Abstract
A characteristic feature of many chronic inflammatory diseases is their persistence and predilection for certain sites. The molecular basis for such tissue tropism and failure of the inflammatory response to resolve has until relative recently remained obscure. Recent studies have strongly implicated fibroblasts as cells which contribute to disease persistence and which help define anatomical location. Therefore fibroblasts make an attractive therapeutic target as they help orchestrate the inflammatory infiltrate. Current anti-inflammatory therapies target immune cells in an attempt to inhibit the production of pro-inflammatory mediators. However an equally important target is the active induction of pro-resolution programmes responsible for the resolution of inflammation. Fibroblasts are likely to be an important source of these anti-inflammatory mediators. Therapeutic manipulation of fibroblasts and their biologically active products is an emerging concept in treating cancer and is likely to provide a novel method to achieve improved control of chronic inflammatory disease.
Keywords: inflammation, fibroblasts, cancer, therapy
An emerging role for fibroblasts in immunity and inflammation
Fibroblasts are ubiquitous cells identified by their morphology, ability to adhere to plastic, production of extracellular matrix (ECM) and lack of epithelial, vascular and leukocyte lineage markers. Despite being the most abundant cells of the stroma, they have until recently remained relatively poorly characterized in molecular terms (Chang et al., 2002). Fibroblasts are primarily responsible for the synthesis and remodelling of ECM in tissues. However their ability to produce and respond to growth factors allows reciprocal paracrine interactions, which maintain the homeostasis of adjacent cell types such as epithelial and endothelial cells (Kalluri and Zeisberg, 2006). These interactions regulate the morphogenesis of epithelial and endothelial structures in tissues and as a consequence, fibroblasts play a critical role during tissue development, differentiation and repair in many organs including lymphoid tissues.
In addition to playing an important structural role in ‘landscaping' the microenvironment, fibroblasts through their production of cytokines and chemokines play a key role in the development and choreography of immune responses in lymphoid tissues. Stromal cells in general and fibroblasts in particular help define distinct anatomical compartments present in the thymus, spleen and lymph nodes (Anderson and Jenkinson, 2001). For example, the compartmentalization of T and B lymphocytes within lymph nodes depends on appropriate interactions between CD4+CD3− lymphoid-like cells (inducers) and VCAM-1 (CD106)-positive fibroblast-like cells (organizers) for their generation and continued existence (Lane et al., 2005). While some of the molecular signals required to initiate lymphoid organogenesis have been elucidated, very little is known about the types of fibroblasts that help demarcate the different functional leukocyte/stromal compartments in these tissues (Mebius, 2003). Even less is known about how these zones are dynamically regulated, and virtually nothing is known about the fibroblast-like cells that are required to support the expansion and contraction of lymphoid tissues following repeated episodes of immune activation.
Recent studies have shown that fibroblasts modify the quality, quantity and duration of the inflammatory infiltrate during the induction of inflammatory responses (Parsonage et al., 2005). At the termination of these responses, fibroblasts contribute to the resolution of inflammation by withdrawing survival signals and normalizing the chemokine gradients, thereby allowing infiltrating leukocytes to undergo apoptosis or leave the tissue through the draining lymphatics (Buckley et al., 2001). A direct role for fibroblasts in suppressing immune responses has been demonstrated in the spleen, where fibroblasts have been shown to drive the development of regulatory dendritic cells, following their activation by infectious agents (Svensson et al., 2004; Zhang et al., 2004).
In this review, we explore the evidence that fibroblasts imprint positional identity and display topographical memory; providing a molecular basis for site-specific inflammatory responses. In chronic inflammation, the normal physiological processes of the death and emigration of unwanted inflammatory cells at the end of the inflammatory response becomes disordered leading to the inappropriate and persistent accumulation of leukocytes within lymphoid aggregates, which resemble those seen in lymphoid tissue. Taken together, these findings suggest that targeting fibroblasts is likely to be an important strategy for future anti-inflammatory therapies.
What are fibroblasts, how are they identified and where do they originate from?
