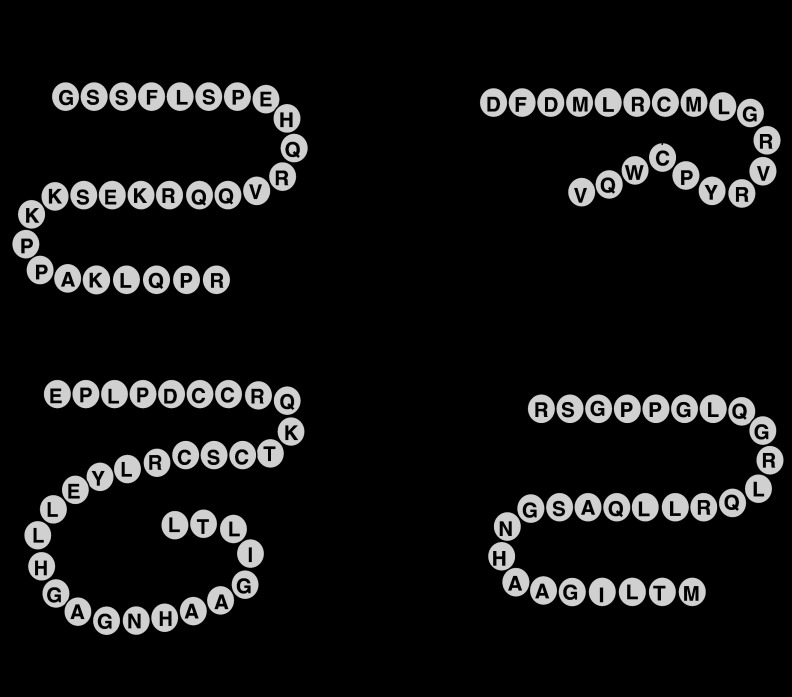Abstract
Orphan G protein-coupled receptors (GPCRs) are receptors lacking endogenous ligands. Found by molecular biological analyses, they became the roots of reverse pharmacology, in which receptors are attempted to be matched to potential transmitters. Later, when high-throughput screening technology was applied to reverse pharmacology, dozens of orphan GPCRs became deorphanized. Furthermore, novel neuropeptides were discovered. This review retraces the history of the orphan GPCRs and of the discoveries of their endogenous ligands, it also discusses the difficulties that the search for new ligands is presently encountering.
Keywords: orphan GPCR, reverse pharmacology, neuropeptides, orexins, MCH, ghrelin, transmitters
Introduction
Most G protein-coupled receptors (GPCRs) were found by sequence similarity. These, however, lack endogenous ligands and thus are orphan GPCRs. Although molecular biological and bioinformatics techniques made the identification of orphan GPCRs amenable, the search for their endogenous ligands has been a challenge. This search has given birth to the reverse pharmacology approach, which uses orphan GPCRs as targets to identify endogenous ligands. This approach was very successful and has led over two decades to the deorphanization of about 300 GPCRs. Many of the ligands that were matched to orphan GPCRs were already known, but a dozen are novel and in particular, nine novel neuropeptide families were discovered. These novel neuropeptides have already enriched our understanding of several important behavioural responses, in particular, the central regulation of food intake. Presently, there exist still 100 orphan GPCRs that may bind endogenous ligands that are not chemosensory. This review recounts the history of orphan GPCR research, discusses some of its successes and its present difficulties.
History of the orphan GPCR research
Orphan GPCRs are receptors lacking endogenous ligands. Their history began with the discovery that the β2-adrenergic receptor and the opsins share a seven-transmembrane domain topology (Dixon et al., 1986). These two receptors were not known to have much in common except that both couple to G proteins to elicit intracellular responses. This gave rise to the concept that all the receptors that couple to G proteins may form a supergene family, thereafter called the GPCRs. This concept gained rapid credence when metabotropic receptors for other neurotransmitters such as acetylcholine and serotonin were shown to be GPCRs (Hall, 1987). It ascertained itself when the receptor for a neuropeptide, substance K, was also found to be a GPCR (Masu et al., 1987).
The discovery of the orphan GPCRs
The GPCR concept had an immediate consequence—nucleic acid-based homology screening approaches could be applied to find new GPCRs. Low-stringency hybridization (Bunzow et al., 1988) and PCR-derived approaches (Libert et al., 1989) were applied in the second part of the 1980s and paved the way for the discoveries of many GPCRs. This was boosted one step higher, 10 years later with the sequence of the genome, when bioinformatic analyses put the number of human GPCRs to about 800 (Vassilatis et al., 2003).
The discovery of new GPCRs found by homology screening suffers from one obvious problem; these receptors lack their pharmacological identities, that is their endogenous ligands. They are all ‘orphan' receptors (Libert et al., 1991). As the vast majority of them are conserved in different species, one could assume that they are functionally active. However, the search for their endogenous ligands seemed to be a formidable, and possibly irresolvable task.
From what had been learned from GPCR cloning in 1986–1987, one could infer that probably all the small molecules that direct intercellular interactions would interact with GPCRs. These are the first messengers, the ‘transmitters', that are released from a cell to carry a message to a second one. They act in endocrine, paracrine or exocrine fashion to allow the organism to react to particular physiological challenges. These transmitters are mostly small molecules although few of them are larger polypeptides. They include biogenic amines, neuropeptides, lipid mediators, nucleotides, amino acid and derivatives, polypeptide hormones and, as will be learned later, chemokines, olfactory and gustatory molecules and some other naturally occurring chemicals such as calcium ions and protons. How could one find which one of these transmitters may bind an orphan GPCR?
