Abstract
The G-protein-coupled receptors (GPCRs) represent one the largest families of drug targets. Upon agonist binding a receptor undergoes conformational rearrangements that lead to a novel protein conformation which in turn can interact with effector proteins. During the last decade significant progress has been made to prove that different conformational changes occur. Today it is mostly accepted that individual ligands can induce different receptor conformations. However, the nature or molecular identity of the different conformations is still ill-known. Knowledge of the potential functionally selective conformations will help to develop drugs that select specific conformations of a given GPCR which couple to specific signalling pathways and may, ultimately, lead to reduced side effects. In this review we will summarize recent progress in biophysical approaches that have led to the current understanding of conformational changes that occur during GPCR activation.
Keywords: G-protein-coupled receptors, 7-TM, conformational changes, functional selectivity, FRET, FlAsH-EDT2
Introduction
The G-protein-coupled receptor (GPCR) family comprises the largest family of cell-surface receptors, which can sense information encoded by diverse stimuli and translate the encoded information into readable signals for the cell (Marinissen and Gutkind, 2001). A large fraction of drugs exert their action via interaction with these receptors (Flower, 1999). Drugs that influence GPCRs can help treat human diseases as different as pain, asthma, hypertension or chronic heart failure. For a long time, receptors were depicted as simple switches for ‘on' and ‘off' states, and ligands were thought to simply stimulate (agonists) or inhibit (antagonists) the receptor by promoting either the ‘on' or the ‘off' state. Accordingly, less efficacious agonists (partial agonists) were only thought to vary in signal strength, but all agonists were assumed to produce qualitatively the same effect as the endogenous agonist(s) (reviewed by Perez and Karnik, 2005). However, a growing body of experimental evidence forced the receptor theories to be constantly updated and led to the inclusion of the ensemble theory in GPCR dynamics (Kenakin, 2002) and the development of the concepts of collateral efficacy and permissive antagonism (Kenakin, 2005) to accommodate different conformations into the receptor theory. Various GPCRs have been studied with respect to different receptor conformations and contributed to the body of evidence supporting this concept. Among them are 5-HT2-serotonin receptors (Berg et al., 1998), α2A-adrenoceptors (Vilardaga et al., 2005; Nikolaev et al., 2006), AT1 receptor (Wei et al., 2003), β2-adrenoceptors (Ghanouni et al., 2001a; Swaminath et al., 2004; Trester-Zedlitz et al., 2005), gonadotropin-releasing hormone receptors (Lu et al., 2007), μ-opioid receptors (Keith et al., 1998; Whistler et al., 1999), parathyroid hormone (PTH) receptors (Bisello et al., 2002) and many others, thus proving that this phenomenon is widespread among GPCRs. Today, it is mostly accepted that different ligands can induce different receptor conformations (Perez and Karnik, 2005; Vauquelin and Van Liefde, 2005; Urban et al., 2007), and this phenomenon has been described by several synonyms such as ‘functional selectivity', ‘agonist-directed trafficking' or ‘biased agonism'. Recently, it has been suggested that the number of different terms be limited by using the term ‘functional selectivity' or ‘ligand-induced differential signalling' to describe this phenomenon (Urban et al., 2007). Besides the mostly phenomenological description of the occurrence of different functionally selective conformations, little is known about the actual distinction at the molecular level. Structural data with high resolution are currently available only for the inactive state of rhodopsin (Palczewski et al., 2000; Schertler, 2005; Ridge and Palczewski, 2007). However, a combined effort from structural biology, molecular pharmacology and computational chemistry may help fill the gap until crystal structural data of an active receptor conformation are made available. To gain information about the switch from an inactive conformation to active receptor conformation(s), several different techniques have been developed and used over the past decade.
In the following sections, the structural information obtained from the rhodopsin system pioneered by the work from Khorana's and Hubbell's groups (Hubbell and Altenbach, 1994), the β2-adrenoceptor system with techniques developed by Kobilka's group (Gether et al., 1995) and the M3 subtype of the muscarinic ACh receptor introduced by the group of Wess (Zeng et al., 1999) will be described in brief. All these techniques use purified and reconstituted GPCRs to investigate the mechanisms of receptor activation. Then, the metal-ion chelator approach that has been widely used by the group of Schwartz (Thirstrup et al., 1996) will be summarized. In comparison to those systems, the recently developed approaches that have made it possible to investigate conformational changes of GPCRs in living cells by means of fluorescence resonance energy transfer (FRET) (Vilardaga et al., 2003; Hoffmann et al., 2005) will be discussed.
The rhodopsin system
The solution structure of rhodopsin has been analysed in great detail, and the work was recently reviewed in the light of the rhodopsin crystal structure (Hubbell et al., 2003). The term ‘solution structure' refers to purified rhodopsin from bovine retina, which has been reconstituted into dodecyl maltoside micelles. This environment is assumed to be a reasonable approximation of the normal lipid bilayer, as it has been shown to conserve functional properties of rhodopsin such as transducin activation (Resek et al., 1993) or receptor phosphorylation by rhodopsin kinase (Thurmond et al., 1997). Three major classes of experiments have been carried out, namely site-directed spin labelling, disulphide cross-linking kinetics and sulphydryl reactivity studies.
Site-directed spin labelling uses cysteine residues that have been substituted for the normal amino acid and can be reacted with nitroxide reagents. This procedure generates nitric oxide side chains that can be utilized in electron paramagnetic resonance and, dependent on the type of experiment, can report on the mobility of the side chain, the accessibility of the side chain in solution and the distance between two side chains if a second paramagnetic group is introduced into the protein of interest (Hubbell et al., 1998). Data from distance measurements by site-directed spin labelling can be complemented by the second technique mentioned, by disulphide cross-linking kinetics. This technique uses the fact that the rate of oxidative formation of disulphide links depends on the distance, relative orientation and flexibility of the side chain to be cross-linked (Falke and Koshland, 1987). Sulphydryl reactivity studies can report about solvent accessibility of the substituted cysteine. In this case, the reaction rate can be followed by measuring the absorption at 324 nm of a stoichiometric by-product of the reaction between the cysteine under investigation and a sulphydryl reagent 4,4′-dithiopyridine. The reaction rate is a measure of the relative accessibility of the respective side chain (Hubbell et al., 2003). By generating more than 100 mutations and various combinations thereof within all of the cytoplasmic domains of rhodopsin, it was possible to achieve a detailed picture of the movements of the rhodopsin structure during the activation process. As the issue of receptor activation itself has been reviewed in great detail by others (Gether, 2000; Bissantz, 2003; Schwartz et al., 2006), only the changes that occur will be briefly described, with emphasis on what is known or can be concluded for the cytoplasmic domains. This issue is focused on, as cytoplasmic domains are the assumed contact points for downstream signalling partners and, hence, are likely to be interesting domains for ligand-selective conformations. Upon receptor activation, transmembrane helix (TM) VI undergoes the largest movement and thus the third intracellular loop close to TMVI should follow the movement and move outward from the structure if viewed from the cytoplasmic side (Hubbell et al., 2003) (Figure 1). Smaller changes were observed for TMIII, and again an outward movement of the second intracellular loop would be the logical consequence (Hubbell et al., 2003). The first intracellular loop appears to be more rigid and does not seem to undergo large-scale movements. However, a slight outward movement can be concluded from distance measurements with different reference points within the rhodopsin molecule (Hubbell et al., 2003). The C-terminal domain is very flexible and totally disordered in solution after position 340 (Cai et al., 1997). Therefore, no information is available for its global movement, but the part close to TMVII should follow the slight outward movement of helix VII, and therefore it can be assumed to move slightly outwards. All in all, the overall movement would open a cleft in the molecule, and the process has been described as a ‘blossoming' of the molecule (Meng and Bourne, 2001), thus allowing interactions with downstream signalling molecules such as transducin or visual arrestin.
