Abstract
A number of human and animal herpes viruses encode G-protein coupled receptors with seven transmembrane (7TM) segments—most of which are clearly related to human chemokine receptors. It appears, that these receptors are used by the virus for immune evasion, cellular transformation, tissue targeting, and possibly for cell entry. In addition, many virally-encoded chemokine 7TM receptors have been suggested to be causally involved in pathogenic phenotypes like Kaposi sarcoma, atherosclerosis, HIV-infection and tumour development. The role of these receptors during the viral life cycle and in viral pathogenesis is still poorly understood. Here we focus on the current knowledge of structure, function and trafficking patterns of virally encoded chemokine receptors and further address the putative roles of these receptors in virus survival and host -cell and/or -immune system modulation. Finally, we highlight the emerging impact of these receptor on virus-mediated diseases.
Keywords: herpesvirus, 7TM receptors, structure–function, signalling, trafficking, disease
Introduction
Herpesviruses appear to have taken advantage of the sophisticated endogenous chemokine system of their hosts (Rosenkilde et al., 2001; Vischer et al., 2006), which comprises over 40 chemokines (chemotactic cytokines, that is, CC and CXC chemokines, one C and one CX3CL1 chemokine) and more than 16 chemokine receptors (CXCRs, CCRs, CX3CR and the Duffy antigen) (Murphy et al., 2000). During evolution, the viruses may have adopted such chemokine genes from their host and modified them as to benefit their own survival and propagation (Ahuja and Murphy, 1999; Rosenkilde et al., 2001; Smit et al., 2003; Michelson, 2004). Hence, herpesviruses have been found to not only encode chemokine receptors, chemokines and chemokine-binding proteins but also to modulate the expression of chemokines and their receptors in the host organism (Rosenkilde, 2005). Thus, studying these ‘pirated' receptors and how they have been shaped by the virus during evolution provides essential knowledge about (i) their role in viral survival strategies and (ii) some basic principles of seven TransMembrane (7TM) receptor function within the rhodopsin-like (class A) receptors.
Pirating the endogenous chemokine receptor system
The majority of virally encoded 7TM receptors are closely related to the family of endogenous human chemokine receptors. Many of these receptors bind endogenous chemokines and subsequently activate downstream signalling pathways. Few receptors, however, do not seem to resemble any of the endogenous 7TM receptor families and are therefore difficult to classify. For these ‘orphan' virally encoded 7TM receptors, no ligand has (yet) been identified. Nevertheless, such orphan receptors may still have a functional role, as exemplified by the BILF1 receptor from human and rhesus Epstein–Barr virus (EBV) (Beisser et al., 2005; Paulsen et al., 2005) or the human cytomegalovirus (HCMV)-encoded receptor unique long region 33 (UL33) (Waldhoer et al., 2002), which both signal in a constitutive—ligand independent—manner.
Rhodopsin-like 7TM receptors: structure and function
Despite the fact that around 50% of all drugs on the market target 7TM receptors (Drews, 2000; Hopkins and Groom, 2002), we still know very little about the structure of these receptors. In fact, until recently, only the crystal structure of bovine rhodopsin in its inactive, dark state, was available (Palczewski et al., 2000; (Li et al., 2004; Okada et al., 2004). However, last month the long awaited crystal structure of a non-rhodopsin 7TM receptor—the beta adrenergic receptor—was published by several groups, also in its inactive state (Cherezov et al., 2007; Rasmussen et al., 2007; Rosenbaum et al., 2007). Despite the lack of a crystal structure of the active form(s) of 7TM receptors, a plethora of functional data—based on biophysical (for example, site-directed spin labelling; Farrens et al., 1996; Hubbell et al., 2003), chemical (for example, cross-linking studies; Jacobsen et al., 2006) or molecular pharmacological approaches (for example, metal-ion site engineering; Elling et al., 1997, 2006; Rosenkilde et al., 1999, 2006b)—have resulted in a generally accepted model for 7TM receptor activation (Schwartz et al., 2006). According to this model, the transmembrane helices 3 (TM-3), TM-6 and TM-7 move away from each other at the intracellular side during receptor activation, creating space for heterotrimeric G proteins, arrestins and/or other adaptor/scaffolding proteins to interfere with the active receptors (Farrens et al., 1996; Shi et al., 2002; Hubbell et al., 2003; Schwartz et al., 2006). On the extracellular side, these three helices approach each other, constrained by either intramolecular bonds within the different helices or intermolecular bonds between the ligand (agonist in this case) and the receptor. A highly conserved Pro in TM-6 serves as a pivot for these helical movements during receptor activation. A tryptophane TrpVI:13/6.48 located two residues upstream of ProVI:15/6.50 in the so-called CWxP motif (see Figure 1) has been dedicated a special role in receptor activation. It functions as a rotamer, such as pointing towards TM-7 in the inactive receptor state, while changing conformation and pointing towards TM-5 in the active receptor state (Lin and Sakmar, 1996; Ballesteros et al., 2001; Elling et al., 2006; Schwartz et al., 2006).
Figure 1.
Serpentine model of a rhodopsin-like 7TM chemokine receptor. Black circles with a white letter represent conserved residues in each helix. Residues important for binding of intracellular adaptor/scaffolding or signalling molecules are indicated in grey. Two generally accepted numbering systems for transmembrane-located residues in 7TM receptors are used: (i) the Schwartz-Baldwin (Baldwin, 1993; Schwartz, 1994) numbering system, where each residue is labelled according to its actual position within a transmembrane helix and (ii) the Ballesteros–Weinstein system, which is based on labelling a highly conserved position in each helix with the number 50 (Ballesteros and Weinstein, 1995). For instance, the conserved arginine in the ‘DRY' motif at the bottom of TM-3 is given the number III:26/3.50, according to the Schwartz/Ballesteros numbering systems, respectively. This figure was adapted from Schwartz and Holst (2003) with permission.
Another region of importance for 7TM receptor activation is the ionic lock formed by interactions between the Arg in the highly conserved D/ERY motif at the intracellular end of TM-3 (ArgIII:26/3.50) and the adjacent Asp/Glu and an additional Asp/Glu in the intracellular loop III (IC-3) (see Figure 1; Ballesteros et al., 2001). However, 30% of rhodopsin-like 7TM receptors—including all chemokine receptors—contain a positively charged residue at the corresponding position in IC-3, which does not support an interaction with the Arg in the DRY motif. In accordance with this, it was recently shown that the introduction of an acidic residue in substitution with the positively charged residue in IC-3 resulted in a highly impaired CCR5 receptor function (Springael et al., 2007). Thus, the molecular interactions locking the inactive state in receptors devoid of this acidic residue in IC-3 must be different.
The highly conserved NPxxY motif in TM-7 (see Figure 1) is involved in receptor regulation through several mechanisms. One is the interaction between the TyrVII:20/7.53 (in the NPxxY motif) with the conserved Phe in helix 8 (located perpendicular to the membrane at the intracellular side) (Fritze et al., 2003). Another mechanism is through the highly conserved Asn (in the NPxxY motif) that is oriented towards TM-6 in the inactive state(s), whereas it is oriented towards the middle of TM-2 (towards Asp in position II:10/2.50) in the active state(s) (Govaerts et al., 2001; Urizar et al., 2005).
Virally encoded 7TM receptors: structure and function
Ligand binding
About 50% of all known virally encoded chemokine receptors have been ‘de-orphanized', that is, their chemokine-binding profile is known. Some of these receptors bind a rich repertoire of endogenous chemokines. For instance, open reading frame74 (ORF74) from HHV8 can be activated by ELR+ CXC chemokines (CXCL1-3; ELR+ CXC chemokines have a Glu-Leu-Arg (ELR) motif preceding the first Cys in the N terminus of the protein and are considered to promote cell proliferation/angiogenesis), and inhibited by ELR− CXC chemokines (CXCL10 and CXCL12, ELR− CXC chemokines lack this motif and are angiostatic chemokines), whereas a third group of ELR+ CXC chemokines have a Glu-Leu-Arg (ELR) motif preceding the first Cys in the N terminus of the protein and are considered to promote cell proliferation/angiogenesis, whereas a third group of ELR+ CXC chemokines (represented by CXCL5, −7 and −8) binds to the receptors with no change in receptor activity (Gershengorn et al., 1998; Rosenkilde et al., 1999). Another example is unique short region 28 (US28) from HCMV, which predominantly binds CC chemokines and the membrane-bound chemokine fractalkine CX3CL (Kledal et al., 1998; Casarosa et al., 2001; Waldhoer et al., 2002). For both of these receptors, chemokine binding was shown to be dependent upon the receptor N terminus (Ho et al., 1999; Rosenkilde et al., 2000; Casarosa et al., 2005). The C terminus, including helix 8, has been shown to be involved in ligand-binding recognition and signalling in ORF74 from HHV8/Kaposi's sarcoma-associated herpesvirus (KSHV) (Schwarz and Murphy, 2001; Liu et al., 2004; Verzijl et al., 2006). In contrast to the broad chemokine-binding pattern of ORF74 and US28, other virally encoded receptors such as the U51 and UL12 receptor families (HHV6 and HHV7) interact with only a few chemokines (Isegawa et al., 1998; Menotti et al., 1999; Bradel-Tretheway et al., 2003; Nakano et al., 2003; Tadagaki et al., 2005, 2007).
The nature of chemokine binding to their cognate receptor(s) is determined by the relatively large size of the chemokines (∼65–95 amino acids). Thus, in contrast to the small endogenous 7TM receptor ligands (for example, monoamines) that activate their cognate receptors by binding deep in the main receptor-binding pocket, chemokines more likely interfere with residues located in the extracellular receptor loops (Jarnagin et al., 1999; Schwarz and Wells, 2002) and the N terminus of the receptor (Schwarz and Wells, 2002; Rosenkilde and Schwartz, 2006). Upon chemokine binding, one of the main signalling pathways activated by these receptors is that of the pertussis toxin-sensitive Gαi/o proteins, resulting in intracellular calcium release and cell migration towards chemokine gradients (Fitzsimons et al., 2006).