The deorphanization of GPCRs
The strategy was to express the orphan GPCR of interest in eukaryotic cells by DNA transfection and to use membranes of these cells as targets for testing the binding abilities of potential ligands. This strategy is now known as reverse pharmacology (Libert et al., 1991; Mills and Duggan, 1994). At the end of the 1980s, there existed about 50 transmitters (in particular, many neuropeptides) that were potential GPCR ligands and had no cognate receptors. The reverse pharmacology approach meant carrying numerous binding assays using a plethora of radioactive ligands, an impossible task in an academic environment. Yet, serendipity and ingenious insights proved to be successful in matching the first orphan GPCRs.
In 1987, G-21 was the first orphan GPCR reported through its sequence similarities to the β2-adrenergic receptors. Attempts at binding adrenergic ligands to G-21 proved unsuccessful, until it was recognized that it binds serotonergic ligands. G-21 is now known as the 5-HT1A receptor (Fargin et al., 1988). We also screened a rat genomic library with a β2-adrenergic probe at low stringency and found numerous hybridizing clones. One in particular, RGB-2, was interesting as it had the seven-transmembrane domain hallmark of the GPCRs, yet was encoded by a gene containing introns and thus evolutionary different from the β2-adrenergic receptors. Moreover, RGB-2 was not only expressed in the CNS but also, at high levels, in the anterior and intermediate lobes of the pituitary. This led us to hypothesize that it may be the dopamine D2 receptor that was then rapidly demonstrated in specific binding assays (Bunzow et al., 1988). It is worth noting that some of the other clones that were found using the low-stringency screening approach led us to report the sequences of the rat β1-adrenergic and 5-HT1A receptors (Albert et al., 1990; Machida et al., 1990).
In 1989, the group of Parmentier and Vassart reported the use of the, at that time somewhat new, PCR technology as a way of finding new GPCRs (Libert et al., 1989). They had recognized that many of the cloned GPCRs contained conserved sequences in their third and sixth transmembrane domains, which allowed for designing DNA probes that could serve as general primers under non-stringent hybridization conditions. Applying this approach, they were able to identify half a dozen new orphan GPCRs. Because of its speed, the PCR-based approach had an important impact on the search for orphan GPCRs (Civelli et al., 2006). It became the method of choice for finding new orphan GPCRs, whereas low-stringency screening remained the most used approach for finding subtypes of cloned GPCRs.
The search for novel transmitters
During the first part of the 90s, application of the reverse pharmacology strategy led to the pharmacological characterization of many GPCRs. Thus, the number of potential orphan GPCR ligands was decreasing. At the same time, random searches for new GPCRs, using PCR-based homology screening approaches were in full swing, thus the overall number of orphan GPCRs was steadily increasing (Marchese et al., 1994, 1999). This led us to conclude that many orphan GPCRs must bind undiscovered ligands.
This conclusion was based on the fact that the vast majority of orphan GPCRs are evolutionarily conserved and thus are expected to be active. Although the seven-transmembrane domain topology has since evolved to include receptors that do not couple to G proteins, and although our understanding of receptor activation has been shown to be complex to include proteins other than the G proteins (Bockaert and Pin, 1999; Angers et al., 2002; Pierce et al., 2002; Kristiansen, 2004), at that time, it was expected that an activated orphan GPCR would couple to a G protein and induce a second messenger response. Consequently, monitoring second messenger levels induced by an orphan GPCR should lead to the identification of its ligand. As there was evidence that GPCRs can be promiscuous and activate more than one second messenger response especially when expressed at high levels as occurs upon heterologous transfection, one did not need to know the natural transduction pathway of an orphan GPCR a priori. Furthermore, by the mid-1990s, assays had been developed that allowed for high-throughput detection of second messenger responses. So reverse pharmacological approaches based on receptor reactivity instead of receptor binding could be applied.
The source of ligands of orphan GPCRs was another issue. From a basic stand point, isolating the natural ligand of an orphan GPCR was seen as most rewarding as it would lead to the identification of a novel transmitter and thus open new doors in our understanding of physiology. Most orphan GPCRs are expressed in the brain indicating that brain extracts would be good starting materials. As purification techniques have been developed that identify small amounts of peptides and as many orphan GPCRs bare the similarities of know neuropeptide GPCRs, the first attempts were directed at identifying novel neuropeptides.
The novel neuropeptides
The first orphan GPCR that was used for discovering a novel transmitter was opioid receptor-like 1, cloned through its homology to the opioid receptors (Henderson and McKnight, 1997). Its activation was monitored by quantifying intracellular decreases in cAMP levels, which could be measured in newly developed scintillation proximity assays. Because its phylogenetic analyses classified opioid receptor-like 1 as a peptidergic GPCR and because opioid receptor-like 1 is expressed in the CNS, peptidergic brain tissue extracts were prepared, purified and fractionated. Fractions were tested for their abilities to inhibit AC activity in cells that were stably transfected with opioid receptor-like 1. A 17-residue long peptide was ultimately isolated, named orphanin FQ or nociceptin (Meunier et al., 1995; Reinscheid et al., 1995). Its structural similarities to the opioid peptides made it an immediate star, yet it has been proven not to bind the opioid receptors (Reinscheid et al., 1998).
The second successful attempt at discovering novel neuropeptides through orphan GPCRs screened over 50 different orphan GPCRs by measuring their abilities to induce intracellular calcium release when subjected to peptidic extracts. One receptor did respond and led to the characterization of two peptides, the orexins (Sakurai et al., 1998), also identified through an RNA subtraction approach as hypocretins (de Lecea et al., 1998). This was immediately followed by the discovery of prolactin-releasing peptide and apelin as the natural ligands of the orphan GPCRs, GPR10 and putative receptor protein related to the angiotensin receptor (AT1), respectively (Hinuma et al., 1998; Tatemoto et al., 1998). Since then, the novel neuropeptides ghrelin, metastin, neuropeptides B/W, prokineticins1/2, neuropeptide S and neuromedin S have been discovered as natural ligands of orphan GPCRs (Civelli et al., 2006).
