Abstract
The A2A-adenosine receptor is a prototypical Gs-coupled receptor. However, the A2A-receptor has several structural and functional characteristics that make it unique. In contrast to the classical model of collision coupling described for the β-adrenergic receptors, the A2A-receptor couples to adenylyl cyclase by restricted collision coupling and forms a tight complex with Gs. The mechanistic basis for this is not clear; restricted collision coupling may arise from the interaction of the receptor with additional proteins or due to the fact that G protein-coupling is confined to specialized membrane microdomains. The A2A-receptor has a long C-terminus (of >120 residues), which is for the most part dispensable for coupling to Gs. It was originally viewed as the docking site for kinases and the β-arrestin family to initiate receptor desensitization and endocytosis. The A2A-receptor is, however, fairly resistant to agonist-induced internalization. Recently, the C-terminus has also been appreciated as a binding site for several additional ‘accessory' proteins. Established interaction partners include α-actinin, ARNO, USP4 and translin-associated protein-X. In addition, the A2A-receptor has also been reported to form a heteromeric complex with the D2-dopamine receptor and the metabotropic glutamate receptor-5. It is clear that (i) this list cannot be exhaustive and (ii) that all these proteins cannot bind simultaneously to the receptor. There must be rules of engagement, which allow the receptor to elicit different biological responses, which depend on the cellular context and the nature of the concomitant signal(s). Thus, the receptor may function as a coincidence detector and a signal integrator.
Keywords: A2A-adenosine receptor, ARNO, MAP kinase, USP4, restricted collision coupling
Adenosine receptor classification
G-protein-coupled receptors (GPCRs) for adenosine, which either inhibited (A1) or stimulated (A2) cAMP accumulation, were first identified in the 1970s (van Calker et al., 1979) and differentiated—by structure–activity relationship and other pharmacological criteria—from the direct action of adenosine at the catalytic centre of AC, the so-called P-site (Londos et al., 1980). The further subclassification of A2-adenosine receptors was introduced later to account for the receptor that required low concentrations of adenosine and adenosine analogues for the stimulation of AC; this was termed the A2A receptor (Bruns et al., 1986). The A2A receptor has one additional close relative the A2B receptor, which also signals via Gs, and two more distant relatives termed A1- and A3-adenosine receptors, which interact with pertussis-toxin-sensitive G proteins of the Gi and Go family. These receptors are encoded by distinct genes and are classified based on their affinities for adenosine analogues and methylxanthine antagonists (Klinger et al., 2002a).
Interest in the A2A receptor has been rekindled by findings that suggest that both agonism at this receptor and its inhibition by specific antagonists may be of therapeutic relevance. Examples are highlighted in the accompanying reviews (Brown, 2008; Sitkovsky, 2008). The A2A-adenosine receptor, however, is also of interest, because it has many features that do not readily fit into the accepted paradigms of GPCR signalling and desensitization; in addition, the receptor binds many proteins other than Gs. This review will focus on these three points.
Precoupling, tight coupling and restricted collision coupling
In contrast to β-adrenergic receptors (or rhodopsin), which engage their signalling cascade by collision coupling, the A2A receptor has long been known to couple to AC by restricted collision coupling. Restricted collision coupling refers to the inability of the receptor to access all Gs molecules and thus activate the full complement of all AC moieties in the cell membrane. Originally, these observations were made with the avian A2 receptor in turkey erythrocytes (Tolkovsky and Levitzki, 1978; Braun and Levitzki, 1979). Restricted collision coupling, however, was also observed in human platelets, which express A2A receptors (Gross and Lohse, 1991; Lohse et al., 1991). The A2A receptor forms a tight complex with Gs; this complex is resistant to dissociation by guanine nucleotides. In addition, the receptor can be solubilized in a complex with Gs in the absence of agonist (Nanoff et al., 1991; Nanoff and Stiles, 1993). Thus, the A2A receptor appears to be precoupled to Gs. Precoupling, that is, formation of a stable complex between receptor and G protein, by definition, gives rise to restricted collision coupling.
It is also worth noting that, when measured directly by fluorescence/Foerster resonance energy transfer (FRET), the kinetics of G protein activation do not differ substantially between β1-adrenergic receptor and A2A-adenosine receptor (Hein et al., 2006). These experiments, however, cannot decide on the existence or absence of precoupling. First, in the ground state (that is, in the absence of agonist), A2A receptor and Gs may be in a complex that does not support efficient FRET. Agonist binding reorients both receptor and Gs into a tightly packed complex, in which energy transfer is supported. Second, when compared to the time constants required for G protein activation (and formation of the high-affinity complex of agonist, receptor and G protein), diffusion of the receptor does not appear to be rate limiting; ternary complexes formed by fusion proteins of receptor and α-subunits of G protein are kinetically indistinguishable from those formed by the individual components (Waldhoer et al., 1999). Although precoupling of the A2A receptor to Gs may provide a mechanistic basis for restricted collision coupling, it remains unclear as to why the A2A receptor would form a tight complex with Gs. Earlier reconstitution experiments did not suggest that the A2A receptor (Nanoff et al., 1994) had a substantially higher affinity for Gs than the β1- or β2-adrenergic receptors (Freissmuth et al., 1991). The formation of a tight receptor/Gs complex and the restricted mobility of the A2A receptor may, however, arise from the interaction with additional proteins (see below); the receptor may, for instance, be concentrated in membrane subdomains, because it is tethered to the actin cytoskeleton via its binding partner α-actinin (Burgueño et al., 2003). Alternatively, the interaction between A2A-adenosine receptor and Gs may be limited to membrane microdomains (‘lipid rafts'). We have examined these conjectures and have observed that the actin cytoskeleton did not account for the restricted mobility of the A2A receptor but that coupling of the A2A receptor to Gs was contingent on the presence of cholesterol. In contrast, activation of an alternative signalling pathway (namely ARNO (ARF-nucleotide binding site opener), the guanine nucleotide exchange factor for ADP-ribosylation factor (ARF)6; Gsandtner et al., 2005) was not impaired by removal of cholesterol (Charalambous et al., manuscript submitted).
