Abstract
Numerous failures in clinical stroke trials have led to some pessimism in the field. This short review examines the following questions: Can experimental models of stroke be validated? How can combination stroke therapies be productively pursued? Can we achieve neuroprotection without reperfusion? And finally, can we move from a pure neurobiology view of stroke towards a more integrative approach targeting all cell types within the entire neurovascular unit? Emerging data from both experimental models and clinical findings suggest that neurovascular mechanisms may provide new opportunities for treating stroke. Ultimately, both bench-to-bedside and bedside-back-to-bench interactions may be required to overcome the translational hurdles for this challenging disease.
Keywords: cerebral ischaemia, cerebral haemorrhage, neuroprotection, blood–brain barrier, animal models, clinical trials
Introduction
It is increasingly clear that a strictly linear bench-to-bedside paradigm may not be optimal for translating basic science findings into clinically effective stroke therapies. There are now numerous examples where neuroprotection has apparently failed in the clinic. However, with each clinical trial, one learns more about the biology of stroke and its response to potential therapies. In this short review, it is proposed that a bedside-back-to-bench paradigm will also be needed if we are to translate into clinical meaning the enormous advances we have made in the basic science of experimental stroke. The following questions will be broadly examined: (a) Can clinical results inform us about the validity of experimental stroke models? (b) Are combination therapies feasible in stroke therapy? (c) Is reperfusion necessary even in the context of ‘pure' neuroprotection? (d) What new targets may be revealed if we shift from a strict ‘neurobiology of stroke' towards a more integrative formulation, wherein we interrogate mechanisms within the entire neurovascular unit?
Experimental stroke models
In the early days of experimental stroke research, invasive surgical techniques predominated, with particular emphasis on transorbital approaches in cats and nonhuman primates where the middle cerebral artery could be accessed and occluded with aneurysm clips, permanent ligation or electrocautery. In the early 1980s, Tamura et al. (1981) described a new approach whereby a craniotomy was performed next to the zygomatic arch to reveal and occlude proximal portions of the middle cerebral artery as it enters the base of the brain in rats. However, the surgery proved challenging. Subsequently, development of the intraluminal filament model triggered an explosion of experimental work with easier-to-use rodent models (Longa et al., 1989; Kawamura et al., 1991). A modified suture could be threaded up the internal carotid artery until the tip occluded the middle cerebral artery, thus inducing focal cerebral ischaemia. Because no surgical invasion of the cranium was needed, the technique was simpler, thus allowing rodent stroke models to be widely used. Another important step was taken when the technique was adapted for mice. Transgenic mice that overexpressed a specific gene or knockouts that lacked a specific gene could be used to ask sophisticated questions about molecular and cellular pathology in the brain after stroke (Hossmann, 2004; Liang et al., 2004). In concert with these mechanical models of arterial occlusion came the development of clot models. Of particular note, Chopp and colleagues (Zhang et al., 1997a, 1997b) pioneered a technique in which homologous blood clots could be placed in the middle cerebral artery in rats and mice via modified catheters. With these models in hand, one may now study novel therapies in combination with tissue plasminogen activator thrombolysis. It is hoped that further development of clot-based embolic models may bring us even closer to questions relevant for clinical stroke.
Multiple clinical trials for the treatment of acute ischaemic stroke were conducted in the 1990s, but all were unsuccessful. Can animal models truly replicate clinical stroke? The answer is complex, and it should be recognized that, in the end, these are only models of stroke, not stroke itself, which is a multifactorial human disease (del Zoppo, 1998; DeGraba and Pettigrew, 2000; Hoyte et al., 2004; Fisher and Tatlisumak, 2005; Kaste, 2005). Use of any model system has to be carefully titrated for questions that make each system relevant and specific. Furthermore, important quality control issues in the animal model literature may exist, and these need to be addressed (Dirnagl, 2006). Nevertheless, although animal models have not directly led to a ‘cure' for stroke, one can find many examples in which these models have correlated quite well with clinical findings in terms of the pathophysiology of stroke.
The first example of experimental and clinical correlation comes from the idea of selective neuronal vulnerability. Models of transient global brain ischaemia in gerbils, rats and mice all show selective and delayed neuron death in the hippocampus (Benveniste, 1991). This matches well with the human experience, whereby neurologic deficits correspond with transient brain ischaemia associated with cardiac arrest (Petito et al., 1987). These selective neuronal responses reflect the basic science concept that active cell death mechanisms become triggered after an ischaemic insult (Lo et al., 2003). Furthermore, these molecular pathways can be experimentally manipulated, and blockade of excitotoxic, free-radical and apoptotic mediators have all been shown to reduce hippocampal damage after transient global cerebral ischaemia.
One such broad-spectrum approach to neuroprotection involves hypothermia, which influences multiple pathways (Corbett and Thornhill, 2000; Lo et al., 2003). Reassuringly, similar results as those seen in animals have been obtained in humans, and effective neurologic salvage by mild-to-moderate hypothermia has been validated by several recent trials in adult cardiac arrest patients (Bernard et al., 2002; Hypothermia after Cardiac Arrest Study Group, 2002), as well as neonates after hypoxia–ischaemia (Shankaran et al., 2005).
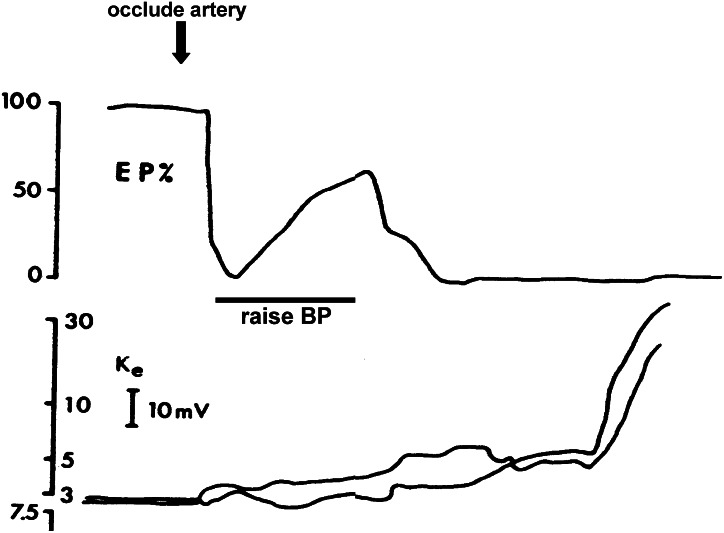
A second example supporting the utility of animal stroke models involves work from the late 1970s by Astrup et al., 1977, which defines what is now recognized as the ischaemic penumbra. Focal occlusion of the middle cerebral artery in nonhuman primate brains resulted in central ‘core' areas of severe ischaemia where tissue damage evolved rapidly. But in peripheral cortex adjacent to the core, there existed areas where blood flow deficits were less severe and energy loss was modest. In these penumbral areas, the ability to fire evoked action potentials was lost, but there was sufficient remnant energy to sustain neuronal resting membrane potentials (Astrup et al., 1977). The reversibility of these penumbral areas was elegantly demonstrated by simply raising blood pressure and presumably enhancing collateral blood flow. With increasing blood pressure, evoked potentials were partially restored (Figure 1). Ultimately, without reperfusion, the penumbra collapsed, resting membrane potentials decayed and the so-called anoxic depolarization occurred. These landmark studies comprised both blood flow (moderate ischaemia) and electrophysiologic (loss of action potential without loss of membrane potential) definitions of the ischaemic penumbra. But it is now clear that multiple manifestations of the penumbra exist. Positron emission tomography studies in stroke patients show areas where loss of blood flow is temporarily compensated via elevated oxygen extraction fraction (Marchal et al., 1996; Powers and Zazulia, 2003). Histologically, at least in animal models, rims of selective neuronal death may surround core areas of pan-necrosis (Kato and Kogure, 1999). Elaborate dissections of the cellular and biochemical pathways of stroke have revealed select profiles of gene activation in focal cerebral ischaemia, constituting a molecular penumbra that may eventually reveal novel targets for intervention (Sharp et al., 2000). It is worth noting that the penumbra may also be a useful concept for haemorrhagic stroke as well. It has been suggested that in perihaematomal areas, neurovascular proteases may be upregulated that mediate oedema, and active cell death mechanisms may concomitantly occur (Matsushita et al., 2000; Felberg et al., 2002; Hoff and Xi, 2003; Lu et al., 2006; Rosell et al., 2006; Tejima et al., 2006). Ultimately, perhaps the only ‘type' of penumbra that matters may be the pharmacologic penumbra, that is whatever can be salvaged after stroke, if one has the correct therapeutic strategies.