Figure 1.
Schematic representation of the helix movements as delineated from experimental data described by the different approaches. The crystal structure data from bovine rhodopsin (PDB access code: 1U19, Okada et al., 2004) were used to generate the figure. Colour code: TMIII, blue; TMVI, green; TMVII, dark orange. Left: view from the cytoplasmic side; right: view from the extracellular side. Helix movements are indicated by arrows and are meant to indicate the general movements as mentioned in the text. Blue arrows: data delineated from the rhodopsin system; green arrows: data delineated from the β2-adrenoceptor system; red arrows: data delineated from the M3 muscarinic ACh receptor system; yellow arrows: data delineated from the metal-ion chelator approach. PDB, Protein Data Bank; TM, transmembrane domain.
Besides the vast amount of information that was achieved by the use of the rhodopsin system about the activation mechanism of GPCRs in general, the system is complicated by the fact that 11-cis-retinal needs to be covalently linked to opsin at position 296 to achieve its full potential as agonist when converted into 11-trans-retinal. Thus, the system is not easy to use for ligand screening to detect functionally selective conformations, although work with alternative ligands has been performed to investigate the role of the retinal ring in receptor activation (Bartl et al., 2005). A lack of the ring structure was found to be without influence on the kinetics of formation of the active conformation metarhodopsin II. However, the lack of the retinal ring was found to not only result in low amounts of metarhodopsin II but also lead to a fast decay of active conformation (Bartl et al., 2005). It was concluded that it has a role in stabilizing the active receptor conformation.
The β2-adrenoceptor system
As two recent reviews have been published on the β2-adrenoceptor as a model system for studying ligand-induced conformational changes (Kobilka, 2007; Kobilka and Deupi, 2007), only the key points that are relevant to the concept of ligand-selective conformational changes will be summarized. In general, the system uses purified and reconstituted β2-adrenoceptors that have been depleted of all but the essential cysteines (Gether et al., 1997). The modified receptor is then reacted with small cysteine-reactive fluorescent probes that can be monitored for several different properties such as mobility, intensity or lifetime. It has been possible to label the receptor construct with probes that provide information about the polar environment in which the actual fluorophore is located (Gether et al., 1997), as well as the dual labelling of the receptor with a fluorophore, such as fluorescein, and a fluorescence quencher in a different position of the receptor, therefore reporting the relative movements of the two groups to each other (Ghanouni et al., 2001b). As already outlined in the section describing the rhodopsin system, this approach has greatly advanced our current knowledge on the molecular details about how GPCRs change their conformation upon activation. These studies have demonstrated movements of TMIII and TMVI during agonist-induced conformational changes (Gether et al., 1995, 1997), which were in good agreement with reports on rhodopsin (Farrens et al., 1996) (Figure 1). In recent years, the system was further developed to allow screening of several different ligands with respect to conformational changes in different positions of the receptor, and it also allowed the detection of intermediate states during the activation process (Swaminath et al., 2004, 2005; Yao et al., 2006). Using a receptor construct that was labelled underneath TMVI with fluorescein and employing fluorescent lifetime measurements as read-out, it was possible to detect different lifetimes for the fluorophore, which were dependent on the ligand that the receptor was exposed to. Full or partial agonists were thus shown to induce different conformational changes (Ghanouni et al., 2001a, 2001b). Kinetic studies followed those initial reports and further refined our understanding of receptor activation. It was shown that the partial agonist dopamine induced a rapid change of the receptor conformation as did the full agonist noradrenaline (Swaminath et al., 2004). However, noradrenaline exhibited a second slower phase that is lacking for dopamine but was observed for the full agonists isoprenaline or adrenaline (Swaminath et al., 2004). As the only structural difference between dopamine and noradrenaline is the lack of the β-hydroxyl group, it was concluded that the presence of the β-hydroxyl group and its interaction with TMVI (Wieland et al., 1996) would be responsible for the induction of the slow phase, whereas the presence of the catechol structure would allow the induction of the rapid phase (Swaminath et al., 2004). This hypothesis was supported by the notion that the partial agonist salbutamol carrying a β-hydroxyl group, but no catechol structure, only induced the slow phase of conformational change and could be complemented by the addition of catechol (Swaminath et al., 2005). The kinetic resolution for receptor activation that has been achieved with this system is in the range of several seconds for the fast phase and almost a minute for the slow phase of receptor activation; therefore, it would not be compatible with the receptor function in biological systems. However, the kinetics of receptor conformational changes improved by careful reconstitution of the receptor (Yao et al., 2006), showing that a more natural environment may lead to kinetic measurements that are more consistent with biological functions. The latest study in this series also addressed the issue of functionally selective receptor conformations at the molecular level (Yao et al., 2006). Two β2-adrenoceptor constructs were used for this study, one that reports upon movements of the TMVI and the second that reports upon changes between TMIII and TMVI. Using a selection of compounds with various degrees of efficacy, it was possible to show that full agonists such as isoprenaline, noradrenaline or adrenaline could induce conformational changes of the receptor in both cases, whereas the partial agonist salbutamol only induced conformational changes in TMVI (Yao et al., 2006). The structural changes of the C terminus have recently been subjected to investigation with the same set of receptor ligands. Again, a complex pattern of movements was observed, which allowed the conclusion that each ligand has the potential to induce individual conformational changes or ligand-selective conformations (Granier et al., 2007). This system has so far been the most informative with respect to molecular changes being induced by ligands of different efficacy.