The stroma is an important structural component of vertebrate animals. It consists of ECM, mesenchymal cells and a scaffold consisting of blood and lymphatic vessels, nerves and inflammatory cells. The most abundant cell type of tissue stroma is the fibroblast. Fibroblasts are ubiquitous cells that provide mechanical strength to tissues by producing ECM, which forms a supporting framework. Recent findings have shown that fibroblasts are not just passive structural landscaping cells, but are active participants in the immune response and influence the switch from acute to chronic inflammation (Kalluri and Zeisberg, 2006).
Fibroblasts are traditionally identified by their spindle-shaped morphology and their ability to adhere to plastic in vitro (Tarin and Croft, 1969). However, a reliable and specific marker for the fibroblast is yet to be found and therefore, the lack of markers for other cell lineages has been used to identify fibroblasts (non-lymphoid, non-endothelium and non-epithelium). Some of the markers that are currently used to identify fibroblasts are highlighted in Table 1.
Table 1. Fibroblast markers. Modified from Kalluri and Zeisberg (2006).
| Marker | Function | Fibroblast types in which it is found | Other cell types in which it is found |
|---|---|---|---|
| Vimentin | Intermediate filament-associated protein | Miscellaneous | Endothelial cells, myoepithelial cells, neurons |
| α-SMA | Intermediate filament-associated protein | Myofibroblasts | Vascular smooth muscle cells, pericytes, myoepithelial cells |
| Desmin | Intermediate filament-associated protein | Skin fibroblasts | Muscle cells, vascular smooth muscle cells |
| FSP1 | Intermediate filament-associated protein | Miscellaneous | Invasive carcinoma cells |
| Discoidin-domain receptor 2 | Collagen receptor | Cardiac fibroblasts | Endothelial cells |
| FAP | Serine protease | Activated fibroblasts | Activated melanocytes |
| α1β1 integrin | Collagen receptor | Miscellaneous | Monocytes, endothelial cells |
| Prolyl 4-hydroxylase | Collagen biosynthesis | Miscellaneous | Endothelial cells, cancer cells, epithelial cells |
| Pro-collagen 1α2 | Collagen-1 biosynthesis | Miscellaneous | Osteoblasts, chondroblasts |
| CD248 | Unknown | Miscellaneous | Pericytes |
| VCAM-1 | Cell adhesion | Miscellaneous | Activated endothelial cells |
Fibroblasts are believed to arise from three distinct cellular origins: primary mesenchyme, local epithelial–mesenchymal transition (EMT) and bone marrow-derived precursors (Postlethwaite et al., 2004). It is widely accepted that the principle origin for fibroblast is from primary mesenchymal cells and that upon appropriate stimulation, fibroblasts can proliferate to generate new fibroblasts. Local EMT is a central mechanism for diversifying cells in the formation of complex tissues (Kalluri and Neilson, 2003). Fibroblasts can be derived by this process in adult tissue following epithelial stress such as inflammation or tissue injury. Essentially, EMT disaggregates epithelial cells and reshapes epithelia for movement. The epithelium loses polarity, adherens junctions, tight junctions, desmosomes and cytokeratin intermediate filaments. They also rearrange their F-actin stress fibres and express filopodia and lamellopodia. A combination of cytokines associated with digestion of the basement membrane is believed to instigate EMT as well as matrix metalloproteinases (MMPs) with transforming growth factor beta (TGF-β) thought to be important in the induction of EMT (Zeisberg et al., 2001, 2003). In tissue inflammation it has been difficult to determine the importance of cytokines involved in EMT, but if EMT can be modulated by anticytokine therapy then this would be an attractive target for disease such as asthma and liver and renal fibrosis.
The EMT hypothesis also contributes to the growing premise that fibroblasts are not a homogeneous population and are a much more active heterogeneous population of cells than was first thought (Garrett and Conrad, 1979; Schor and Schor, 1987; Dugina et al., 1998). More recently, it has been suggested that fibroblasts can be derived from bone marrow-derived, precursor cells, which circulate in the blood termed fibrocytes (Phillips et al., 2004). These cells represent ∼0.05% of circulating blood cells that are believed to arise from CD14+ peripheral blood monocytes. All of these processes appear to contribute to the pathological accumulation of fibroblasts.
