Abstract
Prostaglandin D2 (PGD2) is produced by mast cells, Th2 lymphocytes and dendritic cells and has been detected in high concentrations at sites of allergic inflammation. PGD2 exerts its inflammatory effects through high affinity interactions with the G protein coupled receptors DP1 and chemoattractant-homologous receptor expressed on Th2 cells (CRTH2, also known as DP2). DP1 and CRTH2 act in concert to promote a number of biological effects associated with the development and maintenance of the allergic response. During the process of allergen sensitization, DP1 activation may enhance polarization of Th0 cells to Th2 cells by inhibiting production of interleukin 12 by dendritic cells. Upon exposure to allergen in sensitized individuals, activation of DP1 may contribute to the long lasting blood flow changes in the target organ. CRTH2 is expressed by Th2 lymphocytes, eosinophils and basophils and may mediate the recruitment of these cell types during the late phase allergic response. The role played by CRTH2 in promoting the production of Th2 cytokines and IgE make antagonism of this receptor a particularly attractive approach to the treatment of chronic allergic disease.
Keywords: prostaglandin D2, DP1, CRTH2, mast cells, Th2 lymphocytes, eosinophils, PI3K, allergic rhinitis, asthma
Introduction
There have been significant improvements in the treatment of allergic diseases over the past 20–30 years. Non-sedating antihistamines have proven to be of great benefit to patients with mild seasonal allergic rhinitis and topical corticosteroids (intranasal for allergic rhinitis and inhaled for asthma) are effective in reducing inflammation and disease severity in the majority of patients with a dramatically improved safety profile compared to systemic steroids. Despite these advances, the incidence of allergy is increasing dramatically and important features of both allergic rhinitis and asthma are resistant to current therapy. In more severe persistent allergic rhinitis antihistamines are less effective, particularly on nasal congestion (Howarth, 1997) and around 5% of asthmatics remain poorly controlled by inhaled steroids (Barnes, 2004). There is therefore a clear need for more effective treatments for allergic disease, particularly if they can be administered safely by the oral route.
The challenge, therefore, is to identify therapeutic approaches that target processes fundamentally important in driving allergic inflammation but with sufficient specificity to have an acceptable side effect profile. Cysteinyl leukotriene receptor (CysLT1) antagonists, most notably montelukast, are the most recently introduced drugs to treat asthma and these drugs specifically block the action of cysteinyl leukotrienes (predominantly LTD4) on the CysLT1 receptor expressed by bronchial smooth muscle and leukocytes without inhibiting the effects of leukotrienes mediated by CysLT2. Since CysLT1 has a limited expression profile and leukotrienes active on CysLT1 are produced predominantly by mast cells and eosinophils, this approach is highly specific and consequently, CysLT1 antagonists have proven to have an acceptable side-effect profile. However, this specificity of action comes at a cost—while CysLT1 antagonists are effective in inhibiting the bronchoconstrictor element of asthma their anti-inflammatory activity and consequently, clinical efficacy, is modest compared to inhaled corticosteroids (Busse et al., 2001). The search therefore continues to identify specific approaches that have improved efficacy and are safe enough for systemic use.
Recent evidence suggests that selective blockade of the action of prostaglandin D2 (PGD2) may provide such an opportunity. Production of PGD2, like that of cysteinyl leukotrienes, is restricted to cells involved in the allergic response (at least in peripheral tissues) and appears to be instrumental in orchestrating interactions between mast cells, Th2 lymphocytes, eosinophils and dendritic cells. The inflammatory effects of PGD2 are mediated by high-affinity interaction with D prostanoid receptor 1 (DP1) and chemoattractant receptor-homologous molecule expressed on T helper type 2 cell (Th2) cells (CRTH2). The purpose of this review is to summarize the emerging evidence that DP1 and CRTH2 contribute to allergic inflammation and to highlight the potential therapeutic utility of antagonizing these receptors.
Production of PGD2 in allergic inflammation
Prostaglandin D2 is the major prostanoid produced by mast cells (Lewis et al., 1982; Peters et al., 1982) and PGD2 and its metabolites have been proposed as selective markers of mast cell activation in vivo (O'Sullivan et al., 1996; Bochenek et al., 2003; Dahlen and Kumlin, 2004). Cross linking of cell surface immunoglobulin E (IgE) by allergen leads to the rapid production of PGD2 by mast cells. PGD2 is derived from liberated arachidonic acid in a two-step process where either COX1 or COX2 catalyses the production of PGH2, which is then metabolized to PGD2 by haematopoietic PGD2 synthase. Using mouse mast cells in vitro, it has been shown that the early phase of PGD2 production is COX1-dependent whereas COX2 is responsible for the more prolonged production of PGD2 (Reddy et al., 1999). In vivo, it appears that PGD2 is produced more rapidly (within minutes) which may reflect the presence of COX2 in these chronically inflamed tissues as has been shown in the airways of aspirin-intolerant asthmatics (Sousa et al., 1997). Allergen challenge has been shown to lead to rapid production of PGD2 in the airways of asthmatics (Murray et al., 1986), the nasal mucosa of allergic rhinitis (Naclerio et al., 1983) and in the skin of patients with atopic dermatitis (Charlesworth et al., 1991).
PGD2 is also produced in biological meaningful quantities by Th2 cells although the levels are around 1/10th of those produced by mast cells on a per cell basis and is more delayed. In response to anti-CD3/anti-CD28, PGD2 is produced by human Th2 cells as a consequence of induction of COX2 and haematopoietic PGD2 synthase (Tanaka et al., 2000). Whether dendritic cells produce PGD2 is more controversial but interestingly, dendritic cells incubated with thymic stromal lymphopoietin (TSLP) create a local environment where Th0 cells polarize to the Th2 phenotype and this process is associated with the upregulation of PGD2 synthase in Th2 cells (Wang et al., 2006). It is likely therefore that Th2 cells may be an important source of biologically active PGD2 in a chronic allergic setting or in situations where the allergic response occurs independently of mast cell activation.
Pharmacological properties of DP1 and CRTH2
D prostanoid receptor 1 was the first receptor that was identified for PGD2. DP1 is a member of the prostanoid receptor family that includes EP1–4, FP, IP and TP. It is coupled to Gαs and its activation leads to elevation of intracellular levels of cAMP. DP1 is expressed by vascular smooth muscle and platelets and has well-characterized effects in mediating vasodilatation (Giles et al., 1989; Walch et al., 1999) and inhibition of platelet aggregation (Whittle et al., 1983). Much of our knowledge of the role of DP1 in biological responses comes from the use of the selective DP1 agonist BW245C and the selective DP1 antagonist BWA868C. Dendritic cells express DP1 and activation of DP1 may play an important role in modulating the function of these cells, particularly in controlling the production of cytokines such as interleukin 12.
Chemoattractant receptor-homologous molecule expressed on Th2 cells was originally identified as an orphan known as GPR44. Subsequently, it was found that CRTH2 is expressed preferentially by Th2 lymphocytes, eosinophils and basophils (Nagata et al., 1999a, 1999b) and it mediated chemotactic responses of Th2 lymphocytes, eosinophils and basophils to PGD2 (Hirai et al., 2001). Although CRTH2 and DP1 bind the same ligand there is very little homology between the two receptors, CRTH2 being most closely related to other chemotactic receptors such as the leukotriene B4 receptors BLT1 and BLT2, the complement C5a receptor and the formyl peptide receptors. CRTH2 is coupled to Gαi and its activation leads to elevation of intracellular calcium and reduction in cAMP (Sawyer et al., 2002). Downstream activation of CRTH2 results in phosphatidylinositol-3-kinase (PI3K)-dependent phosphorylation of AKT, phosphorylation of GSK-3β and nuclear translocation of NFAT (Xue et al., 2007). Phosphatidylinositol-3-kinase-dependent phosphorylation of GSK-3β leads to inactivation of GSK-3β which, in turn, leads to reduction in the phosphorylation of NFAT (Xue et al., 2007).
