Abstract
IL-6-type cytokines bind to plasma membrane receptor complexes containing the common signal transducing receptor chain gp130 that is ubiquitously expressed in most tissues including the heart. The two major signalling cascades activated by the gp130 receptor, SHP2/ERK and STAT pathways, have been demonstrated to play important roles in cardiac development, hypertrophy, protection and remodelling in response to physiological and pathophysiological stimuli. Experimental data, both in vivo and in vitro, imply beneficial effects of gp130 signalling on cardiomyocytes in terms of growth and survival. In contrast, it has been reported that elevated serum levels of IL-6 cytokines and gp130 proteins are strong prognostic markers for morbidity and mortality in patients with heart failure or after myocardial infarction. Moreover, it has been shown that the local gp130 receptor system is altered in failing human hearts. In the present review, we summarize the basic principles of gp130 signalling, which requires simultaneous activation of STAT and ERK pathways under the tight control of positive and negative intracellular signalling modulators to provide a balanced biological outcome. Furthermore, we highlight the key role of the gp130 receptor and its major downstream effectors in the heart in terms of development and regeneration and in response to various physiological and pathophysiological stress situations. Finally, we comment on tissue-specific diversity and challenges in targeted pharmacological interference with components of the gp130 receptor system.
Keywords: heart, gp130 receptor, signalling diversification, hypertrophy, cardioprotection, regeneration
Introduction
The glycoprotein-130 (gp130), the central signal transducer of the interleukin-6 (IL-6)-related cytokines, is widely expressed in the mammalian organism, including the developing and adult heart. Activation of gp130 in the heart by IL-6 type cytokines occurs during embryogenesis, in response to inflammation, pressure overload and ischaemic injury thereby inducing signalling via three major pathways: (1) the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway, (2) the Ras/mitogen-activated protein kinase (MAPK and extracellular signal-regulated kinase(ERK)) signalling pathway and (3) the phosphatidylinositol-3-kinase-dependent (PI3K)/Akt pathway (Figure 1) (Hirano et al., 1997; Hirota et al., 1999; Funamoto et al., 2000; Heinrich et al., 2003; Fischer and Hilfiker-Kleiner, 2007).
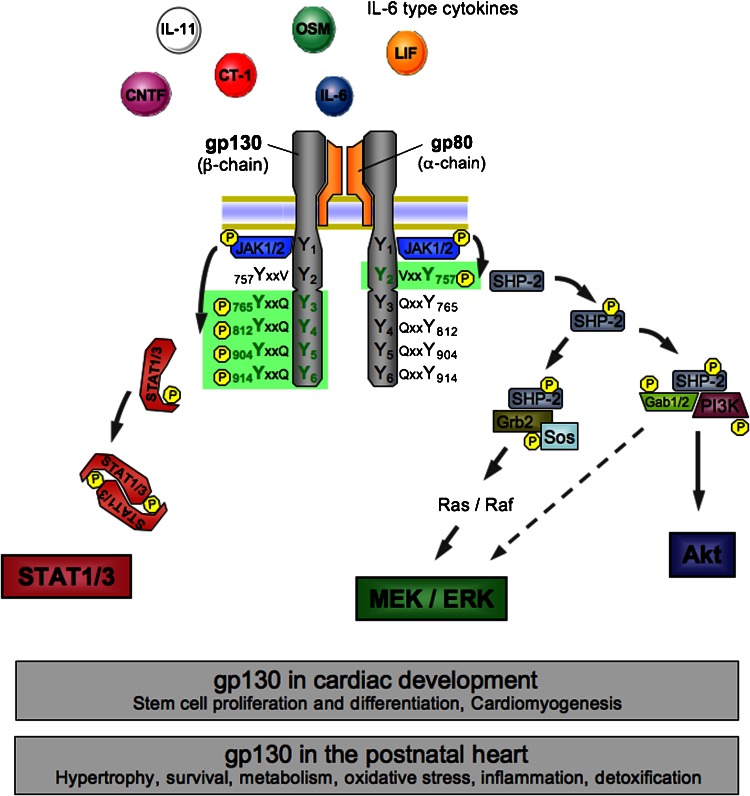
Figure 1.
Role of glycoprotein 130 (gp130) downstream signalling in the heart. Scheme of gp130 receptor activation showing interleukin-6 (IL-6) type cytokines binding to their transmembrane receptor α-subunits, which induces homodimerization of the gp130 receptor β-subunits or heterodimerization of gp130 with either one of the structurally and functionally similar leukaemia inhibitory factor (LIF) receptor and oncostatin M (OSM) receptor β-subunits. Following dimerization of the gp130 receptor complex, Janus kinase-1/2 (JAK1/2) constitutively connected to the intracytoplasmatic membrane proximal regions of the receptor subunits are catalytically activated and themselves transphosphorylate tyrosine residues in the gp130 receptor intracellular domain. Subsequently, two major intracellular signalling cascades are triggered, the signal transducer and activator of transcription (STAT)-1/3 pathway and the SH2 domain-containing cytoplasmic protein tyrosine phosphatase (SHP2)/MEK/extracellular signal-regulated kinase (ERK) pathway. Via the formation of complexes with growth factor receptor binding protein-2 (Grb2)-associated binding protein-1/2 (Gab1/2) and phosphatidylinositol-3-kinase (PI3K), SHP2 activation furthermore initiates the Akt pathway and additionally enhances MEK/ERK signalling. Activation of gp130-mediated signalling plays essential roles in stem cells and cardiomyogenesis. In the adult heart, it is involved in numerous physiological and pathophysiological processes. MEK, mitogen-activated protein kinase (MAPK)/ERK kinase.
In recent years, it has been reported that the serum concentration of IL-6 increases after myocardial infarction (Rattazzi et al., 2003), and that levels of circulating IL-6 and soluble IL-6 receptor (sIL-6R) are elevated in patients with congestive heart failure (Tsutamoto et al., 1998; Buzas et al., 2004), as well as in hypertrophic and dilative cardiomyopathy (Buzas et al., 2004). Furthermore, systemic IL-6 levels correlate with the severity of left ventricular dysfunction and are strong independent predictors of subsequent clinical outcomes in patients with heart failure and after myocardial infarction (Roig et al., 1998; Tsutamoto et al., 1998; Maeda et al., 2000; Orus et al., 2000; Birks et al., 2001; Rattazzi et al., 2003). Similarly, plasma levels of the IL-6 type cytokine, leukaemia inhibitory factor (LIF) and soluble gp130 receptor significantly increase in association with the severity of heart failure (Hirota et al., 2004). More recently, our laboratory has explored the IL-6/gp130/JAK/STAT signalling cascade in patients with end-stage heart failure and showed that this signalling cascade is altered at the levels of ligands, receptors and downstream signalling molecules (Podewski et al., 2003). For example, cardiac expression of IL-6 is reduced whereas the expression of LIF is elevated (Eiken et al., 2001; Podewski et al., 2003). The expression of cardiotrophin-1 (CT-1), another member of the IL-6 cytokine family, is not altered (Podewski et al., 2003). At the receptor level, gp130 expression is not different, but failing hearts display enhanced gp130 activation (tyrosine phosphorylation). The expression of JAK2 and tyrosine kinase-2, the next downstream signalling molecules of the gp130 receptor, are also not altered, but surprisingly their activation state (tyrosine phosphorylation) is diminished in failing hearts. Most strikingly, both expression and activation of the effector signalling molecule of the IL-6/gp130/JAK/STAT cascade, STAT3, is severely reduced in failing hearts (Podewski et al., 2003).
Until recently, only limited information is available on the expression in failing human hearts of the suppressor of cytokine signalling (SOCS) proteins that serve as negative regulators of gp130/JAK/STAT signalling. SOCS3 is transcriptionally upregulated by STAT transcription factors and, in line with lower STAT3 activity, its expression appeared to be attenuated in terminally failing human hearts (Podewski et al., 2003).
In general, observations taken from patient studies are mainly descriptive and do not identify the causative functional roles of individual factors or signalling pathways. In this regard, the biological consequence of IL-6 type cytokine expression and its respective downstream signalling mechanisms in human cardiac pathophysiology has not yet been resolved. It has been suggested that systemically increased IL-6 type cytokine signalling may contribute to the progression of heart failure and its thrombotic complications (Chin et al., 2003) pointing to a deleterious role of IL-6 type cytokine signalling in patients. In contrast, a growing body of experimental work suggests that IL-6-related cytokine signalling contributes to compensatory hypertrophy, provides cardioprotection and promotes neovascularization (Hirota et al., 1999; Negoro et al., 2000; Yamauchi-Takihara and Kishimoto, 2000, 2002; Jacoby et al., 2003; Hilfiker-Kleiner et al., 2004). In particular, the analysis of transgenic mice harbouring knockouts for specific components of the gp130 receptor system shed light on the specific roles of the gp130 receptor system for various cardiovascular cell types and the myocardium itself.
In the current review, we highlight the key role of the gp130 receptor and its major downstream signalling mediators in the heart in terms of cardiac development and postnatal physiology. Moreover, we summarize the basic principle of gp130 signalling and try to place experimental findings and clinical observations in relation to each other to explain potential discrepancies of expected beneficial versus observed detrimental roles of gp130 signalling within the pathophysiological situations of the cardiovascular system.
The gp130 receptor signalling system
The IL-6 type cytokines, comprising IL-6, IL-11, LIF, oncostatin M (OSM), ciliary neurotrophic factor, CT-1 and cardiotrophin-like cytokine are a family of mediators with pleiotropic effects in the organism. They are involved in inflammatory and immunologic processes (May et al., 2003) as well as in haematopoiesis, liver and neuronal regeneration, embryonic development and cardiovascular physiology as reviewed by Heinrich et al. (1998), Ernst and Jenkins (2004) and Fischer and Hilfiker-Kleiner (2007). Hence, they activate target genes involved in growth, differentiation, survival, apoptosis and proliferation. With the shared use of the gp130 receptor, the biological functions of IL-6 family cytokines are largely overlapping as reviewed by Ernst and Jenkins (2004).
Interleukin-6 type cytokines bind to plasma membrane receptor complexes containing the common signal-transducing receptor chain gp130. IL-6, IL-11 and ciliary neurotrophic factor first bind specifically to their respective α-receptor subunits, and only the complex of cytokine and α-receptor efficiently recruits the signalling receptor subunits (Pennica et al., 1995), which was reviewed by Heinrich et al. (1998), Ernst and Jenkins (2004) and Fischer and Hilfiker-Kleiner (2007). The binding of a ligand to its receptor induces homodimerization of gp130 (upon binding of IL-6 and IL-11) or heterodimerization of gp130 with LIF receptor (following binding of LIF, ciliary neurotrophic factor, cardiotrophin-like cytokine and CT-1) or OSM receptor (for OSM), respectively (Pennica et al., 1995), which was reviewed by Fischer and Hilfiker-Kleiner (2007). Receptor binding of all IL-6 cytokines induces dimerization of gp130 receptor β-subunits, which triggers activation of non-overlapping intracellular signalling cascades by phosphorylation of multiple, highly conserved tyrosine residues (Y) within the cytoplasmic domain of the gp130 receptor via constitutively associated JAK family members (Figure 1). Subsequent signal transduction via gp130 involves three major downstream pathways: (1) the JAK/STAT1/3 pathway, (2) the Ras/ERK1/2 signalling pathway and (3) the PI3K/Akt pathway (Figure 1) (Kunisada et al., 1996; Hirano et al., 1997; Oh et al., 1998; Hirota et al., 1999; Heinrich et al., 2003). Phosphorylation of a single-membrane proximal Y residue (Y757) within the cytoplasmic domain of the gp130 receptor is necessary and sufficient for recruitment of the SH2 domain-containing cytoplasmic protein tyrosine phosphatase (SHP2), its subsequent tyrosine phosphorylation and activation of the Ras/ERK1/2 cascade (Figure 1). In this regard, tyrosine phosphorylation of SHP2 provides docking elements for the adaptor protein growth factor receptor binding protein-2, which is constitutively associated with the Ras/GTP exchange factor, Sos as reviewed by Ernst and Jenkins (2004). In addition, tyrosine-phosphorylated SHP2 can form a complex with the scaffolding proteins growth factor receptor binding protein-2-associated binding protein-1/2 and the p85 subunit of PI3K, which leads to activation of the Akt pathway and might also feed into the Ras/ERK1/2 cascade (Figure 1) as reviewed by Ernst and Jenkins (2004). By contrast, docking of STAT proteins (mainly STAT1 and 3) to the C-terminal phosphotyrosine residues within the gp130 receptor results in tyrosine phosphorylation of the STAT1/3 proteins by the activated JAKs with subsequent homo- and heterodimerization and nuclear translocation where STAT transcription factor complexes eventually induce transcription of specific sets of target genes (Figure 1) (Funamoto et al., 2000; Negoro et al., 2001a; Osugi et al., 2002), which was reviewed by Fischer and Hilfiker-Kleiner (2007).