Figure 1.
The original definition of the ischaemic penumbra as an electrophysiologic phenomenon. In areas of moderate blood flow loss, evoked potentials (upper panel) are affected whereas membrane potentials (lower panel) are maintained. With improved blood flow, partial recovery of evoked potentials is possible. Without reperfusion, the membrane potential ultimately collapses as anoxic depolarization occurs. Adapted from Astrup et al. (1977), with permission from Stroke and the American Heart Association.
Does the penumbra exist in clinical stroke? Advances in neuroimaging suggest that it does. As mentioned above, positron emission tomography scanning seems to identify a metabolic penumbra (Heiss et al., 1994; Marchal et al., 1996; Powers and Zazulia, 2003). Magnetic resonance imaging (MRI) shows gradients of blood flow deficits spreading across severely ischaemic core and adjacent brain tissue (Bradley et al., 1989; Kucharczyk et al., 1993; Schlaug et al., 1999). In the early 1990s, Moseley et al. (1990, 1995) pioneered a novel set of MRI tools termed diffusion-weighted imaging, whereby changes in the apparent diffusion coefficients of water are reduced very early during stroke evolution. Today, perfusion-weighted MRI and diffusion-weighted imaging are widely used to assess acute stroke patients. Wherever perfusion deficits are larger than areas of diffusion lesions, it is presumed that a penumbra exists. Of course, many caveats remain. The so-called core of elevated water diffusion may be reversible under some conditions. Also, quantitation of larger perfusion defects may depend on the types of analysis used including mean transit time profiles, peak bolus magnitudes, area under first-pass curves or calculated volume or flow mapping. Although the pathophysiologic underpinnings of these perfusion–diffusion mismatches remain to be fully elucidated, these imaging assays may at least provide clinically useful surrogate estimates of the penumbra (Kidwell et al., 2000; Røhl et al., 2001; Warach, 2001).
Can neuroimaging help in the design of clinical trials? In theory, at least some of the variation in these trials may be due to the fact that stroke patients comprise a heterogeneous population. If one can ‘serotype' patients to find the best responders, the statistics might improve. An initial example may be found in the Desmoteplase In Acute Ischemic Stroke trial that tested the efficacy of the vampire bat plasminogen activator (Hacke et al., 2005). Perhaps use of strict neuroimaging criteria might have helped to extend the therapeutic window for reperfusion (Davis et al., 2005). However, at the time of writing of this paper, initial reports suggest that Desmoteplase In Acute Ischemic Stroke trial 2 might have failed to replicate the extended treatment window phenomenon. Many questions will have to be carefully addressed as the data are processed. But initial questions may include potential differences between MR versus computed tomography-based perfusion imaging, or the disturbing possibility that using perfusion–diffusion mismatch criteria may inadvertently capture subsets of patients with spontaneous recovery as well. Perhaps a simple comparison of perfusion and diffusion is not powerful enough. Many groups are now investigating sophisticated ways to calculate MRI ‘signatures' that may allow one to better select patients for experimental therapies (Jacobs et al., 2001; Wu et al., 2001, 2005; Mitsias et al., 2005), as well as following their recovery outcomes in the future (Chopp et al., 2007).
Combination stroke therapy
How do brain cells die after stroke? A large body of basic research over the past 2 decades implicates mechanisms involving excitotoxicity, oxidative stress and programmed cell death (Lipton, 1999; Chan, 2001; Graham and Chen, 2001). Briefly, when oxygen and glucose supplies are interrupted after stroke, energy failure occurs, ionic gradients are lost and dysregulated glutamate release–reuptake mediates calcium and generalized ionic imbalance. Multiple proteases (both intra- and extracellular) are activated that degrade cellular integrity. Mitochondrial functions such as oxidative phosphorylation fail, and free radicals, including reactive oxygen and reactive nitrogen species, are released that may attack proteins, lipids and nucleic acids. Families of ‘executioner' molecules, for example, caspases, disrupt multiple cell processes in cytoplasm and the nucleus to promote cell death by programmed mechanisms resembling apoptosis. Necrosis may proceed by analogous programmed pathways as well (Driscoll and Gerstbrein, 2003; Degterev et al., 2005).
However, despite overwhelming experimental evidence, selective targets derived from each of these three major cell death pathways have not directly led to a ‘cure.' Although there are surely multiple reasons why (De Keyser et al., 1999; Gladstone et al., 2002), an important part of the equation may be that the molecular pathways triggered after stroke are highly nonlinear and interconnected. Because of the presence of feedback loops and crosstalk, redundant mechanisms of cell death are likely to emerge, so that targeting a single point in a single pathway may not yield sufficient neuroprotection. Recent experimental studies have provided many examples of crosstalk. Tymianski's group (Aarts et al., 2003) has shown that free radicals can further amplify deleterious calcium currents via neuronal transient receptor potential channels of the melastatin family. Nicotera's group (Bano etal., 2005) has defined mechanisms whereby initial calcium-activated calpain in turn cleaves the sodium–calcium exchanger, thus exacerbating ionic imbalance and excitotoxicity. Lipton's group (Bossy-Wetzel et al., 2004) has demonstrated that crosstalk between nitric oxide and p38 stress-activated protein kinase may augment neuronal demise after hypoxia. A recent survey of how stroke research has targeted cell death mechanisms reveals that the overwhelming majority of studies are focused on single pathways (Lo et al., 2005). On the basis of the complexity of pro-life and pro-death signalling and the involvement of multiple cell types in brain, a shift in emphasis will be needed towards targets that mediate crosstalk between multiple cell death mechanisms. From the basic perspective of cell and molecular biology, a motivation for combination stroke therapy is compelling.
Are there examples of combination therapies that work? Many examples can be drawn from experimental stroke models. Whereas hypothermia will surely activate many neuroprotective mechanisms simultaneously, it has been demonstrated that combining temperature control with thrombolysis results in synergistic protection in rat embolic clot models of focal ischaemia (Meden et al., 1994). Similarly, Ma et al. (1998) have shown that combining caspase inhibitors with either MK801 to block NMDA receptors or fibroblast growth factor (Ma et al., 2001) can yield additional neuroprotection in rodent models of stroke. More recently, it has been suggested that recombinant tissue plasminogen activator (rt-PA) may have pleiotropic actions beyond its intended use as a clot lysis agent (Benchenane et al., 2004; Kaur et al., 2004; Lo et al., 2004). Within blood, rt-PA dissolves fibrin clots, but at the neurovascular interface, rt-PA may augment NMDA currents (Nicole et al., 2001), as well as trigger upregulation in extracellular matrix metalloproteinase (MMP) systems (Wang et al., 2003). Chopp, Lawrence, and colleagues (Zhang et al., 2002) have shown that combining rt-PA with a neuronal rt-PA inhibitor neuroserpin can in fact extend therapeutic windows and improve reperfusion efficacy. A similar finding has also been obtained when rt-PA is combined with a GPIIbIIIa antagonist (Zhang et al., 2003).