The other issue is why should have receptors evolved to differ in their coupling modes, that is, collision coupling versus restricted collision coupling. This question could simply be considered an academic preoccupation of little relevance. However, it is evident that collision coupling and restricted collision coupling confer different outcomes to adaptive changes; if a receptor operates by collision coupling, increasing the number of receptors primarily augments the sensitivity of the system. In other words, the concentration–response curve for agonists is shifted to the left, because all receptors have unrestricted access to all G proteins. In restricted collision coupling, where the receptor is allocated its particular lot of G proteins, there are no spare receptors. Changes in receptor number, therefore, translate in changes of the maximum response Emax rather than in EC50 values. Actually, this is precisely the finding that was obtained when the number of A2A receptors was decreased by inactivation (Lohse et al., 1991) or raised by facilitating its export from the endoplasmic reticulum (Milojevic et al., 2006).
Desensitization and internalization of the A2A receptor
For GPCRs, desensitization and receptor trafficking is important for regulation of the temporal and spatial aspects of their signalling properties. Both desensitization and internalization function to control signal termination and rely on the intracellular binding of GPCR kinases (GRKs) and arrestins. The basic mechanisms underlying desensitization of GPCRs is understood in considerable detail. The major insights have originally been obtained with two model GPCRs, rhodopsin and the β2-adrenergic receptor; the agonist stabilizes the active conformation of the receptor, which is recognized by GRKs (GRK2–6, with GRK1 being the specialized rhodopsin kinase). Phosphorylation by GRKs of clustered serine/threonine residues creates docking sites that allow the recruitment of arrestins. The general arrestins, arrestin 2 and arrestin 3, are also referred to as β-arrestin 1 and β-arrestin 2, whereas cone and rod arrestins are confined to the visual system. Binding of arrestin 2 and arrestin 3 via its N-terminal phosphate sensor triggers a structural rearrangement that exposes their C terminus, which recruits components of the clathrin coat. Accordingly, binding of arrestins favours endocytosis of receptors via the clathrin-dependent pathway (reviewed by Gurevich and Gurevich, 2006). It is a matter of debate whether the A2A receptor undergoes a desensitization/resensitization cycle under physiological conditions; short-term desensitization can be observed in various cell culture models (reviewed by Olah and Stiles, 2000). The structural requirements of the receptor that may be necessary for the rapid desensitization have been examined in receptor mutagenesis studies in chinese hamster ovary cells (Palmer and Stiles, 1997). The removal of 95 amino acids, including 10 potential phosphorylation sites, from the cytoplasmic tail of the A2A receptor had no effect on the ability of the receptor to undergo rapid desensitization. Although a simultaneous mutation of Thr298 and Ser305 to Ala residues attenuated the desensitization observed in response to short-term agonist treatment, it did not block the ability to desensitize after long-term agonist exposure. Analysis of the individual mutation of these residues revealed that mutation of Thr298 alone was sufficient to diminish both short-term desensitization and agonist-stimulated receptor phosphorylation. This is remarkable because efficient recruitment of arrestins typically requires a cluster of phosphates, which interact with the N-terminal phosphate sensor and thereby trigger the structural rearrangement that leads to the tight interaction between arrestin and receptor (Gurevich and Gurevich, 2006). This issue may be explained by the following two alternative scenarios: (i) negatively charged amino acids in the vicinity of Thr298 may substitute for phosphoserines/phosphothreonines and thus obviate the requirement for multiple phosphorylated residues for arrestin recruitment or (ii) phosphorylation of Thr298 may induce a conformational change, which, per se, suffices to disrupt G protein coupling; in this scenario, recruitment of arrestin would be dispensable. A precedent for this latter model is provided by the observation that PK-A-mediated desensitization of the β2-adrenergic receptor is apparently not dependent on arrestin recruitment (Vaughan et al., 2006).
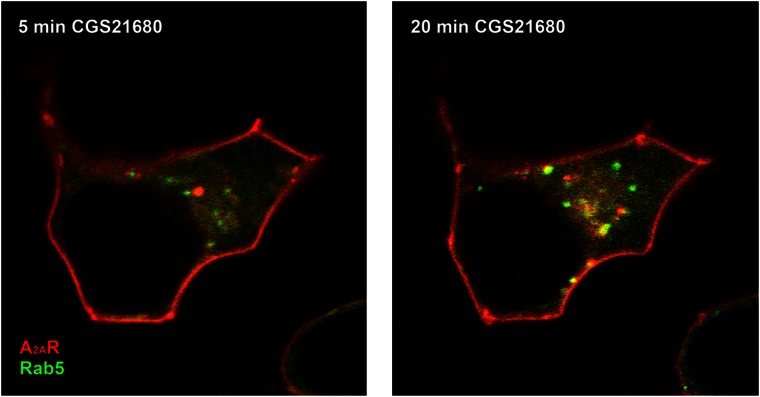
Manipulations in the level of GRK2 affect the extent of desensitization of the A2A receptor (Mundell et al., 1997, 1998). However, the relevance of these observations has been questioned for the following reasons: (i) adenosine appears to signal tonically via the A2A receptor; in vivo, many actions of adenosine are accounted for by the retaliatory metabolite concept that hypoxia and tissue damage lead to the accumulation and release of adenosine; adenosine acts via its receptors to orchestrate a concerted protective response, where the A2A receptor plays a prominent role (Linden, 2005). From a teleological perspective, rapid desensitization is of questionable value in a situation where sustained signalling is vital to support long-term tissue repair. (ii) In most instances, it has been difficult to visualize internalization in vivo (Fredholm et al., 2001). A representative experiment is shown in Figure 1, where the yellow fluorescent protein-labelled A2A receptor was visualized together with cyan fluorescent protein-tagged Rab5 (a marker for early endocytotic vesicles). Even continuous administration of agonist did not result in any appreciable increase in basal A2A receptor endocytosis. There are, however, reports that suggest that rapid internalization upon agonist binding can occur via an interaction with the actin-binding protein α-actinin (Burgueño et al., 2003). α-Actinin is a component of the actin cytoskeleton that plays a central role by directly crosslinking actin molecules; so the presence of a complex involving A2A receptor and α-actinin suggests that α-actinin may mediate receptor association with the actin cytoskeleton. The precise regulatory role of this interaction remains to be established.
