Abstract
Preventing death and limiting handicap from ischaemic stroke are major goals that can be achieved only if the pathophysiology of infarct expansion is properly understood. Primate studies showed that following occlusion of the middle cerebral artery (MCA)––the most frequent and prototypical stroke, local tissue fate depends on the severity of hypoperfusion and duration of occlusion, with a fraction of the MCA territory being initially in a ‘penumbral' state. Physiological quantitative PET imaging has translated this knowledge in man and revealed the presence of considerable pathophysiological heterogeneity from patient to patient, largely unpredictable from elapsed time since onset or clinical deficit. While these observations underpinned key trials of thrombolysis, they also indicate that only patients who are likely to benefit should be exposed to its risks. Accordingly, imaging-based diagnosis is rapidly becoming an essential component of stroke assessment, replacing the clock by individually customized management. Diffusion- and perfusion-weighted MR (DWI-PWI) and CT-based perfusion imaging are increasingly being used to implement this, and are undergoing formal validation against PET. Beyond thrombolysis per se, knowledge of the individual pathophysiology also guides management of variables like blood pressure, blood glucose and oxygen saturation, which can otherwise precipitate the penumbra into the core, and the oligaemic tissue into the penumbra. We propose that future therapeutic trials use physiological imaging to select the patient category that best matches the drug's presumed mode of action, rather than lumping together patients with entirely different pathophysiological patterns in so-called ‘large trials', which have all failed so far.
Keywords: stroke, pathophysiology, ischaemic, penumbra, imaging, therapy, PET, MRI
Introduction
Stroke is a leading cause of death and disability worldwide with far reaching consequences for the society (Feigin et al., 2003). The World Health Organization defines stroke as a rapidly developing focal (or global) brain dysfunction of vascular origin lasting more than 24 h, thus encompassing ischaemic stroke, intracerebral haemorrhage, subarachnoid haemorrhage and cerebral venous sinus thrombosis (Brown et al., 2006).
Ischaemic stroke is by far the most common type of stroke, constituting around 80% of all strokes (Feigin et al., 2003), of which 60% are attributable to large-artery ischaemia. The current understanding of its pathophysiology has dramatically evolved over the past three decades, from early beginnings in animal studies through to the current wealth of information provided by various imaging techniques. This has transformed stroke care and ended decades of nihilism (Saver, 2006). Positron emission tomography (PET) has been particularly instrumental in these developments (Baron, 2005b) and continues to be the gold standard in stroke imaging. Other modalities such as computed tomography (CT), single photon emission CT and magnetic resonance imaging (MRI) have also assumed important roles both in the investigation of stroke pathophysiology and in applying its complex concepts to everyday clinical practice (Table 1). This article reviews the main pathophysiological models of ischaemic stroke and the role of imaging in formulating and implementing them and in the development of new therapeutic strategies.
Table 1. Imaging modalities used to identify the ischaemic penumbra and core.
| Imaging modality | Assessed parameters | Definition of penumbra | Advantages | Limitations |
|---|---|---|---|---|
| CT | ||||
| Perfusion | CBF, CBV, MTT, TTP | Relative CBF <66%a or MTT >145%a and CBV >2 ml 100 g−1 | Cheap, available, fast | Limited brain coverage, not sensitive in posterior circulation, no direct visualization of core |
| Xe | CBF | CBF 7–20 ml 100 g−1 min−1 | Quantitative | Technically complex, not validated, pharmacologic effects of Xe |
| MR | ||||
| DWI–PWI | CBF, CBV, MTT, TTP, ADC | Relative TTP (or MTT) delay >4 sa, relative CBF <37%a & relative ADC above 50%a | Practical, fast, increasingly available, no radiation involved, directly visualizes severely ischaemic tissue | Uncertainties regarding validity of mismatch concept, sensitive to head motion |
| Spectroscopy | NAA, lactate | Elevated lactate and normal NAA | Biochemically characterizes tissue | Not widely available, not validated, poor resolution |
| PET | ||||
| Multi-tracer 15O2 | CBF, CBV, MTT, CMRO2, OEF | CBF 7–22 ml 100 g−1 min −1 and CMRO2>39 μmol 100 g−1 min−1 and OEF>70% | Quantitative, validated | Complex, time consuming, not widely available, expensive |
| 11C-FMZ (+H215O) | Tracer binding | Relative binding ratio>3.4b and CBF <14 ml 100 g−1 min−1 | Based on physiological neuronal integrity | As above + only suitable for grey matter, requires additional perfusion imaging |
| 18F-FMISO | Tracer uptake | Uptake ratio>1.3a | Produces a direct positive image of viable hypoxic tissue | As above + validation incomplete, long imaging time, not practical in acute setting |
| SPECT | ||||
| 99mTc-HMPAO | CBF | Relative CBF 40–70%a | Cheap and relatively available | Thresholds still uncertain, limited spatial resolution |
Relative to mean contralateral hemisphere value.
Relative to contralateral healthy white matter.
Abbreviations: ADC, apparent diffusion coefficient; CBF, cerebral blood flow; CBV, cerebral blood volume; CMRO2, cerebral metabolic rate of oxygen; CT, computed tomography; DWI, diffusion-weighted imaging; FMISO, fluoromisonidazole; FMZ, flumazenil; HMPAO, hexamethylpropyleneamine oxime; MR, magnetic resonance; MTT, mean transit time; NAA, N-acetylaspartate; OEF, oxygen extraction fraction; PET, positron emission tomography; PWI, perfusion-weighted imaging; SPECT, single photon emission computed tomography; TTP, time to peak.
Basic concepts
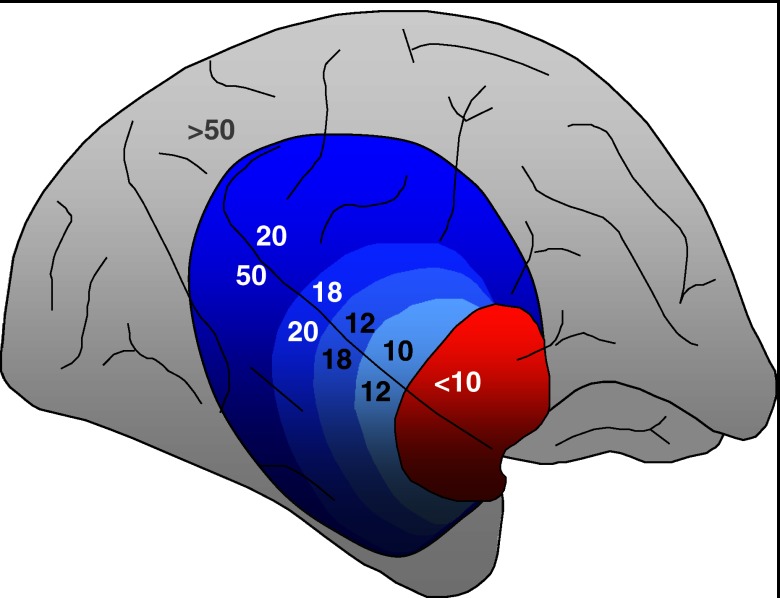
Most experimental and clinical research have focused on proximal occlusion of the middle cerebral artery (MCA). Interruption of blood flow to the supplied basal ganglia, white matter and cortex causes a gradient of hypoperfusion to emerge (Figure 1), rather than complete and homogeneous ischaemia of the entire MCA territory (Astrup et al., 1981). Regions suffering the most severe degrees of hypoperfusion rapidly progress to irreversible damage, representing the ‘ischaemic core'. On multi-tracer 15O PET, this tissue exhibits very low cerebral blood flow (CBF), cerebral blood volume (CBV) and metabolic rates of oxygen and glucose (Marchal et al., 1999a). The remaining hypoperfused tissue exhibits impairment of the normal blood flow autoregulatory mechanisms and is pathophysiologically divided relative to a well-defined perfusion threshold into two compartments, namely, the ‘penumbra' and ‘oligaemia'. In the penumbra, oxygen metabolism is preserved relative to CBF, the oxygen extraction fraction (OEF) is elevated (severe ‘misery perfusion') and the CBV is normal or elevated. Tissue within the penumbra is potentially salvageable, yet its extent decreases over time by gradual recruitment into the core and as such, represents a key target for therapeutic intervention (Baron et al., 1995). This course of events varies from patient to patient, but most exhibit substantial volumes of penumbra for many hours (Baron, 1999) or exceptionally, days after stroke onset (Perez et al., 2006). The oligaemic compartment, on the other hand, suffers a milder degree of hypoperfusion with normal oxygen consumption and elevated CBV and OEF, and is not normally at risk of infarction. If the occlusion persists, however, secondary events such as systemic hypotension, intracranial hypertension or hyperglycaemia may topple this delicate balance and force the oligaemia into a penumbral state and eventually recruitment into the necrotic core.
Figure 1.
The spatial pattern of cerebral blood flow (CBF) reduction following middle cerebral artery (MCA) occlusion in the baboon brain, demonstrating a gradient from ischaemic core (red) through to penumbra and oligaemia (blue) to normally perfused cortex (grey). Values indicate approximate CBF in ml 100 g−1 min−1.
The ischaemic core
The ischaemic core is by definition beyond therapeutic rescue. It is electrically silent and its volume is highly correlated to, and explains part of the severity of admission neurological deficit (Marchal et al., 1999a). On the cellular level, irreversible damage is heralded by depletion of energy metabolites and failure of the cell membrane to maintain its physiologic gradients with dramatic disruption of ion and water homoeostasis (Moustafa and Baron, 2007). This manifests as massive efflux of K+ and reciprocal influx of Na+, water and Ca++ with consequent anoxic depolarization (Branston et al., 1977; Harris et al., 1981) and eventually cell death. Additionally, large slow voltage shifts occur at the borders of the core and propagate as spreading depolarization waves that compromise tissue survival, including the penumbra (Selman et al., 2004).