These successes proved not only the validity of the orphan GPCR approach for finding novel transmitters but also the impact of high-throughput screening of orphan GPCRs. It is therefore not surprising that the pharmaceutical industry became its major proponent (Hinuma et al., 1999). Consequently, orphan GPCRs began to be screened randomly against large libraries of synthetic ligands (Wise et al., 2004). These libraries contained all the ligands that had not been matched to any receptor molecules but also many molecules that are known to exist in the cells. This led in a few years to the deorphanization of some 40 GPCRs (Saito and Civelli, 2005).
Impact of the orphan GPCR research
As described above, the search for new GPCRs by homology screening coincides with the start of the orphan GPCR research. It is this search that led to the discovery of practically all the GPCRs that are studied today. But it is the deorphanization of GPCRs that are of importance and continue to be of importance because it impacts our understanding of the organism's function.
The most striking examples came from the discoveries of the novel neuropeptides. These neuropeptides have been implicated in an increasing number of physiological responses such as stress, sleep and circadian rhythm. Three of these neuropeptides, in particular, have had a direct impact on our understanding of the central regulation of food intake (Figure 1).
Figure 1.
Amino-acid sequences of human ghrelin, MCH, orexin A and orexin B. The third residue of ghrelin, serine, is n-octanoylated. MCH possesses an intrachain disulphide bond. Orexin A possesses an N-terminal pyroglutamyl residue and two intrachain disulphide bonds. Both orexins are C-terminally amidated. MCH, melanin-concentrating hormone.
Impact on nutritional homoeostasis
The orexin system
Orexins were identified as endogenous ligands for orphan GPCRs (Sakurai et al., 1998), although they were also discovered by using mRNA subtraction strategy (de Lecea et al., 1998). The orexins (orexin A and B) activate two related GPCRs, but are encoded by one precursor peptide, prepro-orexin, which is exclusively synthesized in the lateral hypothalamus. This pattern of expression indicated that the orexins may be regulators of feeding behaviour. Indeed it was found that fasting upregulated prepro-orexin mRNA gene and that central administration of orexins increases spontaneous food intake (Sakurai et al., 1998). Orexin-expressing neurons are also activated by peripheral metabolic signals, such as leptin, ghrelin and glucose, indicating that these neurons integrate energy-related signals coming from the periphery to the brain (Yamanaka et al., 2003). Consistent with these data, mice lacking prepro-orexin or mice in which orexin neurons have been ablated exhibit a hypophagic phenotype (Willie et al., 2001). Yet, mice lacking orexin neurons develop late-onset obesity in spite of being hypophagic, which indicates that the orexin system regulates energy expenditure as well.
It is noteworthy that orexin deficiency also caused a narcoleptic phenotype in humans and animals, indicating a role of the orexin system in the maintenance of wakefulness (Chemelli et al., 1999; Lin et al., 1999; Peyron et al., 2000; Thannickal et al., 2000). These two characteristics, increase feeding and maintenance of wakefulness, point at the orexin system as being important in arousal during food searching. Maintenance of arousal during food searching is important for survival. Daily restricted feeding induces an anticipatory locomotor activity. Consequently, it was shown that the activity of orexin neurons is markedly increased during the food-anticipatory period under restricted feeding (Akiyama et al., 2004; Mieda et al., 2004). Furthermore, mice lacking orexin neurons display, under restricted feeding, reduced food-anticipatory increases in wakefulness and locomotor activity (Akiyama et al., 2004). These observations indicate that the orexin system is important in conveying the information about the metabolic status to increase wakefulness and search for food. The orexin system thus provides a link between energy homoeostasis and wakefulness.
The MCH system
Melanin-concentrating hormone (MCH) had been known for 20 years as a peptide that induces paling of the skin in fish (Kawauchi et al., 1983) and since the mid-1990s, as a hypothalamic neuropeptide that is upregulated by fasting in rats (Qu et al., 1996). However, the full impact of the MCH system could not be studied until an orphan GPCR, SLC-1, was shown to be the MCH receptor (Bachner et al., 1999; Chambers et al., 1999; Lembo et al., 1999; Saito et al., 1999; Shimomura et al., 1999).
Acute central administrations of MCH increase food intake whereas chronic injections increase body weight, white adipose tissue mass and liver mass (Qu et al., 1996; Della-Zuana et al., 2002). Mice lacking MCH are lean due to hypophagia and an increase in metabolic rate (Shimada et al., 1998). MCH receptor knockout mice are also lean, yet they are hyperactive and hyperphagic (Marsh et al., 2002). To understand if MCH contributes to the hyperphagic phenotype of ob/ob mice, double-null mice lacking both MCH and leptin were generated (Segal-Lieberman et al., 2003). Surprisingly, these mice are still hyperphagic but present less body fat suggesting that the MCH system is critical for regulating energy expenditure and not for the maintenance of the hyperphagic phenotype in ob/ob mice. MCH may regulate energy expenditure through the hindbrain (Zheng et al., 2005). MCH administration into the fourth ventricle with the intent to target the nucleus tractus solitarus decreases body temperature without changing physical activity and feeding. This indicates that MCH has an inhibitory role in energy expenditure through hindbrain region (Zheng et al., 2005).
Although the MCH1 receptor is predominantly expressed in the brain, significant levels are also found in peripheral organs such as the pancreas, the skeletal muscle, the eye and the fat cells (Saito et al., 1999; Hill et al., 2001). The MCH1 receptor is expressed in insulin-producing cells and in the islets of Langerhans, where MCH significantly stimulates insulin secretion. This insulinotropic effect may contribute to the regulation of metabolism and energy balance (Tadayyon et al., 2000). The MCH1 receptor is also present in rat adipocytes and stimulates leptin production (Bradley et al., 2000). Together, these data show that the MCH system, although prominently viewed as a central system regulating energy homoeostasis, also has peripheral actions that impact on leptin or insulin release.