Figure 1.
Human embryonic kidney cells were transiently transfected with plasmids coding for the fluorescence-tagged versions of the A2A receptor and Rab5. Cells were then stimulated with the A2A-specific agonist CGS2168. Images were taken at the indicated times using a confocal laser microscope. For better visual distinction in the overlay, fluorescent A2A receptors were converted to red and Rab5 to green.
Sustained G-protein-independent signalling via ARNO
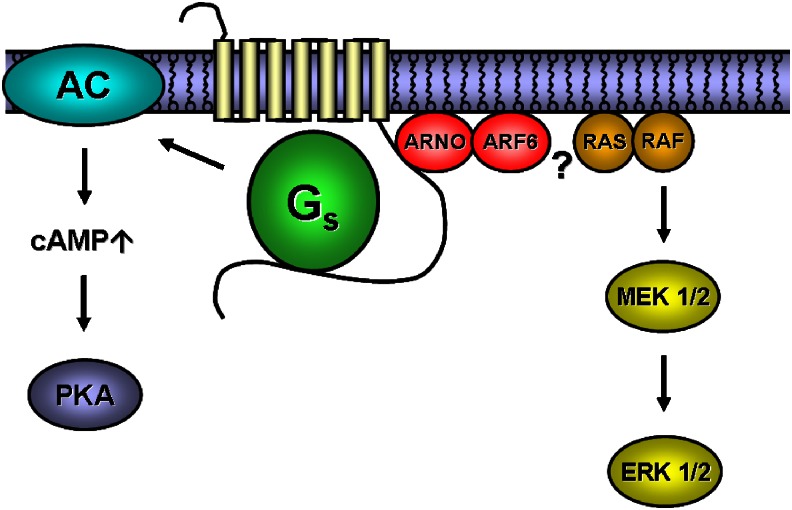
The action of GRKs on agonist-liganded receptors and the subsequent binding of arrestins to the receptors are necessary to initiate desensitization and a second wave of long-term signalling events, the persistent stimulation of mitogen-activated protein kinase (MAP kinase) being a particularly well-studied example (Lefkowitz and Shenoy, 2005). The A2A-adenosine receptor was first shown to cause a Gs-independent sustained stimulation of MAP kinase in human macrovascular endothelial cells (Sexl et al., 1997), which is presumably related to its capacity to support endothelial cell proliferation (Sexl et al., 1995). It was later appreciated that depending on the cell type studied, there was a Gs- and cAMP-dependent mechanism that supported MAP kinase activation, for example, in PC12 cells (Seidel et al., 1999) and that required src-like kinases (Klinger et al., 2002b), as well as a Gs-independent pathway that required p21ras (Seidel et al., 1999). Heterotrimeric G proteins were, in fact, dispensable for the latter signal, as was dynamin-dependent internalization (Klinger et al., 2002c). Clearly, based on these findings, it is safe to conclude that the A2A receptor does not conform to the paradigm established for the β2-adrenergic receptor. More recently, the Gs-independent signalling pathway was found to comprise ARNO and ARF6 (schematically represented in Figure 2); ARNO/cytohesin-2 was identified as an interactor that bound to the C terminus of the A2A receptor in an yeast two-hybrid screen (Gsandtner et al., 2005).
Figure 2.
Schematic illustration of the signalling pathways of the A2A-adenosine receptor. The link between ARF6 and Ras remains to be elucidated.
ARNO contains a sec7 domain, which acts as the guanine nucleotide exchange factor for ARF-family members. These small G proteins have been recently recognized to play a prominent role in endocytosis of GPCRs; many but not all receptors require ARF6 (originally identified as the cofactor necessary for cholera-toxin-mediated ADP-ribosylation of Gαs) for internalization (Lahuna et al., 2005; Houndolo et al., 2005). ARF6 differs from the other members of the ARF family because it is primarily found at the cell membrane, where it regulates endocytosis through both the clathrin/dynamin-dependent and a less well-understood clathrin/caveolae-independent pathway. Two mechanistically conflicting models have been proposed, both involving the exchange factor ARNO. During internalization of the β2-adrenergic receptor, arrestin binding precedes that of ARNO; in fact, ARNO is recruited by arrestin (Claing et al., 2001; Lefkowitz and Shenoy, 2005). In contrast, for the luteinizing hormone/human chorionic gonadotropin receptor, arrestin binding is contingent on prior stimulation of ARNO, the ensuing activation of ARF6 required for the release of membrane-associated arrestin (Hunzicker-Dunn et al., 2002). The A2A receptor differs from these two paradigmatic examples because it binds ARNO/cytohesin-2 directly (Gsandtner et al., 2005). Truncation experiments showed that the proximal 22 amino acids of the C terminus are sufficient for the interaction (highlighted in Figure 3). The classical pathway, activation of AC, was not altered by ARNO, and this was also true for agonist-induced desensitization. In contrast, expression of dominant-negative ARNO and dominant-negative ARF6 abrogated the sustained phase of MAP-kinase stimulation induced by the A2A receptor. Thus, the binding of ARNO to the proximal portion of the C terminus provides one of the missing links to support the alternative—heterotrimeric G-protein-independent—signalling pathway of A2A receptor.