In summary, the expression pattern of IL-6 cytokines may vary among different developmental stages, cell types and stress situations; however, their biological functions in terms of gp130 receptor-mediated signalling seem to be largely overlapping.
Modulation of the cellular response to gp130-mediated signalling: fine-tuning JAK/STAT and SHP2/ERK signalling is the key issue
There is evidence that balanced versus unbalanced activation of gp130 downstream signalling may play a key role between beneficial organ protection and regeneration, and detrimental tumorigenesis and organ failure. For example, prolonged gp130-mediated STAT3 activation promotes liver progenitor cell proliferation, whereas gp130-mediated ERK1/2 activation suppresses this process, suggesting that gp130 signalling acts as an important switch between liver regeneration and tumorigenesis (Yeoh et al., 2007). In the heart, it has been reported that early blockade of gp130-mediated STAT3 signalling negatively affects susceptibility to viral infection (Yajima et al., 2006). Moreover, sufficient activation of gp130/STAT3 signalling plays an important role for increasing cardiomyocyte cell survival until specific immune responses begin to clear the virus (Yajima et al., 2006). Along these lines, it has been shown that gp130-mediated STAT3 activation is transducing important hypertrophic and cytoprotective effects in the heart (Hirota et al., 1999; Jacoby et al., 2003). In contrast, overexpression of the negative regulator of gp130/STAT activation, SOCS3, in the pressure-overloaded heart has been shown to promote the transition between cardiac hypertrophy and failure, further supporting the important role of an intact gp130/STAT cascade for cardioprotection (Yasukawa et al., 2001). Additional data from transgenic mouse lines imply that gp130-mediated JAK/STAT signalling is required throughout life, because systemic ablation of gp130-mediated JAK/STAT activation (gp130ΔSTAT) leads to reduced life spans, impaired humoral and mucosal immune and hepatic acute phase responses, failure of blastocyst implantation, impaired gastrointestinal wound healing, degenerative joint disease, increased numbers of haematopoietic progenitor cells, thrombocytopaenia, leukocytosis and Th2-biased immune response (Ernst and Jenkins, 2004).
As found in mice with defective gp130-mediated JAK/STAT activation, mice with systemic deficiency for gp130-mediated SHP2/ERK (gp130Y757) activation develop various systemic defects, which are, however, of a different nature. These mutant mice develop splenomegaly, lymphadenopathy, enhanced hepatic acute phase and thymus-dependent immune responses, Th1-biased immune response and autoimmune arthritis (Jenkins et al., 2005). Interestingly, it is thought that the majority of organ defects in gp130Y757 mice are not direct consequences of deficient gp130-mediated ERK activation, but rather result from sustained gp130-mediated STAT1/3 activation (Ernst and Jenkins, 2004). This feature is explained by the fact that the Y757 residue serves not only for SHP2 activation, but also for the recruitment of SOCS3 protein to the gp130 receptor (Figure 2) (Ernst and Jenkins, 2004; Johnston, 2004). Specifically, SOCS3 competes with SHP2 for the phosphorylated Y757 docking site, thereby reducing the availability of the phosphorylated tyrosine of the SHP2 docking site and, as a consequence, attenuating gp130-mediated ERK signalling. In addition, as mentioned previously, SOCS proteins serve as negative regulators of JAK/STAT signalling. They harbour an SH2 domain and a COOH-terminal SOCS box and inhibit JAK signalling upon binding to phosphorylated tyrosine residues of the cytokine receptors such as gp130 and/or directly to JAK. Moreover, SOCS proteins are implicated in destabilizing their interacting partners as they can function as the substrate-recruiting component of ubiquitin ligases, thereby not only affecting the activation state of STATs, but also their protein levels (Johnston, 2004).
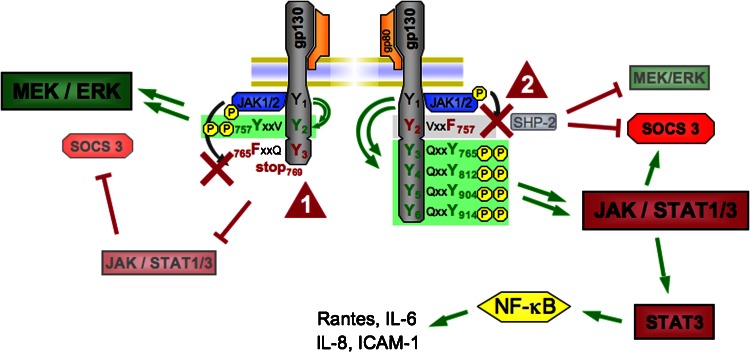
Figure 2.
Mutations in the glycoprotein 130 (gp130) receptor lead to an imbalance in downstream signalling pathways. Detailed schematic view of the gp130 receptor structure shows tyrosine 757 (Y757) necessary for SH2 domain-containing cytoplasmic protein tyrosine phosphatase (SHP2) phosphorylation and suppressor of cytokine signalling (SOCS)-3 binding. A point mutation (Y757 → F757, tyrosine → phenylalanine) abolishes binding of SHP2 and SOCS3 (arrowhead 1) and leads to uncontrolled Janus kinase (JAK)/signal transducer and activator of transcription (STAT) activation with exaggerated STAT3 activation. As a consequence, proinflammatory pathways, that is nuclear factor-κB (NF-κB) activation, are promoted. The left side displays a truncated gp130 intracellular domain incapable of binding and activating the STAT1/3 pathway (arrowhead 2) from its membrane-distal phosphotyrosine residues. This mutant form is characterized by the substitution of Y765 by F765 and is truncated at the C-terminal side of amino acid 768. Deficient STAT1/3 signalling in this model is accompanied by enhanced signalling via the SHP2/MEK/extracellular signal-regulated kinase (ERK) pathway. ICAM, intercellular adhesion molecule-1. MEK, mitogen-activated protein kinase (MAPK)/ERK kinase.
The lack of STAT3 protein degradation in the absence of SOCS3 binding together with the notion that the JAK/STAT1/3 axis upregulates STAT3 expression explains at least in part the hyperactive state of JAK/STAT signalling in gp130Y757 mice. Moreover, even when gp130 receptor activation ceases, increased levels of unphosphorylated STAT3 protein can drive a second, delayed wave of gene expression in the absence of tyrosine phosphorylation, including chemokine, CC motif, ligand-5; IL-6; intercellular adhesion molecule-1 and IL-8 that do not respond directly to phosphorylated STAT3 (Figure 2) (Yang et al., 2005). Here, a transcription factor complex with unphosphorylated nuclear factor-κB (NF-κB) activates many genes responsive to unphosphorylated STAT3. These κB-dependent genes are activated upon accumulation of the complex of unphosphorylated STAT3 and NF-κB in the nucleus binding to the κB elements of promoters and may partially account for the proinflammatory phenotype resulting from prolonged and excessive STAT3 activation as observed in gp130Y757 mice. Moreover, interactions among phosphorylated and unphosphorylated forms of STAT3 and NF-κB as well as binding of unphosphorylated STAT3 to various other transcription factors such as c-Jun and the glucocorticoid response element have been reported. It may, therefore, be that the upregulation of unphosphorylated STAT3 is important for the entire physiological functions of the group of cytokines that use the common gp130 receptor subunit to phosphorylate STAT3 (Yang et al., 2007a).
Taken together, the simultaneous activation of STAT and ERK pathways by gp130 receptor stimulation appears essential for a balanced biological outcome in many physiological and pathophysiological settings, especially as STAT and ERK signalling induce partly contradictory or at least conflicting responses (Ernst and Jenkins, 2004; Jenkins et al., 2005; Yeoh et al., 2007). Disturbance of this ‘signalling orchestration' may lead to an exaggerated activation of a single pathway causing an unrestrained biological response with ultimately pathophysiological outcomes.
The gp130 receptor system plays a crucial role in early heart development and might therefore be important for cardiac regeneration
The heart is among the first organs to be formed and to function during development. Embryos with a homozygous null mutation for gp130 die between 12.5 days postcoitum and term (Yoshida et al., 1996). On 16.5 days postcoitum and later, they show hypoplastic ventricular myocardium without septal and trabecular defect. The subcellular ultrastructures in cardiomyocytes deficient in gp130 appear normal, but the cardiac phenotype indicates a role in the expansion of the compact layer of the ventricular myocardium. The gp130 ligands LIF and CT-1 are expressed at high levels during the course of cardiogenesis and promote proliferation and survival of embryonic cardiomyocytes implicating that the gp130 receptor system plays an essential role for the cardiac development, potentially by regulating cardiomyogenesis, cardiomyocyte survival and growth (Sheng et al., 1996).
In recent years, the nature of the postnatal heart as a terminally differentiated organ which—due to the negligible rates of cardiomyocyte proliferation and the quantitatively insignificant replenishment by endogenous progenitor cells—is incapable of endogenous repair and myocardial regeneration, generated the objective of replacing damaged myocardial tissue by ex vivo expanded and differentiated stem or progenitor cells. Current research focuses on suitable cell types and modes of transplantation, the determination of the cardiac stem cell niche microenvironment and, importantly, the inducers and pathways of stem cell proliferation and directed differentiation into the main myocardial cell types including cardiomyocytes. In this regard, it is worth mentioning that upregulation of IL-6 and activation of STAT3 have been implicated in the G0/G1 cell cycle phase transition and induction of proliferation of hepatocytes after hepatectomy. Mice deficient in either IL-6 or the transcription factor NF-IL-6 display a disturbed proliferative response during liver regeneration partly resulting in liver failure associated with lack of STAT3 activation and decreased expression of adaptor-related protein complex-1, Myc and cyclin D1 mRNA (Streetz et al., 2000; Wallenius et al., 2000). However, it is currently unclear whether knowledge of regenerative aspects of gp130-mediated signalling in the liver can, at least in part, help to decipher modulation of cell cycle progression and cellular division in other organs in the adult, such as the heart.