In proximal MCA occlusion, the striato-capsular and opercular/insular regions are often the earliest to exhibit irreversible damage (Stoeckel et al., 2007). Subsequently, as the penumbra is recruited into the core, the latter progressively expands to other areas, including the cortical mantle. The maximum extent of the core will become the final infarct volume.
Haemodynamically, the core is defined as the tissue that exists below the perfusion threshold of infarction. This threshold was determined to be 5–8 ml 100 g−1 min−1 within the first few hours after stroke onset, but rises progressively over time to reach the penumbra threshold (around 22 ml 100 g−1 min−1) (Baron, 1999). Consequently, infarct expansion occurs earlier in tissue suffering more severe hypoperfusion. The corresponding infarction threshold defined by the cerebral metabolic rate of oxygen is around 39 μmol 100 g−1 min−1 (Marchal et al., 1999a), and in contrast to CBF, does not appear to vary over time.
There are several other means of depicting the core (Table 1) that are gradually being validated against PET to allow application in everyday clinical practice. MRI diffusion-weighted imaging (DWI) is of particular importance, since it has exquisite sensitivity for acute ischaemia and can be positive within a few minutes from onset (Hjort et al., 2005). The DWI signal reflects restriction of the random motion of water in tissue and decline of its apparent diffusion coefficient and although the exact biological correlates of this phenomenon are incompletely understood, cytotoxic oedema and subsequent shrinkage of the extracellular space have been proposed (Nicoli et al., 2003). The volume of DWI abnormality correlates well with both admission and outcome neurological deficit as well as with final infarct volume (Baird et al., 2000). Yet studies in animals and humans document the potential reversibility of DWI lesions and normalization of apparent diffusion coefficient, thus arguing against its equivalence to the core (Li et al., 1999; Kidwell et al., 2000). Predictors of such normalization include thrombolytic therapy and recanalization, particularly within the 3-h time window (Fiehler et al., 2004), suggesting that the DWI lesion may include penumbral tissue. This has also recently been further confirmed in human PET-MR studies (Guadagno et al., 2006).
The core as a therapeutic target
Within the necrotic core, the vasculature may also be severely damaged, exposing it to the risk of undergoing haemorrhagic transformation (HT). This is especially the case with extensive infarction and with the use of thrombolytics, potentially causing further worsening of the clinical condition and outcome, and therefore outweighing the benefit of salvaging the ischaemic penumbra (Fiehler et al., 2005). Thus, although the core is essentially not recoverable, prediction and prevention of HT within it represent important therapeutic goals. For instance, new thrombolytic agents that do not interfere with endothelial function or induce matrix metalloproteinase dysregulation (Wang et al., 2004) might reduce the incidence of thrombolysis-associated haemorrhage. More generally, vascular protectants and agents that modulate matrix proteolysis may also be beneficial in preventing HT within the infarct (Lee et al., 2004).
In clinical trials, HT is often divided into grades based on radiologic appearance into small or confluent petechiae within the infarction (HI-1 and HI-2, respectively); parenchymal haematoma occupying <30% of the infarcted area, with a mild space-occupying effect (PH-1) and parenchymal haematoma in >30% of the infarcted area with a significant space-occupying effect (PH-2) (Larrue et al., 2001). These are further qualified as symptomatic and asymptomatic.
The pathophysiology of HT remains to be proven, but early reperfusion with injury to the microvasculature and disruption of the blood–brain barrier has been suggested. Indeed, some studies (Latour et al., 2004; Warach and Latour, 2004) have used delayed gadolinium enhancement of cerebrospinal fluid space on MR fluid-attenuated inversion recovery images as a marker of blood–brain barrier disruption and demonstrated its dependency on reperfusion and its association to HT and worse clinical outcomes. It is still not entirely clear, however, if these markers will be useful in clinical decision-making or in the development of new therapeutic strategies for HT.
A large parenchymal hypodensity on acute CT statistically predicts the risk of thrombolysis-associated haemorrhage, hence the widespread notion of withholding this treatment if hypodensity exceeds one-third of the MCA territory (Hacke et al., 1998). Studies using MRI have shown that areas of HT have significantly lower apparent diffusion coefficient, CBF and CBV than other areas with perfusion abnormality on initial imaging, and that reperfusion occurs in almost all cases showing HT (Selim et al., 2002; Alsop et al., 2005). Nonetheless, the results of these studies were not analysed separately for symptomatic and asymptomatic grades of HT, and the relevance of these associations to clinical outcome is not identified. Lansberg et al. (2007a) investigated predictors of any haemorrhage causing symptomatic deterioration and found only a large DWI lesion to be an independent predictor in multivariate analysis. Thomalla et al. (2007) further argue that the HT is merely a frequent epiphenomenon related to reperfusion and that it is of little clinical consequence, distinguishing it from parenchymal haemorrhage related to IV thrombolytic therapy as a relevant target for investigation and prevention.
The ischaemic penumbra
The penumbra was originally described on electrophysiological basis as the tissue existing between the thresholds of electrical failure and ion pump failure (Astrup et al., 1981). Thereafter, a haemodynamic and metabolic approach based on multi-tracer 15O PET has defined it as tissue that exists between the threshold of infarction and the penumbral threshold (Baron, 2001a). Operationally, penumbral tissue must satisfy criteria of being (i) functionally impaired viable hypoperfused tissue with undetermined fate that is at-risk of infarction if not salvaged; (ii) contributing to the clinical deficit and that (iii) its resolution is associated with proportional recovery of neurologic function.
Using multi-tracer PET, substantial volumes of cortical penumbra have been reported to decline over time, being present in over 50% of the patients studied within 9 h, and in about one-third of the patients studied between 5 and 18 h (Wise et al., 1983; Heiss, 1992; Marchal et al., 1996). This confirms that the temporal window of opportunity for therapy is protracted in some patients, but is rapidly shrinking in others, thus emphasizing the urgency of acute stroke management. Some patients develop an extensive necrotic infarct core within a few hours of stroke onset, whereas spontaneous reperfusion is seen in the remaining subgroup (Marchal et al., 1993).
The demise of the penumbra is signalled by a decline in cerebral metabolic rate of oxygen, with further decline or stabilization of the CBF (Wise et al., 1983; Heiss, 1992; Marchal et al., 1996) and a dramatic fall in the OEF, from initially very high to sometimes exceedingly low values heralding the exhaustion of the tissue's oxygen needs. Early reperfusion can reverse this grim outcome as shown in studies in baboons (Touzani et al., 1995, 1997) and humans (Heiss et al., 1998), reporting that large volumes of tissue with penumbral levels of CBF escape necrosis if arterial recanalization is achieved. Ample evidence from 15O (Furlan et al., 1996; Heiss et al., 1998) and 18F-fluoromisonidazole PET studies (Read et al., 2000; Markus et al., 2004) also indicates that survival of the penumbra has a definite and predictable benefit on subsequent neurological recovery in man. Less predictably, a better correlation also exists with 2-month recovery scores, suggesting that survival of the penumbra also influences late recovery, possibly through allowing subsequent peri-infarct neuronal reorganization (Furlan et al., 1996).
Similar findings have also been demonstrated using multi-modal MRI combining DWI and perfusion-weighted imaging (PWI). Maps of CBF, CBV and mean transit time are generated by this latter technique, reflecting the perfusion status of brain tissue. Comparison of the perfusion deficit depicted on PWI with the DWI lesion (assumed to denote the core) thus yields either (i) a mismatch pattern (PWI>DWI); (ii) a matched lesion pattern (PWI=DWI) or (iii) a reperfusion pattern indicating recanalization (DWI>PWI). The mismatch pattern is taken to indicate the existence of salvageable at-risk tissue and is found in about 70% of patients with anterior-circulation stroke scanned within 6 h of onset (Barber et al., 1999). Its presence is strongly associated with proximal MCA occlusion and its resolution on reperfusion is associated with neurological recovery (Staroselskaya et al., 2001; Singer et al., 2004). Moreover, successful reperfusion prevents further expansion of the DWI lesion into the area of mismatch (Jansen et al., 1999). Nonetheless, as outlined earlier, uncertainties exist regarding the physiologic accuracy of the DWI lesion and corresponding uncertainties also exist regarding PWI, particularly in the selection of parameters for defining the tissue at risk and in the choice of arterial input function (Heiss et al., 2004; Rose et al., 2004; Sobesky et al., 2004). Thus, although the DWI–PWI mismatch concept is a very clinically useful tool, it may overestimate the penumbra by including oligaemic or even normally perfused but autoregulated tissue, that is not at-risk (Sobesky et al., 2005; Muir et al., 2006a). These questions also become particularly relevant when defining the management of matched DWI–PWI lesions, since response to recanalization largely depends on whether or not it includes penumbral tissue and therefore still likely to progress (Kane et al., 2007).
A simple alternative approach suggests inferring the presence of salvageable tissue by a mismatch between clinical stroke severity (National Institutes of Health Stroke Scale score ⩾8) and the size of the DWI lesion. Studies during the past few years reported that this ‘clinical–DWI mismatch' predicted infarct growth, neurologic deterioration (Davalos et al., 2004) and even the DWI–PWI mismatch (Prosser et al., 2005). A similar clinical–CT mismatch has also been proposed, yet recent evidence has cast doubt on the validity of both these approaches in reliably reflecting the presence of penumbra and in selecting suitable candidates for thrombolytic therapy (Kent et al., 2005; Lansberg et al., 2007b; Messe et al., 2007).