Receptor activation
Virally encoded chemokine receptors essentially bear the same structural hallmarks important for receptor activation as endogenous 7TM receptors. However, detailed mutational studies of the ORF74 and US28 receptor families have greatly expanded our understanding of the impact of these different structural motifs on receptor activation. For instance, ORF74 encoded by the equine herpesvirus 2 possesses a ‘DTW' (Asp, Thr, Trp) instead of the well-conserved ‘DRY' motif at the end of TM-3. Here, this motif is dispensable for ligand-induced as well as constitutive receptor activity (Rosenkilde et al., 2005). Nevertheless, other virally encoded receptors—for example, US28 and the UL33-like receptors—strongly depend upon the Arg for proper signalling (Gruijthuijsen et al., 2002; Waldhoer et al., 2003). Furthermore, mutational and depletion studies of the C-terminal tail—including the ‘helix 8' motif (see Figure 1)—of these receptors have revealed its general importance in the regulation of ligand-binding selectivity, putatively through changes in receptor conformation as such. In addition, the C-terminal tail is also implicated in regulating receptor activation, G-protein recruitment and trafficking patterns of the receptors (Schwarz and Murphy, 2001; Mokros et al., 2002; Waldhoer et al., 2003; Liu et al., 2004; Verzijl et al., 2006).
Signalling
The constitutive signalling activity observed for ∼60% of virally encoded 7TMs stands in stark contrast to endogenous 7TM receptors, where constitutive activity may occur in some instances (for example, the ghrelin, the somatostatin and the EBI2 receptor; Seifert and Wenzel-Seifert, 2002; Rosenkilde et al., 2006a). In addition, the constitutive activity displayed by these roughly one-third of rhodopsin-like 7TM receptors seems to be highly dependent on assay conditions (for example, variations in receptor expression, availability of accessory G proteins and the choice of cell type; Seifert and Wenzel-Seifert, 2002). However, constitutive signalling activity is not a common trait for endogenous chemokine receptors (Rosenkilde et al., 2001; Thelen, 2001; Smit et al., 2003). Most of the virally encoded receptors activate a plethora of different pathways in a constitutive manner, that is, at the level of G proteins, MAP kinases and transcription factors (Waldhoer et al., 2002; Rosenkilde, 2005; Smit et al., 2007). One such example is the ORF74 receptor encoded by HHV8. This receptor signals constitutively through multiple pathways, including the activation of various G proteins, like, Gαq, Gαi and Gα12/13 (Bais et al., 1998; Munshi et al., 1999; Rosenkilde et al., 1999; Sodhi et al., 2000), MAP kinases including p38, p44/p42 and JNK/SAPK and transcription factors nuclear factor-κB, CREB and NFAT (McLean et al., 2004). The multitude of these actions ultimately leads to cellular transformation and production of angiogenic and inflammatory factors (Bais et al., 1998; Munshi et al., 1999; Sodhi et al., 2000; Couty et al., 2001; Montaner et al., 2001; Schwarz and Murphy, 2001; Smit et al., 2002). In addition, vascularized tumours develop when ORF74-expressing cells are injected into nude mice (Bais et al., 1998) and transgenic expression of ORF74 results in Kaposi's sarcoma (KS)-like lesions in mice (Yang et al., 2000; Guo et al., 2003; Montaner et al., 2003). Other examples of such promiscuously and constitutively signalling receptors are the UL33 receptor family (UL33, M33 and R33 from human, murine and rat CMV, respectively) and US28 from HCMV (Kledal et al., 1998; Casarosa et al., 2001, 2003; Waldhoer et al., 2002; McLean et al., 2004; Sherrill and Miller, 2006).
Trafficking of virally encoded chemokine receptors
Expression–Endocytosis–Recycling–Degradation of 7TM receptors: to live or to die
7TM receptor-mediated signalling is extensively regulated to guarantee an appropriate cell surface receptor density in a given physiological setting. First, receptors have to be properly expressed on the cell surface, hence, a quality control system monitoring the proper folding of these proteins and their transport from the endoplasmic reticulum to the cell surface needs to be in place (Duvernay et al., 2005). Once expressed on the cellular surface, receptors bind to ligands and set off intracellular signalling cascades, whereupon they are subjected to the process of endocytosis/internalization. Many 7TMs are endocytosed by a mechanism involving receptor phosphorylation, interaction with β-arrestins and concentration in clathrin-coated pits (Ferguson et al., 1998). However, the functional consequences of 7TM receptor endocytosis through this conserved cellular mechanism are diverse. Trafficking of internalized 7TMs by a rapid recycling pathway restores the complement of functional receptors in the plasma membrane and promotes re-sensitization of receptor-mediated signal transduction. In contrast, the sorting of internalized 7TMs to lysosomes promotes proteolytic downregulation of receptors, leading to a prolonged attenuation of cellular signal transduction (Tsao et al., 2001; Bartlett et al., 2005). Thus, the sorting of individual receptors between recycling and degradative fates is a fundamental mechanism and highly regulated process that controls the signalling capacity of 7TM receptors. Disturbance of such a tightly controlled system can manifest itself in the onset of diseases and has been described for constitutively active mutant signalling receptors (Parnot et al., 2002). Most of these constitutively active receptors are generally also constitutively internalized. To date, for most ligands targeting 7TM receptors, their effects on the trafficking properties of their cognate receptor are unknown. For virally encoded receptors, however, a few studies have addressed this issue in detail (see below; Waldhoer et al., 2003).
Several proteins have been identified that specifically target 7TM receptors—typically by interaction with their C-terminal domains—to either recycling (Cao et al., 1999; Gage et al., 2001; Hanyaloglu et al., 2005; Hanyaloglu and von, 2007; Wang et al., 2007) or degradative pathways (for reviews see also, Brady and Limbird, 2002; Bockaert et al., 2004). One such sorting protein is the GPCR-associated sorting protein-1 (GASP-1), which targets 7TM receptors to the degradative pathway (Whistler et al., 2002; Bartlett et al., 2005; Enquist et al., 2007; Martini et al., 2007; Tappe-Theodor et al., 2007; Thompson et al., 2007), whereas receptors that do not interact with GASP-1 recycle to the cell surface. In addition, whereas sorting nexin 1—such as GASP-1—has been reported to be involved in targeting 7TM receptors to the lysosomal pathway (Gullapalli et al., 2006), the N-ethylmaleimide-sensitive factor induces recycling of receptors back to the membrane (Gage et al., 2005).
Structural determinants involved in viral 7TM receptor trafficking
It has been shown for many 7TM receptors that their cytoplasmic tail is involved in specifying a receptor's cellular localization, scaffolding and/or trafficking through a variety of mechanisms. For proper surface expression, endoplasmic reticulum export signals have been identified in the C termini of many 7TM receptors (Duvernay et al., 2005). For instance, the lack of C-terminal residues R327 and R328 in the rat CMV-encoded receptor R33 results in an intracellularly retained receptor. These RR residues in the so-called RxxxxCxxGxLxxRRxxL motif are conserved among UL33 family members (Gruijthuijsen et al., 2002). It has been suggested that these positively charged basic residues are involved in the interaction of 7TMs with negatively charged phospholipid heads in the cellular membrane and/or these motifs may play a role in the interaction between 7TMs and proteins that guide correct 7TM receptor expression.
In addition, the N terminus and extracellular loops of 7TM receptors may require glycosylation to be exported to the cell surface (Duvernay et al., 2005). For the murine CMV-encoded receptor M33, it has been demonstrated that a 10 amino-acid stretch at the far N terminus of the receptor needs to be glycosylated to be expressed properly and perform its constitutive signalling properties (Sherrill and Miller, 2006).
Typically, ligand-induced receptor activation results in the phosphorylation of the C terminus, and in most cases, the subsequent recruitment of β-arrestins initiates receptor endocytosis (Ferguson et al., 1998). As for the US28 receptor, the C terminus has a high number of serine/threonine residues, providing a broad range of putative phosphorylation sites. Indeed, US28 is a constitutively and highly phosphorylated protein (Mokros et al., 2002; Miller et al., 2003) and its cytoplasmic tail per se is able to confer constitutive receptor endocytosis to even other 7TM receptors (Waldhoer et al., 2003; Huttenrauch et al., 2005). Interestingly, C-terminal-deficient mutants of US28 display higher constitutive activity than the wild-type receptor and are—in contrast to the wild-type receptor—predominantly expressed on the cell surface (Waldhoer et al., 2003). These findings suggest that the cellular localization of US28 (membrane vs intracellular) dictates the degree of its signalling capacity.
Taking a detour: virally encoded 7TMs traffic beyond ‘classical' pathways
Despite their similar signalling properties (that is, constitutive activity), virally encoded 7TM receptors can differ dramatically in their cellular localization and trafficking patterns. For instance, whereas ORF74 from HHV8 and murine herpesvirus 68 seem to be located predominantly on the cell surface (Wakeling et al., 2001; Waldhoer et al., 2003), other viral receptors, such as US28, US27 and UL33, from HCMV can be found in up to 80% in intracellular compartments (Fraile-Ramos et al., 2001; Waldhoer et al., 2003). The majority of these receptors are found in the membranes of intracellular organelles that are components of the endocytotic pathway, in particular multivesicular late endosomes/lysosomes or multivesicular bodies (Fraile-Ramos et al., 2001, 2002). For US28, numerous pathways have been suggested to be involved in its lysosomal targeting. For instance, although US28 is able to recruit arrestins (Miller et al., 2003), it has been shown to be constitutively endocytosed completely independent of β-arrestins (Fraile-Ramos et al., 2003; Droese et al., 2004). Moreover, US28 seems to be able to utilize caveolae/lipid rafts instead of a solely clathrin-dependent pathway (Droese et al., 2004) and recent data suggest that GASP-1 might be involved in the lysosomal targeting of this receptor (Heydorn et al., 2004 and personal observation).
Functional consequences of viral receptor trafficking
The multiple routes of internalization utilized by a virally encoded 7TM receptor highlight yet again the profound adaptational strategies of viruses: taking such a ‘cellular detour'—that is, skipping the most generally regulated processes of receptor internalization by β-arrestins/clathrin-dependent pathways—has not been demonstrated for many 7TM receptors. It was recently demonstrated that HCMV virions were budding into the membranes of these multivesicular endosomes, and it was suggested that this is the place where the viral receptors are incorporated into the viral membranes during the final stages of HCMV assembly (Fraile-Ramos et al., 2002). Hence, one might speculate that it poses an advantage for the virus to employ multiple pathways for its proteins to be targeted to places of virion assembly.
In addition, the cellular localization of a virally encoded 7TM receptor can influence the receptor-activating properties of a ligand. More specifically, depending on the receptor's cellular localization, the chemokine fractalkine/CX3CL1 acts either as an inverse agonist or an agonist on US28. Whereas CX3CL1 is a partial inverse agonist on the wild-type receptor, it acts as a partial agonist on C-terminal-deficient mutants of US28. This suggests that CX3CL1 is in fact an agonist on US28, but its agonism is masked by the constitutive endocytosis of the wild-type receptor. By blocking receptor endocytosis (that is, truncation of the C terminus) and consequently increasing the number of receptors on the cell surface at a given time, the true agonist properties of CX3CL1 are revealed (Waldhoer et al., 2003). Indeed, it is tempting to speculate that it might be equally advantageous for a viral protein to be expressed predominantly on the cell surface. For instance, Streblow et al. (1999) suggested that US28 mediates the migration of SMCs in response to the chemokines RANTES/CCL5 and MCP-1/CCL2. In this study, US28 was concentrated at the leading edge on the cell surface of SMCs when exposed to a chemokine gradient.
