Abstract
The effects of nicotine on dopamine transmission from mesostriatal dopamine neurons are central to its reinforcing properties. Only recently however, has the influence of presynaptic nicotinic receptors (nAChRs) on dopaminergic axon terminals within striatum begun to be understood. Here, rather than simply enhancing (or inhibiting) dopamine release, nAChRs perform the role of a presynaptic filter, whose influence on dopamine release probability depends on presynaptic activity in dopaminergic as well as cholinergic neurons. Both mesostriatal dopaminergic neurons and striatal cholinergic interneurons play key roles in motivational and sensorimotor processing by the basal ganglia. Moreover, it appears that the striatal influence of dopamine and ACh cannot be fully appreciated without an understanding of their reciprocal interactions. We will review the powerful filtering by nAChRs of striatal dopamine release and discuss its dependence on activity in dopaminergic and cholinergic neurons. We will also review how nicotine, acting via nAChR desensitization, promotes the sensitivity of dopamine synapses to activity. This filtering action might provide a mechanism through which nicotine promotes how burst activity in dopamine neurons facilitates goal-directed behaviour and reinforcement processing. More generally, it indicates that we should not restrict our view of presynaptic nAChRs to simply enhancing neurotransmitter release. We will also summarize current understanding of the forms and functions of the diverse nAChRs purported to exist on dopaminergic axons. A greater understanding of nAChR form and function is imperative to guide the design of ligands with subtype-selective efficacy for improved therapeutic interventions in nicotine addiction as well as Parkinson's disease.
Keywords: acetylcholine, nicotine, α6-containing nicotinic receptor, β2-containing nicotinic receptor, dopamine neuron, burst firing, striatum, cholinergic interneuron, Parkinson's disease, smoking
Introduction: nicotinic acetylcholine receptors and the striatum
Nicotinic acetylcholine receptors (nAChRs) are important modulators of neuronal excitability throughout the central nervous system (CNS). The most widely observed role of nAChRs in the CNS is to influence the release of neurotransmitters through presynaptically located ‘heteroreceptors'. Presynaptic nAChRs have been found to modify the release of nearly every neurotransmitter examined through mechanisms that include modifying preterminal membrane excitability, transmembrane Ca2+ permeability or intracellular Ca2+ signalling, as reviewed elsewhere (Dani, 2001; Dajas-Bailador and Wonnacott, 2004; Dani and Bertrand, 2007). nAChRs can also participate in fast postsynaptic neurotransmission as a small excitatory input in several areas including hippocampus, and subcortical areas including ventral tegmental area (Dani, 2001; Mansvelder et al., 2002; Mameli-Engvall et al., 2006). However, this role is far from equivalent to the role of nAChRs in fast excitatory synaptic neurotransmission outside the CNS, for example, as primary mediators of postjunctional excitability in ganglia and neuromuscular junctions, and is no match within the CNS for the postsynaptic effects of excitatory glutamatergic transmission. By contrast, the modulation of neurotransmitter release by presynaptically located nAChRs is among the most powerful yet seen for any receptor family, and it is this presynaptic role for nAChRs that we will discuss further in this review.
Neuronal nAChRs belong to a superfamily of ligand-gated ion channels, which include γ-aminobutyric acid (GABA; A and C), serotonin and glycine receptors, and have cation-selective permeability through receptor-channel complexes formed by pentameric oligomers (Sargent, 1993; McGehee and Role, 1995; Karlin, 2002; Dani and Bertrand, 2007). In the CNS, nAChRs are formed from a portfolio of some 12 different α- and β-subunits (α2–α10 and β2–β4) (Corringer et al., 2000; Le et al., 2002). The association of different subunits can confer distinct structural and functional properties to the resultant nAChR, including different agonist affinity, and kinetics of activation, closure, desensitization, resensitization and even internalization (Ramirez-Latorre et al., 1996; Chavez-Noriega et al., 1997; Fenster et al., 1997; Quick and Lester, 2002; Giniatullin et al., 2005; Nashmi and Lester, 2006). The potential neuron-specific expression of the diverse array of possible conformations of nAChRs could offer varied and discrete neuromodulatory roles to nAChRs in specific neurons. However, the discrete function(s) that might be offered by the expression of any one of the heteromeric nAChR subtypes in situ remain largely unresolved. Moreover, in most neurons, there is coexpression of multiple diverse nAChR subtypes (Gotti et al., 2006), which could therefore have complex outcomes on neurotransmitter release probability. It remains a key challenge to identify the form and function of nAChR types differentially expressed in each brain region and neuron type. If this challenge can be met, we could gain valuable insights into the underlying computational function afforded by presynaptic nAChRs, and moreover, into which of these receptors might be affected or lend itself to a target for drug design in human neurological or neuropsychiatric disorders (Sher et al., 2004). Indeed, nicotinic ligands have already been suggested to hold promise for pharmacological intervention in a large range of disorders, including Parkinson's disease, Alzheimer's disease, epilepsy, schizophrenia and nicotine addiction (Dani, 2001; Quik and McIntosh, 2006; Dani and Bertrand, 2007). Significant progress has been made in identifying the nAChRs expressed by dopamine neurons and in dopamine axon terminal fields within striatum as will be reviewed here; intriguingly however, the details of their influence on dopamine function remains poorly defined.
The influence of presynaptic nAChRs on dopamine neurotransmission in the striatum is particularly important as a model system for nAChR function for several reasons. Anatomically, the striatum is a large subcortical structure that contains the densest innervation of dopaminergic and cholinergic axons seen anywhere in the brain (Bjorklund and Lindvall, 1984; Butcher and Woolf, 1984; Parent et al., 2000; Zhou et al., 2002). Functionally, both dopaminergic and cholinergic systems are central to the role of the striatum, which, as the main input nucleus to the basal ganglia, is central to the processing of motivational and sensorimotor information and to the dysfunction seen in Parkinson's disease and drug addiction. Moreover, dopaminergic axons contain a large diversity of subtypes of heteromeric nAChRs (see later for review of up to seven proposed types), that as a population permits striatal ACh to operate a sophisticated and powerful neuromodulatory control over the release of striatal dopamine (Zhou et al., 2001; Rice and Cragg, 2004; Zhang and Sulzer, 2004). In overview, nAChRs do not simply facilitate presynaptic excitability and/or neurotransmitter release, but rather more dynamically, they act in a capacity as a presynaptic filter to differentially govern how variable activity in dopamine neurons is reported to the striatum by the release of dopamine. More intriguing yet, the cholinergic interneurons that supply the acetylcholine (ACh) tone at striatal nAChRs themselves undergo phasic changes in activity that are time-locked to the phasic changes in dopamine neuron activity. Without appreciating the concurrent activity in dopaminergic and striatal cholinergic neurons, the consequences on striatal signal integration of neuronal activity in either population alone can only be poorly calculated. Thus, the striatum provides an outstanding example of how we should not simply consider the actions of presynaptic nAChRs to be tonic enablers of neurotransmitter release. Both the outcome of nAChR activity and the underlying nAChR activity itself are highly dynamic. These insights require us to rethink our understanding of the scope of the role of presynaptic nAChRs.
Here, we will review our current understanding of the presynaptic nAChR control of dopamine neurotransmission in the striatum. We will discuss the dynamic dependence of nAChR control on the state of presynaptic activity in both the output (dopamine) and input (ACh) synapses. We will also review how the actions of nicotine on striatal dopamine neurotransmission contribute a link and not a contradiction between nicotine-induced nAChR desensitization and postulated mechanisms of drug reinforcement. In addition, we will discuss the known diversity and function of nAChR subunits and pentamers within dopaminergic axon terminal fields. Finally, we will assess whether the field is yet ripe for therapeutic exploitation and conclude with some of the many outstanding questions in nAChR research.
Dopamine and acetylcholine systems in the striatum
The dorsal striatum and ventral striatum collectively participate in a large range of motivational, associative- and sensorimotor-related brain functions (for example, Albin et al., 1989; Haber et al., 2000; Gerdeman et al., 2003; Voorn et al., 2004; Everitt and Robbins, 2005; and Schultz, 2006), among which are the following commonly attributed (and not necessarily mutually exclusive) processes: natural reinforcement, drug reinforcement, conditioned reinforcement, reward prediction, reward-prediction errors, appetitive behaviour, motivational values, stimulus significance, motor response selection, procedural or stimulus–response learning, habit formation and task set-shifting. Dopamine inputs to striatum arise from midbrain dopamine neurons located in the ventral tegmental area (VTA) and substantia nigra pars compacta (SNc), which project in a topographic pattern to differentially innervate the ventral striatum (nucleus accumbens (NAc)) and dorsal striatum (caudate–putamen (CPu)), respectively (Bjorklund and Lindvall, 1984; Gerfen et al., 1987; McFarland and Haber, 2000; Voorn et al., 2004). The dopaminergic innervation to striatum forms large, highly branched arbours with a high density of axonal varicosities (Pickel et al., 1981; Bouyer et al., 1984; Doucet et al., 1986; Sesack et al., 1994; Descarries et al., 1996; Descarries and Mechawar, 2000) and forms dopaminergic synapses at an incidence estimated at 1 in every 10–20 μm3 in the rat (Descarries et al., 1996; Arbuthnott and Wickens, 2007). Although dopamine axons can form synapses, dopamine receptors and the dopamine uptake transporter are found extrasynaptically (Nirenberg et al., 1996, 1997; Pickel, 2000), and dopamine can spill over from sites of release to mediate extrasynaptic or ‘volume' transmission (Fuxe and Agnati, 1991; Garris et al., 1994; Gonon, 1997; Cragg and Rice, 2004). The location of dopamine synapses on the necks of spines on GABAergic medium spiny projection neurons (MSNs) adjacent to corticostriatal glutamatergic input (Freund et al., 1984; Smith and Bolam, 1990; Groves et al., 1994), and the presence of D1-like and/or D2-like dopamine receptors on MSNs, striatal interneurons as well as dopamine axons in striatum (Gerfen, 1992; Sesack et al., 1994; Hersch et al., 1995; Surmeier et al., 1996; Alcantara et al., 2003), ensures dopamine is well placed to modulate striatal function.
The cholinergic input to the striatum arises solely from cholinergic striatal interneurons (Woolf, 1991; Contant et al., 1996; Calabresi et al., 2000). Like the mesostriatal dopamine neurons, striatal ACh interneurons (or ‘tonically active neurons', TANs) (Wilson et al., 1990; Aosaki et al., 1995; Bennett and Wilson, 1999; Zhou et al., 2002) appear pivotal for signalling unexpected primary rewards as well as the learning and signalling of environmental cues that predict reward (or more generally, events of high salience) (Calabresi et al., 2000; Schultz, 2002; Berridge and Robinson, 2003; Centonze et al., 2003; Wickens et al., 2003; Wise, 2004). These large, aspiny neurons form a small fraction (∼2–5%) of the total number of neurons in the neostriatum (Oorschot, 1996; Descarries and Mechawar, 2000; Zhou et al., 2002), but they provide an extensive axonal arborization within dorsal and ventral striatum reminiscent of dopaminergic arbours (Bolam et al., 1984; Graybiel et al., 1986; Phelps and Vaughn, 1986; Zahm and Brog, 1992; Contant et al., 1996; Holt et al., 1997; Calabresi et al., 2000; Descarries and Mechawar, 2000; Zhou et al., 2001, 2002, 2003). The density of ACh varicosities is estimated as similar to dopamine varicosities (Descarries and Mechawar, 2000), and both dopaminergic and ACh arbours are denser in the striatum than elsewhere in the brain. Furthermore, like dopaminergic neurons, striatal cholinergic interneurons form synapses primarily onto distal dendrite shafts and spine necks (Bolam et al., 1984; Phelps et al., 1985). Interestingly, the majority of cholinergic varicosities (>90%) may not form synapses (Descarries et al., 1997; Descarries and Mechawar, 2000), indicating that ACh like DA may also influence striatal function primarily via extrasynaptic, ‘volume' transmission (Descarries et al., 1997).