Other imaging strategies to detect the penumbra include single photon emission CT, xenon-CT and perfusion computed tomography, and their findings are generally consistent with other techniques (Muir et al., 2006a). Perfusion computed tomography has attracted special interest as it is similar in principle to MR PWI but has the practical advantage of being more widely available and cheaper than MR. Recent studies on perfusion computed tomography in acute stroke demonstrated that tissue with CBV <2 ml 100 g−1 represents the core, while a relative mean transit time above 145% of the normal hemisphere with CBV >2 ml 100 g−1 best outlines at-risk tissue (Wintermark et al., 2006). Perfusion computed tomography parameters correlate very well with MR DWI–PWI and accurately predict final infarct volume and clinical recovery, corroborating its potential utility in selecting patients for thrombolysis (Eastwood et al., 2003; Muir et al., 2006b; Wintermark et al., 2007) even when the time of onset is not clear (Hellier et al., 2006).
The penumbra as a therapeutic target
Reperfusion therapy in the 3-h window
In the mid-1990s, recombinant tissue plasminogen activator (alteplase) emerged as the first effective therapy aimed at rescuing at-risk tissue in the first hours of stroke. Large trials demonstrated that IV recombinant tissue plasminogen activator therapy affords at least a 30% increase in the likelihood of a good outcome when administered within 3 h, yet carries a 6–7% risk of symptomatic intracranial haemorrhage (Hacke et al., 2004). Those trials were based on plain (non-contrast) CT and thus did not distinguish stroke subtypes or objectively demonstrate the existence of penumbra, which suggests that more can be gained from thrombolysis by better selection of potential responders to treatment and exclusion of those who may not benefit or may be harmed. A multi-modal imaging approach based on MRI demonstration of DWI–PWI mismatch is thus increasingly being advocated as the investigation of choice in acute stroke though delay in treatment remains the primary concern (Kang et al., 2005). This is probably balanced by the added diagnostic accuracy and that shorter door-to-needle times can be achieved through omitting CT and tailoring MRI protocols to suit hyperacute stroke patients (U-King-Im et al., 2005).
Reperfusion beyond 3 h
The seminal recombinant tissue plasminogen activator trials undoubtedly revolutionized stroke therapy, yet they created an artificial cutoff at 3 h that may not apply to all patients (Baron et al., 1995). Indeed the pathophysiological model outlined earlier suggests that reperfusion can be beneficial beyond 3 h through salvage of the penumbra in appropriate patients. Extension of the therapeutic window is thus an attractive goal that is currently being pursued cautiously with the use of physiologic imaging.
The Diffusion and perfusion imaging Evaluation for Understanding Stroke Evolution study demonstrated a better clinical response among patients with small DWI lesion and substantial MR mismatch treated with alteplase between 3 and 6 h, than in other subgroups, including the matched DWI–PWI lesion, the small DWI and PWI lesion and the large DWI lesion subgroups (Albers et al., 2006). Thus, this important study highlighted the importance of considering not only the presence of DWI–PWI mismatch but also the size of the DWI lesion in the decision-making process. The ongoing EPITHET trial further addresses this question by randomizing patients to alteplase or placebo 3–6 h after stroke onset regardless of baseline MRI findings, testing the hypothesis that in retrospective analysis patients with mismatch will have derived greater benefit than those without (Butcher et al., 2005).
Studies comparing MRI-based alteplase treatment within 3–6 h to conventional CT-based treatment within 3 h have demonstrated similar recanalization rates and functional outcomes (Rother et al., 2002; Ribo et al., 2005). Moreover, MRI-based treatment in the 0–6 h time frame also shows similar or superior safety and efficacy to CT-based treatment within 3 h, when compared directly (Kohrmann et al., 2006) or to data from meta-analyses (Thomalla et al., 2006). Preliminary findings from pooling of results from 1210 patients further strengthen these conclusions (Schellinger et al., 2007).
MR-based selection has also been used in studies testing the new thrombolytic agent desmoteplase (Hacke et al., 2005), where the presence of MR DWI–PWI mismatch of 20% or higher was used to select patients for thrombolysis in the 3–9 h window. A more favourable clinical outcome was demonstrated in patients who experienced reperfusion than in those who did not (52.5 vs 24.6%), and the treatment effect was independent of the duration from onset to treatment. Nonetheless, a further phase 3 trial on desmoteplase (The Desmoteplase in Acute Ischemic Stroke Trial II) (Hacke and Furlan, 2007) has failed to reproduce the same results, which arguably casts doubt on the use of the mismatch model to select patients for treatment. However, the negative results also suggest that the drug's efficacy may have been overestimated in previous studies or that this treatment window is too late. The small number of participants in each arm of the trial (n∼60 in each group) may have also contributed to the lack of a detectable benefit over placebo. Pro-urokinase is another thrombolytic agent that has been investigated for use in acute stroke. The PROACT II study used catheter angiography and plain CT to select patients with MCA occlusion for intra-arterial pro-urokinase treatment up to 6 h from onset (Furlan et al., 1999). The findings were strongly in favour of a beneficial effect on clinical outcome, and subsequently it was shown that detailed analysis of the patients' CT scans (Hill et al., 2003) may further improve patient selection for this treatment. Other thrombolytic agents (such as tenecteplase) and other intra-arterial reperfusion techniques are also under investigation in acute stroke and imaging is increasingly being used to monitor their therapeutic effects (Molina and Saver, 2005).
Neuroprotection
When tested in humans, neuroprotective agents designed to limit the demise of at-risk tissue have consistently failed to produce the effects observed in animal studies (Savitz and Fisher, 2007; Shuaib et al., 2007). These agents targeted critical interlinked events that occur in ischaemic tissue before or after reperfusion ending in necrotic cell death (the ‘ischaemic cascade'). Their failure is variously attributed, among many possibilities, to inadequate preclinical data or therapeutic targets, and the choice of ineffective compounds (Green, 2002). Importantly, physiologic imaging has only been employed in very few of these trials (for example, Warach et al., 2000), so the grouping together of patient populations who may not even have the substrates targeted by such therapies (for example, penumbra) is another likely factor for the failure of translation to humans.
A further reason that is proposed is that most neuroprotective drugs were designed to specifically reduce damage to the cortical grey matter rather than the white matter (Dewar et al., 1999). Several studies using PET and MRI have demonstrated that substantial volumes of potentially salvageable tissue existed in white matter several hours after onset of stroke and that it is at least as resistant to ischaemia as grey matter (Falcao et al., 2004; Koga et al., 2005; Simon et al., 2005). Furthermore, an MRI study by Bristow et al. (2005) quantitatively demonstrated that grey matter was at risk of infarction at higher CBF values and at shorter mean transit time delays than white matter. These findings thus emphasize the necessity of devising approaches that target not only grey matter but also white matter ischaemia when considering novel strategies to neuroprotection.
Another potential strategy for neuroprotection is pre-hospital administration of treatment that can essentially ‘buy time' until imaging can be undertaken and definitive therapy instituted. Such a neuroprotectant clearly has to be safe and tolerable in both ischaemic and haemorrhagic strokes, and should be simple to administer by ambulance personnel. Magnesium sulphate is one such candidate and is currently being tested within 2 h of stroke onset in a large clinical trial (Saver et al., 2004).
On a more general standpoint, the failure of clinical trials on agents targeting solely the neurons together with the evolving understanding of the key roles played by other cell types, support the concept of an integrative approach to neuroprotection that replaces the prevailing neurocentric paradigm (Lee et al., 2004). This approach addresses the various interacting components that make up the neurovascular unit, namely, the neuronal tissue, the glial tissue and supporting matrix, and the cerebral vasculature (Singhal et al., 2005b).
Oxygen therapy
The scarcity of blood supply within the penumbra relative to its oxygen needs means that it is hypoxic and its function is reversibly affected by the reduction in tissue partial pressure of oxygen rather than hypoperfusion per se. This carries two main implications: (i) that increasing the partial pressure of oxygen in inspired air may be an effective therapeutic option and (ii) that mapping of the tissue partial pressure of oxygen in the brain would provide a direct way of depicting salvageable penumbral tissue, either alone or in conjunction with CBF measurements. One such approach is the use of the PET tracer 18F-fluoromisonidazole and other nitroimidazoles. Tissue identified using this technique shares the operational criteria of the ischaemic penumbra (see above) and its fate correlates to clinical outcome up to 48 h from stroke onset (Markus et al., 2004). Nonetheless, among other drawbacks, validation is not yet complete and the long scanning time required for this technique still precludes its use outside the research setting.
Studies on oxygen therapy initially focused on administration of hyperbaric oxygen, but this later became replaced by normobaric oxygen owing to its wider availability, ease of administration and safety (Singhal, 2007). Almost all experimental studies on normobaric oxygen therapy showed significant reduction of infarct size, and recently further demonstrated that it improves CBF and oxygenation, at least in part, by inhibiting peri-infarct depolarization waves and hence reducing oxygen demand (Shin et al., 2007).
Promising results also exist from small clinical trials. In one pilot study (Singhal et al., 2005a), MRI DWI–PWI mismatch was used to select acute stroke patients to receive either 100% oxygen or room air for 8 h. Oxygen-treated patients improved clinically during therapy and at 24 h, with smaller MR DWI lesions than in control subjects. Moreover, oxygen therapy was associated with an increase in relative CBF and CBV within the perfusion (mean transit time) abnormality, consistent with earlier observations of a vasodilatory response to hyperoxia in ischaemic brain tissue rather than the vasoconstriction induced in normal brain tissue (Nakajima et al., 1983). Another small trial (Chiu et al., 2006) did not use an imaging end point but showed that 40% venturi mask oxygen therapy reduced mortality and morbidity in patients with large stroke. Larger trials using similar methodologies and physiological imaging are awaited.