Figure 3.
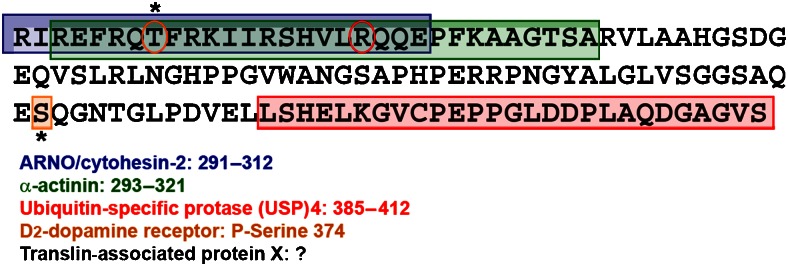
Scheme of interaction sites on the C terminus of the A2A-adenosine receptor. Binding sites for α-actinin, ARNO, the D2 receptor and USP4 have been tentatively assigned in Burgueño et al. (2003), Gsandtner et al. (2005), Woods and Ferré (2005) and Milojevic et al. (2006), respectively. The orange circle identifies a threonine important for short-term desensitization (Palmer and Stiles, 1997). The red circle depicts the arginine unique to the A2A receptor. All other adenosine receptors have a cysteine in this position, which in most GPCRs is palmitoylated and thus stabilizes helix 8.
Accessory proteins
In the last 15 years, it has been appreciated that GPCRs bind many additional proteins; these are being referred to as ‘accessory proteins' for lack of a better description. The list includes proteins involved in selective retention in specialized membrane compartments (for example, dendritic spines) or components of signalling cascades (for example, PKs of the Src and Jak family, adapter proteins like Grb2 and Shc, and enzymes generating second messengers such as PLCβ1 and eNOS; for a detailed list, see Bockaert et al., 2004). The A2A-adenosine receptor can also claim a respectable list of accessory proteins (Gsandtner and Freissmuth, 2006) that bind to various portions of its C terminus (Figure 3). In addition, A2A receptors, like other GPCRs, can form a heteromeric complex with at least one GPCR, namely, the D2-dopamine receptor (see below), and it can transactivate the neurotrophin receptors TrkA and TrkB (Lee and Chao, 2001). The term transactivation of TK has been coined by analogy to its original use in the description of gene regulation: GPCRs can act in trans, that is, recruit signalling components by activation of TK receptors in the absence of cognate ligands of the latter (in this instance, in the absence of nerve growth factor and related neurotrophins). The molecular basis of transactivation is poorly understood, and it is specifically not clear whether the A2A receptor forms a heteromeric complex with TrkA and/or TrkB.
Interaction of the A2A-adenosine receptor with other GPCRs (D2-dopamine receptor, mGluR5)
The first indirect evidence for a membrane-delimited interaction between the A2A receptor and the D2-dopamine receptor came from binding experiments that suggested that activation of the A2A receptor interfered with coupling of the D2-dopamine receptor to its cognate G proteins, presumably a mixture of Gi and Go isoforms (Ferré et al., 1991). Confocal laser microscopy showed that A2A receptors and D2-dopamine receptors colocalized to a large extent in the cell membranes of stably transfected neuroblastoma cells and in cultured striatal neurons; in addition, the existence of heteromeric complexes between these two receptors was confirmed in co-immunoprecipitation experiments (Hillion et al., 2002). Evidence for a direct and specific interaction between A2A and D2 receptors was also obtained with a quantitative bioluminescence resonance energy transfer analysis and sensitized emission FRET as well as acceptor photobleaching FRET analysis (Canals et al., 2003; Kamiya et al., 2003). This phenomenon may therefore constitute the molecular basis of the A2A/D2 receptor interactions, which give rise to mutual antagonism in vitro and in vivo (Fuxe et al., 2005, 2007). These findings led to the idea that blockade of the A2A receptor could be of value in the treatment of Parkinson's disease. In fact, istradefylline, a selective A2A receptor antagonist has shown (modest) efficacy in phase II trials (Hauser et al., 2003), which were considered promising enough to justify phase III clinical trials (Schapira et al., 2006); related selective antagonists are also being tested (Schapira et al., 2006). The metabotropic glutamate receptor 5 (mGluR5) has also been suggested to exist in a complex with the A2A receptor in the striatopallidal neurons (Ferré et al., 2002). This is thought to explain the complex interaction of mGluR5 and A2A receptor ligands; the beneficial action of an mGluR5 antagonist is enhanced by blockage of A2A receptor, but it is nevertheless contingent on the presence of A2A receptors (and D2 receptors) (Kachroo et al., 2005). It has to be stressed that the interaction of D2 and A2A receptors does not uniformly result in mutual antagonism. Under appropriate conditions, the two receptors can also signal synergistically (Kudlacek et al., 2003), and this has been proposed to be important for enhanced rewarding action of adenosine in the nucleus accumbens (Yao et al., 2002). Finally, heterodimerization may affect the kinetics of desensitization; this has, for instance, been documented for the trafficking pattern of V1a- and V2-vasopressin receptors (Terrillon et al., 2004). In fact, the A2A receptor has been shown to co-internalize with the D2-dopamine receptor (Hillion et al., 2002), suggesting that the D2-dopamine receptor confers a classical GRK/arrestin-dependent internalization mechanism to the A2A-adenosine receptor.