The M3 muscarinic ACh receptor system
Using the rat M3 subtype of the muscarinic ACh receptor, an in situ disulphide cross-linking strategy was developed by the group of Wess. To develop this system, an M3 muscarinic ACh receptor construct was generated that lacked most of the native cysteine residues and had the third intracellular loop largely truncated (Zeng et al., 1999). Into the third intracellular loop, two factor Xa cleavage sites were introduced to allow selective digestion of the proteins into two fragments. The first fragment consists of the N-terminal part of the receptor till TMV and the N-terminal part of the intracellular loop 3, whereas the second fragment consists of the C-terminal part of intracellular loop 3 plus TMVI, TMVII and the normal C terminus of the M3 receptor. By selective introduction of two cysteine residues, one in each fragment mentioned above, it is possible to cross-link the two fragments by oxidation with Cu(II)-phenanthroline if the two cysteine residues are in close enough proximity and appropriate orientation. Cross-linked fragments can then be detected by western blotting under non-reducing conditions as judged by the appearance of a full-length receptor band at the appropriate molecular weight, using an antibody against the very C terminus of the M3 receptor. The initial studies used solubilized receptors from membranes of COS-7 cells expressing the receptor construct (Zeng et al., 1999). However, it was recently reported that solubilized receptors in some rare cases might allow promiscuous cross-linking, and the approach was further developed to the use of native membranes (Ward et al., 2006) to prevent this problem. A series of publications have demonstrated the usefulness and strength of this approach. Consistent with previous publications on rhodopsin (Farrens et al., 1996) or the β2-adrenoceptor (Gether et al., 1997), it was demonstrated that the cytoplasmic end of TMVI undergoes a rotational movement upon receptor activation (Ward et al., 2002, 2006) (Figure 1). Furthermore, it was found that agonist activation leads to an increase in distance between the cytoplasmic ends of TMI and TMVII (Han et al., 2005a), whereas the extracellular ends of TMIII and TMVII move closer to each other upon agonist stimulation (Han et al., 2005b), proving that this technique is not limited to studies on the intracellular site of the receptor. In the latest publication from this series, the issue of ligand-selective conformations was addressed. Agonists with different efficacy, such as full, partial or inverse agonists, were studied with respect to conformational changes induced by the respective ligand (Li et al., 2007). It was found that agonists such as carbachol would increase the distance between the C-terminal part of helix VIII and the cytoplasmic end of TMI, whereas inverse agonists such as atropine would decrease the distance in these parts of the receptor (Li et al., 2007). The opposing effects on conformational changes for agonists or inverse agonists have also been demonstrated for the α2A-adrenoceptor by means of FRET (Vilardaga et al., 2005).
A great advantage of the in situ disulphide cross-linking strategy technique compared to the studies for rhodopsin mentioned above is the possibility to include several different ligands in the assay, thus having the potential to pin down different conformational changes for different classes of ligands at the molecular level. One minor drawback of this technique is the fact that only those conformational changes can be observed that will lead to cross-linking of the two fragments; therefore, the design of the fragments itself may limit the approach, as no changes within the fragment TMI–TMV, or the respective intracellular loops 1 and 2, can be investigated. As all measurements are carried out under equilibrium conditions, this approach also does not lead to any information with respect to kinetic resolution of the conformational changes.
Metal-ion chelator sites and receptor activation
An alternative approach using the introduction of histidine side chains to complex metal-ions has contributed greatly to the understanding of conformational changes that occur during receptor activation; these techniques have been reviewed recently (Schwartz et al., 2006). The basic principle of this technique is to introduce histidine residues that can complex Zn(II) or Cu(II), thus forming a bridge between different residues and thereby allowing the study of helix–helix interactions. A variation of this technique uses metal-ions that have been chelated with bipyridine or phenanthroline as aromatic chelators. This allows investigation of the surroundings of the attachment site within the helical bundle in more detail, and the organic metal-ion complex has been shown to mimic nicely small molecule agonists at chemokine receptors (Schwartz et al., 2006).
The metal-ion chelator approach has initially been applied in an inhibitory mode by cross-linking two helices and thus inhibiting receptor activation. Those studies were performed using a variety of receptors, including the κ-opioid receptor (Thirstrup et al., 1996), rhodopsin (Sheikh et al., 1996), the β2-adrenoceptor (Sheikh et al., 1999) and the M1-ACh receptor (Lu and Hulme, 2000). The approach was also successfully used to activate the receptors of interest, and this was first achieved for the β2-adrenoceptor (Elling et al., 1999) and the NK1 receptor (Holst et al., 2000). Using the metal-ion chelator strategy for the PTH receptor, it could be demonstrated that different receptor conformations would be required for G-protein activation and β-arrestin interaction (Vilardaga et al., 2001). A great and detailed work using this strategy has been performed by the group of Schwartz and has led to the development of the ‘global toggle switch' model for receptor activation (Elling et al., 2006; Schwartz et al., 2006). This model suggests an outward movement of the intracellular segments (‘blossoming' of the molecule in the section The rhodopsin system) and an inward movement of the extracellular segments of the transmembrane bundle (Figure 1). According to this model, the extracellular segments of TMVI and TMVII are bent inward towards TMIII, and this conformation would be stabilized by small ligands within the helical bundle. This type of movement has recently been confirmed for the CXCR3 receptor (Rosenkilde et al., 2007). Large agonists such as peptides or proteins could stabilize a similar active conformation by contacting the extracellular ends of the helices or by interacting with the extracellular loops. This type of movement is consistent with a large body of data that was acquired by the different approaches outlined in this review and could be an explanation for a common activation mechanism for GPCRs.