Thus, specific cellular environments could determine the expression and sorting of viral 7TM receptors and ultimately elucidate the functional relevance of this sorting process in viral infection.
Virally encoded 7TM receptors and their link to disease
As outlined in the previous sections, virally encoded 7TM receptors have acquired ‘skills' that might contribute significantly to virus-induced pathology, including the following: (i) binding of a broad spectrum of chemokines, (ii) displaying high constitutive receptor activity through promiscuous G-protein coupling or (iii) constitutively trafficking to intracellular compartments favouring virus assembly. Although our knowledge about the structural, signalling and trafficking capacities of these receptors in vitro accumulates, their actual role in virus-induced pathology in vivo remains vastly unknown (Table 1).
Table 1. Overview of virus-encoded chemokine (and ‘orphan') 7TM receptors.
| Family of virus | Virus | Gene name | Receptor type | Pharmacology | Known or presumed function | References |
|---|---|---|---|---|---|---|
| β-herpesviruses | Human CMV | US27 | Receptor (CCR-like) | Constitutively internalizing, no constitutive signalling, no ligands identified | Located on viral envelope | Fraile-Ramos et al. (2002); Waldhoer et al. (2002); Margulies and Gibson (2007) |
| US28 | CC and CX3C receptor | Constitutively signalling and internalizing CC and CX3C receptor | Chemokine scavenger function, virus attachment and cell fusion, smooth muscle cell migration, possible oncogene | Kledal et al. (1998); Streblow et al. (1999); Casarosa et al. (2001); Waldhoer et al. (2003); Maussang et al. (2006) | ||
| UL33 | Receptor (CCR-like) | Constitutively signalling and internalizing, no ligands identified | Expressed in viral particles and virus-infected cells | Margulies et al. (1996); Waldhoer et al. (2002) | ||
| UL78 | Putative receptor | Unknown | Unknown | Bankier DNA Seq. 1991 2:1 | ||
| Mouse CMV | M33 | Receptor (CCR-like) | Constitutively signalling, no ligands identified | Virulence factor, important for viral replication in salivary glands | Davis-Poynter et al. (1997); Waldhoer et al. (2002); Sherill et al. (2006) | |
| M78 | Putative receptor | Unknown | Virulence and replication | Oliveira and Shenk (2001) | ||
| Rat CMV | R33 | Receptor (CCR-like) | Constitutively signalling, no ligands identified | Virulence factor, important for viral replication in salivary glands | Beisser et al. (1998); Gruijthuijsen et al. (2002); Casarosa et al. (2003) | |
| R78 | Putative receptor | Unknown | Virulence and replication | Beisser et al. (1999) | ||
| HHV6 | U12 | CCR | Binds CC chemokines, not constitutively active | Unknown | Gompels et al. (1995); Isegawa et al. (1998) | |
| U51 | CCR | Binds CC chemokines, constitutively signalling | Downregulation of CCL5/RANTES expression | Milne et al. (2000); Fitzsimons et al. (2006) | ||
| HHV7 | U12 | Receptor (CCR-like) | Binds CC chemokines | Unknown | Nicholas et al. (1996); Tadagaki et al. (2005, 2007) | |
| U51 | Receptor (CCR-like) | Binds CC chemokines, not constitutively active | Unknown | Nicholas et al. (1996) Tadagaki et al. (2005, 2007) | ||
| γ1-herpesviruses | Epstein–Barr virus | BILF/A5 | Rhodopsin-like 7TM receptor | Constitutively signalling, no ligands identified | Expressed during lytic infection | Paulsen et al. (2005); Beisser et al. (2005) |
| γ2-herpesviruses | HHV8 (KSHV) (Kaposi's sarcoma virus) | ORF74-HHV8 | CXC receptor | Binding of and regulation by CXC chemokines Constitutively signalling, weakly constitutively internalizing | Lytic expression, possibly involved in Kaposi's sarcoma formation, antiapoptotic, angiogenic, tumorigenic | Bais et al. (1998); Rosenkilde et al. (1999); Yang et al. (2000) |
| Herpesvirus Saimiri (HVS) | ECRF3 | CXC-receptor | Broad-spectrum CXC chemokine binding, constitutively signalling | Unknown | Ahuja et al. (1993); Rosenkilde et al. (2004) | |
| Ateles herpesvirus (AtHV) | ORF74-AtHV | Receptor (CXC-like) | Unknown | Unknown | Albrecht et al. (2000) | |
| Mouse HV68 (MHV68) | ORF74-MHV68 | CXC receptor | Broad-spectrum CXC chemokine binding, not constitutively active | Lytic expression, reactivation from latency | Rochford et al. (2001); Lee et al. (2003); Moorman et al. (2003); Verzilj et al. (2004) | |
| Equine HV2 (EHV2) | E1 | CC receptor | Binds CCL11/eotaxin, not constitutively active | Unknown | Camarda et al. (1999) | |
| E6 | Receptor | Unknown | Unknown | Telford et al. (1995) | ||
| ORF74-EHV2 | Receptor (CXC-like) | Constitutively signalling, binds CXCL6 | Unknown | Rosenkilde et al. (2005) |
Abbreviations: CMV, cytomegalovirus; ORF, open reading frame; UL, unique long region.
Systematic description of virus family, virus, name of gene, receptor type, pharmacology, known or presumed function and selected references.
Viruses ‘turn' on their hosts
Herpesviruses are widespread pathogens, which establish a life-long latent and persistent infection after primary infection. Although primary infection in immunocompetent hosts is often asymptomatic, reactivation can lead to serious pathological conditions and in some cases to fatal diseases (Vischer et al., 2006). For instance, HCMV can cause febrile illness (mononucleosis-like symptoms in seldom cases) (Mocarski, 1995), but in immunocompromised recipients, such as HIV patients, bone marrow and organ transplant recipients, reactivation of HCMV may have a severe impact on lung (interstitial pneumonitis), brain and retinal physiology (Mocarski, 1995; Gandhi and Khanna, 2004). HCMV has also been associated with chronic diseases, including for example, vascular diseases (Stassen et al., 2006) and malignancies (Cobbs et al., 2002; Harkins et al., 2002). Whereas the primary infection of KSHV is asymptomatic, it is associated with three proliferative diseases, that is, KS, primary effusion lymphomas and some forms of multicentric Castleman's disease.
As for EBV, the primary infection generally occurs at an early age and is usually asymptomatic (for references see, Vischer et al., 2006). EBV infection acquired during adolescence or later may cause severe—but benign—infectious mononucleosis. The presence of EBV in various stages of B-cell development and its ability to infect certain epithelial cells have been shown to contribute to the development of diverse lymphomas (Hodgkin's disease, Burkitt's lymphoma) and carcinomas (nasopharyngeal and gastric carcinoma) in immunocompetent individuals (Middeldorp et al., 2003).
Primary infections with HHV6b and HHV7 often occur in early years of childhood and result in acute febrile illness, which—in some cases—is followed by the appearance of a mild rash. Clinical complications include febrile seizures, but may also cause meningo-encephalitis, encephalopathy and multiple sclerosis (De Bolle et al., 2005).
Cellular chemokine receptors not only control differentiation and trafficking of leukocytes but also appear to be implicated in various chronic inflammatory and vascular diseases and oncogenesis (Muller et al., 2001; Balkwill, 2004). In view of their crucial role in regulation of the immune system and pathological conditions, a role for the virus-encoded receptors in virus-induced pathology is anticipated.
A delicate balance between host and virus
To establish a persistent infection, the maintenance of a delicate balance between the host's immune system, limiting production of virus particles, and the virus is essential. A disturbed balance, for example, induced by inflammatory processes, can lead to reactivation and propagation of herpesviruses and the initiation and/or progression of, in some cases, fatal diseases (for references, see Rosenkilde et al., 2001; Vischer et al., 2006). Interestingly, most herpesvirus-encoded receptors bind a broad spectrum of chemokines, and some are able to internalize and deplete chemokines from the cellular surroundings (Billstrom et al., 1999). This suggests that these receptors may facilitate immune evasion. In some cases, the expression of viral receptors even leads to the migration of cells towards a chemokine gradient, thus likely contributing to viral dissemination (Streblow et al., 1999; Tadagaki et al., 2005). Moreover, by means of their constitutive activity and ability to bind chemokines, these receptors may also enhance inflammatory processes, including vascular disease and cancer.
Vascular diseases
Atherosclerosis is no longer considered a disorder of lipid accumulation, but is instead an inflammatory disease accompanied with the infiltration of macrophages, T lymphocytes and increased expression of adhesion molecules and cytokines (Paoletti et al., 2004). Increasing evidence indicates an HCMV link in vascular diseases, such as atherosclerosis, restenosis and vascular allograft rejection (Zhou et al., 1996; Hsich et al., 2001). HCMV is believed to exert its influence on vascular onset of the disease through activation of inflammatory mechanisms.
Vascular SMC migration is of critical importance for the development of atherosclerosis and other vascular diseases. Interestingly, HCMV infection of primary arterial SMCs results in significant cellular migration, which is dependent on US28 expression and CC chemokines (Streblow et al., 1999). This might provide a molecular basis for the correlative evidence that links HCMV to the acceleration of vascular disease. Likewise, the murine CMV-encoded M33 receptor appears to be essential for vascular SMC migration. Using siRNAs to eliminate M33 in infected cells leads to an effective attenuation of cell migration (Melnychuk et al., 2005). Moreover, the rat CMV-encoded R33 receptor seems to be critical in rat CMV-accelerated transplant vascular sclerosis and chronic rejection, as well as vascular SMC migration (Streblow et al., 2005).
HIV
Various epidemiological studies have shown a positive synergism between HIV and CMV infection (Kovacs et al., 1999). In early stages of HIV infection, the endogenous chemokine receptor CCR5 is used as co-entry factor, whereas CXCR4 is required in later stages (Ray and Doms, 2006). Interestingly, US28 also exhibits HIV co-receptor activity for both R5 and X4 HIV strains when co-expressed with CD4 (Pleskoff et al., 1997).