The ghrelin system
Ghrelin, a 28-amino-acid peptide with an n-octanoylation, was isolated as the endogenous ligand for the growth hormone secretagogue (GHS) receptor (Kojima et al., 1999). Growth hormone secretagogues are small synthetic molecules that act on the pituitary gland and the hypothalamus to stimulate growth hormone release (Howard et al., 1996; McKee et al., 1997).
Ghrelin is synthesized in the stomach. Peripheral and central administrations of ghrelin were shown to stimulate GH secretion and increase food intake and body weight (Wren et al., 2000, 2001). In rats, fasting increases plasma ghrelin levels, whereas feeding decreases them (Wren et al., 2001). In humans, plasma ghrelin levels rise before a meal and fall after the meal (Cummings et al., 2001). Ghrelin is therefore a regulator of energy homoeostasis, which carries its message from the periphery to the CNS. It is worth noting that ghrelin is synthesized as part of a larger precursor that has been reported to encode another bioactive peptide obestatin (Zhang et al., 2005). Obestatin was heralded as having the opposite effects of ghrelin, yet its role and even existence is doubtful (Lauwers et al., 2006; Seoane et al., 2006; Yamamoto et al., 2007).
Systemic administration of ghrelin activates Fos expression in the arcuate nucleus in the brain pointing to this nucleus as the site of circulating ghrelin action (Hewson and Dickson, 2000). Central administration of ghrelin activates Fos immunoreactivity in several brain regions, primarily in regions implicated in the regulation of feeding behaviour, including neuropeptide Y (NPY)/agouti-related protein (AGRP)-expressing neurons in the arcuate nucleus (Nakazato et al., 2001). The arcuate nucleus is a crucial target of leptin action. Leptin is the satiety signal that is released from adipocytes upon feeding and reaches the hypothalamus where it inhibits the activity of NPY/AGRP neurons (Stephens et al., 1995; Schwartz et al., 1996). Concordantly, it was found that leptin inhibits the orexigenic effects of ghrelin whereas ghrelin reverses the anorexic effect of leptin (Nakazato et al., 2001). However, although ghrelin is thought to be able to cross the blood–brain barrier to act on the brain, peripheral injections of ghrelin to vagotomized mice neither stimulated feeding nor activated NPY/AGRP neurons (Date et al., 2002). This on the other hand suggests that the pathway relaying ghrelin's signals to the brain may not be through the arcuate nucleus but via the gastric vagal afferents.
The discovery of the ghrelin system revealed how appetite-regulating factor secreted from peripheral organs can control energy homoeostasis via the CNS. In contrast to leptin, which is secreted from adipocytes as an appetite suppressing signal for the brain, ghrelin is secreted from the stomach as a hunger signal for the brain. Unlike the most orexigenic neuropeptides found in hypothalamus, such as NPY, AGRP, orexin, MCH or galanin, ghrelin is the first circulating neuropeptide that enhances feeding following systemic administration.
Impact on drug discovery
The matching of orphan GPCRs to previously known transmitters did not lead to the discovery of novel signalling molecules. Yet it has opened the opportunity to combine anatomical studies on the site of synthesis of the transmitters with that on their sites of action and thus to gain a full understanding of the localization of the system. Most often, the sites of the orphan GPCR expression serve as primary indication of the role of the system. Genetic ablations of the orphan GPCRs have also helped to understand these new receptor systems. Moreover, the matching of orphan GPCRs to known transmitters has had a major impact on drug discovery.
G protein-coupled receptors are one of the most important drug targets for the pharmaceutical industry as evidenced by the fact that approximately 30% of all marketed drugs act on them (Hopkins and Groom, 2002). The deorphanization successes allowed the pharmaceutical industry to initiate drug screening projects directed at the respective receptors. That is to say, transmitter systems that previously could not be targeted in drug discovery became instantaneously amenable to compound screening. This had and continues to have a profound impact on drug development. Many deorphanized GPCR systems have entered drug design programs, in particular, directed at the three deorphanized systems discussed above. Numerous MCH1 receptor antagonists have been reported and several lines of preclinical studies have indicated that blockade of the MCH1 receptor serves as a promising pharmacological target for the treatment of obesity as well as depression and anxiety (McBriar, 2006; Shimazaki et al., 2006). Indeed, currently, an MCH1 receptor antagonist (NGD-4715; Neurogen Corp., Branford, CT, USA) is undergoing clinical trials. Furthermore, ghrelin (GTP-200; Gastrotech Pharma A/S, Copenhagen K, Denmark) and orexin receptor antagonists (ACT-078573; Actelion Pharmaceuticals Ltd, GSK649868; GlaxoSmithKline plc., Brentford, UK) are also potential drugs and are currently in phase II clinical trials for the treatment of catabolic and sleep disorders, respectively.
So there is no doubt that deorphanized GPCR systems will be future therapeutic targets. In Table 1, we describe a list of deorphanized GPCRs that are in pharmaceutical development, although this evidently may not include all of them. Recognizing the important effort that the pharmaceutical industry has made in espousing the deorphanization of GPCRs, we can expect that deorphanized GPCR systems will be at the origin of several of the drugs in the future.