Studies in living cell by fluorescence resonance energy transfer
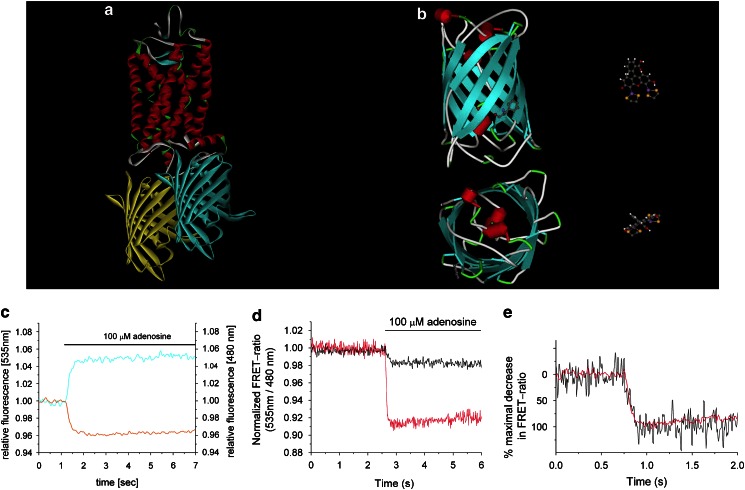
With the introduction of the green fluorescent protein (GFP) and its colour variants in the late 1990s (Tsien, 1998), it became possible to create fusion proteins of almost any kind with genetically encoded fluorescent markers. The introduction of GFP into cell biological work significantly expanded the accessibility of proteins to studies in living cells. It had already been possible for a long time to use FRET to study protein function (Stryer, 1978), but usually isolated proteins had to be chemically labelled and needed to be injected back into the cell to study protein function in living cells (Adams et al., 1991). Such experiments became significantly easier by the availability of GFP. Many biological processes suddenly became ‘visible' (Miyawaki and Tsien, 2000) and could be monitored in real time (Miyawaki, 2003). Activation of a GPCR was monitored by the use of GFP already in 1997 (Barak et al., 1997). However, the approach was indirect, as a β-arrestin–GFP fusion protein was used as a downstream readout for receptor activation, and it did not monitor the changes at the level of the receptor itself. The first approach to directly measure receptor activation by conformational changes in living cells was published in 2003 (Vilardaga et al., 2003). Two receptors from different subclasses of GPCRs were used for this study: the α2A-adrenoceptor and the PTH receptor. The receptor constructs had been modified in a way that a cyan variant of GFP (CFP) was inserted into the third intracellular loop, whereas a yellow variant (YFP) had been fused to the C terminus, or vice versa (Figure 2a). The relative distance of both fluorophores was optimized by using several different truncations at the C terminus or the third intracellular loop, thus allowing optimization of the signal amplitude. The positions for insertion into intracellular loop 3 were concluded from the studies of rhodopsin (Farrens et al., 1996) and the β2-adrenoceptor (Gether et al., 1997), which had shown significant movements of TMVI during receptor activation. Thus, it was assumed that the third intracellular loop would undergo a conformational change upon receptor activation and that the relative distance of the two fluorophores would change and give rise to a change in the FRET signal. These theoretical considerations were confirmed by actual experiments. When the α2A-adrenoceptor was stimulated by an agonist, the change in the FRET signal occurred at a millisecond time scale, whereas the PTH receptor responded much slower with a τ of about 1 s (Vilardaga et al., 2003). The timescale for receptor activation was much faster than that which had been reported for the β2-adrenoceptor but still slower than those reported for rhodopsin (see also Lohse et al., 2007 for a more in-depth discussion of the kinetic aspects). Several control experiments were performed to ensure that the observed signal did arise from a single GPCR subunit and not from potential rearrangements within receptor dimers, or that it was caused by interactions with G-proteins (Vilardaga et al., 2003). Thus, the first system was available to monitor conformational changes induced by direct agonist binding to the receptor in living cells. For the α2A-adrenoceptor, agonist stimulation caused a decrease in the FRET signal, whereas inverse agonists had the opposite effect, exhibiting an increase in the FRET signal of the receptor construct (Vilardaga et al., 2005). Taking into account that the FRET signal is distance-dependent, one could conclude that agonist stimulation leads to an increase in distance, whereas inverse agonists would cause a decrease in distance between the C terminus and intracellular loop 3. In line with this finding is the recent report from the rat M3 receptor. Here, it was shown that agonists increase the distance between helix VIII and TMI, whereas inverse agonists lead to a decrease in distance (Li et al., 2007). Those movements would be consistent with the ‘blossoming' receptor as mentioned above. An extension of this approach using CFP and YFP as fluorophores was reported in 2005 (Hoffmann et al., 2005). For the adenosine A2A and the α2A-adrenoceptor, an alternative approach was realized using CFP in combination with the small fluorescein-derived analogue FlAsH (fluorescein arsenical hairpin binder) (Adams et al., 2002). This fluorescent tag is only 700 Da in size and thus significantly smaller than GFP (27 kDa) (Figure 2b), and it needs only six amino acids (CCPCCC) to be able to bind to the molecule of interest (Adams et al., 2002). A side-by-side comparison of both FRET approaches was made and showed that the kinetics of receptor activation were independent of the fluorophores used for detection of the signal (Hoffmann et al., 2005). The adenosine A2A receptor was activated with a rate constant about 50 ms similar to the α2A-adrenoceptor, showing that small ligand receptors might respond faster than peptide hormone receptors such as the PTH receptor. The FlAsH-based FRET approach showed a larger signal amplitude (Figures 2c–e) and better functionality of the receptor construct (Hoffmann et al., 2005). For the α2A-adrenoceptor, the signal amplitude was improved fourfold and this allowed a much more detailed study of receptor response to ligands with varying efficacy (Nikolaev et al., 2006). Several structurally distinct ligands, such as noradrenaline, dopamine, clonidine and others, were shown to induce kinetically distinct conformational changes of the receptor—a notion that is similar to the finding for the β2-adrenoceptor (Ghanouni et al., 2001a), proving that different ligand-induced conformations do occur in living cells and are not produced by reconstituted systems. Another point proved by this study is the readiness of this system to screen for a larger number of compounds. The approach to monitor conformational changes of a GPCR with CFP and YFP fusion constructs was rapidly adapted by other groups and applied to monitor conformational changes of the bradykinin B2 receptor in endothelial cells, showing that the conformational dynamics of the receptor were altered under fluid shear stress in real time (Chachisvilis et al., 2006). The first report on receptors of a class C GPCR used the mGluR1α receptor as a model system for receptor activation (Tateyama et al., 2004). However, the general concept of this study was different, as the authors investigated movements within receptor dimers rather than movements within individual subunits of a receptor dimer. In this study, no agonist-dependent conformational change within a receptor dimer subunit was observed when the fluorophores were inserted into the intracellular loops 1 or 2 in combination with the C terminus. However, if receptor constructs were used that were solely tagged within the second or first intracellular loop, the outcome was different. Under those conditions, an increase of FRET was observed between the second intracellular loops, whereas a decrease of FRET was observed between the first intracellular loops. The data suggest that rearrangements within receptor dimers may occur during receptor activation. However, these data are not fully conclusive with respect to movements of the intracellular loop 1 or 2 and the C terminus within a receptor dimer subunit, as the receptor constructs were non-functional with respect to calcium signalling and may therefore have been non-responsive to the agonist even though binding was demonstrated.
Figure 2.