Differential gp130 signalling as a switch in directing stem cell fate
It is known that murine embryonic stem (ES) cells can be maintained in an undifferentiated state on a feeder layer of mouse embryonic fibroblasts or in the presence of LIF. Murine ES cells require LIF-mediated gp130 activation or alternatively induced gp130 dimerization (Stuhlmann-Laeisz et al., 2006) for self renewal and, most importantly, STAT3 downstream signalling has been identified as the key pathway in the gp130 system that prevents differentiation of ES cells and keeps them in an undifferentiated state (Matsuda et al., 1999). Likewise, gp130 and activation of its downstream mediator STAT3 have been proven to be mainly responsible for the expansion of haematopoietic precursor cells in the fetal mouse aorto-gonado-mesonephros region (Takizawa et al., 2003). However, it is very likely that other (gp130-independent) paracrine signalling pathways play additional roles in the maintenance of ‘stemness' and the determination of stem cell fate (Dani et al., 1998). In line with their murine counterparts, human ES cells have a functioning gp130 receptor system that displays robust activation of its major signalling pathways JAK/STAT, MEK (MAPK/ERK kinase)/ERK and PI3K/AKT in response to IL-6 type cytokine stimulation. However, activation of gp130-dependent signalling and the JAK/STAT3 pathway in human ES cells leads to the loss of markers of pluripotency, indicating that the maintenance of an undifferentiated state in human ES cells is independent of STAT3 signalling (Daheron et al., 2004; Sumi et al., 2004) and potentially depends on pathways not related to the gp130 receptor system (Dani et al., 1998). Although inhibition of the gp130 receptor-triggered MAPK pathway stabilizes the STAT3-mediated conservation of stemness in murine ES cells, activation of the ERK1/2 pathway in turn leads to the loss of pluripotency and the onset of differentiation. For example, concurrent addition of bone morphogenetic protein-2 and LIF induces the expression of cardiomyocyte markers such as connexin-43, Mef2c, Tbx5, Nkx2.5, GATA-4 and myosin heavy chain (MHC) in murine ES cells as early as 2–4 days in culture, with STAT3 and ERK1/2 playing prominent roles in this determination of cell fate (Rajasingh et al., 2007). From these experimental data, it is possible that the balance and cross talk between STAT3 and MAPK signalling account for the induction of cardiomyocyte differentiation in ES cells (Burdon et al., 2002; Humphrey et al., 2004).
A potentially important role of gp130 signalling may also exist in adult stem cells sources. In this regard, it has recently been shown that spermatogonial stem cells are able to differentiate into cardiomyocytes and are therefore viewed as potential candidates to regenerate the injured heart (Guan et al., 2006, 2007). Spermatogonial stem cells continuously divide in the testis to support spermatogenesis throughout the life of adult male animals. As very few spermatogonial stem cells are present in vivo, it is necessary to expand these cells in vitro for potential therapeutic use. A recent report found that the addition of LIF to newborn testis cell cultures enhanced the formation of germ cell colonies. Interestingly, ciliary neurotrophic factor, but not OSM, had the same effect (Kanatsu-Shinohara et al., 2007). No phenotypic or functional difference was found between spermatogonial stem cells that had been maintained in the absence of LIF, indicating that LIF per se does not alter the stemness of spermatogonial stem cells (Kanatsu-Shinohara et al., 2007). These data suggest that LIF/gp130 signalling could be useful in the initiation and expansion of spermatogonial stem cell cultures for later therapeutic use.
Interestingly, recent data also hint at a role of differential gp130 receptor downstream signalling in adult tissue-committed cell plasticity. Specifically, it was found that OSM induces epithelial-to-mesenchymal transition of proximal tubular cells into myofibroblasts during tubulointerstitial kidney fibrosis in a gp130-dependent manner, involving phosphorylation of STAT1 and STAT3 as well as activation of ERK1, ERK2 and ERK5 (Schramek et al., 2003; Nightingale et al., 2004; Pollack et al., 2007). The promotion of myofibroblast differentiation from resident, tissue-specific cells might also be induced by other cytokines that activate STAT3 or the MAPK pathway and are produced during inflammatory reactions. Moreover, this transdifferentiation mechanism of tissue-committed cells could play an important role in remodelling processes in response to pathological influences such as ischaemia in various organs and tissues, including the heart.
Thus, knowledge of the influence of the gp130 receptor system on embryonic development, cell proliferation or differentiation decisions generated in embryonic or adult stem cells may help to elucidate the mechanisms to expand stem cells and to drive cell fate determination. In addition, it will be of major interest to characterize the role of gp130-dependent signalling in the microenvironment of organ-specific stem cell niches, especially the composition of gp130-dependent paracrine factors that regulate adult stem cell biology and tissue/organ homeostasis to optimize regeneration processes.
Deciphering gp130-mediated effects in cardiomyocytes: hypertrophy, survival, metabolic regulation
The activation of gp130 signalling by distinct members of the IL-6 type family of cytokines, namely CT-1, LIF and potentially IL-11, promotes cardiomyocyte survival (Fischer and Hilfiker-Kleiner, 2007; Kimura et al., 2007) and induces cardiomyocyte hypertrophy in vitro with a predominant increase in myocardial cell length by addition of new sarcomeric units in series (Wollert et al., 1996; Wollert and Chien, 1997; Wang et al., 2001; Ancey et al., 2003). Current data suggest that there is some ligand specificity in terms of gp130-mediated downstream signalling in cultured cardiomyocytes. LIF-induced gp130-mediated cardiomyocyte hypertrophy has been mainly ascribed to the activation of Ras/ERK1/2 signalling (Kodama et al., 2000). In contrast, growth factor receptor binding protein-2-associated binding protein 1/SHP2 interaction appears to play a crucial role in gp130-dependent hypertrophic stimulation of cardiomyocytes through the activation of ERK5 (Nakaoka et al., 2003). In line with this observation, CT-1-induced cardiomyocyte hypertrophy strongly depends on MEK5/ERK5 signal transduction, and appears only partly based on JAK/STAT and Ras/ERK1/2 pathways (Takahashi et al., 2005), and is completely independent of PI3K/AKT signalling (Tian et al., 2004).
Apart from its role in cardiomyocyte hypertrophy and survival, gp130 signalling upon LIF stimulation seems to alter cardiomyocyte metabolism. Indeed, it was reported that LIF induces contractile dysfunction and an increase in anaerobic energy metabolism in isolated cardiomyocytes, in part mediated by reduced expression of components in the ATP synthase complex and insulin-like growth factor-binding proteins 1 and 6 (Florholmen et al., 2004a, 2004b), thereby suggesting an involvement of gp130 signalling in maladaptive responses of the cardiac muscle. In contrast, oxidative stress is likely to impair protective IL-6 type cytokine signalling. In detail, it was shown that the generation of reactive oxidant species in cardiomyocytes blocks STAT3 signalling of the IL-6 type cytokines by targeting JAK1 (Kurdi and Booz, 2007). Specifically, activation of JAK1 is reduced and JAK1 association with both gp130 and LIF receptor is impaired in the presence of oxidative stress, leading to decreased phosphorylation of LIF receptor and gp130. These findings indicate that IL-6 type cytokine signalling may be redox-sensitive, thereby hinting at a new pathophysiologically relevant regulative mechanism in gp130 downstream pathways in cardiomyocytes.
Cardiac gp130 signalling under physiological conditions
Cardiomyocyte gp130 receptor signalling is not essential for normal postnatal cardiac growth, but plays an essential role in the ageing myocardium
To study the role of gp130 in adult organs and to circumvent embryonic and fetal lethality, mice with conditional knockouts or cell-specific overexpression are generated. In the heart, organ-specific overexpression or knockouts can be achieved by driving the gene of interest under a cardiomyocyte-specific promoter, such as the α-myosin heavy chain (α-MHC) or the myosin light chain 2 (mlc-2v) promoter.
Mice with cardiomyocyte-specific overexpression of the gp130 receptor do not develop a specific cardiac phenotype, at least no hypertrophy or cardiac failure have been described to date in these mice, despite a slightly increased activation state of downstream signalling molecules STAT3 and ERK1/2 (Tone et al., 2000). Mice with a cardiomyocyte-specific deficiency for gp130 receptor during fetal (Hirota et al., 1999) or in early postnatal development (α-MHC-Cretg/− and gp130flox/flox) (Fischer and Hilfiker-Kleiner, 2007) are viable and display normal postnatal cardiac development and cardiac function up to the age of 6 months. We have recently shown that gp130 activity in cardiomyocytes is required to protect the mice from age-related heart failure (Fischer and Hilfiker-Kleiner, 2007). In line with a cardioprotective role of gp130-mediated STAT activation, mice with a cardiomyocyte-specific deletion of STAT3 display spontaneous age-related heart failure and increased mortality beyond 12 months of age (Jacoby et al., 2003; Hilfiker-Kleiner et al., 2004).
Taken together, these observations suggest that certain endogenous gp130 ligands are present in the unstressed adult heart and might be responsible for baseline activation of the gp130 receptor under normal conditions. This baseline activation does not induce cardiac hypertrophy but seems essential for cardiac protection in ageing mice.
Role of gp130 signalling in physiological cardiac hypertrophy
Physiological cardiac hypertrophy occurs in response to growth, exercise or pregnancy, where increase in cardiomyocyte size is paralleled by a proportional growth of the vasculature and the capillary network and is usually not accompanied by cardiac fibrosis (Olson, 2004). Regular exercise induces anti-inflammatory effects with elevated levels of IL-6, which is thought to suppress oxidative stress and the production of proinflammatory cytokines, that is, tumour-necrosis factor-α (TNF-α) via activation of gp130-mediated signalling (Petersen and Pedersen, 2006). In addition, exercise-induced production and release of IL-6 from myofibres may contribute to abrogate an atherogenic lipid profile, which is often associated with chronic cardiac diseases (Petersen and Pedersen, 2006). However, there is little information on a direct role for gp130 in the heart during exercise.
Reversible physiological growth of heart also takes place in women during pregnancy, where ventricular hypertrophy, diastolic dysfunction and longer QT-interval dispersion develop as a result of volume overload as well as increased stretch and force demand (Eghbali et al., 2005). Sex hormones, for example, oestrogens, seem to play an important role in adaptation processes of the maternal heart during pregnancy (Eghbali et al., 2005). Serum levels of components of the IL-6/gp130 receptor system are also altered during pregnancy. For example, serum levels of OSM, the soluble LIF receptor sgp190 and of the sIL-6R are increased during pregnancy (Matsuzaki et al., 1995; Pitard et al., 1998; Ogata et al., 2000). Furthermore, we have recently shown that activation of gp130 downstream mediator STAT3 plays an essential role for the protection of the maternal heart from postpartum cardiomyopathy (Hilfiker-Kleiner et al., 2007). We therefore closely monitored female mice harbouring cardiomyocyte-specific deletions of the gp130 receptor for signs of peri- or postpartal heart failure and mortality. We did, however, not observe any pregnancy-associated mortality in these female mice (D Hilfiker-Kleiner, unpublished data), suggesting that gp130 might not be the main mediator of myocardial STAT3 activation during remodelling processes in the peripartal phase.
Role of gp130 signalling in cardiac pathophysiology
Activation of gp130 signalling modulates cardiomyocyte hypertrophy and survival in vivo
Continuous activation of gp130 in the heart, achieved by combined overexpression of both IL-6 and its receptor, is capable of inducing cardiac hypertrophy in vitro, but also in vivo without additional stimuli (Hirota et al., 1995). Importantly, STAT3, the major downstream signalling molecule of gp130, is sufficient to induce cardiomyocyte hypertrophy, and thereby plays a key role for gp130-mediated cardiomyocyte hypertrophy in vitro (Kunisada et al., 1998) and in vivo (Hirota et al., 1999; Kunisada et al., 2000) as determined in transgenic mice with cardiac-specific overexpression of the STAT3 gene. Although the activation of the MEK5/ERK5 and p38 MAPK pathways has been ascribed to the mediation of eccentric, maladaptive cardiac hypertrophy, the gp130/JAK/STAT axis is implicated in concentric hypertrophy (Miyamoto et al., 2004).