The oligaemia
Oligaemic tissue exists at a CBF range above the penumbra threshold and though it shows a mild degree of misery perfusion (high OEF) on PET, it is not normally at risk of infarction (Furlan et al., 1996). Thus, misery perfusion should not be equated with penumbra in acute stroke.
Cellular changes that occur in the oligaemic blood flow range in acute stroke are limited to differential induction and inhibition of protein synthesis. This likely represents the activation of a cellular stress response in which heat-shock proteins, unfolded proteins, endoplasmic reticulum kinases, caspases and many others are involved (DeGracia, 2004). Prolonged persistence of cellular stress responses initiates apoptotic programmed cell death and hence may explain the selective loss of neurons in areas remote from the penumbra and core of cerebral ischaemia (Paschen and Mengesdorf, 2005). Current therapeutic interventions do not specifically target the oligaemic compartment, apart from prevention of secondary insults such as systemic hypotension and hyperglycaemia which may threaten the oligaemia and incorporate it into the at-risk compartment (Baron, 2001b).
Secondary events and contributors
Imaging has been used to identify secondary contributors to ischaemic injury and investigate their influence on tissue outcome. Multi-modal MRI constitutes the main tool in these studies because of its relative availability and tolerability in the acute setting.
Hyperglycaemia
Parsons et al. (2002) explored the association of hyperglycaemia with the fate of at-risk hypoperfused tissue. They showed that acute hyperglycaemia was independently correlated with reduced tissue survival and that higher blood glucose levels were also strongly correlated with larger final infarct sizes and worse functional outcomes. Furthermore, using proton MR spectroscopy, they demonstrated that higher acute blood glucose in patients with DWI–PWI mismatch was associated with greater lactate production, which, in turn, was independently associated with the reduced salvage of mismatched tissue. Baird et al. (2003) similarly demonstrated that high acute blood glucose levels were strongly correlated with infarct expansion on serial MRI and with worsening of functional outcomes. It is, however, still not certain if hyperglycaemia is in itself detrimental in acute stroke or that it is rather a manifestation of a more fundamental and injurious process such as sympathetic activation or hypercortisolism. This is emphasized by the failure of a large trial of intensive insulin therapy to detect any functional benefit despite achieving sustained euglycaemia (Gray et al., 2007). Yet this trial had a number of shortcomings. First, physiological imaging was not employed in selecting patients; the trial was prematurely terminated due to slow recruitment and a wide range of plasma glucose levels were considered abnormal (6–16 mM) and actively treated for a target capillary glucose of 4–7 mM. This may not have been an adequate goal, and given that glucose–potassium–insulin infusions also effected a significant drop in systemic blood pressure in the treatment group, the results ought not be considered definitive.
Haematocrit
Elevated blood haematocrit has been shown to associate with infarct expansion and reduced penumbral tissue salvage (Allport et al., 2005). These effects are probably mediated by increased blood viscosity and impairment of capillary flow. Haemodilution therapy was previously a popular approach in addressing this problem, but enthusiasm has faded following the failure of several trials to demonstrate substantial clinical benefit (Asplund, 1989).
Systemic blood pressure
Demonstration of high OEF or DWI–PWI mismatch in the setting of acute stroke implies that autoregulation of CBF is impaired in the affected territory. Thus, any lowering of the systemic arterial pressure is likely to further reduce the cerebral perfusion pressure and in turn the CBF in the affected tissue, which can be harmful for the penumbra as well as the oligaemia. Accordingly, blood pressure reductions in acute ischaemic stroke have frequently been associated with worse outcome (Ahmed et al., 2000), especially with iatrogenic lowering of reactive hypertension. Conversely, observing hyperperfusion, particularly if early oedema is demonstrated by CT or MRI, may provide rationale for treating hypertension as it is suggested that hyperperfusion in necrotic tissue may promote the development of malignant brain swelling (Marchal et al., 1999b). Several trials are currently underway to address these questions and assess the optimum management of blood pressure in the acute stage, including the UK-based Controlling Hypertension and Hypotension Immediately Post-Stroke Trial (Potter et al., 2005); The Continue or Stop post-Stroke Antihypertensives Collaborative Study (COSSACS) (2005) and the international multi-centre ‘Efficacy of Nitric Oxide in Stroke' study. Another large international study (INTERACT) is exploring the optimal approach to managing blood pressure specifically after intra-cerebral haemorrhage.
Vasogenic oedema
Vasogenic oedema usually develops in the first 2–3 days following the onset of stroke causing swelling of the brain tissue. Oedema is usually of modest clinical impact unless associated with a large rapidly developing space-occupying MCA infarction. This is known as malignant MCA infarction (MMI) owing to its very poor prognosis under standard therapy, with a case-fatality rate approaching 80% (Vahedi et al., 2007). The reasons behind the development of MMI are not clearly understood, but some evidence points to factors beyond the size of infarction, such as inflammation and blood–brain barrier breakdown, as instrumental mechanisms (Serena et al., 2005). Substantial vasogenic oedema increases local tissue pressure and thus reduces the effective perfusion pressure. This, in turn, can lead the penumbra to progress to infarction and hence lead to further infarct expansion with development of more oedema and a vicious cycle ensues.
Predicting the development of MMI as early as possible is important to allow timely institution of therapy. Imaging-based predictors include occlusion of the proximal MCA, carotid T occlusion, involvement of both the superficial and deep MCA territories, inadequate circle of Willis and involvement of other vascular territories (Jaramillo et al., 2006). PET and single photon emission CT allow accurate prediction of MMI (Marchal et al., 1995; Berrouschot et al., 1998), but the more clinically available DWI MRI is also of considerable potential. A large DWI lesion volume (>145 ml within 14 h or >82 ml within 6 h) reportedly predicts MMI with 100% sensitivity and 94% specificity (Oppenheim et al., 2000; Thomalla et al., 2003).
Anecdotal clinical reports of decompressive surgery for MMI prompted experimental studies that demonstrated a beneficial effect on infarct size and outcome (Forsting et al., 1995). Eventually, several clinical trials have shown that decompressive surgery, in the form of wide hemicraniectomy and duraplasty performed within 48 h of stroke onset reduces mortality by an absolute 50% and improves functional outcome in the survivors, although less impressively (Vahedi et al., 2007). Early decompression prevents life-threatening brain herniation and probably also reduces the detrimental effects of raised intracranial pressure on tissue perfusion pressure. Induction of moderate hypothermia (around 33 °C) has also been used in the treatment of MMI and some small open studies showed a beneficial effect on clinical outcome (Schwab et al., 2001; De Georgia et al., 2004), though carrying the risks of pneumonia and rebound increase in intracranial pressure on re-warming. On the other hand, osmotically active agents (for example, mannitol) and steroids offer little benefit in limiting the progression of MMI, and hence devising novel pharmacological approaches to brain oedema remains an area of potential future development.
Inflammation
Inflammation is thought to contribute to the pathophysiology of neuronal cell death by several mechanisms, including apoptosis (Price et al., 2003). Within minutes of ischaemia, proinflammatory genes are upregulated and adhesion molecules are expressed on the vascular endothelium. Neutrophils then migrate from the blood into the brain parenchyma within hours after reperfusion (Emerich et al., 2002), followed by macrophages and monocytes within a few days. The vast majority of macrophages in the infarct area appear to be derived from local microglia that are activated before macrophage infiltration from the blood (Schilling et al., 2003), although the temporal pattern is not entirely clear. Animal studies suggest that microglial activation also extends beyond the core and could contribute to peri-infarct neuronal death (Mabuchi et al., 2000). Conversely, some evidence also exists for a beneficial or protective role for inflammatory cell recruitment and activation in the ischaemic process (Danton and Dietrich, 2003), including promotion of plasticity and modulation of neurotrophic factors (Lalancette-Hebert et al., 2007). Consequently, it is still unclear whether or not strategies to nonspecifically downregulate this response would in fact improve outcome and limit neuronal damage.
The advances in in vivo imaging of inflammation in stroke have expanded the investigation of this phenomenon, particularly in the clinical setting. The PET tracer 11C-PK11195 binds to the peripheral benzodiazepine receptor, which is abundant on brain-derived activated microglia, and thus has been employed in experimental and clinical studies addressing ischaemia-related inflammation. In general, the results of these studies agree that microglial activation becomes significant after a few days of stroke and persists for over 30 days, with a peak around 2 weeks from stroke onset (Sette et al., 1993; Gerhard et al., 2005; Price et al., 2006). Notably, the spatial distribution of PK11195 binding evolves to include not only the core but also the rescued penumbra where inflammation may be secondary (or contributes) to selective neuronal damage (Baron, 2005a). Increased PK11195 binding may also be seen in brain regions distant from the infarct, including the contralateral hemisphere, possibly representing remote Wallerian degeneration (Pappata et al., 2000). These findings suggest that potential targets for therapy may still exist for weeks after stroke onset. A paramagnetic MRI contrast agent (ultrasmall superparamagnetic iron oxide), which is primarily taken up by blood-derived macrophages, has also been used to demonstrate parenchymal macrophage infiltration in animals and humans following ischaemic stroke (Kleinschnitz et al., 2003; Saleh et al., 2004; Wiart et al., 2007). The effect was prominent in the second week after stroke and was independent of blood–brain barrier disruption and lesion size (Nighoghossian et al., 2007). PK11195 and ultrasmall superparamagnetic iron oxide thus target two potentially overlapping components of the brain inflammatory response and further work using these two attractive techniques can be expected to expand the understanding of inflammation in stroke and guide therapeutic innovation.