Table 1. Deorphanized GPCRs reported to be in drug discovery.
| Orphan receptor | Ligand | Therapeutic indication | Action of the compounds | References |
|---|---|---|---|---|
| ORL-1 (NOP) | Nociceptin/Orphanin FQ | Stress and pain | Agonist | Shoblock (2007) |
| Edg1, 3, 5, 6, 8 | S1P | Autoimmune diseases | Agonist/antagonist | Chiba (2005); Foss et al. (2007) |
| H3 | Histamine | Dementia | Antagonist/inverse agonist | Hancock (2006) |
| Orexin1, 2 | OrexinA and B | Sleep disorders | Antagonist | Bingham et al. (2006) |
| SLC-1 (MCH1) | MCH | Obesity, anxiety and depression | Antagonist | McBriar (2006); Shimazaki et al. (2006) |
| GHSR | Ghrelin | Catabolic disorders | Agonist | Davenport et al. (2005) |
| GPR38 | Motilin | Gastroparesis and irritable bowl syndrome | Agonist | McCallum et al. (2007) |
| GPRv53 (H4) | Histamine | Inflammation | Antagonist | Dunford et al. (2007) |
| P2Y12 | ADP | Platelet aggregation | Antagonist | van Giezen and Humphries (2005) |
| GPR16 (BLT1) | LTB4 | Inflammation and rheumatoid arthritis | Antagonist | Ding et al. (2005); Lundeen et al. (2006) |
| BLT2 | LTB4 | Inflammation and rheumatoid arthritis | Antagonist | Ding et al. (2005); Lundeen et al. (2006) |
| HG55 (CysLT1) | LTD4 | Bronchoconstriction | Antagonist | Nayak (2004) |
| GPR40 | Medium and long fatty acids | Diabetes | Agonist | Song et al. (2007) |
| HM74A, B | Nicotinic acid | Dyslipidaemia | Agonist | Offermanns (2006) |
The future of orphan GPCR research
The number of orphan GPCRs is still large. Of the ∼800 GPCRs, some 500 are chemosensory; they include the chemokines and chemoattractant GPCRs, which have mostly been deorphanized and the olfactory and gustatory GPCRs, which in large part remain orphans. The transmitter GPCRs, the focus of this review, account for about 360 GPCRs of which 100 are still orphans. The rate at which transmitter GPCRs were deorphanized at the turn of the century has drastically decreased. At that time, some 10 GPCRs were deorphanized per year, whereas very few have been deorphanized since 2004. Moreover, no novel transmitter has been discovered since then. This raises the question of whether orphan GPCR research has reached the end of its road and, if so, what its causes are. There are several issues that account for this.
The first issue concerns the fact that we have depleted the pool of known transmitters. Practically, all the possible transmitters have been matched to GPCRs and, consequently, the remaining orphan GPCRs can only bind unknown transmitters. Finding these as described above is a risky, lengthy and demanding endeavour that cannot be undertaken if immediate results are desired. It is thus not surprising that many have abandoned this route. One had also hoped that the sequencing of the human genome may reveal candidate bioactive peptides. But thus far, only the family of the QRFP peptides has been found using a bioinformatic approach. (Fukusumi et al., 2003). In this respect, it should also be mentioned that one expects that only 20% of the remaining orphan GPCRs will be neuropeptide receptors, whereas the rest will bind nucleotides, lipid mediators and neurotransmitters. Thus bioinformatic approaches can only have a limited impact on the deorphanization of the still orphan GPCRs.
The second issue relies on the finding that GPCRs exist not as monomers but as dimers or higher oligomers (Bulenger et al., 2005). It is now accepted that many GPCRs associate not only to themselves but also to other GPCRs to activate their signalling cascade. Heteromers carry a different pharmacological profile than the monomers. It is thus possible that some of the remaining orphan GPCRs do not act alone but in conjunction with other GPCRs. They would not induce their own second messenger pathway but would modulate that of other GPCRs. Indeed, such a possibility has been described in the case of a melatonin-like orphan GPCR, which has been shown to act as a negative modulator of the melatonin 1 receptor (Levoye et al., 2006). If orphan GPCRs heterodimerize with other GPCRs, finding their role becomes an about impossible task as they may associate with any of the 260 deorphanized GPCRs. Related to this issue is also the possibility that some orphan GPCRs may require the expression of accesory proteins for their activity. This has been shown in the case of the calcitonin GPCR, which necessitate the presence of RAMPs for inducing its signalling pathway (McLatchie et al., 1998; Hay et al., 2006).
The third issue concerns the fact that presently, the reverse pharmacological approach relies on monitoring changes in second messenger levels and thus a knowledge of that pathway. Although expect that GPCRs induce second messenger responses via G proteins, there are indications that some may link to different transducing pathways, some of which may not have been found. If this was the case, the deorphanization of the remaining GPCRs will await until these pathways have been defined.
The fourth issue concerns the concentrations of the transmitters in their natural environment. It is possible that some transmitters are only expressed at a particular time in the life of the organism or under particular conditions. If this is the case, then knowledge of those transmitter biology will be required before matching to their orphan GPCRs can be undertaken.
But, in spite of these caveats, it remains that the search for the ligands of orphan GPCRs will continue to serve as an important approach in discovering novel transmitters. Any one of these opens the door for a different understanding of particular physiological responses and for uncharted therapeutic developments.
Acknowledgments
This work was supported by NIH Grants MH60231, DK63001, DK70619 and the Eric L and Lila D Nelson Chair in Neuropharmacology.
Glossary
- AGRP
agouti-related protein
- GPCRs
G protein-coupled receptors
- MCH
melanin-concentrating hormone
- NPY
neuropeptide Y
Footnotes
Conflict of interest
The authors state no conflict of interest.
