Abstract
Almost all G-protein coupled receptors (GPCRs) are regulated by phosphorylation and this process is a key event in determining the signalling properties of this receptor super-family. Receptors are multiply phosphorylated at sites that can occur throughout the intracellular regions of the receptor. This diversity of phospho-acceptor sites together with a lack of consensus phosphorylation sequences has led to the suggestion that the precise site of phosphorylation is not important in the phosphorylation-dependent regulation of GPCR function but rather it is the increase in bulk negative charge of the intracellular face of the receptor which is the significant factor. This review investigates the possibility that the multi-site nature of GPCR phosphorylation reflects the importance of specific phosphorylation events which mediate distinct signalling outcomes. In this way receptor phosphorylation may provide for a flexible regulatory mechanism that can be tailored in a tissue specific manner to regulate physiological processes. By understanding the flexible nature of GPCR phosphorylation if may be possible to develop agonists or allosteric modulators that promote a subset of phosphorylation events on the target GPCR and thereby restrict the action of the drug to a particular receptor mediated signalling response.
Keywords: G-protein-coupled receptor, phosphorylation, G-protein-coupled receptor kinase, casein kinase, arrestin, internalization, desensitization, GRK, Akt/PKB, insulin receptor
Introduction
It has been known for more than 20 years that G-protein-coupled receptors (GPCRs) are regulated by phosphorylation (Lefkowitz, 2004). From the outset it was clear that for the non-visual GPCRs, the process of stimulus-dependent receptor phosphorylation was mediated by more than one protein kinase family. Studies on the β2-adrenoceptor determined that both protein kinase A (PKA) (Benovic et al., 1985) and β-adrenoceptor kinase (Benovic et al., 1986), later to be renamed G-protein-coupled receptor kinase 2 (GRK2), could mediate receptor phosphorylation. At this early stage it was clear that these different protein kinases, activated by distinct mechanisms, were able to phosphorylate different sites on the receptor and that this resulted in different signalling outcomes (Lohse, 1993).
Over the intervening years, however, many of the important concepts illustrated in these early studies have been lost in an attempt to generate a unifying hypothesis that would account for the regulation of GPCR function across the entire GPCR superfamily. This search for common regulatory mechanisms has resulted in a clear understanding of the role played by the GRK family and arrestins in universal processes such as receptor desensitization and internalization (Ferguson, 2001). GPCRs are, however, a remarkably diverse and widespread protein family involved in the regulation of nearly every biological response (Kristiansen, 2004; Maudsley et al., 2005). The same receptor subtype is often expressed on very different tissues where it regulates defined physiological responses. It seems likely, therefore, that GPCRs will be regulated differentially in the various tissues in which they are expressed and that this will be in line with the tissue-specific function of the receptor.
In this review, we will be considering the possibility that receptor phosphorylation might be mediated by a range of receptor kinases able to phosphorylate distinct sites on GPCRs. This site-specific phosphorylation would result in a specific signalling outcome. In this way, receptor phosphorylation might act as a dynamic and flexible regulatory process that could be tailored in a cell type-specific manner.
The role of phosphorylation in GPCR interaction with arrestin
GPCRs essentially function as dynamic scaffolding proteins interacting with an array of signalling molecules in a manner that can be influenced by conformational changes in the receptor induced by agonist occupation (Milligan and White, 2001; Kreienkamp, 2002; Bockaert et al., 2004). The publication of the keenly anticipated crystal structures of the β2-adrenoceptor in complex with an inverse agonist (Cherezov et al., 2007; Rasmussen et al., 2007) provides hope that in the near future a definitive structural model of an active GPCR will be possible. In the mean time the consensus view is that agonist binding results in a change in the relative positions of transmembrane α-helices III and VI, which results in a re-orientation of the third intracellular loop in a manner that allows for G-protein coupling (Kobilka, 2002). This agonist-induced conformational change is also thought to unmask sites on the intracellular domains that can be modified by phosphorylation (Pitcher et al., 1998). This combined process of conformational change and post-translational modification allows for the receptor to interact with proteins that previously were unable to interact with the receptor. Alternatively, the conformational change in the receptor following agonist binding can induce a conformational change in interacting partners that are present in a pre-assembled complex with the receptor and in so doing ‘activate' the signalling properties of the partners. Among a growing list of GPCR interacting proteins (Milligan and White, 2001; Kristiansen, 2004), the arrestin family is the most extensively studied and represents the archetypical phosphorylation-dependent interacting partner.
There are four members of the arrestin family. Arrestin1 and arrestin4 are the so-called visual arrestins expressed only in rod and cone photoreceptors where the interaction with activated and phosphorylated rhodopsin and cone opsins results in termination of phototransduction (Luttrell and Lefkowitz, 2002). The remaining two arrestins, arrestin2 and arrestin3, are able to regulate the activity of the many hundreds of non-visual GPCRs (Gurevich and Gurevich, 2006). Early studies determined that arrestin binding is a two-component process where both the phosphorylated receptor and the ligand-activated conformation of the receptor were recognized by distinct regions on the arrestin molecule (Gurevich and Benovic, 1993). This cooperative binding served to change the conformation of the arrestin molecule to an ‘active' state with a high affinity for the receptor (Hirsch et al., 1999). Modelling studies carried out primarily by the Gurevich group demonstrated how the association of arrestin with multiple sites on the intracellular regions of GPCRs results in a complex that effectively ‘caps' the GPCR and hence precludes G-protein coupling (Gurevich and Gurevich, 2006). In this way, arrestin is able to uncouple or desensitize the receptor from G-protein-dependent signalling pathways (Krupnick and Benovic, 1998). It has also become clear that in addition to sterically hindering G-protein interaction, arrestins also act as scaffolding proteins for molecules, such as src and components of the mitogen-activated protein kinase cascade and through this route are able to activate G-protein-independent signalling (Lefkowitz and Whalen, 2004; Lefkowitz and Shenoy, 2005).
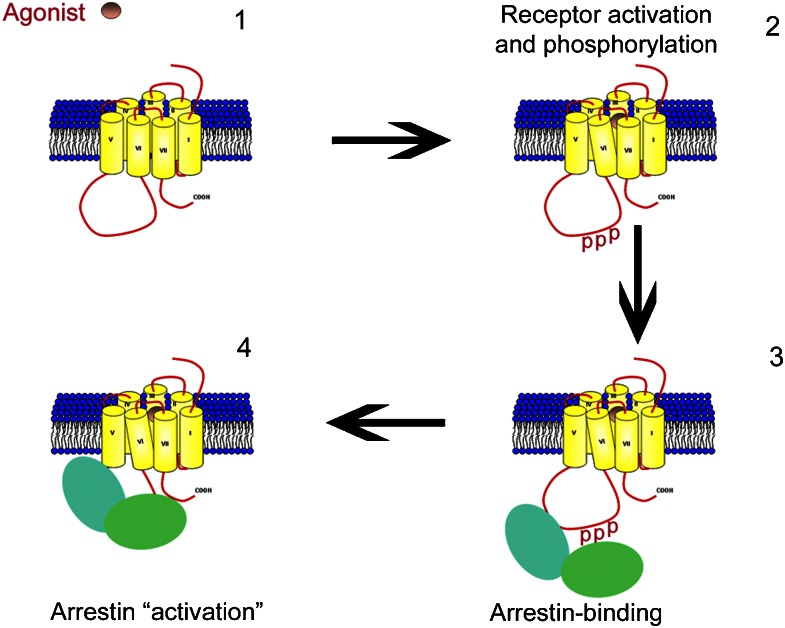
The relationship between receptor phosphorylation and arrestin binding for the visual arrestins is relatively clear. In this case, phosphorylation of serines and threonines contained in a cluster in the C-terminal tail of rhodopsin (Ohguro et al., 1993, 1996) results in a 10-fold increase in the affinity of arrestin1 for the light-activated receptor (Gurevich et al., 1995). The phosphate sensor on arrestin lies buried in a polar core and consists primarily of a salt bridge between a conserved arginine (R175 in arrestin1) and an aspartate (D296) (Vishnivetskiy et al., 1999, 2002). Phosphates contained on the receptor phospho-acceptor sites are proposed to be ‘guided' to the polar core on arrestin by interaction with lysine residues on the concave surface of the arrestin molecule (Gurevich and Gurevich, 2006). The disruption of the salt bridge at the polar core together with the driving force derived from multiple interactions of the concave surface of arrestin with the activated receptor conformation (the so-called activation sensor) (Vishnivetskiy et al., 2004) results in the conformational change in arrestin that allows for high-affinity receptor binding (Hirsch et al., 1999; Gurevich and Gurevich, 2006) (Figure 1).