Interestingly, it could recently be demonstrated that IL-6 is an essential component of angiotensin II (AngII)-mediated hypertension and activation of the myocardial gp130/STAT3 axis leading to cardiac hypertrophy (Coles et al., 2007). However, activation of STAT3 and development of cardiac hypertrophy in response to AngII stimulation appear to depend on classic IL-6 signalling via the membrane-bound cognate IL-6 receptor, whereas AngII-mediated hypertension is mediated by agonistic complexes of IL-6 with the sIL-6R that bind to the gp130 receptor, a mechanism referred to as IL-6 trans-signalling (Coles et al., 2007).
Remarkably, recent data hint on further influences that impact on the phenotypic peculiarity of gp130-mediated hypertrophy. For instance, CT-1-induced hypertrophy was found to be markedly modified by the renin–angiotensin system in a STAT3-dependent manner. Furthermore, it appears that the gp130 receptor system and the renin–angiotensin system compound one another in their hypertrophic effects in vivo, with the extent of gp130-mediated hypertrophy being determined by the level of endogenous hypertensive preactivation, probably resulting in an exaggerated induction of angiotensinogen expression in response to CT-1 stimulation (Lopez et al., 2006).
Moreover, the gp130 receptor system plays an important role in the transition of left ventricular hypertrophy to overt heart failure. Intriguingly, Lopez et al. (2007) found that the loss of LIF receptor abundance in cardiomyocytes from failing hearts of spontaneously hypertensive rats compared with non-failing hearts with left ventricular hypertrophy accounts for diminished CT-1-mediated cytoprotective signalling and protection from apoptotic and non-apoptotic cell death induced by AngII. In this framework, neurohumoral agonists such as AngII, aldosterone and noradrenaline seem to be causally involved in LIF receptor downregulation, which proved to be sufficient for reduction of protective gp130-dependent mechanisms (Zvonic et al., 2005).
Cardiac adaptation to pressure overload depends on gp130 signalling
While initial in vivo studies foreshadowed the involvement of gp130 activation and downstream JAK/STAT signalling in pressure overload-induced hypertrophy (Pan et al., 1998), the pivotal role of gp130 in initiation of cardiac hypertrophy in response to biomechanical stress was demonstrated in mouse models with cardiomyocyte-specific deletion of gp130. Although the function and structure of gp130-deficient hearts is normal under basal conditions, acute pressure overload induces a rapid progress to cardiac dilation and dysfunction due to the absence of compensatory hypertrophy and intense loss of contractile myocardium caused by massive cardiomyocyte apoptosis (Hirota et al., 1999). Similarly, the expression of a dominant-negative mutant of gp130 in the heart leads to marked attenuation of pressure overload (thoracic aortic constriction)-induced cardiac hypertrophy and a reduction of concomitant STAT3 activation (Uozumi et al., 2001).
Closely associated with the myocardial STAT3 activation pattern in response to pressure overload is the biphasic induction of SOCS3, a gene transcriptionally upregulated by an activated gp130/JAK/STAT signalling cascade (Yasukawa et al., 2001). As described before, SOCS3 subsequently interrupts JAK/STAT (Ernst and Jenkins, 2004), but it also attenuates the activity of two other gp130-mediated signalling pathways, Ras/ERK and PI3K/Akt (Johnston, 2004). Therefore, upregulation of SOCS3 in the pressure-overloaded heart represents a key modulator of gp130-mediated cardiomyocyte hypertrophy and survival (Yasukawa et al., 2001). Moreover, it can be hypothesized that in a balanced system, inhibition of gp130 signalling by SOCS3 in cardiomyocytes may ensure the termination of the hypertrophic growth without interfering with survival. In unbalanced situations, that is in SOCS3 overexpressing mice, insufficient hypertrophic growth, disturbed sarcomeric organization and enhanced cardiomyocyte apoptosis may result. In turn, attenuated SOCS3 expression due to insufficient activation of STAT3 may cause enhanced Ras/ERK and PI3K/Akt signalling, leading to exaggerated and unlimited cardiomyocyte hypertrophy and subsequent heart failure.
In conclusion, activation of gp130-mediated signalling upon stress-induced ligand stimulation in cardiomyocytes seems to be a key determinant of hypertrophic growth, survival, metabolisms and function both in vitro and in vivo. Moreover, current experimental data strongly suggest that a well-balanced gp130 receptor system is indispensable for compensatory hypertrophic growth, cardioprotection and prevention of heart failure in response to neurohumoral and mechanical stress factors.
In response to ischaemia, gp130 drives cardioprotective signalling
In post-ischaemic myocardial remodelling, gp130 activation through several cytokines of the IL-6 type family was proven to confer beneficial effects. For instance, persistent activation of the gp130 receptor by administration of the IL-6/sIL-6 receptor complex inhibits cardiomyocyte apoptosis and restricts infarct sizes after ischaemia followed by reperfusion (Matsushita et al., 2005). In addition, LIF and CT-1 not only promote cardiomyocyte hypertrophy and survival (Kodama et al., 1997; Yasukawa et al., 2001), but also seem to be involved in myocardial wound healing and neovascularization (Zou et al., 2003; Freed et al., 2005; Takahashi et al., 2005), activation of cardiac fibroblasts (Tsuruda et al., 2002) and restriction of the post-infarction remodelling process (Wang et al., 2002). As a result, overexpression of LIF in experimental models resulted in preserved left ventricular function and geometry following myocardial infarction (Zou et al., 2003; Berry et al., 2004). Likewise, IL-11 administration was shown to reduce cardiac ischaemia/reperfusion injury by exerting both hypertrophic as well as cytoprotective signals via the gp130 downstream targets STAT3 and ERK1/2 (Kimura et al., 2007). Interestingly, it has also been assumed that LIF released by infiltrating macrophages activated by cardiac tissue degradation products might play a cardioprotective role in the early inflammatory reaction following ischaemia (Trial et al., 2004).
Different gp130 downstream pathways are involved in the cardioprotective effects following myocardial infarction. Mainly an efficient STAT3 signalling appears to be essential for the restriction of cardiac damage induced by ischaemia/reperfusion injury and myocardial infarction (Negoro et al., 2000; Hilfiker-Kleiner et al., 2004). Indeed, myocardial expression of IL-6 is rapidly induced following myocardial ischaemia (Yamauchi-Takihara et al., 1995; Roy et al., 2006) and the gp130/JAK2/STAT3 axis is triggered within minutes after ischaemia and remains activated up to 7 days post myocardial infarction (Fischer and Hilfiker-Kleiner, 2007). The gp130/STAT3-mediated cytoprotective signalling during ischaemia/reperfusion is to a considerable part based upon the scavenging of reactive oxygen species generation via the induction of the antioxidative enzyme manganese superoxide dismutase (Negoro et al., 2001a). Further, protective mechanisms of gp130/STAT3 signalling initiated by IL-6, LIF or OSM include the induction of vascular endothelial growth factor expression and subsequent neovascularization in response to tissue ischaemia or inflammation (Funamoto et al., 2000; Weiss et al., 2003; Rega et al., 2007).
Despite these positive effects of the gp130/STAT3 cascade, it has also been reported that the JAK/STAT axis is involved in unfavourable changes after infarction, such as alteration of gene activity that may underlie early diastolic dysfunction (that is, upregulation of phosphatase 1 and downregulation of p16-phospholamban) as well as downregulation of Kv4.2 gene expression that may underlie increased arrhythmogenicity of the post-infarction heart (El-Adawi et al., 2003). It was recently shown that IL-6 is a causative factor in the development of depressed cardiac function and increased proinflammatory signalling following trauma–haemorrhage injury. In this setting, both blockade of endogenous IL-6 and administration of antibodies against the IL-6 receptor significantly attenuated cardiac NF-κB, intercellular adhesion molecule-1, CINC-1, -3 and myeloperoxidase activity and resulted in an improvement of cardiac output (Yang et al., 2006, 2007b). These studies suggest again that only a precise control of gp130 signalling ensures a physiological outcome not only in the pressure-overloaded heart, but also after myocardial infarction and in the haemorrhagically damaged heart. Indeed, SOCS proteins are upregulated after myocardial ischaemia and one study emphasizes a positive role of SOCS3 to prevent early post-ischaemic myocardial dysfunction by modulating TNF-α-induced IL-6/gp130/STAT3 activity (Wang et al., 2006). This report shows that a deficiency of TNF-α receptor-1 prevented TNF-α-mediated cardiac dysfunction and was associated with reduced IL-6 levels, attenuated STAT3 phosphorylation and upregulated SOCS3 (Wang et al., 2006).
The gp130 receptor system and inflammatory diseases of the heart
Deduced from mouse models, gp130-mediated STAT3 activation seems to play a major role in the preservation of cell survival during virally conditioned myocardial inflammation as cardiac-specific gp130 knockout mice display an increase in susceptibility to viral infection of cardiomyocytes (Yajima et al., 2006). Furthermore, it has been shown that gp130-mediated stimulation of cardiomyocytes has a cytoprotective effect against virus infection in culture that can be attenuated by SOCS3 (Yajima et al., 2006).
In fact, a rather more detrimental role of SOCS proteins emerged from studies in myocarditis models, where, for example, transgenic mice with cardiomyocyte-specific overexpression of SOCS3 display a marked increase in susceptibility to viral infection despite an intact interferon (IFN)-mediated antiviral response (Yajima et al., 2006). In addition, cardiomyocyte-specific transgenic expression of SOCS1 inhibits enterovirus-induced signalling of JAK/STAT, with accompanying increase in viral replication, cardiomyopathy and mortality in Coxsackie virus-infected mice (Yasukawa et al., 2003). The major difference from SOCS3 is that SOCS1 is not induced by the IL-6 type cytokines, but is strongly induced by IFN-γ and modulates the effects of IFN-γ in vivo (Hanada et al., 2006; Qin et al., 2006; Zimmerer et al., 2007; Zitzmann et al., 2007). Considering the essential role of IFN-γ for host defence mechanisms, cardiac SOCS1 may play a particularly important role in virally induced heart disease (Metcalf et al., 2000). In this regard, SOCS1/JAB is likely to mediate the protective effect of CT-1 against lipopolysaccharide-induced left ventricular dysfunction in vivo (Tanimoto et al., 2005). In addition, SOCS1 does not seems to affect the gp130-mediated Ras/ERK and PI3K/Akt signalling, suggesting that gp130/JAK/STAT signalling plays the major protective role in cardiac inflammatory disease. In line with the notion of STAT3 as an important host defence pathway, mice with cardiomyocyte-specific deletion of STAT3 display increased susceptibility to endotoxin (lipopolysaccharide)-induced myocardial inflammation, resulting in extensive fibrosis and increased cardiomyocyte apoptosis (Jacoby et al., 2003).
Thus, gp130/JAK/STAT signalling appears to play an important role in protecting the heart from viral and bacterial infection by preserving adult cardiomyocytes until specific immune responses begin to clear the virus. However, it has been reported that during viral myocarditis, continuous and excessive production of IL-6 promotes myocardial injury by breaking down both cytokine networks and viral clearance (Tanaka et al., 2001), again suggesting the necessity for a tight regulation of the gp130 receptor system.