Interactors that bind to the C terminus of the A2A receptor
In contrast to many other GPCRs and, in particular, to the other members of the adenosine receptor family, the A2A receptor has an unusually long intracellular C-terminal tail (122 amino acids in man compared with only 34 residues in the C terminus of the A1-adenosine receptor). The juxtamembrane segment immediately adjacent to the seventh transmembrane helix is required for proper folding of the receptor. The rest of the C terminus (100 amino acids) is dispensable for ligand binding (Piersen et al., 1994) and for G protein coupling (Klinger et al., 2002c). The vast majority of group I (rhodopsin-like) GPCRs carry one (or two) palmitoylated cysteine(s) within the proximal portion of their C terminus (typically, approximately 20 residues away from the end of the seventh transmembrane helix). The palmitate thioester is thought to act as an additional anchor. This stabilizes the proximal segment in an α-helical conformation (referred to as helix 8 in the rhodopsin structure, which is oriented in a manner parallel to the membrane and perpendicular to helix 7). The A2A receptor does not have any cysteine residue in the proximal segment; there is only a single cysteine in position 394 in the human receptor and this is absent in other species orthologues (for example, of rat and mouse). Instead of the canonical cysteine, the A2A receptor carries an arginine residue in position 309 (circled in Figure 3). Thus, one is tempted to speculate that the C terminus of the A2A receptor is more flexible because it is not constrained by a lipid anchor. These two features, relative length and flexibility, may combine to afford the interaction of the A2A receptor with many additional proteins, other than G protein arrestins and kinases, that is, the so-called ‘accessory' proteins.
In recent years, several interaction partners were identified in yeast two-hybrid interaction screens using the C terminus of the A2A-adenosine receptor as a bait and the list is growing rapidly. Figure 3 gives an overview of accessory proteins that have been found to bind to the A2A receptor. Although the C terminus may exist in an extended conformation and thus provide a lot of room for interaction, the size of the individual binding partners makes it unlikely that the C terminus can accommodate several partners simultaneously. This is highlighted in Figure 3 by the overlapping binding sites for α-actinin (Burgueño et al., 2003) and ARNO (Gsandtner et al., 2005). Thus, individual interactions are likely to be only transient. The nature of the regulatory inputs that promote and terminate these interactions is unknown, but Thr298 (highlighted by an orange circle in Figure 3) is strategically placed to regulate binding of ARNO and α-actinin. It is therefore conceivable that the interaction of the receptor with ARNO and/or α-actinin is regulated by phosphorylation of Thr298. Similarly, the hetero-oligomer between D2 and A2A receptors has been proposed to be stabilized by binding of the phosphorylated Ser374 (boxed in orange Figure 3) to an arginine-rich segment in the third intracellular loop of the D2 receptor (Woods and Ferré, 2005). However, the authors used only synthetic peptides to show the stability of the arginine–phosphate electrostatic interaction. It remains to be demonstrated that the phosphorylation of Ser374 of the A2A receptor occurs in vivo and that it is a prerequisite for complex formation between A2A and D2 receptors.
Finally, it is also conceivable that ubiquitin-specific protease (USP)4 may also participate in regulating interactions. The binding site of deubiquitinating enzyme USP4 on the A2A receptor has been located within the last 50 amino acids of the C terminus and USP4 is required for export of the receptor from the endoplasmic reticulum (Milojevic et al., 2006). It is, however, conceivable that USP4 may also regulate later events in the trafficking of the receptor. The endocytotic routing of GPCRs has been known to depend on the extent of their ubiquitination (Wojcikiewicz, 2004; Lefkowitz and Shenoy, 2005). Extensive ubiquitination favours trafficking of endosomes to late endosomes and eventual lysosomal degradation over recycling (Marchese and Benovic, 2001; Shenoy et al., 2001). It is evident that for recycling receptors both phosphorylation and ubiquitination must be reversed. Dephosphorylation is thought to be accomplished in the endosome by protein phosphatase 2A. Very little is known about the enzymes responsible for deubiquitinating (Millard and Wood, 2006). If one examines the sequence of the C terminus of the A2A receptor in Figure 3, it is evident that the number of lysine residues to which ubiquitin may be attached is limited. Two of the three lysine residues fall into the region where ARNO and α-actinin are thought to bind. It is evident that the addition of a 76-amino-acid moiety may not be conducive for the interaction with either ARNO or α-actinin given that neither has an ubiquitin-interaction domain.
The presence of multiple binding sites for signalling molecules, scaffolding proteins and adapter proteins may allow the C terminus of the A2A receptor to serve as a coincidence detector (the binding of agonist and a second signal must occur simultaneously for interactor recruitment or release), as a signal integrator (several inputs must accumulate sequentially before interactor recruitment or release) or as an alternative switch (depending on where the receptor resides or which accessory proteins are available, the receptor may employ different signalling pathways to elicit distinct biological responses). The presence of translin-associated protein X, for instance, allows the A2A receptor to recruit p53-mediated cell cycle arrest and thus mediate differentiation of PC12 cells in a manner independent of Gs and the cAMP cascade (Sun et al., 2006). At this point, recruitment of ARNO by the A2A receptor is presumably counterproductive, as ARNO inhibits the hallmark of neuronal differentiation, the sprouting of dendrites and axons (Hernandez-Deviez et al., 2002, 2004).