In contrast to the many examples of successful combination therapy in the experimental literature, achieving success in clinical stroke is much more difficult. There may be inherent regulatory and commercial barriers involved, and the statistical powering of such combination trials is challenging. The recent SAINT I and SAINT II trials attempted to tackle this difficult issue, and subsets of patients received both rt-PA as well as the tested free radical spin trap NXY-059. Initial data from SAINT I seemed promising. A secondary analysis comparing patients receiving rt-PA alone versus rt-PA plus NXY-059 suggested that the incidence of rt-PA-associated intracerebral haemorrhage appeared to be reduced by NXY-059 (Lees et al., 2006). These initial findings matched the preclinical data quite well. Previous studies in rabbit and hypertensive rat models of embolic stroke demonstrated that nitrone-based spin traps may significantly reduce the severity of rt-PA-associated haemorrhagic conversion (Asahi et al., 2000; Lapchak et al., 2002). Unfortunately, however, this early promise was not replicated in the follow-up SAINT II trial (Shuaib et al., 2007). Many issues remain to be addressed including dose–response profiles in human versus animal studies, and the question as to whether the free radicals were being targeted in vascular versus parenchymal compartments. Nevertheless, these initial signals in SAINT trials remain suggestive and provides proof-of-concept that these admittedly challenging combination therapy trials can be performed. Adding neuroprotectants to thrombolytics may be a required way forward in the future.
Reperfusion versus neuroprotection
If the primary cause of cell death in ischaemic stroke is lack of blood flow, then how can one expect to protect brain tissue without re-establishing cerebral perfusion? Can we have neuroprotection without reperfusion? A quick survey of the experimental animal model literature suggests that evidence for neuroprotection in animal models of permanent occlusion is less robust than that for transient focal cerebral ischaemia. A formal meta-analysis of FK506 in the published literature demonstrated that the odds ratio for neuroprotection is significantly better for models of transient as opposed to permanent cerebral artery occlusions (Macleod et al., 2005). Perhaps not surprisingly, one may initially conclude that transient ischaemic brain tissue is easier to protect, no matter what the target might be.
Magnetic resonance imaging investigations of perfusion deficits versus diffusion lesions in experimental stroke models support these ideas. Over time, the evolution of brain damage corresponds with progressive changes in the presumed surrogate measurements of penumbra, that is areas of perfusion–diffusion mismatch collapses over time. Reperfusion stops the process, and clearly the therapeutic window measured from the time of stroke onset would therefore be much longer in transient versus permanent arterial occlusions. Adjunct stroke therapies might therefore seek to prolong these reperfusion windows. For example, data suggest that normobaric hyperoxia may transiently sustain penumbral tissue and ‘buy time' for reperfusion (Singhal et al., 2002a, 2002b, 2005; Kim et al., 2005). Intriguingly, the use of normobaric hyperoxia have been shown to work in both animal models as well as small sets of clinical stroke patients.
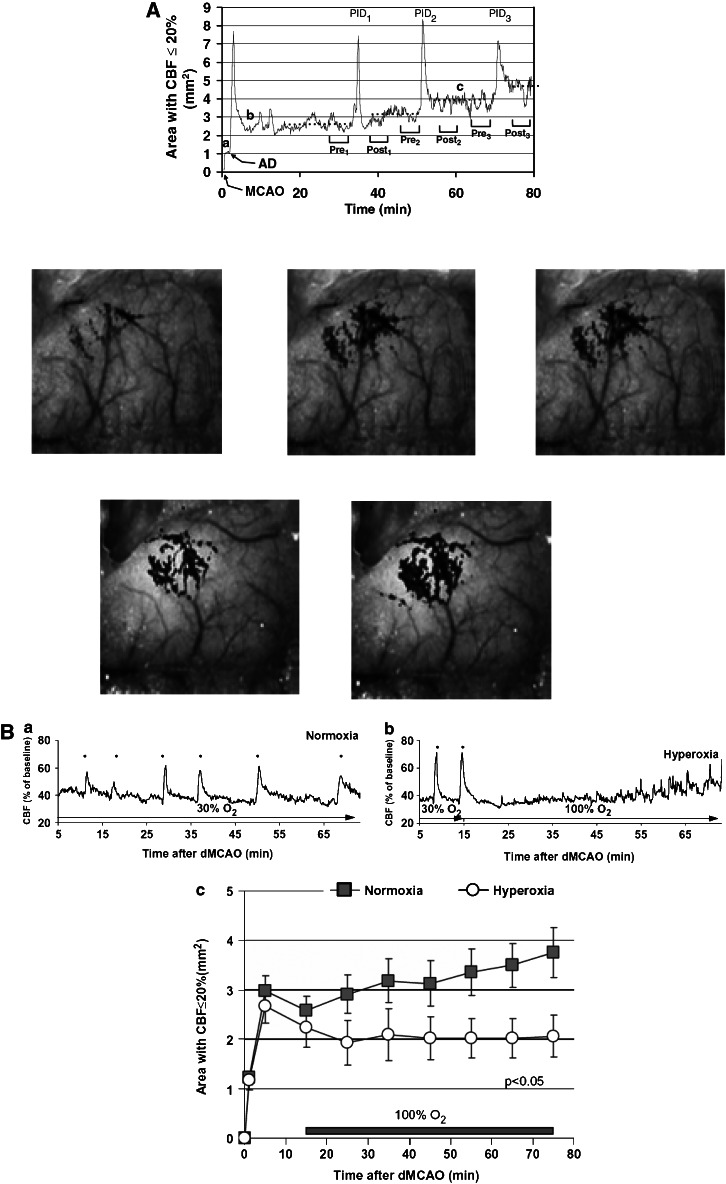
From a purely conceptual perspective, how would one expect to salvage tissue in the long term when it remains ischaemic? The answer may be derived from experimental focal ischaemia models where waves of peri-infarct depolarizations are detected, emanating from the core through the penumbral areas (Shin et al., 2006, 2007). With each wave of spreading depression, energetic stress occurs and peri-infarct tissue is subjected to added pressure. These depolarizing waves may also trigger biochemical and molecular cell death pathways that go on to degrade initially ‘normal' tissue. Thus over time, damaged brain areas expand. Within this conceptual framework, one can then envisage scenarios where neuroprotection is theoretically possible even without reperfusion because one would be targeting areas beyond the initial region of reduced blood flow caused by vessel occlusion. In fact, neuroprotection with NMDA antagonists was demonstrated to be correlated with reductions in these waves of peri-infarct depolarizations (Lauritzen and Hansen, 1992). More recently, advanced laser speckle imaging technologies allow high-resolution mapping of these neurovascular events after permanent cerebral arterial occlusions in animal models (Figure 2) (Ayata et al., 2004; Shin et al., 2006, 2007). Even in permanent ischaemia, waves of damaging neurovascular perturbations spread out to harm ‘at-risk' brain tissue. Re-establishing blood flow is admittedly the logical approach to relieve ischaemia, but reperfusion may not necessarily be the gold standard therapy for stroke, at least from these theoretical considerations.
Figure 2.
(A) Spreading waves of cortical depression emanate from the ischaemic focus in mice subjected to focal occlusion of the middle cerebral artery. Each passage of these peri-ischaemic waves appears to expand areas of severely hypoperfused cortex, thus inducing a growth of the lesion over time. Upper panels show quantified data. Lower panels depict speckle contrast images of cerebral perfusion collected during representative experiment. Adapted from Shin et al. (2006), with permission from Annals of Neurology. (B) Normobaric hyperoxia suppresses spreading waves of cortical depression in mice subjected to focal occlusion of the middle cerebral artery (upper panel), prevents the collapse of penumbral tissue and ameliorates the expansion of critically ischaemic brain tissue (lower panel). Adapted from Shin et al. (2007), with permission from Brain.
