Abstract
Glycogen synthase kinase 3 (GSK3, of which there are two isoforms, GSK3α and GSK3β) was originally characterized in the context of regulation of glycogen metabolism, though it is now known to regulate many other cellular processes. Phosphorylation of GSK3α(Ser21) and GSK3β(Ser9) inhibits their activity. In the heart, emphasis has been placed particularly on GSK3β, rather than GSK3α. Importantly, catalytically-active GSK3 generally restrains gene expression and, in the heart, catalytically-active GSK3 has been implicated in anti-hypertrophic signalling. Inhibition of GSK3 results in changes in the activities of transcription and translation factors in the heart and promotes hypertrophic responses, and it is generally assumed that signal transduction from hypertrophic stimuli to GSK3 passes primarily through protein kinase B/Akt (PKB/Akt). However, recent data suggest that the situation is far more complex. We review evidence pertaining to the role of GSK3 in the myocardium and discuss effects of genetic manipulation of GSK3 activity in vivo. We also discuss the signalling pathways potentially regulating GSK3 activity and propose that, depending on the stimulus, phosphorylation of GSK3 is independent of PKB/Akt. Potential GSK3 substrates studied in relation to myocardial hypertrophy include nuclear factors of activated T cells, β-catenin, GATA4, myocardin, CREB, and eukaryotic initiation factor 2Bɛ. These and other transcription factor substrates putatively important in the heart are considered. We discuss whether cardiac pathologies could be treated by therapeutic intervention at the GSK3 level but conclude that any intervention would be premature without greater understanding of the precise role of GSK3 in cardiac processes.
Keywords: cardiac myocytes, transgenic mouse models, myocardial hypertrophy, glycogen synthase kinase 3, protein kinase B/Akt, mitogen-activated protein kinases, transcription factors, nuclear factor of activated T cells, β-catenin
Introduction
Glycogen synthase kinase 3 (GSK3) is emerging as an important therapeutic target in a variety of pathologies (Cohen and Goedert, 2004). Here, we will be principally concerned with its role in cardiac growth and heart failure, particularly with that occurring during myocardial hypertrophy. GSK3 may also play an important role in the cardioprotection afforded by ischaemic preconditioning (Tong et al., 2002), but this is beyond the scope of this review. GSK3 was originally identified as a Ser/Thr protein kinase that phosphorylates glycogen synthase on multiple sites in its C-terminal region (Figure 1a) to inhibit its activity (Embi et al., 1980). It is a key signalling protein in the regulation of glycogen synthesis and the activity of GSK3 itself is regulated by phosphorylation–dephosphorylation, phosphorylation being inhibitory (Figure 1b). Phosphorylation of GSK3 is increased by insulin and this represents a major mechanism by which insulin promotes glycogen accumulation (Cohen, 2006).
Figure 1.
Regulation of glycogen metabolism by glycogen synthase kinase 3 (GSK3). (a) Casein kinase 2 (CK2) phosphorylates a priming Ser residue in the C terminus of glycogen synthase (Ser657 in the muscle isoform of Homo sapiens) to initiate a relay of GSK3-catalysed phosphorylations, as described in the text. (b) Glycogen synthase activity is regulated by a phosphorylation–dephosphorylation cycle. In the preprandial state, plasma insulin concentrations are low and GSK3 is active. GSK3 phosphorylates glycogen synthase and converts it to the less-active (glucose-6-phosphate (G6P) dependent) form, thus inhibiting glycogen synthesis. When plasma insulin concentrations rise after feeding, insulin promotes phosphorylation of GSK3 and inhibits GSK3 activity. Dephosphorylation of glycogen synthase by protein phosphatases (GS phosphatase) subsequently increases the activity of glycogen synthase and promotes glycogen synthesis. (c) GSK3α and GSK3β exhibit a high degree of homology in their kinase domains, diverging at their N- and C-terminal regions. GSK3α contains an N-terminal Gly-rich domain of unknown function. However, Gly-rich domains are classically associated with binding of nucleotides (Bossemeyer, 1994), though this domain is distinct from the ATP-binding site in the kinase domain. Both isoforms are constitutively phosphorylated on a Tyr residue in the kinase domain and this phosphorylation is required for activity. Phosphorylation of a conserved N-terminal Ser residue inhibits the activity of GSK3.
Since its discovery around 25 years ago, GSK3 has been implicated in the regulation of many additional biological processes apart from glycogen metabolism. Particular emphasis has been placed on its role in the phosphorylation of transcriptional regulators and translation initiation factors, and the consequent effects on gene and protein expression. It is also notable that GSK3 regulates metazoan development by modulating transcriptional activity in the context of the ‘canonical' Wnt pathway (Logan and Nusse, 2004; Cadigan and Liu, 2006; Gordon and Nusse, 2006). The potential role of GSK3 in diseases, including diabetes mellitus and neurodegenerative disorders, has led to the proposal that inhibition of GSK3 may be therapeutically useful (Cohen and Goedert, 2004). However, as discussed by Cohen and Goedert (2004), long-term pharmacological inhibition of GSK3 may result in increased malignancies because active GSK3 generally restrains gene expression. Analogously, in myocardial hypertrophy (which also involves altered patterns and rate of gene expression), the active forms of GSK3 are thought to act as a restraint. Thus, systemic inhibition of GSK3 may also promote anomalous hypertrophic growth of the heart possibly leading to heart failure. Conversely, systemic activation of GSK3 should reduce hypertrophic growth but might promote diabetes and neurodegeneration. We will return to the topic of GSK3 as a point for therapeutic intervention subsequently.
General properties and regulation of GSK3
Mammalian cells express two isoforms of GSK3, GSK3α (molecular mass ∼51 kDa) and GSK3β (molecular mass ∼47 kDa). The catalytic domains of GSK3 exhibit a high degree of sequence similarity, but the isoforms diverge in their N- and C-terminal sequences (Figure 1c). GSK3α and GSK3β are each phosphorylated on at least two residues (Figure 1c). In terms of their diurnal regulation by hormones, such as insulin, probably the most significant residues are GSK3α(Ser21) and GSK3β(Ser9), phosphorylation of which inhibits their protein kinase activities (Ali et al., 2001; Cohen and Frame, 2001; Frame and Cohen, 2001; Woodgett, 2001; Cohen and Goedert, 2004). In addition, ‘maturational' or ‘facilitative' phosphorylations of GSK3α(Tyr279) and GSK3β(Tyr216) are required for GSK3 activity and may play a structural role (Wang et al., 1994; Cole et al., 2004). They probably involve intramolecular autophosphorylation catalysed by GSK3 itself and there is no consistent evidence that changes in the degree of phosphorylation of GSK3α(Tyr279) or GSK3β(Tyr216) play any role in the diurnal regulation of its in vivo activity (Cohen and Goedert, 2004; Cole et al., 2004). In other words, GSK3α(Tyr279) and GSK3β(Tyr216) are always stoichiometrically phosphorylated under physiological conditions.
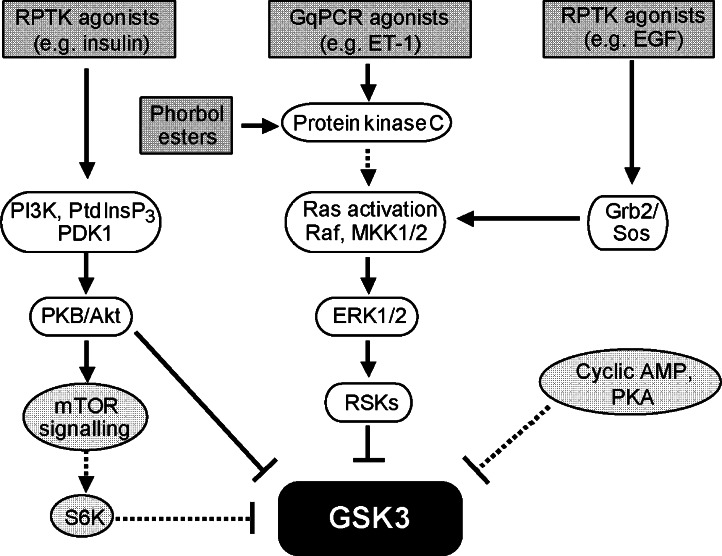
The protein kinases that phosphorylate and regulate GSK3α(Ser21) and GSK3β(Ser9) are reasonably well established (Figure 2; Ali et al., 2001; Cohen and Frame, 2001; Frame and Cohen, 2001; Woodgett, 2001; Cohen and Goedert, 2004). These sites of phosphorylation lie within an established consensus sequence for cyclic AMP-dependent protein kinase B/Akt (PKB/Akt), namely Arg-Xaa-Arg-Xaa-Xaa-Ser(P)/Thr(P), the optimal site being Arg-Lys-Arg-Xaa-Arg-Thr-Tyr-Ser(P)-Phe-Gly as defined by peptide library screening (Obata et al., 2000). Within Homo sapiens GSK3, the sequences are Ser-Gly-Arg-Ala-Arg-Thr-Ser-Ser(P)-Phe-Ala in GSK3α and Ser-Gly-Arg-Pro-Arg-Thr-Thr-Ser(P)-Phe-Ala in GSK3β. In terms of insulin signalling and glycogen accumulation, PKB/Akt is activated by phosphorylation downstream from phosphoinositide 3′ kinases (PI3Ks), phosphatidylinositol 3,4,5-trisphosphate (PtdIns(3,4,5)P3) and 3-phosphoinositide-dependent kinase 1 (Vanhaesebroeck and Alessi, 2000; Cohen, 2006), and this pathway probably represents the most important regulatory route to inhibition of GSK3 by insulin (Cohen, 2006). Other kinases also phosphorylate the Arg-Xaa-Arg-Xaa-Xaa-Ser(P)/Thr(P) motif and, for GSK3, the best studied are the p70/p85-S6 kinases (S6Ks) and the p90-ribosomal S6 kinase (RSK) family (Frödin and Gammeltoft, 1999; Cohen and Frame, 2001; Frame and Cohen, 2001; Cohen and Goedert, 2004). Other kinases including serum/glucocorticoid-regulated kinases (Kobayashi and Cohen, 1999) and the RSK-related mitogen- and stress-activated protein kinases (MSKs, Frödin and Gammeltoft, 1999) also phosphorylate GSK3. Whereas S6Ks are activated downstream from PKB/Akt, RSKs are activated downstream from the best characterized of the mitogen-activated protein kinases (MAPKs), the extracellular signal-regulated kinases 1/2 (ERK1/2). MSKs are activated by both ERK1/2 and p38-MAPKs. In addition, cell membrane-permeating cyclic AMP analogues, agents which raise cyclic AMP or overexpression of the cyclic AMP-dependent protein kinase A (PKA) catalytic subunit stimulate phosphorylation of GSK3α(Ser21) and GSK3β(Ser9) (Fang et al., 2000). Furthermore, PKA physically associates with GSK3β in cell cultures ex vivo, and the PKA catalytic subunit phosphorylates GSK3α(Ser21) and GSK3β(Ser9) in vitro. The GSK3α(Ser21) and GSK3β(Ser9) phosphorylation sites lie within an ‘adequate' consensus sequence for PKA, the optimum being Arg-Arg-Arg-Arg-Ser(P)-Ile-Hydrophobic-Hydrophobic-Ile as defined by peptide library screening (Songyang et al., 1994), that is basic (Arg-) residues lying N-terminal, hydrophobic residues lying C-terminal to the phosphorylated Ser-residue. Effects of cyclic AMP/PKA on GSK3 phosphorylation have been identified sporadically in non-cardiomyocytic cells and tissues (Li et al., 2000; Jensen et al., 2007; Taurin et al., 2007). However, to quote Frame and Cohen (2001) directly, who were admittedly writing some years ago, ‘the physiological relevance (of these observations) is not yet clear'. As a major function of cyclic AMP is to promote glycogen breakdown rather than glycogen synthesis, the phosphorylation of GSK3 by PKA in the context of glycogen metabolism is somewhat dissonant.
Figure 2.
Signalling pathways leading to the phosphorylation and inhibition of glycogen synthase kinase 3 (GSK3). The following scheme is a simplification of the events and only the salient features of the pathways are outlined. Receptor protein tyrosine kinase (RPTK) agonists (for example, insulin) stimulate the activity of phosphoinositide 3′ kinase (PI3K), which phosphorylates the membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2), to produce phosphatidylinositol 3,4,5-trisphosphate (PtdInsP3). PtdInsP3 remains in the plane of the membrane, activating 3-phosphoinositide-dependent kinase 1 (PDK1) and also causing translocation of protein kinase B/Akt (PKB/Akt) to the membrane where it is phosphorylated on Thr308 and partially activated by PDK1. Phosphorylation of PKB/Akt(Ser473) by the mammalian target-of-rapamycin complex 2 (not shown) fully activates the kinase. PKB/Akt subsequently phosphorylates the two GSK3 isoforms on a Ser-residue in each of their N-terminal domains to inhibit their activities (see Figure 1c). Gq-protein-coupled receptor (GqPCR) agonists (for example, endothelin-1 (ET-1)) stimulate phospholipase Cβ which hydrolyses PtdIns(4,5)P2 to produce diacylglycerol and inositol 1,4,5-trisphosphate. The former is the physiological activator of the diacylglycerol-regulated protein kinase C isoforms (which can also be activated by the tumour-promoting phorbol esters), whereas the latter is important in the regulation of intracellular Ca2+ movements. Protein kinase C activates the extracellular signal-regulated kinases 1/2 (ERK1/2) cascade, which involves the small guanine nucleotide-binding protein Ras. Conversion of Ras.GDP to its biologically active form, Ras.GTP, leads to the hierarchical activation of three levels of protein kinases. Raf phosphorylates and activates mitogen-activated protein kinase kinases 1/2 (MKK1/2), which then phosphorylate and activate ERK1/2. The signalling pathway then diverges. One consequence of activation of ERK1/2 is their phosphorylation and activation of the p90-ribosomal subunit S6 kinases (RSKs), which phosphorylate and inhibit GSK3. Some RPTK agonists (for example, epidermal growth factor, EGF), in addition to activating PKB/Akt (Clerk et al., 2006), activate the ERK1/2 cascade by stimulating GTP/GDP exchange on Ras via the Grb2/Sos guanine nucleotide exchange factor system, and thus activate Ras. In addition, PKB/Akt also activates the mammalian target-of-rapamycin complex 1 (mTOR). This leads to phosphorylation and activation of p70/p85-ribosomal subunit S6 kinases (S6Ks), thence to phosphorylation and inhibition of GSK3. Agents that raise cyclic AMP concentrations and activate cyclic AMP-dependent protein kinase A (PKA) may bring about phosphorylation and inhibition of GSK3, though the physiological significance of this mode of regulation is somewhat obscure.