References
- Akiyama M, Yuasa T, Hayasaka N, Horikawa K, Sakurai T, Shibata S. Reduced food anticipatory activity in genetically orexin (hypocretin) neuron-ablated mice. Eur J Neurosci. 2004;20:3054–3062. doi: 10.1111/j.1460-9568.2004.03749.x. [DOI] [PubMed] [Google Scholar]
- Albert PR, Zhou QY, Van Tol HH, Bunzow JR, Civelli O. Cloning, functional expression, and mRNA tissue distribution of the rat 5-hydroxytryptamine1A receptor gene. J Biol Chem. 1990;265:5825–5832. [PubMed] [Google Scholar]
- Angers S, Salahpour A, Bouvier M. Dimerization: an emerging concept for G protein-coupled receptor ontogeny and function. Annu Rev Pharmacol Toxicol. 2002;42:409–435. doi: 10.1146/annurev.pharmtox.42.091701.082314. [DOI] [PubMed] [Google Scholar]
- Bachner D, Kreienkamp H, Weise C, Buck F, Richter D. Identification of melanin concentrating hormone (MCH) as the natural ligand for the orphan somatostatin-like receptor 1 (SLC-1) FEBS Lett. 1999;457:522–524. doi: 10.1016/s0014-5793(99)01092-3. [DOI] [PubMed] [Google Scholar]
- Bingham MJ, Cai J, Deehan MR. Eating, sleeping and rewarding: orexin receptors and their antagonists. Curr Opin Drug Discov Devel. 2006;9:551–559. [PubMed] [Google Scholar]
- Bockaert J, Pin JP. Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J. 1999;18:1723–1729. doi: 10.1093/emboj/18.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RL, Kokkotou EG, Maratos-Flier E, Cheatham B. Melanin-concentrating hormone regulates leptin synthesis and secretion in rat adipocytes. Diabetes. 2000;49:1073–1077. doi: 10.2337/diabetes.49.7.1073. [DOI] [PubMed] [Google Scholar]
- Bulenger S, Marullo S, Bouvier M. Emerging role of homo- and heterodimerization in G-protein-coupled receptor biosynthesis and maturation. Trends Pharmacol Sci. 2005;26:131–137. doi: 10.1016/j.tips.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Bunzow JR, Van Tol HH, Grandy DK, Albert P, Salon J, Christie M, et al. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988;336:783–787. doi: 10.1038/336783a0. [DOI] [PubMed] [Google Scholar]
- Chambers J, Ames RS, Bergsma D, Muir A, Fitzgerald LR, Hervieu G, et al. Melanin-concentrating hormone is the cognate ligand for the orphan G-protein-coupled receptor SLC-1. Nature. 1999;400:261–265. doi: 10.1038/22313. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Chiba K. FTY720, a new class of immunomodulator, inhibits lymphocyte egress from secondary lymphoid tissues and thymus by agonistic activity at sphingosine 1-phosphate receptors. Pharmacol Ther. 2005;108:308–319. doi: 10.1016/j.pharmthera.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Civelli O, Saito Y, Wang Z, Nothacker HP, Reinscheid RK. Orphan GPCRs and their ligands. Pharmacol Ther. 2006;110:525–532. doi: 10.1016/j.pharmthera.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport AP, Bonner TI, Foord SM, Harmar AJ, Neubig RR, Pin JP, et al. International Union of Pharmacology. LVI. Ghrelin receptor nomenclature, distribution, and function. Pharmacol Rev. 2005;57:541–546. doi: 10.1124/pr.57.4.1. [DOI] [PubMed] [Google Scholar]
- Della-Zuana O, Presse F, Ortola C, Duhault J, Nahon JL, Levens N. Acute and chronic administration of melanin-concentrating hormone enhances food intake and body weight in Wistar and Sprague–Dawley rats. Int J Obes Relat Metab Disord. 2002;26:1289–1295. doi: 10.1038/sj.ijo.0802079. [DOI] [PubMed] [Google Scholar]
- Ding XZ, Talamonti MS, Bell RH, Adrian TE. A novel anti-pancreatic cancer agent, LY293111. Anticancer Drugs. 2005;16:467–473. doi: 10.1097/00001813-200506000-00001. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Kobilka BK, Strader DJ, Benovic JL, Dohlman HG, Frielle T, et al. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986;321:75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Dunford PJ, Williams KN, Desai PJ, Karlsson L, McQueen D, Thurmond RL. Histamine H4 receptor antagonists are superior to traditional antihistamines in the attenuation of experimental pruritus. J Allergy Clin Immunol. 2007;119:176–183. doi: 10.1016/j.jaci.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Fargin A, Raymond JR, Lohse MJ, Kobilka BK, Caron MG, Lefkowitz RJ. The genomic clone G-21 which resembles a beta-adrenergic receptor sequence encodes the 5-HT1A receptor. Nature. 1988;335:358–360. doi: 10.1038/335358a0. [DOI] [PubMed] [Google Scholar]
- Foss FW, Snyder AH, Davis MD, Rouse M, Okusa MD, Lynch KR, et al. Synthesis and biological evaluation of gamma-aminophosphonates as potent, subtype-selective sphingosine 1-phosphate receptor agonists and antagonists. Bioorg Med Chem. 2007;15:663–677. doi: 10.1016/j.bmc.2006.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukusumi S, Yoshida H, Fujii R, Maruyama M, Komatsu H, Habata Y, et al. A new peptidic ligand and its receptor regulating adrenal function in rats. J Biol Chem. 2003;278:46387–46395. doi: 10.1074/jbc.M305270200. [DOI] [PubMed] [Google Scholar]
- Hall ZW. Three of a kind: the [beta]-adrenergic receptor, the muscarinic acetylcholine receptor, and rhodopsin. Trends Neurosci. 1987;10:99–101. [Google Scholar]
- Hancock AA. The challenge of drug discovery of a GPCR target: analysis of preclinical pharmacology of histamine H3 antagonists/inverse agonists. Biochem Pharmacol. 2006;71:1103–1113. doi: 10.1016/j.bcp.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Hay DL, Poyner DR, Sexton PM. GPCR modulation by RAMPs. Pharmacol Ther. 2006;109:173–197. doi: 10.1016/j.pharmthera.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Henderson G, McKnight AT. The orphan opioid receptor and its endogenous ligand—nociceptin/orphanin FQ. Trends Pharmacol Sci. 1997;18:293–300. [PubMed] [Google Scholar]