Figure 1.
Interaction of arrestin with an activated and phosphorylated G-protein-coupled receptor. (1) Agonist binding to the receptor results in a conformational change that particularly affects the orientation of transmembrane helices III and VI. (2) This conformational change is thought to result in as yet undefined changes in the intracellular loop regions and C-terminal tail that unmasks phosphorylation sites and results in multi-site phosphorylation (P). (3) The active conformation of the receptor is detected by the activation sensor on arrestin. The activation sensor consists of multiple interaction sites situated on the concave surface of the arrestin molecule that are able to engage with the active conformation of the receptor. Also, phosphorylated residues are shown initially interacting with residues (lysines) situated on the outer surface of the arrestin molecule. (4) Arrestin binds with high affinity to the receptor and adopts an active conformation through further interactions with the activated receptor via the activation sensor and by phosphates interacting with the phosphate sensor situated in the polar core. The active conformation of arrestin is proposed to involve re-orientation of the two domains of arrestin.
Whereas the role of rhodopsin phosphorylation in recruiting arrestin1 is clear, this is not the case for the non-visual GPCRs. Unlike the visual system where rhodopsin phosphorylation dramatically increases the affinity of the activated receptor for arrestin, the phosphorylation of non-visual GPCRs has only a small effect on arrestin affinity, often amounting to no more than a 2- to 3-fold increase (Gurevich and Gurevich, 2006). This modest increase in affinity occurs despite the fact that the essential features of the molecular mechanism of receptor/arrestin binding appear to be conserved between the visual and non-visual systems. Thus, arrestin2 and 3 are thought to make multiple contacts with the ligand-occupied receptor through residues on the concave surface of arrestin that act as the activation sensor. This operates cooperatively with the interaction of receptor phosphates at the phosphate sensor situated at the polar core (Gurevich and Gurevich, 2006). The issue that faces arrestin2 and 3 is that these two molecules are regulators of 600–800 GPCR subtypes, which show very little sequence homology in their intracellular domains and that are phosphorylated at multiple sites within different receptor regions. Thus, GPCRs have been shown to be phosphorylated on the C-terminal tail (Bouvier et al., 1988; Fredericks et al., 1996; Seibold et al., 2000), third intracellular loop (Budd et al., 2001; Kim et al., 2001; Trester-Zedlitz et al., 2005; Torrecilla et al., 2007), first intracellular loop (Nakamura et al., 1998) and possibly on the second intracellular loop (Kim et al., 2001). Receptor phosphorylation can also be mediated by very different protein kinases (Hausdorff et al., 1989; Torrecilla et al., 2007), which in some cases have been demonstrated to promote arrestin binding (Benovic et al., 1987; Lohse et al., 1992; Gurevich et al., 1995; Krasel et al., 2005) and in others to have no effect on arrestin interaction (Lohse et al., 1992). The result of this heterogeneity is a range of receptor–arrestin affinities where certain receptors such as, V2 vasopressin receptor, have a sufficiently high affinity for the arrestin–receptor complex to remain intact during the trafficking of the receptor to intracellular compartments following agonist stimulation (Oakley et al., 1999). Such receptors have been classified as Class B receptors (Oakley et al., 2000). This compares with Class A receptors, such as β2-adrenoceptor, that show a relatively low affinity for arrestin and where the receptor–arrestin complexes are much harder to visualize (Oakley et al., 1999, 2000).
It is also clear that for some receptors, phosphorylation is not an absolute requirement for arrestin binding. This is seen for the substance P (Richardson et al., 2003), lutropin (Mukherjee et al., 2002) and leukotriene B4 (Jala et al., 2005) receptors where phosphorylation-deficient mutants still maintain the ability to interact with arrestin. Furthermore, arrestin binding is maintained in mutants of the oxrexin-1 (Milasta et al., 2005) and protease-activated receptor-2 receptors (Stalheim et al., 2005) where the putative phosphorylation sites had been removed. It is possible that the multiple interactions of the receptor with the activator sensors on arrestin are sufficient to allow for a stable complex in the absence of phosphorylation. This notion has been extended to suggest that each elementary interaction between the receptor and arrestin has low affinity in the micromolar range (Wu et al., 1997; Cen et al., 2001; Liu et al., 2004). It is the cooperative binding of these elementary interactions that results in the overall high-affinity interaction of the receptor with arrestin. In this model, elements of arrestin interaction could be lost (that is, phosphorylation-dependent interaction) with a consequent loss in the affinity of the receptor for arrestin. However, the reduction in affinity may not be sufficient to completely prevent arrestin binding (Gurevich and Gurevich, 2006).
Phosphorylation-independent binding of some receptors may, however, be explained by the presence of negatively charged residues that act as phospho-mimetics. An acidic cluster in the C-terminal tail of the chemokine receptor D6, which appears constitutively bound to arrestin, plays a key role in arrestin interaction (Galliera et al., 2004). Similarly, an aspartate in the third intracellular loop of the lutropin receptor is thought to interact with the phosphate sensor in arrestin (Mukherjee et al., 2002) and that this interaction drives phosphorylation-independent receptor internalization. Given the diversity of GPCR supergene family, it is likely that the D6 and lutropin receptors are not the only examples of receptors that mediate arrestin binding via charged amino-acid residues.
Does site-specific phosphorylation contribute to specific properties of receptor–arrestin interaction?
It is remarkable that arrestin binding to the phosphorylated form of non-visual GPCRs can tolerate the diverse nature of GPCR phosphorylation. This flexibility in arrestin binding has prompted the hypothesis that arrestin can interact with negative charge placed at a number of different positions on the receptor. It has been suggested that it is the amount of negative charge introduced through phosphorylation rather than the precise position or site of phosphorylation, which is the important factor in recruiting arrestin to the receptor (Gurevich and Gurevich, 2006). Thus, arrestin binding appears to require at least two phosphorylated residues in close proximity (Gurevich and Benovic, 1993). The phospho-acceptor sites on GPCRs very often occur at serine/threonine clusters where more than one of the residues are phosphorylated (Wilden and Kuhn, 1982; Smith et al., 1998; Guo et al., 2000; Lee et al., 2000b; Braun et al., 2003). Because these phosphorylation sites appear in different intracellular domains (C-terminal tail, third, second and first intracellular loops), it has been speculated that it is the ‘bulk' negative charge that is important rather then the specific amino-acid sequence of the phospho-acceptor sites (Gurevich and Gurevich, 2006).
It appears, however, that not all phosphorylation events are equal. The first indication of this was that phosphorylation of the β2-adrenoceptor at the PKA/protein kinase C (PKC) site in the distal portion of the third intracellular loop and the PKA site at the proximal region of the C-terminal tail (Yuan et al., 1994; Moffett et al., 1996; Tran et al., 2004) did not result in the recruitment of arrestin (Lohse et al., 1992). In contrast, GRK-mediated phosphorylation at the C-terminal tail mediated arrestin binding. Although, both phosphorylation by the GRKs and PKA/PKC results in β2-adrenoceptor desensitization the mechanisms employed by these receptor kinases are distinct (Roth et al., 1991; Lefkowitz, 1998; Pitcher et al., 1998).