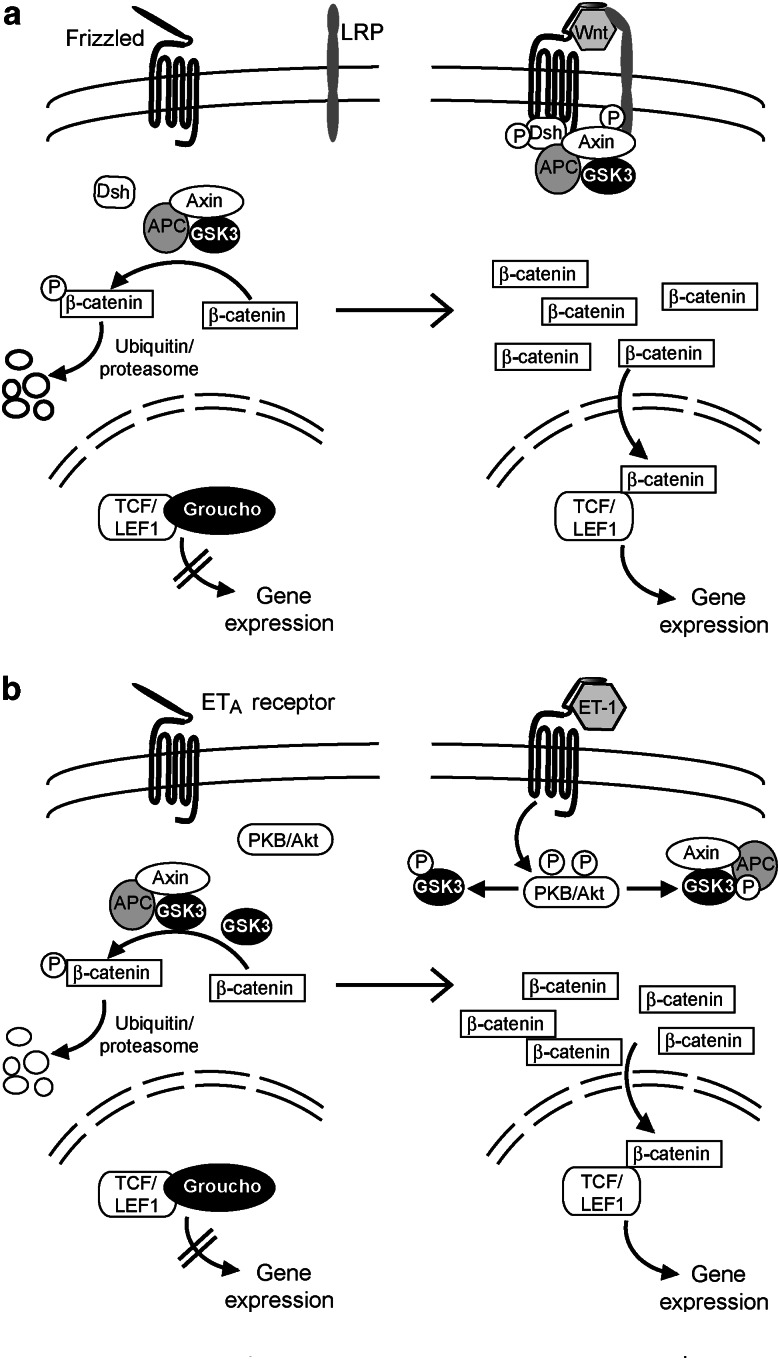
GSK3 is also regulated through the so-called ‘canonical' Wnt pathway (Figure 3a), which is important in metazoan development (Figure 3a; Logan and Nusse, 2004; Cadigan and Liu, 2006; Gordon and Nusse, 2006; see also http://www.stanford.edu/~rnusse/wntwindow.html). Wnts are a family of extracellular palmitoylated glycoproteins encoded by 19 genes in H. sapiens. It is still not entirely clear how Wnts are presented to their target cells, two possibilities being presentation by a ‘donor' cell in the form of cell surface-tethered molecules or presentation in a diffusible form. On presentation to its target cell, Wnt interacts with heptahelical (seven transmembrane spanning) receptors known collectively as Frizzled (10 genes in H. sapiens), and with single transmembrane-pass co-receptors known as low-density lipoprotein receptor-related proteins (LRPs). In the absence of Wnt, GSK3 is catalytically active in a complex with axin and the adenomatous polyposis coli (APC) protein and phosphorylates β-catenin. The pool of β-catenin, which is phosphorylated by GSK3, seems to be specifically involved in regulating gene expression, and a separate additional pool is involved in cell–cell interactions at adherens junctions (Perez-Moreno and Fuchs, 2006). Phosphorylation of β-catenin by GSK3 is a signal for its ubiquitination and degradation by the ubiquitin–proteasome system. In the presence of Wnt, the intracellular scaffolding protein Dishevelled associates with the transmembrane Wnt–Frizzled–LRP complex (Figure 3a). The axin–APC–GSK3 complex is then recruited, with coordinate phosphorylation of Dishevelled and of the intracellular domain of LRP. In this context, GSK3 is unable to phosphorylate β-catenin, dephospho-β-catenin accumulates and subsequently migrates into the nucleus. In the nucleus, T-cell-specific transcription factor/lymphoid enhancer binding factor 1 (TCF/LEF1) is normally maintained in a repressed state by association with Groucho. β-Catenin displaces Groucho and, with TCF/LEF1, forms a complex that activates transcription of TCF/LEF1-regulated genes.
Figure 3.
Regulation of the transcriptional co-regulator β-catenin. (a) Glycogen synthase kinase 3 (GSK3) participates in the regulation of the canonical Wnt pathway. In the absence of Wnt, a pool of GSK3 is present in a complex with axin and the adenomatous polyposis coli (APC) protein. In this complex, GSK3 is catalytically active and it phosphorylates previously ‘primed' β-catenin (that is, phosphorylated by casein kinase 1 on Ser45) at three further sites: Thr41, Ser37 and Ser33. This polyphospho-β-catenin is then degraded through the ubiquitin–proteasome system. When Wnts engage to the extracellular domain of their transmembrane receptors (Frizzled) and to the lipoprotein receptor-related protein (LRP) co-receptor, the axin–APC–GSK3 complex in association with the scaffold protein Dishevelled (Dsh) binds to the intracellular domains of Frizzled and LRP, and phosphorylations occur. GSK3 is now unable to phosphorylate β-catenin, which accumulates and enters the nucleus. Here, it displaces the co-repressor Groucho from its complex with T-cell-specific transcription factor/lymphoid enhancer binding factor 1 (TCF/LEF1), initiating gene expression. (b) It is suggested (Shevtsov et al., 2006) that a similar scheme may operate when endothelin-1 (ET-1) binds to its receptor in cardiac myocytes (the ETA receptor seems to be the principal endothelin-1 receptor). This leads to phosphorylation and activation of protein kinase B/Akt (PKB/Akt) (Figure 2) which then phosphorylates and inhibits GSK3 promoting β-catenin accumulation. It is however unclear which pool(s) of GSK3 is/are involved in this particular phosphorylation of β-catenin.
Substrates of GSK3: general points
GSK3 preferentially phosphorylates substrates on Ser/Thr residues in a ‘relay' fashion. A ‘priming' phosphorylation on a Ser/Thr residue is catalysed by a protein kinase distinct from GSK3. This increases the rate of phosphorylation by GSK3 at a Ser/Thr residue −4 to the priming phosphorylation (that is, the fourth residue lying N-terminal to the priming phosphorylation, for example, Ser-Xaa-Xaa-Xaa-Ser(P)). The first GSK3-mediated phosphorylation may serve to prime a second phosphorylation at any Ser/Thr residue lying −4 relative to this. The extent of such relays may be limited and consist of only two residues as in the transcription factor c-Jun (residues 239 and 243 for H. sapiens c-Jun, the former being the GSK3 site and the latter being the priming site; Cohen and Goedert, 2004). In contrast, a relay system of up to five Ser residues between residues 641 and 657 (the latter representing the priming site; Figure 1a) potentially operates in the N terminus of H. sapiens muscle glycogen synthase. Individual priming phosphorylations are catalysed by one of a number of different protein kinases, including casein kinases (for example, casein kinase 2 catalyses the priming phosphorylation in glycogen synthase), PKA, cyclin-dependent protein kinases, MSKs and dual specificity tyrosine phosphorylated and regulated kinases (Cohen and Goedert, 2004). The crystal structure of GSK3β accounts for the requirement for a priming phosphorylation and for the inhibitory effect of phosphorylation of Ser9 (Dajani et al., 2001; Frame et al., 2001). Thus, GSK3β(Arg96) interacts with the priming phosphate in a substrate, orientating it for phosphorylation at the −4 position. Phosphorylation of GSK3β(Ser9) causes an intramolecular interaction with Arg96, and the N terminus of the enzyme then serves as a pseudosubstrate to block both the binding site for the priming phosphate and access of any substrate to the catalytic pocket.
A brief overview of myocardial/cardiac myocyte hypertrophy
In this review, we use the term ‘hypertrophy' to indicate an increase in heart or cardiac myocyte size above that which would normally be achieved at any given stage of postnatal mammalian growth (Dorn et al., 2003; Dorn, 2007), though the boundaries are not absolute as discussed later. Cardiac myocytes terminally differentiate during the perinatal period and are unable to undergo any further complete cycles of cell division. They therefore grow as the organism grows, a process that we call ‘eutrophy' (Dorn et al., 2003). They are additionally capable of considerable growth on suitable stimulation (hypertrophy). In vivo, myocyte hypertrophy is an important adaptational response that allows the heart to maintain an adequate cardiac output with improved cardiac contractility in a variety of physiological conditions. ‘Physiological', ‘compensated' or ‘adaptive' (all are synonymous) hypertrophy such as is seen in pregnancy (Eghbali et al., 2005), endurance exercise, for example, voluntary running (Allen et al., 2001) or post-prandially in Burmese pythons (Andersen et al., 2005) is probably beneficial (or at least not disadvantageous) and is reversible upon removal of the stimulus. Post-prandial hypertrophy is perhaps an area which is perhaps semantically difficult to differentiate from eutrophy, because, in Burmese pythons, it represents normal behaviour. Even in ‘pathological' conditions (for example, hypertension or following a survived myocardial infarction), the heart may initially adapt beneficially. However, such a beneficial consequence may ultimately prove detrimental, leading to heart failure (Opie et al., 2006). This is the stage when cardiac hypertrophy becomes symptomatic, thus in a clinical context the term is usually associated with heart failure and the hypertrophy is ‘pathological', ‘decompensated' or ‘maladaptive', possibly because of ancillary changes (for example, fibrosis, reduced contractile performance and chamber dilatation/wall thinning). It is not clear whether, in pathological conditions, the compensated phase is a compulsory waypoint on the path to decompensation or whether it is dispensable.
A number of extracellular stimuli and receptors are implicated in the development of myocyte hypertrophy (Sugden and Clerk, 1998; Heineke and Molkentin, 2006). These include particularly Gq-protein-coupled receptor (GqPCR) agonists (for example, endothelin-1, α-adrenergic stimuli), peptide growth factors that bind to receptor protein tyrosine kinases (for example, epidermal growth factor, platelet-derived growth factor, fibroblast growth factor) and cytokines binding to gp130 family receptors (for example, cardiotrophin-1, leukemia inhibitory factor). β-Adrenergic receptors may also promote hypertrophy, though whether this is through classical coupling to GsPCRs or GiPCRs is less clear. The signalling pathways activated by the various stimuli and the role(s) of each pathway in the hypertrophic response have been subject to considerable debate over recent years (Sugden and Clerk, 1998; Heineke and Molkentin, 2006). These pathways frequently operate through phosphorylation and/or dephosphorylation events, and a number of protein kinases and phosphatases may be involved. In the context of hypertrophy, much emphasis has been placed on cyclic AMP-dependent protein kinase C (PKC), the four principal MAPK cascades (ERK1/2, ERK5, p38-MAPKs and c-Jun N-terminal kinases), calcineurin (protein phosphatase 2B) and PKB/Akt. Other protein kinases may be involved in a specific context. For example, Janus kinases are predominantly activated by cytokine receptors (Haan et al., 2006), whereas activation of Ca2+/calmodulin-dependent kinases may be a particularly important response to increased nuclear Ca2+ concentrations (Wu et al., 2006). The dogma is that activation of intracellular signalling pathways leads to changes in the phosphorylation status of transcription and translation factors, and these modulate cardiac myocyte gene and protein expression. Such changes lead to and are ultimately responsible for the hypertrophic state.