Oncogenic herpesviruses
It is well established that γ herpesviruses possess oncogenic potential, as they are able to transform cells upon infection (Flore et al., 1998). KSHV is the aetiological agent of KS (Ganem, 1997), primary effusion lymphomas and multicentric Casle's disease. EBV is associated with Burkitt's lymphoma and Hodgkin's disease (Young and Murray, 2003). Both γ herpesviruses encode for 7TM receptors, which display constitutively active signalling properties (Arvanitakis et al., 1997; Beisser et al., 2005). ORF74 is believed to act as a viral oncogene and considered a key determinant in the pathology of KS, as it possesses proliferative, angiogenic and antiapoptotic properties, recognized to drive the cell-transforming properties of KSHV. Vascularized tumours are formed when NIH-3T3 cells expressing ORF74 are injected into nude mice (Bais et al., 1998). Furthermore, transgenic mice expressing ORF74 within haematopoietic cells develop KS-like lesions in multiple organs (Yang et al., 2000; Holst et al., 2001; Guo et al., 2003; Montaner et al., 2003; Jensen et al., 2005). ORF74 was found to trigger a complex angiogenic programme in vivo (for example, placental growth factor, platelet-derived growth factor B and inducible NO synthase) contributing to formation of KS lesions in a paracrine manner (Grisotto et al., 2006).
Although a strong causative link between ORF74 and KS has been identified, expression of ORF74 is only limited in a subset of lytically infected cells in KS lesions (Kirshner et al., 1999; Sun et al., 1999). A so-called ‘hit and run' mechanism has been proposed, involving ORF74-induced autocrine immortalization of lytically infected endothelial cells, followed by a loss of ORF74 expression and paracrine vascular endothelial growth factor receptor2 activation (Bais et al., 2003). Alternatively, dysregulated expression of the viral programme may lead to non-lytic ORF74 expression (Sodhi et al., 2004). Recent data suggest that paracrine secretion of angiogenic factors from ORF74-expressing cells is required for sustained tumour growth (Montaner et al., 2006). Downregulation of ORF74 expression or targeting its downstream signalling components—such as Akt/PKB or haem oxygenase-1 (Marinissen et al., 2006)—in transgenic ORF74 mice results in the regression of KS lesions (Grisotto et al., 2006). Hence, it is feasible to target ORF74 or linked signalling pathways for potential treatment of KS.
The EBV-encoded receptor BILF1 has only recently been identified. Its ability to constitutively activate signalling pathways linked to proliferation (nuclear factor-κB), as well as its ability to downmodulate cellular antiviral responses, suggests oncogenic potential (Beisser et al., 2005; Paulsen et al., 2005). However, detailed analysis of this receptor in, for example, various B-cell stages and preferably transgenic animal models expressing BILF1 is yet required to evaluate its contribution to EBV-associated lymphomas and carcinomas.
Unlike these oncogenic viruses, HCMV infection fails to transform susceptible cells. Nevertheless, HCMV has been suggested to possess onco-modulatory properties (Soderberg et al., 1996; Cinatl et al., 2004). Although the causative role for HCMV in the development of malignancies remains to be established, various HCMV proteins have been detected in tumour tissues with high frequency (Cobbs et al., 2002; Harkins et al., 2002). HCMV enhances the malignant behaviour of tumour cells by upregulating different growth factors and cytokines, resulting in increased cell survival, proliferation and angiogenesis (Cinatl et al., 2004). Hence, HCMV is considered an onco-modulatory rather than an oncogenic virus.
Recent studies revealed that the HCMV-encoded receptor US28 induces a pro-angiogenic and transformed cellular phenotype in NIH-3T3 fibroblasts by upregulating the expression of the vascular endothelial growth factor. In vivo, US28-expressing fibroblasts promote tumorigenesis in a nude mouse model (Maussang et al., 2006). However, the constitutively inactive mutant R129A of US28 (Waldhoer et al., 2003) induces delayed and attenuated tumour formation, indicating the importance of constitutive receptor signalling activity in the early onset of tumour development (Maussang et al., 2006). In addition, US28 expression resulted in an angiogenic phenotype in HCMV-infected glioblastoma cells (Maussang et al., 2006). The constitutive activity of US28 and its ability to bind a variety of CC chemokines might facilitate tumour progression after infection, as chemokine levels have been shown to be markedly increased in certain types of cancer (Balkwill, 2004). However, the expression of US28 does not induce or enhance transformation in all cell types. For instance, US28 induces rapid apoptosis in a variety of cell types (Pleskoff et al., 2005), indicating that the cellular context dictates the behaviour of this viral receptor. Moreover, US28—when expressed in two different melanoma cell lines—prevents (Seidl et al., 2006; Schaider et al., 2007) rather then promotes (Maussang et al., 2006) tumour formation in mice.
Translational studies—spanning from detailed characterization of virus receptor signalling and ligand binding to the putative roles of these receptors in virus-induced pathology—are required to gain further insights into this intriguing class of receptors. Given the huge knowledge—and the many successes—in drug discovery and development within the rhodopsin-like 7TM receptors, it is tempting to suggest that the receptors encoded by human herpesviruses may serve as innovative and novel drug targets.
Acknowledgments
We thank Thue W Schwartz and Rob Leurs for initiating and nourishing our passion to study virally encoded chemokine receptors. We are grateful to all members of the Waldhoer-lab for critically reading this manuscript and Henry Vischer for the design of Figure 2. MMR was supported by the Danish Medical Research Council, the Novo Nordisk Foundation, the Carlsberg Foundation and the European Community's Sixth Framework Program Grant LSHB-CT-2005-518167. MJS was supported by the Netherlands Organization for Scientific Research. MW was supported by FWF Grant P18723-B11 of the Austrian Science Foundation, the ÖNB Grant 12552 from the Austrian National Bank and by the LANYAR foundation.
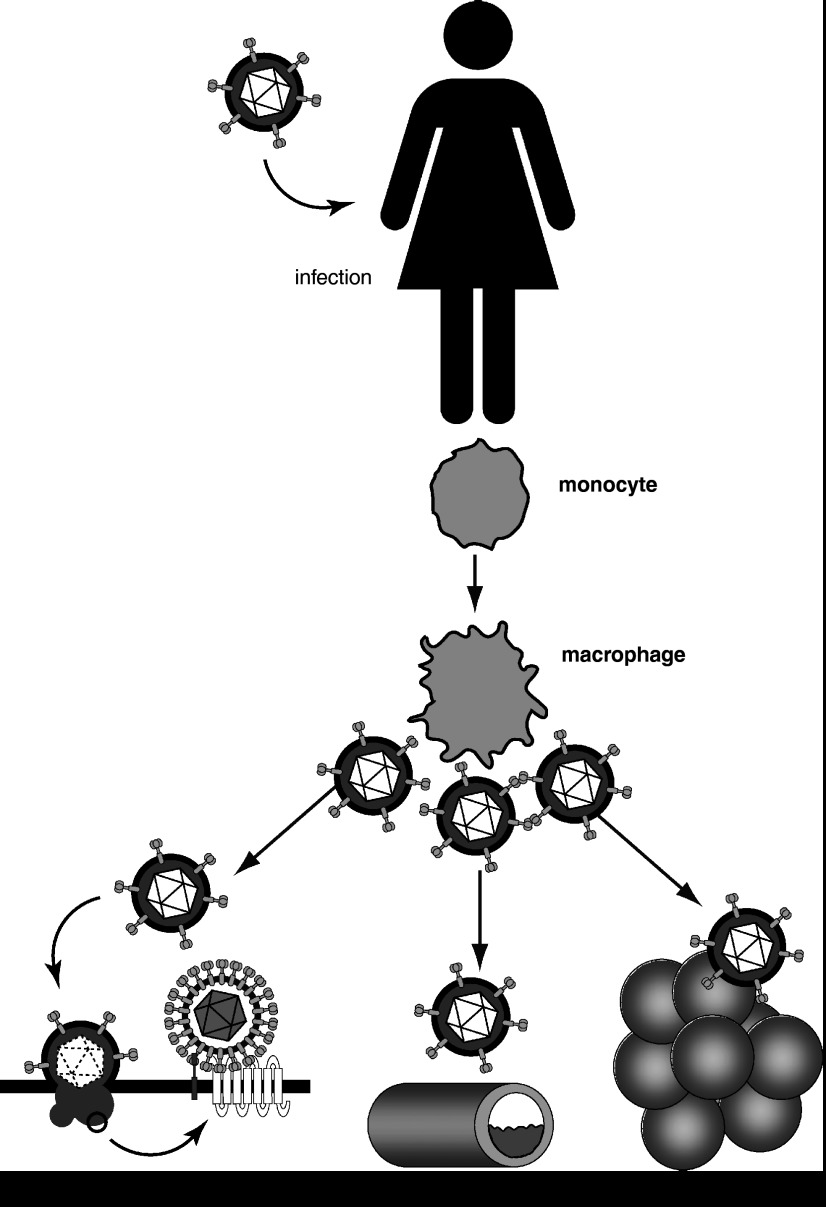
Figure 2.
Herpesviruses encode one or more 7TM receptors that are expressed in host cells after viral infection. Some of these receptors act as a HIV co-receptor, whereas others might play a role in the progression of cardiovascular diseases or cancer.
Glossary
- KSHV
Kaposi's sarcoma-associated herpesvirus
- ORF
open reading frame
- UL
unique long region
- US
unique short region
Footnotes
Conflict of interest
The authors state no conflict of interest.