(a) Schematic representation of a GPCR modified with the cyan and yellow fluorescent protein. Crystal structure data from bovine rhodopsin and GFP were used to generate the figure. (b) Size comparison of GFP (left) and FlAsH (right). A phenylalanine side chain of GFP is shown to indicate that FlAsH was matched to size. Side and top view of both fluorophores are shown. (c) Changes in the relative fluorescence of CFP or FlAsH in response to 100 μM adenosine from a single HEK-293 cell expressing the A2A-FlAsH3-CFP construct. (d) Comparison of FRET signals in FlAsH/CFP- and CFP/YFP-labelled receptors. Normalized FRET ratios in response to 1 mM adenosine from single HEK-293 cells expressing A2A-FlAsH3-CFP (red) or A2A-CFP-YFP (black) are shown. (e) Same data, as in panel d, with the amplitude (response to 1 mM adenosine) set to 100% for both traces. Figure is reproduced with permission from Hoffmann et al. (2005). FlAsH, fluorescein arsenical hairpin binder; FRET, fluorescence resonance energy transfer; GFP, green fluorescent protein; GPCR, G-protein-coupled receptor.
A recent study using the β1-adrenoceptor linked conformational changes induced by inverse agonists with polymorphisms occurring naturally at the receptor (Rochais et al., 2007). Bisoprolol, metoprolol and carvedilol function as inverse agonists at the human β1-adrenoceptor (Hoffmann et al., 2004) and are used to treat chronic heart failure. Using two β1-adrenoceptor variants (Gly389 and Arg389) as FRET sensors, it was demonstrated that only carvedilol differed in its ability to induce conformational changes and that the change was larger for the Arg389 variant. This was consistent with a larger reduction of intracellular cAMP levels for carvedilol treatment of the Arg389 variant (Rochais et al., 2007). No such difference was observed for bisoprolol or metoprolol. As the Arg389 polymorphism is associated with a poor prognosis for heart failure (Mialet Perez et al., 2003) or at least with a different responsiveness to β-blockers (Lohse, 2004), these findings could have possible consequences for the clinical use of the β-blockers.
Outlook
The biophysical and biochemical approaches outlined in this review have led to a great increase in our understanding of what happens structurally when a GPCR is activated. A very different question arises from inverse agonism, and the clinical expectations for inverse agonists have recently been highlighted (Gilchrist and Blackmer, 2007). Two current papers have shown that the strength of inverse agonism or the change from agonism to inverse agonism is determined by rather subtle changes in the binding mode of the ligand (Holst et al., 2007; Miura et al., 2007). However, it is ill known how an active receptor conformation differs structurally with respect to different ligands, and this is also especially true for inverse agonists. Therefore, the quest for molecular explanations of functionally selective conformations is open.
The described FRET approach in living cells using FlAsH in combination with CFP is very promising, compared to the bulky YFP, as it allows a more detailed investigation of conformational changes that could occur in the third intracellular loop during agonist-induced receptor activation, a question that has not been addressed with any of the other approach yet. To our understanding, the different approaches described here are rather complementary than competitive, as all approaches have their strengths or weaknesses. For example, the FRET approaches have good kinetic resolution but currently provide little structural information, whereas the latter is true for the structural approaches. The common weakness of all approaches is currently the lack of a correlate of the observed effects with biological functions. More studies addressing the effects of different compounds on different biological functions will be needed to address this issue. The recently published work on the β2-adrenoceptor (Galandrin and Bouvier, 2006) or the D2 receptor (Lane et al., 2007) should encourage colleagues to follow that path. A significant input could also come from the industrial partners by data mining their archives for valuable information from different screening assays and promising compounds as research tools.
Acknowledgments
We apologize to all authors whose work is not mentioned or cited in this review, but who still made significant contributions to our current understanding of GPCR functions. This is solely due to space limitations. This work was supported by the DFG ‘Sonderforschungsbereich' SFB487 ‘Regulatory membrane proteins'.
Glossary
- FlAsH
fluorescein arsenical hairpin binder
- FRET
fluorescence resonance energy transfer
- GFP
green fluorescent protein
- GPCR
G-protein-coupled receptor
- TM
transmembrane domain
Footnotes
Conflict of interest
The authors state no conflict of interest.
References
- Adams SR, Campbell RE, Gross LA, Martin BR, Walkup GK, Yao Y, et al. New biarsenical ligands and tetracysteine motifs for protein labeling in vitro and in vivo: synthesis and biological applications. J Am Chem Soc. 2002;124:6063–6076. doi: 10.1021/ja017687n. [DOI] [PubMed] [Google Scholar]
- Adams SR, Harootunian AT, Buechler YJ, Taylor SS, Tsien RY. Fluorescence ratio imaging of cyclic AMP in single cells. Nature. 1991;349:694–697. doi: 10.1038/349694a0. [DOI] [PubMed] [Google Scholar]
- Barak LS, Ferguson SS, Zhang J, Caron MG. A beta-arrestin/green fluorescent protein biosensor for detecting G-protein-coupled receptor activation. J Biol Chem. 1997;272:27497–27500. doi: 10.1074/jbc.272.44.27497. [DOI] [PubMed] [Google Scholar]
- Bartl FJ, Fritze O, Ritter E, Herrmann R, Kuksa V, Palczewski K, et al. Partial agonism in a G-protein-coupled receptor: role of the retinal ring structure in rhodopsin activation. J Biol Chem. 2005;280:34259–34267. doi: 10.1074/jbc.M505260200. [DOI] [PubMed] [Google Scholar]
- Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol. 1998;54:94–104. [PubMed] [Google Scholar]