More recently, studies on the angiotensin AT1A and vasopressin V2 receptors have demonstrated that GRK2 is the protein kinase primarily responsible for receptor phosphorylation and arrestin recruitment in transfected HEK293 cells (Oppermann et al., 1996; Kim et al., 2005; Ren et al., 2005). These receptors stimulate a biphasic extracellular-regulated protein kinase (ERK) response where the initial peak response is dependent on G-protein coupling and the prolonged response is mediated by the recruitment of arrestin (Wei et al., 2003; Kim et al., 2005; Ren et al., 2005). In this case, it might be predicted that disrupting GRK2-mediated receptor phosphorylation and arrestin recruitment would disrupt the prolonged phase of ERK activity. Surprisingly, inhibition of GRK2 activity by siRNA had little effect on AT1A and V2 receptor-mediated ERK activity (Wei et al., 2003; Ren et al., 2005). In contrast, inhibition of GRK-5 and 6, despite having no detectable effect on receptor phosphorylation, reduced arrestin recruitment by 30–50% and inhibited the prolonged (arrestin-dependent) phase of the ERK response (Kim et al., 2005; Ren et al., 2005). Other arrestin-mediated processes such as receptor internalization and desensitization were, however, attributed to GRK2-mediated phosphorylation and not GRK5/6 activity (Kim et al., 2005; Ren et al., 2005). One possible interpretation of these data is that GRK2 phosphorylates the AT1A and V2 receptors at different sites from those phosphorylated by GRK5/6. As a result, it has been suggested that the arrestin bound to the receptor phosphorylated by GRK2 could adopt a conformation that is different from that adopted when arrestin is bound to the receptor phosphorylated by GRK5/6 (Wei et al., 2003; Kim et al., 2005). In the case of the AT1A and V2 receptors, the arrestin conformation adopted in response to GRK2 phosphorylation would be important for G-protein uncoupling and that adopted following GRK5/6 phosphorylation would be important in coupling the receptor to the ERK pathway (Wei et al., 2003; Kim et al., 2005; Ren et al., 2005).
Further experiments on the β2-adrenoceptor have demonstrated that the recruitment of arrestin itself may be dependent on the isotype of GRK involved in receptor phosphorylation. Individual siRNA knockdown of GRK2, 3, 5 and 6 had differential effects on the kinetics of arrestin recruitment to the receptor (Violin et al., 2006). This could be interpreted as the GRKs mediating differential phosphorylation of the receptor and this in turn affecting different rates of arrestin recruitment. A cautionary note sounded by the authors of these experiments is that there is currently no evidence from intact cells that the various members of the GRK family can phosphorylate receptors differentially (Kim et al., 2005; Ren et al., 2005; Violin et al., 2006).
Further evidence that the specific site on which a receptor is phosphorylated is able to mediate specific signalling outcomes has emerged from studies on the M2-muscarinic receptor. In this case, mutation of serines/threonines to alanines in two regions of the third intracellular loop known as the N- and C-clusters prevented agonist-dependent receptor phosphorylation, internalization and desensitization (Pals-Rylaarsdam and Hosey, 1997). The preferred phosphorylation sites are at the C-cluster where phosphorylation mediates the interaction of the receptor with arrestin (Lee et al., 2000a). Removal of the C-cluster prevents the association of wild-type arrestin with the M2-muscarinic receptor, but surprisingly it also prevented interaction with the phosphorylation-independent arrestin mutant arrestin-R169E (Kovoor et al., 1999). This arrestin construct, which is mutated in the phosphate sensor, is constitutively active adopting a conformation that mimicks that of the phosphate-docked arrestin (Kovoor et al., 1999). Deletion of a 16 amino-acid region in the M2-muscarinic receptor, which included at its heart the C-cluster, restored the ability of the M2-muscarinic receptor to interact with both wild-type arrestin and arrestin-R169E (Lee et al., 2000b). These results indicated that far from the C-cluster containing phosphorylation sites that allowed for the receptor to interact with the phosphate sensor in arrestin in a conventional way, the C-cluster rather was at the centre of a region that acted as a brake on arrestin binding. Phosphorylation of the C-cluster released the brake and allowed the receptor to interact with arrestin. This was the first demonstration of such a brake mechanism that appears to be in operation in a number of other receptor types, including the δ-opioid (Whistler et al., 2001) and follitropin receptors (Kishi et al., 2002).
It appears, therefore, that the precise sites of phosphorylation on any one particular receptor subtype can be important in the interaction of the receptor with arrestin. Hence, although bulk negative charge can be seen to contribute to arrestin binding it is very likely that, at least for some receptors, the amino-acid context in which the phosphorylation takes place will also be important in dictating the role of phosphorylation in regulating the signalling properties of the receptor.
Regulation of signalling associated with site- specific phosphorylation of the β2-adrenoceptor
It appears that desensitization of the adenylate cyclase response mediated by the β2-adrenoceptor involves both uncoupling from Gs protein and also increased degradation of cyclic AMP. One of the multitude of arrestin-binding partners are isotypes of phosphodiesterases responsible for the hydrolysis of cyclic-AMP (Perry et al., 2002). Recruitment of phosphodiesterases to the activated receptor provides a negative feedback loop, the components of which are assembled at the site of agonist-stimulated adenylate cyclase activity. This regulatory process has recently been linked with the controversial (see Friedman et al., 2002; Lefkowitz et al., 2002) proposal that the β2-adrenoceptor is able to switch between Gs- and Gi-protein signalling via site-specific phosphorylation mediated by PKA (Daaka et al., 1997). Thus, it appears that phosphorylation may have a complex regulatory role in β2-adrenoceptor adenylate cyclase signalling. GRK-mediated phosphorylation of the β2-adrenoceptor allows for the recruitment of arrestin that uncouples the receptor from Gs protein and recruits phosphodiesterases that enhances the degradation of cyclic AMP. This negative feedback loop operates in an apparently simultaneous manner with PKA-mediated phosphorylation of the β2-adrenoceptor which uncouples the receptor from Gs protein (in a manner distinct from GRK-mediated desensitization) and switches signalling to Gi proteins (Baillie et al., 2003).
An example of a specific GRK-mediated phosphorylation event resulting in signal regulation has been reported for GRK5 in the interaction of Na+/H+-exchanger regulatory factor EBP50 with the β2-adrenoceptor. The PDZ domain of EBP50 interacts with the C-terminal tail of the β2-adrenoceptor and this interaction is important in the intracellular sorting of the receptor following agonist-stimulated internalization (Hall et al., 1998; Cao et al., 1999). An essential element in the interaction is serine 411 in the C-terminal tail of the receptor where mutation to an alanine or the phospho-mimetic residue aspartate is seen to disrupt the interaction with EBP50 (Cao et al., 1999). This residue (serine 411) is known to be phosphorylated specifically by GRK5 in vitro (Fredericks et al., 1996) and therefore the suggestion has been made that GRK5-mediated phosphorylation of serine 411 regulates the interaction of EBP50 with the receptor and in so doing regulates receptor sorting and recycling. A caveat to this conclusion is that subsequent in vivo analysis of the agonist-mediated phosphorylation sites on the β2-adrenoceptor has not established serine 411 as a GRK site in intact cells (Seibold et al., 2000; Trester-Zedlitz et al., 2005).
Multi-site nature of GPCR phosphorylation
Mutagenesis (Bouvier et al., 1988; Eason et al., 1995; Prossnitz et al., 1995; Malecz et al., 1998; Smith et al., 1998; Guo et al., 2000; Mendez et al., 2000; Seibold et al., 2000), mass spectrometry (Papac et al., 1993; Karoor and Malbon, 1996; Trester-Zedlitz et al., 2005), phospho-peptide maps (Giannini et al., 1995; Ozcelebi and Miller, 1995; Blaukat et al., 2001; Torrecilla et al., 2007) and antibody studies (Tran et al., 2004) on a wide range of receptor types have demonstrated that GPCRs are multiply phosphorylated. Early studies on rhodopsin demonstrated that this phosphorylation was sequential or hierarchical (Ohguro et al., 1993). There is now a growing body of evidence indicating that this is also the case for the non-visual GPCRs. The chemokine N-formyl peptide C5a receptor is phosphorylated in the basal state by an unknown protein kinase (Giannini et al., 1995), which appears to prime the receptor for ligand-induced phosphorylation that may (Prossnitz et al., 1995) or may not (Milcent et al., 1999) proceed via the GRKs. The primary site for agonist-dependent phosphorylation of the δ-opioid receptor is Ser363 on the C-terminal tail. Mutation of this serine to alanine prevented ligand-dependent phosphorylation at other sites on the C-terminal tail (Kouhen et al., 2000). In vitro phosphorylation of the third intracellular loop of the M3-muscarinic receptor by protein kinase CK2 revealed that mutation of one CK2 consensus site resulted in the loss of a number of phosphorylation events, suggesting that this kinase was mediating multiple hierarchical phosphorylation (Torrecilla et al., 2007). An elegant study of the bradykinin B2 receptor revealed that basal phosphorylation at Ser346 on the C-terminal tail is followed by multiple phosphorylations that were both time and ligand concentration dependent. This complex phosphorylation pattern is due to the multiple kinases that are employed in the phosphorylation of this receptor, which include GRKs, PKC and at least one unidentified protein kinase (Blaukat et al., 2001).