Cholinergic receptors in the striatum are of both the metabotropic muscarinic (muscarinic acetylcholine receptor (mAChR)) and ionotropic nicotinic (nAChR) families. Muscarinic receptor types, M1, M2 and M4, appear to be the dominant muscarinic striatal subtypes (Zhang et al., 2002; Zhou et al., 2003), with evidence for modulation of excitability of striatal neurons that include MSNs (Calabresi et al., 2000), corticostriatal terminals (Malenka and Kocsis, 1988; Pakhotin and Bracci, 2007; Surmeier et al., 2007), GABAergic and cholinergic interneuron outputs (Koos and Tepper, 2002; Zhang et al., 2002), and cholinergic interneurons (Yan and Surmeier, 1996; Bernard et al., 1998; Calabresi et al., 1998). There is no convincing evidence that mAChRs are present on dopamine axon terminals to modulate dopamine release, although indirect striatal circuits may play a role in mAChR-mediated ACh-dopamine interactions (Zhang et al., 2002; Zhou et al., 2003). By contrast, the role of nicotinic AChRs appears less widespread across neuron types within striatum generally; there is some evidence for nAChR modulation of GABAergic interneurons (Koos and Tepper, 2002). Most strikingly however, nAChRs located on dopaminergic axon terminals (Jones et al., 2001) play a major role in the modulation of striatal dopamine release by endogenous ACh (Zhou et al., 2001; Rice and Cragg, 2004; Zhang and Sulzer, 2004).
Acetylcholine-dopamine crosstalk: antagonistic or cooperative?
Apart from their separate actions within striatum, interactions between dopamine and ACh are fundamental to the operation of the striatum. There is a long-standing hypothesis of an antagonistic balance between dopamine and ACh (for reviews see Calabresi et al., 2000; Zhou et al., 2002; Pisani et al., 2003; Centonze et al., 2003). The ACh/dopamine balance hypothesis arose from the alleviation of the debilitating motor dysfunctions of Parkinson's disease by pro-dopaminergic treatments on the one hand, and anticholinergic on the other (Barbeau, 1962; Pisani et al., 2003). This hypothesis is borne out to some extent on the postsynaptic cellular level: dopamine and ACh can have opposing effects on acute excitability of striatal output neurons as well as on long-term corticostriatal plasticity (Calabresi et al., 1998, 2000, 2007; Kerr and Wickens, 2001; Reynolds et al., 2001; Reynolds and Wickens, 2002; Centonze et al., 2003; Pisani et al., 2003; Zhou et al., 2003; Morris et al., 2004). Note that this long-term plasticity in corticostriatal synaptic efficacy is thought to represent reward-related learning (including the acquisition of incentive value by previously neutral stimuli, learning of stimulus–response associations, and development of habits including addiction at the synaptic level) (Reynolds et al., 2001; Reynolds and Wickens, 2002; Gerdeman et al., 2003; Robinson and Berridge, 2003; Robinson and Kolb, 2004) and that this learning subsequently governs the likelihood that reward-related signals are translated into contextually appropriate behavioural responses (reviewed elsewhere, for example, Wickens et al., 2003; Pisani et al., 2005).
This antagonism is less simple on a presynaptic level however. ACh and dopamine interact directly at a presynaptic level. Dopamine can either limit or promote ACh release: local and systemic striatal D1 agonists can enhance striatal ACh release in vivo, while D2 agonists reduce (DeBoer and Abercrombie, 1996; Ikarashi et al., 1997). The availability of dopamine in the striatum appears, however, to promote the long-term acquisition of conditioned inhibition or ‘pauses' in ACh interneuron activity (Maurice et al., 2004; Reynolds et al., 2004) that are thought to signal reward-related information (see later section). Moreover, as we will see, ACh acting at nAChRs can either enhance or inhibit dopamine release within striatum.
Key to the debate of how ACh and dopamine interact is how this interaction functions during the context of physiologically relevant and highly dynamic neuron activity. Clearly, dopamine and ACh may act in opposition (see above). Yet, these systems do not necessarily oppose each other during the context of physiologically relevant changes in dopamine and ACh neurons; the balance of their actions in a physiological context will depend on the dynamic changes in neuron activity and the consequent availability of each neurotransmitter (Cragg, 2006). Importantly, there are coincident changes in the physiological activity of dopaminergic and cholinergic neurons, which together with their consequences for ACh-dopamine interactions indicate a powerful functional cooperativity between dopaminergic and cholinergic systems. It is important that we first consider the underlying physiology in dopamine and ACh neurons before we can begin to appreciate the presynaptic function of striatal nAChRs.
Coincident activity in acetylcholine and dopamine neurons
Both dopamine neurons and striatal cholinergic interneurons participate in reward-related signalling and reinforcement learning by carrying information about the predicted availability, or errors in the prediction as well as the receipt, of primary rewards (Aosaki et al., 1994; Knowlton et al., 1996; Jog et al., 1999; Matsumoto et al., 1999; Reynolds and Wickens, 2000, 2002; Reynolds et al., 2001; Packard and Knowlton, 2002; Schultz, 2002, 2007; Centonze et al., 2003; Gerdeman et al., 2003; Schultz et al., 2003; Wickens et al., 2003). These systems do not by contrast signal the hedonic value of rewards (see Berridge and Robinson, 1998, 2003; Schultz, 2002; Pecina et al., 2003; Robinson et al., 2005), which may be processed by other regions, for example, human orbitofrontal cortex (Kringelbach, 2005). Rather, the reward-related functions of DA and ACh neurons are hypothesized to inform the corticostriatal system of the discrepancy between the prediction of a reward and its actual occurrence. In simple form, if the reward obtained after an action or environmental cue is fully predicted, then the reward prediction error signalled by these neurons at the time of the reward is zero; if however, the reward is unpredicted or greater (or less) than predicted, these neurons signal a positive (or negative) prediction error. These signals can then be used to teach or update the value of that predictor (action or cue), and thus, the likelihood that the action will be repeated or the cue attended to in the future. This underlies reinforcement learning, which ultimately allows an animal to respond or perform optimally in the same environment in the future.
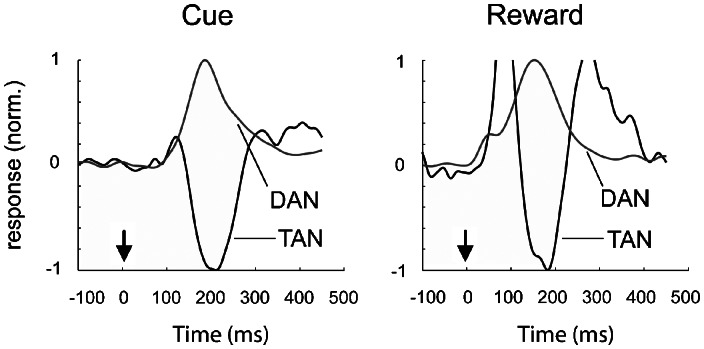
The characteristic responses of dopamine neurons and striatal ACh neurons to such unexpected primary rewards and conditioned reward-predicting cues in the environment can be summarized as follows. Mesostriatal dopamine neurons signal these events by a switch from tonic firing rates (typically 2–5 Hz) to either a phasic increase (burst, 15–100 Hz) or a decrease (pause) according to the presentation or omission, respectively, of a reward (Hyland et al., 2002; Schultz, 2002; Fiorillo et al., 2003; Tobler et al., 2003, 2005; Morris et al., 2004; Bayer and Glimcher, 2005). The responses of striatal cholinergic interneurons (TANs) in contrast, usually (but see Ravel et al., 2001; Yamada et al., 2004) involves a brief pause in tonic rates of activity following reward-related events whether ‘rewarding' or ‘punishing' (Aosaki et al., 1994; Shimo and Hikosaka, 2001; Morris et al., 2004; Apicella, 2007). Moreover, these transient reward-related responses of dopamine and ACh neurons appear time-locked with each other: when recorded in the same task, reward-related bursts in dopamine neurons occur simultaneously with pauses in ACh neurons (similar latency and duration, approximately 100–200 ms) (Figure 1) (Morris et al., 2004). The coincident timing of these events points to a dynamism in the interaction of dopamine and ACh in striatum. Notably, it suggests that if we are to appreciate the function of presynaptic nAChRs in striatum, we must consider highly variable changes in dopamine neuron activity and the coincident reduction in ACh tone that will result from a pause in ACh neuron activity. In other words, we should not confine our understanding of nAChR function to any single effect of ACh; rather, we should consider a range of possible effects of ACh across the range of firing frequencies observed in dopamine neurons, and furthermore, understand the outcome of a loss of resting ACh tone.
Figure 1.
Synchronization of dopaminergic and cholinergic neuron responses to behavioural events. Mean responses of population of dopamine neurons (DAN, grey) and cholinergic interneurons (tonically active neuron (TAN), black) to a visual cue (left) and reward (right) presented to Macaque monkeys in an instrumental conditioning task at t=0 (arrow). The increase in dopamine neurons firing coincides with the same latency and duration as the pause in tonically active cholinergic interneuron firing. Population responses are baseline-subtracted, averaged for all probabilities in the experiment, and normalized to the peak of each response (for DANs) or the maximum trough (for TANs). The different scales in each panel reveal different degrees of changes for positive and negative errors (see text for discussion of negative prediction errors). Figure taken from (Cragg, 2006) and originally adapted from (Morris et al., 2004) with permission.
Presynaptic nAChRs filter the dynamic probability of dopamine release according to dopamine neuron activity
How a given pattern and frequency of incoming dopamine neuron activity is relayed into striatal dopamine release transients will depend on the processing of dopamine neuron activity at the release site. Dopamine release does not depend linearly on the frequency of activity in dopamine neurons (Chergui et al., 1994) owing to use-dependent short-term changes, or ‘plasticity', in dopamine release probability (Cragg, 2003; Montague et al., 2004) as well as neuromodulation by dopamine and ACh transmitters acting at auto- and heteroreceptors on dopaminergic axons (Kennedy et al., 1992; Benoit-Marand et al., 2001; Schmitz et al., 2002, 2003; Rice and Cragg, 2004; Zhang and Sulzer, 2004).