- Bisello A, Chorev M, Rosenblatt M, Monticelli L, Mierke DF, Ferrari SL. Selective ligand-induced stabilization of active and desensitized parathyroid hormone type 1 receptor conformations. J Biol Chem. 2002;277:38524–38530. doi: 10.1074/jbc.M202544200. [DOI] [PubMed] [Google Scholar]
- Bissantz C. Conformational changes of G-protein-coupled receptors during their activation by agonist binding. J Recept Signal Transduct Res. 2003;23:123–153. doi: 10.1081/rrs-120025192. [DOI] [PubMed] [Google Scholar]
- Cai K, Langen R, Hubbell WL, Khorana HG. Structure and function in rhodopsin: topology of the C-terminal polypeptide chain in relation to the cytoplasmic loops. Proc Natl Acad Sci USA. 1997;94:14267–14272. doi: 10.1073/pnas.94.26.14267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chachisvilis M, Zhang YL, Frangos JA. G-protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci USA. 2006;103:15463–15468. doi: 10.1073/pnas.0607224103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elling CE, Frimurer TM, Gerlach LO, Jorgensen R, Holst B, Schwartz TW. Metal ion site engineering indicates a global toggle switch model for seven-transmembrane receptor activation. J Biol Chem. 2006;281:17337–17346. doi: 10.1074/jbc.M512510200. [DOI] [PubMed] [Google Scholar]
- Elling CE, Thirstrup K, Holst B, Schwartz TW. Conversion of agonist site to metal-ion chelator site in the beta(2)-adrenergic receptor. Proc Natl Acad Sci USA. 1999;96:12322–12327. doi: 10.1073/pnas.96.22.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke JJ, Koshland DE., Jr Global flexibility in a sensory receptor: a site-directed cross-linking approach. Science. 1987;237:1596–1600. doi: 10.1126/science.2820061. [DOI] [PubMed] [Google Scholar]
- Farrens DL, Altenbach C, Yang K, Hubbell WL, Khorana HG. Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science. 1996;274:768–770. doi: 10.1126/science.274.5288.768. [DOI] [PubMed] [Google Scholar]
- Flower DR. Modelling G-protein-coupled receptors for drug design. Biochim Biophys Acta. 1999;1422:207–234. doi: 10.1016/s0304-4157(99)00006-4. [DOI] [PubMed] [Google Scholar]
- Galandrin S, Bouvier M. Distinct signaling profiles of beta1 and beta2 adrenoceptor ligands toward adenylyl cyclase and mitogen-activated protein kinase reveals the pluridimensionality of efficacy. Mol Pharmacol. 2006;70:1575–1584. doi: 10.1124/mol.106.026716. [DOI] [PubMed] [Google Scholar]
- Gether U. Uncovering molecular mechanisms involved in activation of G-protein-coupled receptors. Endocr Rev. 2000;21:90–113. doi: 10.1210/edrv.21.1.0390. [DOI] [PubMed] [Google Scholar]
- Gether U, Lin S, Ghanouni P, Ballesteros JA, Weinstein H, Kobilka BK. Agonists induce conformational changes in transmembrane domains III and VI of the beta2 adrenoceptor. EMBO J. 1997;16:6737–6747. doi: 10.1093/emboj/16.22.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gether U, Lin S, Kobilka BK. Fluorescent labeling of purified beta 2 adrenoceptor. Evidence for ligand-specific conformational changes. J Biol Chem. 1995;270:28268–28275. doi: 10.1074/jbc.270.47.28268. [DOI] [PubMed] [Google Scholar]
- Ghanouni P, Gryczynski Z, Steenhuis JJ, Lee TW, Farrens DL, Lakowicz JR, et al. Functionally different agonists induce distinct conformations in the G protein coupling domain of the beta 2 adrenoceptor. J Biol Chem. 2001a;276:24433–24436. doi: 10.1074/jbc.C100162200. [DOI] [PubMed] [Google Scholar]
- Ghanouni P, Steenhuis JJ, Farrens DL, Kobilka BK. Agonist-induced conformational changes in the G-protein-coupling domain of the beta 2 adrenoceptor. Proc Natl Acad Sci USA. 2001b;98:5997–6002. doi: 10.1073/pnas.101126198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist A, Blackmer T. G-protein-coupled receptor pharmacology: examining the edges between theory and proof. Curr Opin Drug Discov Devel. 2007;10:446–451. [PubMed] [Google Scholar]
- Granier S, Kim S, Shafer AM, Ratnala VR, Fung JJ, Zare RN, et al. Structure and conformational changes in the C-terminal domain of the beta2-adrenoceptor: insights from fluorescence resonance energy transfer studies. J Biol Chem. 2007;282:13895–13905. doi: 10.1074/jbc.M611904200. [DOI] [PubMed] [Google Scholar]
- Han SJ, Hamdan FF, Kim SK, Jacobson KA, Bloodworth LM, Li B, et al. Identification of an agonist-induced conformational change occurring adjacent to the ligand-binding pocket of the M(3) muscarinic acetylcholine receptor. J Biol Chem. 2005b;280:34849–34858. doi: 10.1074/jbc.M506711200. [DOI] [PubMed] [Google Scholar]
- Han SJ, Hamdan FF, Kim SK, Jacobson KA, Brichta L, Bloodworth LM, et al. Pronounced conformational changes following agonist activation of the M(3) muscarinic acetylcholine receptor. J Biol Chem. 2005a;280:24870–24879. doi: 10.1074/jbc.M500379200. [DOI] [PubMed] [Google Scholar]
- Hoffmann C, Gaietta G, Bunemann M, Adams SR, Oberdorff-Maass S, Behr B, et al. A FlAsH-based FRET approach to determine G-protein-coupled receptor activation in living cells. Nat Methods. 2005;2:171–176. doi: 10.1038/nmeth742. [DOI] [PubMed] [Google Scholar]
- Hoffmann C, Leitz MR, Oberdorf-Maass S, Lohse MJ, Klotz KN. Comparative pharmacology of human beta-adrenoceptor subtypes—characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:151–159. doi: 10.1007/s00210-003-0860-y. [DOI] [PubMed] [Google Scholar]
- Holst B, Elling CE, Schwartz TW. Partial agonism through a zinc-ion switch constructed between transmembrane domains III and VII in the tachykinin NK(1) receptor. Mol Pharmacol. 2000;58:263–270. doi: 10.1124/mol.58.2.263. [DOI] [PubMed] [Google Scholar]
- Holst B, Mokrosinski J, Lang M, Brandt E, Nygaard R, Frimurer TM, et al. Identification of an efficacy switch region in the ghrelin receptor responsible for interchange between agonism and inverse agonism. J Biol Chem. 2007;282:15799–15811. doi: 10.1074/jbc.M609796200. [DOI] [PubMed] [Google Scholar]
- Hubbell WL, Altenbach C. Investigation of structure and dynamics in membrane proteins using site-specific labeling. Curr Opin Struct Biol. 1994;4:566–573. [Google Scholar]