Involvement of multiple protein kinases in GPCR phosphorylation
The multi-site phosphorylation of GPCRs likely reflects the action of a number of protein kinase families (Figure 2). Although the role of PKA/PKC and the GRKs in GPCR phosphorylation has been known for two decades, evidence that other protein kinase families are involved has been slow to emerge. Probably the clearest example that receptor kinases other than PKA/PKC and the GRKs have a role in GPCR phosphorylation was the discovery that some GPCRs are tyrosine phosphorylated (Paxton et al., 1994; Fan et al., 2001). In particular, the discovery that the β2-adrenoceptor was phosphorylated on tyrosine demonstrated the importance of investigating GPCR regulation in the correct cellular context.
Figure 2.
Summary of protein kinases known to phosphorylate G-protein-coupled receptors (GPCRs). Shown are protein kinases that have been demonstrated to phosphorylate GPCRs and the main functional consequences of the phosphorylation event.
Glucose metabolism is controlled primarily by insulin that stimulates glycogen synthesis, glucose uptake and lipid storage, whereas adrenaline counteracts these actions by promoting glycogen breakdown, gluconeogenesis and lipolysis (White and Kahn, 1994; Saltiel and Kahn, 2001). The actions of insulin are augmented by the ability of the insulin receptor to stimulate the tyrosine phosphorylation and internalization of the β2-adrenoceptor (Karoor and Malbon, 1996; Karoor et al., 1998). Further studies established an intricate mechanism whereby insulin receptor activation of PI-3 kinase drives the activity of Akt/PKB (protein kinase B), which was able to directly phosphorylate the β2-adrenoceptor at serines 345/346 on the C-terminal tail (Doronin et al., 2002). The ability of Akt/PKB to phosphorylate the receptor was obligate on insulin-mediated tyrosine 350 phosphorylation (Doronin et al., 2002). This combined hierarchical phosphorylation process was essential in the internalization of the β2-adrenoceptor. The fact that the Akt/PKB sites of phosphorylation on the β2-adrenoceptor are different from those of the GRK sites (Seibold et al., 2000; Doronin et al., 2002) suggested that the mechanism of receptor internalization was distinct from that mediated by the GRKs. This view was supported by the fact that receptor internalization mediated by insulin and that mediated via the GRKs showed different sensitivities to agents that disrupt cellular cytoskeletal networks (Shumay et al., 2004). One proposed mechanism was that phosphorylation of tyrosine 350 creates an SH2 (src homology domain 2)-binding motif (Karoor et al., 1998) that is able to promote the association of c-Src, Grb2 and dynamin (Karoor et al., 1998; Fan et al., 2001; Gurevich and Gurevich, 2006). The creation of an SH2 domain appears to act in concert with Akt/PKB phosphorylation to mediate receptor internalization in response to insulin (Gavi et al., 2006). One interesting proposition that comes from the study of insulin regulation of the β2-adrenoceptor is that via site-specific phosphorylation of the receptor by a number of protein kinases the receptor can firstly access unique internalization and recycling mechanisms, but that also by the creation of a protein interaction motif, which in this case is a tyrosine-phosphorylated SH2 domain, the receptor acts as a scaffold protein able to signal to pathways such as the mitogen-activated protein kinase pathway in the absence of its own ligand (Wang et al., 2000). Thus in a physiological context, insulin can effectively counter-regulate β2-adrenoceptor signalling through receptor internalization and also is able to enhance its own signalling potential by recruiting the β2-adrenoceptor as a scaffolding protein that augments insulin-mediated ERK activity.
The involvement of Akt/PKB in directly phosphorylating GPCRs is not restricted to the β2-adrenoceptor. Insulin like growth factor-I has been proposed to stimulate Akt/PKB-mediated phosphorylation of the β1-adrenoceptor at serine 412 and this mediates functional antagonism of the β1-adrenoceptor (Gavi et al., 2006). Furthermore, the finding that the α1A-adrenoceptor is desensitized following phosphorylation by Akt/PKB (Garcia-Sainz, 2005) and that sphingosine-1-phosphate-stimulated cell migration is regulated by Akt/PKB-mediated S1P1 (EDG-1) receptor phosphorylation (Lee et al., 2001) has highlighted the importance of this serine/threonine kinase in the regulation of a number of GPCRs.
Recent evidence has pointed to a role of the casein kinase family in GPCR phosphorylation. Studies on the mating factor receptors of the budding yeast Saccharomyces cerevisiae have demonstrated an essential role for CK1 in the phosphorylation of serines in the C-terminal tail. These phosphorylation events drive mono-ubiquitination of lysine residues downstream of the phosphorylation sites and this in turn mediates receptor internalization (Panek et al., 1997; Hicke et al., 1998; Roth and Davis, 2000; Feng and Davis, 2000). Our own studies on the M3-muscarinic receptor have demonstrated by the use of cell-free assays and a CK1α-dominant-negative mutant that CK1α was responsible, at least in part, for the agonist-stimulated phosphorylation of the M1- (Waugh et al., 1999; Mou et al., 2006) and M3-muscarinic receptors (Tobin et al., 1997; Budd et al., 2001). In the case of the M3-muscarinic receptor, this phosphorylation event appeared to regulate the ability of the receptor to couple to the ERK-1/2 pathway (Budd et al., 2001).
The first indication that protein kinase CK2 might be involved in GPCR phosphorylation came from studies on the thyrotropin-releasing hormone receptor where mutation of putative protein kinase CK2 sites in the C-terminal tail resulted in a decrease in receptor phosphorylation and a loss of arrestin-mediated internalization (Hanyaloglu et al., 2001). In this case, it appeared that at least three sites in a tight cluster (T365, T371 and S383) were required to be phosphorylated to allow for arrestin-dependent receptor internalization. Notably, internalization mediated by the phosphorylation-independent mutant, arrestin-R169E, was not affected by removal of these phosphorylation sites. Thus, in the case of the thyrotropin-releasing hormone receptor CK2 appeared to substitute for the GRKs in driving receptor phosphorylation of a region that was involved in arrestin activation and receptor internalization (Hanyaloglu et al., 2001).
Our studies on the M3-muscarinic receptor have demonstrated that protein kinase CK2 inhibition through siRNA knockdown or pharmacological agents could partly reduce agonist-mediated receptor phosphorylation (Torrecilla et al., 2007). Phospho-peptide maps revealed that CK2 was likely to phosphorylate just a subset of phospho-acceptor sites in the third intracellular loop of the receptor (Torrecilla et al., 2007). Thus, protein kinase CK2 can be added to a growing list of protein kinases, which includes GRK2/6 (Willets et al., 2002, 2003) and CK1α (Tobin et al., 1997; Budd et al., 2001), that are known to phosphorylate the M3-muscarinic receptor in an agonist-dependent manner. Interestingly, protein kinase CK2-mediated phosphorylation did not appear to result in receptor internalization nor regulation of the ERK-1/2 pathways (Torrecilla et al., 2007). Rather, CK2 inhibition resulted in enhanced coupling of the receptor to the JUN kinase pathway (Torrecilla et al., 2007). Combined these data suggested that protein kinase CK2 phosphorylated a subset of phospho-acceptor sites and that these were involved in the specific regulation of receptor coupling to the JUN kinase pathway and not involved in other phosphorylation-dependent processes such as receptor internalization and ERK activation.