Ligands for nAChRs (including nicotine) have long been known to influence striatal dopamine release (Di Chiara and Imperato, 1988; Dajas-Bailador and Wonnacott, 2004). Importantly, we now appreciate that the control of dopamine release probability by presynaptic nAChRs and endogenous ACh in striatum is dynamic and multifaceted (Zhou et al., 2001; Rice and Cragg, 2004; Zhang and Sulzer, 2004). Typically, dopamine release in striatum is accompanied by a use-dependent, short-term depression of release probability at rapidly successive pulses (Abeliovich et al., 2000; Cragg, 2003; Montague et al., 2004). Studies in striatal slices, using fast (subsecond) voltammetric detection of dopamine, indicate that the ACh released from spontaneously active striatal cholinergic interneurons (Bennett and Wilson, 1999; Zhou et al., 2001, 2003) acts at β2-subunit-containing (β2*)-nAChRs on striatal dopamine axon terminals to maintain a high probability of dopamine release evoked by single action potentials (Zhou et al., 2001; Rice and Cragg, 2004). ACh thus contributes to consequent short-term depression. A reduction in ACh action at nAChRs, for example, using competitive antagonists, reduces initial dopamine release probability (Zhou et al., 2001; Rice and Cragg, 2004), and as a direct consequence, short-term depression becomes relieved (Cragg, 2003; Rice and Cragg, 2004). Thus, inhibition of nAChRs can suppress release by single stimuli but correspondingly facilitate release by a burst (Figure 2) (Rice and Cragg, 2004).
Figure 2.
Cartoon of outcome of nicotinic acetylcholine receptors (nAChRs) active and inactive on dopamine transients released by burst-like and nonburst activity in dopamine neurons. Left, tonic acetylcholine (ACh) tone at nAChRs on dopaminergic axon terminals. Right, nAChR tone switched off (or reduced) by either a pause in ACh interneuron activity, nAChR desensitization by nicotine or block by nAChR antagonists.
Moreover, this reorganization of dopamine release probabilities by nAChR inhibition depends on the frequency of dopamine neuron activity (Rice and Cragg, 2004; Zhang and Sulzer, 2004): the shorter the interpulse interval, that is, the higher the dopamine neuron frequency, the greater the relief from short-term depression. In other words, antagonism of nAChR activity reduces initial dopamine release probability but in turn permits a high-frequency pass filtering that facilitates burst release. Consequently, reduced nAChR activity can (1) enhance dopamine signals by dopamine neuron bursts at high frequencies that is those that accompany the presentation of rewards or conditioned reward-predicting stimuli (20–100 Hz), while it can also (2) diminish further the reduced dopamine signals accompanying reductions in dopamine neuron activity, as occurs at times of omission of expected rewards (Rice and Cragg, 2004; Cragg, 2006). Thus, a major impact of this filtering will be an increase in contrast in dopamine signals when dopaminergic neurons switch from tonic activity to either a high frequency, reward-related burst or a pause (Cragg, 2006).
Turning the nAChR filter on and off: a pause in TANs and ACh tone?
These data suggest that a reduction in endogenous ACh tone at striatal nAChRs following a TAN pause would similarly enhance the contrast in dopamine signalling when dopamine neurons concurrently change firing rate (Figure 2). A pause response of the TANs to reward presentation- and omission-related events could amplify both of the bipolar effects of these cues on dopamine neuron activity (bursts and pauses) (Cragg, 2006). These findings suggest that synchronization of phasic changes in activity in dopamine and acetylcholine neurons could serve to promote the sensitivity of dopamine synapses to dopamine neuron activity and enhance the contrast between dopamine transients released by different activities, bursts or pauses (Cragg, 2006). These data illustrate the importance of considering the dynamic activity not only in the dopamine neurons, but also in the TANs when considering the function of presynaptic nAChRs on dopamine signalling. The function of presynaptic nAChRs on striatal dopamine transmission (for example, during reward-related tasks) might be completely different in the presence of tonic TAN activity (and nAChR tone) versus a concomitant TAN pause.
Turning the nAChR filter on and off: the effects of nicotine via nAChR desensitization
Nicotine has long been known to enhance the net release of dopamine in vivo measured over a timescale of minutes with microdialysis after system injection (Di Chiara and Imperato, 1988; Corrigall et al., 1992, 1994; Nisell et al., 1994b), and nicotine also acts as a secretagogue at high concentrations (μM) to evoke release of preloaded [3H]-dopamine from striatal synaptosomes (Kulak et al., 1997; Kaiser et al., 1998; Grady et al., 2002; Dajas-Bailador and Wonnacott, 2004). But until recently, much less has been known about how nicotine governs the subsecond release of endogenous striatal dopamine by dynamic patterns of physiological action potentials and where endogenous striatal ACh tone is intact. The effects of nicotine on these dynamic properties of dopamine release at concentrations of nicotine seen in smokers have recently been identified, and have simultaneously offered insights into several aspects of nicotine's properties ranging molecular through to systemic properties, three of which (1–3) will be discussed here.
1. Desensitization versus agonism?
Nicotinic AChRs are notoriously susceptible to desensitization, a reversible or temporary inactivation of response, following sustained administration of nicotinic receptor ligands including nicotine (Pidoplichko et al., 1997; Zhou et al., 2001; Mansvelder et al., 2002; Quick and Lester, 2002; Wooltorton et al., 2003; Giniatullin et al., 2005). The molecular mechanisms underlying nAChR desensitization remain incompletely resolved; candidate mechanisms and their role in modulating receptor function generally are reviewed elsewhere (Quick and Lester, 2002; Giniatullin et al., 2005).
Nicotine, when applied to the brain at concentrations seen in smokers, readily desensitizes β2*-nAChRs in the VTA and in the striatum (Pidoplichko et al., 1997; Zhou et al., 2001; Mansvelder et al., 2002). Note that although α7-receptors are known to have the fastest onset of desensitization (at saturating doses of agonist) of any nAChR, the low, smoking-related concentrations of nicotine preferentially desensitize the higher affinity β2*-nAChRs (see Quick and Lester, 2002). It is this nAChR desensitization rather than simple receptor activation that appears critical to the outcome on dopamine neuron excitability and ultimately, on presynaptic nAChR control of dopamine release in striatum. In the VTA, a greater susceptibility to desensitization of β2*- versus α7*-nAChRs reduces direct cholinergic excitation of dopamine neurons as well as GABAergic inhibition to dopamine neurons but appears to leave relatively intact the α7*-nAChR activation of glutamatergic input to dopamine neurons (Mansvelder et al., 2002). The net effect of sustained nicotine in VTA is therefore an enhanced excitability of dopamine neurons (Mansvelder and McGehee, 2002; Mansvelder et al., 2002). In the striatum however, where action potential-evoked dopamine release is under the control of β2*- but not α7*-nAChRs (Zhou et al., 2001; Rice and Cragg, 2004; Zhang and Sulzer, 2004), nicotine-induced desensitization of tonically active striatal nAChRs, reduces initial dopamine release probability, and consequently, facilitates release by bursts in the same manner as nAChR antagonists (Zhou et al., 2001; Rice and Cragg, 2004; Zhang and Sulzer, 2004). Thus, nicotine dramatically enhances how dopamine is released by reward-related burst activity compared to non-reward-related tonic activity (Figure 2).
Thus, desensitization of nAChRs plays an essential facilitatory role in both the VTA and directly within the striatum in the ability of nicotine to enhance striatal dopamine neurotransmission. We should not limit our appreciation of the action of nicotine on the brain to its effects at nAChRs as a simple agonist. Rather, loss of function of striatal nAChRs can be a gain of function to dopamine transmission.
2. VTA versus striatum?
The desensitization of nAChRs by nicotine in striatum also sheds light on why the striatum was long overlooked as a site of action of nicotine; the long-held notion was that the VTA but not the striatum was important for nicotine's effects on striatal dopamine transmission. For example, previous experiments identified that local administration of nAChR blockers into the VTA but not striatum prevents the self-administration of systemic nicotine (Corrigall et al., 1994) or prevent increases in accumbal dopamine measured with microdialysis following systemic nicotine injection (Nisell et al., 1994b). Furthermore, nicotine infused continuously into the VTA but not striatum has been reported to sustain striatal dopamine release (measured by microdialysis) (Nisell et al., 1994a). Recent insights into nicotine's desensitizing action within striatum now reveal several possible alternative explanations for why the effects of nicotine in the striatum may previously have been underestimated. For example, since nicotine's effects within striatum appear to involve switching nAChRs off, nAChR antagonists in striatum are unlikely to reverse nicotine's effects, but might in fact substitute for nicotine. In contrast in the VTA, where the effects of nicotine require both desensitization and agonism (Mansvelder et al., 2002), nAChR antagonists would be expected to obscure nicotine actions. Furthermore, since nicotine's effects in striatum involve enhancing signal contrast on a subsecond basis by both reducing and enhancing striatal dopamine release depending on dopamine neuron (and TAN) firing rate, it is possible that the net effect of nicotine in striatum might be an undetectable net zero change when measured over a long microdialysis sample period. In addition, nicotine's striatal effects also depend on changes in underlying dopamine neuron firing rate, for example, changes that accompany nicotine reaching the VTA, and may not in contrast be exposed in vivo by striatal administration only. The striatal mechanisms reviewed here are distinct from (but additive with) those actions of nicotine on dopamine neuron excitability at the somatodendritic level. Together, axonal effects in combination with somatodendritic effects (Grenhoff et al., 1986; Pidoplichko et al., 1997; Mansvelder et al., 2002; Champtiaux et al., 2003; Maskos et al., 2005; Mameli-Engvall et al., 2006) will provide a two-step amplification by nicotine of dopamine signalling in striatum. Our improved understanding of the consequences of nicotine in striatum (via nAChR desensitization) on physiological subsecond dopamine signals, now allows us to appreciate the striatum as an important site for nicotine action.
3. Primary reinforcer or enhancer of secondary reinforcement?
The striatal effects of nicotine also offer a neuropharmacological correlate for some of nicotine's less well-understood psychopharmacological properties. A key question in nicotine addiction is to what extent the reinforcing effects of nicotine result from its intrinsic reinforcing properties, that is, as a primary or unconditioned reward, versus its ability to enhance the reinforcing efficacy of other primary and secondary reinforcers (Caggiula et al., 2001; Donny et al., 2003; Olausson et al., 2003). It has been questioned whether the primary, or unconditioned, reinforcing effects of nicotine are sufficient to explain the maintenance of smoking behaviour in humans (Perkins et al., 2003). Rather, sustained nicotine self-administration, in rats as well as in human smokers, may be dependent on associated environmental cues (for example, visual or olfactory), which, through becoming conditioned reinforcers, can take on critical incentive properties (Caggiula et al., 2001). Notably, nicotine can noncontingently enhance the reinforcing efficacy of previously neutral cues (Chaudhri et al., 2003; Donny et al., 2003). A principal means through which novel, behaviourally relevant stimuli and conditioned cues are signalled to the brain is via a switch in dopamine neuron firing activity to a brief high-frequency burst (Schultz, 2002, 2007). Since nicotine via nAChR desensitization in striatum enhances the dopamine signalling by burst versus nonburst activity in dopamine neurons, the pharmacological effects of nicotine in striatum reviewed here may therefore offer a neurochemical correlate for the nicotine enhancement of the reinforcing efficacy of any reward-related stimuli, including non-nicotine conditioned stimuli (for example, predictive visual cues) that support nicotine self-administration (Caggiula et al., 2001; Chaudhri et al., 2003; Donny et al., 2003).
The diversity of nAChR subunits in dopamine neurons
What do we understand of the nAChRs responsible for the control of dopamine neurotransmission by ACh and by nicotine in striatum? Dopamine neurons express a large range of nAChR subunits and several stoichiometric configurations are proposed to exist in dopamine axons and regulate striatal dopamine neurotransmission.