- Hewson AK, Dickson SL. Systemic administration of ghrelin induces Fos and Egr-1 proteins in the hypothalamic arcuate nucleus of fasted and fed rats. J Neuroendocrinol. 2000;12:1047–1049. doi: 10.1046/j.1365-2826.2000.00584.x. [DOI] [PubMed] [Google Scholar]
- Hill J, Duckworth M, Murdock P, Rennie G, Sabido-David C, Ames RS, et al. Molecular cloning and functional characterization of MCH2, a novel human MCH receptor. J Biol Chem. 2001;276:20125–20129. doi: 10.1074/jbc.M102068200. [DOI] [PubMed] [Google Scholar]
- Hinuma S, Habata Y, Fujii R, Kawamata Y, Hosoya M, Fukusumi S, et al. A prolactin-releasing peptide in the brain. Nature. 1998;393:272–276. doi: 10.1038/30515. [DOI] [PubMed] [Google Scholar]
- Hinuma S, Onda H, Fujino M. The quest for novel bioactive peptides utilizing orphan seven-transmembrane-domain receptors. J Mol Med. 1999;77:495–504. doi: 10.1007/s001090050403. [DOI] [PubMed] [Google Scholar]
- Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1:727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- Kawauchi H, Kawazoe I, Tsubokawa M, Kishida M, Baker BI. Characterization of melanin-concentrating hormone in chum salmon pituitaries. Nature. 1983;305:321–323. doi: 10.1038/305321a0. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Kristiansen K. Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol Ther. 2004;103:21–80. doi: 10.1016/j.pharmthera.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Lauwers E, Landuyt B, Arckens L, Schoofs L, Luyten W. Obestatin does not activate orphan G protein-coupled receptor GPR39. Biochem Biophys Res Commun. 2006;351:21–25. doi: 10.1016/j.bbrc.2006.09.141. [DOI] [PubMed] [Google Scholar]
- Lembo PM, Grazzini E, Cao J, Hubatsch DA, Pelletier M, Hoffert C, et al. The receptor for the orexigenic peptide melanin-concentrating hormone is a G-protein-coupled receptor. Nat Cell Biol. 1999;1:267–271. doi: 10.1038/12978. [DOI] [PubMed] [Google Scholar]
- Levoye A, Dam J, Ayoub MA, Guillaume JL, Couturier C, Delagrange P, et al. The orphan GPR50 receptor specifically inhibits MT1 melatonin receptor function through heterodimerization. EMBO J. 2006;25:3012–3023. doi: 10.1038/sj.emboj.7601193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert F, Parmentier M, Lefort A, Dinsart C, Van Sande J, Maenhaut C, et al. Selective amplification and cloning of four new members of the G protein-coupled receptor family. Science. 1989;244:569–572. doi: 10.1126/science.2541503. [DOI] [PubMed] [Google Scholar]
- Libert F, Vassart G, Parmentier M. Current developments in G-protein-coupled receptors. Curr Opin Cell Biol. 1991;3:218–223. doi: 10.1016/0955-0674(91)90142-l. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Lundeen KA, Sun B, Karlsson L, Fourie AM. Leukotriene B4 receptors BLT1 and BLT2: expression and function in human and murine mast cells. J Immunol. 2006;177:3439–3447. doi: 10.4049/jimmunol.177.5.3439. [DOI] [PubMed] [Google Scholar]
- Machida CA, Bunzow JR, Searles RP, Van Tol H, Tester B, Neve KA, et al. Molecular cloning and expression of the rat beta 1-adrenergic receptor gene. J Biol Chem. 1990;265:12960–12965. [PubMed] [Google Scholar]
- Marchese A, Docherty JM, Nguyen T, Heiber M, Cheng R, Heng HH, et al. Cloning of human genes encoding novel G protein-coupled receptors. Genomics. 1994;23:609–618. doi: 10.1006/geno.1994.1549. [DOI] [PubMed] [Google Scholar]
- Marchese A, George SR, Kolakowski LF, Jr, Lynch KR, O'Dowd BF. Novel GPCRs and their endogenous ligands: expanding the boundaries of physiology and pharmacology. Trends Pharmacol Sci. 1999;20:370–375. doi: 10.1016/s0165-6147(99)01366-8. [DOI] [PubMed] [Google Scholar]
- Marsh DJ, Weingarth DT, Novi DE, Chen HY, Trumbauer ME, Chen AS, et al. Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc Natl Acad Sci USA. 2002;99:3240–3245. doi: 10.1073/pnas.052706899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masu Y, Nakayama K, Tamaki H, Harada Y, Kuno M, Nakanishi S. cDNA cloning of bovine substance-K receptor through oocyte expression system. Nature. 1987;329:836–838. doi: 10.1038/329836a0. [DOI] [PubMed] [Google Scholar]
- McBriar MD. Recent advances in the discovery of melanin-concentrating hormone receptor antagonists. Curr Opin Drug Discov Devel. 2006;9:496–508. [PubMed] [Google Scholar]
- McCallum RW, Cynshi O, US INVESTIGATIVE TEAM Efficacy of mitemcinal, a motilin agonist, on gastrointestinal symptoms in patients with symptoms suggesting diabetic gastropathy: a randomized, multi-center, placebo-controlled trial. Aliment Pharmacol Ther. 2007;26:107–116. doi: 10.1111/j.1365-2036.2007.03346.x. [DOI] [PubMed] [Google Scholar]
- McKee KK, Palyha OC, Feighner SD, Hreniuk DL, Tan CP, Phillips MS, et al. Molecular analysis of rat pituitary and hypothalamic growth hormone secretagogue receptors. Mol Endocrinol. 1997;11:415–423. doi: 10.1210/mend.11.4.9908. [DOI] [PubMed] [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Mieda M, Williams SC, Sinton CM, Richardson JA, Sakurai T, Yanagisawa M. Orexin neurons function in an efferent pathway of a food-entrainable circadian oscillator in eliciting food-anticipatory activity and wakefulness. J Neurosci. 2004;24:10493–10501. doi: 10.1523/JNEUROSCI.3171-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills A, Duggan MJ. Orphan seven transmembrane domain receptors: reversing pharmacology. Trends Biotechnol. 1994;12:47–49. doi: 10.1016/0167-7799(94)90099-X. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Nayak A. A review of montelukast in the treatment of asthma and allergic rhinitis. Expert Opin Pharmacother. 2004;5:679–686. doi: 10.1517/14656566.5.3.679. [DOI] [PubMed] [Google Scholar]