In vivo or ex vivo, hypertrophy is characterized by increased myocyte size and sarcomerogenesis, and changes in the expression of characteristic index genes (for example, increased expression of atrial natriuretic factor, B-type natriuretic peptide, β-myosin heavy chain), though there is discussion about how closely these are linked to hypertrophy as opposed to heart failure (Dorn et al., 2003). Other variables are assessed frequently in whole animals, for example, heart (ventricular) weight to body weight ratio (HW/BW). In cases of specific regional hypertrophy (left or right ventricular), these weights are used as numerators. Sometimes, tibial length (TL) is used as a denominator as it is less sensitive to dietary state than BW. In vivo, the extent of fibrosis can also be assessed (unchanged in compensated hypertrophy, increased in decompensated hypertrophy) as can the contractile properties of the heart (increased contractility in compensated hypertrophy, decreased contractility in decompensated hypertrophy). Indeed, the decreased contractility may be linked to the increased fibrosis. Ex vivo, increases in the rate of protein synthesis (difficult to measure in vivo) are often used. In many publications, authors choose to examine only a limited number of aspects of the hypertrophic response to delineate ‘hypertrophy' (for example, increased atrial natriuretic factor expression or increased rates of protein synthesis). Whether a single index is sufficient to define hypertrophy is a different matter, but here we use the term ‘hypertrophy' without any attempt at more detailed definition.
GSK3 and cardiac myocyte hypertrophy
As outlined above, the canonical Wnt pathway (which involves GSK3) is important in developmental growth of the heart (Eisenberg and Eisenberg, 2007). In addition, a number of studies suggest that GSK3 (but GSK3β in particular) plays a pivotal role in cardiac hypertrophy (Hardt and Sadoshima, 2002; Kerkelä et al., 2007). In essence, active GSK3 prevents hypertrophic growth of cardiac myocytes, probably through phosphorylation and inhibition of transcriptional regulators including β-catenin, nuclear factor of activated T cells (NFATs), GATA4, etc., and the translational regulator eukaryotic initiation factor 2Bɛ (eIF2Bɛ), as described below. However, in H. sapiens, although inhibition of GSK3β is seen in end-stage heart failure resulting from coronary artery disease or idiopathic dilated cardiomyopathy, it is not observed in chronic hypertrophy (Haq et al., 2001). This may simply reflect the late stage at which samples were examined. We will now review the data on GSK3 and cardiac hypertrophy (some of which is very recent) and also highlight specific questions which remain.
Inhibition of GSK3 in cardiac myocyte hypertrophy
Hypertrophic agonists such as endothelin-1, or α- or β-adrenoceptor ligands increase phosphorylation of GSK3β(Ser9), resulting in a 40–60% inhibition of GSK3β activity (Haq et al., 2000; Morisco et al., 2000). It is notable that these particular studies do not provide any data for phosphorylation or activity of GSK3α. However, GSK3α is expressed in the heart and, at least with respect to the regulation of glycogen synthase, plays a similar though less prominent role to GSK3β (Mora et al., 2005). In neonatal rat cardiac myocytes, we detect both GSK3α and GSK3β in approximately equal abundance (though the former appears less active) and we also observe phosphorylation of both GSK3α(Ser21) and GSK3β(Ser9) in response to insulin or endothelin-1 (Markou et al., 2008). This suggests that GSK3α should not be ignored and, indeed, a very recent publication (Zhai et al., 2007) has gone some way in rectifying this omission.
Inhibition of GSK3 activity with Li+ (as LiCl) promotes features of the hypertrophic response (Haq et al., 2000) and is likely to affect both isoforms. It is also of note that LiCl is not a particularly effective inhibitor of GSK3. The IC50 is only ∼1–2 mM and, at the concentration normally used (10 mM), inhibition is only about 50% (Stambolic et al., 1996; Davies et al., 2000). Furthermore, specificity may be a problem as casein kinase 2 and two protein kinases activated by p38-MAPK are also at least partially inhibited by Li+ (Davies et al., 2000). This is pertinent as p38-MAPK is activated by endothelin-1 or α-adrenergic agonism in cardiac myocytes (Clerk et al., 1998), and casein kinase 2 sometimes catalyses the priming phosphorylation of GSK3 substrates (for example, glycogen synthase; Picton et al., 1982; Cohen and Goedert 2004). Thus, any effects of Li+ in isolated cells or tissues may result from inhibition of both casein kinase 2 and GSK3. However, as discussed in depth by Davies et al. (2000), Li+ may be a more selective inhibitor of GSK3 (as opposed to casein kinase 2) at physiological K+ concentrations and at the lower physiological Mg2+ concentrations than those that were used in vitro. Even at these K+ and Li+ concentrations, the IC50 of Li+ for GSK3 is only ∼2 mM, suggesting that Li+ should be used at 10 mM. Li+ also powerfully inhibits inositol monophosphatase (Hallcher and Sherman, 1980), and this may influence inositol phosphoinositide signalling.
It will be interesting to determine whether more selective inhibitors of GSK3, which have been recently developed (Cohen and Goedert, 2004), have the same effect on cardiac myocyte hypertrophy as Li+. As a first step here, we have shown that a relatively high concentration (10 μM) of the GSK3 inhibitor, 1-azakenpaullone (Kunick et al., 2004), increases myocyte area but produces an ‘elongated' phenotype compared with the α-adrenergic agonist, phenylephrine (Markou et al., 2008). Like all work with inhibitors, we should add the caveat that 1-azakenpaullone may not be specific. It does also inhibit cyclin-dependent kinase 1/cyclin B and cyclin-dependent kinase 5/p25, but the IC50 is some two orders of magnitude greater (Kunick et al., 2004). Results with Li+ were less convincing in our hands. Furthermore, the acute changes in transcript abundances induced by 1-azakenpaullone differ from those induced by endothelin-1. A more detailed examination of the ‘pro-hypertrophic' effects of 1-azakenpaullone and other GSK3 inhibitors is probably justified. Interestingly, another GSK3 inhibitor, 6-bromoindirubin-3′-oxime, promotes proliferation (hyperplasia) of adult or neonatal rat cardiac myocytes, with loss of phenotype in the case of the adult cell (Tseng et al., 2006). This is somewhat confusing because the mammalian cardiac myocyte is thought to undergo terminal differentiation and withdraw from the proliferative cell cycle during the perinatal period. However, the results are perhaps consonant with the ability of 6-bromoindirubin-3′-oxime to maintain pluripotency of embryonic stem cells through activation of the ‘canonical' Wnt pathway (Sato et al., 2004).
Phosphorylation of GSK3 in ex vivo cardiac myocyte hypertrophy and in vivo cardiac hypertrophy
In all studies relating to phosphorylation and inhibition of GSK3 by physiological means in the heart, the presumption seems to be that the signal is propagated through PKB/Akt. For insulin or insulin-like growth factor 1 (IGF 1), which potently activates PKB/Akt but not ERK1/2 in cardiac myocytes (Clerk et al., 2006), this is indeed likely to be the case (Markou et al., 2008). However, as discussed above, GSK3 may be phosphorylated by other protein kinases. In relation to phosphorylation of GSK3 by β-adrenergic agonism, Morisco et al. (2000) demonstrated that PKB/Akt is phosphorylated/activated in response to β-adrenoceptor stimulation, but the link from PKB/Akt to phosphorylation of GSK3β was not made. Indeed, the time course for activation of PKB/Akt is delayed relative to phosphorylation of GSK3β(Ser9), suggesting that other pathways are involved. An input from Ca2+/calmodulin-dependent kinase into GSK3 phosphorylation was also detected, though any possible direct input from PKA was not considered. With respect to endothelin-1, it is interesting that Haq et al. (2000) inhibited the increase in phosphorylation of GSK3β(Ser9) with the PI3K inhibitors, LY294002 or wortmannin, indicating that endothelin-1 probably signals to GSK3β by activating PKB/Akt. In our hands, endothelin-1 does not substantially activate PKB/Akt (Clerk et al., 2006). Furthermore, the increases in phosphorylation of GSK3β(Ser9) and GSK3α(Ser21) induced by endothelin-1 in cardiac myocytes are unaffected by LY294002 but are suppressed by inhibitors of the ERK1/2 cascade (Markou et al., 2008). This suggests that ERK1/2 and thus possibly RSKs (which lie downstream from and are activated by ERK1/2) are required in this context rather than PI3K–PKB/Akt. In vivo, pro-hypertrophic manipulations such as increasing left ventricular pressure by transverse aortic constriction (TAC) rapidly (within 10 min) increase phosphorylation of GSK3 (Brancaccio et al., 2003), but the signalling pathways are ill defined. By 4 weeks, these differences are no longer detectable, c.f. in man (Haq et al., 2001), and the abundance and activity of GSK3α, but not GSK3β, may even be increased (Zhai et al., 2007).
Some comments on insulin and IGF 1 in relation to cardiac hypertrophy and GSK3
Although perhaps tangential to the main topic of this review, we thought that it might be useful to include some comments on the effects of insulin (and IGF 1) on the growth of the myocardium, given that insulin is probably the agonist most effective in inducing GSK3 phosphorylation. It has been known for many years that insulin stimulates cardiac protein synthesis (Morgan et al., 1971; Smith et al., 1986) and inhibits cardiac protein degradation (Rannels et al., 1975; Sugden and Smith, 1982). Indeed, it is one of the most powerful modulators of these processes yet identified, and decreased plasma insulin concentrations probably contribute to the loss of cardiac protein mass that occurs in starvation (Preedy et al., 1984). IGF 1 stimulates cardiac myocyte protein synthesis to the same extent as insulin and inhibits protein degradation (Fuller et al., 1992; Decker et al., 1995). With either agonist, there is a net accumulation of protein in cardiac myocytes ex vivo (Decker et al., 1995), suggesting that they participate in the regulation of cardiac mass. Inducible, conditional targeted deletion of the cardiac myocyte insulin receptor gene in mice decreases heart size, but increases expression of the supposed hypertrophic index gene, β-myosin heavy chain (Belke et al., 2002), though it should be added that others have failed to detect significant differences in the expression of this gene in wild-type and insulin receptor-null mice (Hu et al., 2003). Although HW and HW/TL were decreased in the insulin receptor-null mice, they exhibited the same absolute extent of hypertrophy (HW/TL) as wild-type animals following TAC and the expression of two hypertrophic index genes was increased. However, after TAC, there was a greater degree of dilatation and fibrosis in the insulin receptor-null mice, and contractility was also decreased, suggesting that they were decompensating (Hu et al., 2003). Overall, though, insulin signalling seemed dispensable for TAC-induced hypertrophy.
In addition to the study of Decker et al. (1995), the effects of IGF 1 on cardiac myocyte hypertrophy (as defined earlier) have been studied both ex vivo and in vivo. Thus, Ito et al. (1993) treated myocytes with IGF 1 and showed that the myocyte cell area was twofold greater than controls. What is not clear is to what extent this difference was a reflection of ‘shrinkage' of control cells (this criticism can be made of most experiments of this type, including our own). There was also evidence of transcriptional changes that were consistent with a hypertrophic response. Duerr et al. (1995) infused IGF 1 in vivo and showed increases in left ventricular weight/TL at ‘medium' or ‘high' rates of infusion and in right ventricular weight/TL at ‘high' rates. McMullen et al. (2004) cardiospecifically overexpressed the IGF 1 receptor and showed that HW/BW and cardiac myocyte size were increased, with some evidence of increased hypertrophic ‘index' gene expression. There is thus evidence that IGF 1 is a bona fide inducer of hypertrophy (as defined above).
At least part of the stimulation of cardiac myocyte protein synthesis by insulin is mediated through PI3K (Pham et al., 2000). Furthermore, insulin and IGF 1 are the most powerful activators of PKB/Akt in cardiac myocytes yet identified (Pham et al., 2000; Wang et al., 2000; Clerk et al., 2006). Insulin induces inhibition and phosphorylation of GSK3α(Ser21) and GSK3β(Ser9), an effect that in cardiac myocytes is mediated largely through the PI3K pathway (Wang et al., 2000; Markou et al., 2008). As summarized in a recent review (Clerk et al., 2007), cardiospecific activation of PI3K or PKB/Akt signalling in transgenic mice generally promotes cardiac growth and cardioprotection (though the resulting phenotype differs significantly between laboratories, possibly because a number of different methodologies have been used to activate PI3K or PKB/Akt signalling). Equally, there is an element of growth reversal in mice expressing ‘dominant-negative' (inhibitory) transgenes.
In contrast to the studies of others (for example, Ito et al., 1993), we have never been able to detect any increases in surface area or sarcomerogenesis in primary cultures of neonatal rat cardiac myocytes treated with insulin or IGF 1 (Clerk et al., 2006), nor have we been able to establish that IGF 1 greatly increased activities of promoters for hypertrophic ‘index' genes using luciferase reporter assays as compared with more established hypertrophic agonism, for example, α-adrenergic agonists (SJ Fuller and PH Sugden, unpublished observations). Thus, while some of the experiments with insulin or IGF 1 are consistent with a primary or exclusive role for GSK3 in cardiac myocyte hypertrophy, the simple interpretation of our own experiments is not. Of course, it is possible that other PI3K- or PKB/Akt-dependent pathways override the input from GSK3 inhibition or that signalling inputs additional to GSK3 may be required, but those are imponderables. Indeed, inhibition of GSK3 by insulin or IGF 1 may contribute to their antiapoptotic effects, as discussed elsewhere in this review. Our view coincides closely with that of Shiojima and Walsh (2006), as expressed in their eloquent review. Thus, insulin couples cardiac protein mass to nutritional status through its pancreatic secretion, whereas IGF 1 controls maturational eutrophy through the hypothalamopituitary axis (Shiojima and Walsh, 2006; Catalucci et al., 2008).
Effects of genetic manipulation of GSK3 signalling on cardiac myocyte hypertrophy ex vivo
Further evidence to support a role for GSK3β in cardiac myocyte hypertrophy derives from overexpression of wild-type GSK3β or expression of a constitutively active mutant form of GSK3β, GSK3β(Ser9Ala), which renders GSK3β resistant to the inhibitory phosphorylation. These approaches prevent or reduce the development of the hypertrophic phenotype induced by endothelin-1 or β-adrenergic agonism in isolated cardiac myocytes (Haq et al., 2000; Morisco et al., 2000). Compensated hypertrophy is usually associated with increased myocyte survival (in part through activation of the ERK1/2 cascade and PKB/Akt) and an enzymically inactive ‘dominant-negative' inhibitor of GSK3, GSK3β(Lys85Met/Lys86Ile), inhibits tumour necrosis factor α/cycloheximide-induced apoptosis (Hirotani et al., 2007). A recent publication by Zhai et al. (2007) likewise concluded that overexpression of GSK3α reduces cardiac myocyte size and decreases the hypertrophic response to phenylephrine but increases apoptosis, whereas ‘knockdown' of GSK3α with shRNA has the opposite effects (increased cell size and reduced apoptosis). These studies suggest that inhibition of GSK3 is pro-hypertrophic but, importantly, is also pro-survival whereas GSK3 activity is antihypertrophic and antisurvival.