To date, 12 mammalian subunits α2–α10 and β2–β4 have been identified and cloned. These subunits can be organized into subfamilies I–IV, according to their gene sequence and structure (Corringer et al., 2000; Le et al., 2002). Only nAChR subunits from subfamilies II (α7) and III (α2–α6, β2–β4) have been identified in mammalian brain (Corringer et al., 2000; Le et al., 2002). These subunits can form homomeric pentamers (all one type of subunit, for example, α7) or heteromeric pentamers, consisting of combinations of various α- and β-type subunits. Dopamine neurons within rodent SNc and VTA express mRNAs for the nAChR subunits α4, α5, α6, β2 and β3, as well as lower levels of α3 and α7 and lower levels yet of β4 (Azam et al., 2002). This diversity of subunit expression has the potential to give rise to multiple types of pentameric receptors in somatodendritic and axon terminal regions of dopamine neurons with a consequent myriad of corresponding functions.
The nAChRs present in both somatodendritic (VTA/SNc) as well as axonal regions (striatum) are believed to be important to the reinforcing effects of nicotine (Grenhoff et al., 1986; Mansvelder and McGehee, 2002; Mansvelder et al., 2002; Zhou et al., 2003; Rice and Cragg, 2004; Zhang and Sulzer, 2004; Ungless and Cragg, 2006; Mameli-Engvall et al., 2006). However, there are differences in the nAChR subunits present in axon terminals compared to somatodendritic regions. Whereas α7- and β4-subunits are present in VTA/SNc, they are not apparently transported to striatal dopaminergic axon terminals (Champtiaux et al., 2003; Quik et al., 2005). The α7-subunit is, however, expressed within striatum by non-dopaminergic neurons, although this is not discussed here. Furthermore, there is a lack of α3-subunits within the striatum in rodents (although not in primates) (Wonnacott et al., 2000; Zoli et al., 2002; Champtiaux et al., 2003; Gotti et al., 2005; Quik et al., 2005). However, the α4-, α5-, α6-, β2- and β3-subunits (and α3-subunits in primate) are found at high density in dopamine axon terminals where they can assemble as functional receptor channels in the form of heteromers.
As heteromeric receptors, these subunits arrange to form two α/β pairs; the interface of each of these pairs is responsible for providing one of the two ACh-binding sites at each nAChR (Gotti and Clementi, 2004). The two α/β pairs that form the ligand-binding sites in dopamine axons in striatum are suggested to be primarily α4/β2 and/or α6/β2 (and/or α3/β2 in primates) (Luetje, 2004; Salminen et al., 2004; Quik et al., 2005). The fifth subunit in the pentamer may consist of any other subunit, including α5 or β3. Several mechanisms are known to control the stoichiometry of pentameric nAChRs during assembly in transfected cell expression systems: nAChR subunit composition and stoichiometry may be influenced by cellular chaperone proteins and intracellular cAMP levels as well as transfected subunit ratio or exposure to nicotine (Zwart and Vijverberg, 1998; Wanamaker et al., 2003; Kuryatov et al., 2005; Vallejo et al., 2005; Exley et al., 2006). These mechanisms will not be discussed further here. However, while we have some understanding of how these characteristics arise in nAChRs in non-neuronal expression systems in vitro, we are still far from understanding their form and function in neurons in vivo.
What is the stoichiometry and function of striatal nAChRs in dopamine axons?
Our current appreciation of the nAChRs that participate in the regulation of striatal dopamine transmission has arisen from the development of specific ligands, subunit-specific knockout (KO) mice and immunoprecipitation studies. We will review our current understanding of the nAChRs present on dopamine axons that is derived primarily from rodent studies with a few valuable insights from primate studies where available.
The β2-subunit was the first shown to be crucial to the reinforcing properties of nicotine in rats (Picciotto et al., 1998). It was later demonstrated that β2-nAChR subunits are widely expressed presynaptically on striatal dopamine axon terminals (Jones et al., 2001). Subsequent immunoprecipitation studies revealed the dominant presence of the β2-subunit within the striatum: all nAChRs on dopamine axon terminals are thought to contain the β2-subunit (Champtiaux et al., 2003; Salminen et al., 2004). The determination of the stoichiometry of these β2*-nAChRs has since been particularly aided by the development of a ligand, which can differentiate alternate α-subunit-containing nAChRs. Specifically, the antagonist α-conotoxin MII (α-CtxMII) (extracted from Conus snails) is selective for α3/α6-subunit-containing nAChRs (Cartier et al., 1996; Whiteaker et al., 2000; McIntosh et al., 2004). This ligand has been used in studies of nicotine-evoked [3H]-dopamine release from synaptosomes to identify two distinct classes of striatal β2*-nAChRs, α-CtxMII-sensitive (α6/α3,β2*-nAChRs) and α-CtxMII-resistant (non-α6/α3,β2*-nAChRs) (Kulak et al., 1997; Kaiser et al., 1998; Quik et al., 2005). These studies suggest that the α-CtxMII-resistant population of nAChRs (that is, non-α6/α3,β2*) can account for up to 60% of nicotine-evoked dopamine release from rodent striatal synaptosomes (Kulak et al., 1997; Kaiser et al., 1998; Salminen et al., 2007). However, the relative importance of α6* versus non-α6-nAChRs in the regulation of DA release by endogenous ACh varies greatly within different functional subterritories of the striatum (Exley et al., in press).
Immunoprecipitation studies have contributed greatly in defining nAChR subunits which could constitute these different α6- and non-α6,β2*-nAChRs in striatal dopaminergic terminals. These studies have shown that in addition to the β2-subunit, both α4- and α6-subunits are also widely present in striatum. In addition, a small portion of α4-subunits are colocalized with α5-subunits (approximately 11%), whereas α6-subunits do not appear to be colocalized with α5 (Champtiaux et al., 2003). The β3-subunit by contrast, appears always to be colocalized with α6-subunits, and a portion of this population can contain α4-subunits (but see later) (Champtiaux et al., 2003; Cui et al., 2003; Gotti et al., 2005). Together, these data have suggested the presence of α4β2- and α4α5β2-nAChRs (α-CtxMII-resistant) as well as α6β2β3- and α6α4β2β3-nAChRs (α-CtxMII-sensitive) (Zoli et al., 2002; Champtiaux et al., 2003; Cui et al., 2003; Luetje, 2004; Salminen et al., 2004, 2005; Gotti et al., 2005). In the primate striatum, there is an additional α3β2*-nAChR which also binds α-CtxMII (Cartier et al., 1996; Quik et al., 2005).
However, immunoprecipitation data also suggest that these four (or five) arrangements may not be the full complement of native nAChRs in dopaminergic axons. Not all α6-containing receptors appear to also contain α4- and β3-subunits: it has recently been shown that α4- and α4β3-subunit-selective KO mice have the capacity to form functional nAChRs that govern dopamine release from synaptosomes with the stoichiometry α6β2β3 and α6β2 (Salminen et al., 2007). Whether or not these additional receptor types can be formed in wild-type mice, however, is still unresolved. Furthermore, there may be a population of α6-containing α-CtxMII-resistant receptors of the arrangement α6α4β2 (Table 1) (Champtiaux et al., 2003; Salminen et al., 2004; Gotti et al., 2005).
Table 1. nAChRs proposed to be expressed on dopaminergic axon terminals in striatum.
| Species | Region |
Receptor stoichiometry |
||||||
|---|---|---|---|---|---|---|---|---|
| α4β2 | α3β2* | α4α5β2 | α6α4β2 | α6α4β2β3 | α6β2 | α6β2β3 | ||
| Mouse | Striatum | Champtiaux et al. (2003); | NA | Champtiaux et al. (2003); | Champtiaux et al. (2003); | Champtiaux et al. (2003); | Champtiaux et al. (2003); | Champtiaux et al. (2003); |
| Gotti et al. (2005); | Gotti et al. (2005); | Salminen et al. (2004) | Gotti et al. (2005); | Salminen et al. (2004) | Gotti et al. (2005); | |||
| Salminen et al. (2004) | Salminen et al. (2004) | Salminen et al. (2004) | ||||||
| CPu | NA | Cui et al. (2003); | Salminen et al. (2007) | Cui et al. (2003); | ||||
| Salminen et al. (2007) | Salminen et al. (2007) | |||||||
| NAc | NA | Cui et al. (2003); | Salminen et al. (2007) | Cui et al. (2003); | ||||
| Salminen et al. (2007) | Salminen et al. (2007) | |||||||
| Rat | Striatum | Zoli et al. (2002) | NA | Zoli et al. (2002) | Zoli et al. (2002) | Zoli et al. (2002) | Zoli et al. (2002) | Zoli et al. (2002) |
| Primate | Striatum | Quik et al. (2005) | Quik et al. (2005) | Quik et al. (2005) | Quik et al. (2005) | |||
Abbreviations: CPu, caudate–putamen (dorsal striatum); NAc, nucleus accumbens (ventral striatum); nAChR, nicotinic acetylcholine receptor. Due to the detectable expression of α3-subunit only in primate but not rodent striatum, the expression of α3β2*-nAChRs are not applicable (NA) to rodents.
Table of studies citing the presence of each proposed nAChR subtype. Note that studies listed in ‘Striatum' do not distinguish between ventral and dorsal territories of striatum.
In summary, our understanding of the subunit composition of striatal nAChRs has increased significantly in the recent years and immunoprecipitation studies currently support the potential existence in dopamine axons of the following nAChRs: α4β2, α4α5β2, α6α4β2β3, α6β2β3, α6β2 and α6α4β2 (Table 1). Intriguingly however, we may not have yet reached a completely accurate picture. One major limitation of immunoprecipitation studies is a limited capacity of the polyclonal antibodies that are typically used to extract each subunit. This has recently been shown by Gotti et al. (2005), who generated a new antiserum that was raised against mouse β3 rather than to rat β3, with a concomitant greater recovery of mouse β3-subunits from mouse tissue than using rat antibodies (Champtiaux et al., 2003). Previous studies may therefore have underestimated subunit colocalization, and therefore, overestimated the types of nAChRs present. Future immunoprecipitation studies may yet refine our understanding of the stoichiometry of nAChRs in striatal dopaminergic axon terminal fields.
Variations in α4β2 stoichiometry and the roles of the fifth subunit position
While we are making great strides in understanding the nAChR subunits that might coexist as pentamers in striatum, it is also important to consider their pentameric arrangements. For example, not all α4β2-nAChRs are equal. When transfected in vitro, α4β2-nAChRs can form two distinct sensitivities for ACh as well as other ligands (Zwart and Vijverberg, 1998; Houlihan et al., 2001; Nelson et al., 2003; Slater et al., 2003). These are believed to arise from the expression of two different α4β2-nAChR stoichiometries: receptors that are (α4)2(β2)3 have high sensitivity, whereas (α4)3(β2)2 have low sensitivity (Nelson et al., 2003; Moroni and Bermudez, 2006). The identity of the fifth subunit changes the properties of the receptor. It remains unresolved which stoichiometry(s) occurs in striatum. Transfected cell lines by contrast preferentially express the low sensitivity nAChR, that is, (α4)3(β2)2, but interestingly after long-term incubation with nicotine, transfected human embryonic kidney cells can selectively increase the expression of high sensitivity (α4)2(β2)3-nAChRs (Buisson and Bertrand, 2001; Nelson et al., 2003; Kuryatov et al., 2005; Sallette et al., 2005; Vallejo et al., 2005). While two pairs of α/β-subunits form the ligand-binding sites, it appears that the fifth subunit is crucial in acting not only as an ‘accessory' subunit (one playing a role in receptor assembly) but also in determining the sensitivity of the receptor to its ligands (Figure 3) (Zwart and Vijverberg, 1998; Tumkosit et al., 2006). This also occurs for other nAChRs.