It is of interest that many of the metabolites of PGD2 including 13,14-dihydro-15-keto-PGD2 (DK-PGD2) (Hirai et al., 2001), Δ12PGD2 (Gazi et al., 2005) Δ12PGJ2 (Heinemann et al., 2003), 15-deoxy-Δ12,14PGD2 and deoxy-Δ12,14PGJ2 (Monneret et al., 2002) retain activity on CRTH2 but are less active on DP1. Δ12PGD2 and Δ12PGJ2 are both major metabolites of PGD2 formed rapidly in plasma (Schuligoi et al., 2007) and so the effects of PGD2 at sites of allergic inflammation is likely to be heavily influenced by metabolism and it is tempting to speculate that in such conditions the effects of CRTH2 will dominate over those of DP1. It has been proposed that Δ12PGJ2 may act as a circulating hormone and cause blood eosinophilia by stimulating eosinophil release from the bone marrow (Heinemann et al., 2003). Some arachidonic acid metabolites formed independently of PGD2 such as 11-dehydro-thromboxane B2 and 9α11β-PGF2 also have CRTH2 agonist activity (Bohm et al., 2004; Sandig et al., 2006). The isoprostane 15R-PGD2 is also a potent CRTH2 agonist (Cossette et al., 2007). This is of particular interest since if 15R-PGD2 is formed in vivo it would be the first evidence of an endogenous CRTH2 agonist formed independently of the cyclooxygenase pathway of arachidonic acid metabolism.
Chemoattractant receptor-homologous molecule expressed on Th2 cells is a much more promiscuous receptor than DP1 and a diverse range of natural and synthetic ligands have been found to interact with this receptor. The NSAID indomethacin is a partial agonist (Hirai et al., 2002; Sawyer et al., 2002; Stubbs et al., 2002) and the indole acetic acid derivative L888,607 is a CRTH2 agonist devoid of cyclooxygenase activity (Gervais et al., 2005). This compound has been proposed as a useful tool to define the role of CRTH2 in vitro and in vivo (Gervais et al., 2005). Indole-3-acetic acid derivatives have been identified that are potent and selective antagonists of CRTH2 (Armer et al., 2005). Ramatroban, originally identified as a TP antagonist (McKenniff et al., 1991) has been demonstrated to be a competitive CRTH2 antagonist (Sugimoto et al., 2003). This observation has led to the identification of ramatroban analogues, which are potent CRTH2 antagonists devoid of TP activity (Ulven and Kostenis, 2005). The only non-acid CRTH2 antagonists so far described are 4-aminotetrahydroquinoline derivatives as exemplified by K117 and K604 (Mimura et al., 2005).
The key biological and pharmacological properties of DP1 and CRTH2 are summarized in Table 1. The molecular pharmacology and signal transduction pathways utilized by DP1 and CRTH2 is reviewed in more detail by Kostenis and Ulven (2006).
Table 1. Comparison of the key pharmacological and biological properties of DP1 and CRTH2.
| CRTH2 | DP1 |
|---|---|
| Endogenous ligands | |
| PGD2 | PGD2 |
| DK-PGD2 | |
| 15R-PGD2 | |
| Δ12PGD2 | |
| Δ12PGJ2 | |
| 15-deoxy-Δ12,14 | |
| PGD2 | |
| 15-deoxy-Δ12,14 | |
| PGJ2 | |
| 9α11βPGF2 | |
| 11-dehydro-thromboxane B2 | |
| Signalling mechanism | |
| GαI | Gαs |
| ↓ cAMP | ↑ cAMP |
| ↑ Ca2+ | |
| P13K-dependent chemotaxis | |
| Calcineurin-dependent cytokine production | |
| Synthetic agonists | |
| Indomethacin | BW245c |
| L-888,607 | |
| Synthetic antagonists | |
| Ramatroban and analogues | BWA868c |
| Indole-3-acetic acids | MK-0524 |
| 4-aminotetrahydroquinoline derivatives K117 and K604 | S-5751 |
| Location | |
| Th2 lymphocytes | Bronchial smooth muscle |
| Eosinophils | Vascular smooth muscle |
| Basophils | Dendritic cells |
| CNS | Platelets |
| CNS | |
| Biological effects | |
| Chemotaxis and activation of Th2 lymphocytes, eosinophils and basophils | Bronchodilatation |
| CNS effects unknown | Vasodilatation |
| Suppression of cytokine production by dendritic cells leading to polarization of Th2 cells | |
| Inhibition of platelet aggregation | |
| Likely involvement in CNS effects e.g. sleep and pain cognition | |
Abbreviations: CRTH2, chemoattractant receptor-homologous molecule expressed on Th2 cells; PGD2, prostaglandin D2; Th2 cell, T helper type 2 cell.
Role of DP1 and CRTH2 in allergic inflammation
Prostaglandin D2 can mimic a number of the key features of allergic responses when it is applied to animals or human volunteers. These effects include blood flow changes, recruitment of eosinophils and Th2 lymphocytes and potentiation of Th2 cytokine production. All of these diverse effects can be explained by discrete actions on DP1 or CRTH2. It has been proposed that PGD2 and its metabolites through interactions with DP1, CRTH2 and other intracellular pathways have opposing roles in regulating leukocyte function during inflammatory responses (Kostenis and Ulven, 2006; Sandig et al., 2007). There is clear evidence that PGD2 that might promote resolution of neutrophilic Th1/Th17-mediated inflammatory responses (Gilroy et al., 1999; Ajuebor et al., 2000; Trivedi et al., 2006) but in the case of eosinophilic Th2-mediated allergic responses the predominant effect of CRTH2 activation appears to be pro-inflammatory. The effects of DP1 activation are more complex but is likely that the ‘anti-inflammatory' effect of DP1-mediated suppression of leukocyte activation, particularly dendritic cells and Th1 cells, can increase allergic responses by promoting Th2 bias, at least during the sensitization phase of an allergic response.
Activation of DP1 contributes to blood flow changes during allergic responses
It has been recognized for some time that PGD2 has the potential to mediate the pathological blood flow changes observed in allergic diseases. In the case of allergic rhinitis, engorgement of the vasculature in the nose contributes to congestion, a troublesome symptom, which is largely resistant to the action of H1 antagonists. The mechanisms of the vascular changes in the mucosa of patients allergic rhinitis is complex but is thought to involve the direct actions of mediators on both the vasculature and neuronal reflexes (Widdicombe, 1990). Blood flow changes contribute to the swelling of the nasal mucosa causing congestion and enhanced leakage of plasma protein, which contributes to nasal secretions. The importance of blood flow changes to the signs and symptoms of rhinitis is evident based on the effectiveness of vasoconstrictors such as pseudoephedrine to reduce ‘stuffiness'.
In human volunteers intravenous administration of PGD2 has been shown to produce nasal congestion associated with intense facial flushing but interestingly, no overt effects on systemic blood pressure or lung function (Heavey et al., 1984). Congestion has also been observed after insufflation of PGD2 in human subjects and it is more effective in this respect than either histamine or bradykinin (Doyle et al., 1990). The ability of PGD2 to induce nasal air flow resistance is blocked by the vasoconstrictor oxymetazoline highlighting the importance of a vascular event in mediating this response (Howarth et al., 1991). Production of PGD2 in response to allergen in pigs has been proposed to mediate a long-lasting component of airway vasodilatation resistant to antihistamines (Alving et al., 1991). This conclusion is based on the ability of PGD2 to mimic the long-lasting airways vasodilatation induced by allergen and the inhibitory effect of the cyclooxygenase inhibitor diclofenac on allergen-induced blood flow changes. The vasoactive effect of PGD2 appears to be more marked in the nose than the lower airways and so be more relevant to allergic rhinitis than asthma. The availability of selective DP1 agonists and antagonists have shown that vasorelaxation of vascular smooth muscle in response to PGD2 is DP1-mediated (Giles et al., 1989; Walch et al., 1999) and the hypotensive effect of PGD2 is inhibited by BW A868C (Hamid-Bloomfield and Whittle, 1989) which suggests that the vascular effects described above are likely to be mediated by DP1. Indeed, the selective DP1 agonist BW245C-induced headache, nasal stuffiness and facial flushing when infused into human volunteers (Al Sinawi et al., 1985). Most recently it has been shown that increased nasal airway resistance induced by intranasal instillation of PGD2 in conscious sheep is completely inhibited by a selective DP1 antagonist (Sturino et al., 2007).