- Offermanns S. The nicotinic acid receptor GPR109A (HM74A or PUMA-G) as a new therapeutic target. Trends Pharmacol Sci. 2006;27:384–390. doi: 10.1016/j.tips.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Higelin J, Henningsen RA, Monsma FJ, Jr, Civelli O. Structures that delineate orphanin FQ and dynorphin A pharmacological selectivities. J Biol Chem. 1998;273:1490–1495. doi: 10.1074/jbc.273.3.1490. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, et al. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Saito Y, Civelli O. G-protein-coupled receptor deorphanizations. Int Rev Neurobiol. 2005;65:179–209. doi: 10.1016/S0074-7742(04)65007-0. [DOI] [PubMed] [Google Scholar]
- Saito Y, Nothacker HP, Wang Z, Lin SH, Leslie F, Civelli O. Molecular characterization of the melanin-concentrating-hormone receptor. Nature. 1999;400:265–269. doi: 10.1038/22321. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Baskin DG, Bukowski TR, Kuijper JL, Foster D, Lasser G, et al. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 1996;45:531–535. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- Segal-Lieberman G, Bradley RL, Kokkotou E, Carlson M, Trombly DJ, Wang X, et al. Melanin-concentrating hormone is a critical mediator of the leptin-deficient phenotype. Proc Natl Acad Sci USA. 2003;100:10085–10090. doi: 10.1073/pnas.1633636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoane LM, Al-Massadi O, Pazos Y, Pagotto U, Casanueva FF. Central obestatin administration does not modify either spontaneous or ghrelin-induced food intake in rats. J Endocrinol Invest. 2006;29:RC13–RC15. doi: 10.1007/BF03344174. [DOI] [PubMed] [Google Scholar]
- Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- Shimazaki T, Yoshimizu T, Chaki S. Melanin-concentrating hormone MCH1 receptor antagonists: a potential new approach to the treatment of depression and anxiety disorders. CNS Drugs. 2006;20:801–811. doi: 10.2165/00023210-200620100-00002. [DOI] [PubMed] [Google Scholar]
- Shimomura Y, Mori M, Sugo T, Ishibashi Y, Abe M, Kurokawa T, et al. Isolation and identification of melanin-concentrating hormone as the endogenous ligand of the SLC-1 receptor. Biochem Biophys Res Commun. 1999;261:622–626. doi: 10.1006/bbrc.1999.1104. [DOI] [PubMed] [Google Scholar]
- Shoblock JR. The pharmacology of Ro 64-6198, a systemically active, nonpeptide NOP receptor (opiate receptor-like 1, ORL-1) agonist with diverse preclinical therapeutic activity. CNS Drug Rev. 2007;13:107–136. doi: 10.1111/j.1527-3458.2007.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song F, Lu S, Gunnet J, Xu JZ, Wines P, Proost J, et al. Synthesis and biological evaluation of 3-aryl-3-(4-phenoxy)-propionic acid as a novel series of G protein-coupled receptor 40 agonists. J Med Chem. 2007;50:2807–2817. doi: 10.1021/jm070130j. [DOI] [PubMed] [Google Scholar]
- Stephens TW, Basinski M, Bristow PK, Bue-Valleskey JM, Burgett SG, Craft L, et al. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature. 1995;377:530–532. doi: 10.1038/377530a0. [DOI] [PubMed] [Google Scholar]
- Tadayyon M, Welters HJ, Haynes AC, Cluderay JE, Hervieu G. Expression of melanin-concentrating hormone receptors in insulin-producing cells: MCH stimulates insulin release in RINm5F and CRI-G1 cell-lines. Biochem Biophys Res Commun. 2000;275:709–712. doi: 10.1006/bbrc.2000.3357. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Giezen JJ, Humphries RG. Preclinical and clinical studies with selective reversible direct P2Y12 antagonists. Semin Thromb Hemost. 2005;31:195–204. doi: 10.1055/s-2005-869525. [DOI] [PubMed] [Google Scholar]
- Vassilatis DK, Hohmann JG, Zeng H, Li F, Ranchalis JE, Mortrud MT, et al. The G protein-coupled receptor repertoires of human and mouse. Proc Natl Acad Sci USA. 2003;100:4903–4908. doi: 10.1073/pnas.0230374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429–458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- Wise A, Jupe SC, Rees S. The identification of ligands at orphan G-protein coupled receptors. Annu Rev Pharmacol Toxicol. 2004;44:43–66. doi: 10.1146/annurev.pharmtox.44.101802.121419. [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50:2540–2547. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- Yamamoto D, Ikeshita N, Daito R, Herningtyas EH, Toda K, Takahashi K, et al. Neither intravenous nor intracerebroventricular administration of obestatin affects the secretion of GH, PRL, TSH and ACTH in rats. Regul Pept. 2007;138:141–144. doi: 10.1016/j.regpep.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- Zhang JV, Ren PG, Avsian-Kretchmer O, Luo CW, Rauch R, Klein C, et al. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin's effects on food intake. Science. 2005;310:996–999. doi: 10.1126/science.1117255. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Morrison C, Banfield BW, Randall JA, Browning KN, et al. Melanin concentrating hormone innervation of caudal brainstem areas involved in gastrointestinal functions and energy balance. Neuroscience. 2005;135:611–625. doi: 10.1016/j.neuroscience.2005.06.055. [DOI] [PubMed] [Google Scholar]