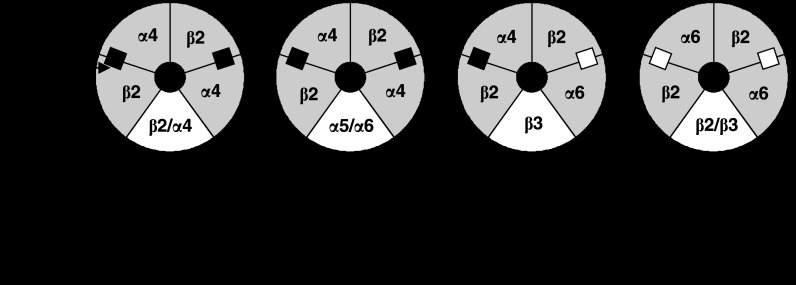
Figure 3.
Nicotinic acetylcholine receptor (nAChR) stoichiometry, α4/β2-binding pairs and the role of the fifth subunit. Both α4/β2 and α6/β2 pairs form ligand-binding domains. For α-conotoxin MII (α-CtxMII)-resistant receptors, the fifth subunit can modulate nAChR ligand affinity. Sensitivity to α-CtxMII is conferred at the α6/β2 domain only and will result from block of either one or two acetylcholine (ACh)-binding sites available to activate the channel. Fifth subunits in α-CtxMII-sensitive receptors may be accessories or affinity modulators. Binding sites for ACh are α-CtxMII-resistant (filled squares) or α-CtxMII-sensitive (unfilled squares).
In β3*-nAChRs (α4α6β2β3- and α6β2β3-nAChRs), α4 and/or α6 will be coupled with β2 to form the two ligand-binding pairs. The β3-subunit is unlikely to be directly coupled to a ligand-binding domain; but nevertheless, it does appear to influence receptor assembly and the binding of α-CtxMII (Cui et al., 2003; Tumkosit et al., 2006). For the α4α5β2 receptor, it appears that the α4/β2 subunits form the ACh-binding site, while the α5-subunit acts as an accessory to occupy the fifth position. Furthermore, inclusion of chick α5-subunit in the human α4β2-nAChR (in Xenopus oocytes) can increase receptor desensitization to nicotine (Ramirez-Latorre et al., 1996). It is interesting to note that inclusion of the human α5-subunit in a different human nAChR, the α3β2 receptor, increases receptor desensitization to nicotine, but also nicotine efficacy (Wang et al., 1996).
In α6*-nAChRs, the α6-subunit predominantly forms a α6/β2 interface, which binds α-CtxMII. However, in rodent striatum, there is a small (∼6%) portion of α6*-nAChRs, which are α-CtxMII-resistant (Gotti et al., 2005). This suggests that some nAChRs have α6-subunits that do not form ligand-binding domains, but rather occupy the fifth non-ACh-binding position. Might the α6-subunit, as seen for the α4 and β2 subunits, be able to take on two different types of roles? One role could confer ligand specificity at an α6/β2 interface in the α6β2-, α6β2β3- and α6α4β2β3-nAChRs (Cui et al., 2003; Salminen et al., 2007), while the other could be to act as an accessory subunit at the fifth position (Figure 3). In summary, even when stoichiometry in striatum is known the nature of the ACh-binding site may depend not only on the four subunits, which constitute the two binding sites, but also the fifth non-binding subunit. We therefore need to improve our understanding of the function and the stoichiometry of the nAChRs that regulate dopamine release, if we are to successfully exploit nAChRs in neurodegeneration and addictive disorders.
Summary and perspective
Our understanding of presynaptic nAChR function in mesostriatal dopamine neurons has been revised significantly by studies in the last 4–5 years. We now understand the need to appreciate how nAChRs can filter, rather than simply enhance, neurotransmitter release from dopamine neurons. It remains a key and open question as to whether this filtering mode of action occurs for presynaptic nAChRs on other neuron types, that is, whether this can be generalized to nAChR function across the brain. Furthermore, the presynaptic filter on dopamine release probability operated by presynaptic nAChRs in striatum depends on activity in both dopamine and striatal cholinergic neurons. Given that synchronized phasic changes in both dopaminergic and cholinergic neurons appear to be the means through which behaviourally relevant information is signalled, it is thus imperative that we appreciate this dynamic interplay. Specifically, we should consider the powerful outcome of turning off nAChR tone during such a pause in cholinergic interneurons, rather than limiting our perspective to the response to nAChR activation. Together, these data suggest that future approaches to finesse our understanding of the function of presynaptic nAChRs on striatal dopaminergic axons should aim to explore dopamine signals using methods that can explore release on a timescale commensurate with the dynamic properties of neuron activity and also consider the outcome of turning ACh tone off. It is also becoming clear that turning off nAChRs may be central to the reinforcing action of nicotine. The desensitization of nAChRs by nicotine is increasingly becoming appreciated as fundamental to nicotine's properties rather than a paradoxical synaptic observation at odds with our expectation of how nicotine might be reinforcing.
We understand much less about why nAChRs are produced in such diverse forms and even exactly how many diverse forms exist within any one neuron type. While we have nonetheless made considerable progress in understanding the possible forms these nAChRs may take in dopaminergic axons, we have yet to obtain insights in to whether these different receptors have different functions in the regulation of discrete dopamine signals by endogenous ACh or by nicotine. We also have yet to establish what the function of any one subtype may be or furthermore whether there is functional redundancy. We have therefore still to bridge a large gap between insights into subunit identity on the one hand, and an understanding of subunit function in the dynamic control of dopamine signalling by endogenous ACh on the other. And yet, if we understood the form and function of nAChR diversity generally, we would not only better grasp the fundamental purpose of nAChRs in the brain, but moreover, we could reveal major potential for selective neuromodulation of targeted neuron types and guide future drug design for targeted nAChR types that could offer major potential therapeutic benefit to many disorders of neuron function that have been suggested to include nicotine addiction, Parkinson's disease, Alzheimer's disease, epilepsy and schizophrenia.
What other unknowns lie between our current understanding of nAChR control of dopaminergic transmission and an ideal goal of therapeutic exploitation, for example, in smoking and Parkinson's disease? To begin with, can this potent presynaptic regulation of DA signalling by nAChRs be demonstrated in behaving animals? To date, real-time electrochemical techniques at carbon-fibre microelectrodes within the striatum have identified that phasic-like DA transients in reward-seeking, behaving animals accompany reward prediction and positive prediction errors (for example, Garris et al., 1999; Phillips et al., 2003; Stuber et al., 2005); however, the effect of ACh on these transients is not currently known. Furthermore, we will need to identify how the adaptive and passive changes in nAChR expression that are well documented after chronic nicotine (Buisson and Bertrand, 2002; Parker et al., 2004; Lai et al., 2005; Pakkanen et al., 2005, 2006; McCallum et al., 2006a, 2006b; Quik et al., 2006; Perry et al., 2007) or in Parkinson's disease (Aubert et al., 1992; Martin-Ruiz et al., 2000; Quik and Jeyarasasingam, 2000; Quik et al., 2001, 2002, 2005; Kulak et al., 2002a, 2002b; Bohr et al., 2005) might influence the underlying nAChR function we would aim to modulate. We will also need a better grasp of subunit-specific function if any nAChR therapy is to achieve regional selectivity. We may still be some way from realizing these potential opportunities for therapeutic avenues in addictive and other neurological disorders but the future now holds significant promise.
Acknowledgments
We thank Professor I Bermudez for comments on the manuscript and acknowledge the Parkinson's Disease Society (UK) for grant support.
Glossary
- ACh
acetylcholine
- CNS
central nervous system
- CPu
caudate–putamen
- α-CtxMII
α-conotoxin MII
- GABA
γ-aminobutyric acid
- KO
knockout
- mAChR
muscarinic acetylcholine receptor
- MSN
medium spiny neuron
- NAc
nucleus accumbens
- nAChR
nicotinic acetylcholine receptor
- SNc
substantia nigra pars compacta
- TAN
tonically active neuron
- VTA
ventral tegmental area
Footnotes
Conflict of interest
SJC has received grant support from Eli Lilly (UK).