While there are no reports on the effects of selective DP1 antagonists on vascular engorgement or congestion in clinical allergy it is of interest that the DP1 antagonist MK-0524 has been reported to reduce facial flushing in human volunteers administered niacin (Cheng et al., 2006). Niacin-induced flushing is mediated by the production of endogenous PGD2 by cells in the skin, probably Langerhans' cells (Maciejewski-Lenoir et al., 2006) which then acts on DP1 to mediate increased blood flow. The relationship between activation of DP1 and nasal blockage has been explored in sensitized guinea pigs where it was found that the selective DP1 antagonist S-5751 inhibited the early phase increase in nasal pressure in response to allergen while the H1 antagonist terfenadine was without effect (Arimura et al., 2001).
It seems likely therefore that at sites of mast activation PGD2 is produced and this mediator contributes to vascular changes leading, in the case of allergic rhinitis, to acute nasal congestion. The contribution of DP1 to congestion associated with more chronic inflammatory changes is less certain, however, and it is possible that activation of CRTH2 may be important in that setting.
DP1 suppresses dendritic cell function: possible relevance to polarization of Th2 cells
DP1 is expressed by dendritic cells and BW245C is able to suppress the ability of these cells to produce cytokines including interleukin 12 (Faveeuw et al., 2003; Hammad et al., 2003; Gosset et al., 2005). It is now well recognized that dendritic cells, in addition to presenting antigen to reactive T cells, also play a central role in controlling the polarization of T cells to either the Th1 or Th2 phenotype (Lambrecht, 2005). Dendritic cells isolated from the human respiratory mucosa preferentially induce polarization to the Th2 phenotype while peripheral blood-derived dendritic cells promote a Th1 pattern of differentiation (Faith et al., 2005). The effect of respiratory mucosal dendritic cells to promote Th2 polarization is associated with low production of IL-12 while high production of this cytokine promotes Th1 differentiation. Dendritic cells from mucosal origins tend to cause an increase in Th2 bias and this effect can be influenced by the local production of soluble factors. Thymic stromal lymphopoietin produced by epithelial cells stimulates dendritic cells to produce an environment which favours polarization and maintenance of CD4+CRTH2+ central memory Th2 cells (Wang et al., 2006). This effect is associated with the induction of PGD2 synthase and may be relevant to the pathogenesis of asthma since thymic stromal lymphopoietin levels in the asthmatic lung correlate with disease severity (Ying et al., 2005). Activated mast cells induce Th2-promoting dendritic cells (Kitawaki et al., 2006), an effect mimicked by PGD2 acting through inhibition of interleukin 12 production (Theiner et al., 2006). It is likely then at sites of mast cell activation the production of PGD2 contributes to the maintenance of Th2 dominance with associated production of IgE which in turn leads to immunological mast cell activation so creating an escalating cycle of increased severity and chronicity. Histamine produced by mast cells is believed to act in a similar manner (Caron et al., 2001; Mazzoni et al., 2001).
The ability of DP1 to control Th2 polarization is a plausible explanation for the reduction in allergic responses afforded by DP1 deficiency (Matsuoka et al., 2000). Compared to wild-type mice, there was a reduction in Th2 cytokine production, eosinophil infiltration, mucus production and airway responsiveness in DP1-null mice. Although enhanced mucus production may be due to a direct action of DP1 (Wright et al., 2000) and DP1-mediated inhibition of eosinophil apoptosis (Gervais et al., 2001) may have contributed to airway eosinophilia, the mouse knockout data are consistent with a role for DP1 in controlling the polarization of Th2 cells and development of the allergic phenotype. However, this view is controversial since it has been shown that local injection of the DP1 agonist BW245C reduces allergic responses in the skin (Angeli et al., 2004) and lungs (Hammad et al., 2007) of mice. In the case of the lung, the effect of BW245C was due to a specific action on DP1 as its effects were ablated in DP1-null mice (Hammad et al., 2007). Furthermore, it was proposed that activation of DP1 by an endogenous ligand plays an important role in suppressing allergic inflammation since selective deficiency in haematopoietic PGD2 synthase enhanced airway inflammation and DP1-deficient dendritic cells demonstrated an enhanced ability to promote Th2 responses in the lung (Hammad et al., 2007). Since lung CD11c+ dendritic cells are essential for the maintenance of Th2-dependent airway inflammation (van Rijt et al., 2005), DP1 activation can lead to suppression of dendritic cell functions that are critical to stimulation of Th2 cells within the allergic airways. It appears that DP1-mediated suppression of dendritic cell function can have disparate effects on Th2-mediated allergic inflammation. On the one hand, DP1-mediated inhibition of IL-12 promotes Th2 polarization during the sensitization phase while suppression of dendritic cell function during the effector phase reduces activation of antigen-specific Th2 cells. Consequently, the effect of DP1 that dominates may vary depending on whether PGD2 is produced during the sensitization or effector phase of the allergic response and on the location (target organ vs lymph node). The role played by DP1 in controlling the Th2 polarization during allergen sensitization may explain why allergic airway responses were reduced in DP1-null mice but it is possible DP1 blockade may exacerbate an established allergic response. The effect of DP1 blockade on airway responses to allergen in sensitized animals has not yet been studied extensively but one study has shown that the selective DP1 antagonist S-5751 reduced, rather than enhanced, airway inflammation in allergic guinea pigs (Arimura et al., 2001). Given the complexity of the effects of DP1 activation it is uncertain whether DP1 antagonists will be effective in asthma and other allergic disorders.
CRTH2 mediates activation of Th2 lymphocytes, eosinophils and basophils
Chemotaxis of Th2 cells, eosinophils and basophils in response to PGD2 is blocked by an anti-CRTH2 antibody whereas Th1 cells, which do not express CRTH2, do not migrate in response to PGD2 (Hirai et al., 2001). Independently it has been shown that PGD2 promotes chemotaxis of eosinophils through a receptor unrelated to DP1 and this was designated DP2 (Monneret et al., 2001). The ability of PGD2 to promote eosinophil accumulation in the airways of preclinical species is mimicked by selective CRTH2 agonists but not DP1 agonists (Almishri et al., 2005; Shiraishi et al., 2005) and inhibited by the CRTH2 antagonist ramatroban (Shiraishi et al., 2005). Injection of PGD2 enhances allergic response in mouse skin and airways and these effects are mimicked by 13,14-dihydro-15-keto-PGD2 but not BW245C (Spik et al., 2005). In addition to promoting increased migration of leukocytes to sites of allergic inflammation activation by PGD2 can lead to production of the Th2 cytokines including interleukin 4, 5 and 13. Overexpression of the human lipocalin-type PGD2 synthase in mice leads to enhanced Th2 cytokine production in response to allergen (Fujitani et al., 2002). PGD2 and 13,14-dihydro-15-keto-PGD2 have been shown to enhance cytokine production by Th2 in response to anti-CD3/anti-CD28 (Tanaka et al., 2004). In fact, PGD2 has unusual ability to induce production of interleukin 4, 5 and 13 in the absence of allergen or costimulation, an effect that is CRTH2-dependent (Xue et al., 2005).