Conclusions
Physiological imaging has elucidated many of the fundamental processes of ischaemic brain injury and demonstrated the substantial heterogeneity among individual stroke patients. The prolonged persistence of salvageable penumbral tissue has been established, and several other potential targets for intervention are gradually emerging. In future trials of therapeutic agents, the use of physiological imaging to select the patient category that best matches the drug's presumed mode of action is recommended, rather than lumping together patients with entirely different pathophysiological patterns in the so-called ‘large trials', which have all failed so far. This approach promises to bring about further significant advances in the treatment of ischaemic stroke. Furthermore, imaging has also highlighted the important roles of the brain vasculature, glia and supporting matrix in stroke, all of which represent potential targets for therapy beyond neuroprotection per se.
Glossary
- CBF
cerebral blood flow
- CBV
cerebral blood volume
- DWI
diffusion-weighted imaging
- HT
haemorrhagic transformation
- MCA
middle cerebral artery
- MMI
malignant middle cerebral artery infarction
- MRI
magnetic resonance imaging
- OEF
oxygen extraction fraction
- PET
positron emission tomography
- PWI
perfusion-weighted imaging
- USPIO
ultrasmall superparamagnetic iron oxide
Footnotes
Conflict of interest
The authors state no conflict of interest.
References
- Ahmed N, Nasman P, Wahlgren NG. Effect of intravenous nimodipine on blood pressure and outcome after acute stroke. Stroke. 2000;31:1250–1255. doi: 10.1161/01.str.31.6.1250. [DOI] [PubMed] [Google Scholar]
- Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the Diffusion and perfusion imaging Evaluation for Understanding Stroke Evolution (DEFUSE) study. Ann Neurol. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- Allport LE, Parsons MW, Butcher KS, Macgregor L, Desmond PM, Tress BM, et al. Elevated hematocrit is associated with reduced reperfusion and tissue survival in acute stroke. Neurology. 2005;65:1382–1387. doi: 10.1212/01.wnl.0000183057.96792.a8. [DOI] [PubMed] [Google Scholar]
- Alsop DC, Makovetskaya E, Kumar S, Selim M, Schlaug G. Markedly reduced apparent blood volume on bolus contrast magnetic resonance imaging as a predictor of hemorrhage after thrombolytic therapy for acute ischemic stroke. Stroke. 2005;36:746–750. doi: 10.1161/01.STR.0000158913.91058.93. [DOI] [PubMed] [Google Scholar]
- Asplund K. Randomized clinical trials of hemodilution in acute ischemic stroke. Acta Neurol Scand Suppl. 1989;127:22–30. doi: 10.1111/j.1600-0404.1989.tb01807.x. [DOI] [PubMed] [Google Scholar]
- Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia—the ischemic penumbra. Stroke. 1981;12:723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- Baird AE, Lovblad KO, Dashe JF, Connor A, Burzynski C, Schlaug G, et al. Clinical correlations of diffusion and perfusion lesion volumes in acute ischemic stroke. Cerebrovasc Dis. 2000;10:441–448. doi: 10.1159/000016105. [DOI] [PubMed] [Google Scholar]
- Baird TA, Parsons MW, Phanh T, Butcher KS, Desmond PM, Tress BM, et al. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke. 2003;34:2208–2214. doi: 10.1161/01.STR.0000085087.41330.FF. [DOI] [PubMed] [Google Scholar]
- Barber PA, Davis SM, Darby DG, Desmond PM, Gerraty RP, Yang Q, et al. Absent middle cerebral artery flow predicts the presence and evolution of the ischemic penumbra. Neurology. 1999;52:1125–1132. doi: 10.1212/wnl.52.6.1125. [DOI] [PubMed] [Google Scholar]
- Baron JC. Mapping the ischaemic penumbra with PET: implications for acute stroke treatment. Cerebrovasc Dis. 1999;9:193–201. doi: 10.1159/000015955. [DOI] [PubMed] [Google Scholar]
- Baron JC. Mapping the ischaemic penumbra with PET: a new approach. Brain. 2001a;124:2–4. doi: 10.1093/brain/124.1.2. [DOI] [PubMed] [Google Scholar]
- Baron JC. Perfusion thresholds in human cerebral ischemia: historical perspective and therapeutic implications. Cerebrovasc Dis. 2001b;11 Suppl 1:2–8. doi: 10.1159/000049119. [DOI] [PubMed] [Google Scholar]
- Baron JC. How healthy is the acutely reperfused ischemic penumbra. Cerebrovasc Dis. 2005a;20 Suppl 2:25–31. doi: 10.1159/000089354. [DOI] [PubMed] [Google Scholar]
- Baron JC. Stroke research in the modern era: images versus dogmas. Cerebrovasc Dis. 2005b;20:154–163. doi: 10.1159/000087199. [DOI] [PubMed] [Google Scholar]
- Baron JC, Von Kummer R, Del Zoppo GJ. Treatment of acute ischemic stroke. Challenging the concept of a rigid and universal time window. Stroke. 1995;26:2219–2221. doi: 10.1161/01.str.26.12.2219. [DOI] [PubMed] [Google Scholar]
- Berrouschot J, Barthel H, Von Kummer R, Knapp WH, Hesse S, Schneider D. 99m technetium-ethyl-cysteinate-dimer single-photon emission CT can predict fatal ischemic brain edema. Stroke. 1998;29:2556–2562. doi: 10.1161/01.str.29.12.2556. [DOI] [PubMed] [Google Scholar]
- Branston NM, Strong AJ, Symon L. Extracellular potassium activity, evoked potential and tissue blood flow. Relationships during progressive ischaemia in baboon cerebral cortex. J Neurol Sci. 1977;32:305–321. doi: 10.1016/0022-510x(77)90014-4. [DOI] [PubMed] [Google Scholar]
- Bristow MS, Simon JE, Brown RA, Eliasziw M, Hill MD, Coutts SB, et al. MR perfusion and diffusion in acute ischemic stroke: human gray and white matter have different thresholds for infarction. J Cereb Blood Flow Metab. 2005;25:1280–1287. doi: 10.1038/sj.jcbfm.9600135. [DOI] [PubMed] [Google Scholar]
- Brown MM, Markus H, Oppenheimer S. Stroke Medicine. Taylor & Francis: Oxon; 2006. [Google Scholar]
- Butcher KS, Parsons M, Macgregor L, Barber PA, Chalk J, Bladin C, et al. Refining the perfusion–diffusion mismatch hypothesis. Stroke. 2005;36:1153–1159. doi: 10.1161/01.str.0000166181.86928.8b. [DOI] [PubMed] [Google Scholar]
- Chiu EH, Liu CS, Tan TY, Chang KC. Venturi mask adjuvant oxygen therapy in severe acute ischemic stroke. Arch Neurol. 2006;63:741–744. doi: 10.1001/archneur.63.5.741. [DOI] [PubMed] [Google Scholar]
- COSSACS (Continue or Stop post-Stroke Antihypertensives Collaborative Study) Rationale and design. J Hypertens. 2005;23:455–458. doi: 10.1097/00004872-200502000-00029. [DOI] [PubMed] [Google Scholar]
- Danton GH, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol. 2003;62:127–136. doi: 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]
- Davalos A, Blanco M, Pedraza S, Leira R, Castellanos M, Pumar JM, et al. The clinical–DWI mismatch: a new diagnostic approach to the brain tissue at risk of infarction. Neurology. 2004;62:2187–2192. doi: 10.1212/01.wnl.0000130570.41127.ea. [DOI] [PubMed] [Google Scholar]
- De Georgia MA, Krieger DW, Abou-Chebl A, Devlin TG, Jauss M, Davis SM, et al. Cooling for Acute Ischemic Brain Damage (COOL AID): a feasibility trial of endovascular cooling. Neurology. 2004;63:312–317. doi: 10.1212/01.wnl.0000129840.66938.75. [DOI] [PubMed] [Google Scholar]
- Degracia DJ. Acute and persistent protein synthesis inhibition following cerebral reperfusion. J Neurosci Res. 2004;77:771–776. doi: 10.1002/jnr.20225. [DOI] [PubMed] [Google Scholar]
- Dewar D, Yam P, Mcculloch J. Drug development for stroke: importance of protecting cerebral white matter. Eur J Pharmacol. 1999;375:41–50. doi: 10.1016/s0014-2999(99)00280-0. [DOI] [PubMed] [Google Scholar]
- Eastwood JD, Lev MH, Wintermark M, Fitzek C, Barboriak DP, Delong DM, et al. Correlation of early dynamic CT perfusion imaging with whole-brain MR diffusion and perfusion imaging in acute hemispheric stroke. Am J Neuroradiol. 2003;24:1869–1875. [PMC free article] [PubMed] [Google Scholar]
- Emerich DF, Dean RL, III, Bartus RT. The role of leukocytes following cerebral ischemia: pathogenic variable or bystander reaction to emerging infarct. Exp Neurol. 2002;173:168–181. doi: 10.1006/exnr.2001.7835. [DOI] [PubMed] [Google Scholar]
- Falcao AL, Reutens DC, Markus R, Koga M, Read SJ, Tochon-Danguy H, et al. The resistance to ischemia of white and gray matter after stroke. Ann Neurol. 2004;56:695–701. doi: 10.1002/ana.20265. [DOI] [PubMed] [Google Scholar]
- Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43–53. doi: 10.1016/s1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- Fiehler J, Knudsen K, Kucinski T, Kidwell CS, Alger JR, Thomalla G, et al. Predictors of apparent diffusion coefficient normalization in stroke patients. Stroke. 2004;35:514–519. doi: 10.1161/01.STR.0000114873.28023.C2. [DOI] [PubMed] [Google Scholar]