References
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alcantara AA, Chen V, Herring BE, Mendenhall JM, Berlanga ML. Localization of dopamine D2 receptors on cholinergic interneurons of the dorsal striatum and nucleus accumbens of the rat. Brain Res. 2003;986:22–29. doi: 10.1016/s0006-8993(03)03165-2. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Kimura M, Graybiel AM. Temporal and spatial characteristics of tonically active neurons of the primate's striatum. J Neurophysiol. 1995;73:1234–1252. doi: 10.1152/jn.1995.73.3.1234. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Tsubokawa H, Ishida A, Watanabe K, Graybiel AM, Kimura M. Responses of tonically active neurons in the primate's striatum undergo systematic changes during behavioral sensorimotor conditioning. J Neurosci. 1994;14:3969–3984. doi: 10.1523/JNEUROSCI.14-06-03969.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella P. Leading tonically active neurons of the striatum from reward detection to context recognition. Trends Neurosci. 2007;30:299–306. doi: 10.1016/j.tins.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Arbuthnott GW, Wickens J. Space, time and dopamine. Trends Neurosci. 2007;30:62–69. doi: 10.1016/j.tins.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Aubert I, Araujo DM, Cecyre D, Robitaille Y, Gauthier S, Quirion R. Comparative alterations of nicotinic and muscarinic binding sites in Alzheimer's and Parkinson's diseases. J Neurochem. 1992;58:529–541. doi: 10.1111/j.1471-4159.1992.tb09752.x. [DOI] [PubMed] [Google Scholar]
- Azam L, Winzer-Serhan UH, Chen Y, Leslie FM. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J Comp Neurol. 2002;444:260–274. doi: 10.1002/cne.10138. [DOI] [PubMed] [Google Scholar]
- Barbeau A. The pathogenesis of Parkinson's disease: a new hypothesis. Can Med Assoc J. 1962;87:802–807. [PMC free article] [PubMed] [Google Scholar]
- Bayer HM, Glimcher PW. Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron. 2005;47:129–141. doi: 10.1016/j.neuron.2005.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BD, Wilson CJ. Spontaneous activity of neostriatal cholinergic interneurons in vitro. J Neurosci. 1999;19:5586–5596. doi: 10.1523/JNEUROSCI.19-13-05586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit-Marand M, Borrelli E, Gonon F. Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo. J Neurosci. 2001;21:9134–9141. doi: 10.1523/JNEUROSCI.21-23-09134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard V, Laribi O, Levey AI, Bloch B. Subcellular redistribution of m2 muscarinic acetylcholine receptors in striatal interneurons in vivo after acute cholinergic stimulation. J Neurosci. 1998;18:10207–10218. doi: 10.1523/JNEUROSCI.18-23-10207.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience. Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Lindvall O.1984Dopamine-containing systems in the CNSIn: Bjorklund A, Hokfelt T (eds).Handbook of Chemical Neuroanatomy Elsevier: New York; 55–122. [Google Scholar]
- Bohr IJ, Ray MA, McIntosh JM, Chalon S, Guilloteau D, McKeith IG, et al. Cholinergic nicotinic receptor involvement in movement disorders associated with Lewy body diseases. An autoradiography study using [(125)I]alpha-conotoxinMII in the striatum and thalamus. Exp Neurol. 2005;191:292–300. doi: 10.1016/j.expneurol.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Wainer BH, Smith AD. Characterization of cholinergic neurons in the rat neostriatum. A combination of choline acetyltransferase immunocytochemistry, Golgi-impregnation and electron microscopy. Neuroscience. 1984;12:711–718. doi: 10.1016/0306-4522(84)90165-9. [DOI] [PubMed] [Google Scholar]
- Bouyer JJ, Joh TH, Pickel VM. Ultrastructural localization of tyrosine hydroxylase in rat nucleus accumbens. J Comp Neurol. 1984;227:92–103. doi: 10.1002/cne.902270110. [DOI] [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Chronic exposure to nicotine upregulates the human (alpha)4(beta)2 nicotinic acetylcholine receptor function. J Neurosci. 2001;21:1819–1829. doi: 10.1523/JNEUROSCI.21-06-01819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Nicotine addiction: the possible role of functional upregulation. Trends Pharmacol Sci. 2002;23:130–136. doi: 10.1016/S0165-6147(00)01979-9. [DOI] [PubMed] [Google Scholar]
- Butcher LL, Woolf NJ.1984Histochemical distribution of acetylcholinesterase in the central nervous system: clues to the localization of cholinergic neuronsIn Björklund A, Hokfelt T, Kuhar MJ (eds).Classical Transmitters and Transmitter Receptors in the CNS, Part II Elsevier: Amsterdam; 1–50. [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, et al. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Blockade of M2-like muscarinic receptors enhances long-term potentiation at corticostriatal synapses. Eur J Neurosci. 1998;10:3020–3023. doi: 10.1111/j.1460-9568.1998.00348.x. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Acetylcholine-mediated modulation of striatal function. Trends Neurosci. 2000;23:120–126. doi: 10.1016/s0166-2236(99)01501-5. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Cartier GE, Yoshikami D, Gray WR, Luo S, Olivera BM, McIntosh JM. A new alpha-conotoxin which targets alpha3beta2 nicotinic acetylcholine receptors. J Biol Chem. 1996;271:7522–7528. doi: 10.1074/jbc.271.13.7522. [DOI] [PubMed] [Google Scholar]
- Centonze D, Gubellini P, Pisani A, Bernardi G, Calabresi P. Dopamine, acetylcholine and nitric oxide systems interact to induce corticostriatal synaptic plasticity. Rev Neurosci. 2003;14:207–216. doi: 10.1515/revneuro.2003.14.3.207. [DOI] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, et al. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Clements L, et al. 2003Enhancement of reinforced operant responding by nicotine and cocaine in rats: analysis of dose and drug-contingencyProgram no. 323.8.2003 Abstract Viewer and Itinerary Planner Society for Neuroscience: Washington, DC; Online. [Google Scholar]
- Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, Johnson EC. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors h alpha 2 beta 2, h alpha 2 beta 4, h alpha 3 beta 2, h alpha 3 beta 4, h alpha 4 beta 2, h alpha 4 beta 4 and h alpha 7 expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1997;280:346–356. [PubMed] [Google Scholar]
- Chergui K, Suaud-Chagny MF, Gonon F. Nonlinear relationship between impulse flow, dopamine release and dopamine elimination in the rat brain in vivo. Neuroscience. 1994;62:641–645. doi: 10.1016/0306-4522(94)90465-0. [DOI] [PubMed] [Google Scholar]
- Contant C, Umbriaco D, Garcia S, Watkins KC, Descarries L. Ultrastructural characterization of the acetylcholine innervation in adult rat neostriatum. Neuroscience. 1996;71:937–947. doi: 10.1016/0306-4522(95)00507-2. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Corringer PJ, Le NN, Changeux JP. Nicotinic receptors at the amino acid level. Annu Rev Pharmacol Toxicol. 2000;40:431–458. doi: 10.1146/annurev.pharmtox.40.1.431. [DOI] [PubMed] [Google Scholar]
- Cragg SJ. Variable dopamine release probability and short-term plasticity between functional domains of the primate striatum. J Neurosci. 2003;23:4378–4385. doi: 10.1523/JNEUROSCI.23-10-04378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg SJ. Meaningful silences: how dopamine listens to the ACh pause. Trends Neurosci. 2006;29:125–131. doi: 10.1016/j.tins.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Cragg SJ, Rice ME. DAncing past the DAT at a DA synapse. Trends Neurosci. 2004;27:270–277. doi: 10.1016/j.tins.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Cui C, Booker TK, Allen RS, Grady SR, Whiteaker P, Marks MJ, et al. The {beta}3 nicotinic receptor subunit: a component of {alpha}-conotoxin MII-binding nicotinic acetylcholine receptors that modulate dopamine release and related behaviors. J Neurosci. 2003;23:11045–11053. doi: 10.1523/JNEUROSCI.23-35-11045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajas-Bailador F, Wonnacott S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol Sci. 2004;25:317–324. doi: 10.1016/j.tips.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Dani JA. Overview of nicotinic receptors and their roles in the central nervous system. Biol Psychiatry. 2001;49:166–174. doi: 10.1016/s0006-3223(00)01011-8. [DOI] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- DeBoer P, Abercrombie ED. Physiological release of striatal acetylcholine in vivo: modulation by D1 and D2 dopamine receptor subtypes. J Pharmacol Exp Ther. 1996;277:775–783. [PubMed] [Google Scholar]
- Descarries L, Gisiger V, Steriade M. Diffuse transmission by acetylcholine in the CNS. Prog Neurobiol. 1997;53:603–625. doi: 10.1016/s0301-0082(97)00050-6. [DOI] [PubMed] [Google Scholar]
- Descarries L, Mechawar N. Ultrastructural evidence for diffuse transmission by monoamine and acetylcholine neurons of the central nervous system. Prog Brain Res. 2000;125:27–47. doi: 10.1016/S0079-6123(00)25005-X. [DOI] [PubMed] [Google Scholar]
- Descarries L, Watkins KC, Garcia S, Bosler O, Doucet G. Dual character, asynaptic and synaptic, of the dopamine innervation in adult rat neostriatum: a quantitative autoradiographic and immunocytochemical analysis. J Comp Neurol. 1996;375:167–186. doi: 10.1002/(SICI)1096-9861(19961111)375:2<167::AID-CNE1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, et al. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Doucet G, Descarries L, Garcia S. Quantification of the dopamine innervation in adult rat neostriatum. Neuroscience. 1986;19:427–445. doi: 10.1016/0306-4522(86)90272-1. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ.2007α6-Containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens Neuropsychopharmacology(in press). [DOI] [PubMed]
- Exley R, Moroni M, Sasdelli F, Houlihan LM, Lukas RJ, Sher E, et al. Chaperone protein 14-3-3 and protein kinase A increase the relative abundance of low agonist sensitivity human alpha 4 beta 2 nicotinic acetylcholine receptors in Xenopus oocytes. J Neurochem. 2006;98:876–885. doi: 10.1111/j.1471-4159.2006.03915.x. [DOI] [PubMed] [Google Scholar]
- Fenster CP, Rains MF, Noerager B, Quick MW, Lester RA. Influence of subunit composition on desensitization of neuronal acetylcholine receptors at low concentrations of nicotine. J Neurosci. 1997;17:5747–5759. doi: 10.1523/JNEUROSCI.17-15-05747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Freund TF, Powell JF, Smith AD. Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience. 1984;13:1189–1215. doi: 10.1016/0306-4522(84)90294-x. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Agnati LF. Volume Transmission in the Brain. Raven Press: New York; 1991. [Google Scholar]
- Garris PA, Ciolkowski EL, Pastore P, Wightman RM. Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. J Neurosci. 1994;14:6084–6093. doi: 10.1523/JNEUROSCI.14-10-06084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Kilpatrick M, Bunin MA, Michael D, Walker QD, Wightman RM. Dissociation of dopamine release in the nucleus accumbens from intracranial self-stimulation. Nature. 1999;398:67–69. doi: 10.1038/18019. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM. It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci. 2003;26:184–192. doi: 10.1016/S0166-2236(03)00065-1. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci. 1992;15:133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Herkenham M, Thibault J. The neostriatal mosaic: II. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J Neurosci. 1987;7:3915–3934. doi: 10.1523/JNEUROSCI.07-12-03915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniatullin R, Nistri A, Yakel JL. Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Trends Neurosci. 2005;28:371–378. doi: 10.1016/j.tins.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Gonon F. Prolonged and extrasynaptic excitatory action of dopamine mediated by D1 receptors in the rat striatum in vivo. J Neurosci. 1997;17:5972–5978. doi: 10.1523/JNEUROSCI.17-15-05972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol. 2004;74:363–396. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Clementi F, Riganti L, McIntosh JM, Collins AC, et al. Expression of nigrostriatal alpha 6-containing nicotinic acetylcholine receptors is selectively reduced, but not eliminated, by beta 3 subunit gene deletion. Mol Pharmacol. 2005;67:2007–2015. doi: 10.1124/mol.105.011940. [DOI] [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Grady SR, Murphy KL, Cao J, Marks MJ, McIntosh JM, Collins AC. Characterization of nicotinic agonist-induced [3H]dopamine release from synaptosomes prepared from four mouse brain regions. J Pharmacol Exp Ther. 2002;301:651–660. doi: 10.1124/jpet.301.2.651. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Baughman RW, Eckenstein F. Cholinergic neuropil of the striatum observes striosomal boundaries. Nature. 1986;323:625–627. doi: 10.1038/323625a0. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Aston-Jones G, Svensson TH. Nicotinic effects on the firing pattern of midbrain dopamine neurons. Acta Physiol Scand. 1986;128:351–358. doi: 10.1111/j.1748-1716.1986.tb07988.x. [DOI] [PubMed] [Google Scholar]