References
- Ahuja SK, Murphy PM.1999Viral mimicry of chemokines and chemokine receptorsIn: Herbert CA (ed).Chemokines in Disease Humana Press Inc.: Totowa, NJ; 235–251. [Google Scholar]
- Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn MC, Cesarman E. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature. 1997;385:347–350. doi: 10.1038/385347a0. [DOI] [PubMed] [Google Scholar]
- Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka EG, Gutkind JS, et al. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391:86–89. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- Bais C, Van GA, Eroles P, Mutlu A, Chiozzini C, Dias S, et al. Kaposi's sarcoma associated herpesvirus G protein-coupled receptor immortalizes human endothelial cells by activation of the VEGF receptor-2/KDR. Cancer Cell. 2003;3:131–143. doi: 10.1016/s1535-6108(03)00024-2. [DOI] [PubMed] [Google Scholar]
- Baldwin JM. The probable arrangement of the helices in G protein-coupled receptors. EMBO J. 1993;12:1693–1703. doi: 10.1002/j.1460-2075.1993.tb05814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- Ballesteros JA, Jensen AD, Liapakis G, Rasmussen SG, Shi L, Gether U, et al. Activation of the beta 2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J Biol Chem. 2001;276:29171–29177. doi: 10.1074/jbc.M103747200. [DOI] [PubMed] [Google Scholar]
- Ballesteros JA, Weinstein H. Receptor Molecular Biology. Academic Press: New York; 1995. Integrated methods for the construction of three-dimensional models and computational probing of structure–function relations in G protein-coupled receptors. In: Sealfon SC (ed) pp. 366–428. [Google Scholar]
- Bartlett SE, Enquist J, Hopf FW, Lee JH, Gladher F, Kharazia V, et al. Dopamine responsiveness is regulated by targeted sorting of D2 receptors. Proc Natl Acad Sci USA. 2005;102:11521–11526. doi: 10.1073/pnas.0502418102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisser PS, Grauls G, Bruggeman CA, Vink C. Deletion of the R78 G protein-coupled receptor gene from rat cytomegalovirus results in an attenuated, syncytium-inducing mutant strain. J Virol. 1999;73:7218–7230. doi: 10.1128/jvi.73.9.7218-7230.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisser PS, Verzijl D, Gruijthuijsen YK, Beuken E, Smit MJ, Leurs R, et al. The Epstein–Barr virus BILF1 gene encodes a G protein-coupled receptor that inhibits phosphorylation of RNA-dependent protein kinase. J Virol. 2005;79:441–449. doi: 10.1128/JVI.79.1.441-449.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisser PS, Vink C, Van Dam JG, Grauls G, Vanherle SJ, Bruggeman CA. The R33 G protein-coupled receptor gene of rat cytomegalovirus plays an essential role in the pathogenesis of viral infection. J Virol. 1998;72:2352–2363. doi: 10.1128/jvi.72.3.2352-2363.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billstrom MA, Lehman LA, Scott Worthen G. Depletion of extracellular RANTES during human cytomegalovirus infection of endothelial cells. Am J Respir Cell Mol Biol. 1999;21:163–167. doi: 10.1165/ajrcmb.21.2.3673. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Fagni L, Dumuis A, Marin P. GPCR interacting proteins (GIP) Pharmacol Ther. 2004;103:203–221. doi: 10.1016/j.pharmthera.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Bradel-Tretheway BG, Zhen Z, Dewhurst S. Effects of codon-optimization on protein expression by the human herpesvirus 6 and 7 U51 open reading frame. J Virol Methods. 2003;111:145–156. doi: 10.1016/s0166-0934(03)00173-3. [DOI] [PubMed] [Google Scholar]
- Brady AE, Limbird LE. G protein-coupled receptor interacting proteins: emerging roles in localization and signal transduction. Cell Signal. 2002;14:297–309. doi: 10.1016/s0898-6568(01)00239-x. [DOI] [PubMed] [Google Scholar]
- Camarda G, Spinetti G, Bernardini G, Mair C, Davis-Poynter N, Capogrossi MC, et al. The equine herpesvirus 2 E1 open reading frame encodes a functional chemokine receptor. J Virol. 1999;73:9843–9848. doi: 10.1128/jvi.73.12.9843-9848.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature. 1999;401:286–290. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- Casarosa P, Bakker RA, Verzijl D, Navis M, Timmerman H, Leurs R, et al. Constitutive signaling of the human cytomegalovirus-encoded chemokine receptor US28. J Biol Chem. 2001;276:1133–1137. doi: 10.1074/jbc.M008965200. [DOI] [PubMed] [Google Scholar]
- Casarosa P, Gruijthuijsen YK, Michel D, Beisser PS, Holl J, Fitzsimons CP, et al. Constitutive signaling of the human cytomegalovirus-encoded receptor UL33 differs from that of its rat cytomegalovirus homolog R33 by promiscuous activation of G proteins of the Gq, Gi, and Gs classes. J Biol Chem. 2003;278:50010–50023. doi: 10.1074/jbc.M306530200. [DOI] [PubMed] [Google Scholar]
- Casarosa P, Waldhoer M, LiWang PJ, Vischer HF, Kledal T, Timmerman H, et al. CC and CX3C chemokines differentially interact with the N terminus of the human cytomegalovirus-encoded US28 receptor. J Biol Chem. 2005;280:3275–3285. doi: 10.1074/jbc.M407536200. [DOI] [PubMed] [Google Scholar]
- Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinatl J, Scholz M, Kotchetkov R, Vogel JU, Doerr HW. Molecular mechanisms of the modulatory effects of HCMV infection in tumor cell biology. Trends Mol Med. 2004;10:19–23. doi: 10.1016/j.molmed.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Cobbs CS, Harkins L, Samanta M, Gillespie GY, Bharara S, King PH, et al. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002;62:3347–3350. [PubMed] [Google Scholar]
- Couty JP, Geras-Raaka E, Weksler BB, Gershengorn MC. Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor signals through multiple pathways in endothelial cells. J Biol Chem. 2001;276:33805–33811. doi: 10.1074/jbc.M104631200. [DOI] [PubMed] [Google Scholar]
- Davis-Poynter NJ, Lynch DM, Vally H, Shellam GR, Rawlinson WD, Barrell BG, et al. Identification and characterization of a G protein-coupled receptor homolog encoded by murine cytomegalovirus. J Virol. 1997;71:1521–1529. doi: 10.1128/jvi.71.2.1521-1529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bolle L, Naesens L, De CE. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. 2005;18:217–245. doi: 10.1128/CMR.18.1.217-245.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews J. Drug discovery: a historical perspective. Science. 2000;287:1960–1964. doi: 10.1126/science.287.5460.1960. [DOI] [PubMed] [Google Scholar]
- Droese J, Mokros T, Hermosilla R, Schulein R, Lipp M, Hopken UE, et al. HCMV-encoded chemokine receptor US28 employs multiple routes for internalization. Biochem Biophys Res Commun. 2004;322:42–49. doi: 10.1016/j.bbrc.2004.07.076. [DOI] [PubMed] [Google Scholar]
- Duvernay MT, Filipeanu CM, Wu G. The regulatory mechanisms of export trafficking of G protein-coupled receptors. Cell Signal. 2005;17:1457–1465. doi: 10.1016/j.cellsig.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Elling CE, Frimurer TM, Gerlach LO, Jorgensen R, Holst B, Schwartz TW. Metal-ion site engineering indicating a global toggle switch model for 7TM receptor activation. J Biol Chem. 2006;281:17337–17346. doi: 10.1074/jbc.M512510200. [DOI] [PubMed] [Google Scholar]
- Elling CE, Thirstrup K, Nielsen SM, Hjorth SA, Schwartz TW. Engineering of metal-ion sites as distance constraints in structural and functional analysis of 7TM receptors. Fold Design. 1997;2:S76–S80. doi: 10.1016/s1359-0278(97)00068-0. [DOI] [PubMed] [Google Scholar]
- Enquist J, Skroder C, Whistler JL, Leeb-Lundberg LM. Kinins promote B2 receptor endocytosis and delay constitutive B1 receptor endocytosis. Mol Pharmacol. 2007;71:494–507. doi: 10.1124/mol.106.030858. [DOI] [PubMed] [Google Scholar]
- Farrens DL, Altenbach C, Yang K, Hubbell WL, Khorana HG. Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science. 1996;274:768–770. doi: 10.1126/science.274.5288.768. [DOI] [PubMed] [Google Scholar]
- Ferguson SS, Zhang J, Barak LS, Caron MG. Molecular mechanisms of G protein-coupled receptor desensitization and resensitization. Life Sci. 1998;62:1561–1565. doi: 10.1016/s0024-3205(98)00107-6. [DOI] [PubMed] [Google Scholar]
- Fitzsimons CP, Gompels UA, Verzijl D, Vischer HF, Mattick C, Leurs R, et al. Chemokine-directed trafficking of receptor stimulus to different G proteins: selective inducible and constitutive signaling by human herpesvirus 6-encoded chemokine receptor U51. Mol Pharmacol. 2006;69:888–898. doi: 10.1124/mol.105.015222. [DOI] [PubMed] [Google Scholar]
- Flore O, Rafii S, Ely S, O'Leary JJ, Hyjek EM, Cesarman E. Transformation of primary human endothelial cells by Kaposi's sarcoma-associated herpesvirus. Nature. 1998;394:588–592. doi: 10.1038/29093. [DOI] [PubMed] [Google Scholar]
- Fraile-Ramos A, Kledal TN, Pelchen-Matthews A, Bowers K, Schwartz TW, Marsh M. The human cytomegalovirus US28 protein is located in endocytic vesicles and undergoes constitutive endocytosis and recycling. Mol Biol Cell. 2001;12:1737–1749. doi: 10.1091/mbc.12.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraile-Ramos A, Kohout TA, Waldhoer M, Marsh M. Endocytosis of the viral chemokine receptor US28 does not require beta-arrestins but is dependent on the clathrin-mediated pathway. Traffic. 2003;4:243–253. doi: 10.1034/j.1600-0854.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- Fraile-Ramos A, Pelchen-Matthews A, Kledal TN, Browne H, Schwartz TW, Marsh M. Localization of HCMV UL33 and US27 in endocytic compartments and viral membranes. Traffic. 2002;3:218–232. doi: 10.1034/j.1600-0854.2002.030307.x. [DOI] [PubMed] [Google Scholar]