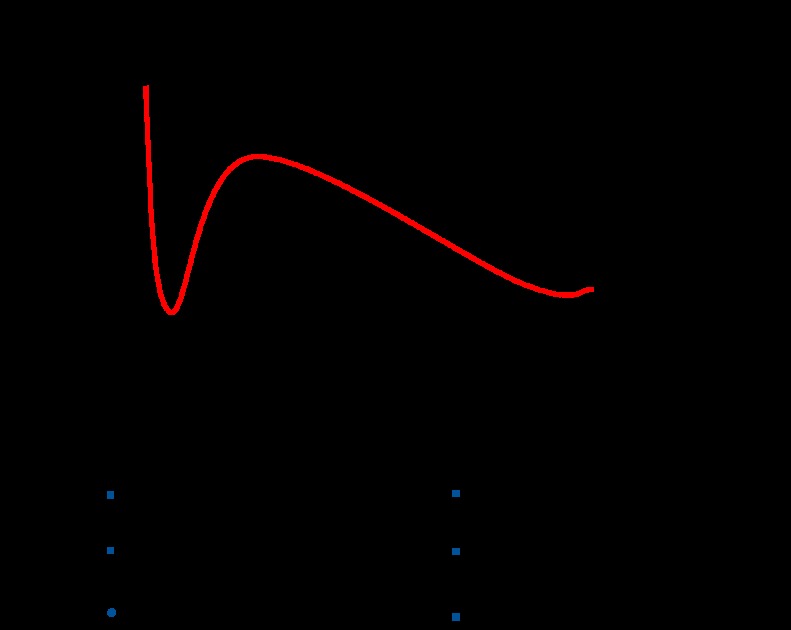
When atopic individuals for example, allergic asthmatics are exposed to allergen there is an early-phase response followed in some cases by a late-phase response (Figure 1). Classically, it is thought that the early phase is mediated by IgE-dependent activation of mast cells and the late-phase response is a consequence of activation of antigen-specific T cells by antigen-presenting cells. This view is supported by a number of findings including the observation that cyclosporine blocks the late phase airway response to allergen but not the early phase (Sihra et al., 1997). Interestingly, though, the anti-IgE antibody omalizumab blocked both the early phase and late-phase airway response (Fahy et al., 1997) suggesting that IgE contributes to the late-phase response as well as the early phase. This effect could be mediated through activation of mast cells or through IgE-facilitated antigen presentation by dendritic cells or both. There is considerable evidence that IgE-facilitated antigen presentation is an important pathological process in allergic disease (van Neerven et al., 2006). However, there is also some evidence that mast cell activation is important in promoting Th2 accumulation independently of antigen presentation by dendritic cells. Studies in IgE transgenic mice have shown that immunological activation of mast cells can lead to recruitment of Th2 cells into the airways in the absence of antigen-specific T-cell activation (Maezawa et al., 2003, 2004). Since it is also known that PGD2 is the major CRTH2 agonist produced by mast cells (Hirai et al., 2001) we have conducted experiments to define the roles of PGD2 and CRTH2 in mediating mast cell-dependent activation of Th2 cells (Gyles et al., 2006). It was found that activation of mast cells with IgE/anti-IgE led to the production of a factor, which promoted increased migration of Th2 lymphocytes. The production of this factor was inhibited by the cyclooxygenase inhibitor diclofenac and its pattern of production was similar to that of PGD2. The effect of the mast cell supernatants on migration of Th2 lymphocytes were blocked by ramatroban (Gyles et al., 2006) as was the effect of mast cell supernatants on activation of eosinophils (unpublished observations). Taken together, these data suggest that mast cell-dependent activation of Th2 cells and eosinophils is mediated by PGD2 acting on CRTH2.
Figure 1.
Development of an early- and late-phase airway response in response to allergen in a sensitized individual. The early phase fall in lung function is due to mast cell-dependent bronchospasm, mediated largely by leukotrienes. The late-phase response is mediated by activated Th2 cells and suppressed by drugs that inhibit T-cell function such as steroids and cyclosporine. It is proposed that CRTH2 is involved in both mast cell-mediated activation of Th2 cells and the amplification of Th2 recruitment that occurs as a result of IgE-facilitated antigen presentation by airway dendritic cells.
CRTH2 also plays an important role in the paracrine activation of Th2 cells (Vinall et al., 2007). Supernatants collected from Th2 cells activated with anti-CD3/anti-CD28 stimulated the migration of naive Th2 cells through a CRTH2-dependent mechanism. Interestingly, there is evidence that Th2 cells produce some CRTH2 agonist activity independently of the cyclooxygenase pathway since this effect was only partially dependent on PGD2 (Vinall et al., 2007). These data suggest that CRTH2 is important in both the early mast cell-dependent recruitment of Th2 cells and in the amplification of Th2 cell accumulation resulting from activation of antigen-specific T cells.
In vivo effects of CRTH2 blockade or deficiency
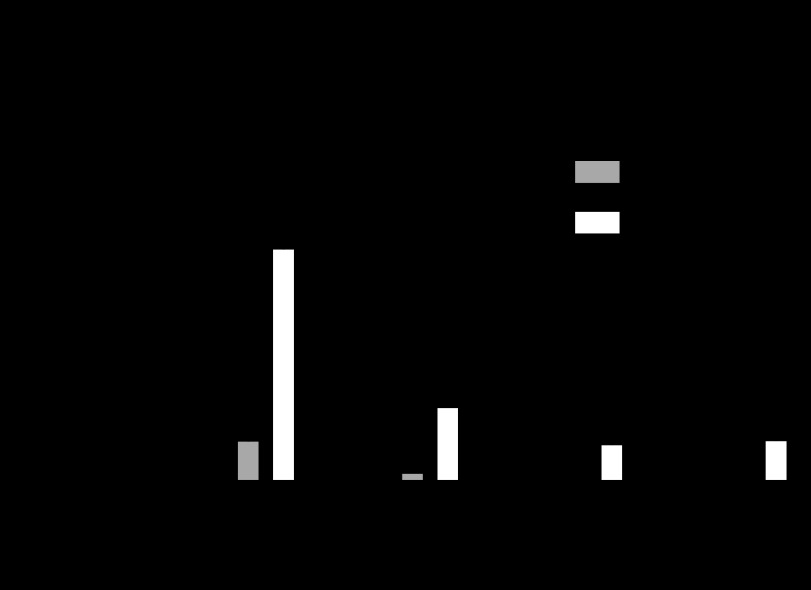
The in vitro effects described above may explain why ramatroban is effective in inhibiting eosinophil accumulation in a number of tissues in response to an allergic challenge, including guinea-pig nasal mucosa (Narita et al., 1996), mouse airways (Nagai et al., 1995) and mouse skin (Takeshita et al., 2004). Recently, it has been shown that a novel ramatroban analogue lacking TP activity is effective in reducing airway eosinophilia and mucus cell hyperplasia in allergic mice (Uller et al., 2007). CRTH2 antagonists that are structurally unrelated to ramatroban are also effective in reducing airway inflammation in preclinical models. A number of different chemical series have been described (Pettipher et al., 2007) and indole acetic acids, in particular, have been identified as highly potent and selective CRTH2 antagonists (Armer et al., 2005). The effect of a compound from an indole acetic acid series has been studied in a guinea-pig model of allergic airway inflammation. When sensitised, guinea pigs were challenged with an aerosol of antigen there was a significant increase in the numbers of leukocytes in the bronchoalveolar lavage fluid. The majority of these leukocytes were eosinophils although increased numbers of monocytes and neutrophils were also detected. Pretreatment with a selective CRTH2 antagonist caused profound inhibition of the recruitment of total leukocytes, including eosinophils (Figure 2).
Figure 2.
Effect of a selective CRTH2 antagonist on airway inflammation 24 h after challenge with antigen in sensitized guinea pigs. The drug was dosed by oral gavage 1 h before and 12 h after allergen exposure. Data are presented as the mean±s.e.mean (n=10 animals per group, *P<0.01 compared to vehicle control).
Studies on knockout mice have apparently produced conflicting results with respect to involvement of CRTH2 in allergic responses. The lack of consistency may be related to differences in immunization procedure and the fact that in the mouse CRTH2 may be involved in the regulation of Th1 function. This is discussed in more detail elsewhere (Pettipher et al., 2007). In situations where CRTH2 does contribute to the development of allergic responses, some interesting observations have been made. In a model of allergic airways inflammation deficiency in CRTH2 is associated with inhibition of interleukin 4 and interleukin 13 production, lower levels of mucus and suppression of airway hyper-responsiveness (Gonzalo et al., 2005). Furthermore, Satoh et al. (2006) have reported that in CRTH2 knockout mice there is a reduction in allergic skin inflammation and production of IgE, an effect most likely to be secondary to inhibition of Th2 cytokine production. Since IgE plays an important role in mast cell activation and in facilitating antigen presentation this observation has important implications for understanding how to prevent the progression of chronic allergic disease, the so-called ‘atopic march', a phrase used to describe the sequential progression of atopy in childhood to progressively more severe allergic disease in adulthood (Hahn and Bacharier, 2005).