- Fiehler J, Remmele C, Kucinski T, Rosenkranz M, Thomalla G, Weiller C, et al. Reperfusion after severe local perfusion deficit precedes hemorrhagic transformation: an MRI study in acute stroke patients. Cerebrovasc Dis. 2005;19:117–124. doi: 10.1159/000083180. [DOI] [PubMed] [Google Scholar]
- Forsting M, Reith W, Schabitz WR, Heiland S, Von Kummer R, Hacke W, et al. Decompressive craniectomy for cerebral infarction. An experimental study in rats. Stroke. 1995;26:259–264. doi: 10.1161/01.str.26.2.259. [DOI] [PubMed] [Google Scholar]
- Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282:2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- Furlan M, Marchal G, Viader F, Derlon JM, Baron JC. Spontaneous neurological recovery after stroke and the fate of the ischemic penumbra. Ann Neurol. 1996;40:216–226. doi: 10.1002/ana.410400213. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Schwarz J, Myers R, Wise R, Banati RB. Evolution of microglial activation in patients after ischemic stroke: a [11C](R)-PK11195 PET study. Neuroimage. 2005;24:591–595. doi: 10.1016/j.neuroimage.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Gray CS, Hildreth AJ, Sandercock PA, O'connell JE, Johnston DE, Cartlidge NE, et al. Glucose–potassium–insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK) Lancet Neurol. 2007;6:397–406. doi: 10.1016/S1474-4422(07)70080-7. [DOI] [PubMed] [Google Scholar]
- Green AR. Why do neuroprotective drugs that are so promising in animals fail in the clinic? An industry perspective. Clin Exp Pharmacol Physiol. 2002;29:1030–1034. doi: 10.1046/j.1440-1681.2002.03767.x. [DOI] [PubMed] [Google Scholar]
- Guadagno JV, Warburton EA, Jones PS, Day DJ, Aigbirhio FI, Fryer TD, et al. How affected is oxygen metabolism in DWI lesions? A combined acute stroke PET–MR study. Neurology. 2006;67:824–829. doi: 10.1212/01.wnl.0000233984.66907.db. [DOI] [PubMed] [Google Scholar]
- Hacke W, Albers G, Al-Rawi Y, Bogousslavsky J, Davalos A, Eliasziw M, et al. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-h window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005;36:66–73. doi: 10.1161/01.STR.0000149938.08731.2c. [DOI] [PubMed] [Google Scholar]
- Hacke W, Donnan G, Fieschi C, Kaste M, Von Kummer R, Broderick JP, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- Hacke W, Furlan A. Results from the phase III study of desmoteplase in acute ischemic stroke trial 2 (DIAS 2) Cerebrovasc Dis. 2007;23:54. [Google Scholar]
- Hacke W, Kaste M, Fieschi C, Von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European–Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- Harris RJ, Symon L, Branston NM, Bayhan M. Changes in extracellular calcium activity in cerebral ischaemia. J Cereb Blood Flow Metab. 1981;1:203–209. doi: 10.1038/jcbfm.1981.21. [DOI] [PubMed] [Google Scholar]
- Heiss WD. Experimental evidence of ischemic thresholds and functional recovery. Stroke. 1992;23:1668–1672. doi: 10.1161/01.str.23.11.1668. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Grond M, Thiel A, Von Stockhausen HM, Rudolf J, Ghaemi M, et al. Tissue at risk of infarction rescued by early reperfusion: a positron emission tomography study in systemic recombinant tissue plasminogen activator thrombolysis of acute stroke. J Cereb Blood Flow Metab. 1998;18:1298–1307. doi: 10.1097/00004647-199812000-00004. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Sobesky J, Hesselmann V. Identifying thresholds for penumbra and irreversible tissue damage. Stroke. 2004;35:2671–2674. doi: 10.1161/01.STR.0000143329.81997.8a. [DOI] [PubMed] [Google Scholar]
- Hellier KD, Hampton JL, Guadagno JV, Higgins NP, Antoun N, Day DJ, et al. Perfusion CT helps decision making for thrombolysis when there is no clear time of onset. J Neurol Neurosurg Psychiatry. 2006;77:417–419. doi: 10.1136/jnnp.2005.067363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MD, Rowley HA, Adler F, Eliasziw M, Furlan A, Higashida RT, et al. Selection of acute ischemic stroke patients for intra-arterial thrombolysis with pro-urokinase by using ASPECTS. Stroke. 2003;34:1925–1931. doi: 10.1161/01.STR.0000082483.37127.D0. [DOI] [PubMed] [Google Scholar]
- Hjort N, Christensen S, Solling C, Ashkanian M, Wu O, Rohl L, et al. Ischemic injury detected by diffusion imaging 11 min after stroke. Ann Neurol. 2005;58:462–465. doi: 10.1002/ana.20595. [DOI] [PubMed] [Google Scholar]
- Jansen O, Schellinger P, Fiebach J, Hacke W, Sartor K. Early recanalisation in acute ischaemic stroke saves tissue at risk defined by MRI. Lancet. 1999;353:2036–2037. doi: 10.1016/S0140-6736(99)01146-0. [DOI] [PubMed] [Google Scholar]
- Jaramillo A, Gongora-Rivera F, Labreuche J, Hauw JJ, Amarenco P. Predictors for malignant middle cerebral artery infarctions: a postmortem analysis. Neurology. 2006;66:815–820. doi: 10.1212/01.wnl.0000203649.60211.0e. [DOI] [PubMed] [Google Scholar]
- Kane I, Sandercock P, Wardlaw J. Magnetic resonance perfusion diffusion mismatch and thrombolysis in acute ischaemic stroke: a systematic review of the evidence to date. J Neurol Neurosurg Psychiatry. 2007;78:485–491. doi: 10.1136/jnnp.2006.100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DW, Chalela JA, Dunn W, Warach S. MRI screening before standard tissue plasminogen activator therapy is feasible and safe. Stroke. 2005;36:1939–1943. doi: 10.1161/01.STR.0000177539.72071.f0. [DOI] [PubMed] [Google Scholar]
- Kent DM, Hill MD, Ruthazer R, Coutts SB, Demchuk AM, Dzialowski I, et al. ‘Clinical–CT mismatch' and the response to systemic thrombolytic therapy in acute ischemic stroke. Stroke. 2005;36:1695–1699. doi: 10.1161/01.STR.0000173397.31469.4b. [DOI] [PubMed] [Google Scholar]
- Kidwell CS, Saver JL, Mattiello J, Starkman S, Vinuela F, Duckwiler G, et al. Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Ann Neurol. 2000;47:462–469. [PubMed] [Google Scholar]
- Kleinschnitz C, Bendszus M, Frank M, Solymosi L, Toyka KV, Stoll G. In vivo monitoring of macrophage infiltration in experimental ischemic brain lesions by magnetic resonance imaging. J Cereb Blood Flow Metab. 2003;23:1356–1361. doi: 10.1097/01.WCB.0000090505.76664.DB. [DOI] [PubMed] [Google Scholar]
- Koga M, Reutens DC, Wright P, Phan T, Markus R, Pedreira B, et al. The existence and evolution of diffusion–perfusion mismatched tissue in white and gray matter after acute stroke. Stroke. 2005;36:2132–2137. doi: 10.1161/01.STR.0000181066.23213.8f. [DOI] [PubMed] [Google Scholar]
- Kohrmann M, Juttler E, Fiebach JB, Huttner HB, Siebert S, Schwark C, et al. MRI versus CT-based thrombolysis treatment within and beyond the 3 h time window after stroke onset: a cohort study. Lancet Neurol. 2006;5:661–667. doi: 10.1016/S1474-4422(06)70499-9. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansberg MG, Thijs VN, Bammer R, Kemp S, Wijman CA, Marks MP, et al. Risk factors of symptomatic intracerebral hemorrhage after tPA therapy for acute stroke. Stroke. 2007a;38:2775–2778. doi: 10.1161/STROKEAHA.106.480475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansberg MG, Thijs VN, Hamilton S, Schlaug G, Bammer R, Kemp S, et al. Evaluation of the clinical–diffusion and perfusion–diffusion mismatch models in DEFUSE. Stroke. 2007b;38:1826–1830. doi: 10.1161/STROKEAHA.106.480145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrue V, Von Kummer RR, Muller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European–Australasian Acute Stroke Study (ECASS II) Stroke. 2001;32:438–441. doi: 10.1161/01.str.32.2.438. [DOI] [PubMed] [Google Scholar]
- Latour LL, Kang DW, Ezzeddine MA, Chalela JA, Warach S. Early blood–brain barrier disruption in human focal brain ischemia. Ann Neurol. 2004;56:468–477. doi: 10.1002/ana.20199. [DOI] [PubMed] [Google Scholar]
- Lee SR, Wang X, Tsuji K, Lo EH. Extracellular proteolytic pathophysiology in the neurovascular unit after stroke. Neurol Res. 2004;26:854–861. doi: 10.1179/016164104X3806. [DOI] [PubMed] [Google Scholar]