Conclusions and perspectives
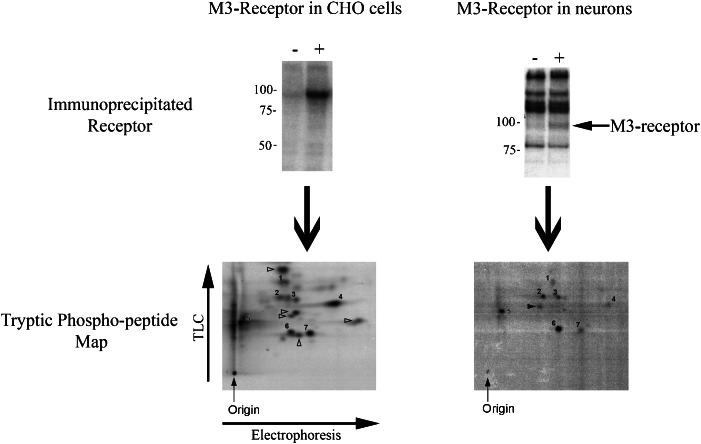
There is now a gathering body of evidence that indicates that GPCR phosphorylation is a complex process involving a range of different protein kinases able to phosphorylate the same receptor at different sites and that this results in differential signalling outcomes (see Figure 2). In this regard, GPCR phosphorylation presents a flexible regulatory process where the recruitment of different protein kinases in cell types could tailor the signalling response of the receptor to suit a particular physiological role. If this were to be true, then one would expect to see different patterns of receptor phosphorylation in different cell types. That this may be the case has emerged from phospho-peptide mapping studies that have produced a ‘phosphorylation signature' of the M3-muscarinic receptor expressed in Chinese hamster ovary (CHO) cells and cerebella granule neurons (Torrecilla et al., 2007) (Figure 3). These maps have determined that some phosphorylation events, marked as numbers in Figure 3, are conserved between the two cell types whereas others, marked with arrows, are exclusive to either the CHO cells or the cerebellar granule neurons (Torrecilla et al., 2007). The question from these studies is whether the common phosphorylation events regulate common receptor functions, such as receptor internalization, and the specific phosphorylations are associated with cell-type-specific signalling. This question is likely to be answered only in the study of the receptors in the experimentally challenging environment of native cell types.
Figure 3.
M3-muscarinic receptors expressed in Chinese hamster ovary (CHO) cells and cerebellar granule neurons are differentially phosphorylated. Cerebellar granule neurons were cultured from 6-day-old neonatal mice and maintained for 6 days before being labelled for phosphorylation studies. CHO cells expressing the recombinant mouse M3-muscarinic receptor or cerebellar granule neurons were labelled with [32P]orthophosphate for 1–2 h (100–200 μCi ml−1). The cells were then stimulated for 5 min with the muscarinic agonist methacholine (100 μM). Cells were then solubilized and the M3-muscarinic receptor immunoprecipitated using a receptor-specific antibody. The immunoprecipitate was resolved by 8% SDS-PAGE and the radioactive band associated with the receptor was excised. The receptor band was then digested with trypsin and the resulting peptides resolved in the first dimension by electrophoresis and in the second dimension by thin layer chromatography. The position of the radioactive phospho-peptides was then determined in a phospho-imager. The numbered peptides are those that run in the same position and represent phosphorylation sites that are the same in the two cell types. The arrows represent phosphorylation events that are specific to the cell in which the receptor is expressed. The experiment shown was adapted from Torrecilla et al. (2007) where details of the methods can be obtained.
This may prove to be a very important point in terms of drug discovery and design. GPCRs are clearly acting in a tissue-specific manner. Currently, little attention is paid to the fact that by identification of tissue-specific processes and targeting these in therapeutic strategies we may limit the side effects common to many GPCR drugs. Phosphorylation may well be one of the tissue-specific phenomena that will allow for more specific drug design. By generating agonists or allosteric modulators that will promote a given subset of phosphorylation events on the target, GPCR pharmaceutical compounds may be made specific to certain signalling pathways (a form of agonist trafficking) or specific to the target GPCR subtype when expressed only in one particular tissue.
Acknowledgments
I thank the Wellcome trust for their support (Grant 047600).
Glossary
- Akt/PKB
protein kinase B
- CHO
Chinese hamster ovary
- ERK
extracellular-regulated protein kinase
- GPCR
G-protein-coupled receptor
- GRK
G-protein-coupled receptor kinase
- PKA
protein kinase A
- PKC
protein kinase C
Footnotes
Conflict of interest
The authors state no conflict of interest.
References
- Baillie GS, Sood A, McPhee I, Gall I, Perry SJ, Lefkowitz RJ, et al. Beta-arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates beta-adrenoceptor switching from Gs to Gi. Proc Natl Acad Sci USA. 2003;100:940–945. doi: 10.1073/pnas.262787199. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Benovic JL, Kuhn H, Weyand I, Codina J, Caron MG, Lefkowitz RJ. Functional desensitization of the isolated beta-adrenergic receptor by the beta-adrenergic receptor kinase: potential role of an analog of the retinal protein arrestin (48-kDa protein) Proc Natl Acad Sci USA. 1987;84:8879–8882. doi: 10.1073/pnas.84.24.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benovic JL, Pike LJ, Cerione RA, Staniszewski C, Yoshimasa T, Codina J, et al. Phosphorylation of the mammalian beta-adrenergic receptor by cyclic AMP-dependent protein kinase. Regulation of the rate of receptor phosphorylation and dephosphorylation by agonist occupancy and effects on coupling of the receptor to the stimulatory guanine nucleotide regulatory protein. J Biol Chem. 1985;260:7094–7101. [PubMed] [Google Scholar]
- Benovic JL, Strasser RH, Caron MG, Lefkowitz RJ. Beta-adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci USA. 1986;83:2797–2801. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaukat A, Pizard A, Breit A, Wernstedt C, Alhenc-Gelas F, Muller-Esterl W, et al. Determination of bradykinin B2 receptor in vivo phosphorylation sites and their role in receptor function. J Biol Chem. 2001;276:40431–40440. doi: 10.1074/jbc.M107024200. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Roussignol G, Becamel C, Gavarini S, Joubert L, Dumuis A, et al. GPCR-interacting proteins (GIPs): nature and functions. Biochem Soc Trans. 2004;32 Part 5:851–855. doi: 10.1042/BST0320851. [DOI] [PubMed] [Google Scholar]
- Bouvier M, Hausdorff WP, De Blasi A, O'Dowd BF, Kobilka BK, Caron MG, et al. Removal of phosphorylation sites from the beta 2-adrenergic receptor delays onset of agonist-promoted desensitization. Nature. 1988;333:370–373. doi: 10.1038/333370a0. [DOI] [PubMed] [Google Scholar]
- Braun L, Christophe T, Boulay F. Phosphorylation of key serine residues is required for internalization of the complement 5a (C5a) anaphylatoxin receptor via a beta-arrestin, dynamin, and clathrin-dependent pathway. J Biol Chem. 2003;278:4277–4285. doi: 10.1074/jbc.M210120200. [DOI] [PubMed] [Google Scholar]
- Budd DC, Willars GB, McDonald JE, Tobin AB. Phosphorylation of the Gq/11-coupled m3-muscarinic receptor is involved in receptor activation of the ERK-1/2 mitogen-activated protein kinase pathway. J Biol Chem. 2001;276:4581–4587. doi: 10.1074/jbc.M008827200. [DOI] [PubMed] [Google Scholar]
- Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature. 1999;401:286–290. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- Cen B, Xiong Y, Ma L, Pei G. Direct and differential interaction of beta-arrestins with the intracellular domains of different opioid receptors. Mol Pharmacol. 2001;59:758–764. doi: 10.1124/mol.59.4.758. [DOI] [PubMed] [Google Scholar]
- Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, et al. High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- Doronin S, Shumay E, Wang HY, Malbon CC. Akt mediates sequestration of the beta(2)-adrenergic receptor in response to insulin. J Biol Chem. 2002;277:15124–15131. doi: 10.1074/jbc.M108771200. [DOI] [PubMed] [Google Scholar]