How do findings from the clinical trials arena relate to these discussions? Strictly speaking, the only positive stroke trial was the National Institutes of Neurological Disorders and Stroke trial testing rt-PA thrombolysis (the National Institute of Neurological Disorders and Stroke, 1995). Reperfusion of ischaemic brain is once again the logical therapy. Initially, positive findings from the SAINT I trial raised hopes that clinical neuroprotection (Lees et al., 2006) might in fact match preclinical studies where NXY-059 was shown to reduce infarction in various animal models of permanent focal cerebral ischaemia (Maples et al., 2004; Green and Ashwood, 2005). However, disappointingly, SAINT II did not replicate these initial findings (Shuaib et al., 2007). Clearly, much more research needs to be done before we can truly reach any form of conclusions.
Can we improve our armamentarium of experimental models for translational stroke research? Admittedly, there are no easy answers. Because models are only models and not the human disease itself, one has to use them to answer specific questions. No single model will capture all aspects of the clinical problem. Some groups have proposed what is now termed the STAIR (Stroke Therapy Academic Industry Roundtable) criteria, where multiple facets of preclinical testing are recommended (STAIR, 1999). Some of these include the need to have a wide therapeutic time window, the inclusion of neurologic and behavioural measures and finally the testing of long-term outcomes. Although these do not provide the final answers, they may help us recognize that use of multiple models and end points will likely be important for a difficult and heterogeneous disease such as stroke. For example, the idea that using nonhuman primate models will solve all our problems may be over-simplistic. The recent sequencing of the rhesus monkey genome demonstrated that even in this evolutionarily close species, up to 200 genes in the normal rhesus monkey corresponded to severe point mutations in humans (Rhesus Macaque Genome Sequencing and Analysis Consortium, 2007). Thus a rhesus monkey will obviously not be a perfect replica of human pathophysiologic responses in stroke. As will be discussed below, instead of looking for a single perfect animal model (which may not exist), perhaps what is needed is a series of linked cell-to-tissue-to-animal platforms where multicellular signals and therapeutic targets are dissected altogether.
Targeting the neurovascular unit
The brain is not only made up of neurons. Although this is an obvious statement, it is somewhat surprising that basic science stroke research has traditionally focused on neuronal responses alone. A recent survey of published papers in the PubMed database revealed that the vast majority of mechanistic studies were performed using neurons, whereas much less emphasis was placed on examining responses in glia and cerebral endothelium (Figure 3a). In 2002, National Institutes of Neurological Disorders and Stroke assembled a Stroke Progress Review Group to reassess stroke research and define new directions and opportunities (Stroke Progress Review Group, 2002). A major recommendation emerging from this working group was to shift the emphasis from a purely neurocentric view of cell death in stroke towards a more integrative approach that considers responses in all brain cells and matrix. The concept of the neurovascular unit (fundamentally comprising endothelium, astrocyte and neuron) provides a modular framework where cell–cell signalling and cell–matrix interactions mediate the overall tissue response to stroke and its treatments (Lo et al., 2003; Iadecola, 2004; Zlokovic, 2005; Abbott et al., 2006; Lok et al., 2007) (Figure 3b). Stroke is neither a purely neuronal disease nor a pure blood disorder. Blood flow and brain homoeostasis are perturbed together. Cerebral endothelium becomes dysfunctional. Astroglial and oligodendroglial processes are affected. Neuroinflammation, including microglial activation, is rapidly triggered. Ultimately, neuronal survival and function are degraded. Altogether, one may hypothesize that stroke can be reinterpreted as a disease in cell–cell signalling at the neurovascular interface. In this context, an ideal therapeutic target for stroke would be one that rescues neurovascular signalling and is active in multiple brain cell types. What we may need for stroke is not neuroprotection but rather full cerebroprotection.
Figure 3.
(a) A search of the PubMed database reveals that the majority of basic cell death research in stroke is focused on neurons alone. The number of papers investigating mechanisms in glia and cerebral endothelium are relatively few. A change from a purely neurocentric focus to a more integrative approach is required to address the entire neurovascular unit in stroke. Adapted from Lo et al. (2005), with permission from Stroke. (b) A schematic of the neurovascular unit emphasizing the importance of cell–cell and cell–matrix signalling between neurons, astrocytes and endothelium. This simplified figure does not include many other cell types, including oligodendrocytes, pericytes and smooth muscle cells. Ultimately, all cell interactions must be considered in whole brain physiology and pathology. Adapted from Lo et al. (2004), with permission from Stroke.
A good example of how the neurovascular unit concept may play a role in stroke comes from a recent reconsideration of rt-PA itself, our only federal drug administration-approved treatment for acute ischaemic stroke. Although rt-PA is traditionally thought of as a blood molecule, that is one that mediates fibrinolysis of offending clots, emerging data suggest that it can do a lot more. Tsirka et al. (1996) first demonstrated that rt-PA knockout mice are protected against kainic acid hippocampal injury and focal cerebral ischaemia (Wang et al., 1998). Within brain parenchyma, rt-PA can amplify deleterious calcium currents via the NMDA channel, thus promoting excitotoxicity (Nicole et al., 2001). Free rt-PA in brain may also degrade extracellular laminin between neurons and mediate anoikis-like cell death (Chen and Strickland, 1997; Melchor and Strickland, 2005). More recently, a signalling connection between rt-PA and the MMP family of extracellular proteases have been proposed (Wang et al., 2003, 2004; Lo et al., 2004). In rat embolic stroke models, clot lysis with rt-PA increases ischaemic brain MMP-9 levels, and cotreatment with MMP inhibitors reduced rt-PA-induced haemorrhagic transformation and brain injury (Aoki et al., 2002; Sumii and Lo, 2002). After focal cerebral ischaemia, MMP-9 levels are lower in rt-PA knockout mice compared with wild-type mice (Wang et al., 2003; Tsuji et al., 2005). In human stroke patients, rt-PA upregulates plasma MMP-9 levels, which are correlated with haemorrhagic conversion after thrombolysis (Montaner et al., 2003; Ning et al., 2006). This rt-PA/MMP hypothesis suggests that rt-PA-induced upregulation of MMPs at the neurovascular interface and degradation of extracellular matrix by these proteases may explain in part the complications of oedema and haemorrhagic conversion during reperfusion injury.
To truly protect brain, a stroke drug has to protect all cell types. Conversely, any stroke drug must be carefully considered in terms of its actions on all cell types and neurovascular function at this interface between blood and brain. Although there are no easy solutions, one might presume that during the experimental drug discovery process, one should screen for safety and efficacy not only in neurons but also in all major brain cell types, including cerebral endothelial cells, astrocytes, oligodendrocytes, microglia and so on. Multiplexed assays such as these should likely include cell survival as well as functional tests.
The concept of the neurovascular unit may apply not only during acute stages of stroke and brain injury, but also during delayed phases of stroke recovery. It is increasingly recognized that endogenous responses in brain are triggered by stroke as the brain attempts to heal itself. Neurogenesis and angiogenesis appears to occur within days to weeks (Parent, 2003). At least in experimental models, one can use pharmacologic approaches such as growth factors, erythropoietin, statins or nitric oxide modulators to augment these neurovascular responses and enhance stroke recovery (Zhang et al., 2005). Of course, whether these approaches will translate well into the clinical arena remains to be determined. Perhaps equally importantly, the idea of neurovascular remodelling after stroke might also influence target selection for acute therapies. It is possible that mediators that are detrimental during early stages of stroke may conversely play beneficial roles during delayed stages where neurovascular recovery begins to take place. For example, neurovascular proteases from the MMP family mediate cell death and blood–brain barrier damage in stroke during acute phases of brain injury. But during delayed phases, MMPs may mediate neurogenesis and angiogenesis (Lee et al., 2006; Wang et al., 2006), and augment trophic factor bioavailability for peri-infarct remodelling (Zhao et al., 2006). Hence, targeting neurovascular proteases for stroke therapy will have to carefully balance the blockade of damaging effects while preserving its beneficial actions during stroke recovery (Zhao et al., 2007).