- Groves PM, Linder JC, Young SJ. 5-hydroxydopamine-labeled dopaminergic axons: three-dimensional reconstructions of axons, synapses and postsynaptic targets in rat neostriatum. Neuroscience. 1994;58:593–604. doi: 10.1016/0306-4522(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, et al. Electron microscopic analysis of D1 and D2 receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neurosci. 1995;15:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Graybiel AM, Saper CB. Neurochemical architecture of the human striatum. J Comp Neurol. 1997;384:1–25. doi: 10.1002/(sici)1096-9861(19970721)384:1<1::aid-cne1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Houlihan LM, Slater Y, Guerra DL, Peng JH, Kuo YP, Lukas RJ, et al. Activity of cytisine and its brominated isosteres on recombinant human alpha7, alpha4beta2 and alpha4beta4 nicotinic acetylcholine receptors. J Neurochem. 2001;78:1029–1043. doi: 10.1046/j.1471-4159.2001.00481.x. [DOI] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Ikarashi Y, Takahashi A, Ishimaru H, Arai T, Maruyama Y. Regulation of dopamine D1 and D2 receptors on striatal acetylcholine release in rats. Brain Res Bull. 1997;43:107–115. doi: 10.1016/s0361-9230(96)00351-6. [DOI] [PubMed] [Google Scholar]
- Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science. 1999;286:1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- Jones IW, Bolam JP, Wonnacott S. Presynaptic localisation of the nicotinic acetylcholine receptor beta2 subunit immunoreactivity in rat nigrostriatal dopaminergic neurones. J Comp Neurol. 2001;439:235–247. doi: 10.1002/cne.1345. [DOI] [PubMed] [Google Scholar]
- Kaiser SA, Soliakov L, Harvey SC, Luetje CW, Wonnacott S. Differential inhibition by alpha-conotoxin-MII of the nicotinic stimulation of [3H]dopamine release from rat striatal synaptosomes and slices. J Neurochem. 1998;70:1069–1076. doi: 10.1046/j.1471-4159.1998.70031069.x. [DOI] [PubMed] [Google Scholar]
- Karlin A. Emerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci. 2002;3:102–114. doi: 10.1038/nrn731. [DOI] [PubMed] [Google Scholar]
- Kennedy RT, Jones SR, Wightman RM. Dynamic observation of dopamine autoreceptor effects in rat striatal slices. J Neurochem. 1992;59:449–455. doi: 10.1111/j.1471-4159.1992.tb09391.x. [DOI] [PubMed] [Google Scholar]
- Kerr JN, Wickens JR. Dopamine D-1/D-5 receptor activation is required for long-term potentiation in the rat neostriatum in vitro. J Neurophysiol. 2001;85:117–124. doi: 10.1152/jn.2001.85.1.117. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Dual cholinergic control of fast-spiking interneurons in the neostriatum. J Neurosci. 2002;22:529–535. doi: 10.1523/JNEUROSCI.22-02-00529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kulak JM, McIntosh JM, Quik M. Loss of nicotinic receptors in monkey striatum after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine treatment is due to a decline in alpha-conotoxin MII sites. Mol Pharmacol. 2002a;61:230–238. doi: 10.1124/mol.61.1.230. [DOI] [PubMed] [Google Scholar]
- Kulak JM, Musachio JL, McIntosh JM, Quik M. Declines in different beta2* nicotinic receptor populations in monkey striatum after nigrostriatal damage. J Pharmacol Exp Ther. 2002b;303:633–639. doi: 10.1124/jpet.102.039347. [DOI] [PubMed] [Google Scholar]
- Kulak JM, Nguyen TA, Olivera BM, McIntosh JM. Alpha-conotoxin MII blocks nicotine-stimulated dopamine release in rat striatal synaptosomes. J Neurosci. 1997;17:5263–5270. doi: 10.1523/JNEUROSCI.17-14-05263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Luo J, Cooper J, Lindstrom J. Nicotine acts as a pharmacological chaperone to up-regulate human alpha4beta2 acetylcholine receptors. Mol Pharmacol. 2005;68:1839–1851. doi: 10.1124/mol.105.012419. [DOI] [PubMed] [Google Scholar]
- Lai A, Parameswaran N, Khwaja M, Whiteaker P, Lindstrom JM, Fan H, et al. Long-term nicotine treatment decreases striatal alpha6* nicotinic acetylcholine receptor sites and function in mice. Mol Pharmacol. 2005;67:1639–1647. doi: 10.1124/mol.104.006429. [DOI] [PubMed] [Google Scholar]
- Le NN, Corringer PJ, Changeux JP. The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J Neurobiol. 2002;53:447–456. doi: 10.1002/neu.10153. [DOI] [PubMed] [Google Scholar]
- Luetje CW. Getting past the asterisk: the subunit composition of presynaptic nicotinic receptors that modulate striatal dopamine release. Mol Pharmacol. 2004;65:1333–1335. doi: 10.1124/mol.65.6.1333. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Kocsis JD. Presynaptic actions of carbachol and adenosine on corticostriatal synaptic transmission studied in vitro. J Neurosci. 1988;8:3750–3756. doi: 10.1523/JNEUROSCI.08-10-03750.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, Changeux JP, et al. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron. 2006;50:911–921. doi: 10.1016/j.neuron.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. J Neurobiol. 2002;53:606–617. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz CM, Piggott M, Gotti C, Lindstrom J, Mendelow AD, Siddique MS, et al. Alpha and beta nicotinic acetylcholine receptors subunits and synaptophysin in putamen from Parkinson's disease. Neuropharmacology. 2000;39:2830–2839. doi: 10.1016/s0028-3908(00)00110-6. [DOI] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–107. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- Matsumoto N, Hanakawa T, Maki S, Graybiel AM, Kimura M. Role of [corrected] nigrostriatal dopamine system in learning to perform sequential motor tasks in a predictive manner. J Neurophysiol. 1999;82:978–998. doi: 10.1152/jn.1999.82.2.978. [DOI] [PubMed] [Google Scholar]
- Maurice N, Mercer J, Chan CS, Hernandez-Lopez S, Held J, Tkatch T, et al. D2 dopamine receptor-mediated modulation of voltage-dependent Na+ channels reduces autonomous activity in striatal cholinergic interneurons. J Neurosci. 2004;24:10289–10301. doi: 10.1523/JNEUROSCI.2155-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum SE, Parameswaran N, Bordia T, Fan H, McIntosh JM, Quik M. Differential regulation of mesolimbic {alpha}3/{alpha}6beta2 and {alpha}4beta2 nicotinic acetylcholine receptor sites and function after long-term oral nicotine to monkeys. J Pharmacol Exp Ther. 2006a;318:381–388. doi: 10.1124/jpet.106.104414. [DOI] [PubMed] [Google Scholar]
- McCallum SE, Parameswaran N, Bordia T, Fan H, Tyndale RF, Langston JW, et al. Increases in alpha4* but not alpha3*/alpha6* nicotinic receptor sites and function in the primate striatum following chronic oral nicotine treatment. J Neurochem. 2006b;96:1028–1041. doi: 10.1111/j.1471-4159.2005.03646.x. [DOI] [PubMed] [Google Scholar]
- McFarland NR, Haber SN. Convergent inputs from thalamic motor nuclei and frontal cortical areas to the dorsal striatum in the primate. J Neurosci. 2000;20:3798–3813. doi: 10.1523/JNEUROSCI.20-10-03798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- McIntosh JM, Azam L, Staheli S, Dowell C, Lindstrom JM, Kuryatov A, et al. Analogs of alpha-conotoxin MII are selective for alpha6-containing nicotinic acetylcholine receptors. Mol Pharmacol. 2004;65:944–952. doi: 10.1124/mol.65.4.944. [DOI] [PubMed] [Google Scholar]
- Montague PR, McClure SM, Baldwin PR, Phillips PE, Budygin EA, Stuber GD, et al. Dynamic gain control of dopamine delivery in freely moving animals. J Neurosci. 2004;24:1754–1759. doi: 10.1523/JNEUROSCI.4279-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni M, Bermudez I. Stoichiometry and pharmacology of two human alpha4beta2 nicotinic receptor types. J Mol Neurosci. 2006;30:95–96. doi: 10.1385/JMN:30:1:95. [DOI] [PubMed] [Google Scholar]
- Morris G, Arkadir D, Nevet A, Vaadia E, Bergman H. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron. 2004;43:133–143. doi: 10.1016/j.neuron.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Lester HA. CNS localization of neuronal nicotinic receptors. J Mol Neurosci. 2006;30:181–184. doi: 10.1385/JMN:30:1:181. [DOI] [PubMed] [Google Scholar]
- Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J. Alternate stoichiometries of alpha4beta2 nicotinic acetylcholine receptors. Mol Pharmacol. 2003;63:332–341. doi: 10.1124/mol.63.2.332. [DOI] [PubMed] [Google Scholar]
- Nirenberg MJ, Chan J, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. Immunogold localization of the dopamine transporter: an ultrastructural study of the rat ventral tegmental area. J Neurosci. 1997;17:5255–5262. doi: 10.1523/JNEUROSCI.17-14-05255.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J Neurosci. 1996;16:436–447. doi: 10.1523/JNEUROSCI.16-02-00436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Infusion of nicotine in the ventral tegmental area or the nucleus accumbens of the rat differentially affects accumbal dopamine release. Pharmacol Toxicol. 1994a;75:348–352. doi: 10.1111/j.1600-0773.1994.tb00373.x. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994b;16:36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Repeated nicotine exposure enhances reward-related learning in the rat. Neuropsychopharmacology. 2003;28:1264–1271. doi: 10.1038/sj.npp.1300173. [DOI] [PubMed] [Google Scholar]
- Oorschot DE. Total number of neurons in the neostriatal, pallidal, subthalamic, and substantia nigral nuclei of the rat basal ganglia: a stereological study using the cavalieri and optical disector methods. J Comp Neurol. 1996;366:580–599. doi: 10.1002/(SICI)1096-9861(19960318)366:4<580::AID-CNE3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Pakhotin P, Bracci E. Cholinergic interneurons control the excitatory input to the striatum. J Neurosci. 2007;27:391–400. doi: 10.1523/JNEUROSCI.3709-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkanen JS, Jokitalo E, Tuominen RK. Up-regulation of beta2 and alpha7 subunit containing nicotinic acetylcholine receptors in mouse striatum at cellular level. Eur J Neurosci. 2005;21:2681–2691. doi: 10.1111/j.1460-9568.2005.04105.x. [DOI] [PubMed] [Google Scholar]
- Pakkanen JS, Stenfors J, Jokitalo E, Tuominen RK. Effect of chronic nicotine treatment on localization of neuronal nicotinic acetylcholine receptors at cellular level. Synapse. 2006;59:383–393. doi: 10.1002/syn.20249. [DOI] [PubMed] [Google Scholar]
- Parent A, Sato F, Wu Y, Gauthier J, Levesque M, Parent M. Organization of the basal ganglia: the importance of axonal collateralization. Trends Neurosci. 2000;23:S20–S27. doi: 10.1016/s1471-1931(00)00022-7. [DOI] [PubMed] [Google Scholar]
- Parker SL, Fu Y, McAllen K, Luo J, McIntosh JM, Lindstrom JM, et al. Up-regulation of brain nicotinic acetylcholine receptors in the rat during long-term self-administration of nicotine: disproportionate increase of the alpha6 subunit. Mol Pharmacol. 2004;65:611–622. doi: 10.1124/mol.65.3.611. [DOI] [PubMed] [Google Scholar]
- Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher ‘wanting' but not ‘liking' for sweet rewards. J Neurosci. 2003;23:9395–9402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins K, Sayette M, Conklin C, Caggiula A. Placebo effects of tobacco smoking and other nicotine intake. Nicotine Tob Res. 2003;5:695–709. doi: 10.1080/1462220031000158636. [DOI] [PubMed] [Google Scholar]
- Perry DC, Mao D, Gold AB, McIntosh JM, Pezzullo JC, Kellar KJ. Chronic nicotine differentially regulates {alpha}6- and {beta}3-containing nicotinic cholinergic receptors in rat brain. J Pharmacol Exp Ther. 2007;322:306–314. doi: 10.1124/jpet.107.121228. [DOI] [PubMed] [Google Scholar]