Effects of genetic manipulation of GSK3 signalling on myocardial hypertrophy in vivo
A number of studies have also been performed in genetically manipulated mice in which transgene expression is placed under the control of the α-myosin heavy chain promoter to ensure a high degree of cardiospecific expression (Palermo et al., 1995). In the ventricles of these animals, the transgene is expressed in myocytes largely from the perinatal period through to adulthood. Overexpression of wild-type GSK3β or expression of GSK3β(Ser9Ala) reduces maturational growth of the heart (Antos et al., 2002; Michael et al., 2004), and reduces the cardiac hypertrophy induced by isoprenaline infusion, TAC or overexpression of constitutively activated calcineurin (Antos et al., 2002). Furthermore, inducible expression of GSK3β(Ser9Ala) in the adult heart reduces the extent of established hypertrophy produced by TAC, as assessed by left ventricular weight/BW (Sanbe et al., 2003). In a mouse model of familial hypertrophic cardiomyopathy resulting from expression of a mutant β-myosin heavy chain transgene (simulating the inheritable human disease to a greater or lesser extent), the increase in cardiac mass was reduced by co-expression of GSK3β(Ser9Ala), although gender and diet were complicating factors (Luckey et al., 2007). These studies suggest that activation of GSK3 may be therapeutically valuable. However, to complicate matters, overexpression of wild-type GSK3β also induces diastolic dysfunction because of disturbances in Ca2+ movements (Michael et al., 2004). The heart becomes unable to regulate its diastolic Ca2+ concentration because of reduced Ca2+ reuptake into the sarcoplasmic reticulum through inhibition of the sarcoplasmic (endoplasmic) reticulum Ca2+ ATPase pump.
Two very recent publications from the group of Junichi Sadoshima have extended these observations. In the first of these, Hirotani et al. (2007) generated mice cardiospecifically expressing ‘dominant-negative' GSK3β(Lys85Met/Lys86Ile). These mice displayed cardiac hypertrophy (as expected) with improved cardiac contractility (adaptive hypertrophy) in the absence of further interventions. Following TAC that was adequate to induce decompensation, there were no differences in terms of relative (to sham-operated controls) hypertrophy of GSK3β(Lys85Met/Lys86Ile) mice compared with non-transgenic mice, but the GSK3β(Lys85Met/Lys86Ile) mice displayed greater cardiac contractility with less fibrosis or apoptosis. Conversely, inducible postnatal cardiospecific overexpression of GSK3β increased GSK3 activity and resulted in the development of left ventricular dysfunction, increased HW/TL, fibrosis and apoptosis, and induced early death. (Quite why cardiomyocyte-specific overexpression of a transgene should lead to fibrosis is unclear. Cardiac myocytes are not normally thought to participate in synthesis of the extracellular matrix. Explanations could include secretion of pro-fibrotic paracrine factors (Kemp et al., 2004) or compulsory coupling of changes in contractility to fibrosis.) The conclusions from these experiments are that inhibition of GSK3 leads to a compensated cardiac phenotype, but perversely activation of GSK3 leads to decompensation (c.f. Michael et al., 2004). In the second publication (Zhai et al., 2007), the focus was GSK3α which, as summarized by Zhai et al. (2007), may differ to some extent from GSK3β in terms of the biological responses which they each provoke. Indeed, TAC induced expression of GSK3α and caused an overall increase in its activity in the absence of changes in GSK3β (Zhai et al., 2007). Cardiospecific overexpression of wild-type GSK3α reduced cardiac growth (c.f. GSK3β; Michael et al., 2004; Hirotani et al., 2007), and reciprocally reduced expression and activity of GSKβ. After TAC, the authors conclude that left ventricular hypertrophy is inhibited in the GSK3α transgenic mice. This is true in absolute terms, but the differences are less clear in relative terms because the GSK3α transgenic mice have lower HW/BW or HW/TL ratios at the outset. This shows the type of consideration that can bedevil such work. Be that as it may, contractile function in the control and GSK3α transgenic mice did not differ at baseline, but was worse in the GSK3α transgenic after TAC, possibly because of increased apoptosis and fibrosis. In other words, the ‘antihypertrophic' effects of GSK3 activation could simply result from increased myocyte loss. On the basis of experiments with inhibitors and GSK3 constructs, the authors devise what, to our minds, is a rather fanciful signalling pathway to account for their findings. They suggest that increased expression/activity of GSK3α in transgenic mice leads to inhibition of ERK1/2 signalling by inhibiting PKCδ and PKCɛ. Assuming that the central roles of ERK1/2 as being pro-hypertrophic and pro-survival are accepted, inhibition of ERK1/2 should decrease hypertrophy and increase apoptosis, but the proposed signalling pathway could also effectively constitute a positive feedback loop, whereby inhibition of ERK1/2 would promote further activation of GSK3 through inhibiting phosphorylation of GSK3α(Ser21) and GSK3β(Ser9), presumably increasing myocyte losses.
As in the ex vivo experiments, these in vivo experiments are consistent with an antihypertrophic role for active GSK3, but also with a potentially undesirable pro-apoptotic role with concurrent reductions in contractility. Thus, the question becomes whether the antihypertrophic action is simply a reflection of the pro-apoptotic effects? Leaving this aside for the time being, it is worth considering the potential effects of GSK3 activation on the best-established substrate for GSK3, glycogen synthase, and on glycogen metabolism generally. Active (dephosphorylated) GSK3 phosphorylates glycogen synthase to inhibit glycogen synthesis, and inhibition (phosphorylation) of GSK3α(Ser21)/GSK3β(Ser9) increases the rate of glycogen synthesis (Figures 1a and b). Overexpression of GSK3 or expression of the GSK3β(Ser9Ala) mutant might therefore be expected to diminish glycogen stores. In the non-stressed heart, changes in GSK3 activity as a consequence of phosphorylation appear to play a minimal role in regulating cardiac glycogen levels, as transgenic mice in which wild-type GSK3α/β genes are replaced by transgenes encoding Ser21Ala/Ser9Ala-mutated forms of GSK3α/β (that is, a GSK3α(Ser21Ala)/GSK3β(Ser9Ala) ‘knock-in') exhibit similar levels of cardiac glycogen as their wild-type littermates (Mora et al., 2005). However, during pathophysiological cardiac stresses (for example, TAC), it is possible that altered glycogen metabolism resulting from GSK3 activation may affect cardiac metabolism and mass and, ultimately, the development of cardiac pathologies. Furthermore, the concentration of glycogen and the activity of the ‘intracellular fuel gauge', AMP-activated protein kinase, are reciprocally related, the link possibly being the glycogen-binding β subunit of AMP-activated protein kinase (Hardie, 2007). A decrease in cardiac glycogen should activate AMP-activated protein kinase, which would lead to inhibition of anabolic processes such as protein accumulation (Hardie et al., 2003; Wojtaszewski et al., 2003; Hardie, 2007). Although such a scheme is hypothetical, it suggests that the consequences of GSK3 activation or inhibition might not simply rely directly on its own ability to regulate transcription and translation (see below) directly. Perhaps unexpectedly, the GSK3α(Ser21Ala)/GSK3β(Ser9Ala) ‘knock-in' mice have significantly higher HW/BW ratios than the wild-type animals in the absence of any significant differences in absolute HW or BW (K Sakamoto, personal communication), though it is not clear that this is attributable to increased myocytic protein mass. Obviously, further experimentation is needed.
Potential downstream targets of GSK3 in cardiac myocyte hypertrophy
As mentioned, GSK3 requires a priming phosphorylation of its substrates to direct it to Ser/Thr residues in the −4 position, but the identities of kinases responsible for the priming phosphorylations are not always clear (Cohen and Goedert, 2004). GSK3 phosphorylates a range of substrates and, generally, inhibits their biological activities (Cohen and Goedert, 2004). Here, we will focus on substrates for which there is evidence of a potential role in hypertrophy. It is entirely possible that several or even all of the protein enumerated may participate in the hypertrophic response. Although most of the substrates identified are transcriptional regulators, the specific changes in transcription associated with changes in GSK3 activity have not been thoroughly characterized.
Nuclear factors of activated T cells
The role of NFATs, which are encoded by five genes (NFATc1–NFATc4, NFAT5) producing multiple alternatively spliced transcripts, is best defined in T-cell activation (Crabtree and Olson, 2002; Gallo et al., 2006). NFATs are phosphorylated on Ser- (and maybe Thr-) residues in their N-terminal regions. These phosphorylations mask nuclear localization signals and retain NFATs in the cytoplasm possibly by binding of 14-3-3 proteins, inhibition of which promotes cardiac myocyte hypertrophy (Liao et al., 2005). T-cell receptor engagement increases intracellular Ca2+, which binds to the Ca2+ sensor protein calmodulin, and the Ca2+–calmodulin complex binds to and activates calcineurin (protein phosphatase 2B). Calcineurin dephosphorylates cytoplasmically localized NFATs, allowing them to migrate into the nucleus to promote gene expression. GSK3 phosphorylates PKA-primed NFATc1 on relays of Ser residues (Figure 4a; Beals et al., 1997; Neal and Clipstone, 2001; Sheridan et al., 2002), and thus inhibits NFATc1-dependent gene expression. Whether PKA is the physiological priming kinase for NFATs is unclear. A ‘systems biology' approach has suggested that dual specificity tyrosine phosphorylated and regulated kinases may catalyse the priming phosphorylation of at least NFATc2 (Gwack et al., 2006). Although these relays are conserved in other NFATs (Figure 4a), the individual identities of the sites phosphorylated are not as well characterized as for NFATc1. However, GSK3 is thought to be involved in the phosphorylation of other NFATs, viz NFATc2 (Okamura et al., 2004), NFATc3 (Nilsson et al., 2006) and NFATc4 (Graef et al., 1999). Equally, it is important to note that GSK3 is not the only kinase that phosphorylates and regulates the localization of NFATs. Casein kinase 1 phosphorylates NFATc2 in a Ser-rich region more N-terminal than the GSK3 sites and in the region of the nuclear localization signal (Okamura et al., 2004). This retains NFATc2 in the cytoplasm and the region is conserved in other NFATs.
Figure 4.
Potential glycogen synthase kinase (GSK3) Ser/Thr-directed phosphorylation relays in nuclear factor of activated T cells (NFATs). (a) Sequences for Homo sapiens NFATs were aligned by CLUSTAL W (1.83). Accession numbers are NFATc1, NP_006153.2 (825 residues); NFATc2, NP_036472.2 (921 residues); NFATc3, NP_004546.1 (1068 residues) and NFATc4, NP_004545.2 (902 residues). Alignment of the ‘atypical' NFAT, NFAT5, has been omitted. The extended and highly conserved Ser/Thr relays (potentially phosphorylated by GSK3) present in the N-terminal regions of NFATc1, NFATc2, NFATc3 and NFATc4 are in underlined, bold italicized type. In NFATc1, these sites are thought to be phosphorylated by GSK3. Other more limited relays are present in individual NFATs and all contain a Ser-rich domain N-terminal to the GSK3 consensus sequences that is phosphorylated by casein kinase 1. (b) GSK3 relays (underlined, bold italicized type) present in GATA4(1–217). (c) GSK3 relays (underlined, bold italicized type) in mouse myocardin (residues 454–466 and 624–636) as identified by Badorff et al. (2005). Identical sequences are present in H. sapiens myocardin. There are also ‘out-of-phase' relays in these regions (shaded, bold and italicized), which are not as strongly conserved in H. sapiens myocardin.
Some years ago, the calcineurin/NFAT system was identified as an important signalling component in cardiac hypertrophy, with NFATc3 (rather than the NFATc4 species studied in the original report) ultimately emerging as a particularly important mediator of the response (Molkentin et al., 1998; Wilkins et al., 2002; Wilkins and Molkentin, 2004). The NFAT isoforms expressed in the heart are not fully defined, partly because of alternative splicing and partly because of the limited specificity of the commercially available antibodies previously available. However, at the mRNA level, NFATc2 transcripts appear to be expressed in considerably greater abundance than the others (LJ De Windt, personal communication). In cardiac myocytes, endothelin-1 reportedly promotes translocation of both GSK3 and NFATs to the nucleus, and the translocation of NFATs is opposed by expression of GSK3β(Ser9Ala) (Haq et al., 2000). Furthermore, in transgenic mice, cardiospecific expression of GSK3β(Ser9Ala) attenuates the increase in nuclear NFAT induced by constitutively activated calcineurin (Antos et al., 2002). These two studies suggest that NFATs may be a target for GSK3 in the heart, and phosphorylation of NFATs by GSK3 may constitute one aspect of the antihypertrophic effect of GSK3. However, the NFAT isoforms involved and the physiological conditions which inhibit GSK3 and promote dephosphorylation of NFATs remain to be fully defined.