Acknowledgments
Work from the authors' laboratory is supported by grants from the Austrian Science Fund FWF.
Glossary
- ARF
ADP-ribosylation factor
- ARNO
ARF-nucleotide binding site opener
- FRET
fluorescence/Foerster resonance energy transfer
- GRK
G-protein-coupled receptor kinase
- GPCR
G-protein-coupled receptor
- MAP kinase
mitogen-activated protein kinase
- mGluR5
metabotropic glutamate receptor 5
- USP4
ubiquitin-specific protease 4
Footnotes
Conflict of interest
The authors state no conflict of interest.
References
- Bockaert J, Fagni L, Dumuis A, Marin P. GPCR interacting proteins (GIP) Pharmacol Ther. 2004;103:203–221. doi: 10.1016/j.pharmthera.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Braun S, Levitzki A. Adenosine receptor permanently coupled to turkey erythrocyte adenylate cyclase. Biochemistry. 1979;18:2134–2138. doi: 10.1021/bi00577a045. [DOI] [PubMed] [Google Scholar]
- Brown RA, Spina D, Page CP.2008Adenosine receptors and asthma Br J Pharmacol 153Suppl 1): S446–S456.(this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns RF, Lu GH, Pugsley TA. Characterization of the A2 adenosine receptor labeled by [3H]NECA in rat striatal membranes. Mol Pharmacol. 1986;29:331–346. [PubMed] [Google Scholar]
- Burgueño J, Blake DJ, Benson MA, Tinsley CL, Esapa CT, Canela EI, et al. The adenosine A2A receptor interacts with the actin-binding protein alpha-actinin. J Biol Chem. 2003;278:37545–37552. doi: 10.1074/jbc.M302809200. [DOI] [PubMed] [Google Scholar]
- Canals M, Marcellino D, Fanelli F, Ciruela F, de Benedetti P, Goldberg SR, et al. Adenosine A2A–dopamine D2 receptor–receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Biol Chem. 2003;278:46741–46749. doi: 10.1074/jbc.M306451200. [DOI] [PubMed] [Google Scholar]
- Claing A, Chen W, Miller WE, Vitale N, Moss J, Premont RT, et al. Arrestin-mediated ADP-ribosylation factor 6 activation and β2-adrenergic receptor endocytosis. J Biol Chem. 2001;276:42509–42513. doi: 10.1074/jbc.M108399200. [DOI] [PubMed] [Google Scholar]
- Ferré S, Karcz-Kubicha M, Hope BT, Popoli P, Burgueno J, Gutierrez MA, et al. Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: implications for striatal neuronal function. Proc Natl Acad Sci USA. 2002;99:11940–11945. doi: 10.1073/pnas.172393799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, von Euler G, Johansson B, Fredholm BB, Fuxe K. Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. Proc Natl Acad Sci USA. 1991;88:7238–7241. doi: 10.1073/pnas.88.16.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Freissmuth M, Selzer E, Marullo S, Schutz W, Strosberg AD. Expression of two human beta-adrenergic receptors in Escherichiacoli: functional interaction with two forms of the stimulatory G protein. Proc Natl Acad Sci USA. 1991;88:8548–8552. doi: 10.1073/pnas.88.19.8548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Ferré S, Canals M, Torvinen M, Terasmaa A, Marcellino D, et al. Adenosine A2A and dopamine D2 heteromeric receptor complexes and their function. J Mol Neurosci. 2005;26:209–220. doi: 10.1385/JMN:26:2-3:209. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Ferré S, Genedani S, Franco R, Agnati LF. Adenosine receptor–dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol Behav. 2007;92:210–217. doi: 10.1016/j.physbeh.2007.05.034. [DOI] [PubMed] [Google Scholar]
- Gross W, Lohse MJ. Mechanism of activation of A2 adenosine receptors. II. A restricted collision-coupling model of receptor–effector interaction. Mol Pharmacol. 1991;39:524–530. [PubMed] [Google Scholar]
- Gsandtner I, Charalambous C, Stefan E, Ogris E, Freissmuth M, Zezula J. Heterotrimeric G protein independent signalling of a G protein-coupled receptor: direct binding of ARNO/cytohesin-2 to the carboxyl terminus of the A2A-adenosine receptor is necessary for sustained activation of the ERK/MAP-kinase pathway. J Biol Chem. 2005;280:31898–31905. doi: 10.1074/jbc.M506515200. [DOI] [PubMed] [Google Scholar]
- Gsandtner I, Freissmuth M. A tail of two signals: the C terminus of the A2A-adenosine receptor recruits alternative signalling pathways. Mol Pharmacol. 2006;70:447–449. doi: 10.1124/mol.106.026757. [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacol Ther. 2006;110:465–502. doi: 10.1016/j.pharmthera.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser RA, Hubble JP, Truong DD, Istradefylline US-001 Study Group Randomized trial of the adenosine A2A receptor antagonist istradefylline in advanced PD. Neurology. 2003;61:297–303. doi: 10.1212/01.wnl.0000081227.84197.0b. [DOI] [PubMed] [Google Scholar]
- Hein P, Rochais F, Hoffmann C, Dorsch S, Nikolaev VO, Engelhardt S, et al. Gs activation is time-limiting in initiating receptor-mediated signalling. J Biol Chem. 2006;281:33345–33351. doi: 10.1074/jbc.M606713200. [DOI] [PubMed] [Google Scholar]
