI] [PubMed] [Google Scholar]
- Pan CS, Yang JH, Cai DY, Zhao J, Gerns H, Yang J, et al. Cardiovascular effects of newly discovered peptide intermedin/adrenomedullin-2. Peptides. 2005;26:1640–1646. doi: 10.1016/j.peptides.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Poyner DR, Sexton PM, Marshall I, Smith DM, Quirion R, Born W, et al. International Union of Pharmacology XXXII: the mammalian calcitonin gene-related peptides, adrenomedullin, amylin and calcitonin receptors. Pharmacol Rev. 2002;54:233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- Prado MA, Evans-Bain B, Oliver KR, Dickerson IM. The role of the CGRP-receptor component protein (RCP) in adrenomedullin receptor signal transduction. Peptides. 2001;22:1773–1881. doi: 10.1016/s0196-9781(01)00517-4. [DOI] [PubMed] [Google Scholar]
- Rademaker MT, Charles CJ, Cooper GJ, Coy DH, Espiner EA, Lewis LK, et al. Combined angiotensin-converting enzyme inhibition and adrenomedullin in an ovine model of heart failure. Clin Sci. 2002;102:653–660. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Ren YS, Ma TG, Wang HB, Yu SQ. Protective effects of CGRP on myocardial cell injury and calcium and magnesium contents following severe hypoxia and simulated reperfusion. Med Sci Res. 1993;21:177–178. [Google Scholar]
- Ren YS, Yang JH, Zhang J, Pan CS, Yang J, Zhao J, et al. Intermedin 1–53 in central nervous system elevates arterial blood pressure in rats. Peptides. 2006;27:74–79. doi: 10.1016/j.peptides.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Rink TJ, Beaumont K, Koda J, Young A. Structure and biology of amylin. Trends Pharmacol Sci. 1993;14:113–118. doi: 10.1016/0165-6147(93)90081-t. [DOI] [PubMed] [Google Scholar]
- Roh J, Chang CL, Bhalla A, Klein C, Hsu SYT. Intermedin is a calcitonin/CGRP family peptide acting through the calcitonin receptor-like receptor activity modifying protein receptor complexes. J Biol Chem. 2004;279:7264–7274. doi: 10.1074/jbc.M305332200. [DOI] [PubMed] [Google Scholar]
- Rosenfeld MG, Mermod JJ, Amara SG, Swanson LW, Sawchenko PE, Rivier J, et al. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 1983;304:129–135. doi: 10.1038/304129a0. [DOI] [PubMed] [Google Scholar]
- Saetrum Opgaard O, Hasbak P, de Vries R, Saxena PR, Edvinsson L. Positive inotropy mediated via CGRP receptors in isolated human myocardial trabeculae. Eur J Pharmacol. 2000;397:373–382. doi: 10.1016/s0014-2999(00)00233-8. [DOI] [PubMed] [Google Scholar]
- Shindo T, Kurihara Y, Nishimatsu H, Moriyama N, Kakoki M, Wang Y, et al. Vascular abnormalities and elevated blood pressure in mice lacking adrenomedullin gene. Circulation. 2001;104:1964–1971. doi: 10.1161/hc4101.097111. [DOI] [PubMed] [Google Scholar]
- Steenbergh PH, Hoppener JWM, Zandberg J, Lips CJM, Jansz HS. A second human calcitonin/CGRP gene. FEBS Lett. 1985;183:403–407. doi: 10.1016/0014-5793(85)80820-6. [DOI] [PubMed] [Google Scholar]
- Szokodi I, Kinnunen P, Tavi P, Weckstrom M, Toth M, Ruskoaho H. Evidence for cAMP-independent mechanisms mediating the effects of adrenomedullin a new inotropic peptide. Circulation. 1998;97:1062–1070. doi: 10.1161/01.cir.97.11.1062. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kikuchi K, Maruyama Y, Urabe T, Nakajima K, Sasano H, et al. Immunocytochemical localisation of adrenomedullin-2/intermedin-like immunoreactivity in human hypothalamus, heart and kidney. Peptides. 2006;27:1383–1389. doi: 10.1016/j.peptides.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Takei Y, Hyodo S, Katafuchi T, Minamino N. Novel fish-derived adrenomedullin in mammals: structure and possible function. Peptides. 2004a;25:1643–1656. doi: 10.1016/j.peptides.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Takei Y, Inoue K, Ogoshi M, Kawahura K, Bannai H, Miyano S. Identification of novel adrenomedullin in mammals: a potent cardiovascular and renal regulator. FEBS Lett. 2004b;556:53–58. doi: 10.1016/s0014-5793(03)01368-1. [DOI] [PubMed] [Google Scholar]