A role for fibroblasts in the persistence of inflammation?
Until recently, fibroblasts have been suggested to play a relatively inert role in the immune system. They have traditionally been seen as more of a structural component mainly involved in the synthesis of ECM. It has now been suggested that fibroblasts not only play an active role in defining the structure of tissue microenvironments but also modulate immune-cell behaviour by conditioning the local cellular and cytokine production. Therefore, the response of the microenvironment can be specifically tailored to the cause of the damage.
Fibroblast activation leads to production of cytokines, chemokines and prostanoids such as prostaglandin E2 (Smith et al., 1997). Fibroblasts can also regulate the behaviour of haematopoietic cells present in damaged tissue via CD40–CD40 ligand interaction, which leads to the activation of the NF-κB family of receptors. This causes fibroblasts to synthesize high levels of IL-6, IL-8, cyclooxygenase-2 and the polysaccharide hyaluronan (Zhang et al., 1998; Pap et al., 2000). This mechanism is similar to the crosstalk that occurs between lymphocytes and antigen-presenting cells and suggests that it may provide crosstalk between fibroblasts and leukocytes. Owing to this role within the immune system and the fact that the cells are relatively long–lived, it is important that fibroblasts are tightly regulated.
Over 20 years ago, fibroblasts isolated from diseased tissues were shown to be phenotypically different from those taken from normal tissue. Fassbender (1983) described subpopulations of fibroblasts within the synovial membrane showing morphological and biological differences. Fibroblasts have also been shown to have a role in cancer at all stages including progression (Ronnov-Jessen et al., 1996), growth (Dvorak, 1986) and metastasis (Schedin and Elias, 2004). Specifically, at the site of a tumour, the surrounding fibroblasts remain continuously activated and it is believed that they may facilitate angiogenesis and cancer progression.
Fibroblast heterogeneity and implications for inflammation
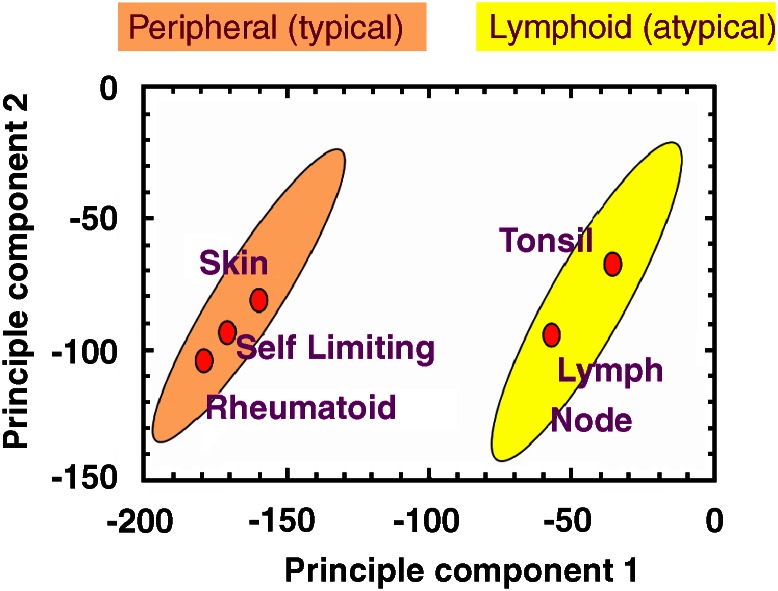
Fibroblasts exhibit site-specific gene expression. Furthermore, fibroblasts isolated from different sites exhibit different functional properties. Functional differences such as migratory capacity, ECM production and degradation and contractility have been observed (Fries et al., 1994). Previous work on human fibroblasts in our group identified two groups of fibroblasts based on their gene and protein expression (Parsonage et al., 2003). These have been named typical (peripheral) and atypical (lymphoid) fibroblasts (Figure 1). We have shown that fibroblast regional identity is not rigidly imprinted but can be transiently modified by inflammatory cytokines. For example, upon stimulation with inflammatory mediators such as TNFα, IL-4 and IFNγ, the fibroblast gene expression can be modified so that the gene expression of one group of fibroblasts (peripheral) can more closely resemble that of the opposite group (lymphoid). This finding suggests that inflammation can, at least transiently, alter a fibroblast's gene-expression profile (Parsonage et al., 2003).