The clinical information on CRTH2 antagonists is limited to data on ramatroban, which is marketed in Japan under the trade name Baynas for the treatment of perennial rhinitis. Although ramatroban is non-selective and possesses only moderate potency, its clinical effects in perennial allergic rhinitis are likely to be due to CRTH2 blockade. In patients with perennial allergic rhinitis, treatment with ramatroban for 4 weeks inhibited chronic nasal swelling and reduced other signs and symptoms (Terada et al., 1998).
Conclusion
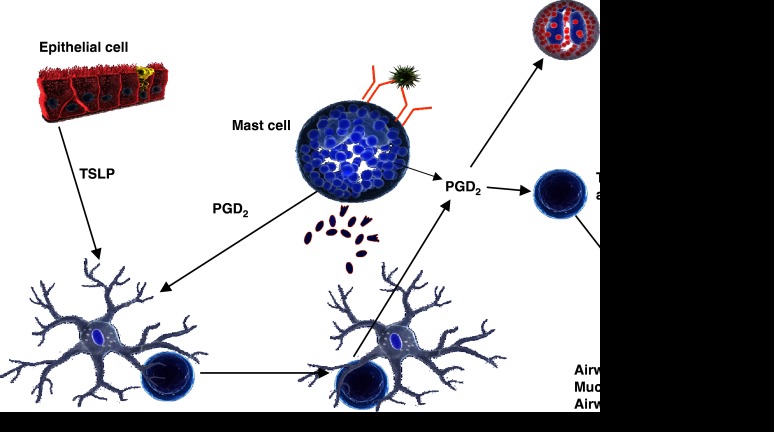
A scheme summarizing the roles of DP1 and CRTH2 in the allergic response is shown in Figure 3.
Figure 3.
Proposed scheme whereby the combined action of DP1 and CRTH2 leads to the polarization and activation of Th2 lymphocytes. PGD2 derived from mast cells acts on dendritic cells to inhibit production of interleukin 12 by an action on DP1 and thereby creates an environment where T cells are polarized to the Th2 phenotype. Thymic stromal lymphopoietin also acts on dendritic cells to promote Th2 polarization, an effect associated with induction of PGD2 synthase. CRTH2 contributes both to mast cell-dependent activation of Th2 cells and eosinophils and to paracrine activation of Th2 cells as might occur during IgE-facilitated antigen presentation.
Although DP1 and CRTH2 are structurally unrelated and have distinct signalling pathways, they share a common ligand, PGD2, and through complementary activities contribute to the development and maintenance of allergic inflammation. In addition to mediating vascular changes associated with the acute allergic response, DP1 may also promote Th2 polarization during allergen sensitization. In contrast, CRTH2 is involved in the recruitment and activation of Th2 lymphocytes and eosinophils that occurs when a sensitized individual encounters allergen. Of particular note is the central role played by CRTH2 in promoting the production of Th2 cytokines and IgE, highlighting the potential utility of CRTH2 antagonists in treating chronic aspects of allergic disease.
Acknowledgments
The guinea-pig allergen challenge experiments described in this review were performed by Aptuit Limited, Riccarton, Scotland.
Glossary
- CRTH2
chemoattractant-homologous receptor expressed on Th2 cells
- CysLT
cysteinyl leukotriene receptor
- DP1
D prostanoid receptor 1
- IgE
immunoglobulin E
- PGD2
prostaglandin D2
- PI3K
phosphatidylinositol-3-kinase
- Th2 cell
T helper type 2 cell
- TSLP
thymic stromal lymphopoietin
Footnotes
Conflict of interest
The author is named as an inventor on patents relating to the use of CRTH2 antagonists in allergic and other diseases.
References
- Ajuebor MN, Singh A, Wallace JL. Cyclooxygenase-2-derived prostaglandin D2 is an early anti-inflammatory signal in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G238–G244. doi: 10.1152/ajpgi.2000.279.1.G238. [DOI] [PubMed] [Google Scholar]
- Al Sinawi LA, Mekki QA, Hassan S, Hedges A, Burke C, Moody SG, et al. Effect of a hydantoin prostaglandin analogue, BW245C, during oral dosing in man. Prostaglandins. 1985;29:99–111. doi: 10.1016/0090-6980(85)90155-8. [DOI] [PubMed] [Google Scholar]
- Almishri W, Cossette C, Rokach J, Martin JG, Hamid Q, Powell WS. Effects of prostaglandin D2, 15-deoxy-Delta12,14-prostaglandin J2, and selective DP1 and DP2 receptor agonists on pulmonary infiltration of eosinophils in Brown Norway rats. J Pharmacol Exp Ther. 2005;313:64–69. doi: 10.1124/jpet.104.079079. [DOI] [PubMed] [Google Scholar]
- Alving K, Matran R, Lundberg JM. Effect of nedocromil sodium on allergen-, PAF-, histamine- and bradykinin-induced airways vasodilatation and pulmonary obstruction in the pig. Br J Pharmacol. 1991;104:452–458. doi: 10.1111/j.1476-5381.1991.tb12450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli V, Staumont D, Charbonnier AS, Hammad H, Gosset P, Pichavant M, et al. Activation of the D prostanoid receptor 1 regulates immune and skin allergic responses. J Immunol. 2004;172:3822–3829. doi: 10.4049/jimmunol.172.6.3822. [DOI] [PubMed] [Google Scholar]
- Arimura A, Yasui K, Kishino J, Asanuma F, Hasegawa H, Kakudo S, et al. Prevention of allergic inflammation by a novel prostaglandin receptor antagonist, S-5751. J Pharmacol Exp Ther. 2001;298:411–419. [PubMed] [Google Scholar]
- Armer RE, Ashton MR, Boyd EA, Brennan CJ, Brookfield FA, Gazi L, et al. Indole-3-acetic acid antagonists of the prostaglandin D2 receptor CRTH2. J Med Chem. 2005;48:6174–6177. doi: 10.1021/jm050519b. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. New drugs for asthma. Nat Rev Drug Discov. 2004;3:831–844. doi: 10.1038/nrd1524. [DOI] [PubMed] [Google Scholar]
- Bochenek G, Nagraba K, Nizankowska E, Szczeklik A. A controlled study of 9alpha,11beta-PGF2 (a prostaglandin D2 metabolite) in plasma and urine of patients with bronchial asthma and healthy controls after aspirin challenge. J Allergy Clin Immunol. 2003;111:743–749. doi: 10.1067/mai.2003.1387. [DOI] [PubMed] [Google Scholar]
- Bohm E, Sturm GJ, Weiglhofer I, Sandig H, Shichijo M, McNamee A, et al. 11-Dehydro-thromboxane B2, a stable thromboxane metabolite, is a full agonist of chemoattractant receptor-homologous molecule expressed on TH2 cells (CRTH2) in human eosinophils and basophils. J Biol Chem. 2004;279:7663–7670. doi: 10.1074/jbc.M310270200. [DOI] [PubMed] [Google Scholar]
- Busse W, Raphael GD, Galant S, Kalberg C, Goode-Sellers S, Srebro S, et al. Low-dose fluticasone propionate compared with montelukast for first-line treatment of persistent asthma: a randomized clinical trial. J Allergy Clin Immunol. 2001;107:461–468. doi: 10.1067/mai.2001.114657. [DOI] [PubMed] [Google Scholar]
- Caron G, Delneste Y, Roelandts E, Duez C, Bonnefoy JY, Pestel J, et al. Histamine polarizes human dendritic cells into Th2 cell-promoting effector dendritic cells. J Immunol. 2001;167:3682–3686. doi: 10.4049/jimmunol.167.7.3682. [DOI] [PubMed] [Google Scholar]
- Charlesworth EN, Kagey-Sobotka A, Schleimer RP, Norman PS, Lichtenstein LM. Prednisone inhibits the appearance of inflammatory mediators and the influx of eosinophils and basophils associated with the cutaneous late-phase response to allergen. J Immunol. 1991;146:671–676. [PubMed] [Google Scholar]
- Cheng K, Wu TJ, Wu KK, Sturino C, Metters K, Gottesdiener K, et al. Antagonism of the prostaglandin D2 receptor 1 suppresses nicotinic acid-induced vasodilation in mice and humans. Proc Natl Acad Sci USA. 2006;103:6682–6687. doi: 10.1073/pnas.0601574103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossette C, Walsh SE, Kim S, Lee GJ, Lawson JA, Bellone S, et al. Agonist and antagonist effects of 15R-prostaglandin (PG) D2 and 11-methylene-PGD2 on human eosinophils and basophils. J Pharmacol Exp Ther. 2007;320:173–179. doi: 10.1124/jpet.106.111062. [DOI] [PubMed] [Google Scholar]