Activation of gp130 protects against the cardiotoxicity of doxorubicin
The anthracycline doxorubicin is an anticancer drug known to cause severe cardiac toxicity by generating free radicals, which accelerate oxidative stress (Shioji et al., 2002). Cardiomyocyte survival in doxorubicin-induced apoptosis through PI3K/Akt phosphorylation and Bcl-xL/caspase-3 interaction is regulated by gp130 (Negoro et al., 2001a). Activation of gp130 inhibits doxorubicin-induced cell death in cardiomyocytes, resulting in the restoration of PI3K/Akt activities and in the inactivation of caspase-3. STAT3 signalling, possibly mediated by gp130 receptor activation, also plays a major protective role in doxorubicin-mediated cardiotoxicity (Kunisada et al., 2000; Jacoby et al., 2003). In general, it is assumed that gp130-mediated protection against doxorubicin-induced cardiotoxicity mainly results from protection against oxidative stress and in a direct upregulation of anti-apoptotic defence mechanisms via STAT3 (Negoro et al., 2001b; Jacoby et al., 2003). It is also conceivable that elevated levels of immunomodulators such as IFN-γ and TNF-α play an additional role in the mediation of cardiotoxic effects under conditions with decreased STAT3 activation (Numata et al., 2007).
Challenges for directed pharmacological interventions in the gp130 receptor system: observing the changes or changing the observed?
The physiological activation of gp130-mediated signalling, especially via its main downstream effector STAT3, in response to various cardiac burdens such as pressure overload, cardiotoxic agents, and ischaemia and its relevance for the cardiac adaptation to these pathophysiological conditions (Hirota et al., 1999; Jacoby et al., 2003; Hilfiker-Kleiner et al., 2004) gave rise to the idea of additionally enhancing gp130 signalling and STAT3 activation via ligand-induced activation following cardiac injury and of enhancing STAT3 function in the heart during the transition from compensated states to cardiac failure. As described previously, administration of the IL-6/sIL-6 receptor complex, but not IL-6 or its soluble receptor alone, was shown to inhibit cardiomyocyte apoptosis and to restrict infarct sizes after cardiac ischaemia/reperfusion injury (Matsushita et al., 2005). This strengthened the possibility that activation of the gp130 receptor by IL-6 might be a future strategy in cardiovascular therapy, even in cases where the targeted receptor might not necessarily be expressed on cardiomyocytes, but may be activated by trans-signalling. However, the finding that the complete knockdown of IL-6 did not influence infarct sizes or left ventricular function, remodelling and survival in mice did not fully support this concept (Fuchs et al., 2003). Other gp130 ligands or factors signalling via JAK/STAT such as angiotensin may either be downregulated or act in a compensatory manner to exert cellular effects within a certain homeostatic range. In contrast, the promotion of myocardial injury by excessive release of IL-6 (Tanaka et al., 2001) suggests that high levels of circulating IL-6 following myocardial infarction or in patients with dilative cardiomyopathy might not only be a marker for the severity of ongoing inflammatory reactions but might also represent an active contributor to sustained damage during myocardial inflammation. Therefore, instead of ligand-induced activation of the gp130 receptor, it might turn out to be more practical to recognize and modulate activity of downstream targets of gp130-mediated pathways, that is, increasing STAT3-dependent manganese superoxide dismutase or vascular endothelial growth factor signalling in the heart, to restrict acute or chronic damage.
It should be taken into account that interventions targeting components of the gp130 receptor system in other organs might also affect its tightly controlled downstream pathways in the heart. For instance, it is known that in contrast to balanced STAT3 signalling, which is required for adaptive responses of cell proliferation, survival and metabolism of various organs to changing environmental influences, persistent STAT3 activation is frequently associated with malignant transformation and is one of the causative molecular abnormalities in oncogenesis as reviewed by Turkson (2004). Aberrant STAT3 activation is known to promote uncontrolled growth and survival through dysregulation of gene expression, including cyclin D1, c-Myc, Bcl-xL and Mcl-1, thereby contributing to oncogenesis (Turkson, 2004). Moreover, recent studies reveal that persistently active STAT3 induces tumour angiogenesis by upregulation of vascular endothelial growth factor and modulates immune functions in favour of tumour immune evasion (Turkson, 2004). Furthermore, IL-6-induced, gp130-mediated activation of STAT3 and the Notch-3 pathway have been found to be critical hallmarks in breast tumorigenesis (Guo et al., 2006; Berishaj et al., 2007; Sansone et al., 2007). Moreover, IL-6 has been ascribed a causative role in STAT3 activation by mutant EGF receptors in lung carcinogenesis (Berishaj et al., 2007). Thus, IL-6 and STAT3 emerged as novel targets for cancer therapy and are currently under investigation (Zaki et al., 2004). Recent studies meanwhile demonstrated that inhibition of STAT3 activation, that is by means of small molecule STAT3 decoys, is capable of inducing apoptosis, and decreasing proliferation and STAT3-mediated gene expression of tumour cells both in vitro and in vivo (Leong et al., 2003; Xi et al., 2005). In addition, Selander et al. (2004) recently showed that blockade of gp130 signalling in a mouse tumour model efficiently inhibited tumour growth and metastasis, emphasizing the use of pharmacological inhibition of gp130 signalling in patients with breast cancer. Taken together, the targeted disruption of gp130-dependent signalling pathways appears to be a promising novel therapeutic strategy for the treatment of cancers characterized by constitutive STAT3 activation. However, one has to take into account the relevance of a tightly controlled gp130 receptor system in the heart while employing therapeutic strategies for other entities such as cancer treatment involving downregulation or inhibition of STAT3 signalling. Here, the maintenance of a balanced gp130 signalling in the heart should be considered equally important and specific treatment strategies should be developed aiming at protection of the heart from adverse side effects associated with systemic downregulation of gp130/STAT3 signalling during antitumour therapy. In turn, displayed against the necessity of an intact, physiologically regulated gp130 receptor system in the heart for the protection against pathophysiological stress such as ischaemia, mechanical stress and cytotoxic agents, a potential oncogenic effect has to be considered and should be carefully investigated.
In the light of these controversies, it is especially important to define further those gp130/STAT3-dependent downstream mediators that may specifically serve as prime targets for novel therapies to treat heart failure, without increasing the risk for malignancies.
Conclusion
The gp130 receptor system plays an essential role for cardiac development, potentially by regulating cardiomyogenesis, cardiomyocyte survival and growth. In experimental models, gp130 receptor signalling has been shown to be essential for cardiac development and for cardioprotection against physiological and pathophysiological stress. In addition, the understanding and directed manipulation of gp130-dependent signal transduction pathways is a prerequisite for cell type-specific pre-commitment that may eventually facilitate stem- and progenitor cell-based therapies for myocardial repair and regeneration.
Importantly, beneficial effects of gp130 signalling seem to require well-balanced circuits of STAT and ERK signalling pathways, where negative feedback mechanisms, that is, SOCS proteins play an important role. Failure in this orchestration of signalling results in loss of cardiac cells, maladaptive hypertrophy and cardiac dysfunction and may explain discrepancies between reported beneficial and protective roles of the gp130 receptor system in experimental models and the association of activated gp130 receptor system components with adverse clinical outcomes in patients with heart failure or after myocardial infarction (Roig et al., 1998; Tsutamoto et al., 1998; Maeda et al., 2000; Orus et al., 2000; Birks et al., 2001; Rattazzi et al., 2003; Buzas et al., 2004; Hirota et al., 2004). The attenuated STAT3 activation and reduced SOCS3 expression observed in patients with end-stage heart failure (Podewski et al., 2003) may reflect disturbance of the physiological activity of the gp130 signalling system. In its consequence, balanced gp130 signalling appears to be cardioprotective, whereas imbalanced gp130 signalling contributes more to maladaptation and heart failure.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft and the Jean Leducq Foundation.
Glossary
- AngII
angiotensin II
- CCL5
chemokine, CC motif, ligand-5
- CT-1
cardiotrophin-1
- ERK
extracellular signal-regulated kinase
- ES cell
embryonic stem cell
- gp130
glycoprotein-130
- IFN-γ
interferon-γ
- IL-6
interleukin-6
- IL-11
interleukin-11
- JAK
Janus kinase
- LIF
leukaemia inhibitory factor
- MAPK
mitogen-activated protein kinase
- MHC
myosin heavy chain
- NF-κB
nuclear factor-κB
- OSM
oncostatin M
- PI3K
phosphatidylinositol-3-kinase
- SHP2
SH2 domain-containing cytoplasmic protein tyrosine phosphatase
- sIL-6R
soluble IL-6 receptor
- SOCS
suppressor of cytokine signalling
- STAT
signal transducer and activator of transcription
- TNF-α
tumour-necrosis factor-α
Footnotes
Conflict of interest
The authors state no conflict of interest.