β-Catenin
In the canonical Wnt pathway (described above), the Wnt–Frizzled–LRP complex in association with Dishevelled prevents GSK3 from phosphorylating β-catenin and thus promotes β-catenin accumulation (Logan and Nusse, 2004; Cadigan and Liu, 2006; Gordon and Nusse, 2006). β-Catenin migrates to the nucleus to promote gene expression via TCF/LEF1. Although this pathway is important in cardiac development (Eisenberg and Eisenberg, 2007), the influence of GSK3 signalling through β-catenin in the context of myocardial hypertrophy is less clear. It has been proposed that hypertrophic G-protein-coupled receptor agonists promote accumulation of β-catenin in cardiac myocytes in a Wnt-independent manner, but which requires phosphorylation and inhibition of GSK3 by PKB/Akt (Figure 3b; Haq et al., 2003; Shevtsov et al., 2006). Consistent with this, the pro-hypertrophic inhibition of GSK3 in the GSK3β(Lys85Met/Lys86Ile) transgenic mouse promotes accumulation of β-catenin (Hirotani et al., 2007). Furthermore, hearts from Dishevelled-1 null mice (three Dishevelled genes are expressed in mice) or from mice with cardiospecific deletion of β-catenin exhibit a reduced hypertrophic response to TAC (Chen et al., 2006; van der Schans et al., 2007). However, in another setting, cardiospecific deletion of β-catenin causes mild cardiac hypertrophy and does not inhibit the cardiac hypertrophy induced by infusion of angiotensin II (Baurand et al., 2007). Indeed, stabilization (and therefore increased protein expression) of β-catenin impairs adaptive hypertrophic growth response to angiotensin II (Baurand et al., 2007; Zelarayan et al., 2007). It would seem that further studies are required to clarify the role of β-catenin in cardiac myocyte hypertrophy and any influence of GSK3 upon this.
GATA4 and myocardin
GATA4 (Liang and Molkentin, 2002) and myocardin (Liu and Olson, 2006; Parmacek, 2007) are two more transcriptional regulators which may be involved in hypertrophy and which are potentially phosphorylated by GSK3 to inhibit their effects on gene expression. The transcriptional transactivating activity of GATA4 is, at least in part, regulated by nucleocytoplasmic shuttling. Thus, GATA4 contains a ‘non-classical' nuclear localization signal lying within residues 270–324, and a nuclear export signal lying within residues 49–54 (Philips et al., 2007). In a transgenic mouse overexpressing constitutively active PKB/Akt, the nuclear pool of GATA4 was increased, though whether this resulted from any GSK3-mediated phosphorylation was not readily amenable to investigation (Condorelli et al., 2002). In the context of stimulation of cardiac myocytes by β-adrenergic receptor agonists, GSK3 phosphorylates GATA4 to promote its export from the nucleus through the exportin, Crm1 (Morisco et al., 2001). Circumstantial evidence from in vitro experiments suggests that the site of phosphorylation lies within the N terminus (2–116) when this region is primed by PKA, but the site(s) has not been clearly defined in vivo (Morisco et al., 2001). There are three potential GSK3 phosphorylation relay systems within GATA4(2–116) (Figure 4b). Although Morisco et al. (2001) do not exclude the possibility that other sites exist in GATA4(117–205), there are no Ser/Thr ‘relays' in this region (Figure 4b). PKA requires an Arg residue(s) within four residues N-terminal to the phosphorylation site and a hydrophobic residue at +1 (Songyang et al., 1994). The most likely site for GSK3-mediated phosphorylation is thus Ser105 with the priming site lying at Ser109. It is not clear whether such a scheme applies in vivo. However, Ser105 can also be phosphorylated by ERK1/2 and, in this instance, phosphorylation of GATA4 increases its transcriptional transactivating activity (Liang et al., 2001). The location of the GSK3 phosphorylation sites in GATA4 awaits resolution.
Myocardin was identified as a muscle-specific transcriptional co-regulator, which modulates gene expression in association with serum response factor (Wang et al., 2001a). It may be phosphorylated by GSK3 on two relays of Ser residues, Ser454–Ser466 and Ser624–Ser636, (Figure 4c; Badorff et al., 2005). Although Ser466 and Ser636 presumably serve as priming phosphorylations, the kinases responsible have not been identified. GSK3 catalyses phosphorylation of myocardin and inhibits its effects on cardiac myocyte gene expression and hypertrophy, but the biochemical mechanisms have not been explored further (Badorff et al., 2005).
The Jun family, CREB and c-Myc
Other transcriptional regulators that are likely to be important in cardiac hypertrophy are phosphorylated by GSK3 in other systems. These include CREB, Jun family proteins (c-Jun, JunB, JunD) and c-Myc (Clerk et al., 2007). Phosphorylation of CREB presents something of a conundrum. Phosphorylation of CREB catalysed by PKA (and possibly a variety of other kinases, for example, especially the RSK-related MSKs, see Deak et al., 1998) at Ser133 or Ser119 in the H. sapiens ‘long' or ‘short' CREB isoform, respectively, was thought originally to increase its transactivating activity, though it now deemed ‘necessary, but not sufficient' (Johannessen and Moens, 2007). The site (Ser129-Arg-Arg-Pro-Ser133(P)-Tyr) is actually closer to a PKA consensus sequence than to one for RSK-related kinases, though GqPCR-coupled hypertrophic agonists certainly induce phosphorylation of CREB(Ser133) in an ERK1/2 cascade-dependent (RSK-dependent?) manner in cardiac myocytes (Harrison et al., 2004). However, phosphorylation of CREB by GSK3 at Ser129 in the ‘long' CREB isoform with a PKA priming phosphorylation at Ser133 decreases its DNA-binding activity (Bullock and Habener, 1998). It would therefore seem that phosphorylation of Ser129 is dependent on phosphorylation of Ser133, but their effects on transcription could be opposite. It has been proposed that reduction in the GSK3-mediated phosphorylation of CREB(Ser129) plays a role in the cardiac myocyte hypertrophy induced by hypoxia/reoxygenation and the inhibition of GSK3 may involve the ERK1/2 cascade, PKB/Akt and/or PKA (El Jamali et al., 2004). Further study of the role of the various CREB phosphorylation sites in relation to cardiac myocyte hypertrophy is warranted.
In non-myocytes, c-Jun, JunB and JunD are phosphorylated by GSK3 close to their DNA-binding domains to inhibit DNA binding and prevent Jun-directed gene expression (Nikolakaki et al., 1993). Phosphorylation of c-Jun by GSK3 also promotes its ubiquitination and degradation (Wei et al., 2005). Similarly, phosphorylation of c-Myc by GSK3 may prime it for proteolysis by the ubiquitin–proteasome system (Henriksson et al., 1993; Sears et al., 2000; Gregory et al., 2003). We have recently shown that 1-azakenpaullone or insulin rapidly increases Jun/Fos binding to the AP-1 consensus sequence (TGAGTCA) in cardiac myocytes, but to a lesser extent than endothelin-1 (Markou et al., 2008). The transcription factor most clearly associated with the AP-1 sequence is JunB. Consistent with this, binding of JunB to the AP-1 sequence is negatively regulated by GSK3, thus inhibition of GSK3 should increase JunB binding to the AP-1 sequence (Nikolakaki et al., 1993). H. sapiens JunB contains a GSK3 relay close to the DNA-binding site between residues 251 and 259 (248–256 in rat) with Ser259 (Ser256 in rat) representing the priming site. Interestingly, expression of JunB protein is also increased by 1-azakenpaullone or insulin, a finding which complicates interpretation of AP-1-binding data because increased binding could simply represent a mass action effect rather than a dephosphorylation event (Markou et al., 2008).
Eukaryotic initiation factor 2B
In addition to changes in gene expression, cardiac myocyte hypertrophy is associated with increases in the rate of protein synthesis. One point of translational regulation involves eIF2 (Proud, 2007). During translational initiation, eIF2.GTP brings the initiator Met-tRNAi to the 40S ribosomal subunit. GTP is hydrolysed during the initiation process, generating eIF2.GDP which is released and must be recharged with GTP before being recycled. eIF2B is the guanine nucleotide exchange factor for eIF2. Of the five eIF2B subunits, the ɛ subunit binds to eIF2 and enhances GTP/GDP exchange. eIF2Bɛ is phosphorylated on at least four sites, including a GSK3 site at Ser535 in rat eIF2Bɛ (Ser540 in H. sapiens; Ser536 in mouse) with a priming site at Ser539 (Ser544 in H. sapiens; Ser540 in mouse) (Wang et al., 2001b). Phosphorylation of rat eIF2Bɛ(Ser535) by GSK3 inhibits its activity, preventing recycling of eIF2 and inhibiting translational initiation. Conversely, inhibition of GSK3 reduces phosphorylation of eIF2Bɛ(Ser535), increases the recycling of eIF2 and hence increases translational initiation. Although dephosphorylation of eIF2Bɛ(Ser535) has the potential to increase the rate of protein synthesis, it is not clearly established that this is the case. Thus, in Chinese hamster ovary cells, dephosphorylation of ‘eIF2Bɛ(Ser535)' (residue numbering for the hamster unknown) occurs in response to insulin (a powerful stimulator of protein synthesis generally and the initiation step in particular) via a PI3K-dependent pathway (Wang et al., 2002). Although GSK3 inhibitors (Li+ and two pharmacological inhibitors) also cause the dephosphorylation of eIF2Bɛ(Ser535), they do not affect overall eIF2B activity (Wang et al., 2002). Thus, the significance of eIF2Bɛ(Ser535) phosphorylation in translational initiation is difficult to assess but again, the GSK3 inhibitors have ‘additional effects' on the protein synthesis machinery, suggesting that the results should be interpreted cautiously.
In rat cardiac myocytes, β-adrenergic receptor agonism decreases phosphorylation of eIF2Bɛ(phospho-Ser535), probably through inhibition of GSK3 (Hardt et al., 2004). (Note that there is some confusion about the residue numbering because the commercial suppliers state that the anti-phospho-eIF2Bɛ antibody is raised against H. sapiens eIF2Bɛ(phospho-Ser539), which is the correct numbering for the GSK3 site if the N-terminal Met-residue is not included.) In the GSK3β(Lys85Met/Lys86Ile) transgenic mouse, pro-hypertrophic inhibition of GSK3 promotes dephosphorylation of the GSK3 phosphorylation site (eIF2Bɛ(phospho-Ser540)) (Hirotani et al., 2007). Thus, eIF2Bɛ may represent a relevant site of regulation in the hypertrophic response.
Relationship of GSK3 signalling to class I histone deacetylases in the heart
Histone acetylation and deacetylation regulate gene expression in eukaryotic cells and tissues, including the heart (Backs and Olson, 2006). Whereas histone acetyltransferases stimulate gene expression by disrupting chromatin, histone deacetylases (HDACs) repress gene expression. However, because individual genes may encode positive or negative regulators of cell processes, histone acetylases and HDACs can each exert individual positive or negative effects. Mammalian HDACs fall into three classes based on their similarity to yeast orthologues. Generally, class II HDACs repress cardiac gene expression and myocyte growth but the situation regarding the classes I and III HDACs is less clear. It has been proposed that class I HDACs might repress antihypertrophic pathways and thus promote the hypertrophic response (Kook et al., 2003). Hence, pharmacologically or genetically induced deficiency in class I HDAC activity leads to an inhibition of the hypertrophic response.
Recently, an interesting connection between the class I HDAC, HDAC2, and GSK3 has been revealed (Trivedi et al., 2007). This does not involve positioning of HDACs downstream from GSK3, but vice versa. The pathway proposes that HDAC2 normally represses expression of the gene encoding inositol polyphosphate-5-phosphatase f. This enzyme (Minagawa et al., 2001) hydrolyses the 5′ phosphate in PtdIns(3,4,5)P3 (Figure 5), the phospholipid signalling molecule controlling the activation of PKB/Akt (Figure 2; Vanhaesebroeck and Alessi, 2000). The proposal (Trivedi et al., 2007) is that a decrease in HDAC2 increases expression of inositol polyphosphate-5-phosphatase f, which decreases the plasma membrane pools of PtdIns(3,4,5)P3. This prevents activation of PKB/Akt and the subsequent inhibition of GSK3, ultimately preventing the development of hypertrophy induced by, for example, TAC. The evidence for involvement of HDAC2 in hypertrophy via GSK3 is primarily genetic or morphological, though the phosphorylation of PKB/Akt and GSK3 is decreased in HDAC2-null mice (Trivedi et al., 2007).
Figure 5.
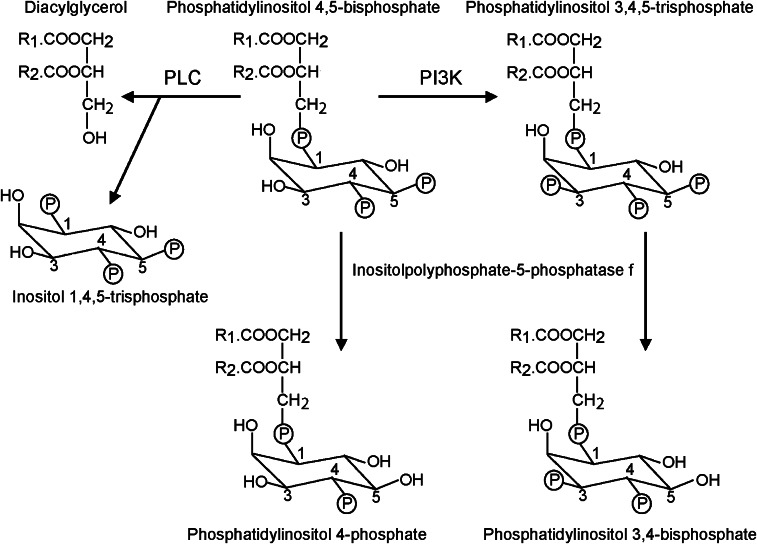
Relevant aspects of phosphoinositide metabolism. Phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) is an important phospholipid in cell signalling producing three separate ‘second messenger' molecules. It is hydrolysed by a variety of phospholipase C (PLC) isoforms to produce diacylglycerol and inositol 1,4,5-trisphosphate. The former is the physiological activator of the diacylglycerol-regulated protein kinase C isoforms (see Figure 2), whereas the latter is important in the regulation of intracellular Ca2+ movements. In cardiac myocytes, the phospholipase Cβ isoforms are best studied in this regard. In a second signalling pathway, PtdIns(4,5)P2 is phosphorylated by phosphoinositide 3′ kinase (PI3K) to form phosphatidylinositol 3,4,5-trisphosphate (PtdIns(3,4,5)P3). This regulates the activity of the 3-phosphoinositide-dependent kinase/protein kinase B (Akt) signalling pathway (see Figure 2). Phosphoinositides are dephosphorylated (hydrolysed) by a number of lipid phosphatases. One such phosphatase which hydrolyses the 5-phosphate of phosphatidylinositol 4,5-bisphosphate and PtdIns(3,4,5)P3 is inositol polyphosphate-5-phosphatase f, which produces phosphatidylinositol 4-phosphate and phosphatidylinositol 3,4-bisphosphate, respectively.