- Li F, Han SS, Tatlisumak T, Liu KF, Garcia JH, Sotak CH, et al. Reversal of acute apparent diffusion coefficient abnormalities and delayed neuronal death following transient focal cerebral ischemia in rats. Ann Neurol. 1999;46:333–342. doi: 10.1002/1531-8249(199909)46:3<333::aid-ana9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Mabuchi T, Kitagawa K, Ohtsuki T, Kuwabara K, Yagita Y, Yanagihara T, et al. Contribution of microglia/macrophages to expansion of infarction and response of oligodendrocytes after focal cerebral ischemia in rats. Stroke. 2000;31:1735–1743. doi: 10.1161/01.str.31.7.1735. [DOI] [PubMed] [Google Scholar]
- Marchal G, Beaudouin V, Rioux P, De La Sayette V, Le Doze F, Viader F, et al. Prolonged persistence of substantial volumes of potentially viable brain tissue after stroke: a correlative PET–CT study with voxel-based data analysis. Stroke. 1996;27:599–606. doi: 10.1161/01.str.27.4.599. [DOI] [PubMed] [Google Scholar]
- Marchal G, Benali K, Iglesias S, Viader F, Derlon JM, Baron JC. Voxel-based mapping of irreversible ischaemic damage with PET in acute stroke. Brain. 1999a;122 Pt 12:2387–2400. doi: 10.1093/brain/122.12.2387. [DOI] [PubMed] [Google Scholar]
- Marchal G, Rioux P, Serrati C, Furlan M, Derlon JM, Viader F, et al. Value of acute-stage positron emission tomography in predicting neurological outcome after ischemic stroke: further assessment. Stroke. 1995;26:524–525. [PubMed] [Google Scholar]
- Marchal G, Serrati C, Rioux P, Petit-Taboue MC, Viader F, De La Sayette V, et al. PET imaging of cerebral perfusion and oxygen consumption in acute ischaemic stroke: relation to outcome. Lancet. 1993;341:925–927. doi: 10.1016/0140-6736(93)91214-7. [DOI] [PubMed] [Google Scholar]
- Marchal G, Young AR, Baron JC. Early postischemic hyperperfusion: pathophysiologic insights from positron emission tomography. J Cereb Blood Flow Metab. 1999b;19:467–482. doi: 10.1097/00004647-199905000-00001. [DOI] [PubMed] [Google Scholar]
- Markus R, Reutens DC, Kazui S, Read S, Wright P, Pearce DC, et al. Hypoxic tissue in ischaemic stroke: persistence and clinical consequences of spontaneous survival. Brain. 2004;127:1427–1436. doi: 10.1093/brain/awh162. [DOI] [PubMed] [Google Scholar]
- Messe SR, Kasner SE, Chalela JA, Cucchiara B, Demchuk AM, Hill MD, et al. CT-NIHSS mismatch does not correlate with MRI diffusion–perfusion mismatch. Stroke. 2007;38:2079–2084. doi: 10.1161/STROKEAHA.106.480731. [DOI] [PubMed] [Google Scholar]
- Molina CA, Saver JL. Extending reperfusion therapy for acute ischemic stroke: emerging pharmacological, mechanical, and imaging strategies. Stroke. 2005;36:2311–2320. doi: 10.1161/01.STR.0000182100.65262.46. [DOI] [PubMed] [Google Scholar]
- Moustafa RR, Baron JC.2007Perfusion thresholds in cerebral ischemiaIn: Donnan GA, Baron JC, Davis SM, Sharp FR (eds).The Ischemic Penumbra Informa Healthcare: New York; 21–35. [Google Scholar]
- Muir KW, Buchan A, Von Kummer R, Rother J, Baron JC. Imaging of acute stroke. Lancet Neurol. 2006a;5:755–768. doi: 10.1016/S1474-4422(06)70545-2. [DOI] [PubMed] [Google Scholar]
- Muir KW, Halbert HM, Baird TA, Mccormick M, Teasdale E. Visual evaluation of perfusion computed tomography in acute stroke accurately estimates infarct volume and tissue viability. J Neurol Neurosurg Psychiatry. 2006b;77:334–339. doi: 10.1136/jnnp.2005.074179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S, Meyer JS, Amano T, Shaw T, Okabe T, Mortel KF. Cerebral vasomotor responsiveness during 100% oxygen inhalation in cerebral ischemia. Arch Neurol. 1983;40:271–276. doi: 10.1001/archneur.1983.04050050039004. [DOI] [PubMed] [Google Scholar]
- Nicoli F, Lefur Y, Denis B, Ranjeva JP, Confort-Gouny S, Cozzone PJ. Metabolic counterpart of decreased apparent diffusion coefficient during hyperacute ischemic stroke: a brain proton magnetic resonance spectroscopic imaging study. Stroke. 2003;34:e82–e87. doi: 10.1161/01.STR.0000078659.43423.0A. [DOI] [PubMed] [Google Scholar]
- Nighoghossian N, Wiart M, Cakmak S, Berthezene Y, Derex L, Cho TH, et al. Inflammatory response after ischemic stroke: a USPIO-enhanced MRI study in patients. Stroke. 2007;38:303–307. doi: 10.1161/01.STR.0000254548.30258.f2. [DOI] [PubMed] [Google Scholar]
- Oppenheim C, Samson Y, Manai R, Lalam T, Vandamme X, Crozier S, et al. Prediction of malignant middle cerebral artery infarction by diffusion-weighted imaging. Stroke. 2000;31:2175–2181. doi: 10.1161/01.str.31.9.2175. [DOI] [PubMed] [Google Scholar]
- Pappata S, Levasseur M, Gunn RN, Myers R, Crouzel C, Syrota A, et al. Thalamic microglial activation in ischemic stroke detected in vivo by PET and [11C]PK1195. Neurology. 2000;55:1052–1054. doi: 10.1212/wnl.55.7.1052. [DOI] [PubMed] [Google Scholar]
- Parsons MW, Barber PA, Desmond PM, Baird TA, Darby DG, Byrnes G, et al. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol. 2002;52:20–28. doi: 10.1002/ana.10241. [DOI] [PubMed] [Google Scholar]
- Paschen W, Mengesdorf T. Endoplasmic reticulum stress response and neurodegeneration. Cell Calcium. 2005;38:409–415. doi: 10.1016/j.ceca.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Perez A, Restrepo L, Kleinman JT, Barker P, Beauchamp N, Wityk RJ. Patients with diffusion–perfusion mismatch on magnetic resonance imaging 48 h or more after stroke symptom onset: clinical and imaging features. J Neuroimaging. 2006;16:329–333. doi: 10.1111/j.1552-6569.2006.00063.x. [DOI] [PubMed] [Google Scholar]
- Potter J, Robinson T, Ford G, James M, Jenkins D, Mistri A, et al. CHHIPS (Controlling Hypertension and Hypotension Immediately Post-Stroke) Pilot Trial: rationale and design. J Hypertens. 2005;23:649–655. doi: 10.1097/01.hjh.0000160224.94220.e7. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wang D, Menon DK, Guadagno JV, Cleij M, Fryer T, et al. Intrinsic activated microglia map to the peri-infarct zone in the subacute phase of ischemic stroke. Stroke. 2006;37:1749–1753. doi: 10.1161/01.STR.0000226980.95389.0b. [DOI] [PubMed] [Google Scholar]
- Price CJ, Warburton EA, Menon DK. Human cellular inflammation in the pathology of acute cerebral ischaemia. J Neurol Neurosurg Psychiatry. 2003;74:1476–1484. doi: 10.1136/jnnp.74.11.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser J, Butcher K, Allport L, Parsons M, Macgregor L, Desmond P, et al. Clinical–diffusion mismatch predicts the putative penumbra with high specificity. Stroke. 2005;36:1700–1704. doi: 10.1161/01.STR.0000173407.40773.17. [DOI] [PubMed] [Google Scholar]
- Read SJ, Hirano T, Abbott DF, Markus R, Sachinidis JI, Tochon-Danguy HJ, et al. The fate of hypoxic tissue on 18F-fluoromisonidazole positron emission tomography after ischemic stroke. Ann Neurol. 2000;48:228–235. [PubMed] [Google Scholar]
- Ribo M, Molina CA, Rovira A, Quintana M, Delgado P, Montaner J, et al. Safety and efficacy of intravenous tissue plasminogen activator stroke treatment in the 3- to 6-hour window using multimodal transcranial Doppler/MRI selection protocol. Stroke. 2005;36:602–606. doi: 10.1161/01.STR.0000155737.43566.ad. [DOI] [PubMed] [Google Scholar]
- Rose SE, Janke AL, Griffin M, Finnigan S, Chalk JB. Improved prediction of final infarct volume using bolus delay-corrected perfusion-weighted MRI: implications for the ischemic penumbra. Stroke. 2004;35:2466–2471. doi: 10.1161/01.STR.0000145199.64907.5a. [DOI] [PubMed] [Google Scholar]
- Rother J, Schellinger PD, Gass A, Siebler M, Villringer A, Fiebach JB, et al. Effect of intravenous thrombolysis on MRI parameters and functional outcome in acute stroke <6 h. Stroke. 2002;33:2438–2445. doi: 10.1161/01.str.0000030109.12281.23. [DOI] [PubMed] [Google Scholar]
- Saleh A, Schroeter M, Jonkmanns C, Hartung HP, Modder U, Jander S. In vivo MRI of brain inflammation in human ischaemic stroke. Brain. 2004;127:1670–1677. doi: 10.1093/brain/awh191. [DOI] [PubMed] [Google Scholar]
- Saver JL. Time is brain—quantified. Stroke. 2006;37:263–266. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- Saver JL, Kidwell C, Eckstein M, Starkman S. Prehospital neuroprotective therapy for acute stroke: results of the Field Administration of Stroke Therapy-Magnesium (FAST-MAG) pilot trial. Stroke. 2004;35:e106–e108. doi: 10.1161/01.STR.0000124458.98123.52. [DOI] [PubMed] [Google Scholar]
- Savitz SI, Fisher M. Future of neuroprotection for acute stroke: in the aftermath of the SAINT trials. Ann Neurol. 2007;61:396–402. doi: 10.1002/ana.21127. [DOI] [PubMed] [Google Scholar]
- Schellinger P, Thomalla G, Kohrmann M. MRI-based thrombolysis is at least as safe and effective as standard CT-based treatment: a multicenter study of 1210 patients. Stroke. 2007;38:454. [Google Scholar]