References
- Ancey C, Menet E, Corbi P, Fredj S, Garcia M, Rucker-Martin C, et al. Human cardiomyocyte hypertrophy induced in vitro by gp130 stimulation. Cardiovasc Res. 2003;59:78–85. doi: 10.1016/s0008-6363(03)00346-8. [DOI] [PubMed] [Google Scholar]
- Berishaj M, Gao SP, Ahmed S, Leslie K, Al-Ahmadie H, Gerald WL, et al. Stat3 is tyrosine-phosphorylated through the interleukin-6/glycoprotein 130/Janus kinase pathway in breast cancer. Breast Cancer Res. 2007;9:R32. doi: 10.1186/bcr1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry MF, Pirolli TJ, Jayasankar V, Morine KJ, Moise MA, Fisher O, et al. Targeted overexpression of leukemia inhibitory factor to preserve myocardium in a rat model of postinfarction heart failure. J Thorac Cardiovasc Surg. 2004;128:866–875. doi: 10.1016/j.jtcvs.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Birks EJ, Latif N, Owen V, Bowles C, Felkin LE, Mullen AJ, et al. Quantitative myocardial cytokine expression and activation of the apoptotic pathway in patients who require left ventricular assist devices. Circulation. 2001;104 12 Suppl 1:I233–I240. doi: 10.1161/hc37t1.094872. [DOI] [PubMed] [Google Scholar]
- Burdon T, Smith A, Savatier P. Signalling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol. 2002;12:432–438. doi: 10.1016/s0962-8924(02)02352-8. [DOI] [PubMed] [Google Scholar]
- Buzas K, Megyeri K, Hogye M, Csanady M, Bogats G, Mandi Y. Comparative study of the roles of cytokines and apoptosis in dilated and hypertrophic cardiomyopathies. Eur Cytokine Netw. 2004;15:53–59. [PubMed] [Google Scholar]
- Chin BS, Blann AD, Gibbs CR, Chung NA, Conway DG, Lip GY. Prognostic value of interleukin-6, plasma viscosity, fibrinogen, von Willebrand factor, tissue factor and vascular endothelial growth factor levels in congestive heart failure. Eur J Clin Invest. 2003;33:941–948. doi: 10.1046/j.1365-2362.2003.01252.x. [DOI] [PubMed] [Google Scholar]
- Coles B, Fielding CA, Rose-John S, Scheller J, Jones SA, O'Donnell VB. Classic interleukin-6 receptor signaling and interleukin-6 trans-signaling differentially control angiotensin II-dependent hypertension, cardiac signal transducer and activator of transcription-3 activation, and vascular hypertrophy in vivo. Am J Pathol. 2007;171:315–325. doi: 10.2353/ajpath.2007.061078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daheron L, Opitz SL, Zaehres H, Lensch WM, Andrews PW, Itskovitz-Eldor J, et al. LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cells. 2004;22:770–778. doi: 10.1634/stemcells.22-5-770. [DOI] [PubMed] [Google Scholar]
- Dani C, Chambers I, Johnstone S, Robertson M, Ebrahimi B, Saito M, et al. Paracrine induction of stem cell renewal by LIF-deficient cells: a new ES cell regulatory pathway. Dev Biol. 1998;203:149–162. doi: 10.1006/dbio.1998.9026. [DOI] [PubMed] [Google Scholar]
- Eghbali M, Deva R, Alioua A, Minosyan TY, Ruan H, Wang Y, et al. Molecular and functional signature of heart hypertrophy during pregnancy. Circ Res. 2005;96:1208–1216. doi: 10.1161/01.RES.0000170652.71414.16. [DOI] [PubMed] [Google Scholar]
- Eiken HG, Oie E, Damas JK, Yndestad A, Bjerkeli V, Aass H, et al. Myocardial gene expression of leukaemia inhibitory factor, interleukin-6 and glycoprotein 130 in end-stage human heart failure. Eur J Clin Invest. 2001;31:389–397. doi: 10.1046/j.1365-2362.2001.00795.x. [DOI] [PubMed] [Google Scholar]
- El-Adawi H, Deng L, Tramontano A, Smith S, Mascareno E, Ganguly K, et al. The functional role of the JAK-STAT pathway in post-infarction remodeling. Cardiovasc Res. 2003;57:129–138. doi: 10.1016/s0008-6363(02)00614-4. [DOI] [PubMed] [Google Scholar]
- Ernst M, Jenkins BJ. Acquiring signalling specificity from the cytokine receptor gp130. Trends Genet. 2004;20:23–32. doi: 10.1016/j.tig.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Fischer P, Hilfiker-Kleiner D. Survival pathways in hypertrophy and heart failure: the gp130-STAT3 axis. Basic Res Cardiol. 2007;102:279–297. doi: 10.1007/s00395-007-0658-z. [DOI] [PubMed] [Google Scholar]
- Florholmen G, Aas V, Rustan AC, Lunde PK, Straumann N, Eid H, et al. Leukemia inhibitory factor reduces contractile function and induces alterations in energy metabolism in isolated cardiomyocytes. J Mol Cell Cardiol. 2004a;37:1183–1193. doi: 10.1016/j.yjmcc.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Florholmen G, Andersson KB, Yndestad A, Austbo B, Henriksen UL, Christensen G. Leukaemia inhibitory factor alters expression of genes involved in rat cardiomyocyte energy metabolism. Acta Physiol Scand. 2004b;180:133–142. doi: 10.1046/j.0001-6772.2003.01245.x. [DOI] [PubMed] [Google Scholar]
- Freed DH, Cunnington RH, Dangerfield AL, Sutton JS, Dixon IM. Emerging evidence for the role of cardiotrophin-1 in cardiac repair in the infarcted heart. Cardiovasc Res. 2005;65:782–792. doi: 10.1016/j.cardiores.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Hilfiker A, Kaminski K, Hilfiker-Kleiner D, Guener Z, Klein G, et al. Role of interleukin-6 for LV remodeling and survival after experimental myocardial infarction. FASEB J. 2003;17:2118–2120. doi: 10.1096/fj.03-0331fje. [DOI] [PubMed] [Google Scholar]
- Funamoto M, Fujio Y, Kunisada K, Negoro S, Tone E, Osugi T, et al. Signal transducer and activator of transcription 3 is required for glycoprotein 130-mediated induction of vascular endothelial growth factor in cardiac myocytes. J Biol Chem. 2000;275:10561–10566. doi: 10.1074/jbc.275.14.10561. [DOI] [PubMed] [Google Scholar]
- Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–1203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- Guan K, Wagner S, Unsold B, Maier LS, Kaiser D, Hemmerlein B, et al. Generation of functional cardiomyocytes from adult mouse spermatogonial stem cells. Circ Res. 2007;100:1615–1625. doi: 10.1161/01.RES.0000269182.22798.d9. [DOI] [PubMed] [Google Scholar]
- Guo W, Pylayeva Y, Pepe A, Yoshioka T, Muller WJ, Inghirami G, et al. Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell. 2006;126:489–502. doi: 10.1016/j.cell.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Hanada T, Kobayashi T, Chinen T, Saeki K, Takaki H, Koga K, et al. IFNgamma-dependent, spontaneous development of colorectal carcinomas in SOCS1-deficient mice. J Exp Med. 2006;203:1391–1397. doi: 10.1084/jem.20060436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374 Part 1:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334 Part 2:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfiker-Kleiner D, Hilfiker A, Fuchs M, Kaminski K, Schaefer A, Schieffer B, et al. Signal transducer and activator of transcription 3 is required for myocardial capillary growth, control of interstitial matrix deposition, and heart protection from ischemic injury. Circ Res. 2004;95:187–195. doi: 10.1161/01.RES.0000134921.50377.61. [DOI] [PubMed] [Google Scholar]
- Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128:589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- Hirano T, Nakajima K, Hibi M. Signaling mechanisms through gp130: a model of the cytokine system. Cytokine Growth Factor Rev. 1997;8:241–252. doi: 10.1016/s1359-6101(98)80005-1. [DOI] [PubMed] [Google Scholar]
- Hirota H, Chen J, Betz UA, Rajewsky K, Gu Y, Ross J, Jr, et al. Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell. 1999;97:189–198. doi: 10.1016/s0092-8674(00)80729-1. [DOI] [PubMed] [Google Scholar]
- Hirota H, Izumi M, Hamaguchi T, Sugiyama S, Murakami E, Kunisada K, et al. Circulating interleukin-6 family cytokines and their receptors in patients with congestive heart failure. Heart Vessels. 2004;19:237–241. doi: 10.1007/s00380-004-0770-z. [DOI] [PubMed] [Google Scholar]
- Hirota H, Yoshida K, Kishimoto T, Taga T. Continuous activation of gp130, a signal-transducing receptor component for interleukin 6-related cytokines, causes myocardial hypertrophy in mice. Proc Natl Acad Sci USA. 1995;92:4862–4866. doi: 10.1073/pnas.92.11.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey RK, Beattie GM, Lopez AD, Bucay N, King CC, Firpo MT, et al. Maintenance of pluripotency in human embryonic stem cells is STAT3 independent. Stem Cells. 2004;22:522–530. doi: 10.1634/stemcells.22-4-522. [DOI] [PubMed] [Google Scholar]
- Jacoby JJ, Kalinowski A, Liu MG, Zhang SS, Gao Q, Chai GX, et al. Cardiomyocyte-restricted knockout of STAT3 results in higher sensitivity to inflammation, cardiac fibrosis, and heart failure with advanced age. Proc Natl Acad Sci USA. 2003;100:12929–12934. doi: 10.1073/pnas.2134694100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins BJ, Roberts AW, Najdovska M, Grail D, Ernst M. The threshold of gp130-dependent STAT3 signaling is critical for normal regulation of hematopoiesis. Blood. 2005;105:3512–3520. doi: 10.1182/blood-2004-09-3751. [DOI] [PubMed] [Google Scholar]
- Johnston JA. Are SOCS suppressors, regulators, and degraders. J Leukoc Biol. 2004;75:743–748. doi: 10.1189/jlb.1003507. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Yoshida S, Toyokuni S, et al. Leukemia inhibitory factor enhances formation of germ cell colonies in neonatal mouse testis culture. Biol Reprod. 2007;76:55–62. doi: 10.1095/biolreprod.106.055863. [DOI] [PubMed] [Google Scholar]
- Kimura R, Maeda M, Arita A, Oshima Y, Obana M, Ito T, et al. Identification of cardiac myocytes as the target of interleukin 11, a cardioprotective cytokine. Cytokine. 2007;38:107–115. doi: 10.1016/j.cyto.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Kodama H, Fukuda K, Pan J, Makino S, Baba A, Hori S, et al. Leukemia inhibitory factor, a potent cardiac hypertrophic cytokine, activates the JAK/STAT pathway in rat cardiomyocytes. Circ Res. 1997;81:656–663. doi: 10.1161/01.res.81.5.656. [DOI] [PubMed] [Google Scholar]
- Kodama H, Fukuda K, Pan J, Sano M, Takahashi T, Kato T, et al. Significance of ERK cascade compared with JAK/STAT and PI3-K pathway in gp130-mediated cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2000;279:H1635–H1644. doi: 10.1152/ajpheart.2000.279.4.H1635. [DOI] [PubMed] [Google Scholar]
- Kunisada K, Hirota H, Fujio Y, Matsui H, Tani Y, Yamauchi-Takihara K, et al. Activation of JAK-STAT and MAP kinases by leukemia inhibitory factor through gp130 in cardiac myocytes. Circulation. 1996;94:2626–2632. doi: 10.1161/01.cir.94.10.2626. [DOI] [PubMed] [Google Scholar]
- Kunisada K, Negoro S, Tone E, Funamoto M, Osugi T, Yamada S, et al. Signal transducer and activator of transcription 3 in the heart transduces not only a hypertrophic signal but a protective signal against doxorubicin-induced cardiomyopathy. Proc Natl Acad Sci USA. 2000;97:315–319. doi: 10.1073/pnas.97.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisada K, Tone E, Fujio Y, Matsui H, Yamauchi-Takihara K, Kishimoto T. Activation of gp130 transduces hypertrophic signals via STAT3 in cardiac myocytes. Circulation. 1998;98:346–352. doi: 10.1161/01.cir.98.4.346. [DOI] [PubMed] [Google Scholar]