- Eason MG, Moreira SP, Liggett SB. Four consecutive serines in the third intracellular loop are the sites for beta-adrenergic receptor kinase-mediated phosphorylation and desensitization of the alpha 2A-adrenergic receptor. J Biol Chem. 1995;270:4681–4688. doi: 10.1074/jbc.270.9.4681. [DOI] [PubMed] [Google Scholar]
- Fan G, Shumay E, Malbon CC, Wang H. c-Src tyrosine kinase binds the beta 2-adrenergic receptor via phospho-Tyr-350, phosphorylates G-protein-linked receptor kinase 2, and mediates agonist-induced receptor desensitization. J Biol Chem. 2001;276:13240–13247. doi: 10.1074/jbc.M011578200. [DOI] [PubMed] [Google Scholar]
- Feng Y, Davis NG. Akr1p and the type I casein kinases act prior to the ubiquitination step of yeast endocytosis: Akr1p is required for kinase localization to the plasma membrane. Mol Cell Biol. 2000;20:5350–5359. doi: 10.1128/mcb.20.14.5350-5359.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- Fredericks ZL, Pitcher JA, Lefkowitz RJ. Identification of the G protein-coupled receptor kinase phosphorylation sites in the human beta2-adrenergic receptor. J Biol Chem. 1996;271:13796–13803. doi: 10.1074/jbc.271.23.13796. [DOI] [PubMed] [Google Scholar]
- Friedman J, Babu B, Clark RB. Beta(2)-adrenergic receptor lacking the cyclic AMP-dependent protein kinase consensus sites fully activates extracellular signal-regulated kinase 1/2 in human embryonic kidney 293 cells: lack of evidence for G(s)/G(i) switching. Mol Pharmacol. 2002;62:1094–1102. doi: 10.1124/mol.62.5.1094. [DOI] [PubMed] [Google Scholar]
- Galliera E, Jala VR, Trent JO, Bonecchi R, Signorelli P, Lefkowitz RJ, et al. Beta-arrestin-dependent constitutive internalization of the human chemokine decoy receptor D6. J Biol Chem. 2004;279:25590–25597. doi: 10.1074/jbc.M400363200. [DOI] [PubMed] [Google Scholar]
- Gavi S, Shumay E, Wang HY, Malbon CC. G-protein-coupled receptors and tyrosine kinases: crossroads in cell signaling and regulation. Trends Endocrinol Metab. 2006;17:48–54. doi: 10.1016/j.tem.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Giannini E, Brouchon L, Boulay F. Identification of the major phosphorylation sites in human C5a anaphylatoxin receptor in vivo. J Biol Chem. 1995;270:19166–19172. doi: 10.1074/jbc.270.32.19166. [DOI] [PubMed] [Google Scholar]
- Guo J, Wu Y, Zhang W, Zhao J, Devi LA, Pei G, et al. Identification of G protein-coupled receptor kinase 2 phosphorylation sites responsible for agonist-stimulated delta-opioid receptor phosphorylation. Mol Pharmacol. 2000;58:1050–1056. doi: 10.1124/mol.58.5.1050. [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Benovic JL. Visual arrestin interaction with rhodopsin. Sequential multisite binding ensures strict selectivity toward light-activated phosphorylated rhodopsin. J Biol Chem. 1993;268:11628–11638. [PubMed] [Google Scholar]
- Gurevich VV, Dion SB, Onorato JJ, Ptasienski J, Kim CM, Sterne-Marr R, et al. Arrestin interactions with G protein-coupled receptors. Direct binding studies of wild type and mutant arrestins with rhodopsin, beta 2-adrenergic and m2 muscarinic cholinergic receptors. J Biol Chem. 1995;270:720–731. doi: 10.1074/jbc.270.2.720. [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacol Ther. 2006;110:465–502. doi: 10.1016/j.pharmthera.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RA, Ostedgaard LS, Premont RT, Blitzer JT, Rahman N, Welsh MJ, et al. A C-terminal motif found in the beta2-adrenergic receptor, P2Y1 receptor and cystic fibrosis transmembrane conductance regulator determines binding to the Na+/H+ exchanger regulatory factor family of PDZ proteins. Proc Natl Acad Sci USA. 1998;95:8496–8501. doi: 10.1073/pnas.95.15.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyaloglu AC, Vrecl M, Kroeger KM, Miles LE, Qian H, Thomas WG, et al. Casein kinase II sites in the intracellular C-terminal domain of the thyrotropin-releasing hormone receptor and chimeric gonadotropin-releasing hormone receptors contribute to beta-arrestin-dependent internalization. J Biol Chem. 2001;276:18066–18074. doi: 10.1074/jbc.M009275200. [DOI] [PubMed] [Google Scholar]
- Hausdorff WP, Bouvier M, O'Dowd BF, Irons GP, Caron MG, Lefkowitz RJ. Phosphorylation sites on two domains of the beta 2-adrenergic receptor are involved in distinct pathways of receptor desensitization. J Biol Chem. 1989;264:12657–12665. [PubMed] [Google Scholar]
- Hicke L, Zanolari B, Riezman H. Cytoplasmic tail phosphorylation of the alpha-factor receptor is required for its ubiquitination and internalization. J Cell Biol. 1998;141:349–358. doi: 10.1083/jcb.141.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JA, Schubert C, Gurevich VV, Sigler PB. The 2.8 Å crystal structure of visual arrestin: a model for arrestin's regulation. Cell. 1999;97:257–269. doi: 10.1016/s0092-8674(00)80735-7. [DOI] [PubMed] [Google Scholar]
- Jala VR, Shao WH, Haribabu B. Phosphorylation-independent beta-arrestin translocation and internalization of leukotriene B4 receptors. J Biol Chem. 2005;280:4880–4887. doi: 10.1074/jbc.M409821200. [DOI] [PubMed] [Google Scholar]
- Karoor V, Malbon CC. Insulin-like growth factor receptor-1 stimulates phosphorylation of the beta2-adrenergic receptor in vivo on sites distinct from those phosphorylated in response to insulin. J Biol Chem. 1996;271:29347–29352. doi: 10.1074/jbc.271.46.29347. [DOI] [PubMed] [Google Scholar]
- Karoor V, Wang L, Wang HY, Malbon CC. Insulin stimulates sequestration of beta-adrenergic receptors and enhanced association of beta-adrenergic receptors with Grb2 via tyrosine 350. J Biol Chem. 1998;273:33035–33041. doi: 10.1074/jbc.273.49.33035. [DOI] [PubMed] [Google Scholar]
- Kim J, Ahn S, Ren XR, Whalen EJ, Reiter E, Wei H, et al. Functional antagonism of different G protein-coupled receptor kinases for beta-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci USA. 2005;102:1442–1447. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KM, Valenzano KJ, Robinson SR, Yao WD, Barak LS, Caron MG. Differential regulation of the dopamine D2 and D3 receptors by G protein-coupled receptor kinases and beta-arrestins. J Biol Chem. 2001;276:37409–37414. doi: 10.1074/jbc.M106728200. [DOI] [PubMed] [Google Scholar]
- Kishi H, Krishnamurthy H, Galet C, Bhaskaran RS, Ascoli M. Identification of a short linear sequence present in the C-terminal tail of the rat follitropin receptor that modulates arrestin-3 binding in a phosphorylation-independent fashion. J Biol Chem. 2002;277:21939–21946. doi: 10.1074/jbc.M110894200. [DOI] [PubMed] [Google Scholar]
- Kobilka BK. Agonist-induced conformational changes in the beta2 adrenergic receptor. J Pept Res. 2002;60:317–321. doi: 10.1034/j.1399-3011.2002.21062.x. [DOI] [PubMed] [Google Scholar]
- Kouhen OM, Wang G, Solberg J, Erickson LJ, Law PY, Loh HH. Hierarchical phosphorylation of delta-opioid receptor regulates agonist-induced receptor desensitization and internalization. J Biol Chem. 2000;275:36659–36664. doi: 10.1074/jbc.M006788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovoor A, Celver J, Abdryashitov RI, Chavkin C, Gurevich VV. Targeted construction of phosphorylation-independent beta-arrestin mutants with constitutive activity in cells. J Biol Chem. 1999;274:6831–6834. doi: 10.1074/jbc.274.11.6831. [DOI] [PubMed] [Google Scholar]
- Krasel C, Bunemann M, Lorenz K, Lohse MJ. Beta-arrestin binding to the beta2-adrenergic receptor requires both receptor phosphorylation and receptor activation. J Biol Chem. 2005;280:9528–9535. doi: 10.1074/jbc.M413078200. [DOI] [PubMed] [Google Scholar]
- Kreienkamp HJ. Organisation of G-protein-coupled receptor signalling complexes by scaffolding proteins. Curr Opin Pharmacol. 2002;2:581–586. doi: 10.1016/s1471-4892(02)00203-5. [DOI] [PubMed] [Google Scholar]