- Phelps PE, Houser CR, Vaughn JE. Immunocytochemical localization of choline acetyltransferase within the rat neostriatum: a correlated light and electron microscopic study of cholinergic neurons and synapses. J Comp Neurol. 1985;238:286–307. doi: 10.1002/cne.902380305. [DOI] [PubMed] [Google Scholar]
- Phelps PE, Vaughn JE. Immunocytochemical localization of choline acetyltransferase in rat ventral striatum: a light and electron microscopic study. J Neurocytol. 1986;15:595–617. doi: 10.1007/BF01611860. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pickel VM. Extrasynaptic distribution of monoamine transporters and receptors. Prog Brain Res. 2000;125:267–276. doi: 10.1016/S0079-6123(00)25016-4. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Beckley SC, Joh TH, Reis DJ. Ultrastructural immunocytochemical localization of tyrosine hydroxylase in the neostriatum. Brain Res. 1981;225:373–385. doi: 10.1016/0006-8993(81)90843-x. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- Pisani A, Bonsi P, Centonze D, Gubellini P, Bernardi G, Calabresi P. Targeting striatal cholinergic interneurons in Parkinson's disease: focus on metabotropic glutamate receptors. Neuropharmacology. 2003;45:45–56. doi: 10.1016/s0028-3908(03)00137-0. [DOI] [PubMed] [Google Scholar]
- Pisani A, Centonze D, Bernardi G, Calabresi P. Striatal synaptic plasticity: implications for motor learning and Parkinson's disease. Mov Disord. 2005;20:395–402. doi: 10.1002/mds.20394. [DOI] [PubMed] [Google Scholar]
- Quick MW, Lester RA. Desensitization of neuronal nicotinic receptors. J Neurobiol. 2002;53:457–478. doi: 10.1002/neu.10109. [DOI] [PubMed] [Google Scholar]
- Quik M, Chen L, Parameswaran N, Xie X, Langston JW, McCallum SE. Chronic oral nicotine normalizes dopaminergic function and synaptic plasticity in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned primates. J Neurosci. 2006;26:4681–4689. doi: 10.1523/JNEUROSCI.0215-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Jeyarasasingam G. Nicotinic receptors and Parkinson's disease. Eur J Pharmacol. 2000;393:223–230. doi: 10.1016/s0014-2999(99)00888-2. [DOI] [PubMed] [Google Scholar]
- Quik M, McIntosh JM. Striatal alpha6* nicotinic acetylcholine receptors: potential targets for Parkinson's disease therapy. J Pharmacol Exp Ther. 2006;316:481–489. doi: 10.1124/jpet.105.094375. [DOI] [PubMed] [Google Scholar]
- Quik M, Polonskaya Y, Kulak JM, McIntosh JM. Vulnerability of 125I-{alpha}-conotoxin MII binding sites to nigrostriatal damage in monkey. J Neurosci. 2001;21:5494–5500. doi: 10.1523/JNEUROSCI.21-15-05494.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Polonskaya Y, McIntosh JM, Kulak JM. Differential nicotinic receptor expression in monkey basal ganglia: effects of nigrostriatal damage. Neuroscience. 2002;112:619–630. doi: 10.1016/s0306-4522(02)00106-9. [DOI] [PubMed] [Google Scholar]
- Quik M, Vailati S, Bordia T, Kulak JM, Fan H, McIntosh JM, et al. Subunit composition of nicotinic receptors in monkey striatum: effect of treatments with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine or L-DOPA. Mol Pharmacol. 2005;67:32–41. doi: 10.1124/mol.104.006015. [DOI] [PubMed] [Google Scholar]
- Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L. Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380:347–351. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- Ravel S, Sardo P, Legallet E, Apicella P. Reward unpredictability inside and outside of a task context as a determinant of the responses of tonically active neurons in the monkey striatum. J Neurosci. 2001;21:5730–5739. doi: 10.1523/JNEUROSCI.21-15-05730.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JN, Hyland BI, Wickens JR. A cellular mechanism of reward-related learning. Nature. 2001;413:67–70. doi: 10.1038/35092560. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Wickens JR. Substantia nigra dopamine regulates synaptic plasticity and membrane potential fluctuations in the rat neostriatum, in vivo. Neuroscience. 2000;99:199–203. doi: 10.1016/s0306-4522(00)00273-6. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Hyland BI, Wickens JR. Modulation of an afterhyperpolarization by the substantia nigra induces pauses in the tonic firing of striatal cholinergic interneurons. J Neurosci. 2004;24:9870–9877. doi: 10.1523/JNEUROSCI.3225-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JN, Wickens JR. Dopamine-dependent plasticity of corticostriatal synapses. Neural Netw. 2002;15:507–521. doi: 10.1016/s0893-6080(02)00045-x. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Robinson S, Sandstrom SM, Denenberg VH, Palmiter RD. Distinguishing whether dopamine regulates liking, wanting, and/or learning about rewards. Behav Neurosci. 2005;119:5–15. doi: 10.1037/0735-7044.119.1.5. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47 Suppl 1:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Sallette J, Pons S, villers-Thiery A, Soudant M, Prado de CL, Changeux JP, et al. Nicotine upregulates its own receptors through enhanced intracellular maturation. Neuron. 2005;46:595–607. doi: 10.1016/j.neuron.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Salminen O, Drapeau J, McIntosh M, Collins AC, Marks MJ, Grady SR. Pharmacology of {alpha}-conotoxin MII-sensitive subtypes of nicotinic acetylcholine receptors isolated by breeding of null mutant mice. Mol Pharmacol. 2007;71:1563–1571. doi: 10.1124/mol.106.031492. [DOI] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, et al. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- Salminen O, Whiteaker P, Grady SR, Collins AC, McIntosh JM, Marks MJ. The subunit composition and pharmacology of alpha-Conotoxin MII-binding nicotinic acetylcholine receptors studied by a novel membrane-binding assay. Neuropharmacology. 2005;48:696–705. doi: 10.1016/j.neuropharm.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Sargent PB. The diversity of neuronal nicotinic acetylcholine receptors. Annu Rev Neurosci. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- Schmitz Y, Benoit-Marand M, Gonon F, Sulzer D. Presynaptic regulation of dopaminergic neurotransmission. J Neurochem. 2003;87:273–289. doi: 10.1046/j.1471-4159.2003.02050.x. [DOI] [PubMed] [Google Scholar]
- Schmitz Y, Schmauss C, Sulzer D. Altered dopamine release and uptake kinetics in mice lacking D2 receptors. J Neurosci. 2002;22:8002–8009. doi: 10.1523/JNEUROSCI.22-18-08002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Changes in behavior-related neuronal activity in the striatum during learning. Trends Neurosci. 2003;26:321–328. doi: 10.1016/S0166-2236(03)00122-X. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Aoki C, Pickel VM. Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J Neurosci. 1994;14:88–106. doi: 10.1523/JNEUROSCI.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher E, Chen Y, Sharples TJ, Broad LM, Benedetti G, Zwart R, et al. Physiological roles of neuronal nicotinic receptor subtypes: new insights on the nicotinic modulation of neurotransmitter release, synaptic transmission and plasticity. Curr Top Med Chem. 2004;4:283–297. doi: 10.2174/1568026043451393. [DOI] [PubMed] [Google Scholar]
- Shimo Y, Hikosaka O. Role of tonically active neurons in primate caudate in reward-oriented saccadic eye movement. J Neurosci. 2001;21:7804–7814. doi: 10.1523/JNEUROSCI.21-19-07804.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater YE, Houlihan LM, Maskell PD, Exley R, Bermudez I, Lukas RJ, et al. Halogenated cytisine derivatives as agonists at human neuronal nicotinic acetylcholine receptor subtypes. Neuropharmacology. 2003;44:503–515. doi: 10.1016/s0028-3908(03)00025-x. [DOI] [PubMed] [Google Scholar]
- Smith AD, Bolam JP. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci. 1990;13:259–265. doi: 10.1016/0166-2236(90)90106-k. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Roitman MF, Phillips PE, Carelli RM, Wightman RM. Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology. 2005;30:853–863. doi: 10.1038/sj.npp.1300619. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, Dickinson A, Schultz W. Coding of predicted reward omission by dopamine neurons in a conditioned inhibition paradigm. J Neurosci. 2003;23:10402–10410. doi: 10.1523/JNEUROSCI.23-32-10402.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307:1642–1645. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- Tumkosit P, Kuryatov A, Luo J, Lindstrom J. Beta3 subunits promote expression and nicotine-induced up-regulation of human nicotinic alpha6* nicotinic acetylcholine receptors expressed in transfected cell lines. Mol Pharmacol. 2006;70:1358–1368. doi: 10.1124/mol.106.027326. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Cragg SJ. A choreography of nicotinic receptors directs the dopamine neuron routine. Neuron. 2006;50:815–816. doi: 10.1016/j.neuron.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Vallejo YF, Buisson B, Bertrand D, Green WN. Chronic nicotine exposure upregulates nicotinic receptors by a novel mechanism. J Neurosci. 2005;25:5563–5572. doi: 10.1523/JNEUROSCI.5240-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wanamaker CP, Christianson JC, Green WN. Regulation of nicotinic acetylcholine receptor assembly. Ann NY Acad Sci. 2003;998:66–80. doi: 10.1196/annals.1254.009. [DOI] [PubMed] [Google Scholar]
- Wang F, Gerzanich V, Wells GB, Anand R, Peng X, Keyser K, et al. Assembly of human neuronal nicotinic receptor alpha5 subunits with alpha3, beta2, and beta4 subunits. J Biol Chem. 1996;271:17656–17665. doi: 10.1074/jbc.271.30.17656. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, McIntosh JM, Luo S, Collins AC, Marks MJ. 125I-alpha-conotoxin MII identifies a novel nicotinic acetylcholine receptor population in mouse brain. Mol Pharmacol. 2000;57:913–925. [PubMed] [Google Scholar]
- Wickens JR, Reynolds JN, Hyland BI. Neural mechanisms of reward-related motor learning. Curr Opin Neurobiol. 2003;13:685–690. doi: 10.1016/j.conb.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J Neurosci. 1990;10:508–519. doi: 10.1523/JNEUROSCI.10-02-00508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Kaiser S, Mogg A, Soliakov L, Jones IW. Presynaptic nicotinic receptors modulating dopamine release in the rat striatum. Eur J Pharmacol. 2000;393:51–58. doi: 10.1016/s0014-2999(00)00005-4. [DOI] [PubMed] [Google Scholar]
- Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol. 1991;37:475–524. doi: 10.1016/0301-0082(91)90006-m. [DOI] [PubMed] [Google Scholar]
- Wooltorton JRA, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci. 2003;23:3176. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Matsumoto N, Kimura M. Tonically active neurons in the primate caudate nucleus and putamen differentially encode instructed motivational outcomes of action. J Neurosci. 2004;24:3500–3510. doi: 10.1523/JNEUROSCI.0068-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Surmeier DJ. Muscarinic (m2/m4) receptors reduce N- and P-type Ca2+ currents in rat neostriatal cholinergic interneurons through a fast, membrane-delimited, G-protein pathway. J Neurosci. 1996;16:2592–2604. doi: 10.1523/JNEUROSCI.16-08-02592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Brog JS. On the significance of subterritories in the ‘accumbens' part of the rat ventral striatum. Neuroscience. 1992;50:751–767. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- Zhang W, Yamada M, Gomeza J, Basile AS, Wess J. Multiple muscarinic acetylcholine receptor subtypes modulate striatal dopamine release, as studied with M1-M5 muscarinic receptor knock-out mice. J Neurosci. 2002;22:6347–6352. doi: 10.1523/JNEUROSCI.22-15-06347.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol. 2002;53:590–605. doi: 10.1002/neu.10150. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Wilson CJ, Dani JA. Muscarinic and nicotinic cholinergic mechanisms in the mesostriatal dopamine systems. Neuroscientist. 2003;9:23–36. doi: 10.1177/1073858402239588. [DOI] [PubMed] [Google Scholar]
- Zoli M, Moretti M, Zanardi A, McIntosh JM, Clementi F, Gotti C. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci. 2002;22:8785–8789. doi: 10.1523/JNEUROSCI.22-20-08785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart R, Vijverberg HP. Four pharmacologically distinct subtypes of alpha4beta2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Mol Pharmacol. 1998;54:1124–1131. [PubMed] [Google Scholar]