It should be noted that inositol polyphosphate-5-phosphatase f will not only deplete the plasma membrane of PtdIns(3,4,5)P3 but also of any 5-phosphoinositides, including phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) (Minagawa et al., 2001). In an analogous paradigm, depletion of PtdIns(4,5)P2 (induced by overexpression of constitutively active Gαq and hence supranormal activation of phospholipase Cβ (Adams et al., 1998) has profound effects in cardiac myocytes and promotes apoptosis (Howes et al., 2003), a phenomenon not examined in detail by Trivedi et al. (2007). The pro-apoptotic effects of PtdIns(4,5)P2 depletion observed by Howes et al. (2003) were attributed to resultant decreases in PtdIns(3,4,5)P3 and repression of the PKB/Akt-dependent pro-survival and -growth pathways. The low level of tonic activity exhibited by PKB/Akt in cardiac myocytes that can be reduced by PI3K inhibitors (Pham et al., 2000) may be relevant because this may be important in diurnal growth and survival. However, it seems unlikely that PKB/Akt and GSK3 would be the sole signalling proteins affected by inositol polyphosphate-5-phosphatase f expression (or by supranormal phospholipase Cβ activation). Depletion of PtdIns(4,5)P2 would ultimately be expected to interfere with GqPCR-mediated activation of phospholipase Cβ, PKC and the ERK1/2 cascade (and maybe with phosphorylation of GSK3 via the ERK1/2 cascade) with consequent repression of hypertrophy, but we are not aware of any studies in myocardial preparations. This is important because GqPCR/Gαq signalling is involved in in vivo hypertrophy induced, for example, by TAC (Akhter et al., 1998). Further examination of paradigms involving modulation of HDCA2 expression (for example, further biochemical evidence from measurement of phosphoinositide concentrations and signalling protein activities) is needed.
GSK3 and apoptosis
GSK3 activity has been associated with both apoptosis and cell survival, though it is perhaps the former as is seen in neuroblastoma cells (Bijur et al., 2000) and cardiac myocytes (Menon et al., 2007) that has attracted more attention (Jope and Johnson, 2004). A pro-apoptotic role of GSK3 in the heart is consistent with the findings of Hirotani et al. (2007) in the GSK3β(Lys85Met/Lys86Ile)-expressing transgenic mouse and in cardiac myocytes, as discussed earlier. Hirotani et al. (2007) also show that expression of the antiapoptotic and rapidly turning-over Bcl2 family member, Mcl-1 (Yang-Yen, 2006), is increased in GSK3β(Lys85Met/Lys86Ile)-expressing mice, though whether increased expression of Mcl-1 is a prime determinant of the improved myocyte survival is unclear. As suggested, a pro-apoptotic role of GSK3 is important because promotion of apoptosis in experimental situations could be equated with inhibition of hypertrophy.
GSK3 research in relation to myocardium hypertrophy: some unanswered questions
Although a number of studies support the notion of an antihypertrophic function for active GSK3 (particularly GSK3β), it is our view that the information is incomplete and a number of issues remain to be addressed. First, there seems to be confusion over likely signalling events leading to GSK3 phosphorylation and inhibition. The presumption is that PKB/Akt (through the PI3K pathway) exclusively regulates GSK3, but other kinases clearly phosphorylate the same inhibitory sites. Second, although a very recent publication (Zhai et al., 2007) has helped to redress the balance, too much emphasis may have been placed on GSK3β as opposed to GSK3α. Our results (Markou et al., 2008) clearly demonstrate phosphorylation of both isoforms and others have identified a role for GSK3α in the regulation of glycogen synthesis in the heart (Mora et al., 2005). Third, although Li+ ions are widely used as inhibitors of GSK3, their use is compromised by their low efficacy and specificity. More selective small molecule inhibitors (Cohen and Goedert, 2004) are becoming increasingly available and should probably be used in preference to Li+. In our experiments (Markou et al., 2008), we have made use of 1-azakenpaullone, as described above. Fourth, if GSK3 is involved with the export of transcription factors from the nucleus or in the regulation of nuclear-localized transcription factors, it should be present in that compartment, as we have observed (Markou et al., 2008). However, glycogen synthesis is primarily a cytoplasmic process. The implication is that at least two pools of GSK3 must exist. There is some evidence of this in skeletal muscle (Jensen et al., 2007), but there is very little information on subcellular compartmentalization of GSK3 signalling within cardiac myocytes. Fifth, even though some transcription factors are already implicated in GSK3 signalling, the integration of transcription factor activities and the genes that they regulate is still unknown. Sixth, the studies in transgenic mice are confusing. Several studies using overexpression of wild-type GSK3β or GSK3α, or expression of GSK3β(Ser9Ala) suggest that catalytically active forms of GSK3 are antihypertrophic in the heart in vivo. However, other studies have suggested that GSK3 activity, although antihypertrophic, is also pro-fibrotic and pro-apoptotic and that it decreases contractile function, with inhibition of GSK3 by transgenesis having the opposite effect (Michael et al., 2004; Hirotani et al., 2007; Zhai et al., 2007). All these studies involve transgene overexpression that is difficult to control (for example, differences in copy number and genetic background between lines can be problematical), and the effects can be difficult to interpret. Further studies with GSK3α(Ser21Ala)/GSK3β(Ser9Ala) ‘knock-in' mice in which single copies of the transgenes are under the control of their endogenous promoters may be more informative, but here, preliminary experiments suggest that HW/BW is increased in the ‘knock-in' mice (K Sakamoto, personal communication). Overall, we consider that the precise molecular mechanisms through which GSK3 participates in the hypertrophic response still need to be determined.
Is manipulation of GSK3 activity a viable myocardial therapy?
In the introduction to this article, we briefly assessed the possible utility of GSK3 activation as an antihypertrophic therapy. This was the most obvious clinical application of the earlier findings (Haq et al., 2000; Antos et al., 2002), but perhaps the utility of such manoeuvres should be reconsidered in the light of more recent work that has shown increased fibrosis, apoptosis and decreased contractility in mice in which GSK3 is activated (Michael et al., 2004; Hirotani et al., 2007; Zhai et al., 2007). Indeed, inhibition may be a better course of action. If activation of GSK3 is beneficial, it is difficult to see how this could be achieved directly using small molecules. In contrast, if inhibition is beneficial, small molecule inhibitors are available and ex vivo experiments have shown that inhibition may represent the philosopher's stone of myocardial therapeutics, namely myocyte regeneration (Tseng et al., 2006). However, as pointed out by Cohen and Goedert (2004), there are fears about the potential oncogenicity of pleiotropic GSK3 inhibition. Currently, the only feasible method which we can envisage whereby therapeutic modulation of GSK3 in the heart could be achieved cardiospecifically is through a ‘gene therapy' approach. To summarize, we just do not know whether to inhibit or activate GSK3, or simply not to interfere. What might be useful would be the further study of a transgenic mouse model involving inducible expression of GSK3α(Ser21Ala) or GSK3β(Ser9Ala) at a stage that would be most clinically relevant, that is, when the heart is already failing. Admittedly, Sanbe et al. (2003) carried out such a study in mice with established TAC-induced left ventricular hypertrophy, but did not examine the contractile function of the hearts. Perhaps this is one model that should be revisited.
Glossary
- APC
adenomatous polyposis coli
- BW
body weight
- eIF
eukaryotic initiation factor
- ERK
extracellular signal-regulated kinase
- GSK3
glycogen synthase kinase 3
- HDAC
histone deacetylase
- HW
heart weight
- IGF 1
insulin-like growth factor 1
- LRP
lipoprotein receptor-related protein
- MAPK
mitogen-activated protein kinase
- MSK
mitogen- and stress-activated protein kinase
- NFAT
nuclear factor of activated T cell
- PtdIns(4,5)P2
phosphatidylinositol 4,5-bisphosphate
- PtdIns(3,4,5)P3
phosphatidylinositol 3,4,5-trisphosphate
- PI3K
phosphoinositide 3′ kinase
- PKA
cyclic AMP-dependent protein kinase A
- PKB
protein kinase B
- PKC
protein kinase C
- RSK
p90-ribosomal S6 kinase
- TAC
transverse aortic constriction
- TCF/LEF1
T-cell-specific transcription factor/lymphoid enhancer binding factor 1
- TL
tibial length
Footnotes
Conflict of interest
The authors state no conflict of interest.
References
- Adams JW, Sakata Y, Davis MG, Sah VP, Wang Y, Liggett SB, et al. Enhanced Gαq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc Natl Acad Sci USA. 1998;95:10140–10145. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter SA, Luttrell LM, Rockman HA, Iaccarino G, Lefkowitz RJ, Koch WJ. Targeting the receptor–Gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science. 1998;280:574–577. doi: 10.1126/science.280.5363.574. [DOI] [PubMed] [Google Scholar]
- Ali A, Hoeflich KP, Woodgett JR. Glycogen synthase kinase-3: properties, functions, and regulation. Chem Rev. 2001;101:2527–2540. doi: 10.1021/cr000110o. [DOI] [PubMed] [Google Scholar]
- Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J Appl Physiol. 2001;90:1900–1908. doi: 10.1152/jappl.2001.90.5.1900. [DOI] [PubMed] [Google Scholar]
- Andersen JB, Rourke BC, Caiozzo VJ, Bennett AF, Hicks JW. Postprandial hypertrophy in pythons. Nature. 2005;434:37–38. doi: 10.1038/434037a. [DOI] [PubMed] [Google Scholar]
- Antos CL, McKinsey TA, Frey N, Kutschke W, McAnally J, Shelton JM, et al. Activated glycogen synthase-3β suppresses cardiac hypertrophy in vivo. Proc Natl Acad Sci USA. 2002;99:907–912. doi: 10.1073/pnas.231619298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backs J, Olson EN. Control of cardiac growth by histone acetylation/deacetylation. Circ Res. 2006;98:15–24. doi: 10.1161/01.RES.0000197782.21444.8f. [DOI] [PubMed] [Google Scholar]
- Badorff C, Seeger FH, Zeiher AM, Dimmeler S. Glycogen synthase kinase 3β inhibits myocardin-dependent transcription and hypertrophy induction through site-specific phosphorylation. Circ Res. 2005;97:645–654. doi: 10.1161/01.RES.0000184684.88750.FE. [DOI] [PubMed] [Google Scholar]
- Baurand A, Zelarayan L, Betney R, Gehrke C, Dunger S, Noack C, et al. Catenin downregulation is required for adaptive cardiac remodeling. Circ Res. 2007;100:1353–1362. doi: 10.1161/01.RES.0000266605.63681.5a. [DOI] [PubMed] [Google Scholar]
- Beals CR, Sheridan CR, Turck CW, Gardner P, Crabtree GR. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997;275:1930–1933. doi: 10.1126/science.275.5308.1930. [DOI] [PubMed] [Google Scholar]
- Belke DD, Betuing S, Tuttle MJ, Graveleau C, Young ME, Pham M, et al. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest. 2002;109:629–639. doi: 10.1172/JCI13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijur GN, De Sarno P, Jope RS. Glycogen synthase kinase-3β facilitates staurosporine- and heat shock-induced apoptosis. Protection by lithium. J Biol Chem. 2000;275:7583–7590. doi: 10.1074/jbc.275.11.7583. [DOI] [PubMed] [Google Scholar]
- Bossemeyer D. The glycine-rich sequence of protein kinases: a multifunctional element. Trends Biochem Sci. 1994;19:201–205. doi: 10.1016/0968-0004(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Brancaccio M, Fratta L, Notte A, Hirsch E, Poulet R, Guazzone S, et al. Melusin, a muscle-specific integrin β1-interacting protein, is required to prevent cardiac failure in response to chronic pressure overload. Nat Med. 2003;9:68–75. doi: 10.1038/nm805. [DOI] [PubMed] [Google Scholar]
- Bullock BP, Habener JF. Phosphorylation of the cAMP response element binding protein CREB by cAMP-dependent protein kinase A and glycogen synthase kinase-3 alters DNA-binding affinity, conformation, and increases net charge. Biochemistry. 1998;37:3795–3809. doi: 10.1021/bi970982t. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Liu YI. Wnt signaling: complexity at the surface. J Cell Sci. 2006;119:395–402. doi: 10.1242/jcs.02826. [DOI] [PubMed] [Google Scholar]
- Catalucci D, Latronico MVG, Ellsingen O, Condorelli G. Physiological myocardial hypertrophy: how and why. Front Biosci. 2008;13:312–324. doi: 10.2741/2681. [DOI] [PubMed] [Google Scholar]