Figure 1.
Fibroblasts can be classified into two main groups based on their gene-transcriptional profiles. The gene-transcriptional profiles of fibroblasts taken from various anatomical sites peripheral (skin, self-limiting synovial and rheumatoid synovial) and lymphoid (tonsil and lymph node) cluster in two main zones in principle component space. Principle component analysis is a means of reducing the dimensionality of array data while maintaining the variation present within the original data set as much as possible. It performs a similar function to hierarchical cluster analysis, which also clusters genes into related groups. If the values for all gene expression were to be plotted in multidimensional space, the axis of the first principle component (usually the x axis) would be the direction that incorporated the maximum variability among all the genes analysed. The second principle component (y axis) must be orthogonal to the first, but capture as much of the remaining variation within the data set as possible. The anatomical origin of the fibroblasts is plotted on the graph as ovals, which represent their position in principle component space as defined by their expression of the most variable genes that make up the principle components (Parsonage et al., 2003).
More extensive analysis of expression profiles from primary human fibroblasts has shown large-scale differences related to three broad anatomical divisions; anterior–posterior, proximal–distal and dermal–nondermal (Rinn et al., 2006). A set of 337 genes was found to vary according to these divisions, including genes involved in pattern formation, cell–cell signalling and matrix remodelling. Interestingly, homeobox (HOX) gene expression was differentially expressed according to the location where the fibroblasts were isolated. These data suggest that the gene-expression profile of adult fibroblasts may play a significant role in assigning positional identity within an organism and has lent support to the proposal of a stromal address code that can direct leukocyte behaviour (Parsonage et al., 2005). In this hypothesis, chemokines, cytokines and adhesion molecules produced by stromal cells control accumulation, retention, survival and differentiation of leukocytes in a site-dependent manner.
The organization of fibroblast gene expression based on anatomical location suggests that fibroblasts could be an important source of molecular landmarks for site-specific differentiation. For example, fibroblasts may construct an identifiable ECM and express structural and cell-surface proteins that provide site-specific signalling for a given anatomic origin. Fibroblasts may also provide important positional cues for wound healing and regeneration of tissues. In addition to their well-known role of producing ECM, it is postulated that fibroblasts provide positional cues for connecting and specifying the regenerated tissues. The long-term positional stability for many passages in vitro of fibroblast gene expression suggests that it may be controlled by epigenetic mechanisms.
Fibroblasts and the inflammatory response in arthritis
Synovial fibroblasts (SFb) taken from patients with rheumatoid arthritis provide a convincing example of how fibroblasts contribute to the persistence of inflammation. Rheumatoid SFb are now known to be direct effectors of tissue injury and remodelling, and display an imprinted phenotype which is stable under in vitro culture conditions, and which extends to functionally important outcomes such as cartilage invasion, as demonstrated in severe combined immunodeficient mouse models (Muller-Ladner et al., 1996). Rheumatoid SFb-mediated erosion of cartilage and bone determine disease outcome for the majority of rheumatoid arthritis patients (Firestein, 1996). Furthermore, evidence suggests that through secretion of cytokines and chemokines, SFb play a role in the persistence of inflammation in the synovium, through recruitment and retention of effector cells of the immune system (Ritchlin, 2000).