Similar considerations might even be possible for targeting free radicals and other reactive species. It has been suggested that the brain is especially vulnerable to oxidative stress because endogenous antioxidant defenses are relatively low in this highly energetic organ (Halliwell, 2006). Targeting oxidative stress is attractive because of the central importance of oxidative stress in the network of cell death mechanisms after stroke, as first demonstrated by the landmark study from the Chan lab where infarction after focal cerebral ischaemia was significantly reduced in transgenic mice overexpressing human superoxide dismutase (Kinouchi et al., 1991). Free radicals are triggered after excitotoxic insults, and potential feedback loops exist whereby reactive species may modulate transient receptor potential channels of the melastatin class and lead to even more ionic imbalance and excitotoxicity (Aarts and Tymianski, 2005). Superoxide and nitric oxide are also potent triggers of apoptotic-like pathways of neuronal death (Sugawara et al., 1999), so there are interactions between oxidative stress and programmed cell death as well. Furthermore, oxidative stress will affect not just neurons but all components of the neurovascular unit, including glia and endothelium. But are there any potential downsides to a potent quenching of all free radicals? Whereas an overgeneration of radicals will surely be damaging, ‘functional' levels of nitric oxide are critical for normal synaptic function and cerebrovascular tone (Iadecola, 1993; Endres et al., 2004). Nitric oxide is a central mediator in neurovascular coupling and function. In addition, hydrogen peroxide is also recognized to play important roles in intracellular redox signalling (Rhee, 2006). Will signalling actions of reactive species also be involved in neurovascular remodelling after stroke? How one can balance the prevention of acute damaging effects of reactive species while preserving or even enhancing potentially beneficial signalling effects within the entire neurovascular unit is a topic that warrants further study. More broadly, it will be critical to characterize the precise spatial gradients and timeframes whereby the transitions occur from acute brain injury into remodelling and repair. A deeper understanding of these profiles should allow us to more logically address the following questions: What targets are we going after? Where do these targets lie in the multicellular spectrum of the brain? How can we reach and affect these targets and for long are the targets active so that we can define treatment windows?
Conclusion
Can experimental targets be validated in human systems? What are the true therapeutic time windows with and without reperfusion? Can we identify with neuroimaging those patients who may benefit the most from various therapies, and can these selection parameters help us refine our experimental approaches? Do combination therapies really work? What cells are we trying to save anyway? Finally, even under the best of circumstances, how much brain can we realistically expect to salvage, and will morphologic rescue always translate into improved clinical outcomes? These are all exceedingly difficult questions, compounded by the fact that stroke is not only a neurodegenerative disorder but also a medical emergency. Timeframes for decision-making become compressed, and therapeutic success is correspondingly challenging. A broader focus on cerebroprotection rather than just neuroprotection may be required. Experimental models should probably encompass linked platforms of multicell-to-tissue-to-animal systems because no single perfect whole animal model exists. Moreover, finally, careful attention should be paid to spatial and temporal transitions between injury and endogenous repair mechanisms.
Because the translational hurdles are enormous, valuable information should be obtained and dissected from the positive or negative results of each clinical trial. Closer communication and collaboration between the clinician and the basic stroke scientist should provide the key as we move ahead. In the United States, National Institutes of Neurological Disorders and Stroke investigators have supported a network of Specialized Programs Of Translational Research In Acute Stroke for several years (http://grants.nih.gov/grants/guide/pa-files/PAR-05-084.html, accessed 8/4/06), and a matching preclinical network for experimental models has also been recently proposed (http://grants.nih.gov/grants/guide/rfa-files/RFA-NS-08-001.html). Similar consortia in Europe have been initiated as well (Meairs et al., 2006). Collaborations between industrial and academic partners should likewise be encouraged to explore boundaries between established targets and novel pathways. Within these multiple networks and consortia, opportunities will emerge for both bench-to-bedside and bedside-back-to-bench interactions, as we collectively search for a widely effective stroke therapy.
Acknowledgments
Supported in part by NIH Grants R01-NS37074, R01-NS48422, R01-NS53560, R01-NS56458, P50-NS10828 and P01-NS55104. Ideas discussed here are based in part on presentations at the 2007 Life Sciences Conference in Glasgow, the 2006 and 2007 International Stroke Conferences and previous reviews in Nat Neurosci Rev 2003, Stroke 2004 and Stroke 2005. Thanks to Sophy Hartdegen for assistance with the manuscript and literature citations. Thanks also to Drs Michael Moskowitz, Gregory del Zoppo, Marc Fisher, Maiken Nedergaard, Bruce Ransom, Kyra Becker and Tom Jacobs for many helpful discussions.
Glossary
- MMP
matrix metalloproteinase
- MRI
magnetic resonance imaging
- rt-PA
recombinant tissue type plasminogen activator
Footnotes
Conflict of interest
The authors state no conflict of interest.
References
- Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, et al. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- Aarts MM, Tymianski M. TRPMs and neuronal cell death. Pflugers Arch. 2005;451:243–249. doi: 10.1007/s00424-005-1439-x. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Aoki T, Sumii T, Mori T, Wang X, Lo EH. Blood–brain barrier disruption and matrix metalloproteinase-9 expression during reperfusion injury: mechanical versus embolic focal ischemia in spontaneously hypertensive rats. Stroke. 2002;33:2711–2717. doi: 10.1161/01.str.0000033932.34467.97. [DOI] [PubMed] [Google Scholar]
- Asahi M, Asahi K, Wang X, Lo EH. Reduction of tissue plasminogen activator-induced hemorrhage and brain injury by free radical spin trapping after embolic focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2000;20:452–457. doi: 10.1097/00004647-200003000-00002. [DOI] [PubMed] [Google Scholar]
- Astrup J, Symon L, Branston NM, Lassen NA. Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke. 1977;8:51–57. doi: 10.1161/01.str.8.1.51. [DOI] [PubMed] [Google Scholar]
- Ayata C, Shin HK, Salomone S, Ozdemir-Gursoy Y, Boas DA, Dunn AK, et al. Pronounced hypoperfusion during spreading depression in mouse cortex. J Cereb Blood Flow Metab. 2004;24:1172–1182. doi: 10.1097/01.WCB.0000137057.92786.F3. [DOI] [PubMed] [Google Scholar]