- Kristiansen K. Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol Ther. 2004;103:21–80. doi: 10.1016/j.pharmthera.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- Lee KB, Ptasienski JA, Bunemann M, Hosey MM. Acidic amino acids flanking phosphorylation sites in the M2 muscarinic receptor regulate receptor phosphorylation, internalization, and interaction with arrestins. J Biol Chem. 2000a;275:35767–35777. doi: 10.1074/jbc.M002225200. [DOI] [PubMed] [Google Scholar]
- Lee KB, Ptasienski JA, Pals-Rylaarsdam R, Gurevich VV, Hosey MM. Arrestin binding to the M(2) muscarinic acetylcholine receptor is precluded by an inhibitory element in the third intracellular loop of the receptor. J Biol Chem. 2000b;275:9284–9289. doi: 10.1074/jbc.275.13.9284. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Thangada S, Paik JH, Sapkota GP, Ancellin N, Chae SS, et al. Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol Cell. 2001;8:693–704. doi: 10.1016/s1097-2765(01)00324-0. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J Biol Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ. Historical review: a brief history and personal retrospective of seven-transmembrane receptors. Trends Pharmacol Sci. 2004;25:413–422. doi: 10.1016/j.tips.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Pierce KL, Luttrell LM. Dancing with different partners: protein kinase a phosphorylation of seven membrane-spanning receptors regulates their G protein-coupling specificity. Mol Pharmacol. 2002;62:971–974. doi: 10.1124/mol.62.5.971. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Whalen EJ. Beta-arrestins: traffic cops of cell signaling. Curr Opin Cell Biol. 2004;16:162–168. doi: 10.1016/j.ceb.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Liu P, Roush ED, Bruno J, Osawa S, Weiss ER. Direct binding of visual arrestin to a rhodopsin carboxyl terminal synthetic phosphopeptide. Mol Vis. 2004;10:712–719. [PubMed] [Google Scholar]
- Lohse MJ. Molecular mechanisms of membrane receptor desensitization. Biochim Biophys Acta. 1993;1179:171–188. doi: 10.1016/0167-4889(93)90139-g. [DOI] [PubMed] [Google Scholar]
- Lohse MJ, Andexinger S, Pitcher J, Trukawinski S, Codina J, Faure JP, et al. Receptor-specific desensitization with purified proteins. Kinase dependence and receptor specificity of beta-arrestin and arrestin in the beta 2-adrenergic receptor and rhodopsin systems. J Biol Chem. 1992;267:8558–8564. [PubMed] [Google Scholar]
- Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci. 2002;115 Part 3:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- Malecz N, Bambino T, Bencsik M, Nissenson RA. Identification of phosphorylation sites in the G protein-coupled receptor for parathyroid hormone. Receptor phosphorylation is not required for agonist-induced internalization. Mol Endocrinol. 1998;12:1846–1856. doi: 10.1210/mend.12.12.0203. [DOI] [PubMed] [Google Scholar]
- Maudsley S, Martin B, Luttrell LM. The origins of diversity and specificity in G protein-coupled receptor signaling. J Pharmacol Exp Ther. 2005;314:485–494. doi: 10.1124/jpet.105.083121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez A, Burns ME, Roca A, Lem J, Wu LW, Simon MI, et al. Rapid and reproducible deactivation of rhodopsin requires multiple phosphorylation sites. Neuron. 2000;28:153–164. doi: 10.1016/s0896-6273(00)00093-3. [DOI] [PubMed] [Google Scholar]
- Milasta S, Evans NA, Ormiston L, Wilson S, Lefkowitz RJ, Milligan G. The sustainability of interactions between the orexin-1 receptor and beta-arrestin-2 is defined by a single C-terminal cluster of hydroxy amino acids and modulates the kinetics of ERK MAPK regulation. Biochem J. 2005;387 Part 3:573–584. doi: 10.1042/BJ20041745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milcent MD, Christophe T, Rabiet MJ, Tardif M, Boulay F. Overexpression of wild-type and catalytically inactive forms of GRK2 and GRK6 fails to alter the agonist-induced phosphorylation of the C5a receptor (CD88): evidence that GRK6 is autophosphorylated in COS-7 cells. Biochem Biophys Res Commun. 1999;259:224–229. doi: 10.1006/bbrc.1999.0758. [DOI] [PubMed] [Google Scholar]
- Milligan G, White JH. Protein–protein interactions at G-protein-coupled receptors. Trends Pharmacol Sci. 2001;22:513–518. doi: 10.1016/s0165-6147(00)01801-0. [DOI] [PubMed] [Google Scholar]
- Moffett S, Adam L, Bonin H, Loisel TP, Bouvier M, Mouillac B. Palmitoylated cysteine 341 modulates phosphorylation of the beta2-adrenergic receptor by the cAMP-dependent protein kinase. J Biol Chem. 1996;271:21490–21497. doi: 10.1074/jbc.271.35.21490. [DOI] [PubMed] [Google Scholar]
- Mou L, Gates A, Mosser VA, Tobin A, Jackson DA. Transient hypoxia induces sequestration of M1 and M2 muscarinic acetylcholine receptors. J Neurochem. 2006;96:510–519. doi: 10.1111/j.1471-4159.2005.03571.x. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Gurevich VV, Preninger A, Hamm HE, Bader MF, Fazleabas AT, et al. Aspartic acid 564 in the third cytoplasmic loop of the luteinizing hormone/choriogonadotropin receptor is crucial for phosphorylation-independent interaction with arrestin2. J Biol Chem. 2002;277:17916–17927. doi: 10.1074/jbc.M110479200. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Hipkin RW, Ascoli M. The agonist-induced phosphorylation of the rat follitropin receptor maps to the first and third intracellular loops. Mol Endocrinol. 1998;12:580–591. doi: 10.1210/mend.12.4.0087. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Association of beta-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem. 1999;274:32248–32257. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- Ohguro H, Palczewski K, Ericsson LH, Walsh KA, Johnson RS. Sequential phosphorylation of rhodopsin at multiple sites. Biochemistry. 1993;32:5718–5724. doi: 10.1021/bi00072a030. [DOI] [PubMed] [Google Scholar]
- Ohguro H, Rudnicka-Nawrot M, Buczylko J, Zhao X, Taylor JA, Walsh KA, et al. Structural and enzymatic aspects of rhodopsin phosphorylation. J Biol Chem. 1996;271:5215–5224. doi: 10.1074/jbc.271.9.5215. [DOI] [PubMed] [Google Scholar]
- Oppermann M, Diverse-Pierluissi M, Drazner MH, Dyer SL, Freedman NJ, Peppel KC, et al. Monoclonal antibodies reveal receptor specificity among G-protein-coupled receptor kinases. Proc Natl Acad Sci USA. 1996;93:7649–7654. doi: 10.1073/pnas.93.15.7649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcelebi F, Miller LJ. Phosphopeptide mapping of cholecystokinin receptors on agonist-stimulated native pancreatic acinar cells. J Biol Chem. 1995;270:3435–3441. doi: 10.1074/jbc.270.7.3435. [DOI] [PubMed] [Google Scholar]
- Pals-Rylaarsdam R, Hosey MM. Two homologous phosphorylation domains differentially contribute to desensitization and internalization of the m2 muscarinic acetylcholine receptor. J Biol Chem. 1997;272:14152–14158. doi: 10.1074/jbc.272.22.14152. [DOI] [PubMed] [Google Scholar]
- Panek HR, Stepp JD, Engle HM, Marks KM, Tan PK, Lemmon SK, et al. Suppressors of YCK-encoded yeast casein kinase 1 deficiency define the four subunits of a novel clathrin AP-like complex. EMBO J. 1997;16:4194–4204. doi: 10.1093/emboj/16.14.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papac DI, Oatis JE, Jr, Crouch RK, Knapp DR. Mass spectrometric identification of phosphorylation sites in bleached bovine rhodopsin. Biochemistry. 1993;32:5930–5934. doi: 10.1021/bi00074a002. [DOI] [PubMed] [Google Scholar]