Type I interferons are produced by the expanded stromal population of SFb and macrophages, resulting in a lack of proliferation, but also a block of the apoptotic signals, which normally result in a coordinated wave of T-lymphocyte death at the conclusion of an inflammatory response (Salmon et al., 1997; Pilling et al., 1999; Buckley et al., 2000). Unexpectedly, T lymphocytes were found to express the chemokine receptor CXCR4 at high levels in the rheumatoid synovium. The ligand for CXCR4 (SDF-1 or CXCL12) is highly expressed on endothelial cells at the sites of T-cell accumulation (Pablos et al., 2003). In addition, stromal-cell-derived TGF-β is responsible for the upregulation of CXCR4 receptors on T cells in the synovium, and may be responsible for the production of a new subset of Il-17-producing cells called TH-17 and which have been strongly implicated in immune-mediated inflammatory diseases (Harrington et al., 2005). Crosstalk between chemokine and cytokine networks may operate to reinforce the retention of T cells by CXCL12, as locally raised IL-1 and TNFα levels cause SFb and macrophages to secrete IL-15, which upregulates CXCR4 on T cells, and may thus also contribute to the retention of T lymphocytes. There is therefore clear evidence in support of the hypothesis that aberrant expression of constitutive chemokines by SFb is responsible for retention of T cells within the Rheumatoid Arthritis (RA) synovium. These studies have been supported by data on the targeting of CXCR4 in murine models of arthritis (Matthys et al., 2001; Tamamura et al., 2004). It is possible that CXCR4 antagonists will be of use in the therapy of rheumatoid arthritis, provided that toxicity issues due to stem cell mobilization from the bone marrow, which depend upon CXCL12/CXCR4 interactions, do not pose a major problem.
The unique, imprinted phenotype of RA SFb bears remarkable phenotypic similarities to stromal cells of the bone marrow concerned with the recruitment and support of haemopoietic cells (Edwards, 2000). Recent studies have suggested that the phenotype of RA SFb is accounted for by accumulation of blood-borne stromal progenitor cells (mesenchymal progenitor cells) (Edwards, 2000). Other possible sources of stromal cells in inflammatory diseases include EMT, a phenomenon observed in inflammatory diseases of the kidney at sites of epithelial injury (Iwano et al., 2002). Targeting of such trans-differentiation processes may prove useful in retarding fibrotic diseases such as systemic sclerosis (Postlethwaite et al., 2004).
The relationship between inflammation and repair: lessons from the cancer paradigm
Wound healing, chronic inflammation and cancer display significant parallels. They are all associated with deposition of new ECM and expansion of adjacent stromal cells including fibroblasts, smooth muscle cells and endothelial cells. Furthermore, many authors have drawn parallels between tumorigenesis and wound healing, classically describing tumours as wounds that fail to heal (Dvorak, 1986). Chang et al. (2004)have recently shown that the transcriptional response of fibroblasts to serum, as seen in wound healing, is also seen in tumour and associated stromal cells. The presence of this ‘serum response' signature in tumour samples was found to be predictive of metastasis and death in common cancers.
Much has been learnt about the communication between networks of stromal cells within tissues, pathological alterations of which are crucial to the process of tumorigenesis. Parrinello et al. (2005) found that senescent fibroblasts were able to disrupt the normal branching morphogenesis of breast epithelial cells by secreted factors including MMPs and epithelial growth factors. When cultured with premalignant cells, this disruption resulted in loss of differentiation, representing malignant transformation. The bystander effect of senescence, itself a means of controlling potentially neoplastic cells, appears paradoxical and has been suggested to account for the age-related incidence of epithelial cancers. It is also an indication that simply inducing apoptosis or senescence of putative inflammatory stromal cells may have adverse effects.
A number of important cytokines and growth factors have been described that contribute to neoplastic transformation of epithelial cells by activated tumour-associated fibroblasts. These include hepatocyte growth factor, TGF-β, MMPs and fibroblast growth factors. Crucially, in contrast to senescent fibroblastic cells, tumour-activated fibroblasts appear to transform normal, in addition to pre-malignant epithelial cells (Bhowmick et al., 2004; Mueller and Fusenig, 2004). Blockade of both TGF-β and the hepatocyte growth factor receptor c-Met have been effective in inhibiting both the development and the metastasis of neoplasms in animal models (Yang et al., 2002; Michieli et al., 2004). Conversely, and confirming the essential organ-like nature of the tumour as both ‘cancer cells' and their surrounding stroma, human breast cancer xenografts are unable to implant successfully into mice unless accompanied by human tumour-derived fibroblasts (Kuperwasser et al., 2004).