- Kurdi M, Booz GW. Evidence that IL-6-type cytokine signaling in cardiomyocytes is inhibited by oxidative stress: parthenolide targets JAK1 activation by generating ROS. J Cell Physiol. 2007;212:424–431. doi: 10.1002/jcp.21033. [DOI] [PubMed] [Google Scholar]
- Leong PL, Andrews GA, Johnson DE, Dyer KF, Xi S, Mai JC, et al. Targeted inhibition of Stat3 with a decoy oligonucleotide abrogates head and neck cancer cell growth. Proc Natl Acad Sci USA. 2003;100:4138–4143. doi: 10.1073/pnas.0534764100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez N, Diez J, Fortuno MA. Differential hypertrophic effects of cardiotrophin-1 on adult cardiomyocytes from normotensive and spontaneously hypertensive rats. J Mol Cell Cardiol. 2006;41:902–913. doi: 10.1016/j.yjmcc.2006.03.433. [DOI] [PubMed] [Google Scholar]
- Lopez N, Varo N, Diez J, Fortuno MA. Loss of myocardial LIF receptor in experimental heart failure reduces cardiotrophin-1 cytoprotection. A role for neurohumoral agonists. Cardiovasc Res. 2007;75:536–545. doi: 10.1016/j.cardiores.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Maeda K, Tsutamoto T, Wada A, Mabuchi N, Hayashi M, Tsutsui T, et al. High levels of plasma brain natriuretic peptide and interleukin-6 after optimized treatment for heart failure are independent risk factors for morbidity and mortality in patients with congestive heart failure. J Am Coll Cardiol. 2000;36:1587–1593. doi: 10.1016/s0735-1097(00)00912-8. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Nakamura T, Nakao K, Arai T, Katsuki M, Heike T, et al. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J. 1999;18:4261–4269. doi: 10.1093/emboj/18.15.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, Iwanaga S, Oda T, Kimura K, Shimada M, Sano M, et al. Interleukin-6/soluble interleukin-6 receptor complex reduces infarct size via inhibiting myocardial apoptosis. Lab Invest. 2005;85:1210–1223. doi: 10.1038/labinvest.3700322. [DOI] [PubMed] [Google Scholar]
- Matsuzaki N, Neki R, Sawai K, Shimoya K, Okada T, Sakata M, et al. Soluble interleukin-6 (IL-6) receptor in the sera of pregnant women forms a complex with IL-6 and augments human chorionic gonadotropin production by normal human trophoblasts through binding to the IL-6 signal transducer. J Clin Endocrinol Metab. 1995;80:2912–2917. doi: 10.1210/jcem.80.10.7559874. [DOI] [PubMed] [Google Scholar]
- May P, Schniertshauer U, Gerhartz C, Horn F, Heinrich PC. Signal transducer and activator of transcription STAT3 plays a major role in gp130-mediated acute phase protein gene activation. Acta Biochim Pol. 2003;50:595–601. [PubMed] [Google Scholar]
- Metcalf D, Di Rago L, Mifsud S, Hartley L, Alexander WS. The development of fatal myocarditis and polymyositis in mice heterozygous for IFN-gamma and lacking the SOCS-1 gene. Proc Natl Acad Sci USA. 2000;97:9174–9179. doi: 10.1073/pnas.160255197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Takeishi Y, Takahashi H, Shishido T, Arimoto T, Tomoike H, et al. Activation of distinct signal transduction pathways in hypertrophied hearts by pressure and volume overload. Basic Res Cardiol. 2004;99:328–337. doi: 10.1007/s00395-004-0482-7. [DOI] [PubMed] [Google Scholar]
- Nakaoka Y, Nishida K, Fujio Y, Izumi M, Terai K, Oshima Y, et al. Activation of gp130 transduces hypertrophic signal through interaction of scaffolding/docking protein Gab1 with tyrosine phosphatase SHP2 in cardiomyocytes. Circ Res. 2003;93:221–229. doi: 10.1161/01.RES.0000085562.48906.4A. [DOI] [PubMed] [Google Scholar]
- Negoro S, Kunisada K, Fujio Y, Funamoto M, Darville MI, Eizirik DL, et al. Activation of signal transducer and activator of transcription 3 protects cardiomyocytes from hypoxia/reoxygenation-induced oxidative stress through the upregulation of manganese superoxide dismutase. Circulation. 2001a;104:979–981. doi: 10.1161/hc3401.095947. [DOI] [PubMed] [Google Scholar]
- Negoro S, Kunisada K, Tone E, Funamoto M, Oh H, Kishimoto T, et al. Activation of JAK/STAT pathway transduces cytoprotective signal in rat acute myocardial infarction. Cardiovasc Res. 2000;47:797–805. doi: 10.1016/s0008-6363(00)00138-3. [DOI] [PubMed] [Google Scholar]
- Negoro S, Oh H, Tone E, Kunisada K, Fujio Y, Walsh K, et al. Glycoprotein 130 regulates cardiac myocyte survival in doxorubicin-induced apoptosis through phosphatidylinositol 3-kinase/Akt phosphorylation and Bcl-xL/caspase-3 interaction. Circulation. 2001b;103:555–561. doi: 10.1161/01.cir.103.4.555. [DOI] [PubMed] [Google Scholar]
- Nightingale J, Patel S, Suzuki N, Buxton R, Takagi KI, Suzuki J, et al. Oncostatin M, a cytokine released by activated mononuclear cells, induces epithelial cell-myofibroblast transdifferentiation via Jak/Stat pathway activation. J Am Soc Nephrol. 2004;15:21–32. doi: 10.1097/01.asn.0000102479.92582.43. [DOI] [PubMed] [Google Scholar]
- Numata K, Kubo M, Watanabe H, Takagi K, Mizuta H, Okada S, et al. Overexpression of suppressor of cytokine signaling-3 in T cells exacerbates acetaminophen-induced hepatotoxicity. J Immunol. 2007;178:3777–3785. doi: 10.4049/jimmunol.178.6.3777. [DOI] [PubMed] [Google Scholar]
- Ogata I, Shimoya K, Moriyama A, Shiki Y, Matsumura Y, Yamanaka K, et al. Oncostatin M is produced during pregnancy by decidual cells and stimulates the release of HCG. Mol Hum Reprod. 2000;6:750–757. doi: 10.1093/molehr/6.8.750. [DOI] [PubMed] [Google Scholar]
- Oh H, Fujio Y, Kunisada K, Hirota H, Matsui H, Kishimoto T, et al. Activation of phosphatidylinositol 3-kinase through glycoprotein 130 induces protein kinase B and p70, S6 kinase phosphorylation in cardiac myocytes. J Biol Chem. 1998;273:9703–9710. doi: 10.1074/jbc.273.16.9703. [DOI] [PubMed] [Google Scholar]
- Olson EN. A decade of discoveries in cardiac biology. Nat Med. 2004;10:467–474. doi: 10.1038/nm0504-467. [DOI] [PubMed] [Google Scholar]
- Orus J, Roig E, Perez-Villa F, Pare C, Azqueta M, Filella X, et al. Prognostic value of serum cytokines in patients with congestive heart failure. J Heart Lung Transplant. 2000;19:419–425. doi: 10.1016/s1053-2498(00)00083-8. [DOI] [PubMed] [Google Scholar]
- Osugi T, Oshima Y, Fujio Y, Funamoto M, Yamashita A, Negoro S, et al. Cardiac-specific activation of signal transducer and activator of transcription 3 promotes vascular formation in the heart. J Biol Chem. 2002;277:6676–6681. doi: 10.1074/jbc.M108246200. [DOI] [PubMed] [Google Scholar]
- Pan J, Fukuda K, Kodama H, Sano M, Takahashi T, Makino S, et al. Involvement of gp130-mediated signaling in pressure overload-induced activation of the JAK/STAT pathway in rodent heart. Heart Vessels. 1998;13:199–208. doi: 10.1007/BF01745045. [DOI] [PubMed] [Google Scholar]
- Pennica D, Shaw KJ, Swanson TA, Moore MW, Shelton DL, Zioncheck KA, et al. Cardiotrophin-1. Biological activities and binding to the leukemia inhibitory factor receptor/gp130 signaling complex. J Biol Chem. 1995;270:10915–10922. doi: 10.1074/jbc.270.18.10915. [DOI] [PubMed] [Google Scholar]
- Petersen AM, Pedersen BK. The role of IL-6 in mediating the anti-inflammatory effects of exercise. J Physiol Pharmacol. 2006;57 Suppl 10:43–51. [PubMed] [Google Scholar]
- Pitard V, Lorgeot V, Taupin JL, Aubard Y, Praloran V, Moreau JF. The presence in human serum of a circulating soluble leukemia inhibitory factor receptor (sgp190) and its evolution during pregnancy. Eur Cytokine Netw. 1998;9:599–605. [PubMed] [Google Scholar]
- Podewski EK, Hilfiker-Kleiner D, Hilfiker A, Morawietz H, Lichtenberg A, Wollert KC, et al. Alterations in Janus kinase (JAK)-signal transducers and activators of transcription (STAT) signaling in patients with end-stage dilated cardiomyopathy. Circulation. 2003;107:798–802. doi: 10.1161/01.cir.0000057545.82749.ff. [DOI] [PubMed] [Google Scholar]
- Pollack V, Sarkozi R, Banki Z, Feifel E, Wehn S, Gstraunthaler G, et al. Oncostatin M-induced effects on EMT in human proximal tubular cells: differential role of ERK signaling. Am J Physiol Renal Physiol. 2007;293:F1714–F1726. doi: 10.1152/ajprenal.00130.2007. [DOI] [PubMed] [Google Scholar]
- Qin H, Wilson CA, Lee SJ, Benveniste EN. IFN-beta-induced SOCS-1 negatively regulates CD40 gene expression in macrophages and microglia. FASEB J. 2006;20:985–987. doi: 10.1096/fj.05-5493fje. [DOI] [PubMed] [Google Scholar]
- Rajasingh J, Bord E, Hamada H, Lambers E, Qin G, Losordo DW, et al. STAT3-dependent mouse embryonic stem cell differentiation into cardiomyocytes: analysis of molecular signaling and therapeutic efficacy of cardiomyocyte precommitted mES transplantation in a mouse model of myocardial infarction. Circ Res. 2007;101:910–918. doi: 10.1161/CIRCRESAHA.107.156786. [DOI] [PubMed] [Google Scholar]
- Rattazzi M, Puato M, Faggin E, Bertipaglia B, Zambon A, Pauletto P. C-reactive protein and interleukin-6 in vascular disease: culprits or passive bystanders. J Hypertens. 2003;21:1787–1803. doi: 10.1097/00004872-200310000-00002. [DOI] [PubMed] [Google Scholar]
- Rega G, Kaun C, Demyanets S, Pfaffenberger S, Rychli K, Hohensinner PJ, et al. Vascular endothelial growth factor is induced by the inflammatory cytokines interleukin-6 and oncostatin m in human adipose tissue in vitro and in murine adipose tissue in vivo. Arterioscler Thromb Vasc Biol. 2007;27:1587–1595. doi: 10.1161/ATVBAHA.107.143081. [DOI] [PubMed] [Google Scholar]
- Roig E, Orus J, Pare C, Azqueta M, Filella X, Perez-Villa F, et al. Serum interleukin-6 in congestive heart failure secondary to idiopathic dilated cardiomyopathy. Am J Cardiol. 1998;82:688–690. doi: 10.1016/s0002-9149(98)00388-9. [DOI] [PubMed] [Google Scholar]
- Roy S, Khanna S, Kuhn DE, Rink C, Williams WT, Zweier JL, et al. Transcriptome analysis of the ischemia-reperfused remodeling myocardium: temporal changes in inflammation and extracellular matrix. Physiol Genomics. 2006;25:364–374. doi: 10.1152/physiolgenomics.00013.2006. [DOI] [PubMed] [Google Scholar]
- Sansone P, Storci G, Giovannini C, Pandolfi S, Pianetti S, Taffurelli M, et al. p66Shc/Notch-3 interplay controls self-renewal and hypoxia survival in human stem/progenitor cells of the mammary gland expanded in vitro as mammospheres. Stem Cells. 2007;25:807–815. doi: 10.1634/stemcells.2006-0442. [DOI] [PubMed] [Google Scholar]
- Schramek H, Feifel E, Marschitz I, Golochtchapova N, Gstraunthaler G, Montesano R. Loss of active MEK1-ERK1/2 restores epithelial phenotype and morphogenesis in transdifferentiated MDCK cells. Am J Physiol Cell Physiol. 2003;285:C652–C661. doi: 10.1152/ajpcell.00463.2002. [DOI] [PubMed] [Google Scholar]
- Selander KS, Li L, Watson L, Merrell M, Dahmen H, Heinrich PC, et al. Inhibition of gp130 signaling in breast cancer blocks constitutive activation of Stat3 and inhibits in vivo malignancy. Cancer Res. 2004;64:6924–6933. doi: 10.1158/0008-5472.CAN-03-2516. [DOI] [PubMed] [Google Scholar]