- Fritze O, Filipek S, Kuksa V, Palczewski K, Hofmann KP, Ernst OP. Role of the conserved NPxxY(x)5,6F motif in the rhodopsin ground state and during activation. Proc Natl Acad Sci USA. 2003;100:2290–2295. doi: 10.1073/pnas.0435715100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage RM, Kim KA, Cao TT, von Zastrow M. A transplantable sorting signal that is sufficient to mediate rapid recycling of G protein-coupled receptors. J Biol Chem. 2001;276:44712–44720. doi: 10.1074/jbc.M107417200. [DOI] [PubMed] [Google Scholar]
- Gage RM, Matveeva EA, Whiteheart SW, von ZM. Type I PDZ ligands are sufficient to promote rapid recycling of G protein-coupled receptors independent of binding to N-ethylmaleimide-sensitive factor. J Biol Chem. 2005;280:3305–3313. doi: 10.1074/jbc.M406934200. [DOI] [PubMed] [Google Scholar]
- Gandhi MK, Khanna R. Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect Dis. 2004;4:725–738. doi: 10.1016/S1473-3099(04)01202-2. [DOI] [PubMed] [Google Scholar]
- Ganem D. KSHV and Kaposi's sarcoma: the end of the beginning. Cell. 1997;91:157–160. doi: 10.1016/s0092-8674(00)80398-0. [DOI] [PubMed] [Google Scholar]
- Gershengorn MC, Geras-Raaka E, Varma A, Clark-Lewis I. Chemokines activate Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor in mammalian cells in culture. J Clin Invest. 1998;102:1469–1472. doi: 10.1172/JCI4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompels UA, Nicholas J, Lawrence G, Jones M, Thomson BJ, Martin MED, et al. The DNA sequence of human herpesvirus-6: structure, coding content, and genome evolution. Virology. 1995;209:29–51. doi: 10.1006/viro.1995.1228. [DOI] [PubMed] [Google Scholar]
- Govaerts C, Lefort A, Costagliola S, Wodak SJ, Ballesteros JA, Van SJ, et al. A conserved Asn in transmembrane helix 7 is an on/off switch in the activation of the thyrotropin receptor. J Biol Chem. 2001;276:22991–22999. doi: 10.1074/jbc.M102244200. [DOI] [PubMed] [Google Scholar]
- Grisotto MG, Garin A, Martin AP, Jensen KK, Chan P, Sealfon SC, et al. The human herpesvirus 8 chemokine receptor vGPCR triggers autonomous proliferation of endothelial cells. J Clin Invest. 2006;116:1264–1273. doi: 10.1172/JCI26666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruijthuijsen YK, Casarosa P, Kaptein SJ, Broers JL, Leurs R, Bruggeman CA, et al. The rat cytomegalovirus R33-encoded G protein-coupled receptor signals in a constitutive fashion. J Virol. 2002;76:1328–1338. doi: 10.1128/JVI.76.3.1328-1338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullapalli A, Wolfe BL, Griffin CT, Magnuson T, Trejo J. An essential role for SNX1 in lysosomal sorting of protease-activated receptor-1: evidence for retromer-, Hrs-, and Tsg101-independent functions of sorting nexins. Mol Biol Cell. 2006;17:1228–1238. doi: 10.1091/mbc.E05-09-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo HG, Sadowska M, Reid W, Tschachler E, Hayward G, Reitz M. Kaposi's sarcoma-like tumors in a human herpesvirus 8 ORF74 transgenic mouse. J Virol. 2003;77:2631–2639. doi: 10.1128/JVI.77.4.2631-2639.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyaloglu AC, McCullagh E, von ZM. Essential role of Hrs in a recycling mechanism mediating functional resensitization of cell signaling. EMBO J. 2005;24:2265–2283. doi: 10.1038/sj.emboj.7600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyaloglu AC, von ZM. A novel sorting sequence in the beta2-adrenergic receptor switches recycling from default to the Hrs-dependent mechanism. J Biol Chem. 2007;282:3095–3104. doi: 10.1074/jbc.M605398200. [DOI] [PubMed] [Google Scholar]
- Harkins L, Volk AL, Samanta M, Mikolaenko I, Britt WJ, Bland KI, et al. Specific localisation of human cytomegalovirus nucleic acids and proteins in human colorectal cancer. Lancet. 2002;360:1557–1563. doi: 10.1016/S0140-6736(02)11524-8. [DOI] [PubMed] [Google Scholar]
- Heydorn A, Sondergaard BP, Ersboll B, Holst B, Nielsen FC, Haft CR, et al. A library of 7TM receptor C-terminal tails. Interactions with the proposed post-endocytic sorting proteins ERM-binding phosphoprotein 50 (EBP50), N-ethylmaleimide-sensitive factor (NSF), sorting nexin 1 (SNX1), and G protein-coupled receptor-associated sorting protein (GASP) J Biol Chem. 2004;279:54291–54303. doi: 10.1074/jbc.M406169200. [DOI] [PubMed] [Google Scholar]
- Ho HH, Du D, Gershengorn MC. The N terminus of Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor is necessary for high affinity chemokine binding but not for constitutive activity. J Biol Chem. 1999;274:31327–31332. doi: 10.1074/jbc.274.44.31327. [DOI] [PubMed] [Google Scholar]
- Holst PJ, Rosenkilde MM, Manfra D, Chen SC, Wiekowski MT, Holst B, et al. Tumorigenesis induced by the HHV8-encoded chemokine receptor requires ligand modulation of high constitutive activity. J Clin Invest. 2001;108:1789–1796. doi: 10.1172/JCI13622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1:727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- Hsich E, Zhou YF, Paigen B, Johnson TM, Burnett MS, Epstein SE. Cytomegalovirus infection increases development of atherosclerosis in apolipoprotein-E knockout mice. Atherosclerosis. 2001;156:23–28. doi: 10.1016/s0021-9150(00)00608-0. [DOI] [PubMed] [Google Scholar]
- Hubbell WL, Altenbach C, Hubbell CM, Khorana HG. Rhodopsin structure, dynamics, and activation: a perspective from crystallography, site-directed spin labeling, sulfhydryl reactivity, and disulfide cross-linking. Adv Protein Chem. 2003;63:243–290. doi: 10.1016/s0065-3233(03)63010-x. [DOI] [PubMed] [Google Scholar]
- Huttenrauch F, Pollok-Kopp B, Oppermann M. G protein-coupled receptor kinases promote phosphorylation and beta-arrestin-mediated internalization of CCR5 homo- and hetero-oligomers. J Biol Chem. 2005;280:37503–37515. doi: 10.1074/jbc.M500535200. [DOI] [PubMed] [Google Scholar]
- Isegawa Y, Ping Z, Nakano K, Sugimoto N, Yamanishi K. Human herpesvirus 6 open reading frame U12 encodes a functional B-chemokine receptor. J Virology. 1998;72:6104–6112. doi: 10.1128/jvi.72.7.6104-6112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen RB, Sale KL, Ayson MJ, Novak P, Hong J, Lane P, et al. Structure and dynamics of dark-state bovine rhodopsin revealed by chemical cross-linking and high-resolution mass spectrometry. Protein Sci. 2006;15:1303–1317. doi: 10.1110/ps.052040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarnagin K, Grunberger D, Mulkins M, Wong B, Hemmerich S, Paavola C, et al. Identification of surface residues of the monocyte chemotactic protein 1 that affect signaling through the receptor CCR2. Biochemistry. 1999;38:16167–16177. doi: 10.1021/bi9912239. [DOI] [PubMed] [Google Scholar]
- Jensen KK, Manfra DJ, Grisotto MG, Martin AP, Vassileva G, Kelley K, et al. The human herpes virus 8-encoded chemokine receptor is required for angioproliferation in a murine model of Kaposi's sarcoma. J Immunol. 2005;174:3686–3694. doi: 10.4049/jimmunol.174.6.3686. [DOI] [PubMed] [Google Scholar]
- Kirshner JR, Staskus K, Haase A, Lagunoff M, Ganem D. Expression of the open reading frame 74 (G-protein-coupled receptor) gene of Kaposi's sarcoma (KS)-associated herpesvirus: implications for KS pathogenesis. J Virol. 1999;73:6006–6014. doi: 10.1128/jvi.73.7.6006-6014.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kledal TN, Rosenkilde MM, Schwartz TW. Selective recognition of the membrane-bound CX3C chemokine, fractalkine, by the human cytomegalovirus-encoded broad-spectrum receptor US28. FEBS Lett. 1998;441:209–214. doi: 10.1016/s0014-5793(98)01551-8. [DOI] [PubMed] [Google Scholar]
- Kovacs A, Schluchter M, Easley K, Demmler G, Shearer W, La RP, et al. Cytomegalovirus infection and HIV-1 disease progression in infants born to HIV-1-infected women. Pediatric pulmonary and cardiovascular complications of vertically transmitted HIV infection study group. N Engl J Med. 1999;341:77–84. doi: 10.1056/NEJM199907083410203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BJ, Koszinowski UH, Sarawar SR, Adler H. A gammaherpesvirus G protein-coupled receptor homologue is required for increased viral replication in response to chemokines and efficient reactivation from latency. J Immunol. 2003;170:243–251. doi: 10.4049/jimmunol.170.1.243. [DOI] [PubMed] [Google Scholar]
- Li J, Edwards PC, Burghammer M, Villa C, Schertler GF. Structure of bovine rhodopsin in a trigonal crystal form. J Mol Biol. 2004;343:1409–1438. doi: 10.1016/j.jmb.2004.08.090. [DOI] [PubMed] [Google Scholar]
- Lin SW, Sakmar TP. Specific tryptophan UV-absorbance changes are probes of the transition of rhodopsin to its active state. Biochemistry. 1996;35:11149–11159. doi: 10.1021/bi960858u. [DOI] [PubMed] [Google Scholar]
- Liu C, Sandford G, Fei G, Nicholas J. Galpha protein selectivity determinant specified by a viral chemokine receptor-conserved region in the C tail of the human herpesvirus 8 g protein-coupled receptor. J Virol. 2004;78:2460–2471. doi: 10.1128/JVI.78.5.2460-2471.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies BJ, Browne H, Gibson W. Identification of the human cytomegalovirus G protein-coupled receptor homologue encoded by UL33 in infected cells and enveloped virus particles. Virology. 1996;225:111–125. doi: 10.1006/viro.1996.0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies BJ, Gibson W. The chemokine receptor homologue encoded by US27 of human cytomegalovirus is heavily glycosylated and is present in infected human foreskin fibroblasts and enveloped virus particles. Virus Res. 2007;123:57–71. doi: 10.1016/j.virusres.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinissen MJ, Tanos T, Bolos M, de Sagarra MR, Coso OA, Cuadrado A. Inhibition of heme oxygenase-1 interferes with the transforming activity of the Kaposi sarcoma herpesvirus-encoded G protein-coupled receptor. J Biol Chem. 2006;281:11332–11346. doi: 10.1074/jbc.M512199200. [DOI] [PubMed] [Google Scholar]