The release of growth factors such as TGF-β, basic fibroblast growth factor, vascular endothelial growth factor (VEGF), platelet-derived growth factor, MMPs and proteolytic enzymes by tumour cells in turn modifies the tumour stroma and its cells to become angiogenic and pro-migratory (Mueller and Fusenig, 2004). The angiogenic properties of tumours are therefore a cooperative product of tumour cells, tumour-associated stromal cells and angiogenic factors released by proteolysis of the ECM. Studies using a skin surface model of tumorigenesis indicated that expression of VEGF was ubiquitous among benign and malignant tumours, however, it was the upregulation by stromal endothelial cells of VEGF receptors that led to tumour invasion, with blockade of such receptors halting tumour progression (Skobe et al., 1997). The concept of tumour stroma ‘normalization' has now become an accepted aspect of new oncology therapies. Clinical studies of angiogenesis inhibitors and antibodies against ECM components such as tenascin have been favourable, while inhibitors of MMPs, overexpression of tissue inhibitors of metalloproteinases and blockade of integrin signalling have all shown promise in pre-clinical trials (Mueller and Fusenig, 2004). Results of studies examining the interactions between endothelial cells and their associated pericytes underline the importance of targeting the stroma as a whole. Bergers et al. (2003) have shown that endothelial cells release platelet-derived growth factor that induce VEGF production from pericytes leading to bidirectional conversations between the two cell types. Interrupting these conversations by using platelet-derived growth factor inhibitors proved to be more effective therapy than using VEGF inhibitors alone. Interestingly, while VEGF inhibitors lost their inhibitory effect in later-stage tumours, targeting of the pericytes helped even late-stage tumours to regress (Bergers et al., 2003). The authors have subsequently shown that pericyte precursors are partly recruited from the bone marrow to tumour perivascular sites (Song et al., 2005). Recent evidence using breast carcinoma models have shown that tumour-associated fibroblasts secrete CXCL12, resulting not only in increased promotion of carcinoma cell growth compared to control fibroblasts, but also to recruitment of endothelial cell precursors (Orimo et al., 2005).
Conclusion
Stromal cells such as fibroblasts have great potential as therapeutic targets. They are responsible for orchestrating and maintaining the presence of inflammatory infiltrates. Although classical approaches using antibody blockade of cytokines such as TNFα have been spectacularly successful, the targeted depletion of tissue-resident cells using cell depletion strategies opens an alternative and exciting new avenue for the treatment of chronic inflammatory diseases.
Acknowledgments
This work was supported by grants from the Medical Research Council (MRC) and Arthritis Research Campaign (arc).
Glossary
- ECM
extracellular matrix
- EMT
epithelial–mesenchymal transition
- SFb
synovial fibroblast
Footnotes
Conflict of interest
CB and MS have received grants and consultancy payments from Wyeth and UCB Celltech.