- Bano D, Young KW, Guerin CJ, Lefeuvre R, Rothwell NJ, Naldini L, et al. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell. 2005;120:275–285. doi: 10.1016/j.cell.2004.11.049. [DOI] [PubMed] [Google Scholar]
- Benchenane K, López-Atalaya JP, Fernández-Monreal M, Touzani O, Vivien D. Equivocal roles of tissue-type plasminogen activator in stroke-induced injury. Trends Neurosci. 2004;27:155–160. doi: 10.1016/j.tins.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Benveniste H. The excitotoxin hypothesis in relation to cerebral ischemia. Cerebrovasc Brain Metab Rev. 1991;3:213–245. [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Talantova MV, Lee WD, Schölzke MN, Harrop A, Mathews E, et al. Crosstalk between nitric oxide and zinc pathways to neuronal cell death involving mitochondrial dysfunction and p38-activated K+ channels. Neuron. 2004;41:351–365. doi: 10.1016/s0896-6273(04)00015-7. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Kent TA, Eisenberg HM, Quast MJ, Ward GA, Campbell GA, et al. Middle cerebral artery occlusion in rats studied by magnetic resonance imaging. Stroke. 1989;20:1032–1036. doi: 10.1161/01.str.20.8.1032. [DOI] [PubMed] [Google Scholar]
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Chen ZL, Strickland S. Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell. 1997;91:917–925. doi: 10.1016/s0092-8674(00)80483-3. [DOI] [PubMed] [Google Scholar]
- Chopp M, Zhang ZG, Jiang Q. Neurogenesis, angiogenesis, and MRI indices of functional recovery from stroke. Stroke. 2007;38:827–831. doi: 10.1161/01.STR.0000250235.80253.e9. [DOI] [PubMed] [Google Scholar]
- Corbett D, Thornhill J. Temperature modulation (hypothermic and hyperthermic conditions) and its influence on histological and behavioral outcomes following cerebral ischemia. Brain Pathol. 2000;10:145–152. doi: 10.1111/j.1750-3639.2000.tb00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SM, Donnan GA, Butcher KS, Parsons M. Selection of thrombolytic therapy beyond 3 h using magnetic resonance imaging. Curr Opin Neurol. 2005;18:47–52. doi: 10.1097/00019052-200502000-00010. [DOI] [PubMed] [Google Scholar]
- De Keyser J, Sulter G, Luiten PG. Clinical trials with neuroprotective drugs in acute ischaemic stroke: are we doing the right thing. Trends Neurosci. 1999;22:535–540. doi: 10.1016/s0166-2236(99)01463-0. [DOI] [PubMed] [Google Scholar]
- DeGraba TJ, Pettigrew LC. Why do neuroprotective drugs work in animals but not humans. Neurol Clin. 2000;18:475–493. doi: 10.1016/s0733-8619(05)70203-6. [DOI] [PubMed] [Google Scholar]
- Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ. Clinical trials in acute stroke: why have they not been successful. Neurology. 1998;51:S59–S61. doi: 10.1212/wnl.51.3_suppl_3.s59. [DOI] [PubMed] [Google Scholar]
- Dirnagl U. Bench to bedside: the quest for quality in experimental stroke research. J Cereb Blood Flow Metab. 2006;26:1465–1478. doi: 10.1038/sj.jcbfm.9600298. [DOI] [PubMed] [Google Scholar]
- Driscoll M, Gerstbrein B. Dying for a cause: invertebrate genetics takes on human neurodegeneration. Nat Rev Genet. 2003;4:181–194. doi: 10.1038/nrg1018. [DOI] [PubMed] [Google Scholar]
- Endres M, Laufs U, Liao JK, Moskowitz MA. Targeting eNOS for stroke protection. Trends Neurosci. 2004;27:283–289. doi: 10.1016/j.tins.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Felberg RA, Grotta JC, Shirzadi AL, Strong R, Narayana P, Hill-Felberg SJ, et al. Cell death in experimental intracerebral hemorrhage: the ‘black hole' model of hemorrhagic damage. Ann Neurol. 2002;51:517–524. doi: 10.1002/ana.10160. [DOI] [PubMed] [Google Scholar]
- Fisher M, Tatlisumak T. Use of animal models has not contributed to development of acute stroke therapies: con. Stroke. 2005;36:2324–2325. doi: 10.1161/01.STR.0000179039.76922.e8. [DOI] [PubMed] [Google Scholar]
- Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- Graham SH, Chen J. Programmed cell death in cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:99–109. doi: 10.1097/00004647-200102000-00001. [DOI] [PubMed] [Google Scholar]
- Green AR, Ashwood T. Free radical trapping as a therapeutic approach to neuroprotection in stroke: experimental and clinical studies with NXY-059 and free radical scavengers. Curr Drug Targets CNS Neurol Disord. 2005;4:109–118. doi: 10.2174/1568007053544156. [DOI] [PubMed] [Google Scholar]
- Hacke W, Albers G, Al Rawi Y, Bogousslavsky J, Davalos A, Eliasziw M, et al. The Desmoteplase in acute ischemic stroke trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005;36:66–73. doi: 10.1161/01.STR.0000149938.08731.2c. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and neurodegeneration: where are we now. J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Graf R, Wienhard K, Löttgen J, Saito R, Fujita T, et al. Dynamic penumbra demonstrated by sequential multitracer PET after middle cerebral artery occlusion in cats. J Cereb Blood Flow Metab. 1994;14:892–902. doi: 10.1038/jcbfm.1994.120. [DOI] [PubMed] [Google Scholar]
- Hoff JT, Xi G. Brain edema from intracerebral hemorrhage. Acta Neurochir Suppl. 2003;86:11–15. doi: 10.1007/978-3-7091-0651-8_3. [DOI] [PubMed] [Google Scholar]
- Hossmann KA. Genetically modified animals in molecular stroke research. Acta Neurochir Suppl. 2004;89:37–45. doi: 10.1007/978-3-7091-0603-7_5. [DOI] [PubMed] [Google Scholar]
- Hoyte L, Kaur J, Buchan AM. Lost in translation: taking neuroprotection from animal models to clinical trials. Exp Neurol. 2004;188:200–204. doi: 10.1016/j.expneurol.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Hypothermia after Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Regulation of the cerebral microcirculation during neural activity: is nitric oxide the missing link. Trends Neurosci. 1993;16:206–214. doi: 10.1016/0166-2236(93)90156-g. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Jacobs MA, Zhang ZG, Knight RA, Soltanian-Zadeh H, Goussev AV, Peck DJ, et al. A model for multiparametric MRI tissue characterization in experimental cerebral ischemia with histological validation in rat: part 1. Stroke. 2001;32:943–949. doi: 10.1161/01.str.32.4.943. [DOI] [PubMed] [Google Scholar]
- Kaste M. Use of animal models has not contributed to development of acute stroke therapies: pro. Stroke. 2005;36:2323–2324. doi: 10.1161/01.STR.0000179037.82647.48. [DOI] [PubMed] [Google Scholar]
- Kato H, Kogure K. Biochemical and molecular characteristics of the brain with developing cerebral infarction. Cell Mol Neurobiol. 1999;19:93–108. doi: 10.1023/a:1006920725663. [DOI] [PubMed] [Google Scholar]
- Kaur J, Zhao Z, Klein GM, Lo EH, Buchan AM. The neurotoxicity of tissue plasminogen activator. J Cereb Blood Flow Metab. 2004;24:945–963. doi: 10.1097/01.WCB.0000137868.50767.E8. [DOI] [PubMed] [Google Scholar]
- Kawamura S, Yasui N, Shirasawa M, Fukasawa H. Rat middle cerebral artery occlusion using an intraluminal thread technique. Acta Neurochir (Wien) 1991;109:126–132. doi: 10.1007/BF01403007. [DOI] [PubMed] [Google Scholar]
- Kidwell CS, Villablanca JP, Saver JL. Advances in neuroimaging of acute stroke. Curr Atheroscler Rep. 2000;2:126–135. doi: 10.1007/s11883-000-0107-z. [DOI] [PubMed] [Google Scholar]
- Kim HY, Singhal AB, Lo EH. Normobaric hyperoxia extends the reperfusion window in focal cerebral ischemia. Ann Neurol. 2005;57:571–575. doi: 10.1002/ana.20430. [DOI] [PubMed] [Google Scholar]