- Sheng Z, Pennica D, Wood WI, Chien KR. Cardiotrophin-1 displays early expression in the murine heart tube and promotes cardiac myocyte survival. Development. 1996;122:419–428. doi: 10.1242/dev.122.2.419. [DOI] [PubMed] [Google Scholar]
- Shioji K, Kishimoto C, Nakamura H, Masutani H, Yuan Z, Oka S, et al. Overexpression of thioredoxin-1 in transgenic mice attenuates adriamycin-induced cardiotoxicity. Circulation. 2002;106:1403–1409. doi: 10.1161/01.cir.0000027817.55925.b4. [DOI] [PubMed] [Google Scholar]
- Streetz KL, Luedde T, Manns MP, Trautwein C. Interleukin 6 and liver regeneration. Gut. 2000;47:309–312. doi: 10.1136/gut.47.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlmann-Laeisz C, Lang S, Chalaris A, Krzysztof P, Enge S, Eichler J, et al. Forced dimerization of gp130 leads to constitutive STAT3 activation, cytokine-independent growth, and blockade of differentiation of embryonic stem cells. Mol Biol Cell. 2006;17:2986–2995. doi: 10.1091/mbc.E05-12-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi T, Fujimoto Y, Nakatsuji N, Suemori H. STAT3 is dispensable for maintenance of self-renewal in nonhuman primate embryonic stem cells. Stem Cells. 2004;22:861–872. doi: 10.1634/stemcells.22-5-861. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Saito Y, Kuwahara K, Harada M, Tanimoto K, Nakagawa Y, et al. Hypertrophic responses to cardiotrophin-1 are not mediated by STAT3, but via a MEK5-ERK5 pathway in cultured cardiomyocytes. J Mol Cell Cardiol. 2005;38:185–192. doi: 10.1016/j.yjmcc.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Takizawa M, Nobuhisa I, Igarashi K, Ueno M, Nakashima K, Kitamura T, et al. Requirement of gp130 signaling for the AGM hematopoiesis. Exp Hematol. 2003;31:283–289. doi: 10.1016/s0301-472x(03)00025-0. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Kanda T, McManus BM, Kanai H, Akiyama H, Sekiguchi K, et al. Overexpression of interleukin-6 aggravates viral myocarditis: impaired increase in tumor necrosis factor-alpha. J Mol Cell Cardiol. 2001;33:1627–1635. doi: 10.1006/jmcc.2001.1428. [DOI] [PubMed] [Google Scholar]
- Tanimoto K, Saito Y, Hamanaka I, Kuwahara K, Harada M, Takahashi N, et al. SOCS1/JAB likely mediates the protective effect of cardiotrophin-1 against lipopolysaccharide-induced left ventricular dysfunction in vivo. Circ J. 2005;69:1412–1417. doi: 10.1253/circj.69.1412. [DOI] [PubMed] [Google Scholar]
- Tian ZJ, Cui W, Li YJ, Hao YM, Du J, Liu F, et al. Different contributions of STAT3, ERK1/2, and PI3-K signaling to cardiomyocyte hypertrophy by cardiotrophin-1. Acta Pharmacol Sin. 2004;25:1157–1164. [PubMed] [Google Scholar]
- Tone E, Kunisada K, Kumanogoh A, Negoro S, Funamoto M, Osugi T, et al. gp130-Dependent signalling pathway is not enhanced in gp130 transgenic heart after LIF stimulation. Cytokine. 2000;12:1512–1518. doi: 10.1006/cyto.2000.0751. [DOI] [PubMed] [Google Scholar]
- Trial J, Rossen RD, Rubio J, Knowlton AA. Inflammation and ischemia: macrophages activated by fibronectin fragments enhance the survival of injured cardiac myocytes. Exp Biol Med (Maywood) 2004;229:538–545. doi: 10.1177/153537020422900612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruda T, Jougasaki M, Boerrigter G, Huntley BK, Chen HH, D'Assoro AB, et al. Cardiotrophin-1 stimulation of cardiac fibroblast growth: roles for glycoprotein 130/leukemia inhibitory factor receptor and the endothelin type A receptor. Circ Res. 2002;90:128–134. doi: 10.1161/hh0202.103613. [DOI] [PubMed] [Google Scholar]
- Tsutamoto T, Hisanaga T, Wada A, Maeda K, Ohnishi M, Fukai D, et al. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol. 1998;31:391–398. doi: 10.1016/s0735-1097(97)00494-4. [DOI] [PubMed] [Google Scholar]
- Turkson J. STAT proteins as novel targets for cancer drug discovery. Expert Opin Ther Targets. 2004;8:409–422. doi: 10.1517/14728222.8.5.409. [DOI] [PubMed] [Google Scholar]
- Uozumi H, Hiroi Y, Zou Y, Takimoto E, Toko H, Niu P, et al. gp130 plays a critical role in pressure overload-induced cardiac hypertrophy. J Biol Chem. 2001;276:23115–23119. doi: 10.1074/jbc.M100814200. [DOI] [PubMed] [Google Scholar]
- Wallenius V, Wallenius K, Jansson JO. Normal pharmacologically-induced, but decreased regenerative liver growth in interleukin-6-deficient (IL-6(−/−)) mice. J Hepatol. 2000;33:967–974. doi: 10.1016/s0168-8278(00)80130-0. [DOI] [PubMed] [Google Scholar]
- Wang F, Seta Y, Baumgarten G, Engel DJ, Sivasubramanian N, Mann DL. Functional significance of hemodynamic overload-induced expression of leukemia-inhibitory factor in the adult mammalian heart. Circulation. 2001;103:1296–1302. doi: 10.1161/01.cir.103.9.1296. [DOI] [PubMed] [Google Scholar]
- Wang F, Trial J, Diwan A, Gao F, Birdsall H, Entman M, et al. Regulation of cardiac fibroblast cellular function by leukemia inhibitory factor. J Mol Cell Cardiol. 2002;34:1309–1316. doi: 10.1006/jmcc.2002.2059. [DOI] [PubMed] [Google Scholar]
- Wang M, Markel T, Crisostomo P, Herring C, Meldrum KK, Lillemoe KD, et al. Deficiency of TNFR1 protects myocardium through SOCS3 and IL-6, but not p38 MAPK or IL-1 beta. Am J Physiol Heart Circ Physiol. 2006;292:H1694–H1699. doi: 10.1152/ajpheart.01063.2006. [DOI] [PubMed] [Google Scholar]
- Weiss TW, Speidl WS, Kaun C, Rega G, Springer C, Macfelda K, et al. Glycoprotein 130 ligand oncostatin-M induces expression of vascular endothelial growth factor in human adult cardiac myocytes. Cardiovasc Res. 2003;59:628–638. doi: 10.1016/s0008-6363(03)00463-2. [DOI] [PubMed] [Google Scholar]
- Wollert KC, Chien KR. Cardiotrophin-1 and the role of gp130-dependent signaling pathways in cardiac growth and development. J Mol Med. 1997;75:492–501. doi: 10.1007/s001090050134. [DOI] [PubMed] [Google Scholar]
- Wollert KC, Taga T, Saito M, Narazaki M, Kishimoto T, Glembotski CC, et al. Cardiotrophin-1 activates a distinct form of cardiac muscle cell hypertrophy. Assembly of sarcomeric units in series VIA gp130/leukemia inhibitory factor receptor-dependent pathways. J Biol Chem. 1996;271:9535–9545. doi: 10.1074/jbc.271.16.9535. [DOI] [PubMed] [Google Scholar]
- Xi S, Gooding WE, Grandis JR. In vivo antitumor efficacy of STAT3 blockade using a transcription factor decoy approach: implications for cancer therapy. Oncogene. 2005;24:970–979. doi: 10.1038/sj.onc.1208316. [DOI] [PubMed] [Google Scholar]
- Yajima T, Yasukawa H, Jeon ES, Xiong D, Dorner A, Iwatate M, et al. Innate defense mechanism against virus infection within the cardiac myocyte requiring gp130-STAT3 signaling. Circulation. 2006;114:2364–2373. doi: 10.1161/CIRCULATIONAHA.106.642454. [DOI] [PubMed] [Google Scholar]
- Yamauchi-Takihara K. Gp130-mediated pathway and left ventricular remodeling. J Card Fail. 2002;8 6 Suppl:S374–S378. doi: 10.1054/jcaf.2002.129254. [DOI] [PubMed] [Google Scholar]
- Yamauchi-Takihara K, Ihara Y, Ogata A, Yoshizaki K, Azuma J, Kishimoto T. Hypoxic stress induces cardiac myocyte-derived interleukin-6. Circulation. 1995;91:1520–1524. doi: 10.1161/01.cir.91.5.1520. [DOI] [PubMed] [Google Scholar]
- Yamauchi-Takihara K, Kishimoto T. A novel role for STAT3 in cardiac remodeling. Trends Cardiovasc Med. 2000;10:298–303. doi: 10.1016/s1050-1738(01)00066-4. [DOI] [PubMed] [Google Scholar]
- Yang J, Chatterjee-Kishore M, Staugaitis SM, Nguyen H, Schlessinger K, Levy DE, et al. Novel roles of unphosphorylated STAT3 in oncogenesis and transcriptional regulation. Cancer Res. 2005;65:939–947. [PubMed] [Google Scholar]
- Yang J, Liao X, Agarwal MK, Barnes L, Auron PE, Stark GR. Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFkappaB. Genes Dev. 2007a;21:1396–1408. doi: 10.1101/gad.1553707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Hu S, Choudhry MA, Rue LW, III, Bland KI, Chaudry IH. Anti-rat soluble IL-6 receptor antibody down-regulates cardiac IL-6 and improves cardiac function following trauma-hemorrhage. J Mol Cell Cardiol. 2007b;42:620–630. doi: 10.1016/j.yjmcc.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Yang S, Hu S, Hsieh YC, Choudhry MA, Rue LW, 3rd, Bland KI, et al. Mechanism of IL-6-mediated cardiac dysfunction following trauma-hemorrhage. J Mol Cell Cardiol. 2006;40:570–579. doi: 10.1016/j.yjmcc.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Yasukawa H, Hoshijima M, Gu Y, Nakamura T, Pradervand S, Hanada T, et al. Suppressor of cytokine signaling-3 is a biomechanical stress-inducible gene that suppresses gp130-mediated cardiac myocyte hypertrophy and survival pathways. J Clin Invest. 2001;108:1459–1467. doi: 10.1172/JCI13939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D, et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;4:551–556. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]
- Yeoh GC, Ernst M, Rose-John S, Akhurst B, Payne C, Long S, et al. Opposing roles of gp130-mediated STAT-3 and ERK-1/2 signaling in liver progenitor cell migration and proliferation. Hepatology. 2007;45:486–494. doi: 10.1002/hep.21535. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Taga T, Saito M, Suematsu S, Kumanogoh A, Tanaka T, et al. Targeted disruption of gp130, a common signal transducer for the interleukin 6 family of cytokines, leads to myocardial and hematological disorders. Proc Natl Acad Sci USA. 1996;93:407–411. doi: 10.1073/pnas.93.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki MH, Nemeth JA, Trikha M. CNTO 328, a monoclonal antibody to IL-6, inhibits human tumor-induced cachexia in nude mice. Int J Cancer. 2004;111:592–595. doi: 10.1002/ijc.20270. [DOI] [PubMed] [Google Scholar]
- Zimmerer JM, Lesinski GB, Kondadasula SV, Karpa VI, Lehman A, Raychaudhury A, et al. IFN-alpha-induced signal transduction, gene expression, and antitumor activity of immune effector cells are negatively regulated by suppressor of cytokine signaling proteins. J Immunol. 2007;178:4832–4845. doi: 10.4049/jimmunol.178.8.4832. [DOI] [PubMed] [Google Scholar]
- Zitzmann K, Brand S, De Toni EN, Baehs S, Goke B, Meinecke J, et al. SOCS1 silencing enhances antitumor activity of type I IFNs by regulating apoptosis in neuroendocrine tumor cells. Cancer Res. 2007;67:5025–5032. doi: 10.1158/0008-5472.CAN-06-2575. [DOI] [PubMed] [Google Scholar]
- Zou Y, Takano H, Mizukami M, Akazawa H, Qin Y, Toko H, et al. Leukemia inhibitory factor enhances survival of cardiomyocytes and induces regeneration of myocardium after myocardial infarction. Circulation. 2003;108:748–753. doi: 10.1161/01.CIR.0000081773.76337.44. [DOI] [PubMed] [Google Scholar]
- Zvonic S, Baugh JE, Jr, Arbour-Reily P, Mynatt RL, Stephens JM. Cross-talk among gp130 cytokines in adipocytes. J Biol Chem. 2005;280:33856–33863. doi: 10.1074/jbc.M508020200. [DOI] [PubMed] [Google Scholar]