References
- Anderson G, Jenkinson EJ. Lymphostromal interactions in thymic development and function. Nat Rev Immunol. 2001;1:31–40. doi: 10.1038/35095500. [DOI] [PubMed] [Google Scholar]
- Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley CD, Amft N, Bradfield PF, Pilling D, Ross E, Arenzana-Seisdedos F, et al. Persistent induction of the chemokine receptor CXCR4 by TGF-beta 1 on synovial T cells contributes to their accumulation within the rheumatoid synovium. J Immunol. 2000;165:3423–3429. doi: 10.4049/jimmunol.165.6.3423. [DOI] [PubMed] [Google Scholar]
- Buckley CD, Pilling D, Lord JM, Akbar AN, Scheel-Toellner D, Salmon M. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 2001;22:199–204. doi: 10.1016/s1471-4906(01)01863-4. [DOI] [PubMed] [Google Scholar]
- Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci USA. 2002;99:12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, Montgomery K, et al. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2004;2:E7. doi: 10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugina V, Alexandrova A, Chaponnier C, Vasiliev J, Gabbiani G. Rat fibroblasts cultured from various organs exhibit differences in alpha-smooth muscle actin expression, cytoskeletal pattern, and adhesive structure organization. Exp Cell Res. 1998;238:481–490. doi: 10.1006/excr.1997.3868. [DOI] [PubMed] [Google Scholar]
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Edwards JC. Fibroblast biology. Development and differentiation of synovial fibroblasts in arthritis. Arthritis Res. 2000;2:344–347. doi: 10.1186/ar110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender HG. Histomorphological basis of articular cartilage destruction in rheumatoid arthritis. Coll Relat Res. 1983;3:141–155. doi: 10.1016/s0174-173x(83)80040-5. [DOI] [PubMed] [Google Scholar]
- Firestein GS. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors. Arthritis Rheum. 1996;39:1781–1790. doi: 10.1002/art.1780391103. [DOI] [PubMed] [Google Scholar]
- Fries KM, Blieden T, Looney RJ, Sempowski GD, Silvera MR, Willis RA, et al. Evidence of fibroblast heterogeneity and the role of fibroblast subpopulations in fibrosis. Clin Immunol Immunopathol. 1994;72:283–292. doi: 10.1006/clin.1994.1144. [DOI] [PubMed] [Google Scholar]
- Garrett DM, Conrad GW. Fibroblast-like cells from embryonic chick cornea, heart, and skin are antigenically distinct. Dev Biol. 1979;70:50–70. doi: 10.1016/0012-1606(79)90006-x. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Neilson EG. Epithelial–mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- Kuperwasser C, Chavarria T, Wu M, Magrane G, Gray JW, Carey L, et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci USA. 2004;101:4966–4971. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane PJ, Gaspal FM, Kim MY. Two sides of a cellular coin: CD4(+)CD3− cells regulate memory responses and lymph-node organization. Nat Rev Immunol. 2005;5:655–660. doi: 10.1038/nri1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthys P, Hatse S, Vermeire K, Wuyts A, Bridger G, Henson GW, et al. AMD3100, a potent and specific antagonist of the stromal cell-derived factor-1 chemokine receptor CXCR4, inhibits autoimmune joint inflammation in IFN-gamma receptor-deficient mice. J Immunol. 2001;167:4686–4692. doi: 10.4049/jimmunol.167.8.4686. [DOI] [PubMed] [Google Scholar]
- Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3:292–303. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
- Michieli P, Mazzone M, Basilico C, Cavassa S, Sottile A, Naldini L, et al. Targeting the tumor and its microenvironment by a dual-function decoy Met receptor. Cancer Cell. 2004;6:61–73. doi: 10.1016/j.ccr.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Mueller MM, Fusenig NE. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- Muller-Ladner U, Kriegsmann J, Franklin BN, Matsumoto S, Geiler T, Gay RE, et al. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am J Pathol. 1996;149:1607–1615. [PMC free article] [PubMed] [Google Scholar]
- Orimo A, Gupta PB, Sgroi DC, Renzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Pablos JL, Santiago B, Galindo M, Torres C, Brehmer MT, Blanco FJ, et al. Synoviocyte-derived CXCL12 is displayed on endothelium and induces angiogenesis in rheumatoid arthritis. J Immunol. 2003;170:2147–2152. doi: 10.4049/jimmunol.170.4.2147. [DOI] [PubMed] [Google Scholar]