- Paxton WG, Marrero MB, Klein JD, Delafontaine P, Berk BC, Bernstein KE. The angiotensin II AT1 receptor is tyrosine and serine phosphorylated and can serve as a substrate for the src family of tyrosine kinases. Biochem Biophys Res Commun. 1994;200:260–267. doi: 10.1006/bbrc.1994.1443. [DOI] [PubMed] [Google Scholar]
- Perry SJ, Baillie GS, Kohout TA, McPhee I, Magiera MM, Ang KL, et al. Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science. 2002;298:834–836. doi: 10.1126/science.1074683. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Kim CM, Benovic JL, Ye RD. Phosphorylation of the N-formyl peptide receptor carboxyl terminus by the G protein-coupled receptor kinase, GRK2. J Biol Chem. 1995;270:1130–1137. doi: 10.1074/jbc.270.3.1130. [DOI] [PubMed] [Google Scholar]
- Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, et al. Crystal structure of the human β2 adrenergic G-protein-coupled receptor. Nature. 2007;450:383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- Ren XR, Reiter E, Ahn S, Kim J, Chen W, Lefkowitz RJ. Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci USA. 2005;102:1448–1453. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MD, Balius AM, Yamaguchi K, Freilich ER, Barak LS, Kwatra MM. Human substance P receptor lacking the C-terminal domain remains competent to desensitize and internalize. J Neurochem. 2003;84:854–863. doi: 10.1046/j.1471-4159.2003.01577.x. [DOI] [PubMed] [Google Scholar]
- Roth AF, Davis NG. Ubiquitination of the PEST-like endocytosis signal of the yeast a-factor receptor. J Biol Chem. 2000;275:8143–8153. doi: 10.1074/jbc.275.11.8143. [DOI] [PubMed] [Google Scholar]
- Roth NS, Campbell PT, Caron MG, Lefkowitz RJ, Lohse MJ. Comparative rates of desensitization of beta-adrenergic receptors by the beta-adrenergic receptor kinase and the cyclic AMP-dependent protein kinase. Proc Natl Acad Sci USA. 1991;88:6201–6204. doi: 10.1073/pnas.88.14.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Seibold A, Williams B, Huang ZF, Friedman J, Moore RH, Knoll BJ, et al. Localization of the sites mediating desensitization of the beta(2)-adrenergic receptor by the GRK pathway. Mol Pharmacol. 2000;58:1162–1173. doi: 10.1124/mol.58.5.1162. [DOI] [PubMed] [Google Scholar]
- Shumay E, Gavi S, Wang HY, Malbon CC. Trafficking of beta2-adrenergic receptors: insulin and beta-agonists regulate internalization by distinct cytoskeletal pathways. J Cell Sci. 2004;117 Part 4:593–600. doi: 10.1242/jcs.00890. [DOI] [PubMed] [Google Scholar]
- Smith RD, Hunyady L, Olivares-Reyes JA, Mihalik B, Jayadev S, Catt KJ. Agonist-induced phosphorylation of the angiotensin AT1a receptor is localized to a serine/threonine-rich region of its cytoplasmic tail. Mol Pharmacol. 1998;54:935–941. doi: 10.1124/mol.54.6.935. [DOI] [PubMed] [Google Scholar]
- Stalheim L, Ding Y, Gullapalli A, Paing MM, Wolfe BL, Morris DR, et al. Multiple independent functions of arrestins in the regulation of protease-activated receptor-2 signaling and trafficking. Mol Pharmacol. 2005;67:78–87. doi: 10.1124/mol.104.006072. [DOI] [PubMed] [Google Scholar]
- Tobin AB, Totty NF, Sterlin AE, Nahorski SR. Stimulus-dependent phosphorylation of G-protein-coupled receptors by casein kinase 1alpha. J Biol Chem. 1997;272:20844–20849. doi: 10.1074/jbc.272.33.20844. [DOI] [PubMed] [Google Scholar]
- Torrecilla I, Spragg EJ, Poulin B, McWilliams PJ, Mistry SC, Blaukat A, et al. Phosphorylation and regulation of a G protein-coupled receptor by protein kinase CK2. J Cell Biol. 2007;177:127–137. doi: 10.1083/jcb.200610018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TM, Friedman J, Qunaibi E, Baameur F, Moore RH, Clark RB. Characterization of agonist stimulation of cAMP-dependent protein kinase and G protein-coupled receptor kinase phosphorylation of the beta2-adrenergic receptor using phosphoserine-specific antibodies. Mol Pharmacol. 2004;65:196–206. doi: 10.1124/mol.65.1.196. [DOI] [PubMed] [Google Scholar]
- Trester-Zedlitz M, Burlingame A, Kobilka B, von Zastrow M. Mass spectrometric analysis of agonist effects on posttranslational modifications of the beta-2 adrenoceptor in mammalian cells. Biochemistry. 2005;44:6133–6143. doi: 10.1021/bi0475469. [DOI] [PubMed] [Google Scholar]
- Violin JD, Ren XR, Lefkowitz RJ. G-protein-coupled receptor kinase specificity for beta-arrestin recruitment to the beta2-adrenergic receptor revealed by fluorescence resonance energy transfer. J Biol Chem. 2006;281:20577–20588. doi: 10.1074/jbc.M513605200. [DOI] [PubMed] [Google Scholar]
- Vishnivetskiy SA, Hirsch JA, Velez MG, Gurevich YV, Gurevich VV. Transition of arrestin into the active receptor-binding state requires an extended interdomain hinge. J Biol Chem. 2002;277:43961–43967. doi: 10.1074/jbc.M206951200. [DOI] [PubMed] [Google Scholar]
- Vishnivetskiy SA, Hosey MM, Benovic JL, Gurevich VV. Mapping the arrestin-receptor interface. Structural elements responsible for receptor specificity of arrestin proteins. J Biol Chem. 2004;279:1262–1268. doi: 10.1074/jbc.M308834200. [DOI] [PubMed] [Google Scholar]
- Vishnivetskiy SA, Paz CL, Schubert C, Hirsch JA, Sigler PB, Gurevich VV. How does arrestin respond to the phosphorylated state of rhodopsin. J Biol Chem. 1999;274:11451–11454. doi: 10.1074/jbc.274.17.11451. [DOI] [PubMed] [Google Scholar]
- Wang H, Doronin S, Malbon CC. Insulin activation of mitogen-activated protein kinases Erk1,2 is amplified via beta-adrenergic receptor expression and requires the integrity of the Tyr350 of the receptor. J Biol Chem. 2000;275:36086–36093. doi: 10.1074/jbc.M004404200. [DOI] [PubMed] [Google Scholar]
- Waugh MG, Challiss RA, Berstein G, Nahorski SR, Tobin AB. Agonist-induced desensitization and phosphorylation of m1-muscarinic receptors. Biochem J. 1999;338 Part 1:175–183. [PMC free article] [PubMed] [Google Scholar]
- Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM, et al. Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci USA. 2003;100:10782–10787. doi: 10.1073/pnas.1834556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistler JL, Tsao P, von Zastrow M. A phosphorylation-regulated brake mechanism controls the initial endocytosis of opioid receptors but is not required for post-endocytic sorting to lysosomes. J Biol Chem. 2001;276:34331–34338. doi: 10.1074/jbc.M104627200. [DOI] [PubMed] [Google Scholar]
- White MF, Kahn CR. The insulin signaling system. J Biol Chem. 1994;269:1–4. [PubMed] [Google Scholar]
- Wilden U, Kuhn H. Light-dependent phosphorylation of rhodopsin: number of phosphorylation sites. Biochemistry. 1982;21:3014–3022. doi: 10.1021/bi00541a032. [DOI] [PubMed] [Google Scholar]
- Willets JM, Challiss RA, Nahorski SR. Endogenous G protein-coupled receptor kinase 6 regulates M3 muscarinic acetylcholine receptor phosphorylation and desensitization in human SH-SY5Y neuroblastoma cells. J Biol Chem. 2002;277:15523–15529. doi: 10.1074/jbc.M111217200. [DOI] [PubMed] [Google Scholar]
- Willets JM, Mistry R, Nahorski SR, Challiss RA. Specificity of G protein-coupled receptor kinase 6-mediated phosphorylation and regulation of single-cell m3 muscarinic acetylcholine receptor signaling. Mol Pharmacol. 2003;64:1059–1068. doi: 10.1124/mol.64.5.1059. [DOI] [PubMed] [Google Scholar]
- Wu G, Krupnick JG, Benovic JL, Lanier SM. Interaction of arrestins with intracellular domains of muscarinic and alpha2-adrenergic receptors. J Biol Chem. 1997;272:17836–17842. doi: 10.1074/jbc.272.28.17836. [DOI] [PubMed] [Google Scholar]
- Yuan N, Friedman J, Whaley BS, Clark RB. cAMP-dependent protein kinase and protein kinase C consensus site mutations of the beta-adrenergic receptor. Effect on desensitization and stimulation of adenylylcyclase. J Biol Chem. 1994;269:23032–23038. [PubMed] [Google Scholar]