- Hernandez-Deviez DJ, Casanova JE, Wilson JM. Regulation of dendritic development by the ARF exchange factor ARNO. Nat Neurosci. 2002;5:623–624. doi: 10.1038/nn865. [DOI] [PubMed] [Google Scholar]
- Hernandez-Deviez DJ, Roth MG, Casanova JE, Wilson JM. ARNO and ARF6 regulate axonal elongation and branching through downstream activation of phosphatidylinositol 4-phosphate 5-kinase α. Mol Biol Cell. 2004;15:111–120. doi: 10.1091/mbc.E03-06-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillion J, Canals M, Torvinen M, Casado V, Scott R, Terasmaa A, et al. Coaggregation, cointernalisation, and codesensitisation of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem. 2002;277:18091–18097. doi: 10.1074/jbc.M107731200. [DOI] [PubMed] [Google Scholar]
- Houndolo T, Boulay P-L, Claing A. G protein-coupled receptor endocytosis in ADP-ribosylation factor 6-depleted cells. J Biol Chem. 2005;280:5598–5604. doi: 10.1074/jbc.M411456200. [DOI] [PubMed] [Google Scholar]
- Hunzicker-Dunn M, Gurevich VV, Casanova JE, Mukherjee S. ARF6: a newly appreciated player in G protein-coupled receptor desensitisation. FEBS Lett. 2002;521:3–8. doi: 10.1016/s0014-5793(02)02822-3. [DOI] [PubMed] [Google Scholar]
- Kachroo A, Orlando LR, Grandy DK, Chen JF, Young AB, Schwarzschild MA. Interactions between metabotropic glutamate-5 and adenosine A2A receptors in normal and parkinsonian mice. J Neurosci. 2005;25:10414–10419. doi: 10.1523/JNEUROSCI.3660-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya T, Saitoh O, Yoshioka K, Nakata H. Oligomerization of adenosine A2A and dopamine D2 receptors in living cells. Biochem Biophys Res Commun. 2003;306:544–549. doi: 10.1016/s0006-291x(03)00991-4. [DOI] [PubMed] [Google Scholar]
- Klinger M, Freissmuth M, Nanoff C. Adenosine receptors: G protein-mediated signalling and the role of accessory proteins. Cell Signal. 2002a;14:99–108. doi: 10.1016/s0898-6568(01)00235-2. [DOI] [PubMed] [Google Scholar]
- Klinger M, Kudlacek O, Seidel MG, Freissmuth M, Sexl V. MAP kinase stimulation by cAMP does not require RAP1 but SRC family kinases. J Biol Chem. 2002b;277:32490–32497. doi: 10.1074/jbc.M200556200. [DOI] [PubMed] [Google Scholar]
- Klinger M, Kuhn M, Just H, Stefan E, Palmer T, Freissmuth M, et al. Removal of the carboxy terminus of the A2A-adenosine receptor blunts constitutive activity: differential effect on cAMP accumulation and MAP kinase stimulation. Naunyn Schmiedebergs Arch Pharmacol. 2002c;366:287–298. doi: 10.1007/s00210-002-0617-z. [DOI] [PubMed] [Google Scholar]
- Kudlacek O, Just H, Korkhov VM, Vartian N, Klinger M, Pankevych H, et al. The human D2 dopamine receptor synergizes with the A2A adenosine receptor to stimulate adenylyl cyclase in PC12 cells. Neuropsychopharmacology. 2003;28:1317–1327. doi: 10.1038/sj.npp.1300181. [DOI] [PubMed] [Google Scholar]
- Lahuna O, Quellari M, Achard C, Nola S, Meduri G, Navarro C, et al. Thyrotropin receptor trafficking relies on the hScrib-betaPIX-GIT1-ARF6 pathway. EMBO J. 2005;24:1364–1374. doi: 10.1038/sj.emboj.7600616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FS, Chao MV. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc Natl Acad Sci USA. 2001;98:3555–3560. doi: 10.1073/pnas.061020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Linden J. Adenosine in tissue protection and tissue regeneration. Mol Pharmacol. 2005;67:1385–1387. doi: 10.1124/mol.105.011783. [DOI] [PubMed] [Google Scholar]
- Lohse MJ, Klotz KN, Schwabe U. Mechanism of A2 adenosine receptor activation. I. Blockade of A2-adenosine receptors by photoaffinity labeling. Mol Pharmacol. 1991;39:517–523. [PubMed] [Google Scholar]
- Londos C, Cooper DM, Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci USA. 1980;77:2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese A, Benovic JL. Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J Biol Chem. 2001;276:45509–45512. doi: 10.1074/jbc.C100527200. [DOI] [PubMed] [Google Scholar]
- Millard SM, Wood SA. Riding the DUBway: regulation of protein trafficking by deubiquitylating enzymes. J Cell Biol. 2006;173:463–468. doi: 10.1083/jcb.200602082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milojevic T, Reiterer V, Stefan E, Korkhov VM, Dorostkar MM, et al. The ubiquitin-specific protease Usp4 regulates the cell surface level of the A2A-receptor. Mol Pharmacol. 2006;69:1083–1094. doi: 10.1124/mol.105.015818. [DOI] [PubMed] [Google Scholar]
- Mundell SJ, Benovic JL, Kelly E. A dominant negative mutant of the G protein-coupled receptor kinase 2 selectively attenuates adenosine A2 receptor desensitisation. Mol Pharmacol. 1997;51:991–998. doi: 10.1124/mol.51.6.991. [DOI] [PubMed] [Google Scholar]
- Mundell SJ, Luty JS, Willets J, Benovic JL, Kelly E. Enhanced expression of G protein-coupled receptor kinase 2 selectively increases the sensitivity of A2A adenosine receptors to agonist-induced desensitisation. Br J Pharmacol. 1998;125:347–356. doi: 10.1038/sj.bjp.0702081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanoff C, Boehm S, Hohenegger M, Schutz W, Freissmuth M. 2′,3′-Dialdehyde GTP as an irreversible G protein antagonist. Disruption and reconstitution of G protein-mediated signal transduction in cells and cell membranes. J Biol Chem. 1994;269:31999–32007. [PubMed] [Google Scholar]