- Pap T, Muller-Ladner U, Gay RE, Gay S. Fibroblast biology. Role of synovial fibroblasts in the pathogenesis of rheumatoid arthritis. Arthritis Res. 2000;2:361–367. doi: 10.1186/ar113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrinello S, Coppe JP, Krtolica A, Campisi J. Stromal–epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci. 2005;118:485–496. doi: 10.1242/jcs.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsonage G, Falciani F, Burman A, Filer A, Ross E, Bofill M, et al. Global gene expression profiles in fibroblasts from synovial, skin and lymphoid tissue reveals distinct cytokine and chemokine expression patterns. Thromb Haemost. 2003;90:688–697. doi: 10.1160/TH03-04-0208. [DOI] [PubMed] [Google Scholar]
- Parsonage G, Filer AD, Haworth O, Nash GB, Rainger GE, Salmon M, et al. A stromal address code defined by fibroblasts. Trends Immunol. 2005;26:150–156. doi: 10.1016/j.it.2004.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling D, Akbar AN, Girdlestone J, Orteu CH, Borthwick NJ, Amft N, et al. Interferon-beta mediates stromal cell rescue of T cells from apoptosis. Eur J Immunol. 1999;29:1041–1050. doi: 10.1002/(SICI)1521-4141(199903)29:03<1041::AID-IMMU1041>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Postlethwaite AE, Shigemitsu H, Kanangat S. Cellular origins of fibroblasts: possible implications for organ fibrosis in systemic sclerosis. Curr Opin Rheumatol. 2004;16:733–738. doi: 10.1097/01.bor.0000139310.77347.9c. [DOI] [PubMed] [Google Scholar]
- Rinn JL, Bondre C, Gladstone HB, Brown PO, Chang HY. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2006;2:e119. doi: 10.1371/journal.pgen.0020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchlin C. Fibroblast biology. Effector signals released by the synovial fibroblast in arthritis. Arthritis Res. 2000;2:356–360. doi: 10.1186/ar112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev. 1996;76:69–125. doi: 10.1152/physrev.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- Salmon M, Scheel-Toellner D, Huissoon AP, Pilling D, Shamsadeen N, Hyde H, et al. Inhibition of T cell apoptosis in the rheumatoid synovium. J Clin Invest. 1997;99:439–446. doi: 10.1172/JCI119178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedin P, Elias A. Multistep tumorigenesis and the microenvironment. Breast Cancer Res. 2004;6:93–101. doi: 10.1186/bcr772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor SL, Schor AM. Clonal heterogeneity in fibroblast phenotype: implications for the control of epithelial–mesenchymal interactions. Bioessays. 1987;7:200–204. doi: 10.1002/bies.950070503. [DOI] [PubMed] [Google Scholar]
- Skobe M, Rockwell P, Goldstein N, Vosseler S, Fusenig NE. Halting angiogenesis suppresses carcinoma cell invasion. Nat Med. 1997;3:1222–1227. doi: 10.1038/nm1197-1222. [DOI] [PubMed] [Google Scholar]
- Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997;151:317–322. [PMC free article] [PubMed] [Google Scholar]
- Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson M, Maroof A, Ato M, Kaye PM. Stromal cells direct local differentiation of regulatory dendritic cells. Immunity. 2004;21:805–816. doi: 10.1016/j.immuni.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Tamamura H, Fujisawa M, Hiramatsu K, Mizumoto M, Nakashima H, Yamamoto N, et al. Identification of a CXCR4 antagonist, a T140 analog, as an anti-rheumatoid arthritis agent. FEBS Lett. 2004;569:99–104. doi: 10.1016/j.febslet.2004.05.056. [DOI] [PubMed] [Google Scholar]
- Tarin D, Croft CB. Ultrastructural features of wound healing in mouse skin. J Anat. 1969;105:189–190. [PubMed] [Google Scholar]
- Yang YA, Dukhanina O, Tang B, Mamura M, Letterio JJ, MacGregor J, et al. Lifetime exposure to a soluble TGF-beta antagonist protects mice against metastasis without adverse side effects. J Clin Invest. 2002;109:1607–1615. doi: 10.1172/JCI15333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg M, Bonner G, Maeshima Y, Colorado P, Muller GA, Strutz F, et al. Renal fibrosis: collagen composition and assembly regulates epithelial–mesenchymal transdifferentiation. Am J Pathol. 2001;159:1313–1321. doi: 10.1016/S0002-9440(10)62518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- Zhang M, Tang H, Guo Z, An H, Zhu X, Song W, et al. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat Immunol. 2004;5:1124–1133. doi: 10.1038/ni1130. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cao HJ, Graf B, Meekins H, Smith TJ, Phipps RP. CD40 engagement up-regulates cyclooxygenase-2 expression and prostaglandin E2 production in human lung fibroblasts. J Immunol. 1998;160:1053–1057. [PubMed] [Google Scholar]