- Chen X, Shevtsov SP, Hsich E, Cui L, Haq S, Aronovitz M, et al. The β-catenin/T-cell factor/lymphocyte enhancer factor signaling pathway is required for normal and stress-induced cardiac hypertrophy. Mol Cell Biol. 2006;26:4462–4473. doi: 10.1128/MCB.02157-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerk A, Aggeli I-K, Stathopoulou K, Sugden PH. Peptide growth factors signal differentially through protein kinase C to extracellular signal-regulated kinase in neonatal cardiomyocytes. Cell Signal. 2006;18:225–235. doi: 10.1016/j.cellsig.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Clerk A, Cullingford TE, Fuller SJ, Giraldo A, Markou T, Pikkarainen S, et al. Signaling pathways mediating cardiac myocyte gene expression in physiological and stress responses. J Cell Physiol. 2007;212:311–322. doi: 10.1002/jcp.21094. [DOI] [PubMed] [Google Scholar]
- Clerk A, Michael A, Sugden PH. Stimulation of the p38 mitogen-activated protein kinase pathway in neonatal rat ventricular myocytes by the G protein-coupled receptor agonists, endothelin-1 and phenylephrine: a role in cardiac myocyte hypertrophy. J Cell Biol. 1998;142:523–535. doi: 10.1083/jcb.142.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. The twentieth century struggle to decipher insulin signalling. Nat Rev Mol Cell Biol. 2006;7:867–873. doi: 10.1038/nrm2043. [DOI] [PubMed] [Google Scholar]
- Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- Cohen P, Goedert M. GSK3 inhibitors: development and therapeutic potential. Nat Rev Drug Discov. 2004;3:479–487. doi: 10.1038/nrd1415. [DOI] [PubMed] [Google Scholar]
- Cole A, Frame S, Cohen P. Further evidence that the tyrosine phosphorylation of glycogen synthase kinase-3 (GSK3) in mammalian cells is an autophosphorylation event. Biochem J. 2004;377:249–255. doi: 10.1042/BJ20031259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condorelli G, Drusco A, Stassi G, Bellacosa A, Roncarati R, Iaccarino G, et al. Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc Natl Acad Sci USA. 2002;99:12333–12338. doi: 10.1073/pnas.172376399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109:S67–S79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- Dajani R, Fraser E, Roe SM, Young N, Good V, Dale TC, et al. Crystal structure of glycogen synthase kinase 3β: structural basis for phosphate-primed substrate specificity and autoinhibition. Cell. 2001;105:721–732. doi: 10.1016/s0092-8674(01)00374-9. [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak M, Clifton AD, Lucocq JM, Alessi DR. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 1998;17:4426–4441. doi: 10.1093/emboj/17.15.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker RS, Cook MG, Behnke-Barclay M, Decker ML. Some growth factors stimulate cultured adult rabbit ventricular myocyte hypertrophy in the absence of mechanical loading. Circ Res. 1995;77:544–555. doi: 10.1161/01.res.77.3.544. [DOI] [PubMed] [Google Scholar]
- Dorn GW., II The fuzzy logic of physiological cardiac hypertrophy. Hypertension. 2007;49:962–970. doi: 10.1161/HYPERTENSIONAHA.106.079426. [DOI] [PubMed] [Google Scholar]
- Dorn GW, II, Robbins J, Sugden PH. Phenotyping hypertrophy: eschew obfuscation. Circ Res. 2003;92:1171–1175. doi: 10.1161/01.RES.0000077012.11088.BC. [DOI] [PubMed] [Google Scholar]
- Duerr RL, Huang S, Miraliakbar HR, Clark R, Chien KR, Ross J., Jr Insulin-like growth factor-1 enhances ventricular hypertrophy and function during the onset of experimental cardiac failure. J Clin Invest. 1995;95:619–627. doi: 10.1172/JCI117706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghbali M, Deva R, Alioua A, Minosyan TY, Ruan H, Wang Y, et al. Molecular and functional signature of heart hypertrophy during pregnancy. Circ Res. 2005;96:1208–1216. doi: 10.1161/01.RES.0000170652.71414.16. [DOI] [PubMed] [Google Scholar]
- Eisenberg LM, Eisenberg CA. Evaluating the role of Wnt signal transduction in promoting the development of the heart. ScentificWorldJournal. 2007;7:161–176. doi: 10.1100/tsw.2007.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Jamali A, Freund C, Rechner C, Scheidereit C, Dietz R, Bergmann MW. Reoxygenation after severe hypoxia induces cardiomyocyte hypertrophy in vitro: activation of CREB downstream of GSK3β. FASEB J. 2004;18:1096–1098. doi: 10.1096/fj.03-1054fje. [DOI] [PubMed] [Google Scholar]
- Embi N, Rylatt DB, Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980;107:519–527. [PubMed] [Google Scholar]
- Fang X, Yu SX, Lu Y, Bast RC, Jr, Woodgett JR, Mills GB. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci USA. 2000;97:11960–11965. doi: 10.1073/pnas.220413597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame S, Cohen P, Biondi RM. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol Cell. 2001;7:1321–1327. doi: 10.1016/s1097-2765(01)00253-2. [DOI] [PubMed] [Google Scholar]
- Frödin M, Gammeltoft S. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol Cell Endocrinol. 1999;151:65–77. doi: 10.1016/s0303-7207(99)00061-1. [DOI] [PubMed] [Google Scholar]
- Fuller SJ, Mynett JR, Sugden PH. Stimulation of cardiac protein synthesis by insulin-like growth factors. Biochem J. 1992;282:85–90. doi: 10.1042/bj2820085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo EM, Canté-Barrett K, Crabtree GR. Lymphocyte calcium signaling from membrane to nucleus. Nat Immunol. 2006;7:25–32. doi: 10.1038/ni1295. [DOI] [PubMed] [Google Scholar]
- Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- Graef IA, Mermelstein PG, Stankunas K, Neilson JR, Deisseroth K, Tsien RW, et al. L-Type calcium channels and GSK-3 regulate the activity of NF-ATc4 in hippocampal neurons. Nature. 1999;401:703–708. doi: 10.1038/44378. [DOI] [PubMed] [Google Scholar]
- Gregory MA, Qi Y, Hann SR. Phosphorylation by glycogen synthase kinase-3 controls c-myc proteolysis and subnuclear localization. J Biol Chem. 2003;278:51606–51612. doi: 10.1074/jbc.M310722200. [DOI] [PubMed] [Google Scholar]
- Gwack Y, Sharma S, Nardone J, Tanasa B, Iuga A, Srikanth S, et al. A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature. 2006;441:646–650. doi: 10.1038/nature04631. [DOI] [PubMed] [Google Scholar]
- Haan C, Kreis S, Margue C, Behrmann I. Jaks and cytokine receptors—an intimate relationship. Biochem Pharmacol. 2006;72:1538–1546. doi: 10.1016/j.bcp.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Hallcher LM, Sherman WR. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem. 1980;255:10896–10901. [PubMed] [Google Scholar]
- Haq S, Choukroun G, Kang ZB, Ranu H, Matsui T, Rosenzweig A, et al. Glycogen synthase kinase-3β is a negative regulator of cardiomyocyte hypertrophy. J Cell Biol. 2000;151:117–129. doi: 10.1083/jcb.151.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq S, Choukroun G, Lim H, Tymitz KM, del Monte F, Gwathmey J, et al. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation. 2001;103:670–677. doi: 10.1161/01.cir.103.5.670. [DOI] [PubMed] [Google Scholar]
- Haq S, Michael A, Andreucci M, Bhattacharya K, Dotto P, Walters B, et al. Stabilization of β-catenin by a Wnt-independent mechanism regulates cardiac myocyte growth. Proc Natl Acad Sci USA. 2003;100:4610–4615. doi: 10.1073/pnas.0835895100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546:113–120. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- Hardt SE, Sadoshima J. Glycogen synthase kinase 3β: a novel regulator of cardiac hypertrophy and development. Circ Res. 2002;90:1055–1063. doi: 10.1161/01.res.0000018952.70505.f1. [DOI] [PubMed] [Google Scholar]
- Hardt SE, Tomita H, Katus HA, Sadoshima J. Phosphorylation of eukaryotic translation initiation factor 2Bɛ by glycogen synthase kinase-3β regulates β-adrenergic cardiac myocyte hypertrophy. Circ Res. 2004;94:926–935. doi: 10.1161/01.RES.0000124977.59827.80. [DOI] [PubMed] [Google Scholar]
- Harrison JG, Sugden PH, Clerk A. Endothelin-1 promotes phosphorylation of CREB transcription factor in primary cultures of neonatal rat cardiac myocytes: implications for the regulation of c-jun expression. Biochim Biophys Acta. 2004;1644:17–25. doi: 10.1016/j.bbamcr.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- Henriksson M, Bakardjiev A, Klein G, Lüscher B. Phosphorylation sites mapping in the N-terminal domain of c-myc modulate its transforming potential. Oncogene. 1993;8:3199–3209. [PubMed] [Google Scholar]
- Hirotani S, Zhai P, Tomita H, Galeotti J, Marquez JP, Gao S, et al. Inhibition of glycogen synthase 3β during heart failure is protective. Circ Res. 2007;101:1164–1174. doi: 10.1161/CIRCRESAHA.107.160614. [DOI] [PubMed] [Google Scholar]
- Howes AL, Arthur JF, Zhang T, Miyamoto S, Adams JW, Dorn GW, II, et al. Akt-mediated cardiac myocyte survival pathways are compromised by Gαq-induced phosphoinositide 4,5-bisphosphate depletion. J Biol Chem. 2003;278:40343–40351. doi: 10.1074/jbc.M305964200. [DOI] [PubMed] [Google Scholar]
- Hu P, Zhang D, Swenson L, Chakrabarti G, Abel ED, Litwin SE. Minimally invasive aortic banding in mice: effects of altered cardiomyocyte insulin signaling during pressure overload. Am J Physiol Heart Circ Physiol. 2003;285:H1261–H1269. doi: 10.1152/ajpheart.00108.2003. [DOI] [PubMed] [Google Scholar]
- Ito H, Hiroe M, Hirata Y, Tsujino M, Adachi S, Shichiri M, et al. Insulin-like growth factor-I induces hypertrophy with enhanced expression of muscle specific genes in cultured rat cardiomyocytes. Circulation. 1993;87:1715–1721. doi: 10.1161/01.cir.87.5.1715. [DOI] [PubMed] [Google Scholar]
- Jensen J, Brennesvik EO, Lai Y-C, Shepherd PR. GSK-3β regulation in skeletal muscles by adrenaline and insulin: evidence that PKA and PKB regulate different pools of GSK-3. Cell Signal. 2007;19:204–210. doi: 10.1016/j.cellsig.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Johannessen M, Moens U. Multisite phosphorylation of the cAMP response element-binding protein (CREB) by a diversity of protein kinases. Front Biosci. 2007;12:1814–1832. doi: 10.2741/2190. [DOI] [PubMed] [Google Scholar]
- Jope RS, Johnson GVM. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Kemp TJ, Aggeli IK, Sugden PH, Clerk A. Phenylephrine and endothelin-1 upregulate connective tissue growth factor in neonatal rat cardiac myocytes. J Mol Cell Cardiol. 2004;37:603–606. doi: 10.1016/j.yjmcc.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Kerkelä R, Woulfe K, Force T. Glycogen synthase kinase-3β—actively inhibiting hypertrophy. Trends Cardiovasc Med. 2007;17:91–96. doi: 10.1016/j.tcm.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Cohen P. Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem J. 1999;339:319–328. [PMC free article] [PubMed] [Google Scholar]
- Kook H, Lepore JJ, Gitler AD, Lu MM, Wing-Man Yung W, Mackay J, et al. Cardiac hypertrophy and histone deacetylase-dependent transcriptional repression mediated by the atypical homeodomain protein Hop. J Clin Invest. 2003;112:863–871. doi: 10.1172/JCI19137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunick C, Lauenroth K, Leost M, Meijer L, Lemcke T. 1-Azakenpaullone is a selective inhibitor of glycogen synthase kinase-3β. Bioorg Med Chem Lett. 2004;14:413–416. doi: 10.1016/j.bmcl.2003.10.062. [DOI] [PubMed] [Google Scholar]
- Li M, Wang X, Meintzer MK, Laessig T, Birnbaum MJ, Heidenreich KA. Cyclic AMP promotes neuronal survival by phosphorylation of glycogen synthase kinase 3β. Mol Cell Biol. 2000;20:9356–9363. doi: 10.1128/mcb.20.24.9356-9363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Q, Molkentin JD. Divergent signaling pathways converge on GATA4 to regulate cardiac hypertrophic gene expression. J Mol Cell Cardiol. 2002;34:611–616. doi: 10.1006/jmcc.2002.2011. [DOI] [PubMed] [Google Scholar]
- Liang Q, Wiese RJ, Bueno OF, Dai YS, Markham BE, Molkentin JD. The transcription factor GATA4 is activated by extracellular signal-regulated kinase 1- and 2-mediated phosphorylation of serine 105 in cardiomyocytes. Mol Cell Biol. 2001;21:7460–7469. doi: 10.1128/MCB.21.21.7460-7469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Wang S, Han C, Zhang Y. 14-3-3 proteins regulate glycogen synthase 3β phosphorylation and inhibit cardiomyocyte hypertrophy. FEBS J. 2005;272:1845–1854. doi: 10.1111/j.1742-4658.2005.04614.x. [DOI] [PubMed] [Google Scholar]
- Liu N, Olson EN. Coactivator control of cardiovascular growth and remodeling. Curr Opin Cell Biol. 2006;18:715–722. doi: 10.1016/j.ceb.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Luckey SW, Mansoori J, Fair K, Antos CL, Olson EN, Leinwand LA. Blocking cardiac growth in hypertrophic cardiomyopathy induces cardiac dysfunction and decreased survival only in males. Am J Physiol Heart Circ Physiol. 2007;292:H838–H845. doi: 10.1152/ajpheart.00615.2006. [DOI] [PubMed] [Google Scholar]
- Markou T, Cullingford TE, Giraldo A, Weiss SC, Alsafi A, Fuller SJ, et al. Glycogen synthase kinases 3α and 3β in cardiac myocytes: regulation and consequences of their inhibition. Cell Signal. 2008;20:206–218. doi: 10.1016/j.cellsig.2007.10.004. [DOI] [PubMed] [Google Scholar]