- Dahlen SE, Kumlin M. Monitoring mast cell activation by prostaglandin D2in vivo. Thorax. 2004;59:453–455. doi: 10.1136/thx.2004.026641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle WJ, Boehm S, Skoner DP. Physiologic responses to intranasal dose-response challenges with histamine, methacholine, bradykinin, and prostaglandin in adult volunteers with and without nasal allergy. J Allergy Clin Immunol. 1990;86:924–935. doi: 10.1016/s0091-6749(05)80156-3. [DOI] [PubMed] [Google Scholar]
- Fahy JV, Fleming HE, Wong HH, Liu JT, Su JQ, Reimann J, et al. The effect of an anti-IgE monoclonal antibody on the early- and late-phase responses to allergen inhalation in asthmatic subjects. Am J Respir Crit Care Med. 1997;155:1828–1834. doi: 10.1164/ajrccm.155.6.9196082. [DOI] [PubMed] [Google Scholar]
- Faith A, McDonald J, Peek E, Richards D, Caulfield J, Chevretton E, et al. Functional plasticity of human respiratory tract dendritic cells: GM-CSF enhances T(H)2 development. J Allergy Clin Immunol. 2005;116:1136–1143. doi: 10.1016/j.jaci.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Faveeuw C, Gosset P, Bureau F, Angeli V, Hirai H, Maruyama T, et al. Prostaglandin D2 inhibits the production of interleukin-12 in murine dendritic cells through multiple signaling pathways. Eur J Immunol. 2003;33:889–898. doi: 10.1002/eji.200323330. [DOI] [PubMed] [Google Scholar]
- Fujitani Y, Kanaoka Y, Aritake K, Uodome N, Okazaki-Hatake K, Urade Y. Pronounced eosinophilic lung inflammation and Th2 cytokine release in human lipocalin-type prostaglandin D synthase transgenic mice. J Immunol. 2002;168:443–449. doi: 10.4049/jimmunol.168.1.443. [DOI] [PubMed] [Google Scholar]
- Gazi L, Gyles S, Rose J, Lees S, Allan C, Xue L, et al. Delta12-prostaglandin D2 is a potent and selective CRTH2 receptor agonist and causes activation of human eosinophils and Th2 lymphocytes. Prostaglandins Other Lipid Mediat. 2005;75:153–167. doi: 10.1016/j.prostaglandins.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Gervais FG, Cruz RP, Chateauneuf A, Gale S, Sawyer N, Nantel F, et al. Selective modulation of chemokinesis, degranulation, and apoptosis in eosinophils through the PGD2 receptors CRTH2 and DP. J Allergy Clin Immunol. 2001;108:982–988. doi: 10.1067/mai.2001.119919. [DOI] [PubMed] [Google Scholar]
- Gervais FG, Morello JP, Beaulieu C, Sawyer N, Denis D, Greig G, et al. Identification of a potent and selective synthetic agonist at the CRTH2 receptor. Mol Pharmacol. 2005;67:1834–1839. doi: 10.1124/mol.104.009068. [DOI] [PubMed] [Google Scholar]
- Giles H, Leff P, Bolofo ML, Kelly MG, Robertson AD. The classification of prostaglandin DP-receptors in platelets and vasculature using BW A868C, a novel, selective and potent competitive antagonist. Br J Pharmacol. 1989;96:291–300. doi: 10.1111/j.1476-5381.1989.tb11816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- Gonzalo J, Qiu Y, Coyle AJ, Hodge MR. CRTH2(DP2) and not DP1 receptor mediate allergen induced mucus production and airway hyperresponsiveness. Am J Respir Crit Care Med. 2005;163:A811. [Google Scholar]
- Gosset P, Pichavant M, Faveeuw C, Bureau F, Tonnel AB, Trottein F. Prostaglandin D2 affects the differentiation and functions of human dendritic cells: impact on the T cell response. Eur J Immunol. 2005;35:1491–1500. doi: 10.1002/eji.200425319. [DOI] [PubMed] [Google Scholar]
- Gyles SL, Xue L, Townsend ER, Wettey F, Pettipher R. A dominant role for chemoattractant receptor-homologous molecule expressed on T helper type 2 (Th2) cells (CRTH2) in mediating chemotaxis of CRTH2+ CD4+ Th2 lymphocytes in response to mast cell supernatants. Immunology. 2006;119:362–368. doi: 10.1111/j.1365-2567.2006.02440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn EL, Bacharier LB. The atopic march: the pattern of allergic disease development in childhood. Immunol Allergy Clin North Am. 2005;25:231–246. doi: 10.1016/j.iac.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Hamid-Bloomfield S, Whittle BJ. Antagonism of PGD2 vasodepressor responses in the rat in vivo by the novel, selective antagonist, BW A868C. Br J Pharmacol. 1989;96:307–312. doi: 10.1111/j.1476-5381.1989.tb11818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad H, de Heer HJ, Soullie T, Hoogsteden HC, Trottein F, Lambrecht BN. Prostaglandin D2 inhibits airway dendritic cell migration and function in steady state conditions by selective activation of the D prostanoid receptor 1. J Immunol. 2003;171:3936–3940. doi: 10.4049/jimmunol.171.8.3936. [DOI] [PubMed] [Google Scholar]
- Hammad H, Kool M, Soullie T, Narumiya S, Trottein F, Hoogsteden HC, et al. Activation of the D prostanoid 1 receptor suppresses asthma by modulation of lung dendritic cell function and induction of regulatory T cells. J Exp Med. 2007;204:357–367. doi: 10.1084/jem.20061196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heavey DJ, Lumley P, Barrow SE, Murphy MB, Humphrey PP, Dollery CT. Effects of intravenous infusions of prostaglandin D2 in man. Prostaglandins. 1984;28:755–767. doi: 10.1016/0090-6980(84)90033-9. [DOI] [PubMed] [Google Scholar]
- Heinemann A, Schuligoi R, Sabroe I, Hartnell A, Peskar BA. Delta 12-prostaglandin J2, a plasma metabolite of prostaglandin D2, causes eosinophil mobilization from the bone marrow and primes eosinophils for chemotaxis. J Immunol. 2003;170:4752–4758. doi: 10.4049/jimmunol.170.9.4752. [DOI] [PubMed] [Google Scholar]
- Hirai H, Tanaka K, Takano S, Ichimasa M, Nakamura M, Nagata K. Cutting edge: agonistic effect of indomethacin on a prostaglandin D2 receptor, CRTH2. J Immunol. 2002;168:981–985. doi: 10.4049/jimmunol.168.3.981. [DOI] [PubMed] [Google Scholar]
- Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K, Takamori Y, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med. 2001;193:255–261. doi: 10.1084/jem.193.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth P, Walsh S, Robinson C. The comparative nasal effects of prostaglandin D2 in normal and rhinitic subjects. Adv Prostaglandin Thromboxane Leukot Res. 1991;21A:449–452. [PubMed] [Google Scholar]
- Howarth PH. Mediators of nasal blockage in allergic rhinitis. Allergy. 1997;52:12–18. doi: 10.1111/j.1398-9995.1997.tb04878.x. [DOI] [PubMed] [Google Scholar]
- Kitawaki T, Kadowaki N, Sugimoto N, Kambe N, Hori T, Miyachi Y, et al. IgE-activated mast cells in combination with pro-inflammatory factors induce Th2-promoting dendritic cells. Int Immunol. 2006;18:1789–1799. doi: 10.1093/intimm/dxl113. [DOI] [PubMed] [Google Scholar]
- Kostenis E, Ulven T. Emerging roles of DP and CRTH2 in allergic inflammation. Trends Mol Med. 2006;12:148–158. doi: 10.1016/j.molmed.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Lambrecht BN. Dendritic cells and the regulation of the allergic immune response. Allergy. 2005;60:271–282. doi: 10.1111/j.1398-9995.2005.00708.x. [DOI] [PubMed] [Google Scholar]