- Martini L, Waldhoer M, Pusch M, Kharazia V, Fong J, Lee JH, et al. Ligand-induced down-regulation of the cannabinoid 1 receptor is mediated by the G-protein-coupled receptor-associated sorting protein GASP1. FASEB J. 2007;21:802–811. doi: 10.1096/fj.06-7132com. [DOI] [PubMed] [Google Scholar]
- Maussang D, Verzijl D, van WM, Leurs R, Holl J, Pleskoff O, et al. Human cytomegalovirus-encoded chemokine receptor US28 promotes tumorigenesis. Proc Natl Acad Sci USA. 2006;103:13068–13073. doi: 10.1073/pnas.0604433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean KA, Holst PJ, Martini L, Schwartz TW, Rosenkilde MM. Similar activation of signal transduction pathways by the herpesvirus-encoded chemokine receptors US28 and ORF74. Virology. 2004;325:241–251. doi: 10.1016/j.virol.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Melnychuk RM, Smith P, Kreklywich CN, Ruchti F, Vomaske J, Hall L, et al. Mouse cytomegalovirus M33 is necessary and sufficient in virus-induced vascular smooth muscle cell migration. J Virol. 2005;79:10788–10795. doi: 10.1128/JVI.79.16.10788-10795.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menotti L, Mirandola P, Locati M, Campadelli-Fiume G. Trafficking to the plasma membrane of the seven transmembrane protein encoded by human herpesvirus 6 U51 gene involves a cell-specific function present in T lymphocytes. J Virol. 1999;73:325–333. doi: 10.1128/jvi.73.1.325-333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson S. Consequences of human cytomegalovirus mimicry. Hum Immunol. 2004;65:465–475. doi: 10.1016/j.humimm.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Middeldorp JM, Brink AA, van den Brule AJ, Meijer CJ. Pathogenic roles for Epstein–Barr virus (EBV) gene products in EBV-associated proliferative disorders. Crit Rev Oncol Hematol. 2003;45:1–36. doi: 10.1016/s1040-8428(02)00078-1. [DOI] [PubMed] [Google Scholar]
- Miller WE, Houtz DA, Nelson CD, Kolattukudy PE, Lefkowitz RJ. G-protein-coupled receptor (GPCR) kinase phosphorylation and beta-arrestin recruitment regulate the constitutive signaling activity of the human cytomegalovirus US28 GPCR. J Biol Chem. 2003;278:21663–21671. doi: 10.1074/jbc.M303219200. [DOI] [PubMed] [Google Scholar]
- Milne RS, Mattick C, Nicholson L, Devaraj P, Alcami A, Gompels UA. RANTES binding and down-regulation by a novel human herpesvirus-6 beta chemokine receptor. J Immunol. 2000;164:2396–2404. doi: 10.4049/jimmunol.164.5.2396. [DOI] [PubMed] [Google Scholar]
- Mocarski ES.1995Cytomegalovirus and their replicationIn: Fields BN, Knipe DM, Howley PM (eds).Fields Virology Lippincott-Raven Publishers: New York; 2447–2492. [Google Scholar]
- Mokros T, Rehm A, Droese J, Oppermann M, Lipp M, Hopken UE. Surface expression and endocytosis of the human cytomegalovirus-encoded chemokine receptor US28 is regulated by agonist-independent phosphorylation. J Biol Chem. 2002;277:45122–45128. doi: 10.1074/jbc.M208214200. [DOI] [PubMed] [Google Scholar]
- Montaner S, Sodhi A, Molinolo A, Bugge TH, Sawai ET, He Y, et al. Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi's sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell. 2003;3:23–36. doi: 10.1016/s1535-6108(02)00237-4. [DOI] [PubMed] [Google Scholar]
- Montaner S, Sodhi A, Pece S, Mesri EA, Gutkind JS. The Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor promotes endothelial cell survival through the activation of Akt/protein kinase B. Cancer Res. 2001;61:2641–2648. [PubMed] [Google Scholar]
- Montaner S, Sodhi A, Ramsdell AK, Martin D, Hu J, Sawai ET, et al. The Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor as a therapeutic target for the treatment of Kaposi's sarcoma. Cancer Res. 2006;66:168–174. doi: 10.1158/0008-5472.CAN-05-1026. [DOI] [PubMed] [Google Scholar]
- Moorman NJ, Willer DO, Speck SH. The gammaherpesvirus 68 latency-associated nuclear antigen homolog is critical for the establishment of splenic latency. J Virol. 2003;77:10295–10303. doi: 10.1128/JVI.77.19.10295-10303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Munshi N, Ganju RK, Avraham S, Mesri EA, Groopman JE. Kaposi's sarcoma-associated herpesvirus-encoded G protein-coupled receptor activation of c-jun amino-terminal kinase/stress-activated protein kinase and lyn kinase is mediated by related adhesion focal tyrosine kinase/proline-rich tyrosine kinase 2. J Biol Chem. 1999;274:31863–31867. doi: 10.1074/jbc.274.45.31863. [DOI] [PubMed] [Google Scholar]
- Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, et al. International Union of Pharmacology. XXII Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- Nakano K, Tadagaki K, Isegawa Y, Aye MM, Zou P, Yamanishi K. Human herpesvirus 7 open reading frame U12 encodes a functional beta-chemokine receptor. J Virol. 2003;77:8108–8115. doi: 10.1128/JVI.77.14.8108-8115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Sugihara M, Bondar AN, Elstner M, Entel P, Buss V. The retinal conformation and its environment in rhodopsin in light of a new 2.2Å crystal structure. J Mol Biol. 2004;342:571–583. doi: 10.1016/j.jmb.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Oliveira SA, Shenk TE. Murine cytomegalovirus M78 protein, a G protein-coupled receptor homologue, is a constituent of the virion and facilitates accumulation of immediate-early viral mRNA. Proc Natl Acad Sci USA. 2001;98:3237–3242. doi: 10.1073/pnas.051629898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, et al. Crystal structure of rhodopsin: a G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- Paoletti R, Gotto AM, Jr, Hajjar DP. Inflammation in atherosclerosis and implications for therapy. Circulation. 2004;109:III-20–III-26. doi: 10.1161/01.CIR.0000131514.71167.2e. [DOI] [PubMed] [Google Scholar]
- Parnot C, Miserey-Lenkei S, Bardin S, Corvol P, Clauser E. Lessons from constitutively active mutants of G protein-coupled receptors. Trends Endocrinol Metab. 2002;13:336–343. doi: 10.1016/s1043-2760(02)00628-8. [DOI] [PubMed] [Google Scholar]
- Paulsen SJ, Rosenkilde MM, Eugen-Olsen J, Kledal TN. Epstein–Barr virus-encoded BILF1 is a constitutively active G protein-coupled receptor. J Virol. 2005;79:536–546. doi: 10.1128/JVI.79.1.536-546.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleskoff O, Casarosa P, Verneuil L, Ainoun F, Beisser P, Smit M, et al. The human cytomegalovirus-encoded chemokine receptor US28 induces caspase-dependent apoptosis. FEBS J. 2005;272:4163–4177. doi: 10.1111/j.1742-4658.2005.04829.x. [DOI] [PubMed] [Google Scholar]
- Pleskoff O, Treboute C, Brelot A, Heveker N, Seman M, Alizon M. Identification of a chemokine receptor encoded by human cytomegalovirus as a cofactor for HIV-1 entry [see comments] Science. 1997;276:1874–1878. doi: 10.1126/science.276.5320.1874. [DOI] [PubMed] [Google Scholar]
- Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, et al. Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature. 2007;450:383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- Ray N, Doms RW. HIV-1 coreceptors and their inhibitors. Curr Top Microbiol Immunol. 2006;303:97–120. doi: 10.1007/978-3-540-33397-5_5. [DOI] [PubMed] [Google Scholar]
- Rochford R, Lutzke ML, Alfinito RS, Clavo A, Cardin RD. Kinetics of murine gammaherpesvirus 68 gene expression following infection of murine cells in culture and in mice. J Virol. 2001;75:4955–4963. doi: 10.1128/JVI.75.11.4955-4963.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, et al. GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function. Science. 2007;318:1266–1273. doi: 10.1126/science.1150609. [DOI] [PubMed] [Google Scholar]
- Rosenkilde MM. Virus-encoded chemokine receptors—putative novel antiviral drug targets. Neuropharmacology. 2005;48:1–13. doi: 10.1016/j.neuropharm.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Rosenkilde MM, Benned-Jensen T, Andersen H, Holst PJ, Kledal TN, Luttichau HR, et al. Molecular pharmacological phenotyping of EBI2. An orphan seven-transmembrane receptor with constitutive activity. J Biol Chem. 2006a;281:13199–13208. doi: 10.1074/jbc.M602245200. [DOI] [PubMed] [Google Scholar]
- Rosenkilde MM, David R, Oerlecke I, Benned-Jensen T, Geumann U, Beck-Sickinger AG, et al. Conformational constraining of inactive and active states of a seven transmembrane receptor by metal ion site engineering in the extracellular end of transmembrane segment V. Mol Pharmacol. 2006b;70:1892–1901. doi: 10.1124/mol.106.027425. [DOI] [PubMed] [Google Scholar]
- Rosenkilde MM, Kledal TN, Bräuner-Osborne H, Schwartz TW. Agonist and inverse agonist for the herpesvirus 8-encoded constitutively active seven-transmembrane oncogene product, ORF-74. J Biol Chem. 1999;274:956–961. doi: 10.1074/jbc.274.2.956. [DOI] [PubMed] [Google Scholar]
- Rosenkilde MM, Kledal TN, Holst PJ, Schwartz TW. Selective elimination of high constitutive activity or chemokine binding in the human herpesvirus 8 encoded 7TM oncogene ORF74. J Biol Chem. 2000;275:26309–26915. doi: 10.1074/jbc.M003800200. [DOI] [PubMed] [Google Scholar]
- Rosenkilde MM, Kledal TN, Schwartz TW. High constitutive activity of a virus-encoded 7TM receptor in the absence of the conserved DRY-motif (Asp-Arg-Tyr) in transmembrane helix 3. Mol Pharmacol. 2005;68:11–19. doi: 10.1124/mol.105.011239. [DOI] [PubMed] [Google Scholar]
- Rosenkilde MM, McLean KA, Holst PJ, Schwartz TW. The CXC chemokine receptor encoded by herpesvirus saimiri, ECRF3, shows ligand-regulated signaling through Gi, Gq, and G12/13 proteins but constitutive signaling only through Gi and G12/13 proteins. J Biol Chem. 2004;279:32524–32533. doi: 10.1074/jbc.M313392200. [DOI] [PubMed] [Google Scholar]
- Rosenkilde MM, Schwartz TW. GluVII:06—a highly conserved and selective anchor point for non-peptide ligands in chemokine receptors. Curr Top Med Chem. 2006;6:1319–1333. doi: 10.2174/15680266106061319. [DOI] [PubMed] [Google Scholar]