- McMullen JR, Shioi T, Huang W-Y, Zhang L, Tarnavski O, Bisping E, et al. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase(p110α) pathway. J Biol Chem. 2004;279:4782–4793. doi: 10.1074/jbc.M310405200. [DOI] [PubMed] [Google Scholar]
- Menon B, Johnson JN, Ross RS, Singh M, Singh K. Glycogen synthase kinase-3β plays a pro-apoptotic role in β-adrenergic receptor-stimulated apoptosis in adult rat ventricular myocytes: role of β1 integrins. J Mol Cell Cardiol. 2007;42:653–661. doi: 10.1016/j.yjmcc.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael A, Haq S, Chen X, Hsich E, Cui L, Walters B, et al. Glycogen synthase kinase-3β regulates growth, calcium homeostasis, and diastolic functions in the heart. J Biol Chem. 2004;279:21383–21393. doi: 10.1074/jbc.M401413200. [DOI] [PubMed] [Google Scholar]
- Minagawa T, Ijuin T, Mochizuki Y, Takenawa T. Identification and characterization of a Sac domain-containing phosphoinositide 5-phosphatase. J Biol Chem. 2001;276:22011–22015. doi: 10.1074/jbc.M101579200. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Lu J-R, Antos CL, Markham B, Richardson J, Robbins J, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora A, Sakamoto K, McManus EJ, Alessi DR. Role of the PDK1–PKB–GSK3 pathway in regulating glycogen synthase and glucose uptake in the heart. FEBS Lett. 2005;579:3632–3638. doi: 10.1016/j.febslet.2005.05.040. [DOI] [PubMed] [Google Scholar]
- Morgan HE, Jefferson LS, Wolpert EB, Rannels DE. Regulation of protein synthesis in heart muscle. II. Effect of amino acid levels and insulin on ribosomal aggregation. J Biol Chem. 1971;246:2163–2170. [PubMed] [Google Scholar]
- Morisco C, Seta K, Hardt SE, Lee Y, Vatner SF, Sadoshima J. Glycogen synthase kinase 3β regulates GATA4 in cardiac myocytes. J Biol Chem. 2001;276:28586–28597. doi: 10.1074/jbc.M103166200. [DOI] [PubMed] [Google Scholar]
- Morisco C, Zebrowski D, Condorelli G, Tsichlis P, Vatner SF, Sadoshima J. The Akt-glycogen synthase kinase 3β pathway regulates transcription of atrial natriuretic factor induced by β-adrenergic receptor stimulation in cardiac myocytes. J Biol Chem. 2000;275:14466–14475. doi: 10.1074/jbc.275.19.14466. [DOI] [PubMed] [Google Scholar]
- Neal JW, Clipstone NA. Glycogen synthase kinase-3 inhibits the DNA binding activity of NFATc. J Biol Chem. 2001;276:3666–3673. doi: 10.1074/jbc.M004888200. [DOI] [PubMed] [Google Scholar]
- Nikolakaki E, Coffer PJ, Hemelsoet R, Woodgett JR, Defize LH. Glycogen synthase kinase 3 phosphorylates Jun family members in vitro and negatively regulates their transactivating potential in intact cells. Oncogene. 1993;8:833–840. [PubMed] [Google Scholar]
- Nilsson J, Nilsson LM, Chen Y-W, Molkentin JD, Erlinge D, Gomez MF. High glucose activates nuclear factor of activated T cells in native vascular smooth muscle. Arterioscler Thromb Vasc Biol. 2006;26:794–800. doi: 10.1161/01.ATV.0000209513.00765.13. [DOI] [PubMed] [Google Scholar]
- Obata T, Yaffe MB, Leparc GG, Piro ET, Maegawa H, Kashiwagi A, et al. Peptide and protein library screening defines optimal substrate motifs for AKT/PKB. J Biol Chem. 2000;275:36108–36115. doi: 10.1074/jbc.M005497200. [DOI] [PubMed] [Google Scholar]
- Okamura H, Garcia-Rodriguez C, Martinson H, Qin J, Virshup DM, Rao A. A conserved docking motif for CK1 binding controls the nuclear localization of NFAT1. Mol Cell Biol. 2004;24:4184–4195. doi: 10.1128/MCB.24.10.4184-4195.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet. 2006;367:356–367. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- Palermo J, Gulick J, Ng W, Grupp IL, Grupp G, Robbins J. Remodeling the mammalian heart using transgenesis. Cell Mol Biol Res. 1995;41:501–509. [PubMed] [Google Scholar]
- Parmacek MS. Myocardin-related transcription factors. Critical coactivators regulating cardiovascular development and adaptation. Circ Res. 2007;100:633–644. doi: 10.1161/01.RES.0000259563.61091.e8. [DOI] [PubMed] [Google Scholar]
- Perez-Moreno M, Fuchs E. Catenins: keeping cells from getting their signals crossed. Dev Cell. 2006;11:601–612. doi: 10.1016/j.devcel.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham FH, Sugden PH, Clerk A. Regulation of protein kinase B and 4E-BP1 by oxidative stress in cardiac myocytes. Circ Res. 2000;86:1252–1258. doi: 10.1161/01.res.86.12.1252. [DOI] [PubMed] [Google Scholar]
- Philips AS, Kwok JC, Chong BH. Analysis of the signals and mechanisms mediating nuclear trafficking of GATA-4. Loss of DNA binding is associated with localization in intranuclear speckles. J Biol Chem. 2007;282:24915–24927. doi: 10.1074/jbc.M701789200. [DOI] [PubMed] [Google Scholar]
- Picton C, Woodgett J, Hemmings B, Cohen P. Multisite phosphorylation of glycogen synthase from rabbit skeletal muscle. Phosphorylation of site 5 by glycogen synthase kinase-5 (casein kinase-II) is a prerequisite for phosphorylation of sites 3 by glycogen synthase kinase-3. FEBS Lett. 1982;150:191–196. doi: 10.1016/0014-5793(82)81332-x. [DOI] [PubMed] [Google Scholar]
- Preedy VR, Smith DM, Kearney NF, Sugden PH. Rates of protein turnover in vivo and in vitro in ventricular muscle of hearts from fed and starved rats. Biochem J. 1984;222:395–400. doi: 10.1042/bj2220395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proud CG. Signalling to translation: how signal transduction pathways control the protein synthetic machinery. Biochem J. 2007;403:217–234. doi: 10.1042/BJ20070024. [DOI] [PubMed] [Google Scholar]
- Rannels DE, Kao R, Morgan HE. Effect of insulin on protein turnover in heart muscle. J Biol Chem. 1975;250:1694–1701. [PubMed] [Google Scholar]
- Sanbe A, Gulick J, Hanks MC, Liang Q, Osinska H, Robbins J. Reengineering inducible cardiac-specific transgenesis with an attenuated myosin heavy chain promoter. Circ Res. 2003;92:609–616. doi: 10.1161/01.RES.0000065442.64694.9F. [DOI] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Sears R, Nuckolls F, Haura E, Taya Y, Tamai K, Nevins JR. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 2000;14:2501–2514. doi: 10.1101/gad.836800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan CM, Heist EK, Beals CR, Crabtree GR, Gardner P. Protein kinase A negatively modulates the nuclear accumulation of NF-ATc1 by priming for subsequent phosphorylation by glycogen synthase kinase-3. J Biol Chem. 2002;277:48664–48676. doi: 10.1074/jbc.M207029200. [DOI] [PubMed] [Google Scholar]
- Shevtsov SP, Haq S, Force T. Activation of β-catenin signaling pathways by classical G-protein-coupled receptors. Mechanisms and consequences in cycling and non-cycling cells. Cell Cycle. 2006;5:2295–2300. doi: 10.4161/cc.5.20.3357. [DOI] [PubMed] [Google Scholar]
- Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006;20:3347–3365. doi: 10.1101/gad.1492806. [DOI] [PubMed] [Google Scholar]
- Smith DM, Fuller SJ, Sugden PH. The effects of lactate, acetate, glucose, insulin, starvation and alloxan-diabetes on protein synthesis in perfused rat hearts. Biochem J. 1986;236:543–547. doi: 10.1042/bj2360543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z, Blechner S, Hoagland N, Hoekstra MF, Piwnica-Worms H, Cantley LC. Use of an orientated peptide library to determine the optimal substrates of protein kinases. Curr Biol. 1994;4:973–982. doi: 10.1016/s0960-9822(00)00221-9. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- Sugden PH, Clerk A. Cellular mechanisms of cardiac hypertrophy. J Mol Med. 1998;76:725–746. doi: 10.1007/s001090050275. [DOI] [PubMed] [Google Scholar]
- Sugden PH, Smith DM. The effects of glucose, acetate, lactate and insulin on protein degradation in the perfused rat heart. Biochem J. 1982;206:467–472. doi: 10.1042/bj2060467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taurin S, Hogarth K, Sandbo N, Yau DM, Dulin NO. Gβγ-mediated prostacyclin production and cAMP-dependent protein kinase activation by endothelin-1 promotes vascular smooth muscle cell hypertrophy through inhibition of glycogen synthase kinase-3. J Biol Chem. 2007;282:19518–19525. doi: 10.1074/jbc.M702655200. [DOI] [PubMed] [Google Scholar]
- Tong H, Imahashi K, Steenbergen C, Murphy E. Phosphorylation of glycogen synthase kinase-3β during preconditioning through a phosphatidylinositol-3-kinase-dependent pathway is cardioprotective. Circ Res. 2002;90:377–379. doi: 10.1161/01.res.0000012567.95445.55. [DOI] [PubMed] [Google Scholar]
- Trivedi CM, Luo Y, Yin Z, Zhang M, Zhu W, Wang T, et al. Hdac2 regulates the cardiac hypertrophic response by modulating Gsk3β activity. Nat Med. 2007;13:324–331. doi: 10.1038/nm1552. [DOI] [PubMed] [Google Scholar]
- Tseng A-S, Engel FB, Keating MT. The GSK-3 inhibitor BIO promotes proliferation in mammalian cardiomyocytes. Chem Biol. 2006;13:957–963. doi: 10.1016/j.chembiol.2006.08.004. [DOI] [PubMed] [Google Scholar]
- van de Schans VAM, van den Borne SWM, Strzelecka AE, Janssen BJ, van der Velden JL, Langen RC, et al. Interuption of Wnt signaling attenuates the onset of pressure overload-induced cardiac hypertrophy. Hypertension. 2007;49:473–480. doi: 10.1161/01.HYP.0000255946.55091.24. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Alessi DR. The PI3K–PDK1 connection: more than just a road to PKB. Biochem J. 2000;346:561–576. [PMC free article] [PubMed] [Google Scholar]
- Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, et al. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 2001a;105:851–862. doi: 10.1016/s0092-8674(01)00404-4. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang X, Proud CG. Activation of mRNA translation in rat cardiac myocytes by insulin involves multiple rapamycin-sensitive steps. Am J Physiol Heart Circ Physiol. 2000;278:H1056–H1068. doi: 10.1152/ajpheart.2000.278.4.H1056. [DOI] [PubMed] [Google Scholar]
- Wang QM, Fiol CJ, DePaoli-Roach AA, Roach PJ. Glycogen synthase kinase-3β is a dual specificity kinase differentially regulated by tyrosine and serine/threonine phosphorylation. J Biol Chem. 1994;269:14566–14574. [PubMed] [Google Scholar]
- Wang X, Janmaat M, Beugnet A, Paulin FEM, Proud CG. Evidence that the dephosphorylation of Ser535 in the ɛ-subunit of eukaryotic initiation factor (eIF) 2B is insufficient for the activation of eIF2B by insulin. Biochem J. 2002;367:475–481. doi: 10.1042/BJ20020677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Paulin FEM, Campbell LE, Gomez E, O'Brien K, Morrice N, et al. Eukaryotic initiation factor 2B: identification of multiple phosphorylation sites in the ɛ-subunit and their functions in vivo. EMBO J. 2001b;20:4349–4359. doi: 10.1093/emboj/20.16.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Jin J, Schlisio S, Harper JW, Kaelin WG., Jr The v-Jun point mutation allows c-Jun to escape GSK3-dependent recognition and destruction by the Fbw7 ubiquitin ligase. Cancer Cell. 2005;8:25–33. doi: 10.1016/j.ccr.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Wilkins BJ, De Windt LJ, Bueno OF, Braz JC, Glascock BJ, Kimball TF, et al. Targeted disruption of NFATc3, but not NFATc4, reveals an intrinsic defect in calcineurin-mediated cardiac growth. Mol Cell Biol. 2002;22:7603–7613. doi: 10.1128/MCB.22.21.7603-7613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins BJ, Molkentin JD. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochem Biophys Res Commun. 2004;322:1178–1191. doi: 10.1016/j.bbrc.2004.07.121. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JFP, MacDonald C, Nielsen JN, Hellsten Y, Hardie DG, Kemp BE, et al. Regulation of 5′AMP-activated protein kinase activity and substrate utilization in exercising human skeletal muscle. Am J Physiol Endocrinol Metab. 2003;284:E813–E822. doi: 10.1152/ajpendo.00436.2002. [DOI] [PubMed] [Google Scholar]
- Woodgett JR. Judging a protein by more than its name: GSK-3. Sci STKE. 2001;2001:RE12. doi: 10.1126/stke.2001.100.re12. [DOI] [PubMed] [Google Scholar]
- Wu X, Zhang T, Bossuyt J, McKinsey TA, Dedman JR, Olson EN, et al. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation–contraction coupling. J Clin Invest. 2006;116:675–682. doi: 10.1172/JCI27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang-Yen HF. Mcl-1: a highly regulated cell death and survival controller. J Biomed Sci. 2006;13:201–204. doi: 10.1007/s11373-005-9064-4. [DOI] [PubMed] [Google Scholar]
- Zelarayan L, Gehrke C, Bergmann MW. Role of β-catenin in adult cardiac remodeling. Cell Cycle. 2007;6:2120–2126. doi: 10.4161/cc.6.17.4632. [DOI] [PubMed] [Google Scholar]
- Zhai P, Gao S, Holle E, Yu X, Yatani A, Wagner T, et al. Glycogen synthase kinase-3α reduces cardiac growth and pressure overload-induced cardiac hypertrophy by inhibition of extracellular signal regulated kinases. J Biol Chem. 2007;282:33181–33191. doi: 10.1074/jbc.M705133200. [DOI] [PubMed] [Google Scholar]