- Kinouchi H, Epstein CJ, Mizui T, Carlson E, Chen SF, Chan PH. Attenuation of focal cerebral ischemic injury in transgenic mice overexpressing CuZn superoxide dismutase. Proc Natl Acad Sci USA. 1991;88:11158–11162. doi: 10.1073/pnas.88.24.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharczyk J, Asgari H, Mintorovitch J, Vexler Z, Rocklage S, Watson A, et al. Cerebrovascular transit characteristics of DyDTPA-BMA and GdDTPA-BMA on normal and ischemic cat brain. AJNR Am J Neuroradiol. 1993;14:289–296. [PMC free article] [PubMed] [Google Scholar]
- Lapchak PA, Araujo DM, Song D, Wei J, Purdy R, Zivin JA. Effects of the spin trap agent disodium- [tert-butylimino)methyl]benzene-1,3-disulfonate N-oxide (generic NXY-059) on intracerebral hemorrhage in a rabbit Large clot embolic stroke model: combination studies with tissue plasminogen activator. Stroke. 2002;33:1665–1670. doi: 10.1161/01.str.0000017145.22806.aa. [DOI] [PubMed] [Google Scholar]
- Lauritzen M, Hansen AJ. The effect of glutamate receptor blockade on anoxic depolarization and cortical spreading depression. J Cereb Blood Flow Metab. 1992;12:223–229. doi: 10.1038/jcbfm.1992.32. [DOI] [PubMed] [Google Scholar]
- Lee SR, Kim HY, Rogowska J, Zhao BQ, Bhide P, Parent JM, et al. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J Neurosci. 2006;26:3491–3495. doi: 10.1523/JNEUROSCI.4085-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees KR, Zivin JA, Ashwood T, Davalos A, Davis SM, Diener HC, et al. NXY-059 for acute ischemic stroke. N Engl J Med. 2006;354:588–600. doi: 10.1056/NEJMoa052980. [DOI] [PubMed] [Google Scholar]
- Liang D, Dawson TM, Dawson VL. What have genetically engineered mice taught us about ischemic injury. Curr Mol Med. 2004;4:207–225. doi: 10.2174/1566524043479194. [DOI] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Lo EH, Broderick JP, Moskowitz MA. tPA and proteolysis in the neurovascular unit. Stroke. 2004;35:354–356. doi: 10.1161/01.STR.0000115164.80010.8A. [DOI] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Lo EH, Moskowitz MA, Jacobs TP. Exciting, radical, suicidal: how brain cells die after stroke. Stroke. 2005;36:189–192. doi: 10.1161/01.STR.0000153069.96296.fd. [DOI] [PubMed] [Google Scholar]
- Lok J, Gupta P, Guo S, Kim WJ, Whalen MJ, van Leyen K, et al. Cell–cell signaling in the neurovascular unit. Neurochem Res. 2007;32:2032–2045. doi: 10.1007/s11064-007-9342-9. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Lu A, Tang Y, Ran R, Ardizzone TL, Wagner KR, Sharp FR. Brain genomics of intracerebral hemorrhage. J Cereb Blood Flow Metab. 2006;26:230–252. doi: 10.1038/sj.jcbfm.9600183. [DOI] [PubMed] [Google Scholar]
- Ma J, Endres M, Moskowitz MA. Synergistic effects of caspase inhibitors and MK-801 in brain injury after transient focal cerebral ischaemia in mice. Br J Pharmacol. 1998;124:756–762. doi: 10.1038/sj.bjp.0701871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Qiu J, Hirt L, Dalkara T, Moskowitz MA. Synergistic protective effect of caspase inhibitors and bFGF against brain injury induced by transient focal ischaemia. Br J Pharmacol. 2001;133:345–350. doi: 10.1038/sj.bjp.0704075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod MR, O'Collins T, Horky LL, Howells DW, Donnan GA. Systematic review and metaanalysis of the efficacy of FK506 in experimental stroke. J Cereb Blood Flow Metab. 2005;25:713–721. doi: 10.1038/sj.jcbfm.9600064. [DOI] [PubMed] [Google Scholar]
- Maples KR, Green AR, Floyd RA. Nitrone-related therapeutics: potential of NXY-059 for the treatment of acute ischaemic stroke. CNS Drugs. 2004;18:1071–1084. doi: 10.2165/00023210-200418150-00003. [DOI] [PubMed] [Google Scholar]
- Marchal G, Beaudouin V, Rioux P, de la Sayette V, Le Doze F, Viader F, et al. Prolonged persistence of substantial volumes of potentially viable brain tissue after stroke: a correlative PET-CT study with voxel-based data analysis. Stroke. 1996;27:599–606. doi: 10.1161/01.str.27.4.599. [DOI] [PubMed] [Google Scholar]
- Matsushita K, Meng W, Wang X, Asahi M, Asahi K, Moskowitz MA, et al. Evidence for apoptosis after intercerebral hemorrhage in rat striatum. J Cereb Blood Flow Metab. 2000;20:396–404. doi: 10.1097/00004647-200002000-00022. [DOI] [PubMed] [Google Scholar]
- Meairs S, Wahlgren N, Dirnagl U, Lindvall O, Rothwell P, Baron JC, et al. Stroke research priorities for the next decade—a representative view of the European scientific community. Cerebrovasc Dis. 2006;22:75–82. doi: 10.1159/000093098. [DOI] [PubMed] [Google Scholar]
- Meden P, Overgaard K, Pedersen H, Boysen G. Effect of hypothermia and delayed thrombolysis in a rat embolic stroke model. Acta Neurol Scand. 1994;90:91–98. doi: 10.1111/j.1600-0404.1994.tb02686.x. [DOI] [PubMed] [Google Scholar]
- Melchor JP, Strickland S. Tissue plasminogen activator in central nervous system physiology and pathology. Thromb Haemost. 2005;93:655–660. doi: 10.1160/TH04-12-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsias PD, Lu M, Silver B, Morris D, Ewing JR, Daley S, et al. MRI-guided, open trial of abciximab for ischemic stroke within a 3- to 24-hour window. Neurology. 2005;65:612–615. doi: 10.1212/01.wnl.0000172862.33641.92. [DOI] [PubMed] [Google Scholar]
- Montaner J, Fernández-Cadenas I, Molina CA, Monasterio J, Arenillas JF, Ribó M, et al. Safety profile of tissue plasminogen activator treatment among stroke patients carrying a common polymorphism (C-1562T) in the promoter region of the matrix metalloproteinase-9 gene. Stroke. 2003;34:2851–2855. doi: 10.1161/01.STR.0000098648.54429.1C. [DOI] [PubMed] [Google Scholar]
- Moseley ME, Butts K, Yenari MA, Marks M, de Crespigny A. Clinical aspects of DWI. NMR Biomed. 1995;8:387–396. doi: 10.1002/nbm.1940080712. [DOI] [PubMed] [Google Scholar]
- Moseley ME, Kucharczyk J, Mintorovitch J, Asgari HS, Wendland MF, Tsuruda J, et al. Diffusion-weighted MR imaging of acute stroke: correlation with T2-weighted and magnetic susceptibility-enhanced MR imaging in cats. AJNR Am J Neuroradiol. 1990;11:423–429. [PMC free article] [PubMed] [Google Scholar]
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET, et al. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat Med. 2001;7:59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- Ning M, Furie KL, Koroshetz WJ, Lee H, Barron M, Lederer M, et al. Association between tPA therapy and raised early matrix metalloproteinase-9 in acute stroke. Neurology. 2006;66:1550–1555. doi: 10.1212/01.wnl.0000216133.98416.b4. [DOI] [PubMed] [Google Scholar]
- Parent JM. Injury-induced neurogenesis in adult mammalian brain. Neuroscientist. 2003;9:261–272. doi: 10.1177/1073858403252680. [DOI] [PubMed] [Google Scholar]
- Petito CK, Feldmann E, Pulsinelli WA, Plum F. Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology. 1987;37:1281–1286. doi: 10.1212/wnl.37.8.1281. [DOI] [PubMed] [Google Scholar]
- Powers WJ, Zazulia AR. The use of positron emission tomography in cerebrovascular disease. Neuroimaging Clin N Am. 2003;13:741–758. doi: 10.1016/s1052-5149(03)00091-1. [DOI] [PubMed] [Google Scholar]
- Report of the Stroke Progress Review Group . http://www.ninds.nih.gov/find_people/gro ups/stroke_prg/04_2002_stroke_prg_report.htm 5-12-2002
- Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- Rhesus Macaque Genome Sequencing and Analysis Consortium Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007;316:222–234. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- Røhl L, Østergaard L, Simonsen CZ, Vestergaard-Poulsen P, Andersen G, Sakoh M, et al. Viability thresholds of ischemic penumbra of hyperacute stroke defined by perfusion-weighted MRI and apparent diffusion coefficient. Stroke. 2001;32:1140–1146. doi: 10.1161/01.str.32.5.1140. [DOI] [PubMed] [Google Scholar]
- Rosell A, Ortega-Aznar A, Alvarez-Sabín J, Fernández-Cadenas I, Ribó M, Molina CA, et al. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke. 2006;37:1399–1406. doi: 10.1161/01.STR.0000223001.06264.af. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Benfield A, Baird AE, Siewert B, Lovblad KO, Parker RA, et al. The ischemic penumbra: operationally defined by diffusion and perfusion MRI. Neurology. 1999;53:1528–1537. doi: 10.1212/wnl.53.7.1528. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic–ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Lu A, Tang Y, Millhorn DE. Multiple molecular penumbras after focal cerebral ischemia. J Cereb Blood Flow Metab. 2000;20:1011–1032. doi: 10.1097/00004647-200007000-00001. [DOI] [PubMed] [Google Scholar]
- Shin HK, Dunn AK, Jones PB, Boas DA, Lo EH, Moskowitz MA, et al. Normobaric hyperoxia improves cerebral blood flow and oxygenation, and inhibits peri-infarct depolarizations in experimental focal ischaemia. Brain. 2007;130:1631–1642. doi: 10.1093/brain/awm071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HK, Dunn AK, Jones PB, Boas DA, Moskowitz MA, Ayata C. Vasoconstrictive neurovascular coupling during focal ischemic depolarizations. J Cereb Blood Flow Metab. 2006;26:1018–1030. doi: 10.1038/sj.jcbfm.9600252. [DOI] [PubMed] [Google Scholar]
- Shuaib A, Lees KR, Lynden P, Grotta J, Davalos A, Davis S, et al. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- Singhal AB, Benner T, Roccatagliata L, Koroshetz WJ, Schaefer PW, Lo EH, et al. A pilot study of normobaric oxygen therapy in acute ischemic stroke. Stroke. 2005;36:797–802. doi: 10.1161/01.STR.0000158914.66827.2e. [DOI] [PubMed] [Google Scholar]
- Singhal AB, Dijkhuizen RM, Rosen BR, Lo EH. Normobaric hyperoxia reduces MRI diffusion abnormalities and infarct size in experimental stroke. Neurology. 2002a;58:945–952. doi: 10.1212/wnl.58.6.945. [DOI] [PubMed] [Google Scholar]
- Singhal AB, Wang X, Sumii T, Mori T, Lo EH. Effects of normobaric hyperoxia in a rat model of focal cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2002b;22:861–868. doi: 10.1097/00004647-200207000-00011. [DOI] [PubMed] [Google Scholar]
- Stroke Therapy Academic Industry Roundtable (STAIR) Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Fujimura M, Morita-Fujimura Y, Kawase M, Chan PH. Mitochondrial release of cytochrome c corresponds to the selective vulnerability of hippocampal CA1 neurons in rats after transient global cerebral ischemia. J Neurosci. 1999;19:1–6. doi: 10.1523/JNEUROSCI.19-22-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke. 2002;33:831–836. doi: 10.1161/hs0302.104542. [DOI] [PubMed] [Google Scholar]
- Tamura A, Graham DI, McCulloch J, Teasdale GM. Focal cerebral ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1981;1:53–60. doi: 10.1038/jcbfm.1981.6. [DOI] [PubMed] [Google Scholar]
- Tejima E, Zhao BQ, Tsuji K, Rosell A, van Leyen K, Gonzalez RG, et al. Astrocytic induction of matrix metalloproteinase-9 and edema in brain hemorrhage. J Cereb Blood Flow Metab. 2006;27:460–468. doi: 10.1038/sj.jcbfm.9600354. [DOI] [PubMed] [Google Scholar]
- Tsirka SE, Rogove AD, Strickland S. Neuronal cell death and tPA. Nature. 1996;384:123–124. doi: 10.1038/384123b0. [DOI] [PubMed] [Google Scholar]
- Tsuji K, Aoki T, Tejima E, Arai K, Lee SR, Atochin D, et al. Tissue plasminogen activator promotes matrix metalloproteinase-9 upregulation after focal cerebral ischemia. Stroke. 2005;36:1954–1959. doi: 10.1161/01.STR.0000177517.01203.eb. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang ZG, Zhang RL, Gregg SR, Hozeska-Solgot A, LeTourneau Y, et al. Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J Neurosci. 2006;26:5996–6003. doi: 10.1523/JNEUROSCI.5380-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lee SR, Arai K, Lee SR, Tsuji K, Rebeck GW, et al. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med. 2003;9:1313–1317. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM, et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35:2726–2730. doi: 10.1161/01.STR.0000143219.16695.af. [DOI] [PubMed] [Google Scholar]
- Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med. 1998;4:228–231. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- Warach S. New imaging strategies for patient selection for thrombolytic and neuroprotective therapies. Neurology. 2001;57:S48–S52. doi: 10.1212/wnl.57.suppl_2.s48. [DOI] [PubMed] [Google Scholar]
- Wu O, Koroshetz WJ, Østergaard L, Buonanno FS, Copen WA, Gonzalez RG, et al. Predicting tissue outcome in acute human cerebral ischemia using combined diffusion- and perfusion-weighted MR imaging. Stroke. 2001;32:933–942. doi: 10.1161/01.str.32.4.933. [DOI] [PubMed] [Google Scholar]
- Wu O, Østergaard L, Sorensen AG. Technical aspects of perfusion-weighted imaging. Neuroimaging Clin N Am. 2005;15:623–637. doi: 10.1016/j.nic.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang ZG, Zhang R, Morris D, Lu M, Coller BS, et al. Adjuvant treatment with a glycoprotein IIb/IIIa receptor inhibitor increases the therapeutic window for low-dose tissue plasminogen activator administration in a rat model of embolic stroke. Circulation. 2003;107:2837–2843. doi: 10.1161/01.CIR.0000068374.57764.EB. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Chopp M. Neurogenesis in the adult ischemic brain: generation, migration, survival and restorative therapy. Neuroscientist. 2005;11:408–416. doi: 10.1177/1073858405278865. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chopp M, Zhang RL, Goussev A. A mouse model of embolic focal cerebral ischemia. J Cereb Blood Flow Metab. 1997a;17:1081–1088. doi: 10.1097/00004647-199710000-00010. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhang L, Yepes M, Jiang Q, Li Q, Arniego P, et al. Adjuvant treatment with neuroserpin increases the therapeutic window for tissue-type plasminogen activator administration in a rat model of embolic stroke. Circulation. 2002;106:740–745. doi: 10.1161/01.cir.0000023942.10849.41. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhang RL, Jiang Q, Raman SB, Cantwell L, Chopp M. A new rat model of thrombotic focal cerebral ischemia. J Cereb Blood Flow Metab. 1997b;17:123–135. doi: 10.1097/00004647-199702000-00001. [DOI] [PubMed] [Google Scholar]
- Zhao BQ, Tejima E, Lo EH. Neurovascular proteases in brain injury, hemorrhage and remodeling after stroke. Stroke. 2007;38:748–752. doi: 10.1161/01.STR.0000253500.32979.d1. [DOI] [PubMed] [Google Scholar]
- Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular mechanisms of Alzheimer's neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]