- Taylor MM, Bagley SL, Samson WK. Intermedin/adrenomedullin-2 acts within the central nervous system to elevate blood pressure and inhibit food and water intake. Am J Physiol. 2005;288:R919–R927. doi: 10.1152/ajpregu.00744.2004. [DOI] [PubMed] [Google Scholar]
- Taylor MM, Bagley SL, Samson WK. Intermedin/adrenomedullin-2 inhibits growth hormone release from cultured primary anterior pituitary cells. Endocrinology. 2006;147:859–864. doi: 10.1210/en.2005-0949. [DOI] [PubMed] [Google Scholar]
- Taylor MM, Samson WK. Stress hormone secretion is altered by central administration of intermedin/adrenomedullin-2. Brain Res. 2005;1045:199–205. doi: 10.1016/j.brainres.2005.03.034. [DOI] [PubMed] [Google Scholar]
- Terata K, Miura H, Liu Y, Loberiza F, Gutterman DD. Human coronary arteriolar dilation to adrenomedullin: role of nitric oxide and K+ channels. Am J Physiol. 2000;279:H2620–H2626. doi: 10.1152/ajpheart.2000.279.6.H2620. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen J, Hansen JL, Smajilovic S, Terwilliger EF, Haunso S, Sheikh SP. Calcium receptor is functionally expressed in rat neonatal ventricular cardiomyocytes. Am J Physiol. 2006;290:H1165–H1171. doi: 10.1152/ajpheart.00821.2005. [DOI] [PubMed] [Google Scholar]
- Totsune K, Takahashi K, Mackenzie HS, Murakami O, Arihara Z, Sone M, et al. Increased gene expression of adrenomedullin and adrenomedullin-receptor complexes, receptor-activity modifying protein (RAMP)2 and calcitonin-receptor-like receptor (CRLR) in the hearts of rats with congestive heart failure. Clin Sci. 2000;99:541–546. [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochme Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Wang X, Fiscus RR. CGRP increases cyclic AMP, tension and rate in rat atria. Am J Physiol. 1989;256:R421–R428. doi: 10.1152/ajpregu.1989.256.2.R421. [DOI] [PubMed] [Google Scholar]
- Wisskirchen FM, Burt RP, Marshall I. Pharmacological characterisation of CGRP receptors mediating relaxation of the rat pulmonary artery and inhibition of twitch responses of the rat vas deferens. Br J Pharmacol. 1998;123:1673–1683. doi: 10.1038/sj.bjp.0701783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JH, Jia YX, Pan CS, Zhao J, Ouyang M, Yang J, et al. Effects of intermedin1-53 on cardiac function and ischemia/reperfusion injury in isolated rat hearts. Biochem Biophys Res Commun. 2005a;327:713–719. doi: 10.1016/j.bbrc.2004.12.071. [DOI] [PubMed] [Google Scholar]
- Yang JH, Pan CS, Jia YX, Zhang J, Zhao J, Pang YZ, et al. Intermedin1–53 activates L-arginine/nitric oxide synthase/nitric oxide pathway in rat aortas. Biochem Biophys Res Commun. 2006;341:567–572. doi: 10.1016/j.bbrc.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Yang JH, Qi YF, Jia YX, Pan CS, Zhao J, Yang J, et al. Protective effects of intermedin/adrenomedullin-2 on ischemia/reperfusion injury in isolated rat hearts. Peptides. 2005b;26:501–507. doi: 10.1016/j.peptides.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Zaidi M, Bevis PJR, Girgis SL, Lynch C, Stevenson JC, McIntyre I. Circulating CGRP comes from the perivascular nerves. Eur J Pharmacol. 1985;117:283–284. doi: 10.1016/0014-2999(85)90616-8. [DOI] [PubMed] [Google Scholar]
- Zatz S, Baylis C. Chronic nitric oxide inhibition model six years on. Hypertension. 1998;32:958–964. doi: 10.1161/01.hyp.32.6.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JF, Jin L, Liu XZ, Sheng SL, Zhang WJ. The effect of CGRP on ischemic reperfusion-induced arrhythmias in rats. Int J Cardiol. 1994;46:33–36. doi: 10.1016/0167-5273(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Zhao YY, Bell D, Smith LR, Zhao L, Devine AB, McHenry EM, et al. Differential effects of components of the cardiomyocyte adrenomedullin/intermedin receptor system following blood pressure reduction in nitric oxide-deficient hypertension. J Pharmacol Exp Ther. 2006;316:1269–1281. doi: 10.1124/jpet.105.092783. [DOI] [PubMed] [Google Scholar]