- Hubbell WL, Altenbach C, Hubbell CM, Khorana HG. Rhodopsin structure, dynamics, and activation: a perspective from crystallography, site-directed spin labeling, sulfhydryl reactivity, and disulfide cross-linking. Adv Protein Chem. 2003;63:243–290. doi: 10.1016/s0065-3233(03)63010-x. [DOI] [PubMed] [Google Scholar]
- Hubbell WL, Gross A, Langen R, Lietzow MA. Recent advances in site-directed spin labeling of proteins. Curr Opin Struct Biol. 1998;8:649–656. doi: 10.1016/s0959-440x(98)80158-9. [DOI] [PubMed] [Google Scholar]
- Keith DE, Anton B, Murray SR, Zaki PA, Chu PC, Lissin DV, et al. mu-Opioid receptor internalization: opiate drugs have differential effects on a conserved endocytic mechanism in vitro and in the mammalian brain. Mol Pharmacol. 1998;53:377–384. [PubMed] [Google Scholar]
- Kenakin T. Drug efficacy at G-protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2002;42:349–379. doi: 10.1146/annurev.pharmtox.42.091401.113012. [DOI] [PubMed] [Google Scholar]
- Kenakin T. New concepts in drug discovery: collateral efficacy and permissive antagonism. Nat Rev Drug Discov. 2005;4:919–927. doi: 10.1038/nrd1875. [DOI] [PubMed] [Google Scholar]
- Kobilka BK. G protein coupled receptor structure and activation. Biochim Biophys Acta. 2007;1768:794–807. doi: 10.1016/j.bbamem.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilka BK, Deupi X. Conformational complexity of G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28:397–406. doi: 10.1016/j.tips.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Lane JR, Powney B, Wise A, Rees S, Milligan G. Protean agonism at the dopamine D2 receptor: (S)-3-(3-hydroxyphenyl)-N-propylpiperidine is an agonist for activation of Go1 but an antagonist/inverse agonist for Gi1, Gi2, and Gi3. Mol Pharmacol. 2007;71:1349–1359. doi: 10.1124/mol.106.032722. [DOI] [PubMed] [Google Scholar]
- Li JH, Han SJ, Hamdan FF, Kim SK, Jacobson KA, Bloodworth LM, et al. Distinct structural changes in a G-protein-coupled receptor caused by different classes of agonist ligands. J Biol Chem. 2007;282:26284–26293. doi: 10.1074/jbc.M704875200. [DOI] [PubMed] [Google Scholar]
- Lohse MJ. Beta-adrenoceptor polymorphisms and heart failure. Trends Mol Med. 2004;10:55–58. doi: 10.1016/j.molmed.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Lohse MJ, Hoffmann C, Nikolaev VO, Vilardaga J-P, Bünemann M. Kinetics analysis of G-protein-coupled receptor signaling using fluorescence resonance energy transfer in living cells. Adv Protein Chem. 2007;74:167–188. doi: 10.1016/S0065-3233(07)74005-6. [DOI] [PubMed] [Google Scholar]
- Lu ZL, Coetsee M, White CD, Millar RP. Structural determinants for ligand–receptor conformational selection in a peptide G-protein-coupled receptor. J Biol Chem. 2007;282:17921–17929. doi: 10.1074/jbc.M610413200. [DOI] [PubMed] [Google Scholar]
- Lu ZL, Hulme EC. A network of conserved intramolecular contacts defines the off-state of the transmembrane switch mechanism in a seven-transmembrane receptor. J Biol Chem. 2000;275:5682–5686. doi: 10.1074/jbc.275.8.5682. [DOI] [PubMed] [Google Scholar]
- Marinissen MJ, Gutkind JS. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol Sci. 2001;22:368–376. doi: 10.1016/s0165-6147(00)01678-3. [DOI] [PubMed] [Google Scholar]
- Meng EC, Bourne HR. Receptor activation: what does the rhodopsin structure tell us. Trends Pharmacol Sci. 2001;22:587–593. doi: 10.1016/s0165-6147(00)01825-3. [DOI] [PubMed] [Google Scholar]
- Mialet Perez J, Rathz DA, Petrashevskaya NN, Hahn HS, Wagoner LE, Schwartz A, et al. Beta 1-adrenoceptor polymorphisms confer differential function and predisposition to heart failure. Nat Med. 2003;9:1300–1305. doi: 10.1038/nm930. [DOI] [PubMed] [Google Scholar]
- Miura SI, Kiya Y, Kanazawa T, Imaizumi S, Fujino M, Matsuo Y, et al. 2007Differential bonding interactions of inverse agonists of angiotensin II type 1 receptor in stabilizing the inactive-state Mol Endocrinol(in press), doi:10.1210/me.2007-312. [DOI] [PMC free article] [PubMed]
- Miyawaki A. Visualization of the spatial and temporal dynamics of intracellular signaling. Dev Cell. 2003;4:295–305. doi: 10.1016/s1534-5807(03)00060-1. [DOI] [PubMed] [Google Scholar]
- Miyawaki A, Tsien RY. Monitoring protein conformations and interactions by fluorescence resonance energy transfer between mutants of green fluorescent protein. Methods Enzymol. 2000;327:472–500. doi: 10.1016/s0076-6879(00)27297-2. [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Hoffmann C, Bunemann M, Lohse MJ, Vilardaga JP. Molecular basis of partial agonism at the neurotransmitter α2A-adrenoceptor and Gi-protein heterotrimer. J Biol Chem. 2006;281:24506–24511. doi: 10.1074/jbc.M603266200. [DOI] [PubMed] [Google Scholar]
- Okada T, Sugihara M, Bondar AN, Elstner M, Entel P, Buss V. The retinal conformation and its environment in rhodopsin in light of a new 2.2 Å crystal structure. J Mol Biol. 2004;342:571–583. doi: 10.1016/j.jmb.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, et al. Crystal structure of rhodopsin: a G-protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- Perez DM, Karnik SS. Multiple signaling states of G-protein-coupled receptors. Pharmacol Rev. 2005;57:147–161. doi: 10.1124/pr.57.2.2. [DOI] [PubMed] [Google Scholar]
- Resek JF, Farahbakhsh ZT, Hubbell WL, Khorana HG. Formation of the meta II photointermediate is accompanied by conformational changes in the cytoplasmic surface of rhodopsin. Biochemistry. 1993;32:12025–12032. doi: 10.1021/bi00096a012. [DOI] [PubMed] [Google Scholar]
- Ridge KD, Palczewski K. Visual rhodopsin sees the light: structure and mechanism of G protein signaling. J Biol Chem. 2007;282:9297–9301. doi: 10.1074/jbc.R600032200. [DOI] [PubMed] [Google Scholar]