- Nanoff C, Jacobson KA, Stiles GL. The A2 adenosine receptor: guanine nucleotide modulation of agonist binding is enhanced by proteolysis. Mol Pharmacol. 1991;39:130–135. [PMC free article] [PubMed] [Google Scholar]
- Nanoff C, Stiles GL. Solubilization and characterization of the A2-adenosine receptor. J Recept Res. 1993;13:961–973. doi: 10.3109/10799899309073703. [DOI] [PubMed] [Google Scholar]
- Olah ME, Stiles GL. The role of receptor structure in determining adenosine receptor activity. Pharmacol Ther. 2000;85:55–75. doi: 10.1016/s0163-7258(99)00051-0. [DOI] [PubMed] [Google Scholar]
- Palmer TM, Stiles GL. Identification of an A2A adenosine receptor domain specifically responsible for mediating short-term desensitisation. Biochemistry. 1997;36:832–838. doi: 10.1021/bi962290v. [DOI] [PubMed] [Google Scholar]
- Piersen CE, True CD, Wells JN. A carboxyl-terminally truncated mutant and nonglycosylated A2A adenosine receptors retain ligand binding. Mol Pharmacol. 1994;45:861–870. [PubMed] [Google Scholar]
- Schapira AH, Bezard E, Brotchie J, Calon F, Collingridge GL, Ferger B, et al. Novel pharmacological targets for the treatment of Parkinson's disease. Nat Rev Drug Discov. 2006;5:845–854. doi: 10.1038/nrd2087. [DOI] [PubMed] [Google Scholar]
- Seidel MG, Klinger M, Freissmuth M, Holler C. Activation of mitogen-activated protein kinase by the A2A-adenosine receptor via a rap1-dependent and via a p21ras-dependent pathway. J Biol Chem. 1999;274:25833–25841. doi: 10.1074/jbc.274.36.25833. [DOI] [PubMed] [Google Scholar]
- Sexl V, Mancusi G, Höller C, Gloria-Maercker E, Schütz W, Freissmuth M. Stimulation of the mitogen-activated-protein kinase (MAP kinase) via the A2A-adenosine receptor in primary human endothelial cells. J Biol Chem. 1997;272:5792–5799. doi: 10.1074/jbc.272.9.5792. [DOI] [PubMed] [Google Scholar]
- Sexl V, Mancusi G, Höller C, Parzer S, Schütz W, Freissmuth M. Stimulation of human umbilical venous endothelial cell proliferation by A2A-adenosine and β2-adrenergic receptors. Brit J Pharmacol. 1995;114:1577–1586. doi: 10.1111/j.1476-5381.1995.tb14942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated β2-adrenergic receptor and β-arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- Sitkovsky M.2008Adenosine A2A receptor antagonists: blockade of adenosinergic effects and T regulatory cells Br J Pharmacol 153Suppl 1): S457–S464.(this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C-N, Cheng H-C, Chou J, Lee S-Y, Lin Y-W, Lai H-L, et al. Rescue of p53 blockage by the A2A adenosine receptor via a novel interacting protein, translin-associated protein X. Mol Pharmacol. 2006;70:454–466. doi: 10.1124/mol.105.021261. [DOI] [PubMed] [Google Scholar]
- Terrillon S, Barberis C, Bouvier M. Heterodimerization of V1a and V2 vasopressin receptors determines the interaction with beta-arrestin and their trafficking patterns. Proc Natl Acad Sci USA. 2004;101:1548–1553. doi: 10.1073/pnas.0305322101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolkovsky AM, Levitzki A. Coupling of a single adenylate cyclase to two receptors: adenosine and catecholamine. Biochemistry. 1978;17:3811–3817. doi: 10.1021/bi00611a021. [DOI] [PubMed] [Google Scholar]
- van Calker D, Muller M, Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979;33:999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]
- Vaughan DJ, Millman EE, Godines V, Friedman J, Tran TM, Dai W, et al. Role of the G protein-coupled receptor kinase site serine cluster in beta2-adrenergic receptor internalization, desensitization, and beta-arrestin translocation. J Biol Chem. 2006;281:7684–7692. doi: 10.1074/jbc.M500328200. [DOI] [PubMed] [Google Scholar]
- Waldhoer M, Wise A, Milligan G, Freissmuth M, Nanoff C. Kinetics of ternary complex formation with fusion proteins composed of the A1-adenosine receptor and G protein α-subunits. J Biol Chem. 1999;274:30571–30579. doi: 10.1074/jbc.274.43.30571. [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz RJH. Regulated ubiquitination of protein in GPCR-initiated signalling pathways. Trends Pharmacol Sciences. 2004;25:35–41. doi: 10.1016/j.tips.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Woods AS, Ferré S. Amazing stability of the arginine–phosphate electrostatic interaction. J Proteome Res. 2005;4:1397–1402. doi: 10.1021/pr050077s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Arolfo MP, Dohrman DP, Jiang Z, Fan P, Fuchs S, et al. Dimers mediate synergy of dopamine D2 and adenosine A2 receptor-stimulated PKA signalling and regulate ethanol consumption. Cell. 2002;109:733–743. doi: 10.1016/s0092-8674(02)00763-8. [DOI] [PubMed] [Google Scholar]