- Lewis RA, Soter NA, Diamond PT, Austen KF, Oates JA, Roberts LJ. Prostaglandin D2 generation after activation of rat and human mast cells with anti-IgE. J Immunol. 1982;129:1627–1631. [PubMed] [Google Scholar]
- Maciejewski-Lenoir D, Richman JG, Hakak Y, Gaidarov I, Behan DP, Connolly DT. Langerhans cells release prostaglandin D2 in response to nicotinic acid. J Invest Dermatol. 2006;126:2637–2646. doi: 10.1038/sj.jid.5700586. [DOI] [PubMed] [Google Scholar]
- Maezawa Y, Nakajima H, Kumano K, Kubo S, Karasuyama H, Iwamoto I. Role of IgE in Th2 cell-mediated allergic airway inflammation. Int Arch Allergy Immunol. 2003;131 Suppl 1:2–6. doi: 10.1159/000070473. [DOI] [PubMed] [Google Scholar]
- Maezawa Y, Nakajima H, Seto Y, Suto A, Kumano K, Kubo S, et al. IgE-dependent enhancement of Th2 cell-mediated allergic inflammation in the airways. Clin Exp Immunol. 2004;135:12–18. doi: 10.1111/j.1365-2249.2004.02337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka T, Hirata M, Tanaka H, Takahashi Y, Murata T, Kabashima K, et al. Prostaglandin D2 as a mediator of allergic asthma. Science. 2000;287:2013–2017. doi: 10.1126/science.287.5460.2013. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Young HA, Spitzer JH, Visintin A, Segal DM. Histamine regulates cytokine production in maturing dendritic cells, resulting in altered T cell polarization. J Clin Invest. 2001;108:1865–1873. doi: 10.1172/JCI13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenniff MG, Norman P, Cuthbert NJ, Gardiner PJ. BAY u3405, a potent and selective thromboxane A2 receptor antagonist on airway smooth muscle in vitro. Br J Pharmacol. 1991;104:585–590. doi: 10.1111/j.1476-5381.1991.tb12473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura H, Ikemura T, Kotera O, Sawada M, Tashiro S, Fuse E, et al. Inhibitory effect of the 4-aminotetrahydroquinoline derivatives, selective chemoattractant receptor-homologous molecule expressed on T helper 2 cell antagonists, on eosinophil migration induced by prostaglandin D2. J Pharmacol Exp Ther. 2005;314:244–251. doi: 10.1124/jpet.104.081539. [DOI] [PubMed] [Google Scholar]
- Monneret G, Gravel S, Diamond M, Rokach J, Powell WS. Prostaglandin D2 is a potent chemoattractant for human eosinophils that acts via a novel DP receptor. Blood. 2001;98:1942–1948. doi: 10.1182/blood.v98.6.1942. [DOI] [PubMed] [Google Scholar]
- Monneret G, Li H, Vasilescu J, Rokach J, Powell WS. 15-Deoxy-delta 12,14-prostaglandins D2 and J2 are potent activators of human eosinophils. J Immunol. 2002;168:3563–3569. doi: 10.4049/jimmunol.168.7.3563. [DOI] [PubMed] [Google Scholar]
- Murray JJ, Tonnel AB, Brash AR, Roberts LJ, Gosset P, Workman R, et al. Release of prostaglandin D2 into human airways during acute antigen challenge. N Engl J Med. 1986;315:800–804. doi: 10.1056/NEJM198609253151304. [DOI] [PubMed] [Google Scholar]
- Naclerio RM, Meier HL, Kagey-Sobotka A, Adkinson NF, Jr, Meyers DA, Norman PS, et al. Mediator release after nasal airway challenge with allergen. Am Rev Respir Dis. 1983;128:597–602. doi: 10.1164/arrd.1983.128.4.597. [DOI] [PubMed] [Google Scholar]
- Nagai H, Takeda H, Yamaguchi S, Tanaka H, Matsuo A, Inagaki N. The effect of a thromboxane A2 receptor antagonist BAY-u-3405 on experimental allergic reactions. Prostaglandins. 1995;50:75–87. doi: 10.1016/0090-6980(95)00111-5. [DOI] [PubMed] [Google Scholar]
- Nagata K, Hirai H, Tanaka K, Ogawa K, Aso T, Sugamura K, et al. CRTH2, an orphan receptor of T-helper-2-cells, is expressed on basophils and eosinophils and responds to mast cell-derived factor(s) FEBS Lett. 1999a;459:195–199. doi: 10.1016/s0014-5793(99)01251-x. [DOI] [PubMed] [Google Scholar]
- Nagata K, Tanaka K, Ogawa K, Kemmotsu K, Imai T, Yoshie O, et al. Selective expression of a novel surface molecule by human Th2 cells in vivo. J Immunol. 1999b;162:1278–1286. [PubMed] [Google Scholar]
- Narita S, Asakura K, Kataura A. Effects of thromboxane A2 receptor antagonist (Bay u 3405) on nasal symptoms after antigen challenge in sensitized guinea pigs. Int Arch Allergy Immunol. 1996;109:161–166. doi: 10.1159/000237215. [DOI] [PubMed] [Google Scholar]
- O'Sullivan S, Dahlen B, Dahlen SE, Kumlin M. Increased urinary excretion of the prostaglandin D2 metabolite 9 α, 11 β-prostaglandin F2 after aspirin challenge supports mast cell activation in aspirin-induced airway obstruction. J Allergy Clin Immunol. 1996;98:421–432. doi: 10.1016/s0091-6749(96)70167-7. [DOI] [PubMed] [Google Scholar]
- Peters SP, Schleimer RP, Kagey-Sobotka A, Naclerio RM, MacGlashan DW, Jr, Schulman ES, et al. The role of prostaglandin D2 in IgE-mediated reactions in man. Trans Assoc Am Physicians. 1982;95:221–228. [PubMed] [Google Scholar]
- Pettipher R, Hansel TT, Armer R. Antagonism of the prostaglandin D2 receptors DP1 and CRTH2 as an approach to treat allergic diseases. Nat Rev Drug Discov. 2007;6:313–325. doi: 10.1038/nrd2266. [DOI] [PubMed] [Google Scholar]
- Reddy ST, Tiano HF, Langenbach R, Morham SG, Herschman HR. Genetic evidence for distinct roles of COX-1 and COX-2 in the immediate and delayed phases of prostaglandin synthesis in mast cells. Biochem Biophys Res Commun. 1999;265:205–210. doi: 10.1006/bbrc.1999.1658. [DOI] [PubMed] [Google Scholar]
- Sandig H, Andrew D, Barnes AA, Sabroe I, Pease J. 9α,11β-PGF2 and its stereoisomer PGF2α are novel agonists of the chemoattractant receptor, CRTH2. FEBS Lett. 2006;580:373–379. doi: 10.1016/j.febslet.2005.11.052. [DOI] [PubMed] [Google Scholar]
- Sandig H, Pease JE, Sabroe I. Contrary prostaglandins: the opposing roles of PGD2 and its metabolites in leukocyte function. J Leukoc Biol. 2007;81:372–382. doi: 10.1189/jlb.0706424. [DOI] [PubMed] [Google Scholar]
- Satoh T, Moroi R, Aritake K, Urade Y, Kanai Y, Sumi K, et al. Prostaglandin D2 plays an essential role in chronic allergic inflammation of the skin via CRTH2 receptor. J Immunol. 2006;177:2621–2629. doi: 10.4049/jimmunol.177.4.2621. [DOI] [PubMed] [Google Scholar]
- Sawyer N, Cauchon E, Chateauneuf A, Cruz RP, Nicholson DW, Metters KM, et al. Molecular pharmacology of the human prostaglandin D2 receptor, CRTH2. Br J Pharmacol. 2002;137:1163–1172. doi: 10.1038/sj.bjp.0704973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuligoi R, Schmidt R, Geisslinger G, Kollroser M, Peskar BA, Heinemann A. PGD2 metabolism in plasma: kinetics and relationship with bioactivity on DP1 and CRTH2 receptors. Biochem Pharmacol. 2007;74:107–117. doi: 10.1016/j.bcp.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Shiraishi Y, Asano K, Nakajima T, Oguma T, Suzuki Y, Shiomi T, et al. Prostaglandin D2-induced eosinophilic airway inflammation is mediated by CRTH2 receptor. J Pharmacol Exp Ther. 2005;312:954–960. doi: 10.1124/jpet.104.078212. [DOI] [PubMed] [Google Scholar]