- Rochais F, Vilardaga JP, Nikolaev VO, Bunemann M, Lohse MJ, Engelhardt S. Real-time optical recording of beta1-adrenoceptor activation reveals supersensitivity of the Arg389 variant to carvedilol. J Clin Invest. 2007;117:229–235. doi: 10.1172/JCI30012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkilde MM, Andersen MB, Nygaard R, Frimurer TM, Schwartz TW. Activation of the CXCR3 chemokine receptor through anchoring of a small molecule chelator ligand between TM-III, -IV, and -VI. Mol Pharmacol. 2007;71:930–941. doi: 10.1124/mol.106.030031. [DOI] [PubMed] [Google Scholar]
- Schertler GF. Structure of rhodopsin and the metarhodopsin I photointermediate. Curr Opin Struct Biol. 2005;15:408–415. doi: 10.1016/j.sbi.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Schwartz TW, Frimurer TM, Holst B, Rosenkilde MM, Elling CE. Molecular mechanism of 7TM receptor activation—a global toggle switch model. Annu Rev Pharmacol Toxicol. 2006;46:481–519. doi: 10.1146/annurev.pharmtox.46.120604.141218. [DOI] [PubMed] [Google Scholar]
- Sheikh SP, Vilardarga JP, Baranski TJ, Lichtarge O, Iiri T, Meng EC, et al. Similar structures and shared switch mechanisms of the beta2-adrenoceptor and the parathyroid hormone receptor. Zn(II) bridges between helices III and VI block activation. J Biol Chem. 1999;274:17033–17041. doi: 10.1074/jbc.274.24.17033. [DOI] [PubMed] [Google Scholar]
- Sheikh SP, Zvyaga TA, Lichtarge O, Sakmar TP, Bourne HR. Rhodopsin activation blocked by metal-ion-binding sites linking transmembrane helices C and F. Nature. 1996;383:347–350. doi: 10.1038/383347a0. [DOI] [PubMed] [Google Scholar]
- Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- Swaminath G, Deupi X, Lee TW, Zhu W, Thian FS, Kobilka TS, et al. Probing the β2 adrenoceptor binding site with catechol reveals differences in binding and activation by agonists and partial agonists. J Biol Chem. 2005;280:22165–22171. doi: 10.1074/jbc.M502352200. [DOI] [PubMed] [Google Scholar]
- Swaminath G, Xiang Y, Lee TW, Steenhuis J, Parnot C, Kobilka BK. Sequential binding of agonists to the beta2 adrenoceptor. Kinetic evidence for intermediate conformational states. J Biol Chem. 2004;279:686–691. doi: 10.1074/jbc.M310888200. [DOI] [PubMed] [Google Scholar]
- Tateyama M, Abe H, Nakata H, Saito O, Kubo Y. Ligand-induced rearrangement of the dimeric metabotropic glutamate receptor 1alpha. Nat Struct Mol Biol. 2004;11:637–642. doi: 10.1038/nsmb770. [DOI] [PubMed] [Google Scholar]
- Thirstrup K, Elling CE, Hjorth SA, Schwartz TW. Construction of a high affinity zinc switch in the kappa-opioid receptor. J Biol Chem. 1996;271:7875–7878. doi: 10.1074/jbc.271.14.7875. [DOI] [PubMed] [Google Scholar]
- Thurmond RL, Creuzenet C, Reeves PJ, Khorana HG. Structure and function in rhodopsin: peptide sequences in the cytoplasmic loops of rhodopsin are intimately involved in interaction with rhodopsin kinase. Proc Natl Acad Sci USA. 1997;94:1715–1720. doi: 10.1073/pnas.94.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trester-Zedlitz M, Burlingame A, Kobilka B, von Zastrow M. Mass spectrometric analysis of agonist effects on posttranslational modifications of the beta-2 adrenoceptor in mammalian cells. Biochemistry. 2005;44:6133–6143. doi: 10.1021/bi0475469. [DOI] [PubMed] [Google Scholar]
- Tsien RY. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Vauquelin G, Van Liefde I. G protein-coupled receptors: a count of 1001 conformations. Fundam Clin Pharmacol. 2005;19:45–56. doi: 10.1111/j.1472-8206.2005.00319.x. [DOI] [PubMed] [Google Scholar]
- Vilardaga JP, Bunemann M, Krasel C, Castro M, Lohse MJ. Measurement of the millisecond activation switch of G protein-coupled receptors in living cells. Nat Biotechnol. 2003;21:807–812. doi: 10.1038/nbt838. [DOI] [PubMed] [Google Scholar]
- Vilardaga JP, Frank M, Krasel C, Dees C, Nissenson RA, Lohse MJ. Differential conformational requirements for activation of G proteins and the regulatory proteins arrestin and G protein-coupled receptor kinase in the G protein-coupled receptor for parathyroid hormone (PTH)/PTH-related protein. J Biol Chem. 2001;276:33435–33443. doi: 10.1074/jbc.M011495200. [DOI] [PubMed] [Google Scholar]
- Vilardaga JP, Steinmeyer R, Harms GS, Lohse MJ. Molecular basis of inverse agonism in a G protein-coupled receptor. Nat Chem Biol. 2005;1:25–28. doi: 10.1038/nchembio705. [DOI] [PubMed] [Google Scholar]
- Ward SD, Hamdan FF, Bloodworth LM, Siddiqui NA, Li JH, Wess J. Use of an in situ disulfide cross-linking strategy to study the dynamic properties of the cytoplasmic end of transmembrane domain VI of the M3 muscarinic acetylcholine receptor. Biochemistry. 2006;45:676–685. doi: 10.1021/bi051503q. [DOI] [PubMed] [Google Scholar]
- Ward SD, Hamdan FF, Bloodworth LM, Wess J. Conformational changes that occur during M3 muscarinic acetylcholine receptor activation probed by the use of an in situ disulfide cross-linking strategy. J Biol Chem. 2002;277:2247–2257. doi: 10.1074/jbc.M107647200. [DOI] [PubMed] [Google Scholar]
- Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM, et al. Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci USA. 2003;100:10782–10787. doi: 10.1073/pnas.1834556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistler JL, Chuang HH, Chu P, Jan LY, von Zastrow M. Functional dissociation of mu opioid receptor signaling and endocytosis: implications for the biology of opiate tolerance and addiction. Neuron. 1999;23:737–746. doi: 10.1016/s0896-6273(01)80032-5. [DOI] [PubMed] [Google Scholar]
- Wieland K, Zuurmond HM, Krasel C, Ijzerman AP, Lohse MJ. Involvement of Asn-293 in stereospecific agonist recognition and in activation of the beta 2-adrenoceptor. Proc Natl Acad Sci USA. 1996;93:9276–9281. doi: 10.1073/pnas.93.17.9276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Parnot C, Deupi X, Ratnala VR, Swaminath G, Farrens D, et al. Coupling ligand structure to specific conformational switches in the beta(2)-adrenoceptor. Nat Chem Biol. 2006;2:417–422. doi: 10.1038/nchembio801. [DOI] [PubMed] [Google Scholar]
- Zeng FY, Hopp A, Soldner A, Wess J. Use of a disulfide cross-linking strategy to study muscarinic receptor structure and mechanisms of activation. J Biol Chem. 1999;274:16629–16640. doi: 10.1074/jbc.274.23.16629. [DOI] [PubMed] [Google Scholar]