- Rosenkilde MM, Waldhoer M, Luttichau HR, Schwartz TW. Virally encoded 7TM receptors. Oncogene. 2001;20:1582–1593. doi: 10.1038/sj.onc.1204191. [DOI] [PubMed] [Google Scholar]
- Schaider H, Seidl H, Quan P, Frank S, Liu Z, Fukunaga M, et al. A signaling mute hCMV chemokine receptor prevents melanoma growth. Exp Dermatol. 2007;16:262–263. [Google Scholar]
- Schwartz TW, Frimurer TM, Holst B, Rosenkilde MM, Elling CE. Molecular mechanism of 7tm receptor activation––a global toggle switch model. Annu Rev Pharmacol Toxicol. 2006;46:481–519. doi: 10.1146/annurev.pharmtox.46.120604.141218. [DOI] [PubMed] [Google Scholar]
- Schwartz TW, Holst B.2003Molecular structure and function of 7TM G-protein-coupled receptorsIn: Foreman JC, Johansen T (eds).Textbook of Receptor Pharmacology CRC Press; 81–110. [Google Scholar]
- Schwarz M, Murphy PM. Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor constitutively activates NF-kappaB and induces proinflammatory cytokine and chemokine production via a C-terminal signaling determinant. J Immunol. 2001;167:505–513. doi: 10.4049/jimmunol.167.1.505. [DOI] [PubMed] [Google Scholar]
- Schwarz MK, Wells TN. New therapeutics that modulate chemokine networks. Nat Rev Drug Discov. 2002;1:347–358. doi: 10.1038/nrd795. [DOI] [PubMed] [Google Scholar]
- Seidl H, Schaider H, Quan P, Frank S, Liu Z, Fukunaga M, et al. The signaling mute hCMV chemokine receptor US28R129A prevents melanoma growth. Pharmacology. 2006;78:149. [Google Scholar]
- Seifert R, Wenzel-Seifert K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:381–416. doi: 10.1007/s00210-002-0588-0. [DOI] [PubMed] [Google Scholar]
- Sherrill JD, Miller WE. G protein-coupled receptor (GPCR) kinase 2 regulates agonist-independent Gq/11 signaling from the mouse cytomegalovirus GPCR M33. J Biol Chem. 2006;281:39796–39805. doi: 10.1074/jbc.M610026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Liapakis G, Xu R, Guarnieri F, Ballesteros JA, Javitch JA. Beta2 adrenergic receptor activation. Modulation of the proline kink in transmembrane 6 by a rotamer toggle switch. J Biol Chem. 2002;277:40989–40996. doi: 10.1074/jbc.M206801200. [DOI] [PubMed] [Google Scholar]
- Smit MJ, Verzijl D, Casarosa P, Navis M, Timmerman H, Leurs R. Kaposi's sarcoma-associated herpesvirus-encoded G protein-coupled receptor ORF74 constitutively activates p44/p42 MAPK and Akt via G(i) and phospholipase C-dependent signaling pathways. J Virol. 2002;76:1744–1752. doi: 10.1128/JVI.76.4.1744-1752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit MJ, Vink C, Verzijl D, Casarosa P, Bruggeman CA, Leurs R. Virally encoded G protein-coupled receptors: targets for potentially innovative anti-viral drug development. Curr Drug Targets. 2003;4:431–441. doi: 10.2174/1389450033491000. [DOI] [PubMed] [Google Scholar]
- Smit MJ, Vischer HF, Bakker RA, Jongejan A, Timmerman H, Pardo L, et al. Pharmacogenomic and structural analysis of constitutive G protein-coupled receptor activity. Annu Rev Pharmacol Toxicol. 2007;47:53–87. doi: 10.1146/annurev.pharmtox.47.120505.105126. [DOI] [PubMed] [Google Scholar]
- Soderberg C, Larsson S, Rozell BL, Sumitran-Karuppan S, Ljungman P, Moller E. Cytomegalovirus-induced CD13-specific autoimmunity—a possible cause of chronic graft-vs-host disease. Transplantation. 1996;61:600–609. doi: 10.1097/00007890-199602270-00015. [DOI] [PubMed] [Google Scholar]
- Sodhi A, Montaner S, Gutkind JS. Does dysregulated expression of a deregulated viral GPCR trigger Kaposi's sarcomagenesis. FASEB J. 2004;18:422–427. doi: 10.1096/fj.03-1035hyp. [DOI] [PubMed] [Google Scholar]
- Sodhi A, Montaner S, Patel V, Zohar M, Bais C, Mesri EA, et al. The Kaposi's sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1alpha [in process citation] Cancer Res. 2000;60:4873–4880. [PubMed] [Google Scholar]
- Springael JY, de PC, Deupi X, Van DJ, Pardo L, Parmentier M. The activation mechanism of chemokine receptor CCR5 involves common structural changes but a different network of interhelical interactions relative to rhodopsin. Cell Signal. 2007;19:1446–1456. doi: 10.1016/j.cellsig.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Stassen FR, Vega-Cordova X, Vliegen I, Bruggeman CA. Immune activation following cytomegalovirus infection: more important than direct viral effects in cardiovascular disease. J Clin Virol. 2006;35:349–353. doi: 10.1016/j.jcv.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Streblow DN, Kreklywich CN, Smith P, Soule JL, Meyer C, Yin M, et al. Rat cytomegalovirus-accelerated transplant vascular sclerosis is reduced with mutation of the chemokine-receptor R33. Am J Transplant. 2005;5:436–442. doi: 10.1111/j.1600-6143.2004.00711.x. [DOI] [PubMed] [Google Scholar]
- Streblow DN, Soderberg-Naucler C, Vieira J, Smith P, Wakabayashi E, Ruchti F, et al. The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell. 1999;99:511–520. doi: 10.1016/s0092-8674(00)81539-1. [DOI] [PubMed] [Google Scholar]
- Sun R, Lin S-F, Staskus K, Gradoville L, Grogan E, Hase A, et al. Kinetics of Kaposi's sarcoma-associated herpesvirus gene expression. J Virol. 1999;73:2232–2242. doi: 10.1128/jvi.73.3.2232-2242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadagaki K, Nakano K, Yamanishi K. Human herpesvirus 7 open reading frames U12 and U51 encode functional beta-chemokine receptors. J Virol. 2005;79:7068–7076. doi: 10.1128/JVI.79.11.7068-7076.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadagaki K, Yamanishi K, Mori Y. Reciprocal roles of cellular chemokine receptors and human herpesvirus 7-encoded chemokine receptors, U12 and U51. J Gen Virol. 2007;88:1423–1428. doi: 10.1099/vir.0.82665-0. [DOI] [PubMed] [Google Scholar]
- Tappe-Theodor A, Agarwal N, Katona I, Rubino T, Martini L, Swiercz J, et al. A molecular basis of analgesic tolerance to cannabinoids. J Neurosci. 2007;27:4165–4177. doi: 10.1523/JNEUROSCI.5648-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford EA, Watson MS, Aird HC, Perry J, Davison AJ. The DNA sequence of equine herpesvirus 2. J Mol Biol. 1995;249:520–528. doi: 10.1006/jmbi.1995.0314. [DOI] [PubMed] [Google Scholar]
- Thelen M. Dancing to the tune of chemokines. Nat Immunol. 2001;2:129–134. doi: 10.1038/84224. [DOI] [PubMed] [Google Scholar]
- Thompson D, Pusch M, Whistler JL. Changes in G protein coupled receptor sorting protein affinity regulate post-endocytic targeting of G protein coupled receptors. J Biol Chem. 2007;282:29178–29185. doi: 10.1074/jbc.M704014200. [DOI] [PubMed] [Google Scholar]
- Tsao P, Cao T, von Zastrow M. Role of endocytosis in mediating downregulation of G-protein-coupled receptors. Trends Pharmacol Sci. 2001;22:91–96. doi: 10.1016/s0165-6147(00)01620-5. [DOI] [PubMed] [Google Scholar]
- Urizar E, Claeysen S, Deupi X, Govaerts C, Costagliola S, Vassart G, et al. An activation switch in the rhodopsin family of G protein-coupled receptors: the thyrotropin receptor. J Biol Chem. 2005;280:17135–17141. doi: 10.1074/jbc.M414678200. [DOI] [PubMed] [Google Scholar]
- Verzijl D, Fitzsimons CP, Van Dijk M, Stewart JP, Timmerman H, Smit MJ, et al. Differential activation of murine herpesvirus 68- and Kaposi's sarcoma-associated herpesvirus-encoded ORF74 G protein-coupled receptors by human and murine chemokines. J Virol. 2004;78:3343–3351. doi: 10.1128/JVI.78.7.3343-3351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzijl D, Pardo L, Van DM, Gruijthuijsen YK, Jongejan A, Timmerman H, et al. Helix 8 of the viral chemokine receptor ORF74 directs chemokine binding. J Biol Chem. 2006;281:35327–35335. doi: 10.1074/jbc.M606877200. [DOI] [PubMed] [Google Scholar]
- Vischer HF, Vink C, Smit MJ. A viral conspiracy: hijacking the chemokine system through virally encoded pirated chemokine receptors. Curr Top Microbiol Immunol. 2006;303:121–154. doi: 10.1007/978-3-540-33397-5_6. [DOI] [PubMed] [Google Scholar]
- Wakeling MN, Roy DJ, Nash AA, Stewart JP. Characterization of the murine gammaherpesvirus 68 ORF74 product: a novel oncogenic G protein-coupled receptor. J Gen Virol. 2001;82:1187–1197. doi: 10.1099/0022-1317-82-5-1187. [DOI] [PubMed] [Google Scholar]
- Waldhoer M, Casarosa P, Rosenkilde MM, Smit MJ, Leurs R, Whistler JL, et al. The carboxyl terminus of human cytomegalovirus-encoded 7 transmembrane receptor US28 camouflages agonism by mediating constitutive endocytosis. J Biol Chem. 2003;278:19473–19482. doi: 10.1074/jbc.M213179200. [DOI] [PubMed] [Google Scholar]
- Waldhoer M, Kledal TN, Farrell H, Schwartz TW. Murine cytomegalovirus (CMV) M33 and human CMV US28 receptors exhibit similar constitutive signaling activities. J Virol. 2002;76:8161–8168. doi: 10.1128/JVI.76.16.8161-8168.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lauffer B, von ZM, Kobilka B, Xiang Y. NSF regulates {beta}2 adrenoceptors trafficking and signaling in cardiomyocytes. Mol Pharmacol. 2007;72:429–439. doi: 10.1124/mol.107.037747. [DOI] [PubMed] [Google Scholar]
- Whistler JL, Enquist J, Marley A, Fong J, Gladher F, Tsuruda P, et al. Modulation of postendocytic sorting of G protein-coupled receptors. Science. 2002;297:615–620. doi: 10.1126/science.1073308. [DOI] [PubMed] [Google Scholar]
- Yang BT, Chen SC, Leach MW, Manfra D, Homey B, Wiekowski M, et al. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi's sarcoma. J Exp Med. 2000;191:445–454. doi: 10.1084/jem.191.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LS, Murray PG. Epstein–Barr virus and oncogenesis: from latent genes to tumours. Oncogene. 2003;22:5108–5121. doi: 10.1038/sj.onc.1206556. [DOI] [PubMed] [Google Scholar]
- Zhou YF, Leon ML, Waclawiw MA, Popma JJ, Yu ZX, Finkel T, et al. Association between prior cytomegalovirus infection and the risk of restenosis after coronary atherectomy. N Engl J Med. 1996;335:624–630. doi: 10.1056/NEJM199608293350903. [DOI] [PubMed] [Google Scholar]