- Sihra BS, Kon OM, Durham SR, Walker S, Barnes NC, Kay AB. Effect of cyclosporin A on the allergen-induced late asthmatic reaction. Thorax. 1997;52:447–452. doi: 10.1136/thx.52.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa A, Pfister R, Christie PE, Lane SJ, Nasser SM, Schmitz-Schumann M, et al. Enhanced expression of cyclo-oxygenase isoenzyme 2 (COX-2) in asthmatic airways and its cellular distribution in aspirin-sensitive asthma. Thorax. 1997;52:940–945. doi: 10.1136/thx.52.11.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spik I, Brenuchon C, Angeli V, Staumont D, Fleury S, Capron M, et al. Activation of the prostaglandin D2 receptor DP2/CRTH2 increases allergic inflammation in mouse. J Immunol. 2005;174:3703–3708. doi: 10.4049/jimmunol.174.6.3703. [DOI] [PubMed] [Google Scholar]
- Stubbs VE, Schratl P, Hartnell A, Williams TJ, Peskar BA, Heinemann A, et al. Indomethacin causes prostaglandin D2-like and eotaxin-like selective responses in eosinophils and basophils. J Biol Chem. 2002;277:26012–26020. doi: 10.1074/jbc.M201803200. [DOI] [PubMed] [Google Scholar]
- Sturino CF, O'Neill G, Lachance N, Boyd M, Berthelette C, Labelle M, et al. Discovery of a potent and selective prostaglandin D2 receptor antagonist, [(3R)-4-(4-chloro-benzyl)-7-fluoro-5-(methylsulfonyl)-1,2,3,4-tetrahydrocy clopenta[b]indol-3-yl]-acetic acid (MK-0524) J Med Chem. 2007;50:794–806. doi: 10.1021/jm0603668. [DOI] [PubMed] [Google Scholar]
- Sugimoto H, Shichijo M, Iino T, Manabe Y, Watanabe A, Shimazaki M, et al. An orally bioavailable small molecule antagonist of CRTH2, ramatroban (BAY u3405), inhibits prostaglandin D2-induced eosinophil migration in vitro. J Pharmacol Exp Ther. 2003;305:347–352. doi: 10.1124/jpet.102.046748. [DOI] [PubMed] [Google Scholar]
- Takeshita K, Yamasaki T, Nagao K, Sugimoto H, Shichijo M, Gantner F, et al. CRTH2 is a prominent effector in contact hypersensitivity-induced neutrophil inflammation. Int Immunol. 2004;16:947–959. doi: 10.1093/intimm/dxh096. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Hirai H, Takano S, Nakamura M, Nagata K. Effects of prostaglandin D2 on helper T cell functions. Biochem Biophys Res Commun. 2004;316:1009–1014. doi: 10.1016/j.bbrc.2004.02.151. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Ogawa K, Sugamura K, Nakamura M, Takano S, Nagata K. Cutting edge: differential production of prostaglandin D2 by human helper T cell subsets. J Immunol. 2000;164:2277–2280. doi: 10.4049/jimmunol.164.5.2277. [DOI] [PubMed] [Google Scholar]
- Terada N, Yamakoshi T, Hasegawa M, Tanikawa H, Maesako K, Ishikawa K, et al. The effect of ramatroban (BAY u 3405), a thromboxane A2 receptor antagonist, on nasal cavity volume and minimum cross-sectional area and nasal mucosal hemodynamics after nasal mucosal allergen challenge in patients with perennial allergic rhinitis. Acta Otolaryngol Suppl. 1998;537:32–37. doi: 10.1080/00016489850182323. [DOI] [PubMed] [Google Scholar]
- Theiner G, Gessner A, Lutz MB. The mast cell mediator PGD2 suppresses IL-12 release by dendritic cells leading to Th2 polarized immune responses in vivo. Immunobiology. 2006;211:463–472. doi: 10.1016/j.imbio.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Trivedi SG, Newson J, Rajakariar R, Jacques TS, Hannon R, Kanaoka Y, et al. Essential role for hematopoietic prostaglandin D2 synthase in the control of delayed type hypersensitivity. Proc Natl Acad Sci USA. 2006;103:5179–5184. doi: 10.1073/pnas.0507175103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uller L, Mathiesen JM, Alenmyr L, Korsgren M, Ulven T, Hogberg T, et al. Antagonism of the prostaglandin D2 receptor CRTH2 attenuates asthma pathology in mouse eosinophilic airway inflammation. Respir Res. 2007;8:16. doi: 10.1186/1465-9921-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulven T, Kostenis E. Minor structural modifications convert the dual TP/CRTH2 antagonist ramatroban into a highly selective and potent CRTH2 antagonist. J Med Chem. 2005;48:897–900. doi: 10.1021/jm049036i. [DOI] [PubMed] [Google Scholar]
- van Neerven RJ, Knol EF, Ejrnaes A, Wurtzen PA. IgE-mediated allergen presentation and blocking antibodies: regulation of T-cell activation in allergy. Int Arch Allergy Immunol. 2006;141:119–129. doi: 10.1159/000094714. [DOI] [PubMed] [Google Scholar]
- van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005;201:981–991. doi: 10.1084/jem.20042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinall SL, Townsend ER, Pettipher R. A paracrine role for chemoattractant receptor-homologous molecule expressed on T helper type 2 cells (CRTH2) in mediating chemotactic activation of CRTH2(+) CD4(+) T helper type 2 lymphocytes. Immunology. 2007;121:577–584. doi: 10.1111/j.1365-2567.2007.02606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch L, Labat C, Gascard JP, de MV, Brink C, Norel X. Prostanoid receptors involved in the relaxation of human pulmonary vessels. Br J Pharmacol. 1999;126:859–866. doi: 10.1038/sj.bjp.0702393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Ito T, Wang YH, Homey B, Watanabe N, Martin R, et al. Maintenance and polarization of human Th2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity. 2006;24:827–838. doi: 10.1016/j.immuni.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Whittle BJ, Moncada S, Mullane K, Vane JR. Platelet and cardiovascular activity of the hydantoin BW245C, a potent prostaglandin analogue. Prostaglandins. 1983;25:205–223. doi: 10.1016/0090-6980(83)90105-3. [DOI] [PubMed] [Google Scholar]
- Widdicombe JG. Nasal pathophysiology. Respir Med. 1990;84 Suppl A:3–9. doi: 10.1016/s0954-6111(08)80001-7. [DOI] [PubMed] [Google Scholar]
- Wright DH, Ford-Hutchinson AW, Chadee K, Metters KM. The human prostanoid DP receptor stimulates mucin secretion in LS174 T cells. Br J Pharmacol. 2000;131:1537–1545. doi: 10.1038/sj.bjp.0703688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Gyles SL, Barrow A, Pettipher R. Inhibition of PI3K and calcineurin suppresses chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2)-dependent responses of Th2 lymphocytes to prostaglandin D2. Biochem Pharmacol. 2007;73:843–853. doi: 10.1016/j.bcp.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Xue L, Gyles SL, Wettey FR, Gazi L, Townsend E, Hunter MG, et al. Prostaglandin D2 causes preferential induction of proinflammatory Th2 cytokine production through an action on chemoattractant receptor-like molecule expressed on Th2 cells. J Immunol. 2005;175:6531–6536. doi: 10.4049/jimmunol.175.10.6531. [DOI] [PubMed] [Google Scholar]
- Ying S, O'Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]