- Schilling M, Besselmann M, Leonhard C, Mueller M, Ringelstein EB, Kiefer R. Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: a study in green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol. 2003;183:25–33. doi: 10.1016/s0014-4886(03)00082-7. [DOI] [PubMed] [Google Scholar]
- Schwab S, Georgiadis D, Berrouschot J, Schellinger PD, Graffagnino C, Mayer SA. Feasibility and safety of moderate hypothermia after massive hemispheric infarction. Stroke. 2001;32:2033–2035. doi: 10.1161/hs0901.095394. [DOI] [PubMed] [Google Scholar]
- Selim M, Fink JN, Kumar S, Caplan LR, Horkan C, Chen Y, et al. Predictors of hemorrhagic transformation after intravenous recombinant tissue plasminogen activator: prognostic value of the initial apparent diffusion coefficient and diffusion-weighted lesion volume. Stroke. 2002;33:2047–2052. doi: 10.1161/01.str.0000023577.65990.4e. [DOI] [PubMed] [Google Scholar]
- Selman WR, Lust WD, Pundik S, Zhou Y, Ratcheson RA. Compromised metabolic recovery following spontaneous spreading depression in the penumbra. Brain Res. 2004;999:167–174. doi: 10.1016/j.brainres.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Serena J, Blanco M, Castellanos M, Silva Y, Vivancos J, Moro MA, et al. The prediction of malignant cerebral infarction by molecular brain barrier disruption markers. Stroke. 2005;36:1921–1926. doi: 10.1161/01.STR.0000177870.14967.94. [DOI] [PubMed] [Google Scholar]
- Sette G, Baron JC, Young AR, Miyazawa H, Tillet I, Barre L, et al. 1993In vivo mapping of brain benzodiazepine receptor changes by positron emission tomography after focal ischemia in the anesthetized baboon Stroke 242046–2057.discussion 2057–2048. [DOI] [PubMed] [Google Scholar]
- Shin HK, Dunn AK, Jones PB, Boas DA, Lo EH, Moskowitz MA, et al. Normobaric hyperoxia improves cerebral blood flow and oxygenation, and inhibits peri-infarct depolarizations in experimental focal ischaemia. Brain. 2007;130:1631–1642. doi: 10.1093/brain/awm071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, et al. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- Simon JE, Bristow MS, Lu H, Lauzon ML, Brown RA, Manjon JV, et al. A novel method to derive separate gray and white matter cerebral blood flow measures from MR imaging of acute ischemic stroke patients. J Cereb Blood Flow Metab. 2005;25:1236–1243. doi: 10.1038/sj.jcbfm.9600130. [DOI] [PubMed] [Google Scholar]
- Singer OC, Du Mesnil De Rochemont R, Foerch C, Stengel A, Sitzer M, Lanfermann H, et al. Early functional recovery and the fate of the diffusion/perfusion mismatch in patients with proximal middle cerebral artery occlusion. Cerebrovasc Dis. 2004;17:13–20. doi: 10.1159/000073893. [DOI] [PubMed] [Google Scholar]
- Singhal AB. A review of oxygen therapy in ischemic stroke. Neurol Res. 2007;29:173–183. doi: 10.1179/016164107X181815. [DOI] [PubMed] [Google Scholar]
- Singhal AB, Benner T, Roccatagliata L, Koroshetz WJ, Schaefer PW, Lo EH, et al. A pilot study of normobaric oxygen therapy in acute ischemic stroke. Stroke. 2005a;36:797–802. doi: 10.1161/01.STR.0000158914.66827.2e. [DOI] [PubMed] [Google Scholar]
- Singhal AB, Lo EH, Dalkara T, Moskowitz MA.2005bAdvances in stroke neuroprotection: hyperoxia and beyond Neuroimaging Clin N Am 15697–720.xii–xiii. [DOI] [PubMed] [Google Scholar]
- Sobesky J, Zaro Weber O, Lehnhardt FG, Hesselmann V, Neveling M, Jacobs A, et al. Does the mismatch match the penumbra? Magnetic resonance imaging and positron emission tomography in early ischemic stroke. Stroke. 2005;36:980–985. doi: 10.1161/01.STR.0000160751.79241.a3. [DOI] [PubMed] [Google Scholar]
- Sobesky J, Zaro Weber O, Lehnhardt FG, Hesselmann V, Thiel A, Dohmen C, et al. Which time-to-peak threshold best identifies penumbral flow? A comparison of perfusion-weighted magnetic resonance imaging and positron emission tomography in acute ischemic stroke. Stroke. 2004;35:2843–2847. doi: 10.1161/01.STR.0000147043.29399.f6. [DOI] [PubMed] [Google Scholar]
- Staroselskaya IA, Chaves C, Silver B, Linfante I, Edelman RR, Caplan L, et al. Relationship between magnetic resonance arterial patency and perfusion–diffusion mismatch in acute ischemic stroke and its potential clinical use. Arch Neurol. 2001;58:1069–1074. doi: 10.1001/archneur.58.7.1069. [DOI] [PubMed] [Google Scholar]
- Stoeckel MC, Wittsack HJ, Meisel S, Seitz RJ. Pattern of cortex and white matter involvement in severe middle cerebral artery ischemia. J Neuroimaging. 2007;17:131–140. doi: 10.1111/j.1552-6569.2007.00102.x. [DOI] [PubMed] [Google Scholar]
- Thomalla GJ, Kucinski T, Schoder V, Fiehler J, Knab R, Zeumer H, et al. Prediction of malignant middle cerebral artery infarction by early perfusion- and diffusion-weighted magnetic resonance imaging. Stroke. 2003;34:1892–1899. doi: 10.1161/01.STR.0000081985.44625.B6. [DOI] [PubMed] [Google Scholar]
- Thomalla G, Schwark C, Sobesky J, Bluhmki E, Fiebach JB, Fiehler J, et al. Outcome and symptomatic bleeding complications of intravenous thrombolysis within 6 h in MRI-selected stroke patients: comparison of a German multicenter study with the pooled data of ATLANTIS, ECASS, and NINDS tPA trials. Stroke. 2006;37:852–858. doi: 10.1161/01.STR.0000204120.79399.72. [DOI] [PubMed] [Google Scholar]
- Thomalla G, Sobesky J, Kohrmann M, Fiebach JB, Fiehler J, Zaro Weber O, et al. Two tales: hemorrhagic transformation but not parenchymal hemorrhage after thrombolysis is related to severity and duration of ischemia: MRI study of acute stroke patients treated with intravenous tissue plasminogen activator within 6 h. Stroke. 2007;38:313–318. doi: 10.1161/01.STR.0000254565.51807.22. [DOI] [PubMed] [Google Scholar]
- Touzani O, Young AR, Derlon JM, Baron JC, Mackenzie ET. Progressive impairment of brain oxidative metabolism reversed by reperfusion following middle cerebral artery occlusion in anaesthetized baboons. Brain Res. 1997;767:17–25. doi: 10.1016/s0006-8993(97)00515-5. [DOI] [PubMed] [Google Scholar]
- Touzani O, Young AR, Derlon JM, Beaudouin V, Marchal G, Rioux P, et al. Sequential studies of severely hypometabolic tissue volumes after permanent middle cerebral artery occlusion. A positron emission tomographic investigation in anesthetized baboons. Stroke. 1995;26:2112–2119. doi: 10.1161/01.str.26.11.2112. [DOI] [PubMed] [Google Scholar]
- U-King-Im JM, Trivedi RA, Graves MJ, Harkness K, Eales H, Joubert I, et al. Utility of an ultrafast magnetic resonance imaging protocol in recent and semi-recent strokes. J Neurol Neurosurg Psychiatry. 2005;76:1002–1005. doi: 10.1136/jnnp.2004.046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6:215–222. doi: 10.1016/S1474-4422(07)70036-4. [DOI] [PubMed] [Google Scholar]
- Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM, et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35:2726–2730. doi: 10.1161/01.STR.0000143219.16695.af. [DOI] [PubMed] [Google Scholar]
- Warach S, Latour LL. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood–brain barrier disruption. Stroke. 2004;35:2659–2661. doi: 10.1161/01.STR.0000144051.32131.09. [DOI] [PubMed] [Google Scholar]
- Warach S, Pettigrew LC, Dashe JF, Pullicino P, Lefkowitz DM, Sabounjian L, et al. Effect of citicoline on ischemic lesions as measured by diffusion-weighted magnetic resonance imaging. Citicoline 010 Investigators. Ann Neurol. 2000;48:713–722. [PubMed] [Google Scholar]
- Wiart M, Davoust N, Pialat JB, Desestret V, Moucharaffie S, Cho TH, et al. MRI monitoring of neuroinflammation in mouse focal ischemia. Stroke. 2007;38:131–137. doi: 10.1161/01.STR.0000252159.05702.00. [DOI] [PubMed] [Google Scholar]
- Wintermark M, Flanders AE, Velthuis B, Meuli R, Van Leeuwen M, Goldsher D, et al. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke. 2006;37:979–985. doi: 10.1161/01.STR.0000209238.61459.39. [DOI] [PubMed] [Google Scholar]
- Wintermark M, Meuli R, Browaeys P, Reichhart M, Bogousslavsky J, Schnyder P, et al. Comparison of CT perfusion and angiography and MRI in selecting stroke patients for acute treatment. Neurology. 2007;68:694–697. doi: 10.1212/01.wnl.0000255959.30107.08. [DOI] [PubMed] [Google Scholar]
- Wise RJ, Rhodes CG, Gibbs JM, Hatazawa J, Palmer T, Frackowiak RS, et al. Disturbance of oxidative metabolism of glucose in recent human cerebral infarcts. Ann Neurol. 1983;14:627–637. doi: 10.1002/ana.410140605. [DOI] [PubMed] [Google Scholar]